Abstract
Addressing coronavirus disease 2019 (COVID-19) vaccine hesitancy and minimizing potential vaccine contraindications are critical to combatting the pandemic. We describe a practical approach to immediate adverse events after the first dose of messenger RNA vaccines for severe acute respiratory syndrome coronavirus 2, focusing on diagnosis and management of allergic reactions.
Keywords: vaccine allergy, graded challenge, polyethylene glycol
True allergic reactions to messenger RNA (mRNA) vaccines for severe acute respiratory syndrome coronavirus 2 are rare, with an initial estimate of 11 events per million doses [1], but these events and the associated media coverage can beget vaccine. The Centers for Disease Control and Prevention guidelines recommend against second-dose administration of either the Pfizer-BioNTech or Moderna vaccine in patients with immediate allergic reactions to the first dose, but they do not distinguish between levels of reaction severity [2]. However, the majority of suspected allergic reactions after vaccine administration are not immunologically mediated; for example, there are several mimics of anaphylaxis including vasovagal syncope and vocal cord dysfunction. Patients with these symptoms may unnecessarily avoid the second dose, raising concern for incomplete immunization and decreased vaccine efficacy.
While it is speculated that polyethylene glycol (PEG) is the culprit allergen in the Pfizer and Moderna vaccines, PEG skin testing for first-dose reactors has low positive and negative predictive values and therefore has poor reliability for guiding clinical decisions [3]. Guidelines from recent years suggest that skin testing should be restricted to settings with high probability of true anaphylaxis [4], and lower-risk patients should be revaccinated with full or split dosing [5]. We describe our institutional experience with immediate reactions to the coronavirus disease 2019 (COVID-19) vaccine, and our protocol for graded- or full-dose vaccine readministration for immediate reactors at low risk for true anaphylaxis.
METHODS
We retrospectively reviewed immediate-onset reactions (≤6 hours) to the first dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccine in healthcare workers at a single institution over a 2-month period (from 17 December 2020 to 16 February 2021). Study subjects were identified though review of occupational health records that documented both management of reactions identified at the vaccine clinic, and reactions later reported by employees via telephone. Any healthcare worker who reported symptoms concerning for allergy within 6 hours of vaccine administration was included. We excluded