Abstract
Background
Vitamin D deficiency has been associated with an increased risk of respiratory infections.
Objectives
The study aimed to evaluate the serum 25-hydroxyvitamin D [25(OH)D] concentration in patients admitted to the intensive care unit (ICU) as a predictor of coronavirus disease 2019 (COVID-19) mortality.
Methods
A single-center retrospective observational study was conducted. Forty adult patients (50% men) with confirmed COVID-19 who were admitted to the ICU were enrolled. The primary endpoint was mortality at day 60. Serum 25(OH)D concentration was measured on the day of admission to the ICU. We used the Mann–Whitney test, Fisher's exact test, Kaplan–Meier analysis, and receiver operator characteristic (ROC) analysis to assess serum 25(OH)D concentration as a predictor of COVID-19 mortality.
Results
All 40 patients had a low median (IQR) serum 25(OH)D concentration at admission [12 (9–15) ng/mL]. The median (IQR) serum 25(OH)D concentration was greater in survivors [13.3 (10.0–17.1) ng/mL, n = 22] than in nonsurvivors [9.6 (7.9–14.2) ng/mL; n = 18], P = 0.044. The area under the ROC curve was 0.69 (95% CI: 0.52, 0.86; P = 0.044). The 60-d mortality rate of those with serum 25(OH)D concentrations ≤9.9 ng/mL (n = 14, 71%) tended to be greater than that of those with concentrations >9.9 ng/mL (n = 26, 31%) (P = 0.065), and they had a 5.6-fold higher risk of death (OR: 5.63; 95% CI: 1.35, 23.45; P = 0.018).
Conclusions
The ICU patients had a low serum 25(OH)D concentration. Serum 25(OH)D concentrations ≤9.9 ng/mL on admission can be used to predict in-hospital mortality in patients with COVID-19.
This trial was registered at clinicaltrials.gov as NCT04450017.
Key words: serum 25(OH)D concentration, SARS-CoV-2, COVID-19, outcome, intensive care unit
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—the virus that causes coronavirus disease 2019 (COVID-19)—pandemic presents a global challenge affecting all aspects of human life and resulting in extensive social, economic, and human losses. Although moderately ill COVID-19 patients generally recover well, this is not the case for critically ill patients, where the results of the therapy are frequently disappointing. In this group of patients, disease progression is often complicated by acute respiratory distress syndrome (ARDS), multiple organ failure, infections, as well as the need for mechanical ventilation and admission to the intensive care unit (ICU) (1). The reported mortality rate in such patients varies significantly across different countries and may reach 85% (2., 3., 4., 5.). The risk factors, including race, age, comorbidities, genetics, and the availability of medical help, may contribute independently and cumulatively to the disease's severity (6).
Higher mortality and hospitalization rates have been previously reported in the northern-latitude regions of the world (6). This has been attributed to the low UV exposure and vitamin D deficiency in the populations in these areas.
Several studies on ICU patients have reported that low vitamin D concentrations are associated with a higher risk of negative outcomes such as death, organ failure, prolonged mechanical ventilation, a higher rate of ventilation-associated pneumonia, and sepsis (7., 8., 9., 10.).
We conducted a single-center retrospective study. The aim was to evaluate the serum 25-hydroxyvitamin D [25(OH)D] concentration in patients admitted to the ICU as a predictor of COVID-19 mortality.
Methods
Study design and participants
The retrospective observational single-center study (NCT04450017) was conducted in the Federal Scientific and Clinical Center of Specialized Types of Medical Care and Medical Technologies of the Federal Medical and Biological Agency of Russia, which was a designated hospital for COVID-19 patients. The diagnosis of COVID-19 was established according to the WHO interim guidance (11). Between 6 and 27 April, 2020, 663 consecutively admitted COVID-19 patients were screened. Of them, 121 were treated in the ICU. Forty patients who had their serum 25(OH)D concentration measured during admission and had a definite outcome (death or discharged) were included.
The study was approved and the requirement for informed consent was waived by the Ethics Commission (record 5 from Protocol No. 5, 2020).
To better define the relation between vitamin D insufficiency and COVID-19, we determined the serum 25(OH)D concentrations in COVID-19 outpatients and non-COVID-19 patients. The study was conducted at the same center through a retrospective analysis of 25 COVID-19 outpatients who were tested for serum 25(OH)D concentrations throughout the course of the disease. Furthermore, we retrospectively selected 100 random patients from the hospital database who represented the general population and had their serum 25(OH)D concentrations determined in our hospital in 2019 before the COVID-19 pandemic. Of these, only 50 patients could be reached and consented to participate in the study. We analyzed serum 25(OH)D concentrations and the incidence of COVID-19 in these patients, who represented 2 age groups (similar to our main study).
