Abstract
Purpose
Preliminary reports suggest that critically ill patients with coronavirus disease 2019 (COVID-19) infection requiring mechanical ventilation may have markedly increased sedation needs compared with critically ill, mechanically ventilated patients without COVID-19. We conducted a study to examine sedative use for this patient population within multiple intensive care units (ICUs) of a large academic medical center.
Methods
A retrospective, single-center cohort study of sedation practices for critically ill patients with COVID-19 during the first 10 days of mechanical ventilation was conducted in 8 ICUs at Massachusetts General Hospital, Boston, MA. The study population was a sequential cohort of 86 critically ill, mechanically ventilated patients with COVID-19. Data characterizing the sedative medications, doses, drug combinations, and duration of administration were collected daily and compared to published recommendations for sedation of critically ill patients without COVID-19. The associations between drug doses, number of drugs administered, baseline patient characteristics, and inflammatory markers were investigated.
Results
Among the study cohort, propofol and hydromorphone were the most common initial drug combination, with these medications being used on a given day in up to 100% and 88% of patients, respectively. The doses of sedative and analgesic infusions increased for patients over the first 10 days, reaching or exceeding the upper limits of published dosage guidelines for propofol (48% of patients), dexmedetomidine (29%), midazolam (7.7%), ketamine (32%), and hydromorphone (38%). The number of sedative and analgesic agents simultaneously administered increased over time for each patient, with more than 50% of patients requiring 3 or more agents by day 2. Compared with patients requiring 3 or fewer agents, patients requiring more than 3 agents were of younger age, had an increased body mass index, had increased serum ferritin and lactate dehydrogenase concentrations, had a lower Pao2:Fio2 (ratio of arterial partial pressure of oxygen to fraction of inspired oxygen), and were more likely to receive neuromuscular blockade.
Conclusion
Our study confirmed the clinical impression of elevated sedative use in critically ill, mechanically ventilated patients with COVID-19 relative to guideline-recommended sedation practices in other critically ill populations.
Keywords: COVID-19, critical illness, mechanical ventilation, sedation
Key Points.
In a study of mechanically ventilated patients with COVID-19–associated acute respiratory distress syndrome in multiple intensive care units of a single medical center, the doses of sedatives and analgesics increased over time, reaching or exceeding the upper dose limits specified in published guidelines.
The number of sedative and analgesic agents simultaneously administered to the study cohort also increased over time.
Compared with patients requiring 3 or fewer sedative or analgesic agents, patients requiring more than 3 agents were, on average, younger, had increased body mass index, had increased serum ferritin and lactate dehydrogenase levels, had worse respiratory function, and were more likely to receive neuromuscular blockade.
Patients with severe coronavirus disease 2019 (COVID-19) frequently develop respiratory failure requiring mechanical ventilation for support. Many of these patients meet criteria for acute respiratory distress syndrome (ARDS), and ensuring a strategy of lung-protective ventilation is important to prevent ventilator-induced lung injury. Sedative agents are commonly administered to promote patient comfort and facilitate lung-protective ventilation, which often includes limiting tidal volumes and plateau pressures, increasing positive end-expiratory pressure, reducing respiratory rates, and avoiding ventilator dysynchrony.1,2 Appropriate use of sedative agents to achieve these goals can reduce the need for use of neuromuscular blocking agents (NMBAs), which can interfere with clinical examination and have been linked to a number of adverse effects, including awareness during neuromuscular blockade and intensive care unit (ICU)–acquired weakness.3
Clinical experience and early reports have suggested that sedation requirements are increased in critically ill, mechanically ventilated patients with COVID-19 compared to other critically ill, mechanically ventilated populations.4,5 Postulated mechanisms for this include increased respiratory drive, exaggerated inflammatory response, hypermetabolic state, and prolonged duration of critical illness associated with COVID-19. Efforts to minimize provider exposure, limited supplies of personal protective equipment, and concern for maintaining patient safety (eg, preventing self-extubation) may also impact sedation practices. Here, we describe a retrospective, observational cohort study of patients with COVID-19 admitted to multiple ICUs in our academic medical center to systematically examine and quantify sedative use in critically ill, mechanically ventilated patients with COVID-19.
Methods
The study was approved by the Massachusetts General Hospital (MGH) institutional review board (MGH-IRB- 2020P001267), with waiver of patient consent. The retrospective, observational cohort study examined 86 consecutive adult patients (≥18 years of age) with COVID-19–associated respiratory failure requiring mechanical ventilation admitted to one of 8 ICUs at MGH from March 14 through April 4, 2020. COVID-19 status was based upon a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction assay performed by the hospital-based clinical laboratory. All patients admitted to a dedicated COVID-19 ICU were cared for by a traditional ICU care team, led by a critical care attending physician, with standard (prepandemic) ICU staffing ratios. There were no critical shortages of ventilators or other critical care equipment, but the institution experienced shortages of cisatracurium and hydromorphone during the study period. ICU physicians made all sedation drug decisions, and sedation was titrated using the Richmond Agitation and Sedation Scale (RASS) by trained ICU practitioners, with specific goals set by those practitioners. At the time of this analysis, there was no sedation guideline across the institution. Decisions regarding use of (NMBAs) were not standardized across ICUs. Depth of paralysis monitoring was performed using train-of-four assessment and titration to ventilator synchrony.
