Abstract
Study Objectives
During the coronavirus disease 2019 (COVID-19) lockdown, there was a worldwide increase in electronic devices’ daily usage. Prolonged exposure to backlit screens before sleep influences the circadian system leading to negative consequences on sleep health. We investigated the relationship between changes in evening screen exposure and the time course of sleep disturbances during the home confinement period due to COVID-19.
Methods
2,123 Italians (mean age ± standard deviation, 33.1 ± 11.6) were tested longitudinally during the third and the seventh week of lockdown. The web-based survey evaluated sleep quality and insomnia symptoms through the Pittsburgh Sleep Quality Index and the Insomnia Severity Index. The second assessment survey inquired about intervening changes in backlit screen exposure in the two hours before falling asleep.
Results
Participants who increased electronic device usage showed decreased sleep quality, exacerbated insomnia symptoms, reduced sleep duration, prolonged sleep onset latency, and delayed bedtime and rising time. In this subgroup, the prevalence of poor sleepers and individuals reporting moderate/severe insomnia symptoms increased. Conversely, respondents reporting decreased screen exposure exhibited improved sleep quality and insomnia symptoms. In this subgroup, the prevalence of poor sleepers and moderate/severe insomniacs decreased. Respondents preserving screen time habits did not show variations of the sleep parameters.
Conclusions
Our investigation demonstrated a strong relationship between modifications of evening electronic device usage and time course of sleep disturbances during the lockdown period. Monitoring the potential impact of excessive evening exposure to backlit screens on sleep health is recommendable during the current period of restraining measures due to COVID-19.
Keywords: COVID-19, lockdown, sleep health, insomnia, electronic devices, evening screen exposure
Statement of Significance.
The present investigation is the first to provide insights on the relationship between changes in evening electronic device usage and time course of sleep disturbances during the coronavirus disease 2019 (COVID-19) lockdown. We demonstrated a strong association between screen time modifications in the hours before falling asleep, development and exacerbation of sleep disturbances, and changes of sleep/wake patterns during home confinement due to the COVID-19 pandemic. To date, hundreds of millions of people are subjected to restraining measures worldwide. Our findings may have large scale implications, considering the inevitable increase in the use of digital devices during the current period of limited physical social interactions. Avoiding evening overexposure to electronic screens may help preserve sleep health during the pandemic emergency.
Introduction
The rapid worldwide spread of the coronavirus disease 2019 (COVID-19) pandemic marked the first months of 2020. During this unprecedented situation, governments across the globe implemented extraordinary measures to reduce the spread of contagion and the pressure on the healthcare systems. From 9 March to 4 May 2020 a total lockdown was imposed in Italy, involving large-scale closure of most work activities, social distancing, and home-based quarantine for the general population. Home confinement measures had a substantial negative impact on global mental health and psychological well-being [1, 2]. The considerable impairment of the daily routine had consistent repercussions on sleep health and circadian rhythms, as documented by several studies [3–6]. However, evidence on the time course of sleep disturbances during the extended period of restraining measures is scarce [7, 8].
The forced social isolation and the limitations of outdoor activities led to a worldwide increase in web-based social communication. In the countries hit hardest by the virus, the total messaging and the time spent on social network increased more than 50%, while the time in video calling increased tenfold [9]. In Italy, during the lockdown, the daily internet traffic volume almost doubled compared to the previous year [10]. Most people spent more time on smartphones and computers [11, 12], and, for example, in the UK adults spent 40% of their day facing a screen during confinement.[13] Electronic devices daily usage increased to compensate for the limited social interactions, fill up free time, and ward off boredom. Furthermore, working from home has become the norm for millions of workers worldwide, and 40% of those currently working in the European Union began to telework full-time due to the pandemic [14]. The implementation of these habits may have helped to cope with the challenging and stressful isolation period. Nevertheless, the increase of screen exposure in the hours before bedtime could have determined adverse consequences on sleep health. Epidemiological and cross-sectional studies indeed showed a strong relationship between the use of electronic devices after sundown and alterations of sleep patterns [15–22]. Firstly, the usage of electronic devices may displace sleep time [23]. Moreover, screen-based activities are related to digital engagement, and the activity type plays a role in the digital media effects on sleep [24]. The screen-mediated contents could be emotionally or psychologically arousing, making it more difficult for individuals to relax before bedtime and, thus, interfering with sleep [23–25]. In particular, portable mobile and media devices allow real-time interactions and hence continuous stimulation [26]. Finally, sleep rhythms are intimately linked with the ambient light, which represents a crucial regulator of the biological clock [27, 28]. The evening exposure to short-wavelength-enriched light emitted from most screens of modern electronic devices (computer, smartphone, tablet, television) can have alerting effects suppressing melatonin release. This assumption has been confirmed by investigations that experimentally manipulated the evening light exposure of tablet [29], eReader [30], and computer screens [31, 32], showing a concomitant decrease of objective and self-reported sleepiness, higher sleep onset latency, and altered sleep architecture.
Therefore, light per se and the stimulating content of electronic devices during the hours preceding habitual bedtime may interfere with sleep patterns intervening on biological and cognitive mechanisms simultaneously [33].
Difficulties in falling asleep (e.g. in insomnia), on the other hand, may lead to longer time spent engaging with screens in the evening hours, establishing a vicious circle.
