Abstract
The recent outbreaks of the Ebola virus (EBOV) in Africa have brought global visibility to the shortage of available therapeutic options to treat patients infected with this or closely related viruses. We have recently computationally identified three molecules which have all demonstrated statistically significant efficacy in the mouse model of infection with mouse adapted Ebola virus (ma-EBOV). One of these molecules is the antimalarial pyronaridine tetraphosphate (IC50 range of 0.82–1.30 μM against three strains of EBOV and IC50 range of 1.01–2.72 μM against two strains of Marburg virus (MARV)) which is an approved drug in the European Union and used in combination with artesunate. To date, no small molecule drugs have shown statistically significant efficacy in the guinea pig model of EBOV infection. Pharmacokinetics and range-finding studies in guinea pigs directed us to a single 300mg/kg or 600mg/kg oral dose of pyronaridine 1hr after infection. Pyronaridine resulted in statistically significant survival of 40% at 300mg/kg and protected from a lethal challenge with EBOV. In comparison, oral favipiravir (300 mg/kg dosed once a day) had 43.5% survival. All animals in the vehicle treatment group succumbed to disease by study day 12 (100% mortality). The in vitro metabolism and metabolite identification of pyronaridine and another of our EBOV active molecules, tilorone, suggested significant species differences which may account for the efficacy or lack thereof, respectively in guinea pig. In summary, our studies with pyronaridine demonstrates its utility for repurposing as an antiviral against EBOV and MARV.
Keywords: Antiviral, Ebola virus disease, Favipiravir, Guinea Pig, Pyronaridine
Introduction
Repurposing drugs for different diseases offers the opportunity to take a molecule that is approved for one clinical use and apply it to another disease, potentially accelerating its application and approval (Bai and Hsu, 2019a; Ekins et al., 2011; Hernandez et al., 2018). There have been many articles on this approach and its successes (Baker et al., 2018). Several examples demonstrate repurposing compounds for the Ebola virus (EBOV) (Bai and Hsu, 2019b; Kouznetsova et al., 2014; Madrid et al., 2015a) which is a member of the virus family Filoviridae and pathogenic in both humans and non-human primates, causing severe hemorrhagic fevers (Kiley et al., 1982) with mortality rates as high as 90% (Formenty et al., 2003; Leligdowicz et al., 2016). The recent outbreaks of EBOV in Africa have highlighted the need for new antiviral drugs for this and other emerging viruses to counter the human and financial cost (Bornholdt et al., 2019; Ekins et al., 2015b). The outbreak in Western Africa in 2014–2016 killed over 11,000 and caused over $53bn in economic damage (Jonas, 2019). The current ongoing outbreak in the Democratic Republic of the Congo, in which well over 2200 people have died to date at the time of writing, and where the current case fatality is ~67% (Ilunga Kalenga et al., 2019)), emphasizes this need for new drugs while there is still no FDA approved drug for this disease. Several small molecule drugs such as favipiravir (Kerber et al., 2019; Sissoko et al., 2016a) and most recently remdesivir (Taylor et al., 2016) have been tested against EBOV in patients, although it is unclear whether any of them demonstrate efficacy (Lee et al., 2019; Mulangu et al., 2019). We have previously used a computational approach with a published high-throughput screen of 868 molecules tested in a viral pseudotype entry assay and an EBOV replication assay (Madrid et al., 2013; Madrid et al., 2015b). This computational model enabled us to virtually screen several thousand compounds and identify three active compounds: tilorone, quinacrine and pyronaridine (Ekins et al., 2015a). All of these molecules inhibited EBOV in HeLa cells but not Vero cells, and they all demonstrated significant in vivo activity in the mouse-adapted EBOV (ma-EBOV) efficacy model (Ekins et al., 2018a; Lane et al., 2019b; Lane et al., 2019c). Pyronaridine (EC50 range of 420 nM-1.14 μM (Anantpadma et al., 2019; Ekins et al., 2015a)) has been previously described in detail (Croft et al., 2012). It is the major component of the EU-approved antimalarial Pyramax, which is a combination antimalarial therapy with artesunate and pyronaridine and is approved for this use in the Democratic Republic of the Congo as well as other countries (e.g. South Korea). Our recent assessment of pyronaridine treated ma-EBOV-infected mice in range-finding studies indicated that a single 75 mg/kg i.p. dose which when given 1hr after infection resulted in 100% survival and statistically significantly reduced viremia on study day 3 (Lane et al., 2019d). Additional studies in ma-EBOV-infected mice demonstrated that we could dose pyronaridine (75 mg/kg) either at 2 or 24 hrs post-exposure and obtain the same level of protection against EBOV (Lane et al., 2019d). This was mirrored in our previous tilorone EBOV mouse study with treatment doses at 30 mg/kg q.d. (Ekins et al., 2018a). The pyronaridine mouse efficacy study also provided preliminary insights into how pyronaridine may possess antiviral activity as cytokine and chemokine panels suggested immunomodulatory actions during an EBOV infection (Lane et al., 2019d). Our recent follow- up studies with the structurally related quinacrine (Lane et al., 2019a) indicated this and many other structurally related antimalarials are active against EBOV in vitro (Lane et al., 2019c) and may have a similar mechanism of action as all are known or suspected to be lysosomotropic amines. Such lysosomotropic compounds can diffuse across the membranes of acidic cytoplasmic organelles in their unprotonated form, then become protonated in the acidic environment, causing substantial accumulation in these organelles (Martin et al., 2009), which has the potential to ultimately impact lysosomal function.
It should be pointed out that there are many FDA approved drugs for which the mechanism is unknown. It is only in recent years that we have started to unravel the mechanism of action of such drugs that were approved decades ago (Camarda et al., 2019; Dziekan et al., 2019). The focus of our current efforts is on assessing pyronaridine and other clinical stage compounds as possible treatments for EBOV. Our studies to date with tilorone, quinacrine and pyronaridine may also provide compounds which could be combined as EBOV therapies for future assessment. While the focus of this study is testing pyronaridine, tilorone was also evaluated alongside favipiravir in the guinea pig model of EBOV infection to assess whether the efficacy observed in mouse would also be observed in this species. In vivo studies in the guinea pig would if successful then lead the way for studies in non-human primates.
Materials and Methods
Ethics statement.
All work with gpEBOV-challenged guinea pigs was approved by the University of Texas Medical Branch’s IACUC (IACUC protocol number 1805041 approved 5th June 2018) and was done in accordance with all applicable sections of the Final Rules of the Animal Welfare Act regulations (9 CFR Parts 1, 2, and 3) and Guide for the Care and Use of Laboratory Animals: Eighth Edition (Institute of Laboratory Animal Resources, National Academies Press, 2011; the Guide). This work was conducted in UTMB’s AAALAC (Association for the Assessment and Accreditation of Laboratory Animal Care)-accredited GNL BSL4 laboratory.
Chemicals and reagents.
Pyronaridine tetraphosphate [4-[(7-Chloro-2-methoxybenzo[b][1,5]naphthyridin-10-yl)amino]-2,6-bis(1-pyrrolidinylmethyl)phenol phosphate (1:4)] (Ekins et al., 2015a) was purchased from BOC Sciences (Shirley NY). Favipiravir was purchased from AdooQ Bioscience (Irvine, CA). Tilorone and pyronaridine was purchased from BOC Sciences. Quinacrine and Chloroquine were purchased from Cayman Chemicals (Ann Arbor, Michigan) and Sigma Aldrich (St. Louis, MO), respectively. All compounds were ≥ 95% purity.
In Vitro ADME assays.
In vitro ADME studies were performed by BioDuro (San Diego, CA) as described below.
In Vitro liver microsome stability assays.
The liver microsome solution (197.5 μL, 0.5 mg/ml protein concentration) (Sekisui Xenotech, Kansas City, KS) was aliquoted into 1.1 ml tubes, to which 2.5 μL of positive control and test compound stock solutions (100 μM in DMSO) were added. The tubes were vortexed gently, pre-incubated for 5 min at 37°C, then 50 μL of 5 mM NADPH or LM buffer (no NADPH buffer) was added into the tubes. For analysis, an aliquot of 30 μL was removed from each tube at 0, 5, 15, 30 and 60 min (without-NADPH reaction:0 and 60 min) and quenched with 300 μL of 5/10 ng/ml terfenadine/tolbutamide in methanol/acetonitrile (1:1, v/v). Samples were vigorously vortexed for 1 min and then centrifuged at 4,000 rpm for 15 min at 4 °C. 100 μL of supernatant from each sample was transferred to tubes for LCMS analysis. The amount of parent compound was determined on the basis of the peak area ratio (compound area to IS area) for each time point (AB SCIEX 4500). Clearance rates were calculated by the equation: t1/2 = Ln(2)/ke and in vitro CLint (μL/min/mg protein) = ke*Incubation volume/Microsomal protein amount, and ke using equation of 1st order kinetics :
In Vitro Metabolite Identification of pyronaridine, quinacrine, chloroquine and tilorone in human, mouse, guinea pig liver microsomes.
A DMSO solution of test compound was spiked into 50 mM KH2PO4 (pH 7.4) buffer containing liver microsome at a concentration of 1 mg/mL (Sekisui Xenotech, Kansas City, KS). The reaction was initiated by the addition of 1.0 mM NADPH to the reaction mixture. The final concentration of the test compound was 1 μM. After 0 min and 60 min incubation at 37°C, an aliquot was removed and the sample were precipitated with a 1:6 acetonitrile, quenching the reaction. The resulting mixture was centrifuged, and the resultant supernatants were dried at N2 stream, the resultant residue were reconstituted with 300 μL 10% acetonitrile/H2O (v/v) (0.1% FA) before LC-MS/MS analysis. The supernatant was used for LC-MS/MS analysis. All separations were performed on a ACQUITY UPLC BEH T3 1.8 μm column (2.1×100 mm) at 25°C with a flow rate of 0.3 mL/min. Mobile phase A consisted of 0.1% formic acid in water and mobile phase B consisted of 0.1% formic acid in acetonitrile. Chromatography used a step gradient by maintaining 1% mobile phase B for 5 min, 10% mobile phase B over 8.0 minutes, 20% mobile phase B over 2.0 min, 90% mobile phase B over 2 minutes, 95% mobile phase B over 2 minutes, then re-equilibration back to 1% B at 20 minutes. The total run time was 22 minutes. For all samples, a 5 μL aliquot of sample was injected. The mass spectrometer (HRMS, Q-Exactive Plus from Thermo Fisher) was operated in highresolution, accurate-mass (HRAM) Orbitrap detection mode.
