ABSTRACT
Background
Many nutrients have powerful immunomodulatory actions with the potential to alter susceptibility to coronavirus disease 2019 (COVID-19) infection, progression to symptoms, likelihood of severe disease, and survival.
Objective
The aim was to review the latest evidence on how malnutrition across all its forms (under- and overnutrition and micronutrient status) may influence both susceptibility to, and progression of, COVID-19.
Methods
We synthesized information on 13 nutrition-related components and their potential interactions with COVID-19: overweight, obesity, and diabetes; protein-energy malnutrition; anemia; vitamins A, C, D, and E; PUFAs; iron; selenium; zinc; antioxidants; and nutritional support. For each section we provide: 1) a landscape review of pertinent material; 2) a systematic search of the literature in PubMed and EMBASE databases, including a wide range of preprint servers; and 3) a screen of 6 clinical trial registries. All original research was considered, without restriction to study design, and included if it covered: 1) severe acute respiratory syndrome coronavirus (CoV) 2 (SARS-CoV-2), Middle East respiratory syndrome CoV (MERS-CoV), or SARS-CoV viruses and 2) disease susceptibility or 3) disease progression, and 4) the nutritional component of interest. Searches took place between 16 May and 11 August 2020.
Results
Across the 13 searches, 2732 articles from PubMed and EMBASE, 4164 articles from the preprint servers, and 433 trials were returned. In the final narrative synthesis, we include 22 published articles, 38 preprint articles, and 79 trials.
Conclusions
Currently there is limited evidence that high-dose supplements of micronutrients will either prevent severe disease or speed up recovery. However, results of clinical trials are eagerly awaited. Given the known impacts of all forms of malnutrition on the immune system, public health strategies to reduce micronutrient deficiencies and undernutrition remain of critical importance. Furthermore, there is strong evidence that prevention of obesity and type 2 diabetes will reduce the risk of serious COVID-19 outcomes. This review is registered at PROSPERO as CRD42020186194.
Keywords: SARS-CoV-2, COVID-19, nutrition, disease risk, disease progression, micronutrients, systematic review
Introduction
The astonishing spread of severe acute respiratory syndrome coronavirus (CoV) 2 (SARS-CoV-2) since late 2019 has resulted in a global pandemic of the coronavirus disease 2019 (COVID-19). Alongside the worldwide effort to deliver a vaccine, there has been a surge of interest in the epidemiological factors that underlie susceptibility to COVID-19, and its progression, in an attempt to explore the most effective preventative and curative options (1–4). Potential interactions between nutritional status and immune function have been widely documented (5–7). As the pandemic unfolds, it exacerbates the risk factors for malnutrition in all its forms (8, 9). Disruption to agricultural production, market linkages, and seasonal labor movements contribute to food price increases (10, 11), making nutritious food even more expensive for those most at risk of micronutrient deficiencies and undernutrition. Cancelled and delayed nutrition counseling, micronutrient distributions, vaccine rounds, and school meal programs accentuate the vulnerability (12–14). Lockdown measures in many countries have increased physical and psychological barriers to healthy eating and exercising, creating an obesogenic environment for many (15, 16).
Understanding the relation between nutritional status and risk of COVID-19 is therefore of critical importance to generate evidence-based recommendations. There may be a potential for nutritional interventions to reduce an individual's susceptibility to infection, progression to symptoms, and likelihood of severe disease (including the use of high- or very-high-dose supplements enterally or intravenously as nutraceuticals).
However, nutrition information has long been miscommunicated to the public (17–19), and nutrition-related myths on COVID-19 protection and treatment are widely prevalent in this pandemic (20). To this end, we have conducted a comprehensive systematic review of journal articles, preprints, and clinical trial registries to provide a robust evidence base of what is currently known and what gaps remain.
Methods
This review considers how malnutrition across all its forms (undernutrition, micronutrient deficiencies, and overnutrition) may influence both susceptibility to, and progression of, COVID-19. We synthesized information on 13 nutrition-related components and their potential interactions with COVID-19: overweight, obesity, and diabetes; protein-energy malnutrition; anemia; vitamins A, C, D, and E; PUFAs; iron; selenium; zinc; antioxidants; and nutritional support. We published our strategy on the PROSPERO database, reference CRD42020186194.