Baseline demographic characteristics as well as baseline inflammation-related laboratory values were collected for each patient. Daily doses of analgesic, sedative, and paralytic medications were collected from the medical record and summed into daily totals; daily averages were calculated for up to 10 days of mechanical ventilation. For the purposes of the study, we excluded bolus as well as oral adjunct medication administrations. As propofol was the primary sedative agent for many patients, daily triglycerides levels were also collected for safety monitoring.
To allow for comparison of dosing data with results of prior sedation studies in the literature, both actual and weight-adjusted doses were calculated when appropriate. Midazolam, hydromorphone, dexmedetomidine, and rocuronium doses were calculated using ideal body weight (IBW). Propofol, ketamine, and fentanyl doses were calculated using adjusted body weight if the patient was obese and actual body weight if the patient was not obese. Cisatracurium doses were calculated using actual body weight. IBW was calculated as follows: 50 kg (male) or 45 kg (female) + 2.3 kg for each inch over 5 feet. Adjusted body weight was calculated as IBW + 0.4(actual body weight – IBW).6
Data were collected from the institution’s electronic medical record (Epic Systems Corporation, Verona, WI), and results are expressed as median with interquartile range (IQR) unless otherwise specified. Baseline characteristics were compared between the 2 groups using 2-sample parametric or nonparametric tests, as appropriate. A 2-sided P value of <0.05 was considered statistically significant. All statistical analysis and graphs were performed using Microsoft Excel (Microsoft Corporation, Redmond, WA) and GraphPad Prism 8 data analysis and statistical software (GraphPad Software, San Diego, CA).
Results
Patient characteristics.
From March 14 through April 4, 2020, 86 adult critically ill patients with COVID-19 were admitted to an ICU with respiratory failure and received continuous sedative infusions to facilitate invasive mechanical ventilation. The baseline demographics are summarized in Table 1. The cohort was predominantly male (65.1%), with a median age of 61 (IQR, 48.5-74.5) years. Of note, a minority of patients had a history of chronic pain (9.3%) and/or substance use disorder (5.8%). The median baseline laboratory results demonstrated elevated inflammatory markers consistent with severe COVID-19. The median ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (Pao2:Fio2) was 193.7 (IQR, 149.0-267.3), corresponding to moderate ARDS by the Berlin definition.7 Fifty-four percent of patients (47 of 86) underwent prone positioning 1 or more times during the study period. No patient in the cohort received extracorporeal membrane oxygenation. Among the 86 patients, 11 were extubated and 9 were deceased prior to the 10th day of mechanical ventilation; we excluded the data from all days after extubation or following death for these patients.
Table 1.
Baseline Characteristics and Laboratory Values in Patient Cohort (n = 86)
| Median (IQR)a | |
|---|---|
| Age, y | 61 (48.5-74.5) |
| Male, No. (%) | 56 (65) |
| Weight, kg | 82 (74.0-102.6) |
| Height, cm | 167.6 (160.0-175.0) |
| Diabetes, No. (%) | 34 (39) |
| Hyperlipidemia, No. (%) | 40 (46) |
| Immunosuppressed, No. (%) | 9 (10) |
| Chronic pain, No. (%) | 8 (9) |
| Substance use disorder, No. (%) | 5 (6) |
| Laboratory values | |
| Creatinine, mg/dL | 168 (114.5-260.0) |
| AST, U/L | 59.50 (44.5-77.25) |
| ALT, U/L | 38.50 (30.25-41.75) |
| ALP, U/L | 69.50 (51.25-81.50) |
| Total bilirubin, mg/dL | 0.50 (0.20-0.50) |
| TG, mg/dL | 168 (114.5-260.0) |
| ESR, mm/h | 48 (28.0-68.0) |
| CRP, mg/L | 147.9 (75.5-226.2) |
| Ferritin, μg/L | 739 (405.0-1,358.0) |
| D-dimer, nL/mL | 1,043.5 (794.0-1,776.0) |
| LDH, U/L | 395 (319.5-539.0) |
| CK, U/L | 152 (86.0-417.0) |
| Pao2:Fio2 | 193.7 (149.0-267.3) |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range; LDH, lactate dehydrogenase; Pao2, arterial partial pressure of oxygen; Fio2, fraction of inspired oxygen; TG, triglycerides.
aUnless otherwise indicated.
Sedative characteristics.