Test article preparation for In Vivo studies.
Vehicle Preparation (Pyronardine study): A solution of 20% Kolliphor HS 15 with Water for injection (WF)I was made to be used for the vehicle. Kolliphor HS 15 was melted at 60 °C. 10 ml of Kolliphor HS 15 was combined with WFI to a final solution volume of 50.0 ml (20% solution) and mixed using a vortex mixer for 30 seconds and then sonicated in an ultrasonic water bath for 25 minutes at 45°C. Test Article Dose Preparation: Dose formulations were prepared by mixing the pyronaridine in the vehicle to achieve the target concentration. The formulation was mixed by inversion 5–6 times and placed on an orbital shaker for 30±5 min. Favipiravir Preparation: A 0.5% solution of methylcellulose was prepared in sterile water. To this, the appropriate amount of Favipiravir was added, and the pH adjusted until the compound goes into solution. Favipiravir was prepared prior to challenge and stored at 4–8 °C.
Guinea pig in vivo dose range-finding toxicity for pyronaridine.
To assess the tolerance of pyronaridine and to select dose groups for pharmacokinetics studies, the drug was given to 5–6-week-old male and female Hartley guinea pigs (Vital River Laboratories) as a single dose by intraperitoneal (i.p.) administration or oral gavage (PO). The compound was formulated in 20% Kolliphor HS 15 (Solutol) in sterile water. There were 8 groups in total (i.p. and oral control groups), with 6 animals per group (3 male, 3 female). I.p. administration was 125, 200 and 300 mg/kg and oral was 125, 300 and 600 mg/kg, each with a dosing volume of 5 ml/kg. Clinical observations were initiated immediately post-dose and once daily up to 168 hrs post-dose.
Guinea pig in vivo pharmacokinetics evaluation of pyronaridine.
Guided by the dose range-finding study, the pharmacokinetics of pyronaridine in guinea pigs were initially assessed at 125 and 600 mg/kg (n=3; male) for i.p. and oral administration, respectively, concentrations at or below the MTD determined by the 7-day study. Pyronaridine for both oral and i.p. administration was solubilized in the same vehicle (20% Kolliphor HS 15). Blood was collected from the treated Guinea pigs at at 1, 4, 8, 24, 72, 168, 264 and 336 hrs post-dose for processing of plasma. All samples were analyzed, and drug levels were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) with a lower limit of quantitation (LLOQ) of 1.0 ng/mL. Notably, in the pyronaridine i.p. dosed, 125 mg/ml group 2 of 3 Guinea pigs were found dead on days 14 and 17 post dose.
Virus strains.
For in vivo experiments, a well-characterized guinea pig-adapted Ebola virus stock (Ebola virus Cavia porcellus/ COD/1976/Mayinga-CDC-808012 (gpaEBOV)) was used for all efficacy studies (Volchkov et al., 2000). All work involving infectious gpa-EBOV was performed at the Galveston National Laboratory (GNL) biosafety level (BSL) 4 laboratory, registered with the Centers for Disease Control and Prevention Select Agent Program for the possession and use of biological select agents.
Initial cell-based testing for inhibition against wild type MARV strain.
MARV expressing GFP was used in testing against viral inhibition as outlined previously (Anantpadma et al., 2016). In short, inhibitors were tested at 8 concentrations for activity. All treatments were done in duplicates, each replicate being on a different plate. Briefly, 4,000 HeLa cells (Ambion, Austin, TX) per well in 25 μl of medium were grown overnight in 384-well tissue culture plates. On the day of assay, test compounds were diluted to 200 μM concentration in complete medium. 25 μl of this mixture was added to the cells already containing 25 μl medium to achieve a concentration of 100 μM. 25 μl of medium was removed from the first wells and added to next well. This type of serial dilution was done 8 times to achieve concentrations of 100, 50, 25, 12.50, 6.25, 3.12, 1.56 and 0.78 μM. One hour after incubating with the compound 25 μl of infection mix containing wild type virus was used to infect cells. This resulted in a final concentration of 50, 25, 12.50, 6.25, 3.12, 1.56, 0.78 and 0.39 μM. Bafilomycin at final a concentration of 10 nM was used as a positive control drug. All virus infections were done in a BSL-4 lab to achieve a MOI of 0.075 to 0.15. Cells were incubated with virus for 24 hours. One day post infection cells were fixed by immersing the plates in formalin overnight at 4°C. Fixed plates were decontaminated and brought out of the BSL-4. Formalin from fixed plates was decanted and plates were washed thrice with PBS. MARV infected plates were immuno-stained using virus specific antibodies. Nuclei were stained using Hoechst at 1:50,000 dilutions. Plates were imaged and nuclei and infected cells were counted using Cell Profiler software.
Cells were permeabilized using 0.1% Triton X-100 (Sigma, Cat#T8787) in PBS and blocked for 1 h in 3.5% bovine serum albumin (Fisher-scientific- Cat#BP9704100), followed by immunostaining. Fixed cells were incubated with an anti-MARV VLP antibody (IBT bioservices, Cat#04–0005, 1:1500 dilution), overnight at 4°C. After 2 washes to remove any excess antibody cells were stained with anti-Rabbit Alexa-546 antibody (Life technologies, Cat#A11035). After 3 washes to remove any non-specific antibody nuclei were stained using Hoechst at 1:50,000 dilution and imaged on a Nikon Ti Eclipse automated microscope. Nuclei and infected cells were counted using CellProfiler software. Relative infection compared to untreated controls was plotted in GraphPad prism 8.2.1 software.
Follow-up cell-based testing against EBOV and MARV strains.
Compounds were tested in vitro against 3 strains of Ebola virus (Kikwit, Makona, Mayinga) and 2 strains of MARV (Angola, Musoke): Ebola virus/H.sapiens-tc/GIN/2014/Makona-C05 (EBOV/Mak, GenBank accession no. KX000398.1), Ebola virus/H.sapiens-tc/COD/1995/Kikwit-9510621 (EBOV/Kik, GenBank accession no. KU182905.1); Ebola virus/H.sapiens-tc/COD/1976/Yambuku-Mayinga (EBOV/May, GenBank accession no. KY425649.1); Marburg virus/H.sapiens-tc/AGO/2005/Ang-1379v (MARV/Ang, BioSample accession no. SAMN05916381); Marburg virus/H.sapiens-tc/KEN/1980/Mt. Elgon-Musoke (MARV/Mus, GenBank accession no. DQ217792). All virus stocks were propagated, and titers were determined by plaque assay on Vero E6 cells obtained from the American Type Culture Collection (Manassas, VA) as previously described (Cong et al., 2016).
The in vitro infection inhibition of the Marburg (Angola and Musoke) and Ebola (Mayinga, Makona and Kikwit) filovirus strains were assessed in HeLa cells.. HeLa cells were seeded at 3 × 104 cells/well in 96-well plates. After 24 hours (h), cells were treated with drugs at 2-fold dilutions starting from 30 μM. Cells were infected with virus 1 hr after the addition of the drugs in BSL4-containment at a multiplicity of infection (MOI) of 0.21 or 0.4. After 48 h, plates were fixed and virus was detected with a mouse antibody specific for EBOV VP40 protein (#B-MD04-BD07-AE11, made by US Army Medical Research Institute of Infectious Diseases, Frederick MD under Centers for Disease Control and Prevention contract) (Cong et al., 2016) or MARV VP40 protein (Cat# IBT0203–012, IBT Bioservices, Rockville, MD) followed by staining with anti-mouse IgG-peroxidase labeled antibody (KPL, Gaithersburg, MD, #074–1802). Luminescence was read on an Infinite® M1000 Pro plate reader (Tecan US, Morrisville, NC). The signal of treated, infected wells was normalized to uninfected control wells and measured (in percent) relative to untreated infected wells. Non-linear regression analysis was performed, and the 50% inhibitory concentrations (EC50s) were calculated from fitted curves (log [agonist] versus response [variable slope] with constraint to remain above 0%) (GraphPad Software, La Jolla, CA). Error bars of dose-response curves represent the standard deviation of three replicates. For quantitation of drug toxicity, HeLa cells were mock infected (no virus) and treated with drug dilutions under the same conditions as the infected cells. After 48 h, cell viability was measured using the CellTiter Glo Luminescent Cell Viability Assay kit according to manufacturer’s protocol (Promega, Madison, WI).
VSV-EBOV-GP pseudotype virus assay.
Vesicular Stomatitis Virus (VSV) pseudotyped with EBOV glycoprotein (GP) expressing a GFP reporter was generously provided by Dr. Wendy Maury (University of Iowa) and has been described previously (Brouillette and Maury, 2017; Quinn et al., 2009). VSV pseudotyped with EBOV glycoprotein was grown by infecting Vero cells (Ambion, Austin, TX) and then harvesting via filtration of the supernatant through 0.4 μM filters 24–30 hours after infection. Virus was then stored at −80 until use.
The cells were tested and imaged using the general methods outlined previously (Anantpadma et al., 2016). In short, HeLa cells (Ambion, Austin, TX) were plated at a density of 20,000 cells/well of a 96 well plate. After attachment overnight, cells were pretreated with compounds for 1 hr at predetermined doses. The dosing series in this case was 25, 12.5, 6.25, 3.12, 1.56, 0.78, 0.39, 0.19, 0.09, 0.04, 0.02 and 0.01 μM. After 1 hr of incubation with compounds, the cells were infected with VSV pseudotyped with EBOV glycoprotein and expressing a GFP reporter. 24 hours after infection, cells were fixed in formalin. After fixation, formalin was washed off, nuclei stained with Hoechst and the cells imaged. Green cells (infected) and blue nuclei (total number of cells) were counted using cell profiler. Relative infection compared to untreated controls was plotted in GraphPad prism 8.2.1 software.
In Vivo efficacy clinical observations and scoring.
Twenty-four (24) experimentally naïve Hartley guinea pigs were assigned to four (4) gender balanced groups. Guinea pigs were anesthetized for dosing (challenge and treatment) via isoflurane inhalation. On study day 0 (SD0) all guinea pigs were challenged with 1000 PFU of gpa-EBOV in 0.2 mL of Minimum Essential Medium (MEM) via intraperitoneal (i.p.) injection. The viral dose administered was verified through plaque assay analysis of the prepared virus suspension.