Laboratory assays and data analysis
Blood samples were collected within 24 h after admission for routine laboratory tests, such as blood count, coagulation profile, and serum biochemical tests (including renal and liver function), in the on-site laboratory. Plasma D-dimer concentration was determined using an ACL TOP 700 automatic coagulation analyzer (Instrumentation Laboratory, CTS Family) on a latex-enhanced photometric immunoassay. The plasma fibrinogen concentration was measured according to a photo-optical Clauss method, with an ACL TOP 700 automatic coagulation analyzer (Instrumentation Laboratory, CTS Family). The serum concentrations of C-reactive protein (CRP) and of ferritin were processed by the immunoturbidimetric method using an Architect c800. The serum concentrations of procalcitonin, IL-6, and troponin were analyzed using an electrochemiluminescence method on a Cobas e411 analyzer (Roche Diagnostics). The serum concentrations of albumin and total bilirubin were measured using an automated colorimetric method on a Cobas 8000 (Roche Diagnostics), whereas the serum creatinine was measured by an enzymatic method with calibration traceable to a reference procedure (isotope dilution-MS) and automatized on a Cobas 8000. Hematology analyses, including blood count, were performed using the flow cytometry method on an ADVIA 2120i (Siemens Healthineers). Immune phenotyping was performed by flow cytometric analysis (ACEA Novocyte flow cytometer, ACEA Bioscience). The serum 25(OH)D concentrations were assessed by chemiluminescence immunoassay on an ARCHITECT i 2000 SR instrument (Abbott Laboratories). The laboratory reference range was 5.0–160.0 ng/mL. All measurements were conducted within 2 h after blood sampling.
Vitamin D status was categorized using cutoffs based on serum 25(OH)D concentrations: <10.0 ng/mL represented severe deficiency, 10.0–19.9 ng/mL represented a deficiency, 20.0–29.9 ng/mL represented insufficiency, and ≥30.0 ng/mL represented sufficient 25(OH)D concentrations (12).
Statistical analysis
The statistical analysis was carried out using SPSS for Mac version 19 (IBM). Continuous and categorical variables were presented as median (IQR) or n (%), as appropriate. A comparison of quantitative characteristics was performed using the Mann–Whitney U test. The χ2 tests (2 × 2) or Fisher's exact test (if there were <10 observations) were performed to assess the significance of the differences between the characteristics according to the categorical variables. The optimal 25(OH)D cutoff was determined using the receiver operator characteristic (ROC) curve. The strength of the association between the serum 25(OH)D concentration and the outcome was defined using the OR. The outcomes were compared using Kaplan–Meier survival analysis. A P value <0.05 was considered statistically significant.
Results
Forty patients [61 (52.5–80) y, 50% male] were enrolled in the present study ( Table 1). All 40 patients had low serum 25(OH)D concentrations during admission to the ICU [12 (9–15) ng/mL]. The BMI was 27.7 (23–31) kg/m2. The all-cause 60-d mortality rate was 45%. The serum 25(OH)D concentration was greater in survivors [13.3 (10.0–17.1) ng/mL; n = 22] than in nonsurvivors [9.6 (7.9–14.2) ng/mL; n = 18], P = 0.044 (Mann–Whitney test).
Table 1.
