Abstract
Recent studies suggest midkine (MDK) is involved in the development and regeneration of the zebrafish retina. We investigate the expression patterns of MDK and related factors, roles in neuronal survival, and influence upon the formation of Müller glia-derived progenitor cells (MGPCs) in chick and mouse model systems. By using single-cell RNA-sequencing, we find that MDK and pleotrophin (PTN), a MDK-related cytokine, are up-regulated by Müller glia (MG) during later stages of development in chick. While PTN is down-regulated, MDK is dramatically up-regulated in mature MG after retinal damage or FGF2 and insulin treatment. By comparison, MDK and PTN are down-regulated by MG in damaged mouse retinas. In both chick and mouse retinas, exogenous MDK induces expression of cFOS and pS6 in MG. In the chick, MDK significantly decreases numbers dying neurons, reactive microglia, and proliferating MGPCs, whereas PTN has no effect. Inhibition of MDK-signaling with Na3VO4 blocks neuroprotective effects with an increase the number of dying cells and negates the pro-proliferative effects on MGPCs in damaged retinas. Inhibitors of PP2A and Pak1, which are associated with MDK-signaling through integrin β1, suppressed the formation of MGPCs in damaged chick retinas. In mice, MDK promotes a small but significant increase in proliferating MGPCs in damaged retinas and potently decreases numbers of dying cells. We conclude that MDK expression is dynamically regulated in Müller glia during embryonic maturation, following retinal injury and during reprogramming into MGPCs. MDK influences glial responses to damage, neuronal survival and the reprogramming of Müller glia into proliferating MGPCs.
Keywords: Midkine, Müller glia, Müller glia derived progenitor cells, scRNA-seq, retinal neuroprotection, Müller glia reprogramming
Introduction
Midkine (MDK) and pleiotrophin (PTN) are secreted factors that belong to a family of basic heparin-binding cytokines (Muramatsu, 2002). The C-terminal domain of MDK interacts with carbohydrate-bindings proteins which facilitate dimerization and cell signaling (Fabri et al., 1993; Iwasaki et al., 1997; Kilpeläinen et al., 2000; Tsutsui et al., 1991). Extracellular matrix proteoglycans that have a high binding-affinity for MDK include protein tyrosine phosphatase-ζ receptor-like 1 (PTPRZ1), syndecans, glypican-2, PG-M/versican, integrin α6β1, low density lipoprotein receptor-related protein (LRP), and neuroglycans (Ichihara-Tanaka et al., 2006; Kojima et al., 1996; Kurosawa et al., 2001; Maeda et al., 1999; Mitsiadis et al., 1995; Muramatsu et al., 2000; Muramatsu et al., 2004; Nakanishi et al., 1997; Zou et al., 2000). MDK forms a complex with these proteoglycans to initiate cell-signaling through receptor tyrosine kinases and activation of second messengers such as src, PI-3K, and PAK1 (Qi et al., 2001; Shen et al., 2015; Thillai et al., 2016).
During development the roles of MDK are conserved across many vertebrate species including fish, mice, and humans (Tsutsui et al., 1991). MDK has different functions including promoting cell survival and proliferation, acting directly on stem cells during normal fetal development and organogenesis (Mitsiadis et al., 1995). MDK has been implicated in the pathogenesis of more than 20 different types of cancers, resistance to chemotherapeutics, increased survival of cancerous cells with acidosis and hypoxia, and elevated levels of MDK have been correlated with poor prognoses (Dai et al., 2009; Kang et al., 2004; Mashima et al., 2009; Mirkin et al., 2005; Reynolds et al., 2004; Salama et al., 2006; Takei et al., 2001; Takei et al., 2006; Tsutsui et al., 1993). In damaged mammalian CNS, MDK expression is elevated and may support neuronal survival (Jochheim-Richter et al., 2006; Kikuchi‐Horie et al., 2004; Miyashiro et al., 1998; Obama et al., 1998; Sakakima et al., 2006). In rodent eyes, subretinal delivery of MDK protects photoreceptors from light-mediated degeneration (Unoki et al., 1994). In sum, MDK has pleotropic functions that are context-dependent.
In fish, retinal regeneration is a robust process that restores neurons and visual function following damage, whereas this process is far less robust in birds and nearly absent in mammals (Hitchcock and Raymond, 1992; Karl et al., 2008; Raymond, 1991). Müller glia (MG) have been identified as the cell-of-origin for progenitors in mature retinas (Bernardos et al., 2007; Fausett and Goldman, 2006; Fausett et al., 2008; Fischer and Reh, 2001; Ooto et al., 2004). In mammalian retina, significant stimulation, such as forced expression of Ascl1, inhibition of histone deacetylases and neuronal damage, is required to reprogram MG into progenitor-like cells (Karl et al., 2008; Pollak et al., 2013, 1; Ueki et al., 2015). In the chick retina, MG readily reprogram into progenitor-like cells that proliferate, but the progeny have a limited capacity to differentiate as neurons (Fischer and Reh, 2001; Fischer and Reh, 2003). Understanding the mechanisms that regulate the proliferation and differentiation of MGPCs is important to harnessing the regenerative potential of MG in warm-blooded vertebrates.
Recent studies in the zebrafish retina have indicated that MDK-a is up-regulated in stem cell niches and by MG during reprogramming into neurogenic progenitor cells (Calinescu et al., 2009). MDK-a is expressed by mitotic retinal progenitors at 30 hrs post-fertilization, then in differentiating MG at 72 hrs post-fertilization and expression is maintained in mature MG (Gramage et al., 2014). During reprogramming of MG into MGPCs, MDK-a regulates cell cycle progression and influences retinal development through the HLH transcription factor Id2a (Luo et al., 2012; Nagashima et al., 2019b). Although PTN is expressed by retinal progenitors during mammalian development, its expression has been correlated with cell cycle exit and differentiation of neurons and glia (Hienola et al., 2004; Jung et al., 2004; Roger et al., 2006). Nothing is known about how MDK influences the process of retinal regeneration in warm-blooded vertebrates. Accordingly, we investigated expression pattern and function of MDK and PTN on glial cells in the chick and mouse retinas in vivo.
Methods and Materials:
Animals:
The use of animals was in accordance with the guidelines established by the National Institutes of Health and IACUC at The Ohio State University. Newly hatched P0 wildtype leghorn chicks (Gallus gallus domesticus) were obtained from Meyer Hatchery (Polk, Ohio). Post-hatch chicks were maintained in a regular diurnal cycle of 12 hours light, 12 hours dark (8:00 AM-8:00 PM). Chicks were housed in stainless-steel brooders at 25°C and received water and Purinatm chick starter ad libitum. Mice were kept on a cycle of 12 h light, 12 h dark (lights on at 6:00 AM). C57BL/6J mice between the ages of P60-P100 were used for all experiments.
Fertilized eggs were obtained from the Michigan State University, Department of Animal Science. Eggs were incubated at a constant 37.5°C, with a 1hr period room temperature cool down every 24hrs. Additionally, the eggs were rocked every 45 minutes, and held at a constant relative humidity of 45%. Embryos were harvested at various time points after incubation and staged according to guidelines established by Hamburger and Hamilton (1951).
Intraocular injections:
Chicks were anesthetized with 2.5% isoflurane mixed with oxygen from a non-rebreathing vaporizer. The technical procedures for intraocular injections were performed as previously described (Fischer et al., 1998). With all injection paradigms, both pharmacological and vehicle treatments were administered to the right and left eye respectively at the same time of day for consecutive daily injections. Drugs were injected in 20 ml of 30% DMSO or sterile saline with 0.05 mg/ml bovine serum albumin added as a carrier. For mice injections, the total volume injected into each eye was 2μl. The specific details of the injected compounds are described (Table S1). Schematic figures for treatment paradigms indicate the days of drug delivery for each eye, and are included for each data-set in figures.
Single Cell RNA sequencing
Retinas were obtained from embryonic, postnatal chick, and adult mouse retinas. Isolated retinas were dissociated in a 0.25% papain solution in Hank’s balanced salt solution (HBSS), pH = 7.4, for 30 minutes, and suspensions were frequently triturated. The dissociated cells were passed through a sterile 70μm filter to remove large particulate debris. Dissociated cells were assessed for viability (Countess II; Invitrogen) and cell-density diluted to 700 cell/μl. Each single cell cDNA library was prepared for a target of 10,000 cells per sample. The cell suspension and Chromium Single Cell 3’ V2 reagents (10X Genomics) were loaded onto chips to capture individual cells with individual gel beads in emulsion (GEMs) using 10X Chromium Controller. cDNA and library amplification for an optimal signal was 12 and 10 cycles respectively. Sequencing was conducted on Illumina HiSeq2500 (Genomics Resource Core Facility, John’s Hopkins University) or HiSeq4000 (Novogene) with 26 bp for Read 1 and 98 bp for Read 2. Fasta sequence files were de-multiplexed, aligned, and annotated using the chick ENSMBL database (GRCg6a, Ensembl release 94) or mouse ENSMBL database (GRCm38.p6, Ensembl release 67) by using Cell Ranger software. Gene expression was counted using unique molecular identifier bar codes, and gene-cell matrices were constructed. Using Seurat toolkits, t-distributed stochastic neighbor embedding (tSNE) plots or Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP) plots were generated from aggregates of multiple scRNA-seq libraries (Butler et al., 2018; Satija et al., 2015). Compiled in each tSNE/UMAP plot are two biological library replicates for each experimental condition. Seurat was used to construct violin/scatter plots. Significance of difference in violin/scatter plots was determined using a Wilcoxon Rank Sum test with Bonferroni correction. Monocle was used to construct unbiased pseudo-time trajectories and scatter plotters for MG and MGPCs across pseudotime (Qiu et al., 2017a; Qiu et al., 2017b; Trapnell et al., 2012). Genes that were used to identify different types of retinal cells included the following: (1) Müller glia: GLUL, VIM, SCL1A3, RLBP1, (2) MGPCs: PCNA, CDK1, TOP2A, ASCL1, (3) microglia: C1QA, C1QB, CCL4, CSF1R, TMEM22, (4) ganglion cells: THY1, POU4F2, RBPMS2, NEFL, NEFM, (5) amacrine cells: ELAVL4, GAD67, CALB2, TFAP2A, (6) horizontal cells: PROX1, CALB2, NTRK1 (only in chick), (7) bipolar cells: VSX1, OTX2, GRIK1, GABRA1, and (7) cone photoreceptors: CALB1 (only in chick), GNAT2, OPN1LW, and (8) rod photoreceptors: RHO, NR2E3, ARR3. The MG have an over-abundant representation in the scRNA-seq databases. This likely resulted from fortuitous capture-bias and/or tolerance of the MG to the dissociation process. Single cell libraries for NMDA and FGF insulin in the mouse and chick generated by our lab have first been reported for cross species comparisons (Hoang et al., 2020), where chick embryonic retinal libraries are first being reported in this study.
Fixation, sectioning and immunocytochemistry:
Retinal tissue samples were formaldehyde fixed, sectioned, and labeled via immunohistochemistry as described previously (Fischer et al., 2008; Fischer et al., 2009a). Antibody dilutions and commercial sources for images used in this study are described (Table S2). Observed labeling was not due to off-target labeling of secondary antibodies or tissue autofluorescence because sections incubated exclusively with secondary antibodies were devoid of fluorescence. Secondary antibodies utilized include donkey-anti-goat-Alexa488/568, goat-anti-rabbit-Alexa488/568/647, goat-anti-mouse-Alexa488/568/647, goat-anti-rat-Alexa488 (Life Technologies) diluted to 1:1000 in PBS and 0.2% Triton X-100.
Fluorescent in situ hybridization (FISH):
The FISH protocol has been adapted from the hybridization chain reaction (HCR) for tissue sections provided by Molecular Instruments. Briefly, the retinal tissue was dissected and fixed in 4% PFA in DEPC water with 2mM EDTA for 4 hrs. Tissues were washed for 30 min in 0.1% Tween-20 PBS (PWT) and set overnight into 30% sucrose. Tissues were cryosectioned and retinal sections were rehydrated in PWT for 15 min on a shaker. Sections were moved to 2x SSCT for 20 minutes. Hybridization buffer was incubated on the slide at 37°C. The buffer was removed and the probe-set diluted 1:50 in hybridization buffer was set to incubate in a humidified chamber at 37°C for 24 hrs. The probe was removed with wash buffer for 15 minutes at 37°C and 5x SSCT for 5 minutes at room temperature. The amplification primers were snap cooled, mixed, and applied to the sections at room temperature for 24hrs in the dark. The primers were washed off with 5x SSCT for 10 minutes, and glass cover slips mounted for imaging.