Dosing for all pyronaridine and all tilorone groups occurred via oral gavage of test/control article on SD0 one hour (± 15 minutes) post-challenge. Favipiravir (300 mg/kg) was given by oral gavage once daily from SD0 through SD7. For the pyronaridine study on SD 3 and during unscheduled euthanasia blood was collected via retro-orbital bleed. For the tilorone study, blood was collected during scheduled and unscheduled euthanasia. Serum was harvested for viremia measurements via plaque assay.
Following challenge, animals were monitored daily by visual examination. Clinical scoring and health assessments were performed and documented at each observation using the scoring system wherein: 1= Healthy; 2= Lethargic and ruffled fur, 3= Sore of 2 + hunched posture an orbital tightening, 4 = Score of 3 + reluctance to move when stimulated, paralysis, unable to access feed and water normally or ≥ 20% body weight loss. Body weights were measured daily during the dosing period (SD0 – SD7) and then every third day until the study was completed. When animals reached a clinical score of 2, the frequency of clinical observations increased to twice daily, 4–6 hours after the initial observation. When the disease progressed, and the clinical score increased to a 3, the frequency of observations was increased to three times daily. All surviving animals were humanely euthanized on Study Day 21.
Viral load determination.
Serum was harvested from guinea pigs that met the euthanasia criteria. Serum harvested for plaque assay analysis was stored frozen (in an ultralow [i.e., −80°C] freezer) until the conclusion of the in-life portion of the animal study, after which samples were batch processed. For this assay, the limit of detection in this assay was 100 PFU/mL. For statistical analysis and graphing all values less than the LOD were assigned a value of one half the LOD.
Results
Testing vs EBOV and MARV strains.
Pyronaridine, tilorone, and quinacrine were all previously discovered using machine learning models for EBOV and tested against the Mayinga strain (Ekins et al., 2015a). We now demonstrate that they block the entry stage of infection in a pseudotype assay (Fig. S1). Even though EBOV and MARV are distantly related (Anantpadma et al., 2016) we also now show these three compounds are active against MARV Musoke strain in HeLa Cells (Fig. S2). These compounds were found by two of our groups to be similarly efficacious against multiple EBOV (Kikwit, Mayinga and Makona, IC50 range of 0.82–1.30 μM) and MARV (Musoke and Angola, IC50 range of 1.01–2.72 μM) (Fig. 1 and Table 1) strains in HeLa cells.
FIG 1.
Pyronaridine, tilorone and quinacrine efficacy and cytotoxicity dose response relationship against multiple strains of EBOV (Kikwit, Mayinga and Makona) and MARV (Musoke and Angola) in HeLa cells. (EBOV/Kik, Mak, May: MOI 0.21; MARV/Ang: MOI 021; MARV/Mus: MOI 0.4).
Table 1.
Pyronaridine, tilorone and quinacrine (± SD) show a similar efficacy against multiple strains of EBOV (Kikwit, Mayinga and Makona) and MARV (Musoke and Angola) in HeLa cells. Analysis via a F-test rejects the hypothesis that the CC50 and the respective IC50 are the same for each of the compounds evaluated (EBOV, Mayinga, tilorone is ambiguous).
| Compound | CC50 (μM)a | Virus | Strain | MOI | IC50 (μM)b | SIc |
|---|---|---|---|---|---|---|
| Kikwit | 0.21 | 1.30 ± 0.42 | 3.2 | |||
| EBOV | Makona | 0.21 | 0.82 ± 0.19 | 5.0 | ||
| Pyronaridine | 4.11 ± 0.50 | Mayinga | 0.21 | 1.01 ± 0.58 | 4.1 | |
| MARV | Angola | 0.21 | 2.72 ± 0.97 | 1.6 | ||
| Musoke | 0.4 | 1.01 ± 0.11 | 4.1 | |||
| Kikwit | 0.21 | 1.48 ± 0.47 | 12.2 | |||
| Tilorone | 18.04 ± 3.04 | EBOV | Makona | 0.21 | 1.14 ± 0.38 | 15.9 |
| Mayinga | 0.21 | 1.21 ± 1.07 | 15.0 | |||
| MARV | Angola | 0.21 | 4.51 ± 1.93 | 4.1 | ||
| Musoke | 0.4 | 2.05 ± 0.28 | 8.8 | |||
| Kikwit | 0.21 | 1.41 ± 0.25 | 6.1 | |||
| EBOV | Makona | 0.21 | 1.05 ± 0.26 | 8.2 | ||
| Quinacrine | 8.62 ± 0.02 | Mayinga | 0.21 | 1.48 ± 0.65 | 5.8 | |
| MARV | Angola | 0.21 | 2.94 ± 0.76 | 2.9 | ||
| Musoke | 0.4 | 1.57 ± 0.17 | 5.5 | |||
Cytotoxicity was determined in HeLa cells that were mock infected. Data represent average of 3 dose response curves with 3 replicates per dose.
Efficacy was determined in HeLa cells infected at an MOI of 0.21. Data represent average of 4–6 dose response curves with 3 replicates per dose.
SI=CC50/IC50
Abbreviations: CC50, 50% cytotoxic concentration; IC50, 50 % inhibitory concentration; SI, selectivity index; MOI, multiplicity of infection.
Metabolic stability across species.
We have previously characterized the in vitro metabolic stability of pyronaridine in mouse, guinea pig, non-human primate and human (Lane et al., 2019d). We have now performed a comparison for tilorone, quinacrine and chloroquine (a known lysosomotropic compound (Homewood et al., 1972)) under similar conditions. Pyronaridine liver microsome (LM) metabolic stability increased in the order of guinea pig, non-human primate, human and then mouse. Tilorone had a similar species-LM stability relationship, with an increase in the order of guinea pig, non-human primate, mouse, followed by human. Chloroquine differed, with LM metabolic stability in the order of mouse, non-human primate, guinea pig and then human. Finally, quinacrine metabolic stability increased in the order of non-human primate, mouse, guinea pig and then human (Table 2). The CYP2D6 substrate probe dextromethorphan metabolism closely paralleled the species differences observed for pyronaridine and was also used to normalize the t1/2 (Table S1 and S2).
Table 2.
Liver microsomal metabolic stability across species
| Species | Pyronaridine | Tilorone | Chloroquine | Quinacrine | |
|---|---|---|---|---|---|
| Mouse | t1/2 (min) | >186 | 102.7 | 47.3 | 12.6 |
| Clint (μL min/mg) |
<7.4 | 13.5 | 29.3 | 110.0 | |
| R2 | 0.65* | 0.91 | 0.96 | 0.98 | |
| Guinea Pig | t1/2 (min) | 66.1 | 12.2 | 132.6 | 17.1 |
| Clint (μL min/mg) |
21.0 | 113.7 | 10.5 | 81.3 | |
| R2 | 0.86 | 1.00 | 0.90 | 0.98 | |
| Non-Human Primate | t1/2 (min) | 89.7 | 94.3 | 106.8 | 10.1 |
| Clint (μL min/mg) |
15.5 | 14.7 | 13.0 | 137.4 | |
| R2 | 0.98 | 0.98 | 0.97 | 0.98 | |
| Human | t1/2 (min) | 122.2 | 127.1 | 201.4 | 27.5 |
| Clint (μL min/mg) |
11.4 | 10.9 | 6.9 | 50.5 | |
| R2 | 0.81 | 0.98 | 0.95 | 0.97 |
Poor fit of the data. Based on previous data (unpublished) using 1 mg LM/rxn, as opposed to 0.5 mg/rx, suggest metabolism in MLM and HLM are very similar
Metabolite identification across species.
We have previously characterized the pyronaridine metabolites produced in mouse microsomes (Lane et al., 2019d). We have now evaluated the metabolites of multiple compounds of interest with in vitro activity against EBOV (pyronaridine, tilorone, quinacrine and chloroquine) across multiple species (human and guinea pig) (Fig. S3–S6). This analysis generated considerable quantities of new previously unpublished metabolism data which for the interests of brevity we will only summarise. The relative peak area abundance (%) for pyronaridine mono-oxygenation was much higher in guinea pig as compared to human liver microsomes. Tilorone N-deethylation and mono-oxygenation was higher in guinea pig relative to both mouse and human. Quinacrine O-demethylation was also 2–3 times higher in guinea pig. In contrast, chloroquine mono-oxygenation was highest in mouse relative to other species. Overall, guinea pig metabolism for these compounds in LMs differed substantially as compared to the other species tested.
Guinea pig dose range-finding toxicity.
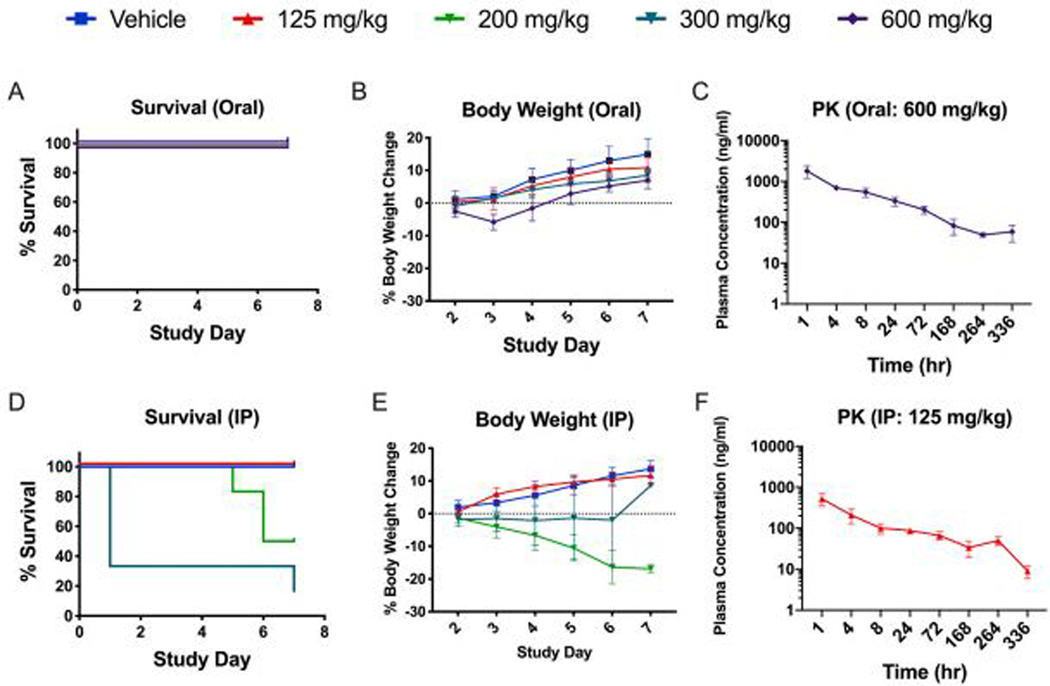
The maximum tolerated dose of pyronaridine was evaluated in Hartley guinea pigs (Fig. 2). In the pyronaridine i.p.-dosed groups the highest dose level of 300 mg/kg was acutely toxic, with 4 of 6 guinea pigs found dead within 30 mins post injection. In addition, one died on day 7 and surprisingly the final surviving guinea pig showed no abnormal clinical observations. For the 200 mg/kg i.p.-dosed guinea pigs, 2 of 6 were found dead on days 5 and 6 and one met criteria for euthanasia on day 6. The remaining surviving guinea pigs from this group were found prostate on day 7 (Fig. 2D). No abnormal clinical observations were noted for guinea pigs administered either 125 mg/kg pyronaridine or vehicle via i.p. administration for the duration of the study. Oral dosing however had drastically reduced toxicity, with only 1 of 6 having any abnormal clinical observations at 600 mg/kg, which was detected directly following administration. This animal was found breathing rapidly for 6 mins, but fully recovered 2 hr post dose. There were no abnormal clinical observations at 300 or 125 mg/kg via oral administration. Based on these results, the maximum tolerated dose (MTD) for a single pyronaridine dose was determined as 125 and >600 mg/kg for i.p. and oral administration, respectively (Fig. 2A). Additionally, the maximum tolerated dose of tilorone was also tested in guinea pigs and it appears well tolerated upto 60mg/kg (Supplemental Methods, Supplemental results, Fig. S7A).