Characteristics of the COVID-19 patients admitted to the ICU1
| Total (n = 40) | 25(OH)D > 9.9 ng/mL subgroup (n = 26) | 25(OH)D ≤ 9.9 ng/mL subgroup (n = 14) | P value2 | |
|---|---|---|---|---|
| Age, y | 61 [52.5–80] | 56.5 [51–69] | 76.5 [61–89] | 0.018 |
| Sex, male | 20 (50) | 13 (50) | 7 (50) | 0.87 |
| BMI, kg/m2 | 27.7 (23.1–31.3) | 28.4 (23.0–31.6) | 26.8 (23.4–29.0) | 0.22 |
| 25(OH)D, ng/mL (N 30–80 ng/mL) | 12.0 [8.7–15.0] | 14.0 [12.1–18.8] | 7.7 [5.2–8.6] | 0.044 |
| Routine tests | ||||
| White blood cell count, 109/L (N 4.5–9 109/L) | 7.4 [6.1–9.6] | 7.1 [5.6–8.9] | 8.7 [6.4–16.0] | 0.18 |
| Neutrophils, 109/L (N 2–7.5 109/L) | 6.4 [4.4–8.7] | 6.1 [4.3–7.6] | 7.6 [5.6–11.4] | 0.14 |
| Lymphocytes, 109/L (N 1.5–4.5 109/L) | 0.9 [0.5–1.1] | 1.0 [0.6–1.2] | 0.7 [0.4–1.0] | 0.06 |
| NLR (N 0.78–3.53) | 7.9 [5.0–13.9] | 6.7 [4.7–10.5] | 12.9 [7.8–20.6] | 0.017 |
| NKT cells (CD3+CD56+CD16+), % (N < 10) | 0.6 [0.3–1.1] | 1.5 [0.8–2.8] | 0.5 [0.2–0.9] | 0.019 |
| NK cells (CD3-CD56+CD16+), % (N 9.9–22.0) | 1.3 [0.9–2.6] | 1.8 [0.9–2.6] | 1.2 [0.9–3.5] | 0.42 |
| Platelets, 109/L (N 150–380 109/L) | 209 [172–290] | 197 [154–285] | 230 [194–290] | 0.25 |
| Hemoglobin, g/L (N 120–170 g/L) | 140 [121–150] | 140 [130–158] | 136 [112–147] | 0.49 |
| Albumin, g/L (N 35–50 g/L) | 34 [32–37] | 35 [33–37] | 32 [26–35] | 0.07 |
| Creatinine, μM/L (N 53–97 μM/L) | 81 [62–101] | 86 [63–99] | 70 [59–118] | 0.69 |
| Total bilirubin, μM/L (N 3.4–17.1 μM/L) | 11.4 [9.3–15.0] | 10.9 [9.0–13.5] | 12.0 [9.2–18.6] | 0.51 |
| Troponin T, pg/mL (N <14 pg/mL) | 13.0 [7.0–32.5] | 10.6 [6.5–26.0] | 21.0 [8.5–51.3] | 0.26 |
| Ferritin, μg/L (N 11–336 μg/L) | 876 [375–1830] | 1004 [486–2030] | 409 [321–990] | 0.09 |
| IL-6, pg/mL (N <7 pg/mL) | 85 [49.8–190] | 85 [50–210] | 88 [47–120] | 0.65 |
| D-dimer, μg/mL (N <0.5 μg/mL) | 0.6 [0.4–1.8] | 0.6 [0.3–1.8] | 1.0 [0.6–2.2] | 0.05 |
| Fibrinogen, g/L (N 2–4 g/L) | 5.2 [4.2–6.4] | 4.9 [4.1–5.9] | 5.9 [4.1–6.7] | 0.27 |
| Procalcitonin, ng/mL (N <0.05 ng/mL) | 0.16 [0.06–0.30] | 0.14 [0.05–0.30] | 0.25 [0.10–0.65] | 0.20 |
| C-reactive protein, mg/L (N <5 mg/L) | 141 [103–210] | 126 [95–180] | 183 [116–240] | 0.23 |
| Outcomes | ||||
| LOS ICU, d | 11 [3–18] | 9 [2–22] | 13 [5–17] | 0.66 |
| Hospital stay, d | 15 [10–22] | 16 [11–25] | 14 [8–19] | 0.14 |
| Nonsurvivors | 18 (45) | 8 (31) | 10 (71) | 0.044 |
Values are median [IQR] for numerical data and n (%) for categorical data. ICU, intensive care unit; LOS, length of stay; N, reference range; NLR, ratio of neutrophils to lymphocytes; 25(OH)D, serum 25-hydroxyvitamin D.
P values were calculated by Mann–Whitney U test, χ² test, or Fisher's exact test, as appropriate.
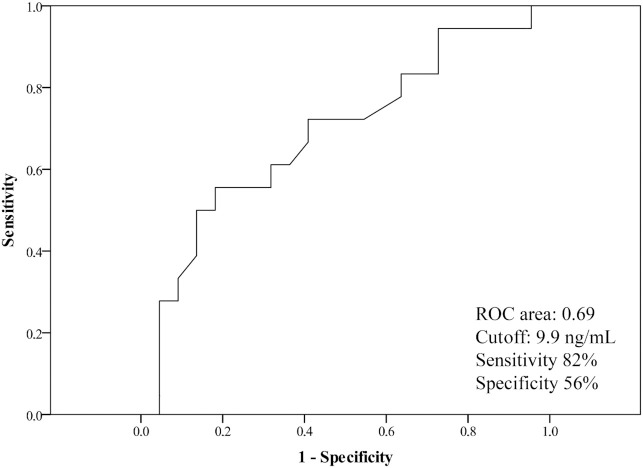
The area under the ROC curve was 0.69 (95% CI: 0.52, 0.86; P = 0.044). The cutoff value for the 25(OH)D concentration was 9.9 ng/mL. The predictive accuracy of mortality in patients with serum 25(OH)D concentrations <9.9 ng/mL had a sensitivity of 82% and a specificity of 56% ( Figure 1).