Based on this evidence, the present study aimed to shed light on the relationship between the longitudinal changes of sleep disturbances between the third (March 25–28, 2020) and the seventh week (April 21–27, 2020) of home confinement in Italy and the retrospectively reported modifications of the exposure to electronic devices before falling asleep during the same lockdown period. We hypothesized that changes in electronic device usage could be a crucial moderator of the lockdown-related sleep alterations over time. We expected that individuals who increased screen exposure in the two hours before sleep onset should have shown the largest sleep impairments and the most marked alterations of the sleep/wake schedule. On the other hand, subjects who reduced evening screen time should have exhibited a positive time course of sleep disturbances.
Methods
Participants and procedure
The present investigation is part of a larger research project aimed to understand the consequences of COVID-19 lockdown on the Italian population [6, 7, 34]. A total of 7,107 Italian citizens were recruited in a web-based survey through a snowball sampling during the third week of lockdown (March 25–31, 2020). The survey comprised a demographic questionnaire (age, gender, education, occupation, and geographical location), the Pittsburgh Sleep Quality Index [35] (PSQI), the Insomnia Severity Index [36] (ISI), and the reduced version of the Morningness–Eveningness Questionnaire [37] (MEQr), in this order. The PSQI is a validated tool to evaluate sleep quality comprising nineteen questions, from which a total score (range 0–19) is calculated. A score >5 identifies poor sleepers. The ISI is a validated instrument used to assess the severity of insomnia symptoms. The total score ranging between 0 and 21, and a score >14 indicates the presence of moderate/severe clinical insomnia condition. The MEQr is a validated questionnaire, and its total score is used to identify the chronotype (4–10 score: evening-type; 11–18 score: intermediate-type; 19–25 score: morning-type). Subsequently, respondents could decide whether continue the compilation of other three questionnaires (Beck Depression Inventory-second edition, BDI-II [38]; 10-item Perceived Stress Scale, PSS-10 [39]; state-anxiety subscale of the State-Trait Anxiety Inventory, STAI-X1 [40]), with the option to stop after each of them. This feature aimed at ensuring higher reliability of the data collected, avoiding false answers in the last questionnaires. The BDI-II is a validated questionnaire used to assess clinical depression symptoms (range 0–63). The PSS-10 is a 10-item questionnaire evaluating the perceived stress following stressful events (range, 0–40). The STAI-X1 is a well-established 20-item scale measuring state anxiety (range, 1–80). In all these questionnaires, higher scores indicate more severe conditions. We included the assessment of depression, perceived stress, and anxiety because they are closely related to the sleep outcomes in the pre-pandemic [41], and pandemic period [5, 42]. In this view, it is important to isolate the effects of the screen exposure changes from the effects of these psychological dimensions to explain the time course of sleep variables.
After four weeks, the website link of the follow-up survey was provided to the participants via email address/telephone number. A total of 2,701 subjects completed the second assessment in a 7-day period (April 21–27, 2020). From this large follow-up sample, we included in the reported analyses only the 2,123 respondents (mean age ± standard deviation, 33.1 ± 11.6; range, 18–82; 401 men, see Table 1) who completed the first survey during the four days preceding the daylight-saving time (March 25–28, 2020; Survey wave 1). This allowed us to avoid interfering and confounding effects at the baseline measurement due to the summertime beginning (for a review, [43]). During the follow-up survey (Survey wave 2), participants completed the same questionnaires of Survey wave 1. Moreover, they were asked to retrospectively evaluate the changes (increase, maintenance, reduction) from the first assessment in the usage duration of electronic devices (smartphone, computer, tablet, television, eReader) in the 2 h before falling asleep.
Table 1.
Sociodemographic composition of the sample participating in both the first and the second measurement (Survey wave 1: March 25–28, 2020; Survey wave 2: April 21–27, 2020)
| Gender | |
|---|---|
| Male | 401 (18.9%) |
| Female | 1,722 (81.1%) |
| Age | |
| 18–30 years | 1,263 (59.5%) |
| 31–50 years | 598 (28.2%) |
| >50 years | 262 (12.3%) |
| Education | |
| Until middle school | 31 (1.5%) |
| High school | 686 (32.3%) |
| Graduated | 1,406 (66.2%) |
| Occupation | |
| Unemployed | 184 (8.7%) |
| Employed | 1,172 (55.2%) |
| Student | 767 (36.1%) |
| Geographical location | |
| Northern Italya | 767 (36.1%) |
| Central Italyb | 593 (27.9%) |
| Southern Italyc | 763 (35.9%) |
| Electronic device usage | |
| Increased | 751 (35.4%) |
| Unchanged | 1,221 (57.5%) |
| Reduced | 151 (7.1%) |
a Northern Italy: Aosta Valley, Emilia Romagna, Friuli-Venezia Giulia, Liguria, Lombardy, Piedmont, Trentino-Alto Adige, and Veneto.
b Central Italy: Lazio, Marche, Tuscany, and Umbria. c Southern Italy: Abruzzo, Apulia, Basilicata, Calabria, Campania, Molise, Sardinia, and Sicily.
At Survey wave 1, a total of 1,783 respondents completed the BDI-II, 1,697 filled in also the PSS-10, and 1,675 completed all the questionnaires, while at Survey wave 2 the number of respondents for the last three optional questionnaires (BDI-II, PSS-10, STAI-X1) was 1,873, 1,811, and 1,789, respectively. The study was approved by the institutional review board of the University of L’Aquila (protocol no. 43066) and carried out according to the principles established by the Declaration of Helsinki. Online informed consent to participate in the whole research was obtained from all the respondents during the first assessment.