Labeling for EdU:
The incorporation of EdU into proliferating nuclei was detected using a copper-catalyzed reaction of 5-ethynyl-2’-deoxyuridine (Click-It Labeling Technology; Thermo Fisher Scientific) with an Azide fluorophore (Thermo Fisher Scientific) to form a stable triazole ring. For experiments where EdU was administered, immunolabeled sections were fixed in 4% formaldehyde in 0.1M PBS pH 7.4 for 5 minutes at room temperature. Samples were washed for 5 minutes with PBS, permeabilized with 0.5% Triton X-100 in PBS for 1 minute at room temperature, and washed twice for 5 minutes in PBS. Sections were incubated for 30 minutes at room temperature in a buffer consisting of 100 mM Tris, 8 mM CuSO4, and 100 mM ascorbic acid in dH2O with an Alexa Fluor 568 Azide (Thermo Fisher Scientific) added to the buffer at a 1:100 dilution.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL):
The TUNEL assay was implemented to identify dying cells by imaging fluorescent labeling of double stranded DNA breaks in nuclei. The In Situ Cell Death Kit (TMR red; Roche Applied Science) was applied to fixed retinal sections as per the manufacturer’s instructions.
Photography, measurements, cell counts and statistics:
Microscopy images of retinal sections were captured with the Leica DM5000B microscope with epifluorescence and the Leica DC500 digital camera. High resolution confocal images were obtained with a Leica SP8 available in The Department of Neuroscience Imaging Facility at The Ohio State University. Representative images are modified to have enhanced color, brightness, and contrast for improved clarity using Adobe Photoshop. In EdU proliferation assays, a fixed region of retina was counted and average numbers of Sox2 and EdU co-labeled cells. The retinal region selected for investigation was standardized between treatment and control groups to reduce variability and improve reproducibility.
Similar to previous reports (Fischer et al., 2009a; Fischer et al., 2009b; Ghai et al., 2009), immunofluorescence was quantified by using ImagePro6.2 (Media Cybernetics, Bethesda, MD, USA) or Image J (NIH). Identical illumination, microscope, and camera settings were used to obtain images for quantification. Retinal areas were sampled from 5.4 MP digital images. These areas were randomly sampled over the inner nuclear layer (INL) where the nuclei of the bipolar and amacrine neurons were observed. Measurements of immunofluorescence were performed using ImagePro 6.2 as described previously (Ghai et al., 2009; Stanke et al., 2010; Todd and Fischer, 2015). The density sum was calculated as the total of pixel values for all pixels within thresholded regions. The mean density sum was calculated for the pixels within threshold regions from ≥5 retinas for each experimental condition. GraphPad Prism 6 was used for statistical analyses.
Measurement for immunofluorescence of cFos in the nuclei of MG/MGPCs were made by from single optical confocal sections by selecting the total area of pixel values above threshold (≥70) for Sox2 or Sox9 immunofluorescence (in the red channel) and copying nuclear cFos from only MG (in the green channel). The MG-specific cFos was quantified (as described below). Measurements of cFOS or pS6 immunofluorescence were made for a fixed, cropped areas (14,000 μm2) of INL, OPL and ONL. Measurements were made for regions containing pixels with intensity values of 70 or greater (0 = black and 255 = saturated). The intensity sum was calculated as the total of pixel values for all pixels within threshold regions. The mean intensity sum was calculated for the pixels within threshold regions from ≥5 retinas for each experimental condition.
To test for normality, we performed a Levine’s test. For statistical evaluation of differences in treatments, a two-tailed paired t-test was applied to account for intra-individual variability where each biological sample provides a control. For two treatment groups comparing assessing significant across inter-individual variability, a two-tailed unpaired t-test was applied. For multivariate analysis, an ANOVA with the associated Tukey Test was used to evaluate any significant differences between multiple groups.
Results:
MDK and PTN are upregulated in maturing MG during chick retinal development
scRNA-seq retina libraries were established at four stages of development including E5, E8, E12, and E15. The aggregation of these libraries yielded 22,698 cells after filtering to exclude doublets, cells with low UMI, and low genes/cell. UMAP plots of aggregate libraries of embryonic retinas formed clustered of cells into patterns that correlated to both developmental stage and cell type (Fig. 1a). Cell types were identified based on expression of well-established markers. Specifically, retinal progenitor cells from E5 and E8 retinas were identified by expression of ASCL1, CDK1, and TOP2A. (Supplemental Fig. 1a,b). Maturing MG were identified by expression of GLUL, RLBP1 and SLC1A3 (Supplemental Fig. 1a,b).
Figure 1.
Maturing MG upregulate MDK, PTN, and CSPG5 in embryonic chick retina. scRNA-seq was used to identify patterns of expression of MDK, PTN and putative receptor CSPG5 among embryonic retinal cells at four stages of development (E5, E8, E12, E15). UMAP-ordered clusters of cells were identified by expression of hallmark genes (a,b). A heatmap of MDK, PTN and CSPG5 illustrates expression profiles in different developing retinal cells (c). Each dot represents one cell and black dots indicate cells with 2 or more genes expressed. The upregulation of MDK and PTN in RPCs and maturing MG is illustrated with violin plot (d). The number on each violin indicates the percentage of expressing cells. The transition from RPC to mature MG is modelled with pseudotime ordering of cells with early RPCs to the far left and maturing MG to the right of the pseudotime trajectory (e). MDK and PTN are up-regulated in MG during maturation as illustrated by the pseudotime heatmap (f) and pseudotime plot (g). Significant difference (*p<0.01, **p<0.0001, ***p<<0.0001) was determined by using a Wilcox rank sum with Bonferoni correction. RPC – retinal progenitor cell, MG – Müller glia, iMG – immature Müller glia, mMG - mature Müller glia.
Elevated levels of MDK expression were observed in MG at E12 and E15, with lower levels of expression in immature MG at E8 and retinal progenitor cells at E5 (Fig. 1c,d). PTN was prominently expressed in immature and mature MG, but was also detected in immature amacrine cells (E8), rod photoreceptors (E12), and cone photoreceptors (E15) (Fig. 1c). Levels of MDK and PTN were significantly higher in maturing MG compared to immature MG and RPCs (Fig. 1d). Putative receptors and signal transducers of MDK and PTN include integrin β1 (ITGB1), receptor-like protein tyrosine phosphatase-ζ (PTPRZ1), chondroitin sulfate proteoglycan 5 (CSPG5) and p21-activated serine/threonine kinase (PAK1). These mRNAs had variable, low levels of expression in embryonic retinal cells. PTPRZ1, ITGB1 and PAK1 were observed in RPCs, immature and mature MG (Supplemental Fig. 1c). CSPG5 was expressed by developing photoreceptors, amacrine, ganglion and bipolar cells (Fig. 1c). Additionally, CSPG5 was expressed at elevated levels by immature and maturing MG compared to levels see in RPCs (Fig. 1c.d). We did not detect expression of anaplastic lymphoma kinase (ALK; ENSGALG00000009034), a putative receptor for MDK and PTN (Stoica et al., 2001) in any type of cell in embryonic or mature chick retina.
Re-embedding of RPCs and MG for pseudotime analysis revealed an ordering of cells with early RPCs and maturing MG at opposite ends of the trajectory (Fig. 1e). Across the pseudotime trajectory levels of GLUL increased, while levels of CDK1 decreased (Supplemental Fig. 1e,f). Similar to the pattern of expression of GLUL, the expression of MDK and PTN increases from retinal progenitors to maturing MG (Fig. 1e,f). Higher levels of expression were observed for both MDK and PTN in maturing MG, with PTN at low levels in retinal progenitors (Fig. 1f,g). Across pseudotime, levels of MDK were high in early progenitors, with a dip in expression during transition phases, and increased in maturing MG (Fig. 1f,g). Collectively, these findings suggest that both PTN and MDK are up-regulated by maturing MG during late stages of embryonic development, and, based on patterns of expression of putative receptors, MDK and PTN may have autocrine and paracrine actions in late-stage embryonic chick retinas.
MDK is upregulated in MG of damaged chick retinas
Chick retinas were damaged with NMDA, an established method of excitotoxic damage that rapidly induces neuronal death and can stimulate MG to de-differentiate and form proliferating MGPCs (Fischer and Reh, 2001). NMDA-induced retinal damage has been widely used to study to formation of MGPCs in the retinas of fish, chicks and rodents (Gallina et al., 2014). Accordingly, we established scRNA-seq libraries for retinal cells at different times after NMDA-treatment (Hoang et al., 2020). These data were analyzed to assess levels of expression of MDK, PTN and putative down-stream signaling genes in retinal neurons and glia following a damage paradigm where MGPCs are formed.
scRNA-seq libraries were aggregated for retinal cells obtained from control and NMDA-damaged retinas respectively at various time points (24, 48 and 72 hrs) after treatment (Fig. 2a). UMAP plots were generated and clusters of different cells were identified based on well-established patterns of expression (Fig. 2a,b). For example, resting MG formed a discrete cluster of cells and expressed high levels of GLUL, RLBP1 and SLC1A3 (Supplemental Fig. 2a,b). After damage, MG down-regulate markers of mature glia as they transition into reactive glial cells and into progenitor-like cells that up-regulate TOP2A, CDK1 and ESPL1 (Supplemental Fig. 2a,b). MDK was expressed at low levels in few resting MG in undamaged retina, unlike maturing MG in late stages of embryonic retinas (Fig. 1c,e), suggesting a down-regulation of MDK in MG as development proceeds after hatching. MDK was detected in a few oligodendrocytes and Non-astrocytic Inner Retinal Glia (NIRGs). NIRG cells are a distinct type of glial cells that has been described in the retinas of birds (Rompani and Cepko 2010; Fischer et al., 2010) and some types of reptiles (Todd et al., 2016). Following NMDA-induced damage, MDK is upregulated in MG and MGPCs at 24hrs, 48hrs, and 72hrs after treatment (Fig. 2c,e). In addition, MGPCs maintain high levels of MDK (Fig. 2c,e). By comparison, PTN was widely expressed in most types of retinal cells and was significantly down-regulated by MG and MGPCs in damaged retinas (Fig. 2c,e). We queried expression of putative receptors for MDK and PTN, including PTPRZ1, CSPG5 and Syndecan 4 (SDC4). Although SDC4 was expressed in few retina cells, SDC4 was low in resting MG and was up-regulated across many MG at 24hrs after NMDA-treatment (Fig. 2d,e). CSPG5 was expressed at high levels in resting MG, and was down-regulated in activated MG at 24hrs after NMDA and in MGPCs, and remained elevated in activated MG at 48 and 72hrs after NMDA (Fig. 2d,e). PTPRZ1 was not detected in MG, but was expressed at high levels in NIRG cells and in a few amacrine and bipolar cells (Fig. 2d). CSPG5, SDC4, and ITGB1 were dynamically expressed by different retinal neurons and NIRG cells in NMDA-damaged retinas (Supplemental Fig. 2a–e), suggesting that MDK may influence NIRG cells and neurons following injury.
Figure 2.
Chick MG robustly upregulate MDK and putative receptors following acute injury. scRNA-seq was used to identify patterns of expression of MDK-related genes among acutely dissociated retinal cells with the data presented in UMAP plots (a–d, f, g, h) and violin plots (e,i). Control and treated scRNA-seq libraries were aggregated from 24hr, 48hr, and 72hr after NMDA-treatment (a). UMAP-ordered cells formed distinct clusters with MG and MGPCs forming distinct clusters (b). Expression heatmaps of MDK, PTN, and receptor genes PTPRZ1, CSPG5, and SDC4 demonstrate patterns of expression in the retina, with black dots representing cells with 2 or more genes (c,d). In addition to NMDA, retinas were treated with insulin and FGF2 and expression levels of MDK, PTN, and CSPG5 were assessed in MG and MGPCs (f–i). UMAP and violin plots illustrate relative levels of expression in MG and MGPCs treated with NMDA alone or NMDA plus insulin and FGF2 (h,i). Violin plots illustrate levels of gene expression and significant changes (*p<0.1, **p<0.0001, ***p<<0.0001) in levels were determined by using a Wilcox rank sum with Bonferroni correction. The number on each violin indicates the percentage of expressing cells. scRNA-seq was validated using fluorescent in-situ hybridization for MDK (green) and PTN (red) before and 24hrs after NMDA-treatment (j). MDK transcripts upregulated after NMDA-treatment colocalized with immunoreactivity for glutamine synthetase (k).