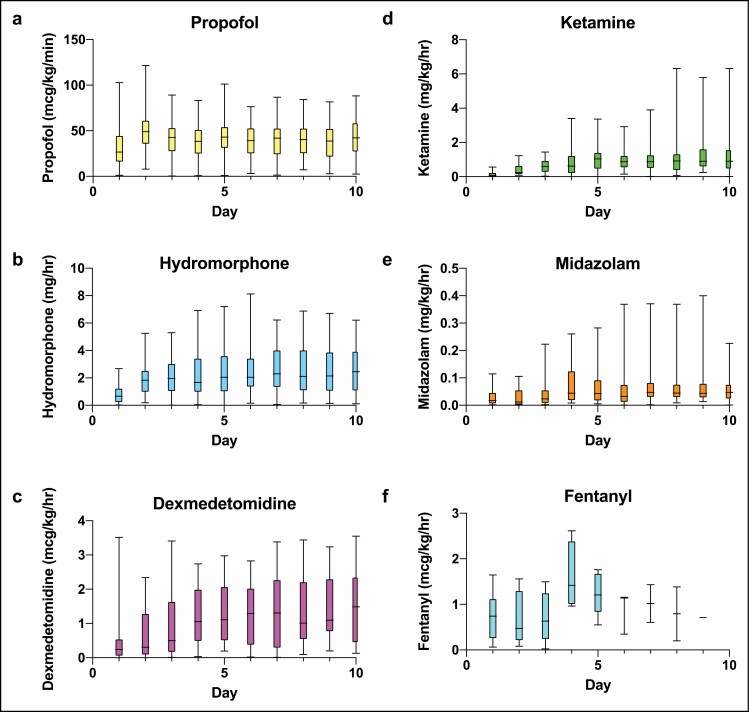
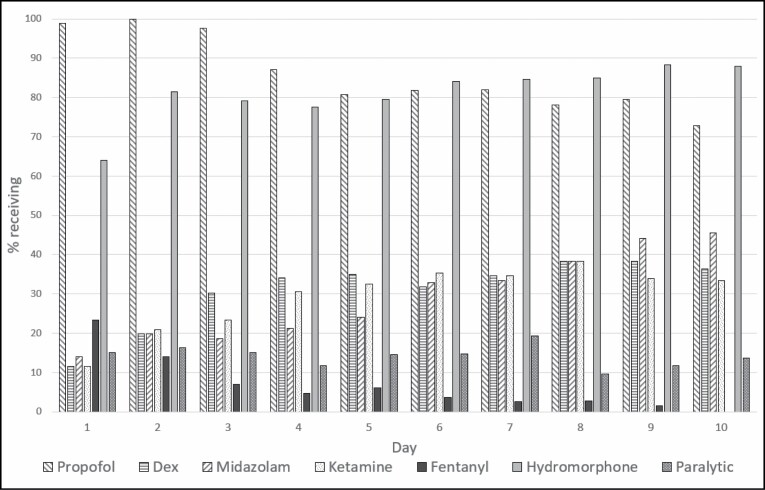
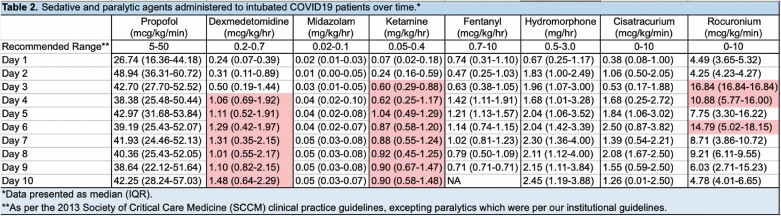
The distribution of sedative and analgesic doses administered as well as the percentage of patients receiving each drug on a given day over the first 10 days of mechanical ventilation are displayed in Figures 1 and 2. Propofol and hydromorphone were most commonly administered in our patient cohort, with up to 100% of the patients receiving propofol and up to 88% receiving hydromorphone on a given day. Ketamine, dexmedetomidine, and midazolam were also frequently administered as adjuncts—in up to 38.4%, 38.4%, and 45.5% of patients, respectively, on any given day. Table 2 displays the median doses of the sedative and analgesic agents administered as well as the upper dose limits recommended by the Society of Critical Care Medicine (SCCM) clinical practice guidelines for management of pain, agitation, and delirium.8 While doses were variable, the percentages of patients for whom doses of propofol, dexmedetomidine, midazolam, ketamine, and/or hydromorphone exceeded the upper limits on any given were 48%, 29%, 8%, 32%, and 38%, respectively (Table 3).
Figure 1.
Weight-adjusted total daily doses of sedative and analgesic agents administered to intubated patients with COVID-19 by day of intubation.
Figure 2.
Percentage of intubated patients with COVID-19 receiving a given sedative, analgesic, or paralytic agent by day of intubation.
Table 2.
aRed shading indicates values that are above published recommended dose ranges.
Table 3.
Percentage of Intubated Patients With COVID-19 Who Received Drug Doses Exceeding Recommended Rangea,b
| Propofol | DEX | Midazolam | Ketamine | Fentanyl | HYDRO | |
|---|---|---|---|---|---|---|
| Day 1 | 16 (18.60) | 2 (2.33) | 1 (1.16) | 1 (1.16) | 0 (0) | 0 (0) |
| Day 2 | 41 (47.67) | 6 (6.98) | 1 (1.16) | 7 (8.14) | 0 (0) | 9 (10.47) |
| Day 3 | 27 (31.40) | 11 (12.79) | 1 (1.16) | 13 (15.12) | 0 (0) | 17 (19.77) |
| Day 4 | 19 (22.35) | 21 (24.71) | 5 (5.88) | 16 (18.82) | 0 (0) | 17 (20.00) |
| Day 5 | 23 (27.38) | 20 (23.81) | 4 (4.76) | 23 (27.38) | 0 (0) | 18 (21.43) |
| Day 6 | 19 (23.17) | 17 (20.73) | 5 (6.10) | 24 (29.27) | 0 (0) | 21 (25.61) |
| Day 7 | 18 (23.08) | 18 (23.08) | 6 (7.69) | 22 (28.21) | 0 (0) | 26 (33.33) |
| Day 8 | 18 (24.66) | 19 (26.03) | 4 (5.48) | 21 (28.77) | 0 (0) | 25 (34.25) |
| Day 9 | 17 (25.00) | 20 (29.41) | 4 (5.88) | 22 (32.35) | 0 (0) | 26 (38.24) |
| Day 10 | 20 (30.30) | 18 (27.27) | 3 (4.55) | 17 (25.76) | 0 (0) | 25 (37.88) |
Abbreviations: DEX, dexmedetomidine; HYDRO, hydromorphone.
