Abstract
Background and Objectives:
Exhaled breath condensate (EBC) represents a potential diagnostic tool for Primary Ciliary Diskinesia (PCD). An increased oxidative stress is present in the airways of children affected and many neutrophil chemoattractants and markers of oxidative stress can be involved. The aim of the study is to evaluate leukotriene B4 (LTB-4), interleukin 8 (IL-8), 8-isoprostane (8-IP) concentration in PCD subjects, investigating their potential role as non-invasive markers of inflammation for the diagnosis and management of PCD.
Methods:
Cross-sectional study. 43 patients were enrolled in the study and divided in two groups: PCD (27) and healthy subjects (16). Physical examination, lung function test, nFeNO measurement and EBC collection were performed in all subjects.
Results:
PCD subjects showed an EBC 8-IP concentration significantly higher than the control group (median value: 11.9 pg/ml; IQR, 5.5–24.0 vs. median, 6.7 pg/ml; IQR, 2.5–11.3, p-value of Wilcoxon rank-sum test 0.0436). LTB4 EBC concentration did not differ between the two group (median, 4.3 pg/ml; IQR, 3.0–8.8 vs. 7.5 pg/ml; IQR, 3.0–9.5; P=0.4901). No significant correlation was found between FEV1 and EBC 8-IP (r=-0.10, P=0.6314) or LTB4 concentration (r=0.03, P=0.8888) in PCD subjects. No significant correlation was found between nFeNO and EBC 8-IP (r=-0.31, P=0.1385) or LTB4 (r=0.04, P=0.8565) in PCD subjects.
Conclusions:
EBC 8-IP levels are significantly increased in PCD subjects, highlighting the role of oxidative stress in airway inflammation. It could have a potential role as a non-invasive marker of inflammation for the diagnosis and management of PCD, although a therapeutic application of this evidence seems far.
KEY WORDS: 8-isoprostane, exhaled breath condensate, primary ciliary dyskinesia
INTRODUCTION
Only two guidelines[1,2] and two consensus recommendations [3,4] were published on the topic of primary ciliary dyskinesia (PCD) in the last 4 years. Definition, symptoms, and signs of disease presentation in different age groups are known.[1] About the PCD diagnosis, both guidelines and consensus recommendations suggest nasal fractional nitric oxide (NO) measurement as a fundamental tool in adults and children >5 years old.[1,2,3,4] PCD genetic testing panels, ciliary biopsy with electron microscopy, functional ciliary beat, and immunofluorescence testing represent further recommended diagnostic testing methods for PCD diagnosis.[2] On the other hand, nasal saccharin testing, visual assessment of ciliary motion without high-speed recording devices, and ciliary beat frequency calculation are no recommended for PCD evaluation because their limitations represented by a high percentage of false-negative and false-positive results, especially in uncooperative children.[2] Exhaled breath condensate (EBC) can represent a useful diagnostic tool in the PCD diagnosis and management.[5] To the best of our knowledge, recent scientific literature has shown no progress in this field in the last 4 years. Specifically, only three articles were published, focusing the attention, especially on metabolomics. EBC represents a matrix in which biomarkers can be identified. Beyond the metabolomics, a lot of biomarkers including chemokines, cytokines, and eicosanoids have been reproducibly validated in EBC.[6] On the basis of the current state of scientific knowledge, EBC does not represent a validated diagnostic tool for PCD diagnosis and management.[6] On the other hand, it is demonstrated that EBC can be analyzed in children with asthma and cystic fibrosis to investigate several inflammatory pathways involved.[7] About PCD, it is demonstrated that NO concentration does not differ between pathological and healthy subjects.[8] On the other hand, an increased oxidative stress, caused by a persistent neutrophilic inflammation, is present in the airways of children with PCD.[5] A lot of neutrophil chemoattractants and markers of oxidative stress, such as leukotriene B4 (LTB-4), interleukin 8 (IL-8), 8-isoprostane (8-IP), and leptin can be involved in the pathogenesis of PCD.[5,9] Zihlif et al. demonstrated a statistically significant increase of 8-IP concentration in EBC of children affected by PCD; on the contrary, no difference in EBC IL-8 and LTB4 was found between pathological and healthy subjects.[5] Continuing along this line of research, the aim of the present study is to evaluate LTB4 and 8-IP EBC concentration in PCD, investigating their potential role as a noninvasive marker of inflammation for the diagnosis and management of PCD.
MATERIAL AND METHODS
Study population
This was a cross-sectional study. 43 patients were enrolled in the study (14 males, 29 females; 6–50 years old) and divided into two groups: PCD (27) and healthy subjects (16).