Fig. 2.
Guinea pig dose range-finding toxicity and Pharmacokinetics profile of Pyronaridine administered via oral gavage (A,B,C) or by intraperitoneal injection (D,E,F).
Guinea pig pharmacokinetics evaluation of pyronaridine.
The pharmacokinetics of pyronaridine was evaluated in Hartley guinea pigs (Fig. 2C, F). After an initial rapid absorption phase, the pyronaridine plasma profile exhibited a distribution phase at about 1hr, then a prolonged phase with plasma drug concentrations remaining essentially unchanged, or slightly higher until about 72hrs. All samples for animals dosed orally and i.p. contained measurable levels of pyronaridine though 336 and 168 hrs, respectively (LLOQ = 1 ng/ml). The plasma drug levels were analyzed using noncompartmental modeling allowing for the calculation of pharmacokinetic parameters (Table 3). Pyronaridine plasma levels reached the peak in the first sample, taken at 1 hr post administration. The elimination-phase t1/2 was calculated as 72.7 and 90.5 hrs for i.p. and oral administration, respectively. This is considerably shorter than the t1/2 found in humans and mice of between 195–251 hrs (Jayaraman et al., 1997; Ramanathan et al., 2005) and 146 hrs (Lane et al., 2019d), respectively. Maximum concentration of unbound drug in plasma (Cmax), area under the concentration-time curve from time zero to the last measurable concentration (AUClast), and area under the concentration-time curve from time zero to infinity (AUCinf) are provided in Table 3. Additionally, the pharmacokinetics of tilorone was also evaluated in guinea pigs (Supplemental Methods, Supplemental results, Fig. S7B, C and S8).
Table 3.
Mean pharmacokinetics data in male guinea pigs treated with pyronaridine
| Cmax (ng/ml) | AUClast (hr*ng/ml) | AUCinf (hr*ng/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | Administration | Sex (n=3) | T1/2 (h) | SE | Tmax (h) | Mean | SE | Mean | SE | Mean | SE |
| 125 | IP | M | 72.7 | 9.3 | 1 | 523 | 175 | 16,565 | 5269 | 17,430 | 5428 |
| 600 | Oral | M | 90.5 | 3.9 | 1 | 1800 | 348 | 50,964 | 4406 | 58,783 | 6712 |
Pyronaridine efficacy and clinical observations.
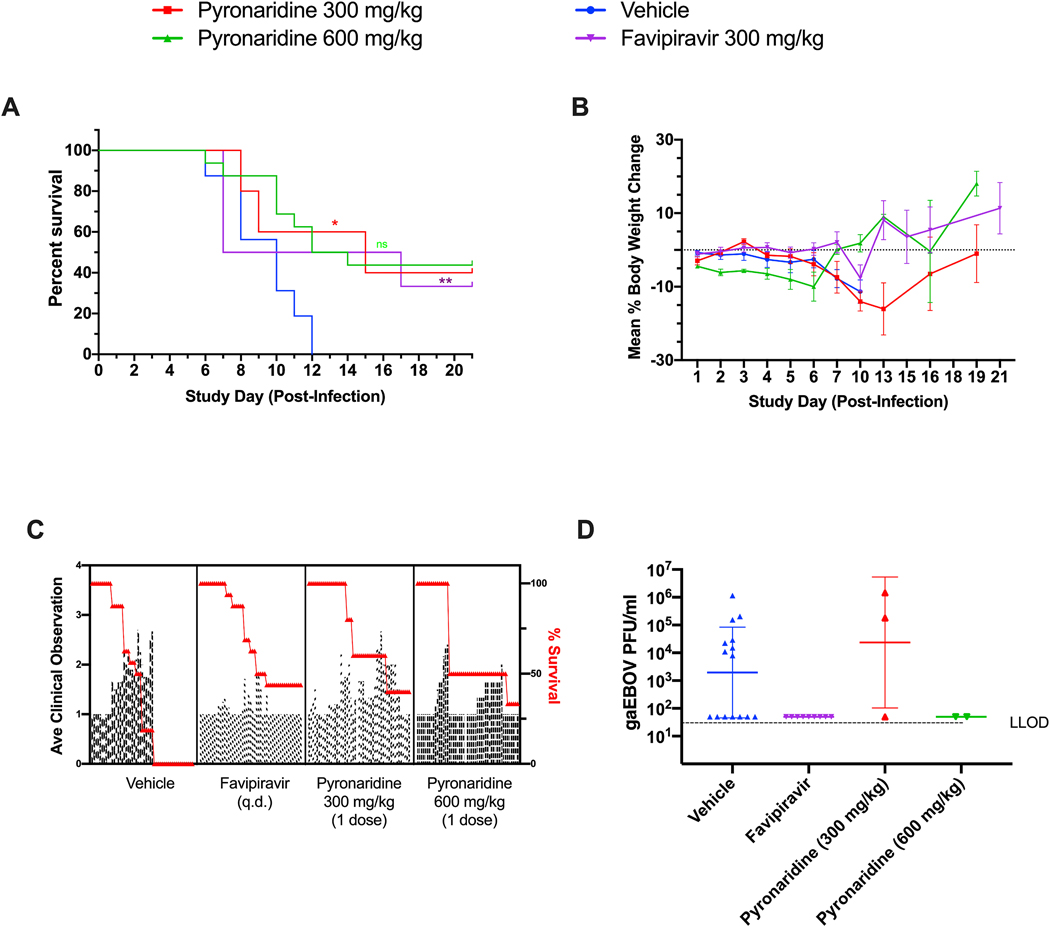
The efficacy of pyronaridine was evaluated in Hartley guinea pigs challenged with guinea pig adapted EBOV (gpa-EBOV). All animals in vehicle treatment (Group 1) succumbed to disease by study day 12 (100% mortality). Group 2 (pyronaridine 300 mg/kg) and Group 3 (pyronaridine 600 mg/kg) resulted in 40% and 33% survival, respectively. Among the 16 animals in the combined Group 4 (favipiravir 300 mg/kg), 7 GPs survived through the end of the 21-day study period; the other 9 guinea pigs succumbed to disease by study day 14 (43.75% survival, Fig. 3A). It should be noted that an animal in Group 2 was euthanized due to a clinical score of 4 on study day 2. Ebola disease in guinea pigs does not progress this rapidly so it is unlikely the animal succumbed to disease and it is more likely the animal incurred esophageal trauma because of the oral gavage technique, therefore this animal has been removed from data analysis.
FIG 3.
Guinea pig dose range-finding efficacy. gaEBOV efficacy data. Data from favipiravir and vehicle-treated (combined, n=16) groups were combined from our own two independent studies in order to strengthen their predictive power. (A) The survival curves between pyronaridine 300 and 600 mg/kg, favipiravir and vehicle. Asterisks represent significant difference from the vehicle (Log-rank (Mantel-Cox) test; Pyronaridine p=0.0307; Favipiravir p=0.0014). B. Mean percent body weight change from SD0 C. Mean clinical scoring results with overlaid percent survival. D. Plaque assay for viable EBOV in sera (GPs sacrificed based on clinical score) with Dunnett’s T3 multiple comparisons test. Statistical significance was calculated with log-transformed plaque assay data using a Dunnett’s T3 multiple comparisons test (Forsythe and Welch ANOVA) with the vehicle designated as the control. The difference from the vehicle was not found to be significant. For the plaque assay gaEBOV viral load had LLOD of 100 PFU/ml. Quantified values below these where set to 0.5 × LLOD. Bars and error-bars represents the geometric mean and geometric SD.
Body weight results for each treatment group are summarized in Fig. 3B. Animals in Group 1 maintained body weight through study day 7 but decreased by study day 10, when all animals succumbed to disease. Group 2 animals body weight continuously decreased through study day 7, with surviving animals returning to pre-challenge body weights by study day 10. Group 3 animals dropped body weight following challenge, but the survivors reverted to pre-challenge body weight by the end of the 21-day study. It should be noted that an animal in this group had developed clinical signs late and succumbed to infection on study day 17. Animals in Group 4 remained at a consistent body weight until study day 10 where the mean body weight began to decrease briefly, but the surviving animals rebounded to pre-challenge weight by study day 13.
Plaque assay results from serum samples taken from animals that met euthanasia criteria during the scheduled observation times from all four groups are presented in Fig. 3D. Only 10 of the 20 terminal samples had detectable levels of viable gpa-EBOV: eight animals from Group 1 (4.87 × 107 geometric mean PFU/mL) and two animals from Group 2 (8.15 × 105 geometric mean PFU/mL). Interestingly, all the guinea pigs treated with favipiravir were below the limit of detection for the plaque assay (Fig. S9).
Clinical scoring results for each guinea pig was assessed on a scale ranging from 1–4, where a score of 1 indicated a healthy animal and a score of 4 was indicative of a moribund animal. All animals in each group succumbed at least partially to disease by study day 8 (Fig. 3A). In each group the mean clinical observation increased inversely with survival, with each pyronaridine-treated group having two independent increases in average clinical observations. Interestingly, during post-study day 8 the average clinical score in the favipiravir group seemed to be independent of survival (Fig. 3C).
