Abstract
Background:
The genomic/cytokine “storm” after severe trauma is well described. However, the differing composition, magnitude and resolution of this response, and its relationship to clinical outcomes remains unclear.
Methods:
This is a secondary analysis of a prospective longitudinal cohort study of severely injured trauma patients in hemorrhagic shock. Peripheral blood sampling was performed at 0.5, 1, 4, 7, 14 and 28 days after injury for measurement of circulating immune biomarkers. K-means clustering utilizing overall mean and trajectory slope of selected immunologic biomarkers were used to identify distinct temporal immunologic endotypes. Endotypes were compared to known clinical trajectories defined as early death [<14 days], chronic critical illness (CCI) [≥14 days ICU LOS + persistent organ dysfunction] and rapid recovery (RAP) [ICU LOS <14 days + organ recovery].
Results:
The cohort included 102 subjects enrolled across two Level 1 trauma centers. We identified 3 distinct immunologic endotypes (iA, iB, iC), each with unique associations to clinical trajectory. Endotype iA (n=47) exhibited a moderate initial proinflammatory response followed by a return to immunologic homeostasis, with a primary clinical trajectory of RAP (n=44, 93.6%). Endotype iB (n=44) exhibited an early hyperinflammatory response with persistent inflammation and immunosuppression, with the highest incidence of CCI (n=10, 22.7%). Endotype iC (n=11) exhibited a similar hyperinflammatory response, but with rapid return to immunologic homeostasis and a predominant trajectory of RAP (n=9, 81.8%). Patients with endotype iB had the highest severity/duration of organ dysfunction, highest incidence of nosocomial infections (50%, p=0.001) and was the predominant endotype of patients that developed CCI (10/13 CCI, 76.9%; p=0.002).
Conclusion:
We identified three distinct immunologic endotypes after severe injury differing the magnitude and duration of the early response. The clinical trajectory of chronic critical illness (CCI) is characterized by an endotype (iB) defined by persistent alteration in inflammation/immunosuppression, and is associated with poor clinical outcomes.
Level of evidence:
III, prognostic
Keywords: Trauma, shock, inflammation, endotypes
BACKGROUND
Advances in trauma resuscitation and critical care have led to a progressive decrease in mortality for severely injured patients. (1, 2) Among patients with initially survivable injury, the severity and duration of multiple organ failure is one of the strongest determinants of outcome. (3, 4) While early mortality from refractory multiple organ failure continues to decline, an increasing number of patients now survive to a state of chronic critical illness (CCI), with prolonged hospitalization, sustainable but persistent organ dysfunction, disposition to long-term care facilities, and poor long-term outcomes. (5, 6)
Severe injury and hemorrhagic shock trigger a “genomic storm” within the host innate immune system with greater than 80% of the circulating leukocyte transcriptome acutely changing. (7) Acute hyperinflammation, quantifiable by pro-inflammatory mediators, is presumed to be the primary driver of persistent organ dysfunction. (8, 9) Recently, we validated a 63-gene genomic metric that when measured within 24 hours of blunt traumatic injury quantifies the magnitude of this early transcriptional dysregulation and has strong predictive value for persistent organ dysfunction. (10, 11) However, it is strongly suspected that the subsequent trajectory of this genomic response (and whether or not there is return to immunologic homeostasis) may play an even more important role in predicting long-term patient outcomes.
There is significant heterogeneity among the early innate immune response to pro-inflammatory insults, such as severe trauma and sepsis. Traditionally, clinical signs and symptoms (phenotype) drive clinical management of these patients. However, clinical phenotypes do not necessarily relate or provide insight into the underlying pathophysiologic mechanisms actually driving the disease process. (12) Thus, there is increasing interest in better defining mechanistic subtypes of disease (endotype) within individuals in order to facilitate a more precise and effective approach to therapy. (13, 14) In this study, we sought to characterize distinct immunologic endotypes based on circulating biomarkers among severely injured patients in hemorrhagic shock. We hypothesized that distinct endotypes that are defined by the magnitude and trajectory of immune biomarkers would offer insight into the heterogeneity of the innate immune response to injury. We also hypothesized these endotypes would be uniquely related to clinical trajectory and outcomes.
METHODS
Study design, inclusion/exclusion criteria and enrollment
This is a secondary analysis of a prospective, observational study conducted at two Level-1 trauma centers (UF Health Shands Hospital, Gainesville, Florida; and Harborview Medical Center, Seattle, Washington). The primary aim of this study was to validate a genomic metric predictive of complicated outcomes among severely injured patients in hemorrhagic shock. (11) The Institutional Review Board (IRB) of each institution granted approval prior to study initiation. Informed consent was obtained from the patient and/or legal proxy within 96 hours of initial enrollment and sampling under an established precedent of delayed consent. (7, 10, 11) Expanded study design and methods description for the parent cohort study can be found within the supplemental digital content (SDC 1).(11) Inclusion and exclusion criteria were similar to those for the Inflammation and Host Response to Injury (“Trauma Glue Grant”) program, which provided the ‘discovery’ cohort for the genomic metric. (7, 10, 11) Inclusion criteria consisted of patients aged 18 years or older with severe blunt traumatic injury, and in hemorrhagic shock. Hemorrhagic shock was defined as systolic blood pressure less than 90 mm Hg or base deficit of greater than or equal to 6 meq/L within sixty minutes of emergency department arrival. Patients deemed likely to die within 48 hours (i.e., refractory shock) were excluded. Additionally, patients with severe traumatic brain injury (TBI; best Glasgow Coma Scale <8, and abnormal head computed tomography) or complete spinal cord injury were excluded to prevent prolonged ventilation due primarily to poor neurologic status from confounding organ dysfunction outcomes. (3, 7, 10, 11) This study is currently registered with clinicaltrials.gov (NCT01810328).
Biomarker analyses
A set of a priori immune biomarkers were selected prior to initiation of the study. As mentioned previously, the primary objective of the study was to validate within 24 hours of injury, the ability of the peripheral blood genomic metric to predict complicated in-hospital trajectories after severe trauma. (11) Secondary objectives (i.e., this analysis) included serial biomarker sampling to characterize the heterogeneity in onset, severity and resolution of the initial host immune response to injury. A broad range of immune biomarkers was selected to assess the magnitude and trajectory of hyper-inflammation and immunosuppression over time. These included interleukin (IL)-6, IL-8, IL-10, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1), interferon-γ-inducible protein 10 (IP-10 [CXCL10]), interleukin-17 alpha (IL-17A) and soluble programmed death ligand 1 (sPD-L1). Peripheral blood samples were collected 0.5, 1, 4, 7, and 14 days after injury, while hospitalized. Biomarker measurements, with the exception of sPD-L1, were performed on plasma samples utilizing the MILLIPLEX® Multiplex (Merck KGaA, Darmstadt, Germany) and Luminex MAGPIX® (Luminex corp., Austin, Texas, U.S.A.) systems. sPD-L1 was measured by enzyme-linked immunoassay (R&D Systems, Minneapolis, MN).