PCD group was made up by 27 subjects (11 males, 16 females; 6–50 years old). Exclusion criteria were represented by a history of smokers (current and past smokers), use of inhaled corticosteroids or nasal decongestant drugs, or adenotonsillectomy.
The control group was made up by 16 subjects (3 males, 13 females; 6–50 years old). All subjects performed a normal spirometry; their exclusion criteria were represented by the same of PCD group; moreover, subjects with a history of chronic respiratory disease and an acute airway infection within 2 weeks before study entry were excluded from the study.
At recruitment, physical examination, lung function test, nasal fractional concentration of exhaled NO (nFeNO) measurement, and EBC collection were performed.
The study was approved by the Ethical Committee CF Centre of Verona (code CE1716, 2009) and all patients gave their informed consent.
Exhaled breath condensate collection
EBC was collected by a condensing device formed by two glass chambers (Incofar Srl, Modena, Italy). The inner glass chamber was cooled by means of ice and suspended in a larger glass chamber. The children were instructed to tidally breathe by the mouth through a two-way nonrebreathing valve for 15 min. To minimize salivary contamination, the two-way valve served as a saliva trap, with a 12 cm banded tube vertically positioned between the mouthpiece and the condenser, while the mouth of the subject remained at a lower position with respect to the inlet of the device. Children were asked to periodically swallow their saliva. EBC samples were stored at –70°C. EBC 8-IP and LTB4 were measured by an immunoassay kit (Catalog No.: KHC0084, BioSource International Inc., Camarillo, California, USA) with the highest assayable concentration of 25 pg/ml and detection limit lower than 100 fg/ml. EBC values were obtained interpolating data from the standard curve (0.18–25 pg/ml). Optical density on EBC samples was significantly different from those obtained in blanks we used (distilled high purity water and reaction buffer).
Lung function measurement
Lung function was measured (forced vital capacity [FVC], forced expiratory volume in 1 s [FEV1], forced expiratory flow at 25 and 75% [FEF25%–75%]) by a Vitalograph Compact spirometer (Vitalograph Ltd, Buckingham, UK). The best value of three maneuvers was accepted and expressed as a percentage of the predicted normal values, according to ATS guidelines.[1] FEV1, FVC, maximum midexpiratory flow (FEF25%–75%), and Tiffeneau index (FEV1/FVC) were considered for the evaluation of PCD and healthy subjects.
Nasal fractional concentration of exhaled nitric oxide measurement
The fractional exhaled NO (FeNO) measurement was performed by an Ecomedics analyzer CLD 88sp (Ecomedics; Durnten, Switzerland) and was performed according to the ERS and ATS guidelines.[1] An analyzer calculated automatically exhaled NO levels at the end of the test.
Statistical analysis
Demographic and spirometric data were expressed as mean ± standard error (x ± SEM) of the mean. The analysis of data showed normal distribution for all the investigated parameters in our study population. Results were expressed as the median and interquartile range (IQR). EBC 8-IP and LTB4 levels in PCD patients and control group were compared by Wilcoxon rank-sum test. Correlations were evaluated by Spearman's rank test. P < 0.05 was considered significant in all cases.
RESULTS
All children performed EBC collection and FeNO measurement. On the other hand, only 23 children performed spirometry.
Exhaled breath condensate 8-isoprostane and LTB4 levels
PCD subjects showed an EBC 8-IP concentration significantly higher than the control group (median value: 11.9 pg/ml; IQR, 5.5–24.0 vs. median, 6.7 pg/ml; IQR, 2.5–11.3, P value of Wilcoxon rank-sum test 0.0436) [Figure 1].
Figure 1.
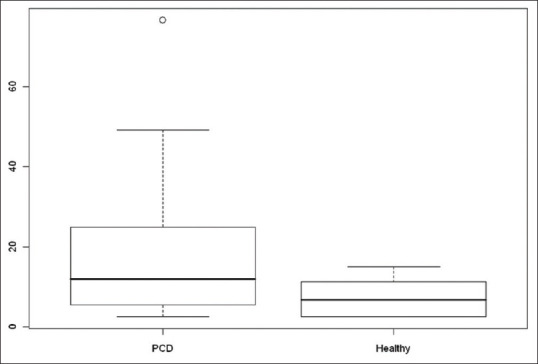
Exhaled breath condensate 8-isoprostane boxplot in primary ciliary dyskinesia and healthy subjects
On the contrary, LTB4 EBC concentration did not differ between the two groups (median, 4.3 pg/ml; IQR, 3.0–8.8 vs. 7.5 pg/ml; IQR, 3.0–9.5; P = 0.4901).
Lung function
Using Spearman's rank correlation, no significant correlation was found between FEV1 and EBC 8-IP (r = −0.10, P = 0.6314) or LTB4 concentration (r = 0.03, P = 0.8888) in PCD subjects.