To validate some of the findings from scRNA-seq we performed fluorescence in situ hybridization (FISH) for MDK and PTN. FISH for MDK demonstrated no signal in undamaged retinas, whereas robust signal for MDK appeared in the INL at 24hrs after NMDA-treatment (Fig 2j). This signal co-localized with glutamine synthetase immunoreactivity in MG (Fig. 2k). In undamaged retinas, FISH for PTN revealed signal within the ONL (photoreceptors), cells in the distal INL (bipolar cells and MG), and a few scattered cells in IPL and GCL (putative NIRG cells) (Fig. 2j). In NMDA-damaged retinas, FISH for PTN appeared unchanged in the ONL, was diminished in the distal INL (damaged or dying bipolar cells), but appeared concentrated in the middle of the INL and overlapped with signal for MDK (Fig. 2j). The FISH for MDK and PTN closely match the patterns of expression from scRNA-seq within retinal cells.
We re-embedded scRNA-seq data to establish a pseudotime trajectory on these datasets to better assess changes in expression of MDK-related genes during the transition of MG to progenitor cells after damage (Supplemental Fig. 3). Analysis of different pseudotime states revealed a branched trajectory with resting MG, proliferating MGPCs, and activated MG from 72hr after NMDA-treatment largely confined to different branches and states (Supplemental Fig. 3a–d). The expression of MDK across pseudotime positively correlates with a transition toward an MGPC-phenotype and up-regulation of progenitor markers, such as CDK1, and inversely correlated to resting glial phenotypes with significant down-regulation of glial markers such as GLUL (Supplemental Fig. 3a–d). By comparison, levels of PTN were decreased across pseudotime, with the largest decrease in PTN in activated MG compared to resting MG (Supplemental Fig. 3a–d). Similar to expression patterns of CDK1, patterns of expression of SDC4 are significantly elevated in pseudotime state 4 populated by activated MG and proliferating MGPCs (Supplemental Fig. 3a–d). In sum, these pseudotime trajectories showed similar patterns of expression for MDK and PTN as those seen in UMAP analyses in Fig 2e, with MDK increasing and PTN decreasing in activated MG and MGPCs after neuronal injury.
When comparing MG and MGPCs from 48hrs after NMDA with and without FGF2 and insulin, the relative levels of MDK and PTN were significantly decreased (Fig. 2f–i). UMAP plots revealed distinct clustering of MG and MGPCs from retinas from 48hrs NMDA alone and 48hrs NMDA plus FGF2 and insulin (Fig. 2f–i). Similarly, levels of GLUL, RLBP1 and CSPG5 were significantly decreased by FGF2 and insulin in damaged retinas in both MG and MGPCs (Fig. 2f–I; Supplemental Fig. 3e–g). By contrast, levels of CDK1 and TOP2A were significantly increased by FGF2 and insulin in MGPCs in damaged retinas (Supplemental Fig. 3e–g). Collectively, these findings suggest expression levels of PTN reflects a resting glial phenotype and levels are decreased by damage and further decreased by FGF2 and insulin. Expression levels of MDK corresponds with acutely activated glia, which is strongly induced by damage, but decreased by FGF2 and insulin in damaged retinas. Furthermore, because FGF2 and insulin are neuroprotective, it is possible that decreased upregulation of MDK, PTN and CSPG5 in MG result secondarily from reduced levels of neuronal death.
Exogenous MDK is neuroprotective and induces cFOS and pS6 in chick MG
The large, significant up-regulation of MDK by MG in NMDA-treated retinas suggests that this growth factor is involved in the responses of retinal cells to acute damage. To determine whether MDK influences retinal cells we probed for the activation of different cell-signaling pathways following a single intraocular injection of recombinant MDK or PTN. Recombinant chick MDK was utilized due to poor conservation with mouse and human homologs (48% and 42% respectively). Four hours after delivery of MDK we found a significant up-regulation of cFOS and pS6 specifically in MG (Fig. 3a–e), suggesting activation of the mTor-pathway. In addition, amacrine cells appeared to significantly up-regulate cFOS and NIRG cells up-regulated pS6 in response to MDK (Fig. 3a–e). To test whether cell signaling was influenced by PP2A-inhibitors, we co-applied fostriecin and calyculin A with MDK. We found that fostriecin and calyculin A significantly reduced levels of pS6 in MDK-treated MG (Fig. 3f,g), where cFOS activation was unaffected and independent of PP2A inhibition (data not shown). There were no detectable changes in levels of pERK1/2, p38 MAPK, pCREB, pSmad1/5/8, pStat3, and nuclear smad2/3 following intravitreal delivery of MDK. We did not detect changes in cell signaling in response to intraocular injections of PTN. Four consecutive daily intraocular injections of MDK or PTN had no significant effect upon MG reactivity or formation of proliferating MGPCs.
Figure 3.
MDK activates cFOS and pS6 mediated cell-signaling in chick MG. A single intraocular injection of MDK was delivered and retinas were harvested 4 hours later. The histogram in a represents the mean percent change (±SD) in intensity sum for cFOS and pS6 immunofluorescence. Each dot represents one biological replicate retina. Significance of difference (**p<0.01, ***p<0.001) was determined by using a paired t-test. Sections of saline (control) and MDK-treated retinas were labeled with antibodies to cFOS (green; b,c), pS6 (green; d,e) and Sox2 (magenta; c,e). Arrows indicate the nuclei of MG, small double-arrows indicate the nuclei of amacrine cells, and hollow arrow-heads indicate the nuclei of presumptive NIRG cells. An identical paradigm with the addition of ITGB1 signaling inhibitors fostriecin & calyculin measured changes in pS6 signaling in MG (f) and was quantified for intensity changes (g). The calibration bar (50 μm) in panel d applies to b and d. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Injections of MDK after NMDA-treatment had no significant effect upon glial reactivity or proliferation of MGPCs, NIRG cells or microglia. Administration of MDK after NMDA lacked significant effects likely because endogenous levels of MDK were very high and MDK-mediated cell-signaling may have been saturated. By comparison, injection of MDK prior to NMDA-treatment significantly reduced the numbers of proliferating MGPCs that accumulated EdU or were immunolabeled for pHisH3 (Fig. 4a–d). We probed for changes in NIRG cells and microglia in the chick retina after damage, as both cell types are known to proliferation and transiently accumulate in the retina after damage (Zelinka et al., 2012). There was a significant reduction in the total number NIRG cells that accumulated in NMDA-damaged retinas (Fig. 4e,f). These cells were identified and quantified by Sox2+/Nkx2.2+ colocalization. In addition, microglial reactivity was influenced by MDK. We measured the area and intensity sum for CD45 immunofluorescence, which is known to be increased in reactive microglia (Fischer et al., 2014). Both the area and the intensity of CD45-immunofluorescence was decreased in response to MDK (Fig. 4g,h). There were no detected MDK-mediated changes in read-outs of different cell-signaling pathways including pS6, pCREB, p38 MAPK, pERK1/2, or pStat3 after damage (data not shown).
Figure 4.
MDK treatment prior to NMDA reduces numbers of proliferating MGPCs, suppresses the accumulation of NIRG cells, and increases neuronal survival. Eyes were injected with MDK or saline at P6 and P7, and NMDA at P8. Some retinas were harvested at P9, whereas other eyes were injected at P10 with EdU and retinas harvested 4hrs later, 24hrs later at P11 or 10 days later at P20. Sections of the retina were labeled for EdU (red) and Sox9 (green; a), phospho-Histone H3 (pHisH3; magenta) and Sox2 (green; c), Nkx2.2 (e), CD45 (g), TUNEL (i), and AP2α (red) and Otx2 (green) or calretinin (red; k). Arrows indicate nuclei of proliferating MGPCs and hollow arrow-heads indicate TUNEL-positive cells. The histogram/scatter-plots b, d, f, j and l illustrate the mean number of labeled cells (±SD). The histogram in h represents the mean percent change (±SD) in density sum and area for CD45 immunofluorescence. Each dot represents one biological replicate. Significance of difference (*p<0.01) was determined by using a paired t-test. The calibration bars panels a, c, e, g, i and k represent 50 μm. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Levels of retinal damage and cell death are known to positively correlate to numbers of proliferating MGPCs (Fischer and Reh, 2001; Fischer and Reh, 2003). Thus, it is possible that reduced numbers of proliferating MGPCs resulted from less cell death with MDK pre-treatment. Using the TUNEL method to labeling dying cells, we found that the administration of MDK before NMDA-damage significantly reduced numbers of dying cells (Fig. 4i,j). Decreased numbers of dying cells were observed at both 24h and 72h after NMDA-treatment with MDK pre-treatment. To complement the cell death studies, we probed for long-term survival of inner retinal neurons. NMDA damage results in death of amacrine and bipolar cells, and survival was measured by cell counts after cell death and clearance. Although there was no change in numbers of AP2α+ amacrine cells, there was a significant increased numbers of surviving calretinin+ cells in retinas treated with MDK (Fig. 4k,i), indicating that MDK promoted the survival of subtypes of amacrine cells.
PTN was significantly down-regulated in MG following NMDA-treatment (Fig. 2). Despite the administration of high doses (1μg/dose) of PTN, intravitreal delivery of PTN with NMDA had no measurable effects on the formation of MGPCs, the reactivity and proliferation of microglia, and accumulation of NIRG cells (data not shown). Although these experiments were conducted using recombinant human PTN, there is high conservation between chick, mouse and human PTN (92% and 93% respectively).
Inhibition of MDK-signaling increases cell death and reduces MGPC formation in chick
MDK-signaling is often upregulated in tissues with proliferating cells, such as tumors, and this proliferation can be suppressed by inhibition of MDK-signaling (Hao et al., 2013; Takei et al., 2006). Since levels of MDK were markedly increased in MG in damaged retinas, we tested whether inhibition of MDK-signaling supressed the formation of proliferating MGPCs. We applied a MDK-expression inhibitor (MDKi), which downregulates protein expression in a dose dependent manner (Masui et al., 2016). However, application of MDKi after NMDA-treatment did not influence MG, NIRG cells, or microglia (data not shown). Alternatively, we applied a PTPRZ inhibitor SCB4380 that targets the intracellular domain of the receptor (Fujikawa et al., 2016). However, this inhibitor did not influence MG, NIRG cells or microglia when applied after NMDA-treatment (not shown). It is likely that MDKi and SCB4380 had poor cellular permeability and these drugs failed to adequately diffuse into the retina.
We next applied an inhibitor of MDK-signaling, sodium orthovanadate (Na3VO4) that suppresses the activity of tyrosine phosphatases, including PTPRZ1 (Qi et al., 2001; Sakaguchi et al., 2003), which was predominantly expressed by NIRG cells, amacrine cells, and some bipolar cells (Fig. 2c). It should be noted that this drug can impact the function of tyrosine phosphatases in addition to PTPRZ1. Application of Na3VO4 after NMDA significantly reduced numbers of proliferating MGPCs (Fig. 5a,b). In addition, treatment with Na3VO4 significantly increased numbers of NIRG cells in the IPL (Fig. 5c,d) and increased numbers of dying cells (Fig. 5e,f). Despite this increase in retinal damage, proliferation of MGPCs was reduced in response to Na3VO4 after NMDA-induced damage (Fig. 5a,b).
Figure 5.
