Abstract
The distribution of the novel Covid-19 vaccines has been on a scale as unprecedented as the pandemic itself. While the vaccines promise to greatly reduce the spread and impact of the disease, encountering side-effects in clinical practice may pose diagnostic dilemmas. In this case report, we describe a patient with known metastatic renal cell carcinoma who presents with axillary lymphadenopathy found on PET/CT imaging after receiving a Covid-19 vaccine, which was subsequently confirmed to be reactive lymphadenopathy following biopsy.
Keywords: Coronavirus, Reactive lymphadenopathy, Vaccination
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has spread world-wide with over 119 million infected worldwide and over 2.6 million dead at the time of publication [1]. Three vaccines have been approved for use in the United States. Two of these, the “Moderna” and “Pfizer-BioNTech” vaccines are mRNA vaccines encoding for the coronavirus “spike protein,” a glycoprotein which is necessary for cellular entry.[2]. As of publication, over 107 million people in the U.S. have received at least one dose of these vaccines [3].
Case report
A 73-year-old with stage IV clear cell renal cell carcinoma with known bone and lung metastases presented for follow-up with his oncologist. The history was notable for a right nephrectomy 25 months prior and unilateral hip replacement due to metastases to the femur. The patient had been maintained on cabozantinib (a receptor tyrosine kinase inhibitor) after tumor progression under treatment with nivolumab. The patient was advised to have a follow-up PET/CT scan. Two days prior to imaging the patient received the second dose of the Moderna COVID-19 vaccine in the left deltoid.
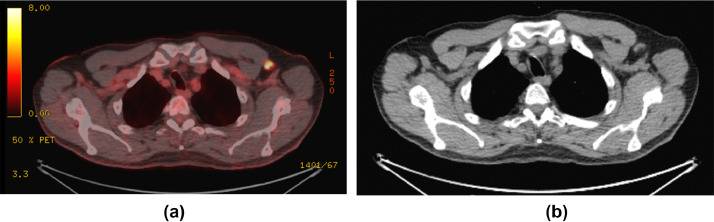
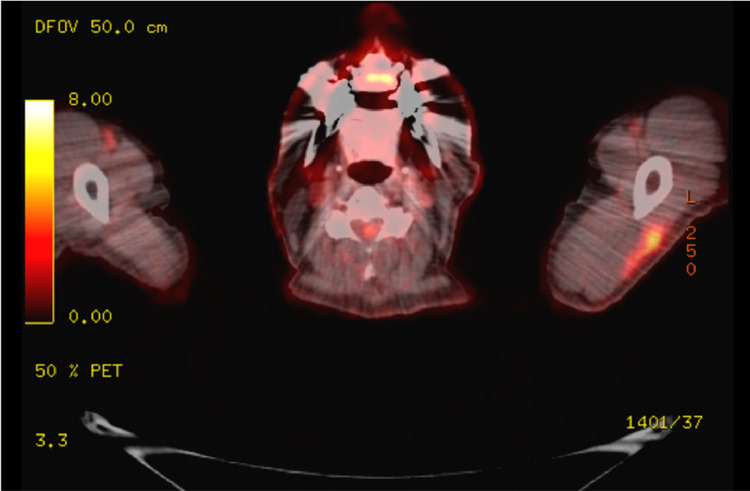
PET/CT demonstrated new left axillary lymph adenopathy. The largest node measured 1.2 × 2.3 cm with a maximum standardized uptake value (SUV) of 9.7 (Fig. 1), with additional sub-centimeter left axillary lymph nodes with a maximum SUV of 4.1. The scan additionally revealed an unchanged 0.8 lingular lung nodule with a SUV of 3.1, a 0.7cm lower lobe nodule with SUV increased to 4.0 from 1.9, a decrease in psoas avidity to 10.1 from 32.2, and focal avid FDG uptake in the left lateral deltoid muscle (Fig. 2).
Fig. 1 –
(A) PET CT scan with intense FDG uptake in a left axillary lymph node. (B) Corresponding CT images from PET scan with left axillary node with cortical thickening.
Fig. 2 –
Focal intense FDG uptake in the left lateral deltoid, corresponding to site of second Moderna Covid-19 vaccine injection.