Favipiravir and Pyronaridine in vivo efficacy against EBOV.
It should be noted that in order to increase the statistical power of our study we combined the favipiravir, positive-control data from our two individual experiments (n=6, n=10) as these used comparable approaches and were performed by the same group. Due to the small group sizes the data for the negative, vehicle controls (n=6, n=10) were also combined with the noted caveat of a variation in frequency of administration.
Favipiravir has been shown to protect guinea pigs from adapted Sudan Virus (Rahim et al., 2018) however in our study it protected ~44% of the animals against gpa-EBOV, with deaths starting on study day 6 and continuing until study day 14 (Fig. 3A). These were not statistically different from the treatment with pyronaridine 300 and 600 mg/kg with 40% and 33% survival, respectively (Log-rank (Mantel-Cox) test). Tilorone at the doses assessed were either comparable or had significantly reduced survival rates as compared to the vehicle (Fig. S9A). In summary, there was a statistically significant difference for both pyronaridine (300 mg/kg) and favipiravir when compared to the control combined from our two studies (Fig. 3).
The results in guinea pig are surprising as previous studies in mouse have shown pyronaridine, tilorone and favipiravir all protect mice infected with ma-EBOV (Ekins et al., 2018b; Lane et al., 2019a; Lane et al., 2019d). Interestingly, virus was only detected in serum samples collected at the time of euthanasia from one and two Guinea pigs from the vehicle and pyronaridine 300 mg/kg, respectively, in the current pyronaridine study. This differed in our tilorone study (Supplemental Methods, Supplemental Results, Fig. S7–S9) where virus was recovered from all but one serum sample harvested from the guinea pigs in the vehicle and tilorone-treated groups (Fig. S9D). Virus was not detected in guinea pigs treated with favipiravir (both survivors and non-survivors) and was statistically significantly reduced from vehicle (Fig. 3D).
Discussion
There have been very few small molecule drugs that have reached the clinic for testing against EBOV, including favipiravir (Sissoko et al., 2016b), GS-5734 (remdesivir) (Taylor et al., 2016) and galidesivir (Taylor et al., 2016). Favipiravir has demonstrated 100% efficacy in the mouse model of EBOV (Oestereich et al., 2014; Smither et al., 2014), 83% protection in the Interferon α/β and γ Double Knockout Mice mouse model (Comer et al., 2019), 17% (Bixler et al., 2018) to 50% (Guedj et al., 2018) survival in the cynomolgous macaque and increased survival and decreased viral load (Bai et al., 2016) or unclear efficacy in humans (Kerber et al., 2019; Sissoko et al., 2016a). Once daily IV dosed remdesivir has demonstrated 100% survival in non-human primates only (Siegel et al., 2017) and on this basis has been tested in humans during the current EBOV outbreak. Recently, a significant portion of data was described (499 individuals) from a clinical trial involving the investigation of multiple therapeutics against EBOV (NCT03719586) with ZMapp (a monoclonal antibody cocktail) (Qiu et al., 2014)), remdesivir, MAb114 (a monoclonal antibody) (Corti et al., 2016)) and REGN-EB3 (monoclonal antibody combination) (Sivapalasingam et al., 2018)). These results showed that the antibodies REGN-EB3 and mAb114 had overall survival rates of 71% and 66%, respectively, and were much more effective with patients with low viremia levels. Both ZMapp and remdesivir were shown to be less effective with a 51% and 47% survival rates, respectively (Mulangu et al., 2019). Ebola generally has a wide variation in its fatality rates of between 25% to 90%, (average ~50%). While these results are promising for the monoclonal antibodies and currently represents the “standard of care”, their high cost to produce, delivery and administration of likely temperature sensitive treatments (unless lyophilized) to remote areas in Africa is a potential issue. A shelf stable, small molecule drug that can be made cheaply, with efficacy against multiple filoviruses could be given orally as a single dose that would be ideal and alleviate some of these logistical challenges that constitute the critical final stage of delivering a therapeutic to the patient.
From this present study, pyronaridine and several other drugs which we have identified have shown activity in several strains of EBOV and MARV in vitro (Fig. 1 and Table 1), indicating they may have a broad-spectrum activity against the virus family Filoviridae. Based on our pseudovirus data these would appear to be preventing entry of the virus (Fig. 1). Two of these drugs were studied further in the guinea pig model of EBOV infection. It is apparent that pyronaridine did not show as substantial of a difference in the survival rate in guinea pig as was observed for mouse (100% survival) (Lane et al., 2019d). This may be because the half-life for pyronaridine is much shorter in the guinea pig, so efficacious plasma levels of drug are likely not maintained long enough (90 hrs). The pharmacokinetics varies in other species, where the half-life in mice and humans is approximately 140 hrs and 200 hrs, respectively. Many other small molecules have failed to progress beyond guinea pig for EBOV due to a lack of significant efficacy (Dowall et al., 2016; Dowall et al., 2015; Dyall et al., 2018; Madrid et al., 2015a; Miller et al., 2016). While antibodies have been successfully used in this animal model (Rijal et al., 2019; Wec et al., 2019), these failures may represent a significant limitation of the guinea pig model to extrapolate small-molecule efficacy against EBOV in humans. We have demonstrated there are substantial metabolic stability differences between mouse, non-human primate, human and guinea pig (Lane et al., 2019d), with the latter having a considerably lower metabolic stability for pyronaridine (Table 2). While to our knowledge this has not been determined for favipiravir, it has been shown that the pharmacokinetics of this compound exhibit nonlinearity over dose and time in non-human primates (Madelain et al., 2017), making these interspecies’ comparisons potentially much more complex. Based on this in vitro data it would also suggest the metabolic stability of pyronaridine in the non-human primate may also be poor, requiring a dose adjustment to retain efficacy and sufficient drug plasma levels in this model. In contrast, antibody therapeutics for EBOV are not likely to be metabolized by the same drug metabolizing enzymes; therefore, they may show more universal efficacy across species. We have also evaluated the metabolism of several drugs of interest in liver microsomes of various species under similar conditions (Table 2). The structurally unrelated tilorone has limited metabolic stability in guinea pig and is much more stable in non-human primate and human liver microsomes. Chloroquine and quinacrine show different metabolic stability patterns across species, in contrast. Our comparison of metabolic stability with the substrate probe dextromethorphan may also point to the role of CYP2D family in the metabolism of pyronaridine. It has previously been shown that pyronaridine inhibits known substrates of CYP2D6 both in vitro (Lane et al., 2019d) and in vivo (Morris et al., 2014), also suggesting that it may be a CYP2D6 substrate as well. Detailed metabolite identification for each of these four compounds have been made available for the first time (Figure S3–6). It is unclear what effect EBOV infection would have on the metabolic enzymes such as the P450’s in the guinea pig. To our knowledge, favipiravir has not previously been tested orally against EBOV in guinea pig (though it has demonstrated survival rates of 83–100% in Sudan virus-infected guinea pigs when dosed subcutaneously (Rahim et al., 2018)), and in this study we also demonstrate efficacy (when dosed orally) on a par with what was observed in non-human primates (Bixler et al., 2018; Guedj et al., 2018). In comparison, survival after pyronaridine (300 and 600 mg/kg) treatment was not significantly different from oral-administered favipiravir in the guinea pig model ((Log-rank (Mantel-Cox) test), suggesting a similar efficacy. There was a statistically significant difference for both pyronaridine (300 mg/kg) and favipiravir when compared to the control combined from our two studies (Fig. 3). The survival rate of the negative controls from multiple EBOV-challenged GP studies (either untreated or oral vehicle treatment, n=55) (Chan et al., 2018; Dowall et al., 2016; Dowall et al., 2015) were combined with the data from our current studies to evaluate the possible statistical significance of the efficacy of pyronaridine and or favipiravir. Evaluation of this “meta-analysis control” also shows a significant difference for both pyronaridine 300 mg/kg and favipiravir (Log-rank (Mantel-Cox) test, (Figure S10)). Our initial dose ranging work showed significant toxicity with pyronaridine when dosed i.p. in guinea pig (accumulation in the abdominal cavity) hence the focus on oral administration in this study. It should be noted that we used only a single dose of pyronaridine for all our efficacy studies, and it is feasible that a single higher dose or more frequent dosing at the same level may overcome the lower half life to result in a higher exposure and subsequent increased survival rate for both drugs tested orally. However, in a BSL4 enviroment it should be noted that more frequent daily dosing would require additional use of anesthesia which may not be ideal as it would increase the risk to the animals.
Developing small molecule drugs for EBOV is extremely challenging. While high throughput screens have readily identified many FDA approved drugs as well as other candidate molecules with in vitro inhibitory activities against EBOV (Johansen et al., 2013; Madrid et al., 2013), when these compounds are tested in guinea pig, to date, all of them have failed. For example, chloroquine (Dowall et al., 2015; Madrid et al., 2015a), azithromycin (Madrid et al., 2015a), amiodarone (Dyall et al., 2018), iminosugars (Miller et al., 2016), BGB324 (Dowall et al., 2016), NCK8 (Dowall et al., 2016) and 17-DMAG (Dowall et al., 2016) were all inactive in the guinea pig in vivo model. Interestingly, tilorone (Fischer et al., 1996), chloroquine (Homewood et al., 1972), azithromycin (Tyteca et al., 2002), amiodarone (Ikeda et al., 2008), BGB324 (Chen et al., 2018) and 17-DMAG (Duvvuri et al., 2006) are all known lysosomotropic compounds and NCK8 is most likely as well (Ghosh et al., 2017). This may indicate that these compounds could have also failed due to a common antiviral mechanism that does not transcend species. In addition, there is recent evidence that the type-I IFN antiviral immune response in guinea pig is significantly different than in mouse or non-human primate (Zhang et al., 2017), therefore guinea pig may not be an appropriate model to universally predict the antiviral response in humans, regardless. This is particularly relevant to EBOV, since viral susceptibility and adaptation to guinea pig was directly linked to differences in the immune response (Chepurnov et al., 2001).
In total, these results for pyronaridine, tilorone and favipiravir may question the need for demonstrating efficacy against gpa-EBOV before expanding to the non-human primate model of Ebola virus infection. They also suggest that larger group sizes are required to show statistical significance and to allow for spontaneous animal deaths due to issues with oral gavage, which ultimately reduces animal group sizes.