aAll data are number (percentage) of patients; the denominator (n) for percentage calculations varied from?? to 86. To allow for more precise comparisons, percentages were not rounded.
bAs per 2013 Society of Critical Care (SCCM) clinical practice guidelines,8 recommended dose ranges are as follows: propofol, 5-50 µg/kg/min; dexmedetomidine, 0.2-0.7 µg/kg/h; midazolam, 0.02-0.10 mg/kg/h; ketamine, 0.05-0.4 mg/kg/h; fentanyl, 0.7-10 µg/kg/h; and hydromorphone, 0.5-3.0 mg/h.
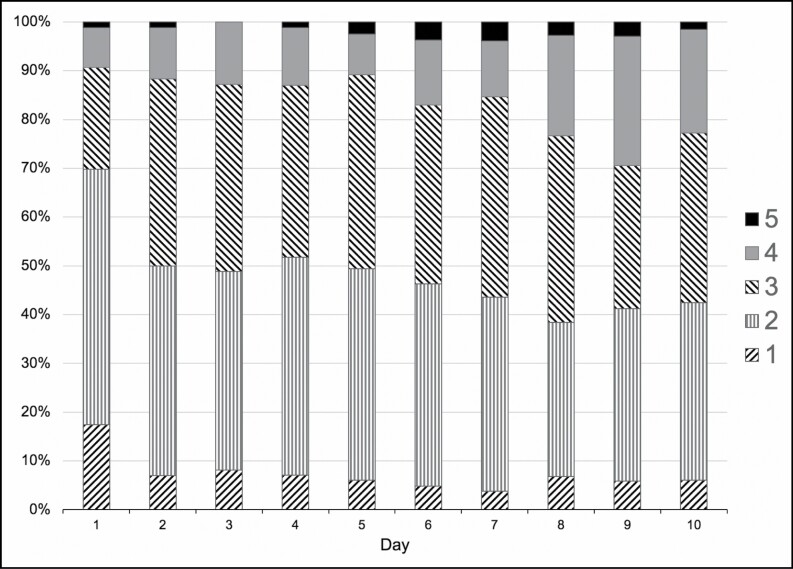
The numbers of sedative agents and analgesic agents simultaneously administered increased rapidly after initiation of mechanical ventilation, with over 50% of patients requiring 3 or more agents by day 2 and up to 5 agents administered to a given patient by the end of the study interval (Figure 3). The median infusion durations were greatest for propofol (9 [IQR, 6-10] days) and hydromorphone (9 [IQR, 8-10] days), followed by ketamine, at 6 (IQR, 3.3-8.0) days (Table 4). The percentage of patients receiving NMBAs was variable over time but was less than 20% on any given day (Figure 4). In total, 37.2% of intubated patients with COVID-19 (32 of 86) received NMBAs over the course of the study.
Figure 3.
Percentage of intubated patients with COVID-19 requiring a given number of sedative and analgesic agents by day of intubation.
Table 4.
Median Duration of Sedative and Analgesic Infusions (in Days) Administered to Intubated Patients with COVID-19a
| Infused Drug | Duration of Infusion |
|---|---|
| Propofol | 9.00 (6.00-10.00) |
| Dexmedetomidine | 4.00 (2.00-7.00) |
| Midazolam | 5.00 (3.00-7.00) |
| Ketamine | 6.00 (3.25-8.00) |
| Fentanyl | 2.00 (1.00-2.00) |
| Hydromorphone | 9.00 (8.00-10.00) |
aData presented as median (interquartile range).
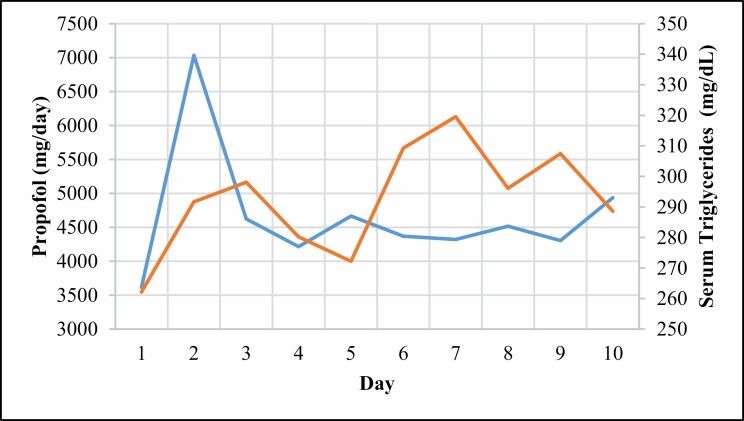
Figure 4.
Median daily propofol dose (blue) versus median serum triglyceride concentration (orange) over first 10 days of intubation.
Sedative requirements and patient characteristics.