Data analysis
In order to control for potential selection bias of the follow-up participants, we performed preliminary mixed model analyses comparing the Survey wave 1 questionnaire scores of respondents who participated only to the first assessment and those who attended both the measurements (Survey wave 1 and Survey wave 2). These control analyses did not highlight significant differences (all p > 0.10).
According to the purpose of the present study, the main variables were the PSQI and ISI scores. Additionally, from the PSQI questionnaire, we extracted other variables such as total sleep time (TST, min), sleep onset latency (SOL, min), bedtime (BT, hh:mm), and rise time (RT, hh:mm). To evaluate the time course of the sleep dimensions as a function of the reported changes of exposure to electronic devices, all the above variables were submitted to mixed model analyses with a random intercept per participant, accounting for the expected intraindividual variability. The models comprised “Survey wave” (Survey wave 1, Survey wave 2), “Screen exposure” (Increased, Unchanged, Reduced), and their interaction as predictors. Additionally, “Gender” (male, female) was included as a factor, and age as a covariate, to control for putative effects of these demographic variables on the main outcomes of the present study. Subsequently, explorative analyses were carried out, adding to the models the Survey wave 1 and Survey wave 2 scores of MEQr, BDI-II, PSS-10, and STAI-X1 as time-varying covariates. These further analyses aimed to control for the effects of chronotype, depression, stress, and anxiety on sleep measures. Mixed model analyses were performed using the “lme4” R package [44]. Models were fitted using REML, using the Satterthwaite approximation to compute p-values. Bonferroni post hoc tests were obtained using the “emmeans” R package [45]. Finally, the validated cut-off scores of PSQI and ISI were used to determine the prevalence of poor sleepers and moderate/severe insomnia condition. Subsequently, McNemar’s tests were performed to evaluate the modifications of the prevalence of sleep disturbances between Survey wave 1 and Survey wave 2 in the three groups characterized by different changes of exposure to electronic devices before falling asleep. For all the analyses, statistical significance was set at p < 0.05, and all tests were two-tailed.
Results
Relationships between screen exposure and sleep variables
The results of the mixed model analyses on the sleep variables [PSQI and ISI scores, total sleep time (TST), sleep onset latency (SOL), bedtime (BT), rise time (RT)] are reported in Table 2. The analyses did not highlight significant effects of the “Survey wave” factor for all the sleep variables (all p ≥ 0.24). “Screen exposure” was significant for all the variables (all p ≤ 0.005). The analyses yielded a significant effect of the interaction between “Survey wave” and “Screen exposure” predictors for all the variables (all p ≤ 0.001). The “Age” covariate was significant for PSQI, TST, SOL, BT, and RT (all p ≤ 0.03), and “Gender” was significant for PSQI, ISI, SOL, BT (all p ≤ 0.001).
Table 2.
Results (F and p) of the mixed model analyses on PSQI score (sleep quality), ISI score (insomnia severity symptoms), total sleep time, sleep onset latency, bedtime, and rise time. The models comprised Survey wave (Survey wave 1, Survey wave 2), Screen exposure (Increased, Unchanged, Reduced) as predictors, their interaction, and age and gender (male, female) as covariates
| PSQI score | ISI score | Total sleep time | Sleep onset latency | Bedtime | Rise time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors and covariates | F | p | F | p | F | p | F | p | F | p | F | p |
| Survey wave | 0.11 | 0.75 | 0.75 | 0.39 | 1.37 | 0.24 | 0.49 | 0.49 | 0.10 | 0.76 | 0.14 | 0.71 |
| Screen exposure | 29.57 | <0.001 | 32.51 | <0.001 | 6.74 | 0.001 | 6.14 | 0.002 | 7.14 | <0.001 | 5.26 | 0.005 |
| Survey wave* Screen exposure | 20.29 | <0.001 | 23.70 | <0.001 | 9.07 | <0.001 | 6.70 | 0.001 | 30.11 | <0.001 | 20.63 | <0.001 |
| Age | 46.64 | <0.001 | 0.95 | 0.33 | 247.53 | <0.001 | 4.60 | 0.03 | 184.86 | <0.001 | 397.50 | <0.001 |
| Gender | 16.62 | <0.001 | 11.64 | <0.001 | 0.89 | 0.35 | 19.14 | <0.001 | 13.01 | <0.001 | 0.02 | 0.89 |
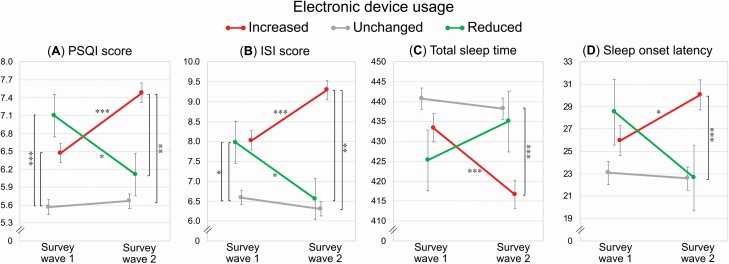
Post hoc comparisons between Survey wave 1 and Survey wave 2 (Figures 1 and 2) suggested that participants who reported an increase of electronic device usage before falling asleep also showed a significant increase over time of PSQI (mean change ± standard error, +1.01 ± 0.15; p < 0.001) and ISI scores (+1.26 ± 0.21; p < 0.001), a reduction of TST (–16.70 ± 3.22 min; p < 0.001), a prolongation of SOL (+4.08 ± 1.31 min; p = 0.03), and delayed BT (+23.08 ± 3.13 min; p < 0.001) and RT (+18.92 ± 2.83 min; p < 0.001). On the other hand, participants who reduced the screen exposure showed concurrent decreases of PSQI (–1.00 ± 0.33; p = 0.04) and ISI scores (–1.44 ± 0.42; p = 0.02), earlier BT (–23.25 ± 6.70 min; p = 0.009), and no changes in TST, SOL, and RT (p = 1.00, p = 0.58, p = 0.11; respectively). No differences in all the variables were obtained for the participant who maintained unchanged electronic device use habits (all p = 1.00). Although the mean PSQI and ISI changes between the two survey waves for the groups increasing or reducing the exposure to electronic devices could appear small, they proved to have a clinical significance, as emerged in the analyses on the prevalence of poor sleepers and clinical insomniacs (see next section).