Fractional concentration of exhaled nitric oxide
No significant correlation was found between nFeNO and EBC 8-IP (r = −0.31, P = 0.1385) or LTB4 (r = 0.04, P = 0.8565) in PCD subjects.
DISCUSSION
In agreement with previous literature,[5] PCD subjects showed an EBC 8-IP concentration significantly higher than the control group. On the other hand, EBC LTB4 concentration did not differ between the two groups and seem to be involved in the pathogenesis of PCD. In addition, we have confirmed previous evidence regarding the absence of correlation between lung function parameters and nFeNO and the assessment of PCD airway inflammation.[8]
All these evidence seem to be related to PCD pathogenesis. Specifically, as in asthma and cystic fibrosis, the chronic airway inflammation represents the main cause of the damage of the airway.[5] PCD subjects are affected by frequent infections and chronic lower airway neutrophilic inflammation because of the impaired mucociliary clearance. This results in an accumulation of mucus in the upper and lower airways.[5] Cytological studies have demonstrated the role of neutrophils against acute infections, such as acute bacterial pneumonia.[10] Acute infections are characterized by a rapid clearing of airway neutrophils after the resolution of the infection.[10] As cystic fibrosis, PCD is characterized by a chronic airway infection and the absence of the resolution of the episodes. Consequently, airway neutrophilia persists, causing a release of tissue-damaging neutrophil products (e.g., neutrophil elastase and myeloperoxidase) and a call of neutrophil chemoattractans, such as LTB4 and IL-8.[10] This results in an oxidative stress, which causes an airway damage, and can be measured through 8-IP.[10]
CONCLUSION
This study demonstrates that EBC 8-IP levels are significantly increased in PCD subjects, highlighting their role of oxidative stress in airway inflammation. The noninvasive method of collection and the direct information on airway inflammation makes it interesting and potentially useful. Moreover, it could have a potential role as a noninvasive marker of inflammation for the diagnosis and management of PCD. Antioxidant treatments might be worth pursuing in this illness, but this assumption seems to be utopian at the present time because the number of studies on this topic is small and their results require a lot of confirmation before therapeutic trials of antioxidants in PCD.
Conflicts of Interest
All authors have report no potential, perceived, or real conflict of interest or financial arrangement (includes involvement with any organization with a direct financial, intellectual, or other interest in the subject of the manuscript).
Financial support and sponsorship
Nil.
REFERENCES
- 1.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AJ, Zariwala MA, Ferkol T, Davis SD, Sagel SD, Dell SD, et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol. 2016;51:115–32. doi: 10.1002/ppul.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2017;49:1601090. doi: 10.1183/13993003.01090-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehlink E, Hogg C, Carr SB, Bush A. Clinical phenotype and current diagnostic criteria for primary ciliary dyskinesia. Expert Rev Respir Med. 2016;10:1163–75. doi: 10.1080/17476348.2016.1242414. [DOI] [PubMed] [Google Scholar]
- 5.Zihlif N, Paraskakis E, Tripoli C, Lex C, Bush A. Markers of airway inflammation in primary ciliary dyskinesia studied using exhaled breath condensate. Pediatr Pulmonol. 2006;41:509–14. doi: 10.1002/ppul.20344. [DOI] [PubMed] [Google Scholar]
- 6.Davis MD, Fowler SJ, Montpetit AJ. Exhaled breath testing - A tool for the clinician and researcher. Paediatr Respir Rev. 2019;29:37–41. doi: 10.1016/j.prrv.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Carraro S, Baraldi E. Exhaled breath condensate in children: Present knowledge and future prospects. J Breath Res. 2008;2:037003. doi: 10.1088/1752-7155/2/3/037003. [DOI] [PubMed] [Google Scholar]
- 8.Csoma Z, Bush A, Wilson NM, Donnelly L, Balint B, Barnes PJ, et al. Nitric oxide metabolites are not reduced in exhaled breath condensate of patients with primary ciliary dyskinesia. Chest. 2003;124:633–8. doi: 10.1378/chest.124.2.633. [DOI] [PubMed] [Google Scholar]
- 9.Bodini A, Tenero L, Sandri M, Maffeis C, Piazza M, Zanoni L, et al. Serum and exhaled breath condensate leptin levels in asthmatic and obesity children: A pilot study. J Breath Res. 2017;11:046005. doi: 10.1088/1752-7163/aa61c5. [DOI] [PubMed] [Google Scholar]
- 10.Kharitonov AS, Barnes PJ. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers. 2002;7:1–32. doi: 10.1080/13547500110104233. [DOI] [PubMed] [Google Scholar]