We evaluated the relationship between sedation requirements and patient characteristics by comparing the subgroup of patients who required more than 3 sedative agents (n = 16) with those who required 3 or fewer agents (n = 70) on day 2. This comparison was selected because institutional data indicated that more than 95% of mechanically ventilated critically ill patients had been managed with 3 or fewer sedative agents prior to the COVID-19 pandemic. Compared with patients requiring 3 or fewer agents, as a group patients requiring more than 3 agents were significantly younger (median [IQR] age, 45 [41-53] years vs 62.5 [53.3-76.8] years; P = 0.002); had higher median (IQR) values for BMI (31 [28.2-36.5] vs 29 [26.2-35.4], P = 0.03), serum ferritin (1,041 [439-1,564] µg/L vs 712 [408-1,304] µg/L, P = 0.02), and serum lactate dehydrogenase (LDH) (473 [274.3-667] U/L vs 382 [330.5-501.5] U/L, P = 0.01); had lower values for Pao2:Fio2 (155 [115-190] vs 201.34 [160-271.8], P = 0.03); and were more likely to receive NMBAs during the study period (68.8% vs 30%, P = 0.02) (Table 5).
Table 5.
Characteristics of Intubated Patients With COVID-19 Requiring ≤3 vs >3 Sedative Agents for Ventilator Synchrony on Day 2 of Intubationa
| ≤3 Sedative Agents on Day 2 (n = 70) | >3 Sedative Agents on Day 2 (n = 16) | P Value | |
|---|---|---|---|
| Age, y | 62.50 (53.25-76.75) | 45.00 (41.00-53.00) | 0.002b |
| BMI (kg/m2) | 29 (26.24-35.36) | 31 (28.22-36.47) | 0.03b |
| Male, No. (%) | 48 (68.57) | 9 (56.25) | 0.57 |
| Chronic pain, No. (%) | 5 (7.14) | 2 (12.50) | 0.64 |
| Substance use disorder, No. (%) | 5 (7.14) | 0 (0.00) | 0.59 |
| Baseline laboratory values | |||
| Creatinine, mg/dL | 0.98 (0.88-1.08) | 0.96 (0.78-1.09) | 0.75 |
| AST, U/L | 38.5 (32.5-47.5) | 29.5 (28-36) | 0.60 |
| ALT, U/L | 66 (54.75-74.5) | 56 (49-80) | 0.40 |
| ALP, U/L | 60.5 (53.75-70.25) | 59 (41-139) | 0.91 |
| Total bilirubin, mg/dL | 0.55 (0.22-0.68) | 0.5 (0.2-0.7) | 0.21 |
| Ferritin, μg/L | 712.00 (408.00-1,304.00) | 1,041.00 (439.00-1,564.00) | 0.02b |
| D-dimer, ng/mL | 1,072.50 (794.00-1,705.00) | 942.00 (751.00-1,343.00) | 0.75 |
| LDH, U/L | 382.00 (330.50-501.50) | 473.50 (274.25-667.00) | 0.01b |
| CK, U/L | 159.00 (91.00-406.00) | 133.00 (81.00-396.00) | 0.78 |
| Baseline Pao2:Fio2 | 201.34 (160.00-271.75) | 155.00 (115.00-190.00) | 0.03b |
| NMBA use during study, No. (%) | 21 (30.00) | 11 (68.75) | 0.02b |
| RASS score | –3.5 (–4.25 to –2.25) | –4 (–5 to –3) | 0.83 |
| CPOT score | 0 (0.0-0.5) | 0 (0-0) | 0.32 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; CPOT, Critical-Care Pain Observation Tool; CRP, C-reactive protein; Fio2, fraction of inspired oxygen; IQR, interquartile range; LDH, lactate dehydrogenase; Pao2, arterial partial pressure of oxygen; RASS, Richmond Agitation Sedation Scale; TG, triglycerides.
aAll data presented as median (IQR) unless otherwise specified.
bStatistically significant difference per a priori definition (P < 0.05).
Discussion
Clinical experience and early reports from our institution and others have suggested that sedation requirements are increased in critically ill, mechanically ventilated patients with COVID-19 compared to other critically ill, mechanically ventilated populations, but published data to support this contention is limited.4,5,9 A study in New York hospitals found sharp increases in aggregate utilization of sedative and analgesic medications during the early weeks of the COVID-19 pandemic, consistent with the surge in patient numbers, but the study did not examine individual patient dosing.10 Our study was the largest study to systematically quantify individual sedation and analgesic dosing requirements for mechanically ventilated, critically ill patients with COVID-19 that has been conducted to date (as of the time of writing). High analgesic and sedative medication requirements were observed in a multi-ICU cohort of mechanically ventilated, critically ill patients with COVID-19. Notably, doses of sedatives and opioids frequently exceeded the upper limits specified in published SCCM guidelines. Furthermore, multiple agents were frequently administered to achieve sedation goals. Patient characteristics such as younger age, higher BMI, and elevations in specific inflammatory markers (serum LDH and ferritin) were associated with increased sedation requirements.