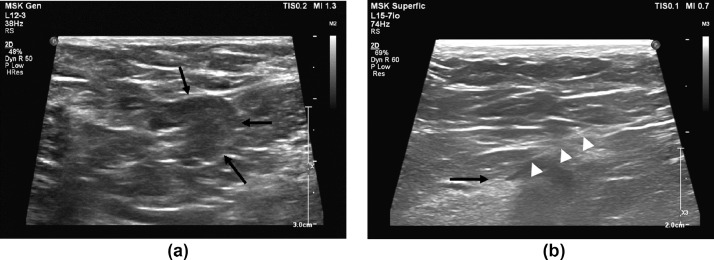
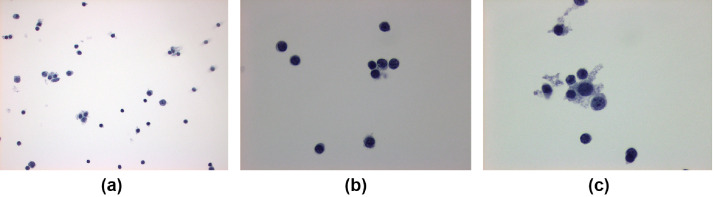
Ultrasound guided fine-needle aspiration biopsy of the left axillary node was performed after discussion with the patient, because of his known history of metastatic disease to lung and bone, with variable PET activity to known metastatic lesions in the lung (Fig. 3). Cytopathology demonstrated a polymorphous lymphoid population compatible with a reactive lymph node and was negative for metastatic carcinoma (Fig. 4).
Fig. 3 –
(A) Transverse images of the enlarged left axillary lymph node with hypoechoic cortical thickening (arrow). (B) Longitudinal imaging during fine needle aspiration biopsy (FNA) with needle tip at the deep margin of the cortical tissue (arrow) and needle shaft (arrow heads)
Fig. 4 –
Lymph node aspirate using a thin preparation with Papanicolaou stain. (A) Polymorphous lymphoid population at 100x magnification. (B) Benign appearing lymphocytes at 400x magnification. (C) Benign lymphocytes with macrophages at 400x magnification.
Discussion
This case report describes a patient with known metastatic clear cell renal cell carcinoma who presents with increased avidity on PET/CT imaging in the axilla ipsilateral to the arm in which a COVID-19 vaccine was administered. Subsequent biopsy demonstrated reactive hyperplasia.
Lymphadenopathy following COVID-19 vaccination is a common event. In clinical trials of the Moderna vaccine, 10.2% of patients had any degree of lymph node enlargement or axillary fullness after the first dose as compared to 4.8% in the placebo group and 14.0% after the second dose vs 3.9% placebo. These rates vary with age, with those above 65 reporting lymphadenopathy more infrequently. Grade 3 lymphadenopathy, defined as preventing daily activity or necessitating the use of prescription pain relievers occurred in 0.3% of patients after the first dose (0.2% placebo) and 0.5% after the second dose (0.1% placebo) [4]. Data is less detailed for the Pfizer-BioNTech vaccine which reported a 0.3% rate of any lymphadenopathy [5].
The COVID-19 mRNA vaccines are not unique in their ability to provoke lymph node enlargement. It is a rare, but known effect of other vaccines, including the bacille Calmette-Guerin, Smallpox, Influenza, HPV, and anthrax vaccines [6], [7], [8], [9], [10]. The high incidence of lymphadenopathy after COVID-19 vaccination in particular poses a challenge to radiographers and other providers who may discover subclinical lymph node enlargement on imaging. This incidental finding lends itself to a differential which includes not only reactive hyperplasia, but also malignancy, systemic infections, and local injury.
This problem has been highlighted in several recent case reports and case series. The majority of these reports describe lymphadenopathy discovered in the setting of routine mammography [11,12]. However, there have been a small number of additional reports of patients imaged for cancer staging or follow-up in which adenopathy is discovered, including in metastatic melanoma, invasive ductal carcinoma, and liposarcoma [12], [13], [14]. Decisions regarding management in the latter of these 2 scenarios must take into account the higher pre-test probability of metastatic spread to a node.