Search strategy
We adopted 3 key approaches for compiling information for each of the 13 sections listed above, as follows:
A landscape review of pertinent material. This section is nonsystematic and covers a brief description of the nutrient/condition vis-à-vis infection and immunity, evidence of any role in viral infections, possible mechanisms, and possible utility in treatment.
A systematic search of the literature in PubMed and EMBASE databases and including a systematic search of a wide range of preprint servers (listed in Supplemental Material 1).
A screen of 6 clinical trial registries, listed in Supplemental Material 1.
For the PubMed and EMBASE database searches, a search string was designed to encompass terms related to the following: 1) SARS-CoV-2, Middle East respiratory syndrome CoV (MERS-CoV), or SARS-CoV viruses; 2) disease susceptibility; 3) disease progression; and 4) the nutritional component of interest. The search string was then built combining the terms for 1 AND (2 OR 3) AND 4. The search string corresponding to components 1–3 was kept consistent between all sections, with component 4 being adapted to the relevant exposure of interest. The clinical trial registry and preprint server searches were restricted to COVID-19. Full search string terms for the PubMed, EMBASE, preprint server, and clinical trial registry searches are provided in Supplemental Material 2.
In the landscape reviews we summarized the insights learned from other viral diseases, where relevant, and included other coronaviruses (MERS-CoV and SARS-CoV) in the systematic searches. From the outset we acknowledge that COVID-19 is behaving differently to other viral diseases, and therefore cautiously extrapolate risk throughout the review.
Inclusion and exclusion criteria
We considered all populations of any sex, age, or nutritional status, with no specific geographic boundaries. We restricted the systematic searches to human populations and studies in English. All original research was considered, without restriction to study design. Systematic reviews were included to search bibliographies. We excluded comments, letters, opinions, and nonsystematic reviews.
Outcomes
Main outcomes for disease susceptibility were related to key concepts such as immunosuppression, inflammation, lymphocyte regulation, oxidative stress, and all forms of immune dysfunction. Main outcomes for disease progression related to viral load, viral replication, viral mutation, and transmission; worsening of respiratory tract and gastrointestinal infections; multiple organ failure; and other pathological features on disease progression to death. As the potential role of nutrition in disease susceptibility and progression is broad, we did not prespecify the measures of effect to consider. Instead, we report the measures of effect that the authors have used in the eligible studies.
Screening and selection
A lead and co-author were assigned to each of the 13 nutrition-related sections of the review. The 2 researchers then performed the PubMed and EMBASE searches for their section. After abstract screening, full texts were retrieved for the potentially eligible studies. The lead author then reviewed these studies and used a standardized template to extract data on the eligible studies. A team of 2 researchers searched and abstract-screened all the preprint servers listed in Supplemental Material 1 for all 13 sections. They exported potentially eligible matches to the lead author of the relevant section for the full screen. At the time of article revision (23 January 2021), we updated the references of any subsequently published preprints, but kept them in their original preprint sections for consistency with the search dates reported in Supplemental Table 1. One researcher searched all of the clinical trial registries for the 13 sections. Details of the potentially eligible clinical trials were sent to the lead author for review and data extraction. Searches took place between 16 May and 11 August 2020. Full details of the search dates by section can be found in Supplemental Table 1. Due to the expected heterogeneity of study types, exposures, and outcomes, we did not undertake a formal risk-of-bias assessment for each included study.
Data synthesis
We were guided by the Synthesis Without Meta-analysis (SWiM) reporting guidelines for systematic reviews (21). Due to the heterogeneity of outcomes related to disease susceptibility and progression we did not attempt to transform results into a standardized metric. For each section of the review we summarized the effect sizes as reported by the authors in the included studies.
Results
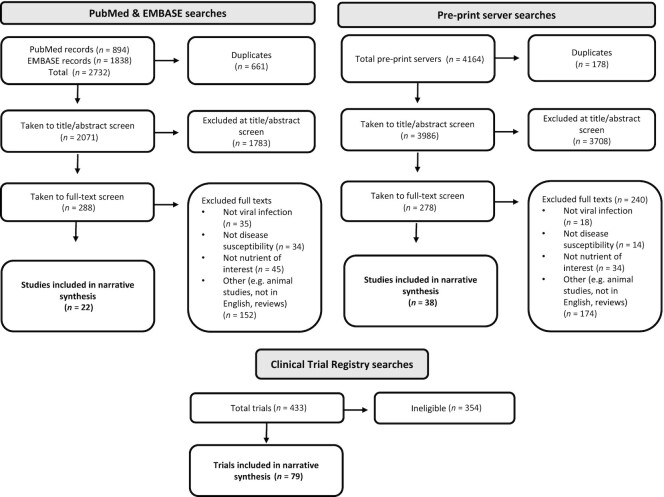
Figure 1 provides the overall flowchart summary of all articles retrieved and included in the narrative synthesis. The detailed flowchart breakdowns per section are given in Supplemental Table 1. Across the 13 searches, a total of 2732 hits from PubMed and EMBASE were returned. After removal of 661 duplicates, 2071 were taken to title/abstract screen and 1783 were deemed ineligible at this stage. A total of 288 articles were taken to the full-text screen and 266 were further excluded. The remaining 22 articles were included in the narrative synthesis and Supplemental Table 2.