Figure 1.
The ROC curve for serum 25(OH)D concentrations as a predictor of mortality risk in COVID-19 patients in the intensive care unit. The area under the ROC curve was 0.69 (95% CI: 0.52, 0.86; P = 0.044). The optimum cutoff value for 25(OH)D concentration was 9.9 ng/mL. The predictive accuracy of mortality in patients with 25(OH)D concentrations ≤ 9.9 ng/mL had a sensitivity of 82% and a specificity of 56%. ROC, receiver operator characteristic; 25(OH)D, 25-hydroxyvitamin D.
These 2 groups [patients with serum 25(OH)D concentrations >9.9 and <9.9 ng/mL] had significantly different ages (P = 0.018), ratios of neutrophils to lymphocytes (P = 0.017), and D-dimer concentrations (P = 0.05).
Our data indicate that fewer NKT cells were found in the circulation in critically ill COVID-19 patients, and the NKT cell count was 67% less in the group of patients having serum 25(OH)D concentrations <9.9 ng/mL (P = 0.019). There were no between-group differences in other routine laboratory tests, including those for white blood cell count, neutrophils, lymphocytes, platelets, NK cells, albumin, total bilirubin, creatinine, troponin, ferritin, IL-6, procalcitonin, CRP, and fibrinogen (Table 1).
The durations of stay in the ICU [9 (2–22) d compared with 13 (5–17) d; P = 0.66] and hospital stay [16 (11–25) d compared with 14 (8–19) d; P = 0.14] did not significantly differ between the groups.
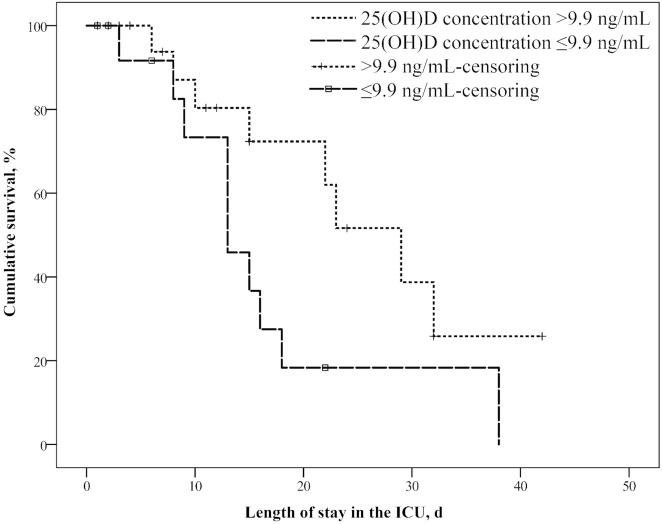
Kaplan–Meier survival curves for the 25(OH)D concentrations on admission to the ICU showed that the 60-d mortality rate was greater in those with serum 25(OH)D concentrations ≤9.9 ng/mL (n = 14, 71%) than in those with concentrations >9.9 ng/mL (n = 26, 31%) (P = 0.065) ( Figure 2). Patients with serum 25(OH)D concentrations ≤9.9 ng/mL had a 5.6-fold higher risk of death than those with concentrations >9.9 ng/mL (OR: 5.63; 95% CI: 1.35, 23.45; P = 0.018).
Figure 2.
Survival curves for COVID-19 patients admitted to the ICU with serum 25(OH)D concentrations > 9.9 and ≤9.9 ng/mL. The Kaplan–Meier survival curve showed that the 60-d mortality rate of those with 25(OH)D concentrations ≤ 9.9 ng/mL (n = 14, 71%) tended to be greater than that of those with concentrations > 9.9 ng/mL (n = 26, 31%) (P = 0.065). ICU, intensive care unit; 25(OH)D, 25-hydroxyvitamin D.