Sodium orthovanadate in NMDA-damaged retinas suppressed the formation of MGPCs, increases numbers of dying cells, and stimulates the accumulation of NIRG cells. Eyes were injected with NMDA and Na3VO4 tyrosine phosphatase inhibitor or vehicle at P8, inhibitor or vehicle at P9, EdU at P10, and retinas harvested at P11. Sections of the retina were labeled for EdU (red) and Sox9 (green; a, g), Nkx2.2 and Sox9 (green; c), or TUNEL (e). Arrows indicate nuclei of proliferating MGPCs (a,g) and hollow arrow-heads indicate NIRG cells (c). The histogram/scatter-plots in b, d, f, h and i illustrate the mean (±SD) number of labeled cells. Each dot represents one biological replicate. Significance of difference (*p<0.05) was determined by using a paired t-test. The calibration bars panels a, c, e and g represent 50 μm. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
We next examined the specificity of Na3VO4, by testing whether Na3VO4 blocked the effects of MDK on damaged retinas. Comparison across treatment groups (NMDA alone, NMDA + Na3VO4, NMDA + MDK, and NMDA + Na3VO4 + MDK) revealed a significant decrease in MGPCs in eyes treated with Na3VO4 and MDK alone (Fig. 5g,h). With the combination of MDK and Na3VO4 there was a significant increase in proliferating MGPCs relative to treatment with MDK or Na3VO4 alone (Fig. 5h). However, this level was not increased relative to levels seen with NMDA alone (Fig. 5h). In addition, the combination of MDK and Na3VO4 resulted in no significant difference in numbers of TUNEL+ dying cells compared to NMDA alone (Fig 5i). These findings suggest that the effects of MDK and Na3VO4 upon proliferating MGPCs and numbers of dying neurons are mediated by overlapping targets.
Inhibitors of signaling though Integrin-Beta (ITGB) reduce MGPC formation in chick retina
MDK has been found to bind and signal through Integrin-Beta 1 (ITGB1) (Muramatsu et al., 2004). ITGB1 signaling occurs through different second messengers including Integrin linked kinase (ILK), p21 activated kinase 1 (PAK1), cell division factor 42 (CDC42), protein phosphatase 2a (PP2A, gene: PPP2CA), and Git/Cat-1 (GIT1) regulated cytoskeleton remodeling, migration, and cellular proliferation (Bagrodia and Cerione, 1999; Ivaska et al., 1999; Kawachi et al., 2001; Kim et al., 2004; Martin et al., 2016; Mulrooney et al., 2000) (see Fig. 11). PAK1 has been implicated as a cell cycle regulator that is downstream of MDK and PTN signaling (Kawachi et al., 2001). PAKs are components of the mitogen activated protein kinase (MAPK) pathway and are believed to regulate small GTP-binding proteins (CDC42 and RAC) (Bagrodia and Cerione, 1999; Frisch, 2000) (see Fig. 11).
Figure 11.
Schematic summary of MDK-signaling in normal and NMDA-damaged retinas. Patterns of expression, determined by scRNA-seq, are shown for Integrin β1, Integrin α, PTPRZ1, PAK1 and MDK in MG, NIRG cells and inner retinal neurons. Although GIT1, ILK, CDC42, and PP2A (PPP2CA) were widely expressed by nearly all retinal cells (according to scRNA-seq data; see Fig 6a), signaling through Integrins is shown only in MG because ITG’s were largely confined to MG. Putative sites of action are shown for small-molecule inhibitors, including IPA3, calyculin A, fostriecin and Na3VO4. Abbreviations: PRL – photoreceptor layer, ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, NFL – nerve fiber layer.
By probing scRNA-seq libraries we found that PAK1 was widely expressed at relatively high levels in resting MG, and levels were significantly reduced in MG at different times after NMDA, and further reduced in MGPCs (Fig. 6a,b). Similarly, levels of PPP2CA, CDC42, GIT1 and ILK were expressed at relatively high levels in resting MG, but were widely expressed at reduced levels in MGPCs and activated MG in damaged retinas (Fig. 6a,b). In addition, PPP2CA, CDC42, GIT1 and ILK were expressed by different types of retinal neurons, NIRG cells and oligodendrocytes (Fig. 6a). We found that MG expressed ITGB1 and other integrin isoforms, including ITGA1, ITGA2, ITGA3 and ITGA6 (Fig. 6a,b). In general, integrins were expressed at high levels in relatively few resting MG, whereas levels were reduced, but more widely expressed among activated MG, and further reduced in MGPCs (Fig. 6a,b). Collectively, the data demonstrate the expression of ITGB1 signaling components in MG, and changes in patterns of expression imply that this pathway is activate in glia in damaged retinas.
Figure 6.
ITGB1 signaling inhibitors reduce the formation of chick MGPCs after NMDA damage. scRNA-seq libraries (Fig. 2) were probed for patterns of expression of integrin alpha/beta isoforms and associated signaling ITGB1 molecules p21 associated kinase-1 (PAK1), protein phosphatase 2a catalytic subunit alpha (PPP2CA), integrin linked kinase (ILK), and ARF GTPase-activating protein (GIT1). tSNE plots demonstrate patterns of expression of PAK1, ITGB1, ITGA1, ITGA2, ITGA3, ITGA6, ITGAV, CAT, CDC42, GIT1, ILK and PPP2CA (a). Violin/scatter plots indicate significant differences (*p<0.01, **p<0.001, ***p<<0.001; Wilcox rank sum with Bonferoni correction) in expression of PAK1, ITGB1, ITGA1, ITGA2, ITGA3, ITGA6, ITGAV, CAT, CDC42, GIT1, ILK and PPP2CA among MG and MGPCs (b). The number on each violin indicates the percentage of expressing cells. PAK1-specific inhibitor IPA3 was injected with and following NMDA (c,d) or before NMDA (e) and analyzed for proliferation of MGPCs. Alternatively, PP2A-specific inhibitors calyculin A or fostriecin were injected with and following NMDA (f–h). Sections of the retina were labeled for EdU (red) and Sox9 (green; c, f). Arrows indicate nuclei of proliferating MGPCs (a,g). The histogram/scatter-plots in d, e, g and h illustrate the mean (±SD) number of labeled cells. Each dot represents one biological replicate. Significance of difference (*p<0.05) was determined by using a paired t-test. Arrows indicate nuclei of proliferating MGPCs (c,f). The calibration bar panels c and f represent 50 μm. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ns – not significant.
We next tested how PAK1 and PP2A influence the formation of MGPCs in NMDA-damaged retinas. MDK-signaling is known to be modulated by the second messenger PAK1 which is up-regulated in proliferating cancerous cells (Kumar et al., 2006). IPA3 is an isoform-specific allosteric inhibitor of PAK1 which prevents autophosphorylation (Deacon et al., 2008). Administration of IPA3 with NMDA significantly decreased numbers of proliferating MGPCs (Fig. 6c,d). By contrast, IPA3 had no significant effect upon the proliferation and accumulation of NIRG cells or microglia (Supplemental Fig. 4). Unlike Na3VO4, IPA3 had no impact on numbers of TUNEL+ cells compared to those seen in retinas treated with NMDA alone (Supplemental Fig. 4). Since PAK1 expression was most prevalent in resting MG and decreased after NMDA damage, we tested whether application of IPA3 prior to NMDA influenced glial cells and neuronal survival. We found that IPA3 prior to NMDA resulted in a significant decrease in proliferating MGPCs (Fig. 6e), whereas there was no significant difference in numbers of dying cells or proliferation of microglia and NIRG cells (Supplemental Fig. 4). Similar to the effects of IPA3, two different inhibitors to PP2A, fostriecin and calyculin A, significantly decreased numbers of proliferating MGPCs in NMDA-damaged retinas (Fig. 6f–h). Fostriecin and calyculin A had relatively little effect upon the accumulation, reactivity, cell death and proliferation of NIRG cells and microglia, with the exception of a small but significant decrease in proliferating microglia with calyculin A-treatment compared to controls (Supplemental Fig. 4). Collectively, these findings suggest that putative signal-transducers of MDK-signaling promote the formation of proliferating MGPCs in damaged retinas.
Insulin and FGF2 increase MDK expression while decreasing PTN expression
In the postnatal chick retina, the formation of proliferating MGPCs can be induced by consecutive daily injections of Fibroblast growth factor 2 (FGF2) and insulin in the absence of neuronal damage (Fischer et al., 2002b). Eyes were treated with two or three consecutive daily doses of FGF2 and insulin and retinas were processed to generate scRNA-seq libraries. Cells were clustered based on their gene expression in UMAP plots and colored by their library of origin (Fig. 7a,b). MG were identified based on collective expression of VIM, GLUL and SLC1A3 and MGPCs were identified based on expression of TOP2A, NESTIN, CCNB2 and CDK1 (Supplemental Fig. 5a,b). Resting MG from saline-treated retinas formed a cluster distinct from MG from retinas treated with two- and three-doses of FGF2+insulin based on unique patterns of gene expression (Fig. 7b; Supplemental Fig. 5a,b). Additionally, MG treated with 2 versus 3 doses of insulin and FGF2 were sufficiently dissimilar to follow different trajectories of gene expression in pseudotime analysis (Supplemental Fig. 5c,d).
Figure 7.
Insulin and FGF2 induce MDK and putative MDK-receptors in chick retinas. scRNA-seq was used to identify patterns of expression of MDK-related genes among cells in saline-treated retinas and in retinas after 2 and 3 consecutive doses of FGF2 and insulin (a,b). In UMAP plots, each dot represents one cell, and expressing cells indicated by colored heatmaps of gene expression for MDK, PTN, PAK1, CSPG5 and ITGB1 (c,d). Black dots indicate cells with expression of two or more genes. (e) Changes in gene expression among UMAP clusters of MG and MGPCs are illustrated with violin plots and significance of difference (*p<0.1, **p<0.0001, ***p<<0.0001) determined using a Wilcox rank sum with Bonferoni correction. The number on each violin indicates the percentage of expressing cells.
Similar to patterns of expression in NMDA-damaged retinas, there was a significant increase in MDK with growth factor-treatment as demonstrated by patterns of expression in UMAP and violin plots, and pseudotime analyses (Fig. 7c–e; Supplemental Fig. 5d–f). By comparison, levels of PTN were significantly decreased in MG following treatment with insulin and FGF2 (Fig. 7c,e; Supplemental Fig. 5d–f). Similarly, levels of PAK1 were decreased in activated MG and MGPCs in response to growth factor treatment (Fig. 7d,e; Supplemental Fig. 5e,f). CSPG5 was widely expressed at high levels in resting MG, and was significantly reduced in MG and MGPCs following treatment with insulin and FGF2 (Fig. 7d,e; Supplemental Fig. 5e, f). ITGB1 expression was detected in many resting MG, and decreased slightly in MG and MGPCs treated with insulin and FGF2 (Fig. 7d,e; Supplemental Fig. 5e,f). In sum, treatment with FGF2 and insulin in the absence of retina damage influenced patterns of expression for MDK-related genes similar to those seen in NMDA-damaged retinas.
We next isolated MG, aggregated and normalized scRNA-seq data from saline-, NMDA-, FGF2+insulin- and NMDA/FGF2+insulin-treated retinas to directly compare levels of MDK, PTN and related factors. UMAP plots revealed distinct clustering of MG from control retinas and MG from 24hrs after NMDA-treatment, whereas MG from retinas at 48 and 72hrs after NMDA and from retinas treated with insulin and FGF2 formed a large cluster with distinct regions (Fig. 8a–e). UMAP and Dot plots revealed distinct patterns of expression of genes associated with resting MG, de-differentiating MG, activated MG and proliferating MGPCs (Fig. 8c–e). Different zones, representing MGPCs in different phases of the cell cycle were comprised of cells from different times after NMDA-treatment and FGF2+insulin-treatment (Fig. 8e). Expression of MDK was most widespread and significantly up-regulated in MG in damaged retinas and MGPCs compared to MG from retinas treated with insulin and FGF2 (Fig. 8f). Compared to levels seen in resting MG, levels of PTN were reduced in activated MG from damaged retinas and MGPCs, and levels were further decreased in MG from normal and damaged retinas that were treated with insulin and FGF2 (Fig. 8f); similar patterns of expression were seen for PAK1, CSPG5, ITGB1, PPP2CA and CDC42. By contrast, levels of SDC4 were highest and most widespread in MG at 24hrs after NMDA-treatment and were relatively reduced in all other groups of MG and MGPCs (Fig. 8f). Collectively, these findings indicate that damaged-induced changes of MDK, PTN and related factors in MG are very dramatic, and these changes in relative expression levels in MG are dampened by insulin and FGF2 whether applied to undamaged or damaged retinas.
Figure 8.