Figure 1.
“Survey wave” × “Screen exposure” interaction for PSQI and ISI scores, total sleep time (min), and sleep onset latency (min).
Mean ± standard error of the PSQI and ISI scores (A, B), total sleep time (C), and sleep onset latency (D) at the two assessments (Survey wave 1, Survey wave 2) for respondents who declared an increase, preservation, or reduction of the electronic device usage duration before falling asleep. Bonferroni significant post hoc comparisons are reported with asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001).
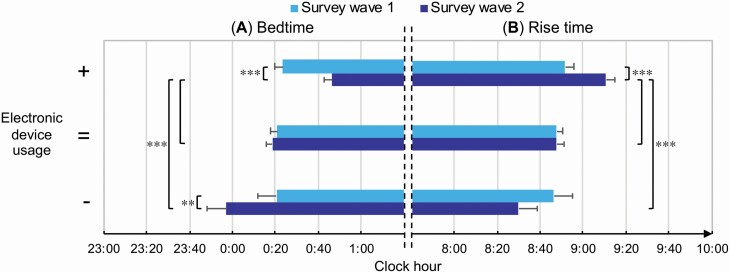
Figure 2.
“Survey wave” × “Screen exposure” interaction for bedtime and rise time (hh:mm).
Mean ± standard error of the bedtime (A) and rise time (B) at the two assessments (Survey wave 1, Survey wave 2) for participants who declared an increase (+), preservation (=), or reduction (–) of the electronic device usage duration before falling asleep. Bonferroni post hoc results are reported with asterisks (** p < 0.01, *** p < 0.001).
At Survey wave 1, there were no differences in PSQI and ISI scores between respondents who later reported an increase or reduction of screen exposure (both p = 1.00). Participants maintaining device use habits showed lower PSQI scores at Survey wave 1 than those who increased or reduced the exposure to backlit screens (both p < 0.001). ISI scores were lower at Survey wave 1 for subjects who did not change the screen exposure than participants who increased or reduced it (p < 0.001, p = 0.04; respectively). The three groups did not differ at Survey wave 1 on TST, SOL, BT, and RT (all p > 0.85).
Participants who reported an increase of screen exposure also showed higher PSQI and ISI scores at Survey wave 2, and delayed BT and RT compared to the other two groups (all p < 0.01), as well as shorter TST and longer SOL compared to the group that did not change the device usage habits (both p < 0.001, see Figure 2). No differences for all the variables were obtained at Survey wave 2 between subjects who reduced or maintained the device usage duration before falling asleep (all p > 0.32).
Further control analyses confirmed the above-reported pattern of results, controlling for the covariance of age, gender, chronotype, depression, perceived stress, and anxiety. In particular, the interaction between “Survey wave” and “Screen exposure” remained significant for all the variables (PSQI: F2,1605.95 = 17.50, p < 0.001; ISI: F2,1685.08 = 14.20, p < 0.001; TST: F2,1694.81 = 8.37, p < 0.001; SOL: F2,1711.04 = 4.53, p = 0.01; BT: F2,1664.94 = 17.11, p < 0.001; RT: F2,1645.32 = 12.70, p < 0.001), confirming the crucial role of the changes in screen exposure in explaining the time course of the sleep outcomes during the lockdown.
Relationships between screen exposure and sleep disturbance prevalence
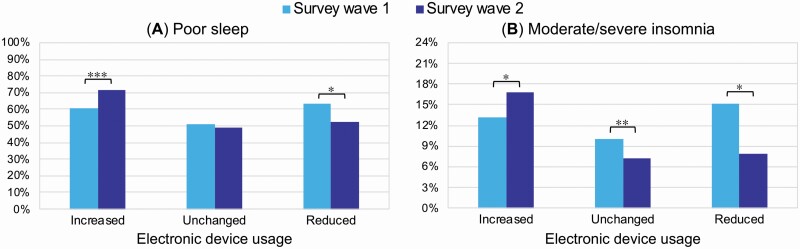
McNemar’s tests highlighted a significant prevalence increase of poor sleepers (+11.4%) and of moderate/severe insomnia condition (+3.6%) in the group of respondents reporting an increased usage of electronic devices before falling asleep (χ2 = 108.23, p < 0.001, Cohen’s g = 0.21; χ2 = 149.73, p = 0.01, Cohen’s g = 0.15; respectively) (Figure 3). On the other hand, there was a significant decrease of poor sleepers (–10.7%) and of moderate/severe insomnia condition (–7.3%) in the group reporting a reduction of the device usage (χ2 = 19.90, p = 0.04, Cohen’s g = 0.18; χ2 = 12.21, p = 0.04, Cohen’s g = 0.24; respectively). Finally, in the group of participants who maintained screen habits unchanged there was a reduction of clinical insomnia prevalence (–2.8%; χ2 = 188.51, p = 0.002, Cohen’s g = 0.13), but not of poor sleepers’ prevalence (–2.3%; χ2 = 200.25, p = 0.17, Cohen’s g = 0.04). According to the standard interpretation of Cohen’s g [46], all the variations in the groups of respondents who increased or reduced the screen exposure was of medium extent, while the effect size of the insomnia condition reduction among those who maintained unchanged the use of electronic devices was small.