A challenge in conducting an analysis of sedation requirements in our study cohort of mechanically ventilated patients with COVID-19 was identifying an appropriate population for comparison. It is important to note that the question of whether “COVID-19 ARDS” can be directly compared to non–COVID-19 ARDS regardless of the severity of hypoxemia remains a topic of controversy.11,12 In comparison to non–COVID-19 ARDS, severe SARS-CoV-2 infection disproportionately causes endothelial damage that disrupts pulmonary vasoregulation, promotes ventilation-perfusion mismatch, and fosters thrombogenesis.13 In addition, there is often a markedly increased respiratory drive in patients with COVID-19 that may, if unchecked, intensify the strain and energy demands placed on highly vulnerable tissue. The desire to depress this respiratory drive so as to minimize further lung injury may be a primary determinant in the administration of sedative and analgesic agents.14 Finally, as compared to non–COVID-19 ARDS, for which (per the Berlin criteria7) there is an onset limit of 1 week post infection, COVID-19 ARDS may have a delayed onset (often 1 week or more after infection).
Even if the population of patients with non–COVID ARDS is an appropriate population for comparison with the COVID-19 ARDS population, additional challenges arise. Despite numerous ARDS studies in the literature, few have provided detailed information on the types and quantities of sedatives administered to the patients enrolled.15 In a recent letter, Kapp et al9 reported the sedation requirements among a small cohort of 19 ventilated and 5 nonventilated patients with COVID-19. Those investigators reported a 3-fold increase in opioid administration in their intubated patients who underwent neuromuscular blockade compared to patients with ARDS enrolled in the OSCILLATE trial16 (median daily dose in oral morphine equivalents, 937 mg vs 289 mg) despite similar durations of intubation and similar Pao2:Fio2 ratios at the time of intubation. The differences in benzodiazepine administration between their patients and the patients in the OSCILLATE trial were less pronounced (median daily dose in oral midazolam equivalents, 225 mg vs 199 mg). In contrast, the opioid administration in our study was approximately 2-fold greater (a median of 600 mg in oral morphine equivalents daily) than in the OSCILLATE trial. The differences in daily opioid administration between the study by Kapp et al9 and ours may be attributed to the “analgesia first” sedation strategy used by Kapp and colleagues and to our lower threshold for use of additional sedative agents, which was implemented for reasons enumerated below.
The selection and dosing of sedative and analgesic agents administered in our study cohort deserve consideration. While propofol and hydromorphone were the most commonly administered sedative and analgesics, there was considerable variability in the threshold for use of an additional agent(s). Perhaps the most predictable determinant for adding agent(s) was the development of elevated triglyceride levels with use of propofol (Figure 4), for which ketamine or midazolam were commonly added. Among the sedatives and analgesics administered, ketamine and dexmedetomidine were the agents for which dosing most consistently exceeded the SCCM pain, agitation, and delirium guidelines. The potent sedative and analgesic properties of ketamine may explain its frequent use. While a recent review reported increased ketamine dosing ranges in the ICU setting (up to 0.9 µg/kg/min),17 even this upper limit was commonly exceeded in our cohort. The early and frequent use of dexmedetomidine was unexpected for a cohort of patients who required deep sedation. Possible explanations for the dexmedetomidine use prior to escalation of other medication doses include a goal of providing multimodal sedation as well as a desire to limit the adverse effects resulting from further escalation of doses of existing medications (eg, ileus with opioid use or delirium with use of benzodiazepines).
There were a number of factors that likely contributed to higher doses of analgesic and sedative medications and higher numbers of drugs administered in our patient cohort. First, a relatively small proportion of patients received NMBAs, and therefore higher drug doses and higher use of drug combinations may have been targeted to achieve ventilator synchrony in an effort to avoid neuromuscular blockade. These doses often appeared to be in excess of doses required to provide patient comfort alone, as reflected in the low RASS and Critical-Care Pain Observation Tool scores (Table 6). The pyrexia and tachypnea characteristic of COVID-19 infection may lead to more patient-ventilator dyssynchrony, necessitating additional sedation. In addition, patients who received NMBAs may have been given higher doses and numbers of drugs to ensure comfort and lack of awareness. Processed electroencephalography (EEG) monitors (bispectral index monitors and/or SedLine monitors [Masimo Corporation, Irvine, CA]) were only used to monitor depth of sedation during paralysis in a small number of cases, but device shortages and lack of familiarity with these monitors may have led providers to prefer to err on the side of providing more sedation.
Table 6.
Sedation and Pain Scores Over First 10 Days of Intubationa
| Day | RASS | CPOT |
|---|---|---|
| 1 | –4.00 (–4.75 to –3.25) | 0 (0-0) |
| 2 | –4.00 (–5.00 to –3.00) | 0 (0-0) |
| 3 | –4.00 (–5.00 to –2.25) | 0 (0-0) |
| 4 | –4.00 (–5.00 to –3.00) | 0 (0-0) |
| 5 | –4.00 (–5.00 to –3.75) | 0 (0-0) |
| 6 | –4.00 (–5.00 to –4.00) | 0 (0-0) |
| 7 | –4.00 (–4.75 to –3.00) | 0 (0-1.5) |
| 8 | –4.00 (–4.75 to –1.25) | 0 (0-1.5) |
| 9 | –4.00 (–4.00 to –4.00) | 0 (0-0) |
| 10 | –4.00 (–4.75 to –4.00) | 0 (0-0.75) |
Abbreviations: CPOT, Critical-Care Pain Observation Tool; RASS, Richmond Agitation and Sedation Scale.
aAll data presented as median (interquartile range).