The serum 25(OH)D concentrations of the COVID-19 outpatients and non-COVID-19 outpatients and the main outcomes are summarized in the Supplemental data. Among the 25 COVID-19 outpatients, 8 (32%) had an insufficiency and 12 (48%) had a deficiency of 25(OH)D. No differences between the serum 25(OH)D concentrations were observed according to age group (P = 0.24). None of the patients were hospitalized for COVID-19 (Supplemental Table 1).
The prevalence of vitamin D insufficiency and deficiency among non-COVID-19 (n = 31) outpatients was 6 (19%) and 10 (32%), respectively. There were no intergroup (young compared with old) differences for any of the outcomes, including the rate of hospitalization due to COVID-19 progression and the incidence of COVID-19 (Supplemental Table 2). There were no differences between the serum 25(OH)D concentrations of the groups of patients with (n = 19) and without (n = 31) COVID-19 (P = 0.94) (Supplemental Table 3). The serum 25(OH)D concentrations of the COVID-19 and non-COVID-19 outpatients were significantly higher than those of the COVID-19 patients admitted to the ICU [22.5 (13.8–32.5) ng/mL and 12.0 (8.7–15.0) ng/mL, respectively; P < 0.001].
Discussion
The main findings of this study were that ICU patients with COVID-19 had deficient serum 25(OH)D concentrations and that a serum 25(OH)D concentration at admission ≤9.9 ng/mL was predictive of in-hospital mortality.
25(OH)D deficiency is common in critical illness with prevalence ranging from 40% to 70% (13). In critically ill patients, a precipitous decline in 25(OH)D concentration was reported to occur owing to compromised 25(OH)D metabolism, downregulation of vitamin D–binding protein and albumin, as well as owing to attenuated 25(OH)D conversion to 1,25-dihydroxyvitamin D [1,25(OH)2D] in the kidneys (7, 14). High-volume fluid infusion therapy in critically ill patients may similarly result in lowering of 25(OH)D and vitamin D–binding protein (15).
Vitamin D deficiency did not appear to be common in similar COVID-19 outpatients and non-COVID-19 outpatients. The serum 25(OH)D concentrations in COVID-19 and non-COVID-19 outpatients were significantly higher than the serum 25(OH)D concentrations in COVID-19 patients admitted to the ICU. It seems that COVID-19 can be a factor of the low serum 25(OH)D.
In our study, the patients with serum 25(OH)D concentrations ≤ 9.9 ng/mL were older. This is consistent with the well-known fact that older adult patients tend to have severe vitamin D deficiency. This occurs owing to the age-related downregulation of expression of vitamin D receptors, the decrease in 1,25(OH)2D production in the kidneys, the decrease in vitamin D production in the skin, dietary insufficiency of vitamin D precursors, and the disruption of calcium metabolism (16). Older age is a common risk factor for vitamin D deficiency and unfavorable outcomes of COVID-19 infection (4, 16). We conducted subgroup analyses based on age to reduce possible confounding by this variable. There were no inter-age-group differences in serum 25(OH)D concentrations between the COVID-19 outpatients and the non-COVID-19 outpatients in the study. Furthermore, we did not reveal the relations between serum 25(OH)D concentrations and COVID-19 progression among COVID-19-positive outpatients and the incidence of COVID-19 in the general population. A larger cohort of COVID-19 patients is required to study the correlation between vitamin D deficiency, hospitalization, and mortality.
Several pathways are affected by vitamin D, and supplementation with vitamin D may reduce the risk of infection and death. Recent studies focused on the role of vitamin D in reducing morbidity due to acute upper respiratory tract infections and uncovered that it affected both cellular and humoral immunity, induced the expression of vitamin D–dependent antimicrobial peptides, and boosted antioxidant production (17, 18). Several studies have also demonstrated that vitamin D deficiency is associated with influenza, respiratory syncytial virus, and tuberculosis infections (19, 20).
Recently, several cytokines have been demonstrated to be associated with severe disease progression in COVID-19 patients (5, 21). Vitamin D is known to display potent immunomodulating activity, and it may affect pro- and anti-inflammatory cytokines. It inhibits monocyte production of proinflammatory cytokines such as IL-1, IL-6, IL-8, IL-12, and TNFα (22). It may also downregulate the production of other proinflammatory cytokines, such as IL-17 and IL-21, and enhance the secretion of anti-inflammatory IL-10 (23). Several studies have demonstrated that vitamin D supplementation may reduce the severity of cytokine release syndrome in the context of flu and Crimean-Congo hemorrhagic fever (24, 25). Silberstein (26) hypothesized that vitamin D deficiency may underlie the variation in the severities of COVID-19, and that treatment with vitamin D may offer a simpler alternative to tocilizumab.