Definition of outcomes
The primary goal was to identify distinct immune endotypes among severely injured trauma patients using unsupervised clustering analysis of the aforementioned biomarkers Once defined, we sought to determine the association of these endotypes with specific baseline clinical characteristics, trajectories and outcomes. Baseline clinical characteristics of interest included age, sex, comorbidities, mechanism and severity of injury, blood product transfusion, and shock severity. Outcomes of interest included the incidence and severity of organ dysfunction, organ dysfunction time to recovery (TTR) (10), intensive care unit (ICU) and hospital length of stay (LOS), infectious complications, and discharge disposition. Overall clinical trajectory was defined as either early death (within 14 days of injury), rapid recovery (RAP; ICU LOS <14 days with organ function recovery), or development of chronic critical illness (CCI; ICU LOS ≥14 days with persistent organ dysfunction). (5)
Statistical/Cluster Analysis
Baseline characteristics and outcomes data are presented as frequency and percentage for categorical variables, mean and standard deviations for normally distributed variables, and median and quartiles for non-normally distributed variables. Group comparisons were performed using either t-test or Kruskal–Wallis test as appropriate. To delineate distinct immunologic endotypes after injury, we performed unsupervised K-means clustering utilizing both overall mean level and temporal trajectory of the log-transformed measured immune biomarker measurements over time. First, a linear regression model was fitted for each biomarker and each patient across all five time points. We then calculated the overall mean and slope of the regression results to represent the temporal mean and trajectory slope of the individual biomarker responses for each individual patient. Additionally, we performed Z-score standardization of the temporal mean and slope values before final clustering analysis. Post-hoc analysis with Elbow method, Gap statistic, and prediction strength statistic were used to determine the optimal number of clusters. (15) We utilized principal component analysis to visually represent the underlying data of the clustering results. All statistical analyses were performed with SAS (v.9.4, Cary, North Carolina, USA) and R (v.3.5.1, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Cohort demographics & outcomes
The parent cohort consisted of 135 severely injured patients enrolled across the two centers over a 3-year period (Oct. 2013 to Aug. 2016). Of these, 102 patients had adequate sample and/or biomarker result availability for cluster analysis and were included in the endotyping cohort. Overall, this cohort consisted of severe blunt trauma patients with multi-system injuries that were predominantly male, of middle age, and with physiologic signs of hemorrhagic shock (Table 1). The primary mechanism of injury was deceleration injury resulting from predominantly motor vehicle collision or a fall from height (Table 1). Overall, injury severity was high as measured by ISS, with clear evidence of hemorrhagic shock as measured by blood product transfusion totals, pre-hospital and emergency department (ED) hypotension, and high peak lactate levels (Table 1).
Table 1.
Baseline patient, injury and shock characteristics.
| Characteristics | Overall (n=102) |
endotype iA (n=47, 46.1%) |
endotype iB (n=44, 43.1%) |
endotype iC (n=11, 10.8%) |
p† |
|---|---|---|---|---|---|
| A. Baseline patient characteritiscs | |||||
| Age, median (25th, 75th) | 46.5 (26, 56) | 44 (26, 55) | 48.5 (29, 57.5) | 43 (25, 54) | 0.48 |
| Male sex (n, %) | 67 (65.69) | 32 (68.09) | 29 (65.91) | 6 (54.55) | 0.73 |
| Race (n, %) | 0.82 | ||||
| White | 91 (89.22) | 40 (85.11) | 40 (90.91) | 11 (100) | |
| African American | 6 (5.88) | 3 (6.38) | 3 (6.82) | 0 (0) | |
| American Indian | 1 (0.98) | 0 (0) | 1 (2.27) | 0 (0) | |
| Pacific Islander | 1 (0.98) | 1 (2.13) | 0 (0) | 0 (0) | |