Various reprogramming treatments induce MDK, ITGB1, CDC4, while repressing PTN, PAK1, and CSPG5 in chick MGPCs. In UMAP and violin plots each dot represents one cell. MG were bioinformatically isolated from 2 biological replicates for control retinas and retinas treated with 2 doses of insulin and FGF2, 3 doses of insulin and FGF2, 24 hrs after NMDA, 48 hrs after NMDA, 48hrs after NMDA + insulin and FGF2, and 72 hrs after NMDA. UMAP analysis revealed distinct clusters of MG which includes control/resting MG, activated MG from retinas 24hrs after NMDA treatment, activated MG from 2 doses of insulin and FGF2, activated MG from 3 doses of insulin FGF2 and NMDA at different times after treatment, activated MG returning toward a resting phenotype from 48 and 72 hrs after NMDA-treatment, and 3 regions of MGPCs. The dot plot in c illustrates some of the pattern-distinguishing genes and relative levels across the different UMAP-clustered MG and MGPCs. UMAP plots illustrate the distinct and elevated expression of GLUL, RLBP, VIM and SLC1A3 in resting MG (d) and CDK1, TOP2A, PCNA and SPC25 in different regions of MGPCs (e). Violin plots in f illustrate relative expression levels for MDK, PTN, SDC4, PAK1, CSPG5, ITGB1, PPP2CA and CDC4 in UMAP-clustered MG and MGPCs. Significance of difference (**p<0.001, ***p<<0.001) was determined by using a Wilcox rank sum with Bonferoni correction. The number on each violin indicates the percentage of expressing cells.
Since growth factor treatment induces significant changes in expression of MDK-related genes, similar to that with NMDA damage, we tested whether treatment with MDK or inhibitors influenced the formation of MGPCs, or the accumulation of NIRG cells or microglia. However, we found no significant effects of MDK or IPA3 when combined with insulin and FGF2 (Supplemental Fig. 7). By comparison, the combination of Na3VO4 with insulin and FGF2 resulted in increased accumulation of NIRG cells and some cell death, but had no influence upon the proliferating MGPCs (Supplemental Fig. 7).
Mdk is down-regulated in MG and promotes MGPC formation in damaged mouse retinas
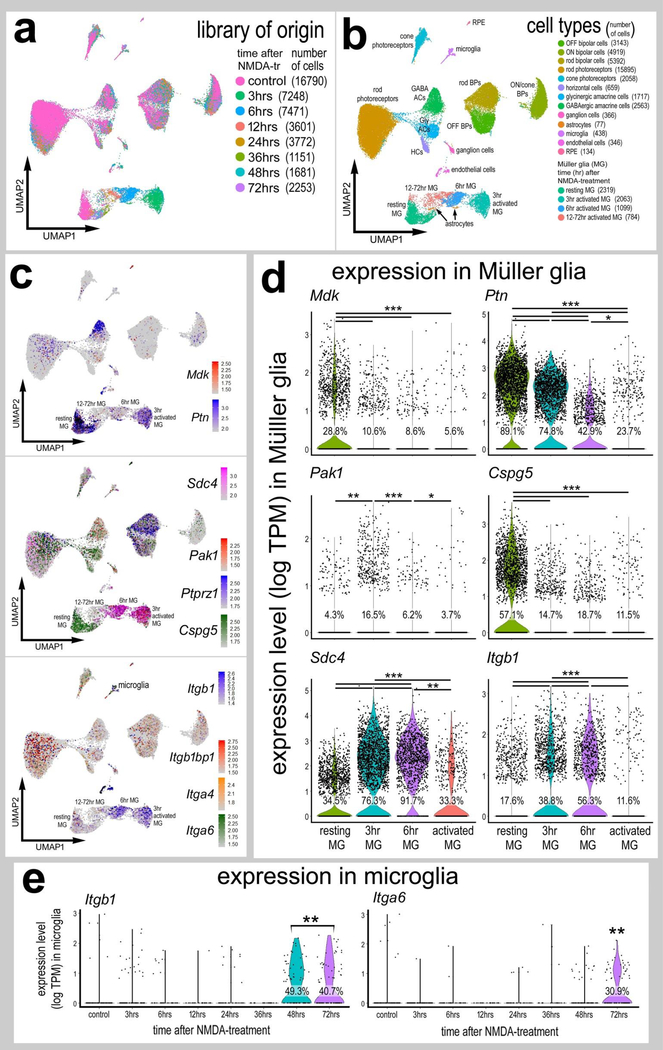
We next sought to assess the expression of Mdk and related factors in normal and NMDA-damaged mouse retinas. Comparison of the different responses of glial cells across species can indicate important factors that promote or inhibit the ability of MG to reprogram into MGPCs (Hoang et al., 2020). UMAP analysis of cells from control and NMDA-damage mouse retinas revealed discrete clusters of different cell types (Fig. 9a). Neuronal cells from control and damaged retinas were clustered together, regardless of time after NMDA-treatment (Fig. 9a). By contrast, resting MG, which included MG from 48 and 72 hrs after NMDA, and activated MG from 3, 6, 12 and 24 hours after treated were spatially separated in UMAP plots (Fig. 9a,b). Pseudotime analysis placed resting MG (control and some MG from 48 and 72 hrs after treatment) to the left, MG from 3 and 6 hrs after treatment to the far right, and MG from 12 and 24 hrs bridging the middle (Supplemental Fig. 6a–d). Unlike chick MG, mouse MG rapidly downregulate Mdk in response to damage and this downregulation is maintained through 72 hrs after treatment (Fig. 9c,d). Similar to MG in the chick, Ptn was rapidly down-regulated at 3hrs, and further down-regulated at 6hrs, and expressed by relatively few MG at 12–48hrs (Fig. 9c,d). Unlike patterns of expression in chick, levels of Pak1 were low in resting MG, and elevated in MG only at 3hrs after NMDA-treatment (Fig. 9c,d). Similar to chick MG, Cspg5 was significantly decreased in activated MG in damaged retinas (Fig. 9c,d). By contrast, there were significant increases in levels of Sdc4 and Itgb1 in MG in damaged retinas (Fig. 9c,d; Supplemental Fig.6eg). We did not detect anaplastic lymphoma kinase (Alk; ENSMUSG00000055471) a putative receptor for midkine and pleotrophin (Stoica et al., 2001) in any type of cell in the mouse retina. We further analyzed the responses of MG in damaged retinas at 48hrs after NMDA ± treatment with insulin and FGF2, which is known to stimulate the proliferation of MG (Karl et al., 2008). Treatment with FGF2 and insulin in damaged retinas significantly reduced levels of Glul, whereas levels of Vim and Gfap were significantly increased (Supplemental Fig. 6h–j). By comparison, levels of Mdk and Sdc4 were significantly increased in MG in retinas treated with NMDA+FGF2/insulin, whereas levels of Ptn, Cspg5 and Itgb1 were unchanged (Supplemental Fig. 6h–j).
Figure 9.
Mouse MG dynamically express Mdk, Ptn and MDK-related genes in response to NMDA damage. Cells were obtained from control retinas and from retinas at 3, 6, 12, 24, 36, 48 and 72hrs after NMDA-treatment and clustered in UMAP plots with each dot representing an individual cell (a). UMAP plots revealed distinct clustering of different types of retinal cells; resting MG (a mix of control, 48hr and 72hr NMDA-tr), 12–72 hr NMDA-tr MG (activated MG in violin plots), 6hrs NMDA-tr MG, 3hrs NMDA-tr MG, microglia, astrocytes, RPE cells, endothelial cells, retinal ganglion cells, horizontal cells (HCs), amacrine cells (ACs), bipolar cells (BPs), rod photoreceptors, and cone photoreceptors (b). Cells were colored with a heatmap of expression of Mdk, Ptn, Sdc4, Pak1, Ptprz1, Cspg5, Itgb1bp1, Itga4 and Itgba6 gene expression (c). Black dots indicate cells with two or more markers. In MG, changes in gene expression are illustrated with violin/scatter plots of Mdk, Ptn, Pak1, Cspg5, Sdc4, and Itgb1 and quantified for significant changes (d) (*p<0.01, **p<0.0001, ***p<<0.001). Similarly, UMAP-clustered microglia were analyzed and genes Itgb1 and Itga6 were detected and quantified in violin plots for cells from each library of origin (e). The number on each violin indicates the percentage of expressing cells.
We next investigated the activation of different cell-signaling pathways in retinal cells in response to intravitreal delivery of MDK. Similar to findings in the chick retina, we did not detect activation of NFkB, pStat3, pSmad1/5/8, pCREB, p38 MAPK or pERK1/2. Similar to findings in the chick retina, a single injection of MDK resulted in a selective and significant up-regulation of cFOS and pS6 in MG in the mouse retina (Fig. 10a–e). Other types of retinal cells did not appear to respond to MDK with up-regulation of cFOS or pS6. We next tested whether intraocular injections of MDK combined with NMDA-induced damage influences the proliferation of MG in the mouse retina. Consistent with previous reports (Karl et al., 2008), there were very few proliferating MG in NMDA-damaged retinas (Fig. 10f,h). By contrast, application of MDK with NMDA resulted in a small, but significant increase in numbers of proliferating MG (Fig. 10f–h). Similar to results seen in the chick retina, application of MDK prior to NMDA significantly reduced numbers of TUNEL+ cells in the INL and GCL (Fig. 10i–l), suggesting that MDK is potently neuroprotective to inner retinal neurons, including ganglion cells.
Figure 10.
MDK activates cFOS and pS6 cell-signaling in MG, stimulates proliferation of MGPCs, and promote neuroprotection in the mouse retina. (a–e) A single intraocular injection of MDK was delivered and retinas were harvested 4 hours later. The histogram in a represents the mean percent change (±SD) in density sum and area for percentage change in intensity sum for cFOS and pS6 immunofluorescence. Vertical sections of saline (control) and MDK-treated retinas were labeled with antibodies to cFOS (green; b,c), pS6 (green; d,e) and Sox2 (magenta; c,e). (f–h) Treatment included intraocular injections of MDK or vehicle at P60, NMDA and MDK/vehicle at P62, MDK or vehicle at P60, EdU was applied daily by intraperitoneal (IP) injections from P62 through P66, and tissues were harvested at P67. The histogram in h represents the mean (±SD) number of EdU+/Sox9+ cells in the INL. (i–j) Treatment included intraocular injections of MDK or vehicle at P60 and P61, NMDA at P62, and tissues were harvested at P63. Sections of the retina were labeled for fragmented DNA using the TUNEL method (i). The histogram in j represents the mean (±SD) number of TUNEL+ cells in the retinal total, only in the GCL, or only in the INL (j). Each dot represents one replicate retina (a,h,j). Significance of difference (*p<0.05, **p<0.001, ***p<0.0001) was determined by using a paired two-way t-test. Arrows indicate the nuclei of MG, arrow-heads indicate EdU+/Sox9− cells (presumptive proliferating microglia), hollow arrow-heads indicate pS6+ inner retinal neurons, and small double-arrows indicate the nuclei of Sox2+ cholinergic amacrine cells. The calibration bar (50 μm) in panel d applies to b and d. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Discussion:
In this study, we use scRNA-seq to investigate the role of MDK and PTN in the transition of MG to MPGCs in chick and mouse retina. The patterns of gene regulation are complex and context dependent, but the dynamic regulation of mRNA is strongly correlated with changes in protein levels and function (Liu et al., 2016). Mouse and chick MG expression differed in their response to damage, and we used small molecule inhibitors of MDK and PTN to determine the functional role of these factors on MG reprogramming.
In the chick retina, MDK expression was upregulated in maturing MG during retinal development and in postnatal MG following injury or growth factor-treatment. High levels of MDK expression were selectively and rapidly induced in MG following damage or treatment with insulin and FGF2, with larger increases in expression seen in damaged tissues. Addition of MDK before damage was neuroprotective and resulted in decreased numbers of proliferating MGPCs. Antagonism of MDK-signaling reduced numbers of proliferating MGPCs and stimulated the accumulation of NIRG cells and increased numbers of dying cells. Na3VO4 and PAK1 antagonist had differential effects on NIRG cells and cell death that were context dependent. In contrast to the findings in chick, we find that Mdk is down-regulated by MG in damaged mouse retinas. In both chick and mouse retinas, exogenous MDK selectively induces mTOR-signaling, expression of cFOS in MG and protects inner retinal neurons against excitotoxic injury. However, inner retinal neurons express PTPRZ1 and MDK may directly act to protect these cells against excitotoxic injury (Fig. 11).