Challenges to entering patient rooms frequently in the setting of personal protective equipment requirements, as well as concerns over patient harm (eg, self-extubation), may have resulted in higher medication doses and reduced downward titrations of continuous infusions. Alterations in drug metabolism resulting from COVID-19–related inflammation might also have contributed to increased sedation needs and rapid development of tolerance over time.18 This possibility would be supported by the positive correlation between the number of drugs administered and elevated markers of inflammation. Increased requirements despite the high prevalence of hepatic and renal impairment in patients with COVID-19 would further support this hypothesis (Table 7).19,20 Finally, the prevalence of obesity and younger age of patients in the study were also likely factors contributing to increased sedation needs.
Table 7.
Markers of Renal and Hepatic Function Over First 10 Days of Intubationa
| Day | Creatinine, mg/dL | ALT, U/L | AST, U/L | ALP, U/L | Total Bilirubin, mg/dL |
|---|---|---|---|---|---|
| 1 | 0.93 (0.88-1.05) | 38.50 (30.25-41.75) | 59.50 (44.50-77.25) | 69.50 (51.25-81.50) | 0.50 (0.20-0.50) |
| 2 | 0.98 (0.78-1.16) | 35.00 (28.75-38.75) | 52.00 (45.00-61.00) | 67.00 (50.25-80.75) | 0.55 (0.38-0.73) |
| 3 | 1.08 (0.96-1.37) | 32.00 (22.75-33.75) | 48.50 (30.75-68.25) | 70.00 (51.50-76.25) | 0.50 (0.43-1.60) |
| 4 | 1.07 (0.88-1.50) | 29.50 (24.75-41.50) | 57.50 (50.25-71.75) | 76.50 (60.5-89.25) | 0.55 (0.33-1.58) |
| 5 | 1.02 (0.93-2.14) | 40.50 (24.75-58.75) | 73.50 (55.00-85.00) | 80.00 (59.00-110.00) | 0.40 (0.33-0.88) |
| 6 | 0.97 (0.89-2.71) | 45.00 (26.00-55.50) | 68.00 (43.50-94.00) | 81.50 (62.25-104.25) | 0.55 (0.30-0.88) |
| 7 | 1.03 (0.95-2.37) | 53.00 (22.50-74.50) | 70.50 (41.25-98.25) | 100.50 (75.75-137.00) | 0.50 (0.35-0.70) |
| 8 | 1.05 (1.00-1.68) | 46.00 (22.00-72.00) | 50.50 (41.00-90.25) | 122.00 (76.50-145.00) | 0.50 (0.33-0.83) |
| 9 | 1.02 (0.90-1.77) | 49.00 (26.50-71.75) | 44.00 (37.00-122.50) | 114.00 (77.75-179.50) | 0.55 (0.40-0.65) |
| 10 | 1.02 (0.90-1.44) | 43.50 (27.50-64.75) | 50.50 (36.25-85.75) | 116.00 (73.25-135.75) | 0.50 (0.30-0.80) |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase.
aAll data presented as median (interquartile range).
Our findings raise a number of clinically important questions regarding the impact of increased sedation administration on patient outcomes. Oversedation has been associated with a number of adverse outcomes in critically ill patients without COVID-19, including increases in length of stay, days of mechanical ventilation, rates of delirium, and neurocognitive impairment.21 These outcomes have also been reported in critically ill patients with COVID-19. While viral infection and consequent immune cascade and inflammation may play a role, it is likely that oversedation also contributes to this effect. While we did not measure these outcomes in our patient population directly, reported length of stay and mortality among mechanically ventilated patients at our institution are comparable to or even lower than those reported at other centers,22 suggesting that our strategy of sedation may not have been profoundly detrimental. Favoring sedation—even with multiple agents at high doses—over neuromuscular blockade appeared to be the practice of many practitioners caring for patients within the study cohort, which is consistent with evidence that early neuromuscular blockade for patients with ARDS is not widely adopted.23
It remains unknown whether early neuromuscular blockade—thereby reducing sedation requirements—would result in improved outcomes. This approach achieves ventilator synchrony but impairs clinical examination, risks undersedation, and predisposes to ICU-acquired weakness. In particular, increased risks of catastrophic neurologic and gastrointestinal complications in critically ill patients with COVID-19 make clinical examination especially important, which may favor a strategy of high sedative administration over paralysis.13,24 If a patient is receiving NMBAs, using processed EEG to guide sedation may help to minimize sedation and thereby reduce the risk of delirium. The tradeoff between muscle weakness with use of NMBAs versus delirium and post-ICU syndrome with prolonged use of high-dose sedation remains unclear. Finally, the optimal drug regimens, combinations, and thresholds for adding additional agents remain unclear. Given the prolonged duration of mechanical ventilation and sedation for this patient population, it seems plausible that sedation rotation strategies may reduce total dose exposure, thus minimizing development of tolerance and reducing adverse effects.25
To address these unanswered questions, further investigation is needed. As a first step, sedation practices should routinely be reported in studies of critically ill patients with COVID-19 so as to allow evaluation of the effects of sedation on outcomes. These differences in practices should be compared in conjunction with meaningful outcome measures to evaluate benefits and harms. Guidelines that provide best practices for providers should be developed. Looking forward, understanding and measuring the cognitive impact of prolonged use of multiple high-dose sedatives in this patient population will be necessary. Future studies should identify and quantify chronic pain, delirium, post-ICU syndrome, and posttraumatic stress disorder in this population. The medical burden of COVID-19 may persist for these patients long after they leave the ICU. In the interim, given the wide heterogeneity in critically ill patients with COVID-19,26 it is important to not forget that “one size does not fit all” 27: Individualized management of sedation and analgesia with the guidance of validated scales remains essential.