| Asian | 2 (1.96) | 2 (4.26) | 0 (0) | 0 (0) | |
| Unknown | 1 (0.98) | 1 (2.13) | 0 (0) | 0 (0) | |
| Hispanic Origin (n, %) | 6 (5.88) | 3 (6.38) | 2 (4.55) | 1 (9.09) | 0.86 |
| BMI, median (25th, 75th) | 27 (24, 30) | 27 (24, 34) | 26 (24, 30) | 27 (24, 30) | 0.61 |
| Number of comorbidities (n, %) | 0.94 | ||||
| 0 | 39 (38.2) | 16 (34.0) | 19 (43.2) | 4 (36.4) | |
| 1 | 35 (34.3) | 17 (36.2) | 14 (31.9) | 4 (36.4) | |
| ≥2 | 28 (27.5) | 14 (29.8) | 11 (25) | 3 (27.3) | |
| B. Injury characteristics | |||||
| Injury mechanism (n, %) | 0.24 | ||||
| Fall | 9 (8.8) | 3 (6.4) | 4 (9.1) | 2 (18.2) | |
| Motor vehicle collision | 86 (84.3) | 42 (89.4) | 37 (84.1) | 7 (63.6) | |
| Other | 7 (6.9) | 2 (4.3) | 3 (6.8) | 2 (18.2) | |
| ISS, median (25th, 75th) | 30 (24, 41) | 27 (22, 41) | 35 (29, 43) | 24 (17, 34) | 0.001 |
| AIS Head | 3 (2, 4) | 3 (2, 3) | 3 (2, 4) | 3.5 (2, 4) | 0.69 |
| AIS Face | 2 (2, 2) | 2 (1, 3) | 2 (2, 2) | 2 (2, 2) | 0.9 |
| AIS Neck | 3 (2.5, 3) | 2.5 (2, 3) | 3 (3, 3) | 3 (3, 3) | 0.33 |
| AIS Thorax | 3 (3, 4) | 3 (3, 4) | 3 (3, 4) | 3 (2.5, 3.5) | 0.093 |
| AIS Abdomen | 3 (2, 4) | 3 (3, 4) | 3 (2, 4) | 3 (3, 4) | 0.75 |
| AIS Spine | 2 (2, 2) | 2 (2, 2) | 2 (2, 2) | 3 (2, 3) | 0.045 |
| AIS Upper Extremity | 2 (2, 2) | 2 (2, 2) | 2 (2, 3) | 2 (2, 2) | 0.46 |
| AIS Lower Extremity | 3 (3, 5) | 3 (3, 5) | 3 (3, 5) | 3 (2.5, 3) | 0.22 |
| C. Shock severity | |||||
| Lowest pre-hospital SBP, median (25th, 75th) | 88 (72, 104) | 99.5 (80, 115) | 80 (72, 93) | 70 (57, 107) | 0.007 |
| Initial ED SBP (mm Hg), median (25th, 75th) | 118 (97, 131) | 121 (104, 137) | 113 (96, 131) | 122 (97, 137) | 0.37 |
| Lowest ED SBP (mmHg), median (25th, 75th) | 85.5 (68, 101) | 89 (74, 105) | 81.5 (64, 96.5) | 88 (61, 105) | 0.42 |
| Initial ED lactate, median (25th, 75th) | 3.8 (2.6, 5.5) | 3.1 (2.5, 4.5) | 4.9 (3.1, 6.2) | 4.6 (2.4, 6.5) | 0.019 |
| Initial ED base deficit, median (25th, 75th) | −6.8 (−9, −3.4) | −4.4 (−7, 1.3) | −8.4 (−12.3, −5.9) | −6.6 (−8.5, −4.4) | <0.001 |
| Max. lactate 24 hrs, median (25th, 75th) | 4.6 (3.3, 6.3) | 4 (3.1, 4.8) | 5.4 (3.8, 8.2) | 4.9 (3.1, 6.6) | 0.005 |
| Max. base deficit 24 hrs, median (25th, 75th) | −7.3 (−11.5, −4) | −6.0 (−8.5, 0.4) | −10.3 (−14.5, −6.7) | −7.4 (−13.3, −5.3) | <0.001 |
| D. Resuscitation requirements | |||||
| PRBC (units) 24 hrs , median (25th, 75th) | 3.8 (0.9, 7.3) | 2.0 (0, 4) | 8.2 (4.1, 13.9) | 4.3 (0, 4.7) | <0.001 |
| FFP (units) 24 hrs, median (25th, 75th) | 1.4 (0, 3.44) | 0 (0, 1.29) | 4 (1.45, 7.31) | 0 (0, 1.57) | <0.001 |
| Crystalloid (L) 24 hrs, median (25th, 75th) | 8.6 (6.6, 12.5) | 6.8 (5.3, 8.7) | 10.7 (8.7, 14.4) | 8.2 (6.9, 13.5) | <0.001 |
| E. Operative interventions | |||||
| Operative procedures, median (25th, 75th) | 2 (1, 4) | 2 (1, 3) | 3 (2, 5) | 1 (1, 2) | 0.001 |
3-group endotype comparison.
BMI, body mass index; ISS, injury severity score; AIS, abbreviated injury score; ED, emergency department; SBP, systolic blood pressure; hrs, hours; PRBC, packed red blood cells; FFP, fresh frozen plasma.
The overall cohort showed significant signs of organ dysfunction, with nearly ninety percent of the cohort required mechanical ventilation and approximately twelve percent meeting criteria for multiple organ failure by Denver MOF criteria (Table 2). Average time to organ dysfunction recovery (TTR) for the overall cohort was approximately eight days, with average ICU and hospital length of stays of 7 and 18 days respectively (Table 2). The majority of patients exhibited a clinical trajectory of rapid recovery (RAP), while approximately 13 percent developed CCI (Table 2). Not unexpectedly given the study criteria excluding patients expected to die within the first 48 hours, overall mortality was low with less than 3 percent of patients suffering an early death (<14 days), and less than 5 percent mortality at 28 days (Table 2).
Table 2.
Clinical outcomes by endotype.
| Outcomes | Overall (n=102) |
endotype iA (n=47, 46.08%) |
endotype iB (n=44, 43.14%) |
endotype iC (n=11, 10.78%) |
p † |
|---|---|---|---|---|---|