PTN signaling in the retina
PTN and MDK are in the same family of growth factors and are both dynamically expressed in the developing, damaged, and growth factor-treated retinas. Although treatment with MDK had varying effects on MG, microglia, NIRGs, and neurons, PTN administration had no detectable effects upon retinal cells. Levels of PTN are high in resting MG and down-regulated in response to neuronal damage or treatment with insulin and FGF2. In principle, PTN acts at the same receptors as MDK, but expression of receptor isoforms may underlie the different cellular responses to MDK and PTN. PTN has preferred binding-affinity for SDC4 and ITGB3, whereas MDK has preferred binding-affinity for SDC3 and ITGB1 (Muramatsu et al., 2004; Raulo et al., 1994; Xu et al., 2014), which are expression by bipolar cells and MG.
PTN and MDK may induce different cellular response at the same receptors. For instance, data suggests that there may be differential receptor activity between PTN and MDK on the PTPRz receptor. Binding of PTN to PTPRz induces oligomerization of the receptor that reduces phosphatase activity (Fukada et al., 2006). Conversely, MDK promotes embryonic neuronal survival in a PTPRz receptor complex, which is inhibited by Na3VO4 (Sakaguchi et al., 2003). Although there were no detectable PTN-mediated effects upon retinal cells, PTN may serve other important biological roles in retinal hometostasis, glial phenotype/functions or neuroprotection in other models of retinal damage.
Receptor expression and cells responding to MDK
The effects of MDK on retinal glia has not been studied in mammals or birds. In acutely damaged chick retina, MG are capable of forming numerous proliferating progenitor cells (MGPCs), but few of the progeny differentiate into neurons (Fischer and Reh, 2001; Fischer and Reh, 2002). The reprogramming of MG into MGPCs can be induced by FGF2 and insulin in the absence of damage through MAPK signaling (Fischer and Reh, 2002; Fischer et al., 2002a; Fischer et al., 2002b). Similarly, IGF1, BMP, retinoic acid, sonic hedgehog, Wnt, and Jak/Stat agonists have been observed to enhance the formation of MGPCs (Fischer et al., 2009a; Gallina et al., 2015; Todd and Fischer, 2015; Todd et al., 2016; Todd et al., 2017; Todd et al., 2018). Consistent across the different signaling pathways that drive the formation of proliferating MGPCs is the up-regulation of cFOS and necessity for mTor-signaling in reprogramming MG (Zelinka et al., 2016). Previous reports have provided many examples of MDK activating cell-signaling pathways that drive proliferation (Reiff et al., 2011; Winkler and Yao, 2014). Accordingly, we propose that MDK-mediated cell-signaling that results in activation of cFOS and mTOR contributes to the network of pathways that drive the formation of proliferating MGPCs in the chick retinas.
Patterns of expression for receptors suggests than glial cells and inner retinal neurons are targets of MDK and PTN. In MG the predominant receptor is ITGB1, whereas NIRGs cells express PTPRZ1, and amacrine and bipolar cells express a combination of PTPRZ1 and SDC4. The mechanism by which ITGB1 and PTPRZ influence cell cycle progression and differentiation are distinctly different. PTPRZ promotes stem cell characteristics and ligand binding inhibits phosphatase function (Fujikawa et al., 2016; Fukada et al., 2006; Kuboyama et al., 2015). Signal transduction through ITGB1 influences cytoskeleton remodeling that is associated with cell migration and proliferation (Muramatsu et al., 2004). ITGB1 can activate or inhibit secondary messengers depending on tyrosine phosphorylation (Kim et al., 2004; Mulrooney et al., 2000; Song et al., 2014). Ligand binding to ITGB1 initiates tyrosine phosphorylation of intracellular domains, and integrin linked kinases (ILKs) activate PP2A and cell cycle kinases, such as CDC42 (Ivaska et al., 1999; Ivaska et al., 2002).It has yet to be investigated how the relative changes in expression between these factors influences reprogramming and if these changes are due to positive or negative signaling feedback. The transcriptional profiles of individual retinal cell types suggest that MDK and PTN likely have autocrine and paracrine actions that are dynamically regulated in resting and damaged retinas, and, in part, manifested through MG in both chick and mouse retinas.
MDK-signaling in MG
Application of MDK prior to NMDA-induced damage decreased numbers of proliferating MGPCs and decreased numbers of dying cells. Levels of retinal damage positively correlate to the proliferative response of MG (Fischer and Reh, 2001; Fischer et al., 2004). We propose that the neuroprotective effects of MDK secondarily influenced the proliferative of MGPCs. It is possible that the addition of MDK to damaged retinas did not influence MGPCs because of “ceiling effects” wherein (i) ligand/receptor interactions are saturated, (ii) the activity of secondary messengers are saturated, or (iii) the massive up-regulation of MDK by MG is not directly involved in driving the formation of proliferating MGPCs.
The phosphatase inhibitor Na3VO4 suppressed the formation of MGPCs and increased cell death, and these effects where blocked by addition of MDK. Unfortunately, this compound likely targeted tyrosine phosphatases in addition to PTPRZ1. However, exogenous MDK reversed the inhibitory effects of Na3VO4 on the proliferation of MGPCs, suggesting that MDK signaling was, in part, targeted by the Na3VO4. We cannot exclude the possibility that some of the Na3VO4 mediated-effects, such as increased cell death and accumulation of NIRG cells, were mediated by inhibition of phosphatases in addition to PTPRZ1. We further investigated second messengers associated with ITGB1 receptors., including PP2A and PAK1. Inhibition of Git/Cat-1/PAK1-signaling is associated with ITGB1-mediated cytoskeleton remodeling during migration and proliferation (Martin et al., 2016; Muramatsu et al., 2004). Consistent with these observations, we found that inhibition of PAK1 and PP2A effectively suppressed the formation of MGPCs in damaged retinas. Further studies are required to identify changes in phosphorylation and expression of targets that are down-stream of PP2A activity. Collectively, these findings suggest that MDK promotes the formation of proliferating MGPCs via different receptors and cell-signaling pathways in the chick retina (Fig. 11).
MDK and cell-signaling inhibitors did not have significant impacts upon MG and microglia in retinas treated with insulin and FGF2. Similar to NMDA-treatment, we see significant changes in expression levels of MDK, PTN, PAK1 and CSPG5, suggesting that MDK-signaling is active in undamaged retinas treated with insulin and FGF2 (see Fig. 7). However, direct comparison of relative expression levels of MDK and related genes in MG across all treatment groups indicated that: (i) although MDK is up-regulated with insulin and FGF2, levels are much less than those seen with damage, (ii) SDC4 is modestly induced in MG by insulin and FGF2, and (iii) levels of PAK1, CSPG5, ITGB1, PPP2CA and CDC4 are further down-regulated by insulin and FGF2 compared to levels in MG in damaged retinas. The diminished levels of MDK-receptors and signal transducers in MG treated with insulin and FGF2, compared to levels in MG in damaged retinas, may underlie the absence of effects of exogenous MDK and inhibitors in undamaged retinas. Collectively, these findings suggest that MDK and down-stream signaling are not required for the formation of MGPCs in undamaged retinas treated with insulin+FGF2. Alternatively, the cell-signaling pathways that are activated by MDK are the same as those activated by insulin+FGF2 and there is no net gain in second messenger activation in MG by combining these factors. This may be unique because many signaling pathways that have been implicated in regulating the formation of MGPCs in the chick retina are active following both NMDA-induced damage and treatment with insulin and FGF2. These pathways include MAPK (Fischer et al., 2009a; Fischer et al., 2009b), mTOR (Zelinka et al., 2016), Notch (Ghai et al., 2010; Hayes et al., 2007), Jak/Stat (Todd et al., 2016), Wnt/b-catenin (Gallina et al., 2015), glucocorticoid (Gallina, 2015), Hedgehog (Todd and Fischer, 2015), BMP/SMAD (Todd et al., 2017), retinoic acid (Todd et al., 2018) and NFkB-signaling (Palazzo et al., 2020).
MDK signaling in NIRG cells
A well-established receptor of MDK is PTPRz (Maeda et al., 1999). PTPRz is a cell-surface receptor that acts as a protein tyrosine phosphatase and is known to promotes proliferation (Fujikawa et al., 2016). This receptor is activated by MDK (Sakaguchi et al., 2003), but is deactivated by the binding of PTN through dimerization and tyrosine phosphorylation (Kuboyama et al., 2015). In the chick retina, the NIRG cells predominantly express PTPRZ1 and the accumulation of these cells in response to damage was decreased with MDK-treatment and increased by treatment with phosphatase inhibitor Na3VO4 (Fig. 11). The accumulation of NIRG cells may result, in part, from migration, as MDK has been associated with migration and process elongation in different cell types (Ichihara-Tanaka et al., 2006; Kuboyama et al., 2015; Qi et al., 2001). Given that nothing is currently known about the specific functions of NIRG cells, it is difficult to infer how MDK-signaling in these glia impacts the reprogramming of MG or function/survival of retinal neurons.
MDK and reprogramming of MG into MGPCs
There has been significant investigation into the different cell-signaling pathways involved in the reprogramming of MG into proliferating MGPCs. IGF1, BMP, retinoic acid, HB-EGF, sonic hedgehog, Wnt, and CNTF are known to enhance the formation of MGPCs (Fischer et al., 2009a; Fischer et al., 2009b; Gallina et al., 2015; Todd and Fischer, 2015; Todd et al., 2016; Todd et al., 2017; Todd et al., 2018). The roles of these different pathways are similar in chick and zebrafish models of retinal regeneration, despite different capacities for neurogenesis (Goldman, 2014; Wan and Goldman, 2016). MDK has been implicated as an important factor for reprogramming and cellular proliferation of MGPCs (Ang et al., 2020; Gramage et al., 2015; Nagashima et al., 2019a; Nagashima et al., 2019b). In the chick model, different inhibitors to PAK1, PP2A and PTPRZ1 had relatively modest impacts on the formation of MGPCs. Although exogenous MDK likely added nothing to already saturated levels in damaged retinas, MDK or PTN alone were not sufficient to induce the formation of MGPCs in the absence of damage when levels of MDK were low. These findings suggest that MDK signaling is not a primary signaling component to drive the formation of MGPCs in chick, unlike the key role for MDK seen in zebrafish (Gramage et al., 2015; Nagashima et al., 2019b). In the chick, our findings suggests that MDK has pleiotropic roles and serves to both minimize neuronal cell death after damage and regulate the accumulation of NIRG cells. Similar to effects seen in chick MG, MDK stimulated mTor-signaling and cFos expression in mouse MG, but only induced a modest increase in numbers of proliferating MGPCs in damaged retinas. The neurogenic potential of these proliferating MG remains to be determined, but is likely to be very low given previous reports of MG-mediated regeneration in the rodent retina (Karl et al., 2008; Ooto et al., 2004).
Conclusions:
MDK and PTN are highly expressed by maturing MG in embryonic retinas. MDK is down-regulated while PTN remains highly expressed by resting MG in the retinas of hatched chicks. Similarly, in mature mouse retinas PTN and MDK were highly expressed by mature resting MG. When MG are stimulated by growth factors or neuronal damage in chick retinas, MDK is robustly up-regulated whereas PTN is down-regulated. By contrast, MDK and PTN are both down-regulated by MG in damaged mouse retinas. Exogenous MDK had significant effects upon proliferating glia, formation of MGPCs, and glial reactivity, whereas we did not detect cellular responses to exogenous PTN. When applied before excitotoxic injury, MDK conveyed survival-promoting effects upon inner retinal neurons in both chick and mouse models. Inhibitors of ITGB1-signaling reduced MGPC formation and phosphatase inhibitor Na3VO4 over-rode the effects of MDK upon neuronal survival and MGPC formation. These effects were limited to damaged retinas, whereas in undamaged retinas treated with insulin+FGF2 these effects were not observed despite dynamic changes in expression of MDK and related genes. Although MDK activated cFOS and mTor-signaling in MG in both chick and mouse retinas, MDK had a very modest effect in stimulating the dedifferentiation and proliferation of MG in damaged mouse retinas. Overall, the up-regulation of MDK in the chick retina is among the largest increases in gene expression detected in MG during maturation, or caused by neuronal damage, implying significant multifactorial functions in the context of development, reprogramming, and glial responses to tissue damage.