In conclusion, the guinea pig in vivo data collected in this study points to ~40% survival for pyronaridine and favipiravir against gpa-EBOV. The accumulated in vitro metabolic data indicates that the guinea pig may be a suboptimal model to predict the efficacy of these compounds to combat EBOV. This could be due to differences in EBOV mechanism and drug metabolism (e.g. species differences in the metabolic enzymes involved (Mankowski et al., 1999; Shimada et al., 1997)). Our combined in vitro and in vivo studies with pyronaridine demonstrate its potential utility for repurposing as an antiviral against different strains of EBOV and MARV. These efforts also provide justification for further in vivo evaluation before possible provision of the combination drug Pyramax for future Ebola outbreaks.
Supplementary Material
Highlights.
We demonstrate in vitro activity of the antimalarial pyronaridine against multiple strains of Ebola and Marburg.
We describe pharmacokinetics (PK) and range-finding studies in guinea pigs for pyronaridine.
We demonstrate pyronaridine (300mg/kg) has statistically significant survival in guinea pig infected with Ebola.
In vitro metabolism of pyronaridine in microsomes suggests significant species differences.
These findings also may be useful for repurposing pyronaridine against other viruses in future.
Acknowledgments
We gratefully acknowledge the team at Bioduro for their considerable efforts on this project and in particular Mr. Dan Contoit for whom we dedicate this article to his memory.
Dr. Joel S. Freundlich is kindly acknowledged for consultations. We acknowledge Elena Postnikova, Janie Liang, and Shuiqing Yu who performed the in vitro testing of compounds against multiple virus strains. Dr. Mupenzi Mumbere is kindly thanked for providing information on drug availability in the Democratic Republic of the Congo.
H.Z., J.D., and M.R.H performed this work as employees of Battelle Memorial Institute (BMI). The findings and conclusions in this report do not necessarily reflect the views or policies of the US Department of Health and Human Services or of the institutions and companies affiliated with the authors.
Funding
We kindly acknowledge NIH funding: R21TR001718 from NCATS (PI – Sean Ekins)., NIAID CONTRACT NO.: HHSN272201700040I, NIAID TASK ORDER NO.: HHSN27200007 (PI - Peter Madrid). This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases (NIAID); Integrated Research Facility (NIAID, Division of Clinical Research); Battelle Memorial Institute’s prime contract with NIAID (Contract # HHSN272200700016I). H.Z., J.D., and M.R.H performed this work as employees of Battelle Memorial Institute (BMI). The findings and conclusions in this report do not necessarily reflect the views or policies of the US Department of Health and Human Services or of the institutions and companies affiliated with the authors.
Footnotes
Conflicts of interest
SE is CEO of Collaborations Pharmaceuticals, Inc. TRL is an employee at Collaborations Pharmaceuticals, Inc. Collaborations Pharmaceuticals, Inc. has obtained FDA orphan drug designations for pyronaridine, tilorone and quinacrine for use against Ebola.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anantpadma M, Kouznetsova J, Wang H, Huang R, Kolokoltsov A, Guha R, Lindstrom AR, Shtanko O, Simeonov A, Maloney DJ, Maury W, LaCount DJ, Jadhav A, Davey RA, 2016. Large-Scale Screening and Identification of Novel Ebola Virus and Marburg Virus Entry Inhibitors. Antimicrobial agents and chemotherapy 60, 4471–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantpadma M, Lane T, Zorn KM, Lingerfelt MA, Clark AM, Freundlich JS, Davey RA, Madrid P, Ekins S, 2019. Ebola Virus Bayesian Machine Learning Models Enable New In Vitro Leads ACS Omega 4, 2353–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai CQ, Mu JS, Kargbo D, Song YB, Niu WK, Nie WM, Kanu A, Liu WW, Wang YP, Dafae F, Yan T, Hu Y, Deng YQ, Lu HJ, Yang F, Zhang XG, Sun Y, Cao YX, Su HX, Sun Y, Liu WS, Wang CY, Qian J, Liu L, Wang H, Tong YG, Liu ZY, Chen YS, Wang HQ, Kargbo B, Gao GF, Jiang JF, 2016. Clinical and Virological Characteristics of Ebola Virus Disease Patients Treated With Favipiravir (T-705)-Sierra Leone, 2014. Clin Infect Dis 63, 1288–1294. [DOI] [PubMed] [Google Scholar]
- Bai JPF, Hsu CW, 2019a. Drug repurposing for Ebola virus disease: principles of consideration and the Animal Rule. J Pharm Sci 108, 798–806. [DOI] [PubMed] [Google Scholar]
- Bai JPF, Hsu CW, 2019b. Drug Repurposing for Ebola Virus Disease: Principles of Consideration and the Animal Rule. J Pharm Sci 108, 798–806. [DOI] [PubMed] [Google Scholar]
- Baker NC, Ekins S, Williams AJ, Tropsha A, 2018. A bibliometric review of drug repurposing. Drug Discov Today 23, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler SL, Bocan TM, Wells J, Wetzel KS, Van Tongeren SA, Dong L, Garza NL, Donnelly G, Cazares LH, Nuss J, Soloveva V, Koistinen KA, Welch L, Epstein C, Liang LF, Giesing D, Lenk R, Bavari S, Warren TK, 2018. Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antiviral Res 151, 97–104. [DOI] [PubMed] [Google Scholar]
- Bornholdt ZA, Herbert AS, Mire CE, He S, Cross RW, Wec AZ, Abelson DM, Geisbert JB, James RM, Rahim MN, Zhu W, Borisevich V, Banadyga L, Gunn BM, Agans KN, Wirchnianski AS, Goodwin E, Tierney K, Shestowsky WS, Bohorov O, Bohorova N, Velasco J, Ailor E, Kim D, Pauly MH, Whaley KJ, Alter G, Walker LM, Chandran K, Zeitlin L, Qiu X, Geisbert TW, Dye JM, 2019. A Two-Antibody Pan-Ebolavirus Cocktail Confers Broad Therapeutic Protection in Ferrets and Nonhuman Primates. Cell Host Microbe 25, 49–58 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette RB, Maury W, 2017. Production of Filovirus Glycoprotein-Pseudotyped Vesicular Stomatitis Virus for Study of Filovirus Entry Mechanisms. Methods Mol Biol 1628, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarda G, Jirawatcharadech P, Priestley RS, Saif A, March S, Wong MHL, Leung S, Miller AB, Baker DA, Alano P, Paine MJI, Bhatia SN, O’Neill PM, Ward SA, Biagini GA, 2019. Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat Commun 10, 3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M, Holtsberg FW, Vu H, Howell KA, Leung A, Van der Hart E, Walz PH, Aman MJ, Kodihalli S, Kobasa D, 2018. Efficacy of Ebola Glycoprotein-Specific Equine Polyclonal Antibody Product Against Lethal Ebola Virus Infection in Guinea Pigs. J Infect Dis 218, S603-S611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Song Q, Yu Q, 2018. Axl inhibitor R428 induces apoptosis of cancer cells by blocking lysosomal acidification and recycling independent of Axl inhibition. Am J Cancer Res 8, 1466–1482. [PMC free article] [PubMed] [Google Scholar]
- Chepurnov AA, Dadaeva AA, Kolesnikov SI, 2001. Study of the pathogenesis of Ebola fever in laboratory animals with different sensitivity to this virus. Bull Exp Biol Med 132, 1182–1186. [DOI] [PubMed] [Google Scholar]
- Comer JE, Escaffre O, Neef N, Brasel T, Juelich TL, Smith JK, Smith J, Kalveram B, Perez DD, Massey S, Zhang L, Freiberg AN, 2019. Filovirus Virulence in Interferon alpha/beta and gamma Double Knockout Mice, and Treatment with Favipiravir. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Dyall J, Hart BJ, DeWald LE, Johnson JC, Postnikova E, Zhou H, Gross R, Rojas O, Alexander I, Josleyn N, Zhang T, Michelotti J, Janosko K, Glass PJ, Flint M, McMullan LK, Spiropoulou CF, Mierzwa T, Guha R, Shinn P, Michael S, Klumpp-Thomas C, McKnight C, Thomas C, Eakin AE, O’Loughlin KG, Green CE, Catz P, Mirsalis JC, Honko AN, Olinger GG Jr., Bennett RS, Holbrook MR, Hensley LE, Jahrling PB, 2016. Evaluation of the Activity of Lamivudine and Zidovudine against Ebola Virus. PLoS One 11, e0166318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D, Misasi J, Mulangu S, Stanley DA, Kanekiyo M, Wollen S, Ploquin A, Doria-Rose NA, Staupe RP, Bailey M, Shi W, Choe M, Marcus H, Thompson EA, Cagigi A, Silacci C, Fernandez-Rodriguez B, Perez L, Sallusto F, Vanzetta F, Agatic G, Cameroni E, Kisalu N, Gordon I, Ledgerwood JE, Mascola JR, Graham BS, Muyembe-Tamfun JJ, Trefry JC, Lanzavecchia A, Sullivan NJ, 2016. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 351, 1339–1342. [DOI] [PubMed] [Google Scholar]
- Croft SL, Duparc S, Arbe-Barnes SJ, Craft JC, Shin CS, Fleckenstein L, Borghini-Fuhrer I, Rim HJ, 2012. Review of pyronaridine anti-malarial properties and product characteristics. Malar J 11, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowall SD, Bewley K, Watson RJ, Vasan SS, Ghosh C, Konai MM, Gausdal G, Lorens JB, Long J, Barclay W, Garcia-Dorival I, Hiscox J, Bosworth A, Taylor I, Easterbrook L, Pitman J, Summers S, Chan-Pensley J, Funnell S, Vipond J, Charlton S, Haldar J, Hewson R, Carroll MW, 2016. Antiviral Screening of Multiple Compounds against Ebola Virus. Viruses 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowall SD, Bosworth A, Watson R, Bewley K, Taylor I, Rayner E, Hunter L, Pearson G, Easterbrook L, Pitman J, Hewson R, Carroll MW, 2015. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J Gen Virol 96, 3484–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvvuri M, Konkar S, Hong KH, Blagg BS, Krise JP, 2006. A new approach for enhancing differential selectivity of drugs to cancer cells. ACS Chem Biol 1, 309–315. [DOI] [PubMed] [Google Scholar]