| A. Organ failure | |||||
| Mechanically ventilated (n, %) | 89 (87.3) | 37 (78.7) | 42 (95.5) | 10 (90.9) | 0.053 |
| Ventilator-free days (28-day), median (25th, 75th) | 25 (19, 27) | 26 (23, 27) | 21 (15, 25) | 27 (25, 27) | <0.001 |
| Max. modified Marshall MOF score , median (25th, 75th) | 2 (0, 3) | 1 (0, 3) | 3 (2, 3.5) | 0 (0, 2) | <0.001 |
| Max. Denver MOF score , median (25th, 75th) | 0.5 (0, 2) | 0 (0, 2) | 2 (0, 2) | 0 (0, 0) | <0.001 |
| MOF (n, %) | 12 (11.8) | 2 (4.3) | 10 (22.8) | 0 (0) | 0.017 |
| Time to recovery1 (days), median (25th, 75th) | 7.5 (4, 25) | 6 (3, 12) | 11 (7, 29) | 4 (2, 29) | 0.001 |
| Cardiovascular recovery | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 1) | 0.26 |
| Hematologic recovery | 3 (1, 6) | 2 (1, 5) | 5 (3, 7) | 1 (1, 2) | <0.001 |
| Hepatic recovery | 1 (1, 2) | 1 (1, 1) | 1 (1, 2) | 1 (1, 1) | 0.17 |
| Renal recovery | 1 (1, 2) | 1 (1, 1) | 1 (1, 3.5) | 1 (1, 1) | 0.016 |
| Respiratory recovery | 3 (1, 8) | 2 (1, 5) | 7 (2.5, 13) | 2 (1, 3) | 0.003 |
| B. Infectious/other complications | |||||
| Nosocomial infections (n, %) | 33 (32.4) | 11 (23.4) | 22 (50) | 0 (0) | 0.001 |
| # infections per patient (n, %) | 0.01 | ||||
| 0 | 69 (67.7) | 36 (76.6) | 22 (50) | 11 (100) | |
| 1 | 23 (22.6) | 8 (17) | 15 (34) | 0 (0) | |
| ≥2 | 10 (9.8) | 3 (6.4) | 7 (15.9) | 0 (0) | |
| Infection source (n, %) | |||||
| Pneumonia | 20 (19.6) | 6 (12.8) | 14 (31.8) | 0 (0) | 0.02 |
| Pseudomembranous colitis | 11 (10.8) | 5 (10.6) | 6 (13.6) | 0 (0) | 0.6 |
| UTI | 7 (6.9) | 2 (4.3) | 5 (11.4) | 0 (0) | 0.36 |
| Blood stream infection | 2 (2) | 0 (0) | 2 (4.6) | 0 (0) | 0.39 |
| Empyema | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Other | 1 (1) | 1 (2.1) | 0 (0) | 0 (0) | 1 |
| Deep venous thrombosis | 4 (3.9) | 2 (4.3) | 2 (4.6) | 0 (0) | 1 |
| Pulmonary embolus | 5 (4.9) | 3 (6.4) | 2 (4.6) | 0 (0) | 1 |
| Cardiac arrest | 6 (5.9) | 2 (4.3) | 4 (9.1) | 0 (0) | 0.48 |
| Rhabdomyolysis | 4 (3.9) | 1 (2.1) | 3 (6.8) | 0 (0) | 0.59 |
| Other | 15 (14.7) | 5 (10.6) | 9 (20.5) | 1 (9.1) | 0.45 |
| C. Clinical trajectory/outcomes | |||||
| Clinical trajectory (n, %) | 0.005 | ||||
| Early death | 3 (2.9) | 0 (0.0) | 1 (2.3) | 2 (18.2) | |
| Chronic critical Illness | 13 (12.8) | 3 (6.4) | 10 (22.7) | 0 (0.0) | |
| Rapid recovery | 86 (84.3) | 44 (93.6) | 33 (75.0) | 9 (81.8) | |
| Hospital LOS, median (25th, 75th) | 18 (11, 24) | 17 (11, 25) | 19 (16, 27.5) | 7 (3, 13) | 0.001 |
| ICU LOS, median (25th, 75th) | 7 (4, 13) | 6 (3, 11) | 11 (7, 16) | 3 (2, 4) | <0.001 |
| Discharge disposition (n, %) | 0.049 | ||||
| “Good” Disposition | |||||
| Inpatient rehabilitation facility | 18 (17.7) | 9 (19.2) | 9 (20.5) | 0 (0) | |
| Home with services | 14 (13.7) | 7 (14.9) | 5 (13.6) | 1 (9.1) | |
| Home | 30 (29.4) | 17 (36.2) | 7 (15.9) | 6(54.6) | |
| “Poor” Disposition | |||||
| SNF | 32 (31.4) | 13 (27.7) | 17 (38.6) | 2 (18.2) | |
| LTAC/Another hospital | 3 (2.9) | 1 (2.1) | 2 (4.6) | 0 (0) | |
| Death (in-hospital) | 5 (4.9) | 0 (0) | 3 (6.8) | 2 (18.2) | |
| 28-day mortality (n, %) | 5 (4.9) | 0 (0) | 3 (6.8) | 2 (18.2) | 0.017 |
3-group endotype comparison.
Time to recovery, see definition in methods section.
MOF, multiple organ failure; UTI, urinary tract infection; ICU, intensive care unit; LOS, length of stay; SNF, skilled nursing facility; LTAC, long-term acute care facility.
Cluster results & Endotype characteristics
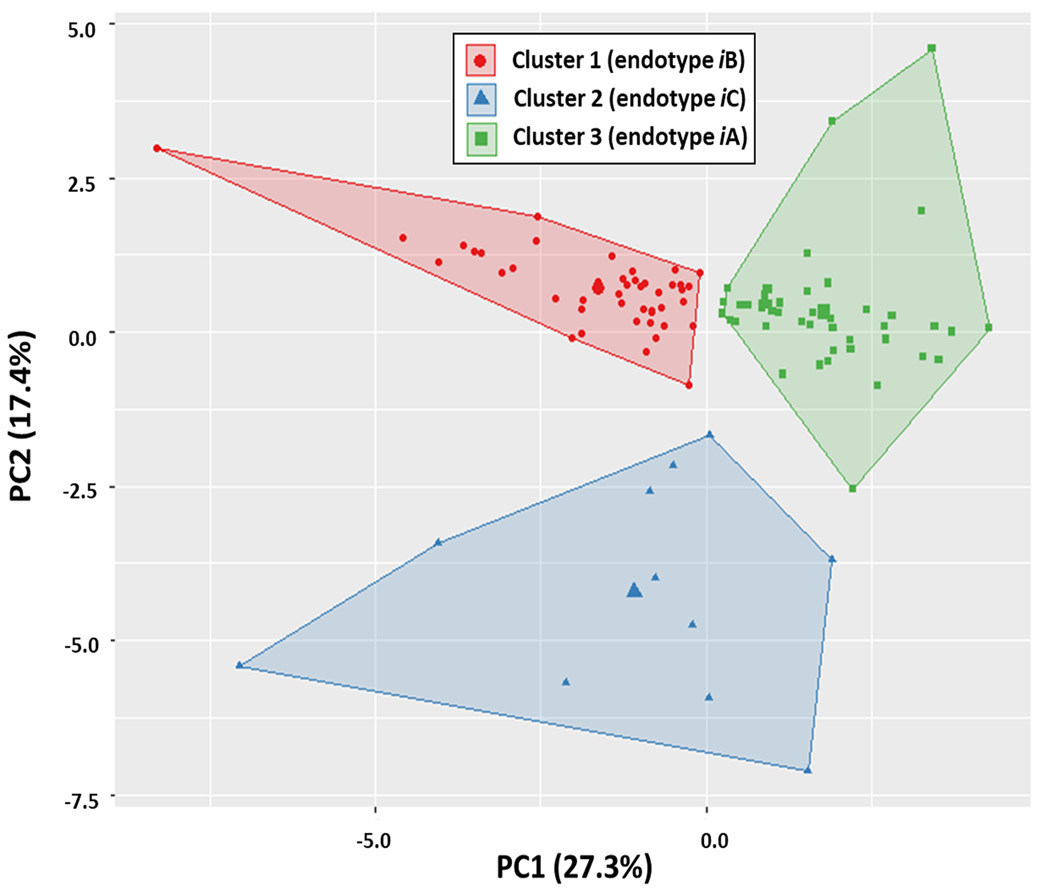
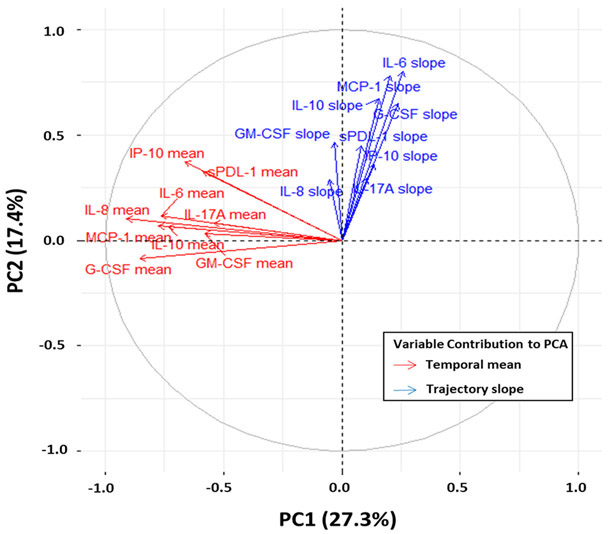
Cluster analysis of the immune biomarkers revealed an optimal number of three clusters, which we subsequently defined as immune endotypes iA, iB and iC (Figure 1, SDC 2). Contribution of each individual biomarker to the overall principal components of the three endotypes derived from the cluster analysis is illustrated in Figure 2. Distance from center offers a visualization of the quantitative contribution of each biomarker to the principal components of the endotypes, with increasing distance representing increasing variable contribution.