delayed reactions with onset later than 6 hours, owing to the limited literature and unknown mechanisms of most delayed vaccine hypersensitivity, and reactions consistent with known vaccine effects (eg, site pain, fatigue, myalgias, and fever). We also excluded persons who had experienced isolated local reactions without systemic symptoms, based on prior reports that these are not contraindications to the second dose [6].
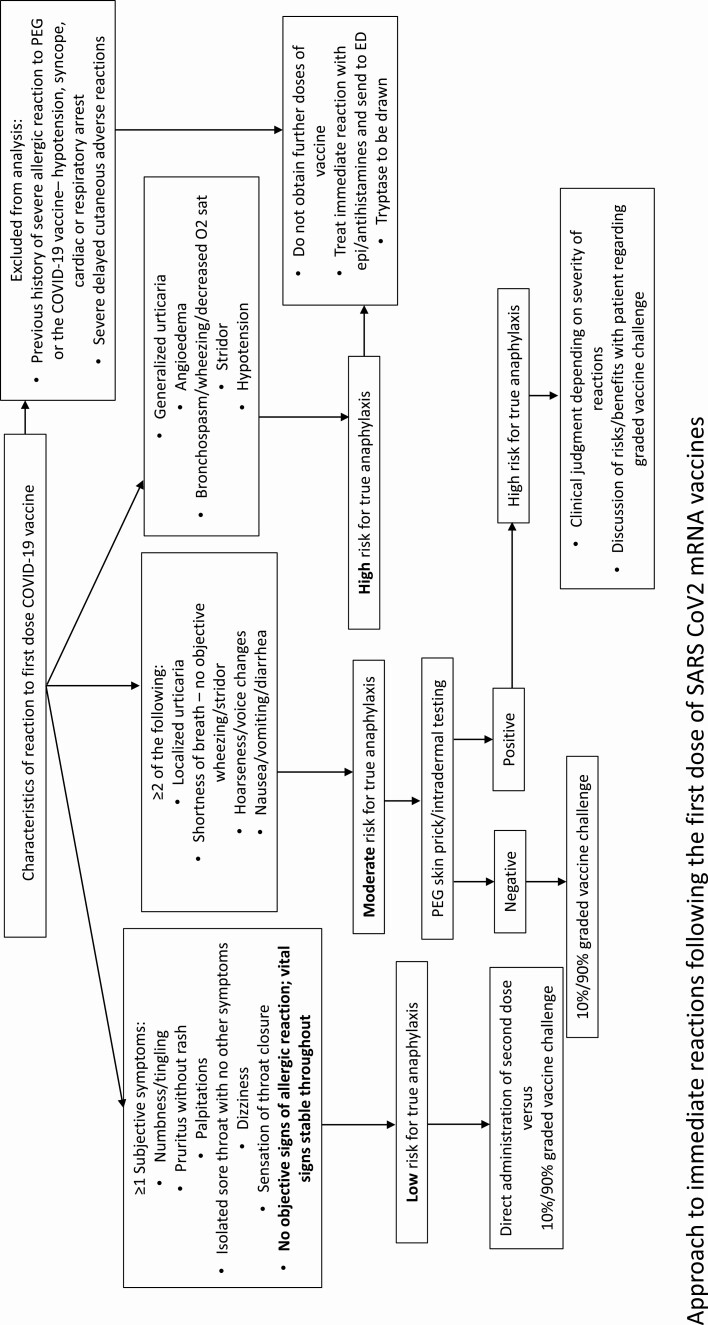
The probability of immunological anaphylaxis was estimated using the Brighton criteria case definition, which defines the level of anaphylaxis based on the likelihood of true reactivity [7]. Brighton level 1 represents the highest level of certainty, and level 3 the lowest. Reactors were classified as high (Brighton level 1), intermediate (Brighton level 2), or low (Brighton level 3) likelihood for true anaphylaxis. After this stratification, the decision was made to proceed with skin testing, followed by graded-dose vaccine challenge if results were negative in the highest-risk cases, graded-dose vaccine challenge without skin testing in intermediate-risk cases, and direct vaccine challenge in low-risk cases (Figure 1). Owing to the low yield of PEG skin testing in patients deemed to have probable anaphylaxis, we transitioned to performing graded-dose challenge alone for Brighton levels 1 and 2. This was at the discretion of a trained allergist in conjunction with an infectious disease specialist, and contingent on the reaction history and the patient’s comfort with proceeding.
Figure 1.
Approach to immediate reactions after the first dose of messenger RNA vaccine for severe acute respiratory syndrome coronavirus 2. Abbreviations: COVID-19, coronavirus disease 2019; ED, emergency department; O2, oxygen; PEG, polyethylene glycol.
Skin testing was performed in a dedicated allergy clinic. Patients underwent skin prick tests with stock solutions of PEG-2000 and methylprednisolone acetate (20 mg/mL), which contains PEG-3350, followed by intradermal testing with a 1:10 solution of methylprednisolone if prick test results were negative. Patients with negative skin test results, as well as those cleared for graded- or full-dose vaccine challenge, went on to receive the second dose. Vaccine challenges were undertaken with either a single full dose or a 2-step graded-dose challenge protocol (10% vaccine dose followed by the remaining 90%). The 10% challenge dose solution was mixed by pharmacists using a full vaccine dose. Patients undergoing challenge were monitored for 30 minutes after the 10% challenge and for 60 minutes after the 90% dose in a monitored environment with on-site emergency facilities.