Figure 3.
Prevalence of poor sleepers (A) and moderate/severe clinical insomnia condition (B) at the two assessments (Survey wave 1, Survey wave 2) for the respondents who increased, maintained unchanged, or reduced the usage of electronic devices before falling asleep. Significant results of the McNemar’s tests are reported with asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001).
Discussion
In the present study, we showed a strong relationship between changes in evening screen exposure and time course of sleep parameters during the COVID-19 lockdown.
In line with the initial assumption, individuals declaring increased electronic device usage before falling asleep showed a general sleep impairment over time (from the third to the seventh week of home confinement). This outcome is exemplified by decreased sleep quality, exacerbation of insomnia symptoms, reduced sleep duration, and longer sleep onset latency. Consistently, we found an increased prevalence of poor sleepers and moderate/severe insomnia condition only within this group of respondents. Increased screen exposure was also linked to delayed bedtime and rising time, outlining the delayed sleep phase across the home confinement period. Furthermore, individuals who increased the device usage showed the poorest sleep quality, the most severe insomnia symptoms, the lowest sleep duration, the highest sleep onset latency, and they went to bed and woke up later compared to the other participants during the seventh week of lockdown.
In addition, participants reporting decreased evening screen exposure showed the opposite time course of sleep disturbances. They indeed exhibited improved sleep quality and mitigation of insomnia symptoms, which turned into a prevalence reduction of poor sleepers and clinical insomnia condition. This group of respondents went to bed earlier after four weeks of home confinement. Finally, the respondents who maintained unchanged electronic device usage habits did not show any modification in all the examined dimensions, except for a prevalence reduction of moderate/severe insomnia conditions.
Remarkably, we obtained the present findings controlling for the effects of gender and age, and they were confirmed also controlling for the covariance of chronotype, depression, stress, and anxiety. Therefore, our results indicate a direct relationship between evening device usage and time course of sleep disturbances during the home confinement period, independent of other psychological and circadian dimensions.
The pattern of results of our longitudinal investigation is consistent with a large pre-outbreak cross-sectional literature addressing the relationship between sleep and evening electronic device usage. In particular, higher screen time has been associated with reduced sleep duration [15, 47–49], prolongation of sleep onset latency [16–19, 49], later sleep onset and waking up [15, 16, 48], poor sleep quality [15–19], and insomnia symptoms [16, 21, 47].
The COVID-19 pandemic has affected all the world, and home confinement constitutes the most widely used measure to contrast the spread of the contagion. In modern societies, the increase of screen-based device usage could represent an unavoidable consequence of the pandemic-related home confinement periods. Indeed, more than one-third of our sample reported an increase in electronic device usage in the two hours before falling asleep. On the other hand, only a small percentage of the sample (7.1%) reduced the evening screen time between the third and the seventh week of lockdown. This evidence suggests that the reduction of screen time and the associated sleep improvement during a prolonged confinement period were rare, while the opposite situation was quite common. Consequently, our findings have substantial large-scale implications when contextualized to the current unprecedented situation.
Adequate sleep quantity/quality is essential to deal with stressful events [50] and preserve mental health [51, 52], and it plays a crucial role in emotional processing [53, 54] and mood regulation [55]. The increased screen time and its consequences on sleep health may negatively affect psychological well-being increasing anxiety, depression and stress symptoms during the current pandemic period. Indeed, aberrant light exposure and excessive screen time were associated with sleep and mental health problems [56, 57]. Consistently, blocking screen-emitted blue light has proved to be effective in promoting both sleep quality and mood [58, 59] and it was proposed as a useful approach to treat both clinical insomnia [59, 60] and mood disorders [56, 61], although the current literature presents inconsistencies [62].
Finally, sleep and the circadian system support the proper functioning of the immune system [63, 64]. Short sleep duration and poor sleep continuity are associated with increased vulnerability to infectious illness, including higher susceptibility to the common cold and greater symptom reporting [65–67]. The largest vaccination campaign in human history is around the corner, and studies have clearly shown that sleep is an important factor in determining the effectiveness of vaccinations, for example, against influenza viruses [68, 69]. In light of these considerations, the relationship between screen time and sleep outcomes has a broad spectrum of implications, configuring a major public health concern during the COVID-19 outbreak.