Our data are consistent with the data of Shojaei et al. (27). The incidence of vitamin D deficiency in septic patients presenting to the emergency department was 60.2%. Age (R = −0.261, P = 0.037) and mortality (R = −0.426, P = 0.025) were significantly correlated with low serum 25(OH)D concentrations. The area under the ROC curve of serum 25(OH)D concentration and 1-mo mortality in their analysis was 0.701 (95% CI: 0.439, 0.964). In a meta-analysis including 1736 patients with sepsis, low 25(OH)D on admission was reported to be independently associated with increased mortality (28).
There are several protective mechanisms for vitamin D in the context of sepsis. First, 1,25(OH)2D increases the expression of tight junction proteins. This strengthens the epithelial barrier (29) and prevents capillary leakage (30); both capillary leakage and a weak epithelial barrier are the hallmarks of sepsis. Second, this acts via the boosted expression of vitamin D–dependent antimicrobial peptides (cathelicidin and defensin) which, in turn, suppresses the growth of Gram-positive and Gram-negative bacteria, fungi, and enveloped and nonenveloped viruses. Thus, vitamin D may help dampen microbial load in patients with sepsis (31).
Vitamin D deficiency is prevalent in ARDS patients (32); furthermore, vitamin D deficiency is associated with increased risk of acute lung injury. The odds of ARDS in patients with 25(OH)D < 20 nmol/L were 3.5-fold those of patients with 25(OH)D ≥ 20 nmol/L (95% CI: 1.06, 11.6; P = 0.040) (33). Retrospective study of a cohort of 1985 critically ill patients identified an association of low prehospitalization concentrations of vitamin D with progression to acute respiratory failure (34).
Several animal studies have indicated that vitamin D demonstrates protective activity in ARDS. Kong et al. (35) reported that vitamin D reduces the severity of LPS-induced lung injury. Similarly, Xu et al. (36) demonstrated that vitamin D may help protect from acute lung injury by modulating expression of the renin–angiotensin system, including that of angiotensin-converting enzyme 2 (ACE-2), renin, and angiotensin II. Vitamin D is a well-known player in the renin–angiotensin system (37), and ACE-2 is the major cell entry receptor of SARS‐CoV‐2. Vitamin D deficiency is accompanied with the activation of the renin–angiotensin system, yet its permanent activation is associated with progression of chronic cardiovascular diseases and lung fibrosis (33).
Vitamin D deficiency is also associated with a number of other diseases that are known risk factors for a severe course and unfavorable outcomes in COVID-19 patients. Low concentration of vitamin D may lead to obesity and insulin resistance (38), and in elderly patients it contributes to insulinemia and glucose intolerance (39). Multiple studies support an association between vitamin D concentrations and heart failure, as well as outcomes in this patient cohort (40, 41).
Low vitamin D concentration was associated with a higher risk of thrombotic complications (42), which are very frequent (≤16.7%) among COVID-19 patients despite the anticoagulation therapy (43). Vitamin D directly controls the expression of >200 target genes (44), many of which may contribute to hypercoagulopathy. The present study has uncovered the association between severe vitamin D deficiency and increased D-dimer concentration.
According to numerous publications, vitamin D modulates immune cells and stimulates immune tolerance (45, 46). In this study, we paid attention to the immune status of patients with severe courses. NK cell concentrations in the peripheral blood inversely correlated with disease severity (47). In patients with moderate and severe COVID-19, lower concentrations of circulating NK cells were observed, and their functions were inhibited (47). During the early stages of COVID-19, NK-cell activation across distinct subsets was elevated in the peripheral blood, mirroring the activation signature of NK cells in the bronchoalveolar lavage fluid of patients with COVID-19. With a severe course of the disease, a sharp decrease in NK cells in the blood was observed. Further research is needed to determine the protective antiviral and the deleterious pathological roles of NK cells in COVID-19 patients (48). Another study demonstrated that in SARS-CoV-2 infection, the NK cells significantly reduced; however, the cells were strongly activated, and this activation correlated with the development of severe disease (49). Our data also indicate that fewer NKT cells were found in the circulation in critically ill COVID-19 patients with serum 25(OH)D concentrations ≤ 9.9 ng/mL. The good effects of vitamin D on protective immunity are due to its effects on the innate immune system. Lee et al. (45) reported that NK cell cytotoxicity and the counts of CD3− NK1.1+ cells were reduced in mice lacking 25(OH)D-upregulated protein 1. With in vitro treatment with 1,25(OH)2D, enhanced lytic activity of NK cells has been observed against target tumor cells (46). Nonetheless, data that describe the opposite effects, namely the negative regulation of NK cell activity and cell differentiation by vitamin D, are available (50). A larger cohort of COVID-19 patients is required to study the correlation between vitamin D deficiency and NK lymphopenia and activation.