Limitations.
The study had a number of limitations. It was conducted in a single center and involved a relatively small number of patients. However, generalizability is improved by the fact the patients were cared for in multiple ICUs by critical care practitioners with widely different sedation practices, with no institutional protocolized sedation strategy in place at the time of analysis. Our study was limited by a lack of historical controls, which prevented comparisons of drug doses within institutional practice. However, the OSCILLATE study16 and the experience of Kapp et al9 provided points of comparison. Mortality and ICU length of stay were not analyzed in our study. However, those outcomes were reported for a subset of our patient sample and were comparable or reduced relative to mortality and ICU length of stay reported by Kapp et al,9 suggesting that our sedation strategy did not have a comparatively detrimental effect on these outcomes. Other outcome measures—such as rate of delirium, hospital length of stay, ICU-acquired weakness, and gastrointestinal dysfunction—were not measured, which prevents further analysis of the impact of drugs, doses, and combinations on these important measures. Finally, data for only the first 10 days of intubation were included in our study. We selected a 10-day timeframe to provide a “snapshot” of sedation and analgesic requirements and their escalation during the initial period of mechanical ventilation, recognizing that the duration of intubation was substantially longer for some patients. While the totality of sedation and analgesic administration is an important question, it was not the focus of the study.
Conclusion
Our study confirmed the clinical impression of increased sedative use in critically ill patients with COVID-19 requiring mechanical ventilation. Reporting of sedation practices from other institutions would be valuable for practice comparisons. Future studies to examine the correlation of different sedation strategies and patient outcomes are needed. Through such studies, best practice guidelines can be developed and outcomes improved for this challenging population of critically ill patients.
Disclosures
Dr. Hanidziar is supported by a Clinical Investigator Award from the National Heart, Lung, and Blood Institute (K08HL141694). The authors have declared no potential conflicts of interest.
References
- 1. Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46:606-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. deBacker J, Hart N, Fan E. Neuromuscular blockade in the 21st century management of the critically ill patient. Chest. 2017;151:697-706. [DOI] [PubMed] [Google Scholar]
- 4. Hanidziar D, Bittner EA. Sedation of mechanically ventilated COVID-19 patients: challenges and special considerations. Letter. Anesth Analg. 2020;131:e40-e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madhok J, Mihm FG. Rethinking sedation during prolonged mechanical ventilation for COVID-19 respiratory failure. Comment. Anesth Analg. 2020;131:e123-e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erstad BL, Barletta JF. Drug dosing in the critically ill obese patient—a focus on sedation, analgesia, and delirium. Crit Care. 2020;24:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526-2533. [DOI] [PubMed] [Google Scholar]
- 8. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263-306. [DOI] [PubMed] [Google Scholar]
- 9. Kapp C, Zaeh S, Niedermeyer S, et al. The use of analgesia and sedation in mechanically ventilated patients with COVID-19 ARDS. Letter. Anesth Analg. 2020;131:e198-e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dabestani A, DeAngelo D, Chhay SR, Larson BJ, Ganio MC. Medication utilization in patients in New York hospitals during the COVID-19 pandemic. Am J Health-Syst Pharm. 2020;77:1885-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grieco DL, Bongiovanni F, Chen L, et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care. 2020;24:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaafarani HMA, El Moheb M, Hwabejire JO, et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020;272:e61-e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruces P, Retamal J, Hurtado DE, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care. 2020;24:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanidziar D, Bittner EA. Re: The use of analgesia and sedation in mechanically ventilated patients with COVID-19 ARDS. In response. Anesth Analg. 2020;131:e200-e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795-805. [DOI] [PubMed] [Google Scholar]
- 17. Hurth KP, Jaworski A, Thomas KB, Kirsch WB, Rudoni MA, Wohlfarth KM. The reemergence of ketamine for treatment in critically ill adults. Crit Care Med. 2020;48:899-911. [DOI] [PubMed] [Google Scholar]
- 18. Martyn JAJ, Mao J, Bittner EA. Opioid tolerance in critical illness. N Engl J Med. 2019;380:365-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phipps MM, Barraza LH, LaSota ED, et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large US cohort. Hepatology. 2020;72:807-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. 2012;185:486-497. [DOI] [PubMed] [Google Scholar]
- 22. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanavia E, Mencía S, Lafever SN, Solana MJ, Garcia M, López-Herce J. Sedative and analgesic drug rotation protocol in critically ill children with prolonged sedation: evaluation of implementation and efficacy to reduce withdrawal syndrome. Pediatr Crit Care Med. 2019;20:1111-1117. [DOI] [PubMed] [Google Scholar]
- 26. Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Payen JF, Chanques G, Futier E, Velly L, Jaber S, Constantin JM. Sedation for critically ill patients with COVID-19: which specificities? one size does not fit all. Anaesth Crit Care Pain Med. 2020;39:341-343. [DOI] [PMC free article] [PubMed] [Google Scholar]