The present results were obtained in an Italian sample, but they could be generalized to other modern societies since the putative underlying mechanisms involve a disruption of circadian physiology due to evening light exposure [29–32], increased arousal caused by the stimulating content of the screen-mediated material before bedtime [23–25], and a direct displacement of sleep time [23]. However, we can not infer the causality of this relationship since this is an observational study, and the measurement of screen exposure changes has been retrospectively reported during the second assessment. Notwithstanding that comprehensive literature supported the detrimental effect of electronic devices’ evening usage on sleep patterns, we can not exclude reverse causation. Nevertheless, the two interpretations are not mutually exclusive, and a bidirectional model of causation has been suggested [70]. We propose that a vicious circle during the confinement period was established, in which the increased screen exposure before falling asleep negatively impacted the sleep parameters, which in turn supported the overuse of electronic devices after the sunset. Notably, participants who did not change the screen exposure during the examined four weeks of lockdown exhibited the lowest PSQI and ISI scores at the first assessment (Survey wave 1). This outcome could be interpretable as a tendency to maintain unchanged screen habits among individuals with fewer sleep disturbances.
In conclusion, our findings corroborate the assumption that the governments should pursue policies aimed at raising public awareness on healthy sleep behaviors during confinement due to the COVID-19 pandemic, discouraging the excessive use of electronic devices before falling asleep [71, 72]. The evening use of blue-light blocking glasses and the application of a blue wavelength light filter (night shift settings) on the electronic screens should be encouraged to mitigate the well-known detrimental consequences of bright light exposure. In addition, the implementation of psychophysiologically and emotionally arousing screen-based activities such as computer work and surfing the Internet [24], playing videogames [25], and overuse of media to obtain information about COVID-19 [73] should be discouraged before the sleep onset.
To date, the feared risk of a second wave of contagion has become a concrete reality, and hundreds of thousands of people are subjected to home confinement measures worldwide. In light of our results, the above-mentioned interventions focused on sleep hygiene are fundamental to counteract the occurrence and exacerbation of sleep disturbances and foster the general well-being during the period of social distancing and restraining measures due to the COVID-19 pandemic.
Limitations
To the best of our knowledge, the present investigation is the first to provide insights into the relationship between electronic device usage and the time course of sleep disturbances during the COVID-19 lockdown. However, it should be acknowledged that we used a non-probabilistic sampling technique, and the sample comprised a higher prevalence of women and young people. Moreover, under-eighteen years-old individuals were not included. However, the relationship between evening screen time and sleep disturbances was widely shown in adolescents [23, 26, 74]. We hypothesize that our results could be generalizable to younger people. Further research focused on the younger population is necessary as children and adolescents are spending increasingly more time on electronic devices during the pandemic emergency [75]. Additionally, the electronic device category of our survey included a broad set of devices, and we can not discern the relationship between the usage of each device (i.e. smartphone, computer, tablet, television, eReader) and the time course of the sleep outcomes. Finally, in our survey, we did not assess the extent of the screen exposure changes, the use of bright/dim screens, the room lighting, and the implementation of blue-light blocking glasses or blue light filter technology, thus we can not estimate their contribution to the present findings. Further research should be performed accounting for these limitations to disentangle the causal relationship between sleep patterns and the increased digital device usage before sleep onset during the current pandemic period. Future longitudinal investigations should include a detailed day-by-day quantification of screen time for each device (e.g., using daily diaries and/or specific applications), an objective estimation of sleep patterns (e.g., through actigraphy), and an evaluation of the screen-mediated contents as well as the use of blue-light blocking approaches.
Acknowledgments
We are grateful to Professor Marco Lauriola for his valuable support in the statistical analysis and to Jasmin Cascioli for her help in data collection. We thank all the Italians who participated in our study and the three anonymous reviewers, whose insightful comments helped improve and significantly clarify this manuscript.
Conceptualization, F.S. and M.F.; Methodology, F.S. and M.F.; Investigation, F.S., G.A., D.C., L.V.; Data curation, F.S.; Formal analysis, F.S.; Writing – original draft, F.S.; Writing – review & editing, F.S., A.D.A., D.T. and M.F.; Supervision, M.F.
Disclosure Statement
Financial Disclosure: none.
Non-financial Disclosure: none.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Rajkumar RP. COVID-19 and mental health: a review of the existing literature. Asian J Psychiatr. 2020;52. doi: 10.1016/j.ajp.2020.102066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vindegaard N, et al. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blume C, et al. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020;30(14):R795–R797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wright KP Jr, et al. Sleep in university students prior to and during COVID-19 Stay-at-Home orders. Curr Biol. 2020;30(14):R797–R798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cellini N, et al. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J Sleep Res. 2020;29(4):e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salfi F, et al. The impact of home confinement due to COVID-19 pandemic on sleep quality and insomnia symptoms among the Italian population. J Sleep Res. 2020;29(S1):73–74. [Google Scholar]
- 7. Salfi F, et al. Gender-related time course of sleep disturbances and psychological symptoms during the COVID-19 lockdown: a longitudinal study on the Italian population. Neurobiol Stress. 2020;13:100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang C, et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. 2020;87:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FACEBOOK. Keeping Our Services Stable and Reliable During the COVID-19 Outbreak. https://about.fb.com/news/2020/03/keeping-our-apps-stable-during-covid-19/. Accessed September 1, 2020.
- 10. AGCOM. Osservatorio sulle telecomunicazioni, Monitoraggio COVID-19 n.2/2020. https://www.agcom.it/documents/10179/20594109/Documento+generico+02-11-2020/a11f575f-b057-4a39-bb5d-35de8006faaf?version=1.0. Accessed December 18, 2020.