- Dyall J, Johnson JC, Hart BJ, Postnikova E, Cong Y, Zhou H, Gerhardt DM, Michelotti J, Honko AN, Kern S, DeWald LE, O’Loughlin KG, Green CE, Mirsalis JC, Bennett RS, Olinger GG Jr., Jahrling PB, Hensley LE, 2018. In Vitro and In Vivo Activity of Amiodarone Against Ebola Virus. J Infect Dis 218, S592-S596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziekan JM, Yu H, Chen D, Dai L, Wirjanata G, Larsson A, Prabhu N, Sobota RM, Bozdech Z, Nordlund P, 2019. Identifying purine nucleoside phosphorylase as the target of quinine using cellular thermal shift assay. Sci Transl Med 11. [DOI] [PubMed] [Google Scholar]
- Ekins S, Freundlich J, Clark A, Anantpadma M, Davey R, Madrid P, 2015a. Machine learning models identify molecules active against Ebola virus in vitro. F1000Res 4, 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Lingerfelt MA, Comer JE, Freiberg AN, Mirsalis JC, O’Loughlin K, Harutyunyan A, McFarlane C, Green CE, Madrid PB, 2018a. Efficacy of Tilorone Dihydrochloride against Ebola Virus Infection. Antimicrobial agents and chemotherapy 62, e01711–01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Lingerfelt MA, Comer JE, Freiberg AN, Mirsalis JC, O’Loughlin K, Harutyunyan A, McFarlane C, Green CE, Madrid PB, 2018b. Efficacy of Tilorone Dihydrochloride against Ebola Virus Infection. Antimicrob Agents Chemother 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Southan C, Coffee M, 2015b. Finding small molecules for the ‘next Ebola’. F1000Res 4, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S, Williams AJ, Krasowski MD, Freundlich JS, 2011. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov Today 16, 298–310. [DOI] [PubMed] [Google Scholar]
- Fischer J, Lullmann H, Lullmann-Rauch R, 1996. Drug-induced lysosomal storage of sulphated glycosaminoglycans. Gen Pharmacol 27, 1317–1324. [DOI] [PubMed] [Google Scholar]
- Formenty P, Libama F, Epelboin A, Allarangar Y, Leroy E, Moudzeo H, Tarangonia P, Molamou A, Lenzi M, Ait-Ikhlef K, Hewlett B, Roth C, Grein T, 2003. [Outbreak of Ebola hemorrhagic fever in the Republic of the Congo, 2003: a new strategy?]. Med Trop (Mars) 63, 291–295. [PubMed] [Google Scholar]
- Ghosh C, Chaubey S, Tatu U, Haldar J, 2017. Aryl-alkyl-lysines: small molecular membrane-active antiplasmodial agents. Medchemcomm 8, 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj J, Piorkowski G, Jacquot F, Madelain V, Nguyen THT, Rodallec A, Gunther S, Carbonnelle C, Mentre F, Raoul H, de Lamballerie X, 2018. Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques. PLoS Med 15, e1002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez HW, Soeung M, Zorn KM, Ashoura N, Mottin M, Andrade CH, Caffrey CR, de Siqueira-Neto JL, Ekins S, 2018. High Throughput and Computational Repurposing for Neglected Diseases. Pharm Res 36, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homewood CA, Warhurst DC, Peters W, Baggaley VC, 1972. Lysosomes, pH and the anti-malarial action of chloroquine. Nature 235, 50–52. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Hirayama M, Hirota Y, Asa E, Seki J, Tanaka Y, 2008. Drug-induced phospholipidosis is caused by blockade of mannose 6-phosphate receptor-mediated targeting of lysosomal enzymes. Biochem Biophys Res Commun 377, 268–274. [DOI] [PubMed] [Google Scholar]
- Ilunga Kalenga O, Moeti M, Sparrow A, Nguyen VK, Lucey D, Ghebreyesus TA, 2019. The Ongoing Ebola Epidemic in the Democratic Republic of Congo, 2018–2019. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Jayaraman SD, Ismail S, Nair NK, Navaratnam V, 1997. Determination of pyronaridine in blood plasma by high-performance liquid chromatography for application in clinical pharmacological studies. J Chromatogr B Biomed Sci Appl 690, 253–257. [DOI] [PubMed] [Google Scholar]
- Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, Hoffstrom BG, DeWald LE, Schornberg KL, Scully C, Lehár J, Hensley LE, White JM, Olinger GG, 2013. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med 5, 190ra179–190ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas O, 2019. Pandemic bonds: designed to fail in Ebola. Nature 572, 285. [DOI] [PubMed] [Google Scholar]
- Kerber R, Lorenz E, Duraffour S, Sissoko D, Rudolf M, Jaeger A, Cisse SD, Camara AM, Miranda O, Castro CM, Akoi Bore J, Raymond Koundouno F, Repits J, Afrough B, Becker-Ziaja B, Hinzmann J, Mertens M, Vitoriano I, Hugh Logue C, Bottcher JP, Pallasch E, Sachse A, Bah A, Cabeza-Cabrerizo M, Nitzsche K, Kuisma E, Michel J, Holm T, Zekeng EG, Cowley LA, Garcia-Dorival I, Hetzelt N, Baum JHJ, Portmann J, Carter L, Yenamaberhan RL, Camino A, Enkirch T, Singethan K, Meisel S, Mazzarelli A, Kosgei A, Kafetzopoulou L, Rickett NY, Patrono LV, Ghebreghiorghis L, Arnold U, Colin G, Juchet S, Marchal CL, Kolie JS, Beavogui AH, Wurr S, Bockholt S, Krumkamp R, May J, Stoecker K, Fleischmann E, Ippolito G, Carroll MW, Koivogui L, Magassouba N, Keita S, Gurry C, Drury P, Diallo B, Formenty P, Wolfel R, Caro AD, Gabriel M, Anglaret X, Malvy D, Gunther S, 2019. Laboratory Findings, Compassionate Use of Favipiravir, and Outcome in Patients With Ebola Virus Disease, Guinea, 2015-A Retrospective Observational Study. J Infect Dis 220, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley MP, Bowen ET, Eddy GA, Isaacson M, Johnson KM, McCormick JB, Murphy FA, Pattyn SR, Peters D, Prozesky OW, Regnery RL, Simpson DI, Slenczka W, Sureau P, van der Groen G, Webb PA, Wulff H, 1982. Filoviridae: a taxonomic home for Marburg and Ebola viruses? Intervirology 18, 24–32. [DOI] [PubMed] [Google Scholar]
- Kouznetsova J, Sun W, Martinez-Romero C, Tawa G, Shinn P, Chen CZ, Schimmer A, Sanderson P, McKew JC, Zheng W, Garcia-Sastre A, 2014. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect 3, e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TR, Comer JE, Freiberg AN, Madrid PB, Ekins S, 2019a. Repurposing Quinacrine Against Ebola Virus Infection In vivo. Antimicrobial agents and chemotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TR, Comer JE, Freiberg AN, Madrid PB, Ekins S, 2019b. Repurposing Quinacrine Against Ebola Virus Infection In vivo. Antimicrobial agents and chemotherapy 63, e01142–01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TR, Massey C, Comer JE, Anantpadma M, Freundlich JS, Davey RA, Madrid PB, Ekins S, 2019c. Repurposing The Antimalarial Pyronaridine Tetraphosphate To Protect Against Ebola Virus Infection PLoS Negl Trop Dis 13, e0007890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TR, Massey C, Comer JE, Anantpadma M, Freundlich JS, Davey RA, Madrid PB, Ekins S, 2019d. Repurposing The Antimalarial Pyronaridine Tetraphosphate To Protect Against Ebola Virus Infection PLoS neglected tropical diseases In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Adhikari NKJ, Kwon HY, Teo K, Siemieniuk R, Lamontagne F, Chan A, Mishra S, Murthy S, Kiiza P, Hajek J, Bah EI, Lamah MC, Kao R, Fowler RA, 2019. Anti-Ebola therapy for patients with Ebola virus disease: a systematic review. BMC Infect Dis 19, 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leligdowicz A, Fischer WA 2nd, Uyeki TM, Fletcher TE, Adhikari NK, Portella G, Lamontagne F, Clement C, Jacob ST, Rubinson L, Vanderschuren A, Hajek J, Murthy S, Ferri M, Crozier I, Ibrahima E, Lamah MC, Schieffelin JS, Brett-Major D, Bausch DG, Shindo N, Chan AK, O’Dempsey T, Mishra S, Jacobs M, Dickson, Lyon GM 3rd, Fowler RA, 2016. Ebola virus disease and critical illness. Crit Care 20, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelain V, Guedj J, Mentre F, Nguyen TH, Jacquot F, Oestereich L, Kadota T, Yamada K, Taburet AM, de Lamballerie X, Raoul H, 2017. Favipiravir Pharmacokinetics in Nonhuman Primates and Insights for Future Efficacy Studies of Hemorrhagic Fever Viruses. Antimicrobial agents and chemotherapy 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid PB, Chopra S, Manger ID, Gilfillan L, Keepers TR, Shurtleff AC, Green CE, Iyer LV, Dilks HH, Davey RA, Kolokoltsov AA, Carrion R Jr., Patterson JL, Bavari S, Panchal RG, Warren TK, Wells JB, Moos WH, Burke RL, Tanga MJ, 2013. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PloS one 8, e60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid PB, Panchal RG, Warren TK, Shurtleff AC, Endsley AN, Green CE, Kolokoltsov A, Davey R, Manger ID, Gilfillan L, Bavari S, Tanga MJ, 2015a. Evaluation of Ebola Virus Inhibitors for Drug Repurposing. ACS Infect Dis 1, 317–326. [DOI] [PubMed] [Google Scholar]
- Madrid PB, Panchal RG, Warren TK, Shurtleff AC, Endsley AN, Green CE, Kolokoltsov AA, Davey RA, Manger ID, Gilfillan L, Bavari S, Tanga MJ, 2015b. Evaluation of Ebola Virus Inhibitors for Drug Repurposing. ACS Inf Dis 1, 317–326. [DOI] [PubMed] [Google Scholar]
- Mankowski DC, Laddison KJ, Christopherson PA, Ekins S, Tweedie DJ, Lawton MP, 1999. Molecular cloning, expression, and characterization of CYP2D17 from cynomolgus monkey liver. Arch Biochem Biophys 372, 189–196. [DOI] [PubMed] [Google Scholar]