Figure 1. Principle component analysis of cluster endotypes.
Results of 3-group cluster analysis defining endotypes iA (n=47), iB (n=44) and iC (n=11). The first two principle components (PC1, PC2) are defined as X and Y-axes of the two-dimension space respectively. The fraction of the variance explained by a principal component to the total variance is shown for PC1 (27.3%) and PC2 (17.4%). Clustering results and endotype assignment of each data point are labeled and shown in the figure key. K-means clustering centroids are also visualized for each cluster.
Figure 2. Contribution of individual biomarkers to cluster endotypes.
Similar to Figure 1, the first two principle components (PC1, PC2) are defined as X and Y-axes of the two-dimension space respectively. The fraction of the variance explained by a principal component to the total variance is shown for PC1 (27.3%) and PC2 (17.4%). Variable contributions to PC1 and PC2 are plotted, with increasing distance representing increased magnitude of variable contribution.
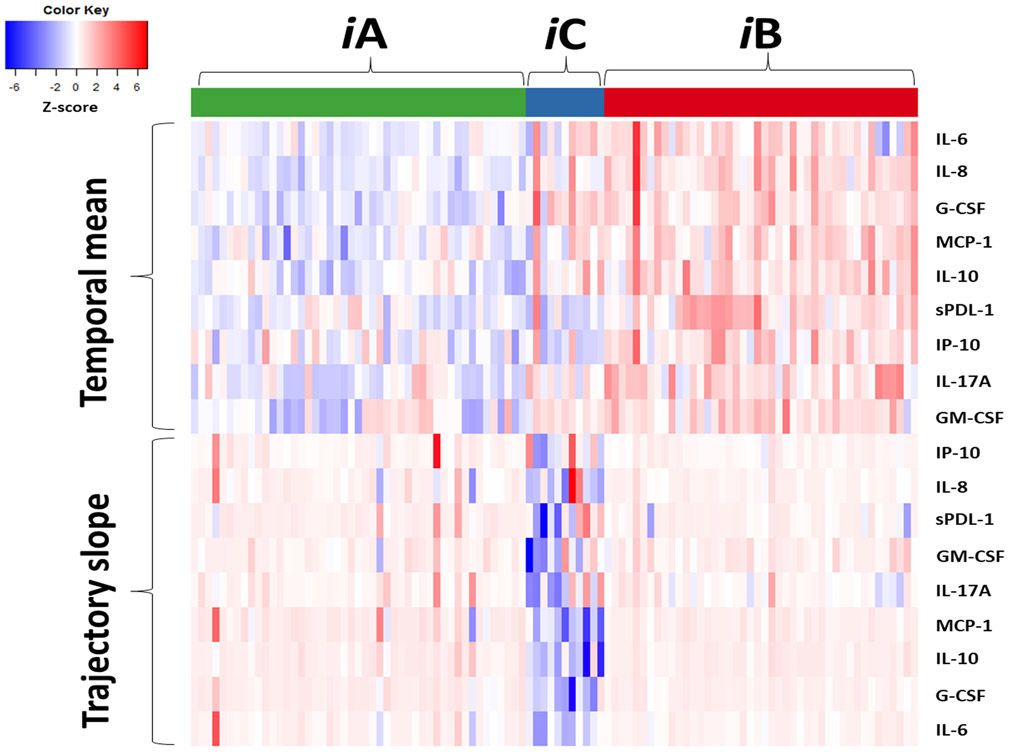
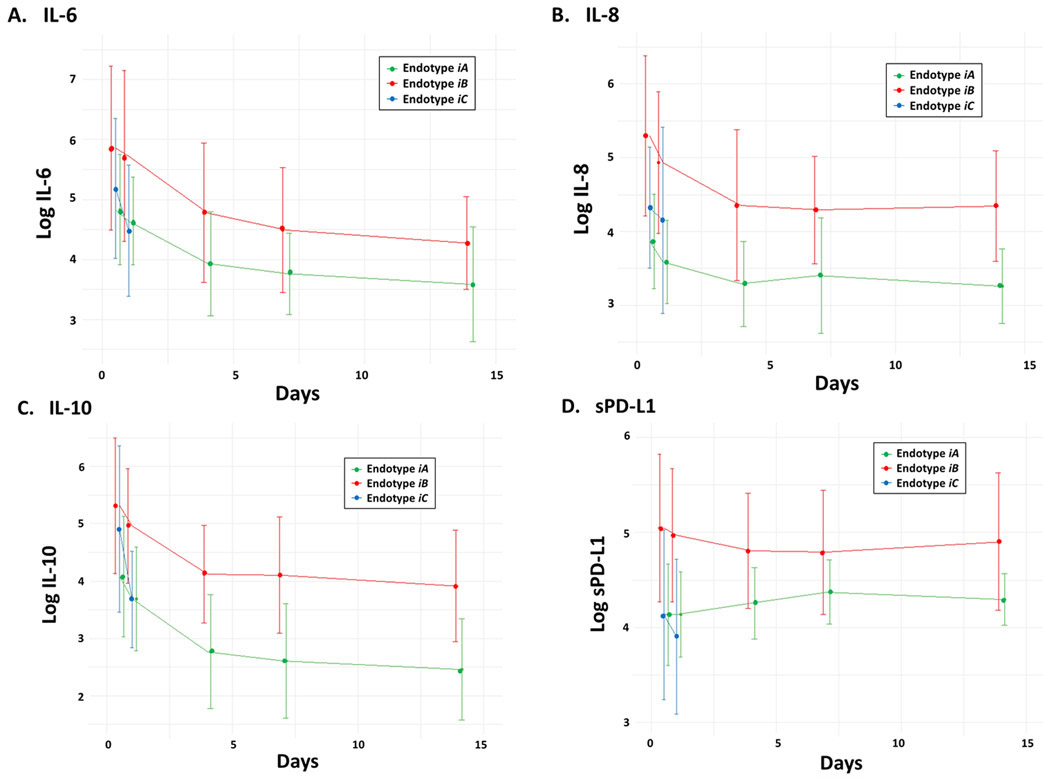
The magnitude of the temporal mean and trajectory slope of the immune biomarkers (at the individual level and grouped by endotype) is represented in the heatmap shown in Figure 3. As log-transformed biomarker values were standardized by Z-score, individual heatmap cells in the red end of the spectrum represents higher than overall cohort average mean/slope, with cells in the blue spectrum lower than cohort average. Thus, heatmap patterns reveal distinct differences between endotypes in regards to the magnitude and trajectory of the post-injury immune response. Endotype iA (n=47) exhibited a restrained initial pro-inflammatory response as measured by temporal mean, followed by a gradual return towards immunologic homeostasis. In contrast, endotype iB (n=44) exhibited an early robust hyperinflammatory response with a subsequent trajectory of persistent inflammation and immunosuppression. Endotype iC (n=11) exhibited a similar robust hyperinflammatory response similar to endotype iB, but in contrast showed a rapid return towards immunologic homeostasis (Figure 3). These patterns can also be seen in the select individual biomarkers shown in Figure 4 that are commonly used to quantify systemic inflammation and immunosuppression (IL-6, IL-8, IL-10 and sPD-L1).