RESULTS
Of 20 657 first doses of Pfizer and Moderna vaccines administered to healthcare workers before 16 February 2021, a total of 138 reactions were reported (0.66%). Twenty patients were excluded owing to isolated local symptoms, mostly numbness at the injection site, or complaints that were unrelated to allergy, and 30 were excluded owing to delayed reactions, >6 hours after dose administration. The remaining 88 patients who reported symptoms (73%) were classified as having immediate reactions, and these patients had a marked female predominance (92%).
Immediate reactions were classified as Brighton level 1 in none of the reports, as level 2 in 7, and as level 3 in 81. Therefore, only 8% of episodes were categorized as likely anaphylactic (Brighton level 2). Thirty-nine of 86 patients (45%) had underlying medication/vaccine allergies; this information was not available for 2 patients.
The most frequently reported primary symptoms among reactors classified as Brighton level 3 were numbness/tingling (46 of 88), sensation of throat closure (24 of 88), lightheadedness (23 of 88), flushing (19 of 88), pruritus (18 of 88), palpitations (16 of 88), subjective swelling of the eyes, lips, or mouth (15 of 88), and other respiratory symptoms (3 of 88). Among the 7 reactors categorized as Brighton level 2, an objective rash or hives was documented in addition to lip swelling (1 of 7) and throat tightness (5 of 7). Fifty-seven (64.7%) of nonanaphylactic first-dose reactions resolved without any intervention, and the remaining patients received antihistamines (29.5%), corticosteroids (4.5%), or short-acting β-agonists (1.1%). Epinephrine was administered only as emergency treatment in 2 cases, despite its status as first-line treatment of anaphylaxis. This may reflect the perceived mild nature of most reactions, for which management with steroids and antihistamines was considered sufficient. Of note, acute tryptase levels were not obtained on site owing to logistical difficulties with procuring blood samples. Fourteen of 88 patients were evaluated in the emergency department, but tryptase was not sent owing to the time elapsed between symptom onset and wait times in the emergency department, which in turn was due to a corresponding COVID-19 case surge.
Of 88 patients, 73 (82.9%) tolerated the second dose of the vaccine, 57 of 88 (64.7%) through full-dose vaccine challenge without precaution and 16 of 88 (18.2%) through graded-dose challenge. Among Brighton level 2 reactors, 4 of 7 underwent graded-dose challenge uneventfully, 2 declined, and 1 was lost to follow-up. Five patients with full-dose administration had recurrent immediate reactions that were either self-limited or resolved with antihistamines or bronchodilator use. Of the reactors who underwent allergist-supervised graded vaccine challenge, skin tests were performed in 4 patients (4.5%), with no positive reactions to any reagents. All (100%) who underwent graded challenges tolerated both the test and the 90% dose, with only few reports of subjective itching. All participants were hemodynamically stable, and none required emergency treatment. Of 88 reactors, 9 (10.2%) did not receive dose 2, and dose information was unavailable for 6 patients (6.8%).
DISCUSSION
We present a narrative review of adverse events with COVID-19 mRNA vaccines at a single institution, our investigational protocol, and outcomes after rechallenge with the second dose. In our cohort of 88 immediate reactors to the first dose, revaccination was administered to approximately 83% of them. This rate is consistent with previous studies of graded drug challenges reporting overall reaction rates between 4% and 12%, but significantly lower true reaction rates [8]. The low reaction rates for single-dose and test-dose protocols indicate the comparable safety of both procedures.
Most immediate reactions after COVID-19 immunization were not suggestive of true immunological anaphylaxis, akin to findings in other studies of vaccine reactions. Despite the concern for an underlying allergic mechanism, only 7 episodes (7.9%) were diagnosed as high risk (Brighton 2) after review of symptom presentation and reaction course. Most of these patients presented with largely subjective symptoms, that can be explained at least partly through non–immune-mediated mechanisms, such as a vasovagal reaction or vocal cord dysfunction. However, the fear of anaphylaxis on revaccination often prompts presumptive allergy diagnosis and precautionary discontinuation of further vaccine doses pending evaluation by an allergist.