Supplementary Material
Midkine Antibodies.
| Antigen | Working Dilution | Host, Clone, or Catalog Number | Source |
|---|---|---|---|
| AP2α | 1:20000 | Mouse, | DSHB |
| Calretinin | 1:1000 | Rabbit, CR 7697 | Swant |
| CD45 | 1:200 | Mouse, | DSHB |
| Draq5 | 1:2000 | 62251 | Thermo Scientific |
| Neurofilament | 1:80 | Mouse, | DSHB |
| Nkx2.2 | 1:50 | Mouse, | DSHB |
| Olig2 | 1:1000 | Goat, | |
| Pax6 | 1:1000 | Rabbit, 901301 | Biolegend |
| pERK | 1:600 | Rabbit, | Cell Signaling Technologies |
| pHH3 | 1:600 | Rabbit | DSHB |
| Sox2 | 1:1000 | Goat, | Sigma |
| Sox9 | 1:2000 | Rabbit, | EMD Millipore |
| Transitin | 1:600 | Mouse, | DSHB |
Main Points.
Midkine and pleotrophin are expressed by Müller glia in chick and mouse retinas. In the chick, midkine is up-regulated by Müller glia during development and reprogramming into proliferating progenitor-like cells, but not in mouse. Midkine, but not pleotrophin, induces cFos and pS6 (mTor-signaling) in Müller glia, protects neurons against excitotoxicty, and promotes the proliferation of Müller glia-derived progenitor cells in both chick and mouse.
Acknowledgements:
This work was supported by RO1 EY022030-08 (AJF) and UO1 EY027267-04 (SB, AJF).
Footnotes
Competing Interests: The authors have no competing interests to declare.
Data availability: RNA-Seq data are deposited in GEO (GSE135406), Github (https://github.com/fischerlab3140/scRNAseq_libraries), and chick scRNA-Seq data can be queried at https://proteinpaint.stjude.org/F/2019.retina.scRNA.html.
References:
- Ang NB, Saera-Vila A, Walsh C, Hitchcock PF, Kahana A, Thummel R and Nagashima M (2020). Midkine-a functions as a universal regulator of proliferation during epimorphic regeneration in adult zebrafish. PLOS ONE 15, e0232308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia S and Cerione RA (1999). PAK to the future. Trends Cell Biol. 9, 350–355. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR and Raymond PA (2007). Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci 27, 7028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E and Satija R (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol 36, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calinescu A-A, Vihtelic TS, Hyde DR and Hitchcock PF (2009). The Cellular Expression of Midkine-a and Midkine-b During Retinal Development and Photoreceptor Regeneration in Zebrafish. J. Comp. Neurol 514, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L-C, Yao X, Wang X, Niu S-Q, Zhou L-F, Fu F-F, Yang S-X and Ping J-L (2009). In vitro and in vivo suppression of hepatocellular carcinoma growth by midkine-antisense oligonucleotide-loaded nanoparticles. World J. Gastroenterol. WJG 15, 1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon SW, Beeser A, Fukui JA, Rennefahrt UEE, Myers C, Chernoff J and Peterson JR (2008). An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol 15, 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri L, Maruta H, Muramatsu H, Muramatsu T, Simpson RJ, Burgess AW and Nice EC (1993). Structural characterisation of native and recombinant forms of the neurotrophic cytokine MK. J. Chromatogr 646, 213–225. [DOI] [PubMed] [Google Scholar]
- Fausett BV and Goldman D (2006). A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci 26, 6303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD and Goldman D (2008). The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J. Neurosci. Off. J. Soc. Neurosci 28, 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ and Reh TA (2001). Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci 4, 247–252. [DOI] [PubMed] [Google Scholar]
- Fischer AJ and Reh TA (2002). Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol 251, 367–79. [DOI] [PubMed] [Google Scholar]
- Fischer AJ and Reh TA (2003). Potential of Muller glia to become neurogenic retinal progenitor cells. Glia 43, 70–6. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RLP, Poon J and Stell WK (1998). Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J. Comp. Neurol 393, 1–15. [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD and Reh TA (2002a). Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development 129, 2283–91. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD and Reh TA (2002b). Insulin and Fibroblast Growth Factor 2 Activate a Neurogenic Program in Müller Glia of the Chicken Retina. J. Neurosci 22, 9387–9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Foster S, Scott MA and Sherwood P (2008). The transient expression of LIM-domain transcription factors is coincident with the delayed maturation of photoreceptors in the chicken retina. J. Comp. Neurol 506, 584–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Ritchey ER and Sherwood P (2009a). Mitogen-activated protein kinase-signaling regulates the ability of Müller glia to proliferate and protect retinal neurons against excitotoxicity. Glia 57, 1538–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA and Tuten W (2009b). Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia 57, 166–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Gallina D, Scott MA and Todd L (2014). Reactive microglia and macrophage facilitate the formation of Müller glia-derived retinal progenitors. Glia 62, 1608–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM (2000). cAMP takes control. Nat. Cell Biol 2, E167–E168. [DOI] [PubMed] [Google Scholar]
- Fujikawa A, Nagahira A, Sugawara H, Ishii K, Imajo S, Matsumoto M, Kuboyama K, Suzuki R, Tanga N, Noda M, et al. (2016). Small-molecule inhibition of PTPRZ reduces tumor growth in a rat model of glioblastoma. Sci. Rep 6, 20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada M, Fujikawa A, Chow JPH, Ikematsu S, Sakuma S and Noda M (2006). Protein tyrosine phosphatase receptor type Z is inactivated by ligand-induced oligomerization. FEBS Lett. 580, 4051–4056. [DOI] [PubMed] [Google Scholar]
- Gallina DZ, Cebulla CP, Fischer CM, A. J. (2015). Activation of glucocorticoid receptors in Müller glia is protective to retinal neurons and suppresses microglial reactivity. Exp Neurol 273, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Todd L and Fischer AJ (2014). A comparative analysis of Müller glia-mediated regeneration in the vertebrate retina. Exp. Eye Res 123, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Palazzo I, Steffenson L, Todd L and Fischer AJ (2015). Wnt/betacatenin-signaling and the formation of Muller glia-derived progenitors in the chick retina. Dev Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C and Fischer AJ (2009). Serotonin released from amacrine neurons is scavenged and degraded in bipolar neurons in the retina. J Neurochem 111, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C and Fischer AJ (2010). Notch signaling influences neuroprotective and proliferative properties of mature Muller glia. J Neurosci 30, 3101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D (2014). Müller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci 15, 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramage E, Li J and Hitchcock P (2014). The expression and function of midkine in the vertebrate retina. Br J Pharmacol 171, 913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramage E, D’Cruz T, Taylor S, Thummel R and Hitchcock PF (2015). Midkine-a Protein Localization in the Developing and Adult Retina of the Zebrafish and Its Function During Photoreceptor Regeneration. PLoS ONE 10,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Maeda Y, Fukazawa T, Yamatsuji T, Takaoka M, Bao X-H, Matsuoka J, Okui T, Shimo T, Takigawa N, et al. (2013). Inhibition of the Growth Factor MDK/Midkine by a Novel Small Molecule Compound to Treat Non-Small Cell Lung Cancer. PLOS ONE 8, e71093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Nelson BR, Buckingham B and Reh TA (2007). Notch signaling regulates regeneration in the avian retina. Dev Biol 312, 300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hienola A, Pekkanen M, Raulo E, Vanttola P and Rauvala H (2004). HB-GAM inhibits proliferation and enhances differentiation of neural stem cells. Mol. Cell. Neurosci 26, 75–88. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF and Raymond PA (1992). Retinal regeneration. Trends Neurosci 15, 103–8. [DOI] [PubMed] [Google Scholar]
- Hoang T, Wang J, Boyd P, Wang F, Santiago C, Jiang L, Yoo S, Lahne M, Todd LJ, Jia M, et al. (2020). Gene regulatory networks controlling vertebrate retinal regeneration. Science 370,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara-Tanaka K, Oohira A, Rumsby M and Muramatsu T (2006). Neuroglycan C Is a Novel Midkine Receptor Involved in Process Elongation of Oligodendroglial Precursor-like Cells. J. Biol. Chem 281, 30857–30864. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kähäri V-M and Heino J (1999). Integrin α2β1 Mediates Isoform-Specific Activation of p38 and Upregulation of Collagen Gene Transcription by a Mechanism Involving the α2 Cytoplasmic Tail. J. Cell Biol 147, 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Nissinen L, Immonen N, Eriksson JE, Kähäri V-M and Heino J (2002). Integrin α2β1 Promotes Activation of Protein Phosphatase 2A and Dephosphorylation of Akt and Glycogen Synthase Kinase 3β. Mol. Cell. Biol 22, 1352–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki W, Nagata K, Hatanaka H, Inui T, Kimura T, Muramatsu T, Yoshida K, Tasumi M and Inagaki F (1997). Solution structure of midkine, a new heparin-binding growth factor. EMBO J. 16, 6936–6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochheim-Richter A, Rüdrich U, Koczan D, Hillemann T, Tewes S, Petry M, Kispert A, Sharma AD, Attaran F, Manns MP, et al. (2006). Gene expression analysis identifies novel genes participating in early murine liver development and adult liver regeneration. Differentiation 74, 167–173. [DOI] [PubMed] [Google Scholar]
- Jung C-G, Hida H, Nakahira K, Ikenaka K, Kim H-J and Nishino H (2004). Pleiotrophin mRNA is highly expressed in neural stem (progenitor) cells of mouse ventral mesencephalon and the product promotes production of dopaminergic neurons from embryonic stem cell-derived nestin-positive cells. FASEB J. 18, 1237–1239. [DOI] [PubMed] [Google Scholar]
- Kang HC, Kim I-J, Park J-H, Shin Y, Ku J-L, Jung MS, Yoo BC, Kim HK and Park J-G (2004). Identification of Genes with Differential Expression in Acquired Drug-Resistant Gastric Cancer Cells Using High-Density Oligonucleotide Microarrays. Clin. Cancer Res 10, 272–284. [DOI] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B and Reh TA (2008). Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U A 105, 19508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi H, Fujikawa A, Maeda N and Noda M (2001). Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase ζ/β by the yeast substrate-trapping system. Proc. Natl. Acad. Sci. U. S. A 98, 6593–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi‐Horie K, Kawakami E, Kamata M, Wada M, Hu J-G, Nakagawa H, Ohara K, Watabe K and Oyanagi K (2004). Distinctive expression of midkine in the repair period of rat brain during neurogenesis: Immunohistochemical and immunoelectron microscopic observations. J. Neurosci. Res 75, 678–687. [DOI] [PubMed] [Google Scholar]
- Kilpeläinen I, Kaksonen M, Kinnunen Tarja §∥, Avikainen H, Fath M, Linhardt RJ, Raulo E and Rauvala H (2000). Heparin-binding Growth-associated Molecule Contains Two Heparin-binding β-Sheet Domains That Are Homologous to the Thrombospondin Type I Repeat. J. Biol. Chem 275, 13564–13570. [DOI] [PubMed] [Google Scholar]
- Kim S-M, Kwon MS, Park CS, Choi K-R, Chun J-S, Ahn J and Song WK (2004). Modulation of Thr Phosphorylation of Integrin β1 during Muscle Differentiation. J. Biol. Chem 279, 7082–7090. [DOI] [PubMed] [Google Scholar]
- Kojima T, Katsumi A, Yamazaki T, Muramatsu T, Nagasaka T, Ohsumi K and Saito H (1996). Human Ryudocan from Endothelium-like Cells Binds Basic Fibroblast Growth Factor, Midkine, and Tissue Factor Pathway Inhibitor. J. Biol. Chem 271, 5914–5920. [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Fujikawa A, Suzuki R and Noda M (2015). Inactivation of Protein Tyrosine Phosphatase Receptor Type Z by Pleiotrophin Promotes Remyelination through Activation of Differentiation of Oligodendrocyte Precursor Cells. J. Neurosci 35, 12162–12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE and Barnes CJ (2006). p21-activated kinases in cancer. Nat. Rev. Cancer 6, 459. [DOI] [PubMed] [Google Scholar]