Figure 3. Immune endotype biomarker heatmap.
Heatmap of biomarker temporal mean and trajectory slope, grouped by endotype (iA, iB, iC). Individual subjects are represented by column and biomarkers by row. Temporal mean and trajectory slope values are calculated based on the log-transformed biomarker measurements and then standardized as Z-scores, with white cell color representing the cohort mean, red spectrum above cohort mean and blue spectrum below cohort mean.
Figure 4. Select inflammatory and immunosuppressive biomarker trajectories by endotype.
Log-transformed circulating (A) IL-6, (B) IL-8, (C) IL-10 and (D) soluble programmed death ligand 1 (sPD-L1) levels measured from peripheral blood at 0.5, 1, 4, 7 and 14 days after injury and classified by endotype (iA, iB, iC).
Baseline injury and shock characteristics by endotype
There were no significant differences in baseline patient characteristics between endotypes including age, sex, race/ethnicity, BMI or comorbidities (Table 1A). The distribution of blunt injury source and pattern (as measured by AIS score) were also similar among endotypes (Table 1B). Uniquely, endotype iC, had higher spine injury severity, likely associated with the non-statistically significant trend of higher frequency of falls as primary injury mechanism (Table 1B).
In contrast to baseline patient characteristics and injury mechanism, there were significant differences among endotypes regarding overall injury severity, physiologic signs of shock severity and initial resuscitation requirements. Overall injury severity was highest among endotype iB, followed by endotype iC and then iA (Table 1B). Consistent with the delineated study inclusion criteria, all endotypes had documented hypotension during their time in the emergency department (Table 1C). However, endotypes iB and iC had evidence of greater degrees of hemodynamic instability in the field or during transport compared to endotype iA (Table 1C). Shock severity was significantly higher in endotypes iB and iC as compared to iA, with endotype iB having the highest lactate and base deficit levels across the first 24 hours after injury (Table 1C). Resuscitation requirements, and specifically blood product volumes, were highest in endotype iB, followed by endotypes iC and iA (Table 1C). Of note, there was a significant difference in the number of major operations between groups, with endotype iB patients undergoing the greatest, and enodtype iC the fewest number of operative procedures (Table 1E).
Organ dysfunction, complications and clinical outcomes by endotype
The three immune endotypes also exhibited unique associations with both severity and duration of organ dysfunction, as well as clinical trajectory and infectious complications. Endotype iB had the highest incidence and severity of organ dysfunction as measured by maximum Denver MOF score, and incidence of meeting Denver MOF criteria (Table 2A). Additionally, organ failure time to recovery (TTR) was significantly longest in endotype iB, in comparison to the rapid recovery in endotypes iA and iC (Table 2A).
Endotype iB had significantly higher incidence and number of infectious complications, driven primarily by a significantly higher rate of pneumonia (Table 2B). Interestingly, while having a similar early hyperinflammatory profile to iB, endotype iC had no nosocomial infections (Table 2B). There were no significant differences between endotypes among other non-infectious complications (Table 2B).
Regarding clinical trajectory, endotype iB had the highest incidence of CCI (23%) as compared to endotypes iA (6%) and iC (0%; Table 2C). Accordingly, endotype iB was the predominant endotype of patients that developed CCI (n=10/13, 77%; p=002). Overall ICU and hospital lengths of stay were longest in endotype iB, and shortest in endotype iC (Table 2C). Endotype iB also had the highest rate of discharge disposition to skilled nursing facilities, long-term acute care facilities, or another hospital (Table 2C). Interestingly, despite having the shortest TTR, inpatient mortality was highest in endotype iC (18%) with two deaths (Table 2C). The underlying cause of those two deaths were acute heart failure and blunt cerebrovascular related stroke. In comparison, the inpatient deaths within endotype iB were all attributable to progressive multiple organ failure.
DISCUSSION
Despite a wide body of knowledge obtained through decades of dedicated research, the innate host response to severe injury and hemorrhagic shock remains incompletely understood. However, the adverse impact on outcomes of an overly robust and dysfunctional immune response to injury is clear. (7, 10, 11, 16, 17) While the early post-injury “genomic storm” has been well described at the population level, the degree of heterogeneity amongst individual patients in the magnitude and trajectory of this response after injury remains unknown. The identification of distinct immunologic endotypes after severe injury could yield significantly deeper insight into the mechanisms that drive persistent organ dysfunction in many of these patients. Additionally, the failure of multiple immunotherapy trials in critical illness (e.g., sepsis) suggests to us that attempting to target individual inflammatory pathways for intervention without a thorough characterization of a given individual’s underlying mechanistic subtype is likely to be futile. (18-20) Attempts to block the early “cytokine storm” in these settings have met with repeated failure, likely due to the complexity and redundancy of the early inflammatory response. (21-23) Furthermore, immune stimulant therapies such as GM-CSF, IL-7 and immune checkpoint inhibitors (e.g., Nivolumab) remain unproven in restoring immune function among sepsis patients that survive the initial pro-inflammatory insult. (24-27) Attempting to reverse immunoparalysis, however, should have wider window of potential efficacy compared to trying to blunt the initial burst of inflammation within the first minutes to hours after injury. (28-30) Regardless, if we are to have any hope of developing effective immunotherapeutic approaches to these conditions, a precision diagnostic approach to stratify patients by immune endotype and select appropriately targeted therapies is necessary.