Our study has several limitations. First, it is a small single-center study. Second, the low serum 25(OH)D concentration may have been influenced by seasonality, because the study was performed in April after a long period of limited sun exposure. Third, despite the observed prognostic potential of the serum 25(OH)D concentration, the effect of adding vitamin D to the therapy of this group of patients, especially the potential increase in therapeutic efficacy, is yet to be established.
In conclusion, we found that the ICU patients had a low serum 25(OH)D concentration on admission. Serum 25(OH)D concentrations ≤ 9.9 ng/mL on admission can be used to predict in-hospital mortality in patients with COVID-19 infection. An intervention study is warranted to elucidate the usefulness of vitamin D supplementation for the treatment and/or prevention of COVID-19.
Acknowledgments
We are grateful for the assistance of the health care personnel of the intensive care units of the Federal Scientific and Clinical Center. We also acknowledge the laboratory personnel for assistance with samples every day. The authors’ responsibilities were as follows—MVB, TVK, VPB, and AVT: designed the research; MVB and SAA: conducted the research; MVB, TVK, and IAM: wrote the paper; NAK and GMY: performed the laboratory analyses; IAM and SAA: analyzed the data; MVB: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Footnotes
The authors reported no funding received for this study.
Author disclosures: the authors report no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Supplementary Material
References
- 1.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, Huang F, Zhou J, Zhang B, Yan F, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panarese A, Shahini E. Letter: Covid-19, and vitamin D. Aliment Pharmacol Ther. 2020;51(10):993–995. doi: 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair P, Lee P, Reynolds C, Nguyen ND, Myburgh J, Eisman JA, Center JR. Significant perturbation of vitamin D–parathyroid–calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39(2):267–274. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 8.Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;42(1):97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 9.Quraishi SA, Bittner EA, Blum L, McCarthy CM, Bhan I, Camargo CA., Jr Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit Care Med. 2014;42(6):1365–1371. doi: 10.1097/CCM.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39(4):671–677. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. WHO clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. [Internet]. Geneva, Switzerland: World Health Organization; 2020; [cited 8 February, 2020]. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 12.Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 13.Amrein K, Papinutti A, Mathew E, Vila G, Parekh D. Vitamin D and critical illness: what endocrinology can learn from intensive care and vice versa. Endocr Connect. 2018;7(12):R304–R315. doi: 10.1530/EC-18-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czarnik T, Czarnik A, Gawda R, Gawor M, Piwoda M, Marszalski M, Maj M, Chrzan O, Said R, Rusek-Skora M, et al. Vitamin D kinetics in the acute phase of critical illness: a prospective observational study. J Crit Care. 2018;43:294–299. doi: 10.1016/j.jcrc.2017.09.179. [DOI] [PubMed] [Google Scholar]
- 15.Quraishi SA, Camargo CA., Jr Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care. 2012;15(6):625–634. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher JC. Vitamin D and aging. Endocrinol Metab Clin North Am. 2013;42(2):319–332. doi: 10.1016/j.ecl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rondanelli M, Miccono A, Lamburghini S, Avanzato I, Riva A, Allegrini P, Faliva MA, Peroni G, Nichetti M, Perna S. Self-care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and Echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid Based Complement Alternat Med. 2018:5813095. [DOI] [PMC free article] [PubMed]
- 18.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry DJ, Hesketh K, Power C, Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106(9):1433–1440. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 20.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1α,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45(3):190–197. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant WB, Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Dermatoendocrinol. 2009;1(4):215–219. doi: 10.4161/derm.1.4.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parlak E, Ertürk A, Çağ Y, Sebin E, Gümüşdere M. The effect of inflammatory cytokines and the level of vitamin D on prognosis in Crimean-Congo hemorrhagic fever. Int J Clin Exp Med. 2015;8(10):18302–18310. [PMC free article] [PubMed] [Google Scholar]