FIGURE 1.
Flowchart summarizing studies and trials included in the systematic review of the role of nutrition in the susceptibility and progression of COVID-19. COVID-19, coronavirus disease 2019.
A total of 4164 hits from across the preprint servers were returned. After removal of 178 duplicates, 3986 were taken to title/abstract screen and 3708 were ineligible. A total of 278 articles were taken to full-text screen and 240 were excluded. The remaining 38 articles were included in the narrative synthesis and Supplemental Table 2.
From the clinical registry searches 433 trials were returned and 354 were ineligible. Seventy-nine trials are detailed in Supplemental Table 3 and further described in a narrative synthesis in Supplemental Material 3.
Protein-energy malnutrition
Landscape review
Protein-energy malnutrition (PEM), also called protein-energy undernutrition or simply “undernutrition,” is a state of nutritional insufficiency attributable to inadequate energy and/or protein intake and is often associated with multiple micronutrient deficiencies (22). According to the 2020 Global Nutrition Report, an estimated 820 million people worldwide (11% of the global population) are hungry or undernourished, and the majority are found in low-and-middle-income countries (LMICs) (23).
Globally PEM affects at least 1 in 5 children <5 y, with the greatest burden in LMICs, predominantly those in sub-Saharan African and South Asia (23). It manifests as stunting [weight-for-age z scores < −2, compared with the WHO Growth Reference Standards (24)], underweight (including low birth weight, weight-for-age z scores < −2), and acute malnutrition (kwashiorkor or wasting, defined as weight-for-height/-length < −2 z scores). The severe form of the latter, severe acute malnutrition, is associated with up to 50% mortality among children admitted to the hospital (25). In 2019, 49.5 million (7.3%) children aged <5 y were wasted and 149 million (22%) were stunted globally (23).
Wasting and stunting often coexist in children in LMICs and both are associated with increased mortality in childhood due to infectious diseases, particularly diarrhea and pneumonia (26). This susceptibility to infections is due to impaired immune function (including weakened gut-barrier function, humoral and cell-mediated immunity) with consequent inadequate nutrient intake due to anorexia and malabsorption (27). This further exacerbates immune suppression and impaired growth while energy and micronutrients are diverted to acute-phase immune responses to combat multiple and often recurrent infections, leading to a chronic systemic inflammatory state and bacterial translocation (28). Indeed, PEM is the primary cause of immune deficiency worldwide, and the vicious cycle of infection (clinical and subclinical) and PEM is well described (29, 30).
In high-income countries PEM is common among hospitalized adults, particularly the elderly, where 23–60% of elderly patients in acute health care settings are malnourished (31) and up to 50% of patients with concurrent morbidities are also affected (32). The causes are commonly poor nutrient intake (e.g., in the elderly due to poor oral health, depression, as a side effect of medication, or inadequate feeding support) and chronic underlying conditions that increase the metabolic demand due to inflammation, resulting in anorexia and increased muscle catabolism (cachexia), such as end-stage renal failure (33, 34). This leads to altered body composition and adverse functional and clinical outcomes. The Global Leadership Initiative on Malnutrition has developed internationally validated diagnostic criteria based on both phenotypic (weight loss, low BMI, reduced muscle mass/sarcopenia) and etiologic (reduced food intake or assimilation, and inflammation or disease burden, including major infections or trauma) criteria to facilitate early identification and management of patients with PEM to avert deaths and adverse outcomes (34).