In this study, we have shown that there are three distinct immunologic endotypes among patients with severe blunt traumatic injury and hemorrhagic shock. Importantly, these endotypes have significant clinical meaning in that: 1) they illustrate that uniquely different mechanistic subtypes are found within a uniformly defined high-risk clinical cohort, and 2) they have unique associations to a complicated clinical course, infectious complications and overall poor outcomes. Additionally, the immunologic patterns revealed within the endotypes offer mechanistic insight into how the trajectory of immune dysfunction is a likely determinant of whether organ dysfunction resolves or persists. We have shown here that persistent inflammation and immune suppression (endotype iB) is a common endotype (>40%) among severely injured patients in hemorrhagic shock, and strongly associated with a trajectory of CCI (persistent organ dysfunction), increased infections (compromised adaptive immunity), and prolonged length of stay (high resource utilization). This is similar to what is found among surgical sepsis patients, in whom a significant subset develop a pathophysiology defined by the persistent inflammation, immunosuppression and catabolism syndrome (PICS). (31-34) We have shown previously that sepsis patients who develop the PICS are readmitted with recurrent infections, fail to rehabilitate their functional status and have high 1-year mortality. (35, 36)
Perhaps the most novel and important finding suggested by our results is that an endotype with prompt return towards immunologic homeostasis and immune recovery may be more important in determining clinical trajectory than the magnitude of the early genomic and inflammatory response. In our study, Endotype iA was characterized by a moderate initial pro-inflammatory response and a gradual but progressive return to baseline. Not surprisingly, this endotype was associated with a clinical trajectory of rapid recovery. However, endotype iC demonstrated an even more rapid return to immunologic homeostasis, even while exhibiting an early pro-inflammatory response of similar to magnitude to the “bad” endotype iB. Despite a more robust initial inflammatory response, endotype iC had lower severity and duration of organ dysfunction, lower incidence of CCI, and shorter average ICU and hospital length of stay than endotype iA. Interestingly, endotype iC had the highest inpatient mortality rate (18%), which was significantly higher even than endotype iB (7%). However, it is important to note that the two deaths in endotype iC were not directly related to acute organ dysfunction, but rather attributable to blunt cerebrovascular injury-related stroke and decompensated heart failure. All deaths within endotype iB were patients that ultimately succumbed to progressive multiple organ failure.
The results of our study also given insight into the question of whether differences in the host innate response are more closely related to inherent patient characteristics, or rather acquired factors such as injury and/or shock severity. Interestingly, there were no significant differences in baseline patient characteristics (i.e., age, sex, race, BMI, comorbidities) between all three immune endotypes. Given the known association of shock severity to the early “genomic storm”, it is not surprising that the “hyperinflammatory” immune endotypes iB and iC were associated with similar base deficit and lactate levels through 24 hours consistent with a severe shock state. However, the factors most distinguishing between these two endotypes was that iB had higher overall injury severity and blood transfusion requirements.
It is important to remember the variability in immune endotypes we identified in this study were found within a study population that is clinically homogenous by typical blunt trauma classification standards. While there is some variance in degree of severity, all patients in the cohort were severely injured patients in hemorrhagic shock. It is unlikely that a clinician in the trauma bay would identify (in real time) the rather subtle differences found in ISS and hemodynamics in a way that would affect management or prognostication of outcome within the first few days of injury. Ultimately, we are not surprised that there are these subtle differences in injury and shock severity between endotype groups. Why? It is likely that the immunologic trajectory after trauma is likely most influenced by two factors: 1) the severity of the overall injury/shock insult, likely driven by the magnitude and chronicity of endogenous “alarmin” (i.e., Damage Associated Molecular Patterns; DAMP) exposure, and 2) host factors; some of which may be easily observed clinical factors (age, comorbidity, ect.), but also biologic/genomic factors which are more difficult to identify at the level of an individual patient without insight at the molecular level. Clearly, our data supports that duration and severity of shock (best represented by total 24 hour blood product requirement) is likely the key insulting determinant of immunologic trajectory. This leads us to propose that an endotype of persistent inflammation and immunosuppression is caused by continuous subacute alarmin exposure from shock-associated organ injury, major blunt tissue injury, repetitive operative insults, recurrent infections, and is the key mechanistic driver of the development of CCI after severe trauma.
Several limitations of this study need to be acknowledged. Being similar in design to the “Trauma Glue Grant” studies, there is a clear element of survival bias in that this study cohort in that it excludes patients in refractory shock and/or expected to die within the first 48 hours after injury. This was by design, since the focus of this line of work is to understand the immune biology of severely injured patients that survive long enough to develop these different immune and clinical trajectories. However, we acknowledge that this may be omitting the identification of a separate immune endotype amongst these very early post-injury deaths. Secondly, although it was performed across two distinctly different and geographically distant trauma centers, the study population was modest in size and relatively homogenous regarding patient population demographics. It will therefore be both critical and of significant scientific value to replicate these findings across a larger, more diverse, multi-center population. Finally, similar to other studies (and despite the widespread adoption of standardized ratio-based massive transfusion protocols), it is also difficult to distinguish whether there is a unique and independent effect of blood transfusion in regards to post-injury immune endotype. A common argument is that transfusion volumes may merely represent the best composite variable for overall injury and shock severity.(3, 5, 37) This remains a challenging confounder to address.
In conclusion, we have identified three distinct immune endotypes among severely injured blunt patients in hemorrhagic shock. Importantly, these endotypes illustrate that there is significant heterogeneity of the innate immune response within a clinically defined cohort known to be at high-risk of multiple organ dysfunction and complicated outcomes. It is our belief that determining the individual endotype of severely injured patients is a key requirement for both a deeper understanding of the mechanisms driving persistent organ dysfunction, and for the future successful application of immunotherapies for this challenging population.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Jennifer Lanz, Laura Hennessy, Ruth Davis, Jillianne Brakenridge, Ashley McCray and Eduard Navarro for their invaluable contribution to the successful execution of this study.
FUNDING & CONFLICT OF INTEREST DISCLOSURES
Supported, in part by National Institute of General medical Sciences (NIGMS) grants: R01 GM040586 and R01 GM104481 (LM), R01 GM113945 (PE), P50 GM111152 (BB, PE, LM, FM, SB), and by postgraduate training grant T32 GM008721 (MC, RH, DD) in burns, trauma, and perioperative injury awarded by NIGMS. Further support in part by National Institute on Aging grant R03 AG056444 (SB). All authors report no conflict of interest.