- Martin RE, Marchetti RV, Cowan AI, Howitt SM, Broer S, Kirk K, 2009. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science 325, 1680–1682. [DOI] [PubMed] [Google Scholar]
- Miller JL, Spiro SG, Dowall SD, Taylor I, Rule A, Alonzi DS, Sayce AC, Wright E, Bentley EM, Thom R, Hall G, Dwek RA, Hewson R, Zitzmann N, 2016. Minimal In Vivo Efficacy of Iminosugars in a Lethal Ebola Virus Guinea Pig Model. PLoS One 11, e0167018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, Pokorny R, Lopez-Lazaro L, Miller RM, Arbe-Barnes S, Duparc S, Borghini-Fuhrer I, Shin JS, Fleckenstein L, 2014. Pharmacokinetic interaction between pyronaridine-artesunate and metoprolol. Antimicrobial agents and chemotherapy 58, 5900–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S, Dodd LE, Davey RT Jr., Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum JJ, Group, P.W., Sivahera B, Camara M, Kojan R, Walker R, Dighero-Kemp B, Cao H, Mukumbayi P, Mbala-Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallee D, Nordwall J, Team PCS, 2019. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med 381, 2293–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestereich L, Ludtke A, Wurr S, Rieger T, Munoz-Fontela C, Gunther S, 2014. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 105, 17–21. [DOI] [PubMed] [Google Scholar]
- Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, Johnson A, Morton J, Swope K, Bohorov O, Bohorova N, Goodman C, Kim D, Pauly MH, Velasco J, Pettitt J, Olinger GG, Whaley K, Xu B, Strong JE, Zeitlin L, Kobinger GP, 2014. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn K, Brindley MA, Weller ML, Kaludov N, Kondratowicz A, Hunt CL, Sinn PL, McCray PB Jr., Stein CS, Davidson BL, Flick R, Mandell R, Staplin W, Maury W, Chiorini JA, 2009. Rho GTPases modulate entry of Ebola virus and vesicular stomatitis virus pseudotyped vectors. J Virol 83, 10176–10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim MN, Zhang Z, He S, Zhu W, Banadyga L, Safronetz D, Qiu X, 2018. Postexposure Protective Efficacy of T-705 (Favipiravir) Against Sudan Virus Infection in Guinea Pigs. J Infect Dis 218, S649-S657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Karupiah S, Nair NK, Olliaro PL, Navaratnam V, Wernsdorfer WH, Mansor SM, 2005. A new and simple solid-phase extraction method for LC determination of pyronaridine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 824, 45–50. [DOI] [PubMed] [Google Scholar]
- Rijal P, Elias SC, Machado SR, Xiao J, Schimanski L, O’Dowd V, Baker T, Barry E, Mendelsohn SC, Cherry CJ, Jin J, Labbe GM, Donnellan FR, Rampling T, Dowall S, Rayner E, Findlay-Wilson S, Carroll M, Guo J, Xu XN, Huang KA, Takada A, Burgess G, McMillan D, Popplewell A, Lightwood DJ, Draper SJ, Townsend AR, 2019. Therapeutic Monoclonal Antibodies for Ebola Virus Infection Derived from Vaccinated Humans. Cell Rep 27, 172–186 e177. [DOI] [PubMed] [Google Scholar]
- Shimada T, Mimura M, Inoue K, Nakamura S, Oda H, Ohmori S, Yamazaki H, 1997. Cytochrome P450-dependent drug oxidation activities in liver microsomes of various animal species including rats, guinea pigs, dogs, monkeys, and humans. Arch Toxicol 71, 401–408. [DOI] [PubMed] [Google Scholar]
- Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, Neville S, Carra E, Lew W, Ross B, Wang Q, Wolfe L, Jordan R, Soloveva V, Knox J, Perry J, Perron M, Stray KM, Barauskas O, Feng JY, Xu Y, Lee G, Rheingold AL, Ray AS, Bannister R, Strickley R, Swaminathan S, Lee WA, Bavari S, Cihlar T, Lo MK, Warren TK, Mackman RL, 2017. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J Med Chem 60, 1648–1661. [DOI] [PubMed] [Google Scholar]
- Sissoko D, Laouenan C, Folkesson E, M’Lebing AB, Beavogui AH, Baize S, Camara AM, Maes P, Shepherd S, Danel C, Carazo S, Conde MN, Gala J-L, Colin G, Savini H, Bore JA, Le Marcis F, Koundouno FR, Petitjean F, Lamah MC, Diederich S, Tounkara A, Poelart G, Berbain E, Dindart JM, Duraffour S, Lefevre A, Leno T, Peyrouset O, Irenge L, Bangoura N, Palich R, Hinzmann J, Kraus A, Barry TS, Berette S, Bongono A, Camara MS, Chanfreau Munoz V, Doumbouya L, Souley H, Kighoma PM, Koundouno FR, Rene L, Loua CM, Massala V, Moumouni K, Provost C, Samake N, Sekou C, Soumah A, Arnould I, Komano MS, Gustin L, Berutto C, Camara D, Camara FS, Colpaert J, Delamou L, Jansson L, Kourouma E, Loua M, Malme K, Manfrin E, Maomou A, Milinouno A, Ombelet S, Sidiboun AY, Verreckt I, Yombouno P, Bocquin A, Carbonnelle C, Carmoi T, Frange P, Mely S, Nguyen VK, Pannetier D, Taburet AM, Treluyer JM, Kolie J, Moh R, Gonzalez MC, Kuisma E, Liedigk B, Ngabo D, Rudolf M, Thom R, Kerber R, Gabriel M, Di Caro A, Wolfel R, Badir J, Bentahir M, Deccache Y, Dumont C, Durant JF, El Bakkouri K, Gasasira Uwamahoro M, Smits B, Toufik N, Van Cauwenberghe S, Ezzedine K, D’Ortenzio E, Pizarro L, Etienne A, Guedj J, Fizet A, Barte de Sainte Fare E, Murgue B, Tran-Minh T, Rapp C, Piguet P, Poncin M, Draguez B, Allaford Duverger T, Barbe S, Baret G, Defourny I, Carroll M, Raoul H, Augier A, Eholie SP, Yazdanpanah Y, Levy-Marchal C, Antierrens A, Van Herp M, Gunther S, de Lamballerie X, Keita S, Mentre F, Anglaret X, Malvy D, Group, J.S., 2016a. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS Med 13, e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissoko D, Laouenan C, Folkesson E, M’Lebing A-B, Beavogui A-H, Baize S, Camara A-M, Maes P, Shepherd S, Danel C, Carazo S, Conde MN, Gala J-L, Colin G, Savini H, Bore JA, Le Marcis F, Koundouno FR, Petitjean F, Lamah M-C, Diederich S, Tounkara A, Poelart G, Berbain E, Dindart J-M, Duraffour S, Lefevre A, Leno T, Peyrouset O, Irenge L, Bangoura NF, Palich R, Hinzmann J, Kraus A, Barry TS, Berette S, Bongono A, Camara MS, Chanfreau Munoz V, Doumbouya L, Souley H, Kighoma PM, Koundouno FR, Réné L, Loua CM, Massala V, Moumouni K, Provost C, Samake N, Sekou C, Soumah A, Arnould I, Komano MS, Gustin L, Berutto C, Camara D, Camara FS, Colpaert J, Delamou L, Jansson L, Kourouma E, Loua M, Malme K, Manfrin E, Maomou A, Milinouno A, Ombelet S, Sidiboun AY, Verreckt I, Yombouno P, Bocquin A, Carbonnelle C, Carmoi T, Frange P, Mely S, Nguyen V-K, Pannetier D, Taburet A-M, Treluyer J-M, Kolie J, Moh R, Gonzalez MC, Kuisma E, Liedigk B, Ngabo D, Rudolf M, Thom R, Kerber R, Gabriel M, Di Caro A, Wölfel R, Badir J, Bentahir M, Deccache Y, Dumont C, Durant J-F, El Bakkouri K, Gasasira Uwamahoro M, Smits B, Toufik N, Van Cauwenberghe S, Ezzedine K, Dortenzio E, Pizarro L, Etienne A, Guedj J, Fizet A, Barte de Sainte Fare E, Murgue B, Tran-Minh T, Rapp C, Piguet P, Poncin M, Draguez B, Allaford Duverger T, Barbe S, Baret G, Defourny I, Carroll M, Raoul H, Augier A, Eholie SP, Yazdanpanah Y, Levy-Marchal C, Antierrens A, Van Herp M, Günther S, de Lamballerie X, Keïta S, Mentre F, Anglaret X, Malvy D, Group, J.S., 2016b. Experimental treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A historically controlled, single-arm proof-of-concept trial in Guinea . PLOS Medicine 13, e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivapalasingam S, Kamal M, Slim R, Hosain R, Shao W, Stoltz R, Yen J, Pologe LG, Cao Y, Partridge M, Sumner G, Lipsich L, 2018. Safety, pharmacokinetics, and immunogenicity of a co-formulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first-in-human phase 1 study. Lancet Infect Dis 18, 884–893. [DOI] [PubMed] [Google Scholar]
- Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS, 2014. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res 104, 153–155. [DOI] [PubMed] [Google Scholar]
- Taylor R, Kotian P, Warren T, Panchal R, Bavari S, Julander J, Dobo S, Rose A, El-Kattan Y, Taubenheim B, Babu Y, Sheridan WP, 2016. BCX4430 - A broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infect Public Health 9, 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyteca D, Van Der Smissen P, Mettlen M, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP, Courtoy PJ, 2002. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res 281, 86–100. [DOI] [PubMed] [Google Scholar]
- Volchkov VE, Chepurnov AA, Volchkova VA, Ternovoj VA, Klenk HD, 2000. Molecular characterization of guinea pig-adapted variants of Ebola virus. Virology 277, 147–155. [DOI] [PubMed] [Google Scholar]
- Wec AZ, Bornholdt ZA, He S, Herbert AS, Goodwin E, Wirchnianski AS, Gunn BM, Zhang Z, Zhu W, Liu G, Abelson DM, Moyer CL, Jangra RK, James RM, Bakken RR, Bohorova N, Bohorov O, Kim DH, Pauly MH, Velasco J, Bortz RH 3rd, Whaley KJ, Goldstein T, Anthony SJ, Alter G, Walker LM, Dye JM, Zeitlin L, Qiu X, Chandran K, 2019. Development of a Human Antibody Cocktail that Deploys Multiple Functions to Confer Pan-Ebolavirus Protection. Cell Host Microbe 25, 39–48 e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Xu WW, Zhang Z, Liu J, Li J, Sun L, Sun W, Jiao P, Sang X, Ren Z, Yu Z, Li Y, Feng N, Wang T, Wang H, Yang S, Zhao Y, Zhang X, Wilker PR, Liu W, Liao M, Chen H, Gao Y, Xia X, 2017. The innate immunity of guinea pigs against highly pathogenic avian influenza virus infection. Oncotarget 8, 30422–30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.