In the current SARS-CoV-2 global pandemic, there is an urgent need to identify PEM-related factors that render individuals vulnerable to succumbing to this infection. As a staggering 11% of the population are likely to have impaired immunity due to PEM (23), many populations, particularly in LMICs, are potentially at risk of developing disease during this pandemic, although the severity of the trajectory is yet to be fully determined. Furthermore, although COVID-19 primarily affects the respiratory tract, patients can also have gastrointestinal symptoms, including diarrhea, nausea, and vomiting and loss of smell, that can have an impact on nutrient intake and assimilation (35). Human enteric CoV causes moderate to severe villous atrophy in animal models, with virus particles visible in enterocytes of the large and small intestine (36, 37). CoV-like particles have also been found in degenerating jejunal epithelial cells of adults in India with histological evidence of malabsorption due to environmental enteric dysfunction and among Aboriginal children with lactose malabsorption post-gastroenteritis (38, 39). However, the exact mechanisms of COVID-19–induced gastrointestinal symptoms of nausea, vomiting, and loss of taste remain elusive (40).
Although there are no current published data on the impact of PEM on the susceptibility and disease progression of SARS-CoV-2 infection in children, extrapolation from other RNA viral infections suggests that undernourished children are likely to have more severe respiratory and gastrointestinal disease. RNA viruses, including influenza A and B, and human metapneumovirus are important pathogens causing pneumonia in children aged <5 y globally (41). PEM has been associated with influenza-related severe acute respiratory illness in under-5s in South Africa [adjusted OR (aOR): 2.4; 95% CI: 1.1, 5.6] (42). In previous pandemics of influenza A(H1N1), such as the one in Guatemala in 2009 where 5 of the 11 deaths among hospitalized patients occurred in under 5s, PEM was thought to have been a key contributing factor (43). Children between 6 mo and 5 y were thus identified as a priority group for vaccination (43). However, to date, children appear to be at lower risk of suffering severe episodes of COVID-19 than adults (44).
In the current pandemic, a similar pattern is being played out to what we have seen in previous pandemics. Patients with PEM, especially among the elderly and those with comorbidities, have been among those with the highest mortality (45). Prolonged intensive care unit (ICU) admission causes or worsens existing PEM with associated sarcopenia (loss of skeletal muscle mass and function), exacerbated by the inflammation associated with the infection (46). Identification and management of PEM is now a key component of managing patients with COVID-19 in Europe to avert adverse outcomes. There are no clinical trial data to guide the design of optimal nutrition-management strategies in the context of COVID-19. The European Society for Clinical Nutrition and Metabolism (ESPEN) has published nutrition rehabilitation guidelines primarily based on consensus and expert opinion using a combination of enteral (EN) and parenteral nutrition (PN) if oral intake is not adequate (46) (see also section on Nutritional Support).
Systematic review
Our systematic search involved terms related to PEM in both children and adults and RNA viruses. The systematic screen of PubMed and EMBASE yielded 120 papers after removing duplicates; 23 were taken to full-text screen and all were excluded as they did not examine the influence of PEM on CoV susceptibility or disease course.
A further search of the preprint servers identified 2 studies that were included. First, Li et al. (47) conducted a cross-sectional study and recruited 182 elderly hospitalized COVID-19 patients ≥65 y in 1 center in Wuhan, China. The authors found that 53% were classified as malnourished using a mini-nutrition assessment (based on recall of dietary intake) and 28% were at risk of malnutrition. There were no statistically significant differences in the triceps skinfold thickness and midarm circumference between those who were nonmalnourished, at risk of malnutrition, or malnourished. However, diabetes mellitus (DM) (OR: 2.12; 95% CI: 1.92, 3.21), low calf circumference (OR: 2.42; 95% CI: 2.29, 3.53), and low albumin (OR: 2.98; 95% CI: 2.43, 5.19) were independent risk factors for malnutrition. Their recommendation was for nutritional support to be enhanced for elderly COVID-19 patients with diabetes, low albumin, and low calf circumference due to their increased risk of becoming malnourished.
Second, a retrospective study that included 141 COVID-19 patients in the analysis explored the risk of adverse clinical outcomes among elderly patients (>65 y) by nutritional status [using validated nutrition risk screening tools for adults including the Nutrition Risk Screening 2002 (NRS-2002), Malnutrition Universal Screening Tool (MUST), Mini Nutrition Assessment - short form (MNA-sf), and Nutrition Risk Index (NRI)] in 1 hospital in China (48). They found that patients at risk of PEM had significantly longer hospital stay, poor appetite, more severe COVID-19 disease, and greater weight loss than patients not at nutritional risk using the NRS-2002, MNA-sf, and NRI-2002. They recommended routine screening of elderly COVID-19 patients for nutrition risk coupled with nutrition interventions to improve clinical outcomes.