- 26.Silberstein M. Vitamin D: a simpler alternative to tocilizumab for trial in COVID-19?. Med Hypotheses. 2020;140:109767. doi: 10.1016/j.mehy.2020.109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shojaei M, Sabzeghabaei A, Valaei Barhagh H, Soltani S. The correlation between serum level of vitamin D and outcome of sepsis patients; a cross-sectional study. Arch Acad Emerg Med. 2019;7(1):e1. [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Ding S. Serum 25-hydroxyvitamin D and the risk of mortality in adult patients with sepsis: a meta-analysis. BMC Infect Dis. 2020;20(1):189. doi: 10.1186/s12879-020-4879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Baylink DJ, Walter MH, Lau KH, Meng X, Wang J, Cherkas A, Tang X, Qin X. Targeted 25-hydroxyvitamin D3 1α-hydroxylase adoptive gene therapy ameliorates DSS-induced colitis without causing hypercalcemia in mice. Mol Ther. 2015;23(2):339–351. doi: 10.1038/mt.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012;12:57. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herr C, Shaykhiev R, Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin Biol Ther. 2007;7(9):1449–1461. doi: 10.1517/14712598.7.9.1449. [DOI] [PubMed] [Google Scholar]
- 32.Dancer RC, Parekh D, Lax S, D'Souza V, Zheng S, Bassford CR, Park D, Bartis DG, Mahida R, Turner AM, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70(7):617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y, Liu T, Yao L, Xing Y, Zhao X, Fu J, Xue X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci Rep. 2017;7(1):3312. doi: 10.1038/s41598-017-03474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thickett DR, Moromizato T, Litonjua AA, Amrein K, Quraishi SA, Lee-Sarwar KA, Mogensen KM, Purtle SW, Gibbons FK, Camargo CA, Jr, et al. Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: a retrospective cohort study. BMJ Open Respir Res. 2015;2(1):e000074. doi: 10.1136/bmjresp-2014-000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong J, Zhu X, Shi Y, Liu T, Chen Y, Bhan I, Zhao Q, Thadhani R, Li YC. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27(12):2116–2125. doi: 10.1210/me.2013-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16(5):7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forman JP, Williams JS, Fisher NDL. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55(5):1283–1288. doi: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann K, Janssen OE. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114(10):577–583. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- 39.Baynes KC, Boucher BJ, Feskens EJ, Kromhout D. Vitamin D, glucose tolerance and insulinaemia in elderly men. Diabetologia. 1997;40(3):344–347. doi: 10.1007/s001250050685. [DOI] [PubMed] [Google Scholar]
- 40.Liu LCY, Voors AA, van Veldhuisen DJ, van der Veer E, Belonje AM, Szymanski MK, Silljé HHW, van Gilst WH, Jaarsma T, de Boer RA. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail. 2011;13(6):619–625. doi: 10.1093/eurjhf/hfr032. [DOI] [PubMed] [Google Scholar]
- 41.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 42.Wu W-X, He D-R. Low vitamin D levels are associated with the development of deep venous thromboembolic events in patients with ischemic stroke. Clin Appl Thromb Hemost. 2018;24(9_suppl):69S–75S. doi: 10.1177/1076029618786574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26(5):662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 45.Lee KN, Kang H-S, Jeon J-H, Kim E-M, Yoon S-R, Song H, Lyu C-Y, Piao Z-H, Kim S-U, Han Y-H, et al. VDUP1 is required for the development of natural killer cells. Immunity. 2005;22(2):195–208. doi: 10.1016/j.immuni.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Al-Jaderi Z, Maghazachi AA. Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins. 2013;5(11):1932–1947. doi: 10.3390/toxins5111932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Market M, Angka L, Martel AB, Bastin D, Olanubi O, Tennakoon G, Boucher DM, Ng J, Ardolino M, Auer RC. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front Immunol. 2020;11:1512. doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alrubayyi A. NK cells in COVID-19: protectors or opponents?. Nat Rev Immunol. 2020;20(9):579. doi: 10.1038/s41577-020-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maucourant C, Filipovic I, Ponzetta A, Aleman S, Cornillet M, Hertwig L, Strunz B, Lentini A, Reinius B, Brownlie D, et al. Natural killer cell immunotypes related to COVID-19 disease severity. Sci Immunol. 2020;5(50):eabd6832. doi: 10.1126/sciimmunol.abd6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weeres MA, Robien K, Ahn Y-O, Neulen M-L, Bergerson R, Miller JS, Verneris MR. The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J Immunol. 2014;193(7):3456–3462. doi: 10.4049/jimmunol.1400698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.