- 11. DATAREPORTAL. Digital 2020: April Global Statshot. https://datareportal.com/reports/digital-2020-april-global-statshot. Accessed September 1, 2020.
- 12. The Washington Post. Our iPhone Weekly Screen Time Reports are Through the Roof, and People are “Horrified.”https://www.washingtonpost.com/technology/2020/03/24/screen-time-iphone-coronavirus-quarantine-covid/. Accessed September 1, 2020.
- 13. Ofcom. Lockdown Leads to Surge in TV Screen Time and Streaming. https://www.ofcom.org.uk/about-ofcom/latest/features-and-news/lockdown-leads-to-surge-in-tv-screen-time-and-streaming. Accessed: September 1st, 2020.
- 14. Eurofound. Living, Working and COVID-19, COVID-19 Series, Publications Office of the European Union, Luxembourg. Accessed December 18, 2020. [Google Scholar]
- 15. Lastella M, et al. Electronic device use in bed reduces sleep duration and quality in adults. Sleep Biol Rhythms. 2020;18:121–129. [Google Scholar]
- 16. Exelmans L, et al. Bedtime mobile phone use and sleep in adults. Soc Sci Med. 2016;148:93–101. [DOI] [PubMed] [Google Scholar]
- 17. Christensen MA, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS One. 2016;11(11):e0165331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rafique N, et al.. Effects of mobile use on subjective sleep quality. Nat Sci Sleep. 2020;12:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Šmotek M, et al. Evening and night exposure to screens of media devices and its association with subjectively perceived sleep: should “light hygiene” be given more attention? Sleep Health. 2020;6(4):498–505. [DOI] [PubMed] [Google Scholar]
- 20. Gradisar M, et al.. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9(12):1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fossum IN, et al. The association between use of electronic media in bed before going to sleep and insomnia symptoms, daytime sleepiness, morningness, and chronotype. Behav Sleep Med. 2014;12(5):343–357. [DOI] [PubMed] [Google Scholar]
- 22. Johnson JG, et al. Association between television viewing and sleep problems during adolescence and early adulthood. Arch Pediatr Adolesc Med. 2004;158(6):562–568. [DOI] [PubMed] [Google Scholar]
- 23. Cain N, et al. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11(8):735–742. [DOI] [PubMed] [Google Scholar]
- 24. Orzech KM, et al. Digital media use in the 2 h before bedtime is associated with sleep variables in university students. Comput Human Behav. 2016;55(A):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higuchi S, et al. Effects of playing a computer game using a bright display on presleep physiological variables, sleep latency, slow wave sleep and REM sleep. J Sleep Res. 2005;14(3):267–273. [DOI] [PubMed] [Google Scholar]
- 26. Carter B, et al. Association between portable screen-based media device access or use and sleep outcomes: a systematic review and meta-analysis. JAMA Pediatr. 2016;170(12):1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown GM. Light, melatonin and the sleep-wake cycle. J Psychiatry Neurosci. 1994;19(5):345–353. [PMC free article] [PubMed] [Google Scholar]
- 28. Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11(6):453–464. [DOI] [PubMed] [Google Scholar]
- 29. Wood B, et al. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl Ergon. 2013;44(2):237–240. [DOI] [PubMed] [Google Scholar]
- 30. Chang AM, et al. Reply to Zeitzer: Good science, in or out of the laboratory, should prevail. Proc Natl Acad Sci USA. 2015;112(13):E1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cajochen C, et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol (1985). 2011;110(5):1432–1438. [DOI] [PubMed] [Google Scholar]
- 32. Green A, et al. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol Int. 2017;34(7):855–865. [DOI] [PubMed] [Google Scholar]
- 33. Woods HC, et al.. Merging the biological and cognitive processes of sleep and screens. Curr Sleep Medicine Rep. 2019;5:150–155. [Google Scholar]
- 34. Salfi F, et al.. Sleeping under the waves: a longitudinal study across the contagion peaks of the COVID-19 pandemic in Italy. J Sleep Res. 2021;00:e13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Curcio G, et al. Validity of the Italian version of the Pittsburgh Sleep Quality Index (PSQI). Neurol Sci. 2013;34(4):511–519. [DOI] [PubMed] [Google Scholar]
- 36. Castronovo V, et al. Validation study of the Italian version of the Insomnia Severity Index (ISI). Neurol Sci. 2016;37(9):1517–1524. [DOI] [PubMed] [Google Scholar]
- 37. Natale V, et al. Validity of the reduced version of the Morningness-Eveningness Questionnaire. Sleep Biol Rhythms. 2006;4:72–74. [Google Scholar]
- 38. Ghisi M, et al.. Beck Depression Inventory-II: Edizione Italiana. Firenze, IT: Giunti Editore; 2006: 1v79. [Google Scholar]
- 39. Mondo M, et al.. Psychometric evaluation of three versions of the Italian Perceived Stress Scale. Curr Psychol. 2021;40:1884–1892. doi: 10.1007/s12144-019-0132-8 [DOI] [Google Scholar]
- 40. Spielberger CD, Gorsuch RL, Lushene RE.. The State-Trait Anxiety Inventory (STAI) Test Manual for Form X. Palo Alto: Consulting Psychologist Press; 1970. tr. it.: Lazzari R, Pancheri P. S.T.A.I. Questionario di autovalutazione dell’ansia di stato e di tratto. Firenze, IT: Organizzazioni Speciali; 1980. [Google Scholar]
- 41. Alvaro PK, et al. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang W, et al.. Sleep disturbance and psychological profiles of medical staff and non-medical staff during the early outbreak of COVID-19 in Hubei Province, China. Front Psychiatry. 2020;11:733. doi: 10.3389/fpsyt.2020.00733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harrison Y. The impact of daylight saving time on sleep and related behaviours. Sleep Med Rev. 2013;17(4):285–292. [DOI] [PubMed] [Google Scholar]
- 44. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 45. Lenth R, et al. Package “emmeans”: Estimated Marginal Means, aka Least-Squares Means. R package version 1.3.0. 2020. https://cran.r-project.org/web/packages/emmeans/emmeans.pdf.