- Kurosawa N, Chen G-Y, Kadomatsu K, Ikematsu S, Sakuma S and Muramatsu T (2001). Glypican-2 binds to midkine: The role of glypican-2 in neuronal cell adhesion and neurite outgrowth. Glycoconj. J 18, 499–507. [DOI] [PubMed] [Google Scholar]
- Liu Y, Beyer A and Aebersold R (2016). On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 165, 535–550. [DOI] [PubMed] [Google Scholar]
- Luo J, Uribe RA, Hayton S, Calinescu A-A, Gross JM and Hitchcock PF (2012). Midkine-A functions upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate retina. Neural Develop. 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T and Noda M (1999). A Receptor-like Protein-tyrosine Phosphatase PTPζ/RPTPβ Binds a Heparin-binding Growth Factor Midkine INVOLVEMENT OF ARGININE 78 OF MIDKINE IN THE HIGH AFFINITY BINDING TO PTPζ. J. Biol. Chem 274, 12474–12479. [DOI] [PubMed] [Google Scholar]
- Martin K, Pritchett J, Llewellyn J, Mullan AF, Athwal VS, Dobie R, Harvey E, Zeef L, Farrow S, Streuli C, et al. (2016). PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat. Commun 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima T, Sato S, Sugimoto Y, Tsuruo T and Seimiya H (2009). Promotion of glioma cell survival by acyl-CoA synthetase 5 under extracellular acidosis conditions. Oncogene 28, 9–19. [DOI] [PubMed] [Google Scholar]
- Masui M, Okui T, Shimo T, Takabatake K, Fukazawa T, Matsumoto K, Kurio N, Ibaragi S, Naomoto Y, Nagatsuka H, et al. (2016). Novel Midkine Inhibitor iMDK Inhibits Tumor Growth and Angiogenesis in Oral Squamous Cell Carcinoma. Anticancer Res. 36, 2775–2781. [PubMed] [Google Scholar]
- Mirkin BL, Clark S, Zheng X, Chu F, White BD, Greene M and Rebbaa A (2005). Identification of midkine as a mediator for intercellular transfer of drug resistance. Oncogene 24, 4965. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Salmivirta M, Muramatsu T, Muramatsu H, Rauvala H, Lehtonen E, Jalkanen M and Thesleff I (1995). Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development 121, 37–51. [DOI] [PubMed] [Google Scholar]
- Miyashiro M, Kadomatsu K, Ogata N, Yamamoto C, Takahashi K, Uyama M, Muramatsu H and Muramatsu T (1998). Midkine expression in transient retinal ischemia in the rat. Curr. Eye Res 17, 9–13. [DOI] [PubMed] [Google Scholar]
- Mulrooney J, Foley K, Vineberg S, Barreuther M and Grabel L (2000). Phosphorylation of the β1 Integrin Cytoplasmic Domain: Toward an Understanding of Function and Mechanism. Exp. Cell Res 258, 332–341. [DOI] [PubMed] [Google Scholar]
- Muramatsu T (2002). Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 132, 359–71. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Zou K, Sakaguchi N, Ikematsu S, Sakuma S and Muramatsu T (2000). LDL Receptor-Related Protein as a Component of the Midkine Receptor. Biochem. Biophys. Res. Commun 270, 936–941. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Zou P, Suzuki H, Oda Y, Chen G-Y, Sakaguchi N, Sakuma S, Maeda N, Noda M, Takada Y, et al. (2004). α4β1- and α6β1-integrins are functional receptors for midkine, a heparin-binding growth factor. J. Cell Sci 117, 5405–5415. [DOI] [PubMed] [Google Scholar]
- Nagashima M, D’Cruz T, Hesse D and Hitchcock PF (2019a). Midkine-a deficiency causes cell cycle arrest and reactive gliosis in zebrafish Müller glia following photoreceptor cell death. Invest. Ophthalmol. Vis. Sci 60, 3115–3115. [Google Scholar]
- Nagashima M, D’Cruz TS, Danku AE, Hesse D, Sifuentes C, Raymond PA and Hitchcock PF (2019b). Midkine-a is required for cell cycle progression of Müller glia glia during neuronal regeneration in the vertebrate retina. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Kadomatsu K, Okamoto T, Ichihara-Tanaka K, Kojima T, Saito H, Tomoda Y and Muramatsu T (1997). Expression of Syndecan-1 and −3 during Embryogenesis of the Central Nervous System in Relation to Binding with Midkine. J. Biochem. (Tokyo) 121, 197–205. [PubMed] [Google Scholar]
- Obama H, Biro S, Tashiro T, Tsutsui J, Ozawa M, Yoshida H, Tanaka H and Muramatsu T (1998). Myocardial infarction induces expression of midkine, a heparin-binding growth factor with reparative activity. Anticancer Res. 18, 145–152. [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y and Takahashi M (2004). Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U A 101, 13654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo I, Deistler K, Hoang TV, Blackshaw S and Fischer AJ (2020). NF-κB signaling regulates the formation of proliferating Müller glia-derived progenitor cells in the avian retina. Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, Levine EM and Reh TA (2013). ASCL1 reprograms mouse Müller glia into neurogenic retinal progenitors. Dev. Camb. Engl 140, 2619–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Ikematsu S, Maeda N, Ichihara-Tanaka K, Sakuma S, Noda M, Muramatsu T and Kadomatsu K (2001). Haptotactic Migration Induced by Midkine INVOLVEMENT OF PROTEIN-TYROSINE PHOSPHATASE ζ, MITOGEN-ACTIVATED PROTEIN KINASE, AND PHOSPHATIDYLINOSITOL 3-KINASE. J. Biol. Chem 276, 15868–15875. [DOI] [PubMed] [Google Scholar]
- Qiu X, Hill A, Packer J, Lin D, Ma Y-A and Trapnell C (2017a). Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA and Trapnell C (2017b). Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulo E, Chernousov MA, Carey DJ, Nolo R and Rauvala H (1994). Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3). J. Biol. Chem 269, 12999–13004. [PubMed] [Google Scholar]
- Raymond PA (1991). Retinal regeneration in teleost fish. Ciba Found Symp 160, 171–86; discussion 186–91. [DOI] [PubMed] [Google Scholar]
- Reiff T, Huber L, Kramer M, Delattre O, Janoueix-Lerosey I and Rohrer H (2011). Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. Development 138, 4699–4708. [DOI] [PubMed] [Google Scholar]
- Reynolds PR, Mucenski ML, Cras TDL, Nichols WC and Whitsett JA (2004). Midkine Is Regulated by Hypoxia and Causes Pulmonary Vascular Remodeling. J. Biol. Chem 279, 37124–37132. [DOI] [PubMed] [Google Scholar]
- Roger J, Brajeul V, Thomasseau S, Hienola A, Sahel J-A, Guillonneau X and Goureau O (2006). Involvement of Pleiotrophin in CNTF-mediated differentiation of the late retinal progenitor cells. Dev. Biol 298, 527–539. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Muramatsu H, Ichihara-Tanaka K, Maeda N, Noda M, Yamamoto T, Michikawa M, Ikematsu S, Sakuma S and Muramatsu T (2003). Receptor-type protein tyrosine phosphatase ζ as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci. Res 45, 219–224. [DOI] [PubMed] [Google Scholar]
- Sakakima H, Kamizono T, Matsuda F, Izumo K, Ijiri K and Yoshida Y (2006). Midkine and its receptor in regenerating rat skeletal muscle after bupivacaine injection. Acta Histochem. 108, 357–364. [DOI] [PubMed] [Google Scholar]
- Salama RHM, Muramatsu H, Zou P, Okayama M and Muramatsu T (2006). Midkine, a heparin-binding growth factor, produced by the host enhances metastasis of Lewis lung carcinoma cells. Cancer Lett. 233, 16–20. [DOI] [PubMed] [Google Scholar]
- Satija R, Farrell JA, Gennert D, Schier AF and Regev A (2015). Spatial reconstruction of single-cell gene expression data. Nat Biotechnol 33, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Xi G, Wai C and Clemmons DR (2015). The Coordinate Cellular Response to Insulin-like Growth Factor-I (IGF-I) and Insulin-like Growth Factor-binding Protein-2 (IGFBP-2) Is Regulated through Vimentin Binding to Receptor Tyrosine Phosphatase β (RPTPβ). J. Biol. Chem 290, 11578–11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhang J, Wang J, Cao Z, Wang J, Guo X and Dong W (2014). β1 integrin modulates tumor growth and apoptosis of human colorectal cancer. Oncol. Rep 32, 302–308. [DOI] [PubMed] [Google Scholar]
- Stanke J, Moose HE, El-Hodiri HM and Fischer AJ (2010). Comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J Comp Neurol 518, 2316–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, Caughey DJ, Wen D, Karavanov A, Riegel AT, et al. (2001). Identification of Anaplastic Lymphoma Kinase as a Receptor for the Growth Factor Pleiotrophin. J. Biol. Chem 276, 16772–16779. [DOI] [PubMed] [Google Scholar]
- Takei Y, Kadomatsu K, Matsuo S, Itoh H, Nakazawa K, Kubota S and Muramatsu T (2001). Antisense Oligodeoxynucleotide Targeted to Midkine, a Heparin-binding Growth Factor, Suppresses Tumorigenicity of Mouse Rectal Carcinoma Cells. Cancer Res. 61, 8486–8491. [PubMed] [Google Scholar]
- Takei Y, Kadomatsu K, Goto T and Muramatsu T (2006). Combinational antitumor effect of siRNA against midkine and paclitaxel on growth of human prostate cancer xenografts. Cancer 107, 864–873. [DOI] [PubMed] [Google Scholar]
- Thillai K, Lam H, Sarker D and Wells CM (2016). Deciphering the link between PI3K and PAK: An opportunity to target key pathways in pancreatic cancer? Oncotarget 8, 14173–14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L and Fischer AJ (2015). Hedgehog signaling stimulates the formation of proliferating Müller glia-derived progenitor cells in the chick retina. Dev. Camb. Engl 142, 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Squires N, Suarez L and Fischer AJ (2016). Jak/Stat signaling regulates the proliferation and neurogenic potential of Müller glia-derived progenitor cells in the avian retina. Sci. Rep 6,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Palazzo I, Squires N, Mendonca N and Fischer AJ (2017). BMP- and TGFβ-signaling regulate the formation of Müller glia-derived progenitor cells in the avian retina. Glia 65, 1640–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Suarez L, Quinn C and Fischer AJ (2018). Retinoic Acid-Signaling Regulates the Proliferative and Neurogenic Capacity of Müller Glia-Derived Progenitor Cells in the Avian Retina. STEM CELLS 36, 392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7, 562–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui J, Uehara K, Kadomatsu K, Matsubara S and Muramatsu T (1991). A new family of heparin-binding factors: Strong conservation of midkine (MK) sequences between the human and the mouse. Biochem. Biophys. Res. Commun 176, 792–797. [DOI] [PubMed] [Google Scholar]
- Tsutsui J, Kadomatsu K, Matsubara S, Nakagawara A, Hamanoue M, Takao S, Shimazu H, Ohi Y and Muramatsu T (1993). A New Family of Heparin-binding Growth/Differentiation Factors: Increased Midkine Expression in Wilms’ Tumor and Other Human Carcinomas. Cancer Res. 53, 1281–1285. [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, Sternhagen K, Simic M, Ullom K, Nakafuku M and Reh TA (2015). Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci 112, 13717–13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki K, Ohba N, Arimura H, Muramatsu H and Muramatsu T (1994). Rescue of photoreceptors from the damaging effects of constant light by midkine, a retinoic acid-responsive gene product. Invest. Ophthalmol. Vis. Sci 35, 4063–4068. [PubMed] [Google Scholar]
- Wan J and Goldman D (2016). Retina regeneration in zebrafish. Curr. Opin. Genet. Dev 40, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C and Yao S (2014). The midkine family of growth factors: diverse roles in nervous system formation and maintenance. Br. J. Pharmacol 171, 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zhu S, Wu M, Han W and Yu Y (2014). Functional Receptors and Intracellular Signal Pathways of Midkine (MK) and Pleiotrophin (PTN). Biol. Pharm. Bull 37, 511–520. [DOI] [PubMed] [Google Scholar]
- Zelinka CP, Scott MA, Volkov L and Fischer AJ (2012). The Reactivity, Distribution and Abundance of Non-Astrocytic Inner Retinal Glial (NIRG) Cells Are Regulated by Microglia, Acute Damage, and IGF1. PLOS ONE 7, e44477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinka CP, Volkov L, Goodman ZA, Todd L, Palazzo I, Bishop WA and Fischer AJ (2016). mTor signaling is required for the formation of proliferating Müller glia-derived progenitor cells in the chick retina. Dev. Camb. Engl 143, 1859–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Muramatsu H, Ikematsu S, Sakuma S, Salama RHM, Shinomura T, Kimata K and Muramatsu T (2000). A heparin-binding growth factor, midkine, binds to a chondroitin sulfate proteoglycan, PG-M/versican. Eur. J. Biochem 267, 4046–4053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.