Footnotes
This manuscript was presented at the 79th Annual Meeting of the American Association for the Surgery of Trauma and Clinical Congress of Acute Care surgery, Sept. 8-18, 2020 (Virtual Meeting).
REFERENCES
- 1.DiMaggio C, Ayoung-Chee P, Shinseki M, Wilson C, Marshall G, Lee DC, Wall S, Maulana S, Leon Pachter H, Frangos S. Traumatic injury in the United States: In-patient epidemiology 2000-2011. Injury. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, Bankey PE, Nathens AB, Cuenca AG, Efron PA, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012;255(5):993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140(5):432–8; discussion 8-40. [DOI] [PubMed] [Google Scholar]
- 5.Mira JC, Cuschieri J, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Loftus TJ, Stortz JA, Raymond SL, Lanz JD, Hennessy LV, et al. The Epidemiology of Chronic Critical Illness After Severe Traumatic Injury at Two-Level One Trauma Centers. Crit Care Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson GH, Hamlat CA, Rivara FP, Koepsell TD, Jurkovich GJ, Arbabi S. Long-term survival of adult trauma patients. JAMA. 2011;305(10):1001–7. [DOI] [PubMed] [Google Scholar]
- 7.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, O'Keefe GE, Cohen MJ, Moldawer LL, Tompkins RG, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med. 2012;40(4):1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuschieri J, Bulger E, Schaeffer V, Sakr S, Nathens AB, Hennessy L, Minei J, Moore EE, O'Keefe G, Sperry J, et al. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 2010;34(4):346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuenca AG, Gentile LF, Lopez MC, Ungaro R, Liu HZ, Xiao WZ, Seok J, Mindrinos MN, Ang D, Baslanti TO, et al. Development of a Genomic Metric That Can Be Rapidly Used to Predict Clinical Outcome in Severely Injured Trauma Patients. Crit Care Med. 2013;41(5):1175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raymond SL, Hawkins RB, Wang Z, Mira JC, Stortz JA, Han F, Lanz JD, Hennessy LV, Brumback BA, Baker HV, et al. Prospective Validation of a Transcriptomic Metric in Severe Trauma. Ann Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Invest. 2019;129(4):1493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scicluna BP, van Vught LA, Zwinderman AH, Wiewel MA, Davenport EE, Burnham KL, Nürnberg P, Schultz MJ, Horn J, Cremer OL, et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir Med. 2017;5(10):816–26. [DOI] [PubMed] [Google Scholar]
- 14.Antcliffe DB, Burnham KL, Al-Beidh F, Santhakumaran S, Brett SJ, Hinds CJ, Ashby D, Knight JC, Gordon AC. Transcriptomic Signatures in Sepsis and a Differential Response to Steroids. From the VANISH Randomized Trial. Am J Respir Crit Care Med. 2019;199(8):980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan M, Ye K. Determining the number of clusters using the weighted gap statistic. Biometrics. 2007;63(4):1031–7. [DOI] [PubMed] [Google Scholar]
- 16.Tompkins RG. Genomics of injury: The Glue Grant experience. J Trauma Acute Care Surg. 2015;78(4):671–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher CJ Jr., Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271(23):1836–43. [PubMed] [Google Scholar]
- 19.Opal SM, Fisher CJ Jr., Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25(7):1115–24. [DOI] [PubMed] [Google Scholar]
- 20.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–64. [DOI] [PubMed] [Google Scholar]
- 21.Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273(12):934–41. [PubMed] [Google Scholar]
- 22.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309(11):1154–62. [DOI] [PubMed] [Google Scholar]
- 23.Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, Demeyer I, Sainati S, Amlot N, Cao C, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38(8):1685–94. [DOI] [PubMed] [Google Scholar]
- 24.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180(7):640–8. [DOI] [PubMed] [Google Scholar]
- 25.Laterre PF, François B, Collienne C, Hantson P, Jeannet R, Remy KE, Hotchkiss RS. Association of Interleukin 7 Immunotherapy With Lymphocyte Counts Among Patients With Severe Coronavirus Disease 2019 (COVID-19). JAMA Netw Open. 2020;3(7):e2016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmelé T, Blood T, Morre M, et al. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, Martin GS, Coopersmith CM, Brakenridge S, Mayr FB, et al. Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559). Crit Care Med. 2019;47(5):632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remy KE, Brakenridge SC, Francois B, Daix T, Deutschman CS, Monneret G, Jeannet R, Laterre PF, Hotchkiss RS, Moldawer LL. Immunotherapies for COVID-19: lessons learned from sepsis. Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Opal S. Immunotherapy for sepsis--a new approach against an ancient foe. N Engl J Med. 2010;363(1):87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371(4):380–3. [DOI] [PubMed] [Google Scholar]
- 31.Horiguchi H, Loftus TJ, Hawkins RB, Raymond SL, Stortz JA, Hollen MK, Weiss BP, Miller ES, Bihorac A, Larson SD, et al. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy. Front Immunol. 2018;9:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit Care Med. 2017;45(2):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat Baslanti T, Wang Z, Ghita GL, Leeuwenburgh C, Segal M, Bihorac A, et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg. 2018. February;84(2):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkins RB, Raymond SL, Stortz JA, Horiguchi H, Brakenridge SC, Gardner A, Efron PA, Bihorac A, Segal M, Moore FA, et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front Immunol. 2018;9:1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brakenridge SC, Efron PA, Cox MC, Stortz JA, Hawkins RB, Ghita G, Gardner A, Mohr AM, Anton SD, Moldawer LL, et al. Current Epidemiology of Surgical Sepsis: Discordance Between Inpatient Mortality and 1-year Outcomes. Ann Surg. 2019;270(3):502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner AK, Ghita GL, Wang Z, Ozrazgat-Baslanti T, Raymond SL, Mankowski RT, Brumback BA, Efron PA, Bihorac A, Moore FA, et al. The Development of Chronic Critical Illness Determines Physical Function, Quality of Life, and Long-Term Survival Among Early Survivors of Sepsis in Surgical ICUs. Crit Care Med. 2019;47(4):566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brakenridge SC, Phelan HA, Henley SS, Golden RM, Kashner TM, Eastman AE, Sperry JL, Harbrecht BG, Moore EE, Cuschieri J, et al. Early blood product and crystalloid volume resuscitation: risk association with multiple organ dysfunction after severe blunt traumatic injury. J Trauma. 2011;71(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.