- 46. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 47. Bhat S, et al. “To sleep, perchance to tweet”: in-bed electronic social media use and its associations with insomnia, daytime sleepiness, mood, and sleep duration in adults. Sleep Health. 2018;4(2):166–173. [DOI] [PubMed] [Google Scholar]
- 48. Gamble AL, et al. Adolescent sleep patterns and night-time technology use: results of the Australian Broadcasting Corporation’s Big Sleep Survey. PLoS One. 2014;9(11):e111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hysing M, et al.. Sleep and use of electronic devices in adolescence: results from a large population-based study. BMJ Open. 2015. doi: 10.1136/bmjopen-2014-006748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leggett A, et al. The impact of sleep disturbance on the association between stressful life events and depressive symptoms. J Gerontol B Psychol Sci Soc Sci. 2016;71(1):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pigeon WR, et al. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):44. [DOI] [PubMed] [Google Scholar]
- 52. Freeman D, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. 2017;4(10):749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tempesta D, et al.. Sleep and emotional processing. Sleep Med Rev. 2018;40:183–195. [DOI] [PubMed] [Google Scholar]
- 54. Tempesta D, et al. The impact of five nights of sleep restriction on emotional reactivity. J Sleep Res. 2020;29(5):e13022. [DOI] [PubMed] [Google Scholar]
- 55. Fairholme CP, et al. Sleep, emotions, and emotion regulation: an overview. sleep affect assess theory clin implic. In: Kimberly A, ed. Sleep and Affect: Assessment, Theory, and Clinical Implications. San Diego, CA: Elsevier Academic Press; 2015: 45v61. doi: 10.1016/B978-0-12-417188-6.00003-7 [DOI] [Google Scholar]
- 56. Bedrosian TA, et al. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 2017;31(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu X, et al. Low physical activity and high screen time can increase the risks of mental health problems and poor sleep quality among Chinese college students. PLoS One. 2015;18(3):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burkhart K, et al. Amber lenses to block blue light and improve sleep: a randomized trial. Chronobiol Int. 2009;26(8):1602–1612. [DOI] [PubMed] [Google Scholar]
- 59. Janků K, et al. Block the light and sleep well: Evening blue light filtration as a part of cognitive behavioral therapy for insomnia. Chronobiol Int. 2020;37(2):248–259. [DOI] [PubMed] [Google Scholar]
- 60. Shechter A, et al. Blocking nocturnal blue light for insomnia: a randomized controlled trial. J Psychiatr Res. 2018;96:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Phelps J. Dark therapy for bipolar disorder using amber lenses for blue light blockade. Med Hypotheses. 2008;70(2):224–229. [DOI] [PubMed] [Google Scholar]
- 62. Heath M, et al. Does one hour of bright or short-wavelength filtered tablet screenlight have a meaningful effect on adolescents’ pre-bedtime alertness, sleep, and daytime functioning? Chronobiol Int. 2014;31(4):496–505. [DOI] [PubMed] [Google Scholar]
- 63. Bryant PA, et al. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457–467. [DOI] [PubMed] [Google Scholar]
- 64. Besedovsky L, et al. Sleep and immune function. Pflugers Arch – Eur J Physiol. 2012;463:121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cohen S, et al. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169(1):62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Prather AA, et al. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38(9):1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Spiegel K, et al. Effect of sleep deprivation on response to immunization. JAMA. 2002;288(12):1471–1472. [DOI] [PubMed] [Google Scholar]
- 69. Zimmermann P, et al. Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev. 2019;32(2):e00084–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Magee CA, et al. Bidirectional relationships between sleep duration and screen time in early childhood. JAMA Pediatr. 2014;168(5):465–470. [DOI] [PubMed] [Google Scholar]
- 71. SleepFoundation.org. Sleep Guidelines During the COVID-19 Pandemic. https://www.sleepfoundation.org/sleep-guidelines-covid-19-isolation. Accessed September 1, 2020.
- 72. Johns Hopkins Medicine. Managing Sleep Problems during COVID-19. https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_Psychiatry_Guide/787390/all/Managing_Sleep_Problems_during_COVID_19. Accessed September 1, 2020.
- 73. Léger D, et al.. Poor sleep associated with overuse of media during the COVID-19 lockdown. Sleep. 2020;43(10):zsaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hale L, et al. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Montag C, et al. Discussing digital technology overuse in children and adolescents during the COVID-19 pandemic and beyond: on the importance of considering Affective Neuroscience Theory. Addict Behav Rep. 2020;12:100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.