Given the diagnostic uncertainty of post-vaccine lymphadenopathy, it is recommended that when possible, patients postpone routine screenings until 4-6 weeks after the completion of their vaccination course [15,16]. In situations where this was not possible and lymphadenopathy is discovered after vaccination, the course of management depends on the clinicians determination of the likelihood that the lymphadenopathy is related to the vaccine. If lymphadenopathy is discovered within 6 weeks of vaccination, is ipsilateral to the site of vaccine injection, and in a patient with no history of cancer, then it may be assumed to be a result of the vaccine. The patient should be reassessed in 6 weeks to confirm resolution. If it persists for more than 6 weeks, is on the contralateral side to the vaccine, or is in the setting of known cancer, then biopsy should be considered [15], [16], [17].
As COVID-19 vaccination becomes more accessible, the rate of discovery of incidental lymphadenopathy will become more common. Care must be taken in clinical decision making to avoid the overuse of biopsies or further imaging. Physicians need to be aware of patients' vaccine status and the location of the injection when interpreting radiologic images and performing physical exams in order to risk-stratify findings in an appropriate manner.
Patient consent
The authors of this report confirm that informed consent for publication of their case was obtained from the patient.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.WHO coronavirus (COVID-19) Dashboard. (n.d.). Retrieved March 15, 2021, from https://covid19.who.int/
- 2.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC COVID data tracker. (n.d.). Retrieved March 15, 2021, from https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 4.Local reactions, Systemic REACTIONS, adverse events, and serious Adverse EVENTS: Moderna Covid-19 Vaccine. (2020). Retrieved March 15, 2021, from https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolger T., O'Connell M., Menon A., Butler K. Complications associated with the bacille Calmette-Guerin vaccination in Ireland. Arch Dis Child. 2006;91(7):594–597. doi: 10.1136/adc.2005.078972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X.C., Bell C.A., Simmonds K.A., Svenson L.W., Russell M.L. Adverse events following HPV vaccination, Alberta 2006–2014. Vaccine. 2016;34(15):1800–1805. doi: 10.1016/j.vaccine.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett J., Borio L., Radonovich L., Mair J.S., O'Toole T., Mair M. Smallpox vaccination in 2003: key information for clinicians. Clin Infect Dis. 2003;36(7):883–902. doi: 10.1086/374792. [DOI] [PubMed] [Google Scholar]
- 9.Sever J.L., Brenner A.I., Gale A.D., Lyle J.M., Moulton L.H., Ward B.J. Safety of anthrax vaccine: an expanded review and evaluation of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) Pharmacoepidemiol Drug Saf. 2004;13(12):825–840. doi: 10.1002/pds.936. [DOI] [PubMed] [Google Scholar]
- 10.Shirone N., Shinkai T., Yamane T., Uto F., Yoshimura H., Tamai H. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 11.Mortazavi S. Coronavirus disease (COVID-19) vaccination associated axillary adenopathy: imaging findings and follow-up recommendations in 23 women. AJR Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25651. [DOI] [PubMed] [Google Scholar]
- 12.Mehta N., Sales R.M., Babagbemi K., Levy A.D., McGrath A.L., Drotman M. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Özütemiz C., Krystosek L.A., Church A.L., Chauhan A., Ellermann J.M., Domingo-Musibay E. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients. Radiology. 2021 doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avner M., Orevi M., Caplan N., Popovtzer A., Lotem M., Cohen J.E. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur J Nucl Med Mol Imaging. 2021:1–2. doi: 10.1007/s00259-021-05278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seely, J. M., & Barry, M. H. (2021). The Canadian Society of Breast Imaging/Canadian Association of Radiologists’ recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination. [DOI] [PubMed]
- 16.Becker A.S., Perez-Johnston R., Chikarmane S.A., Chen M.M., El Homsi M., Feigin K.N. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: Radiology scientific expert panel. Radiology. 2021 doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehman C.D., Mendoza D.P., Succi M.D., Kambadakone A., Lamb L.R. Unilateral lymphadenopathy post COVID-19 vaccination: a practical management plan for radiologists across specialties. J Am Coll Radiol. 2021;18(6):843–852. doi: 10.1016/j.jacr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]