There is also increasing evidence that direct or split-dose vaccine challenge may be a sufficiently cautious approach to reimmunization, even without antecedent skin testing. In a recent pediatric study of potential immunoglobulin E–mediated vaccine reactions, 71 of 73 direct challenges to the vaccine had negative reactions [9]. Allergist referral for skin testing should be restricted to those patients whose reactions are compatible with true anaphylaxis, and full- or split-dose revaccination protocols considered for low-risk patients, as performed without incident in a case series of immediate reactors to the Moderna vaccine [10].
Our study was limited by its retrospective nature and protocol adaptations that resulted in some nonuniformity of the diagnostic process. Another limitation was the high percentage of patients in whom second-dose tolerance could not be confirmed owing to patient refusal. We therefore may have underestimated the number of patients confirmed as tolerant. Additional challenges to more widespread implementation of our protocol would include the necessity of trained allergists for assessment of risk, the desire to perform challenges in an environment more monitored than a typical vaccine clinic, and the need for some minimal vaccine waste to perform graded-dose challenges.
Our experience highlights the importance of evaluating COVID-19 vaccine adverse events to ensure that patients without a history of true anaphylaxis can proceed to the second vaccine dose. A history of mild immediate symptoms after the first vaccine dose should not preclude second-dose administration, in order to maintain this vital public health intervention.
Note
Potential conflicts of interest. F. E. H. L. reports grants from the National Institutes of Health (grants P01AI125180, U01AI045969, and R01AI121252), the Bill & Melinda Gates Foundation, and Genentech; and service on the advisory board for AstraZeneca and the scientific advisory board for Be Biopharma. She was also a founder of MicroBplex and has stock/stock options in Be Biopharma, outside the submitted work. J. S. is president-elect of the Allergy, Asthma & Immunology Society of Georgia. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. CDC.gov. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:125–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html#Contraindications. Accessed 15 March 2021.
- 3. Pitlick MM, Sitek AN, Kinate SA, Joshi AY, Park MA. Polyethylene glycol and polysorbate skin testing in the evaluation of coronavirus disease 2019 vaccine reactions. Ann Allergy Asthma Immunol 2021; 126:P735–8. Available at: https://www.annallergy.org/issue/S1081-1206(21)X0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zafack JG, De Serres G, Rouleau I, et al. . Clinical approach used in medical consultations for allergic-like events following immunization: case series report in relation to practice guidelines. J Allergy Clin Immunol Pract 2017; 5:718–27.e1. [DOI] [PubMed] [Google Scholar]
- 5. Kelso JM, Greenhawt MJ, Li JT, et al. . Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol 2012; 130:25–43. [DOI] [PubMed] [Google Scholar]
- 6. Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med 2021; 384:1273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joshi D, Alsentzer E, Edwards K, Norton A, Williams SE. An algorithm developed using the Brighton collaboration case definitions is more efficient for determining diagnostic certainty. Vaccine 2014; 32:3469–72. [DOI] [PubMed] [Google Scholar]
- 8. Iammatteo M, Ferastraoaru D, Koransky R, et al. . Identifying allergic drug reactions through placebo-controlled graded challenges. J Allergy Clin Immunol Pract 2017; 5:711–17 e2. [DOI] [PubMed] [Google Scholar]
- 9. Cheung A, Choo S, Perrett KP. Vaccine allergy? skin testing and challenge at a tertiary pediatric hospital in Melbourne, Australia. J Allergy Clin Immunol Pract 2019; 7:1541–49. [DOI] [PubMed] [Google Scholar]
- 10. Mustafa SS, Ramsey A, Staicu ML. Administration of a second dose of the Moderna COVID-19 vaccine after an immediate hypersensitivity reaction with the first dose: two case reports. Ann Intern Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]