Overweight, obesity, and DM
Landscape review
Obesity is a recognized risk factor for type 2 DM, and both have been associated with an increased burden of respiratory tract infections (RTIs) (49). A systematic analysis found a U-shaped relation between body size and risk of RTIs (50), and DM has also been found to increase susceptibility to, as well as severity of, respiratory infections in general (51). It is therefore not understood if they independently contribute to this increased morbidity and mortality risk (52).
Obesity is causally related to, and potentiates, cardiovascular and metabolic derangements such as hyperglycemia and DM (53). This reduces the protective cardiorespiratory reserve and potentiates the immune dysregulation that appears, at least in part, to mediate the progression to critical illness and organ failure in a proportion of patients with severe respiratory infections including COVID-19 (53, 54).
Several cellular mechanisms that may increase the susceptibility of DM patients to respiratory infections have also been described, including greater affinity of SARS-CoV-2 for cell binding and entry, reduced viral clearance (55), inhibited lymphocyte proliferative response to different kinds of stimuli (56), as well as impaired monocyte/macrophage and neutrophil functions (57).
Systematic review
The systematic literature search yielded a total of 1331 articles; 947 were taken to title and abstract screen after 384 duplicates were removed. A total of 115 articles were considered for full-text screening and 6 papers met the inclusion criteria for obesity and 12 for diabetes. The preprint server search for obesity and diabetes yielded a total of 154 articles. Thirty-four were considered for full-text screening and 29 of these met the inclusion criteria. Since included studies were numerous, and largely confirmed the same key messages of increased risk of severe disease progression, we did not extract all studies to Supplemental Table 2 but do refer to all included studies in the following narrative synthesis.
Obesity
Obesity is a frequent finding in hospitalized COVID-19 patients, with the prevalence varying between studies: 10% in China (58), 41.7% (59) and 47.5% (60) in the United States, and 75.8% in France (61). A study compared 44 ICU COVID-19 patients in France with a historical control group of 39 consecutive acute respiratory distress syndrome (ARDS) patients admitted to the ICU just before the COVID-19 crisis and found obesity to be the most frequent comorbidity among patients [n = 32 (73%) vs. n = 11 (28%) in controls; P < 0.001] (62).
Obesity is generally associated with poor COVID-19 outcomes and this has been confirmed in all studies included in this systematic review. The contributory mechanisms, as has been suggested by Zhang et al. (63), are aggravated inflammatory response, enhanced cardiac injury, and increased coagulation activity. Their study, which included 13 young patients who died of COVID‐19 and 40 matched survivors, found a higher BMI among deceased individuals (OR: 1.35; 95% CI: 1.08, 1.70) (63). Another study has suggested that increased angiotensin-converting enzyme 2 (ACE2) expression in the bronchial epithelium of obese individuals may contribute to poor outcome (64).
Obesity has been associated with a higher risk of severe COVID-19 disease in many populations and across age brackets. A study by Cai et al. (58) found that patients with a BMI (kg/m2) >28 had significantly higher odds of developing severe disease (aOR: 3.40; 95% CI: 1.40, 2.86). Klang et al. (65), in a study of 3406 patients, found poor outcomes in different age groups (young: <50 y; old: ≥50 y). For the younger population, a BMI >40 was independently associated with mortality (aOR: 5.1; 95% CI: 2.3, 11.1). For the older population, a BMI >40 was also independently associated with mortality but to a lesser extent (aOR: 1.6; 95% CI: 1.2, 2.3). In a cohort of 46 pregnant women, 15 had severe COVID-19, with the majority being either overweight or obese (80%) (66). Another study also found that obesity (BMI >30) was associated with increased risk of ICU admission or death [risk ratio (RR): 1.58; P = 0.002], whereas being underweight was not (RR: 1.04; P = 0.892) (67).
Obese patients were more likely to require invasive mechanical ventilation, with severe obesity (BMI ≥35) found to be associated with ICU admission (aOR: 5.39; 95% CI: 1.13, 25.64) (60). Similar findings of adverse outcomes were found in other studies (61, 68). Hur et al. (69) found that obese patients with COVID-19 had a decreased chance of extubation compared with nonobese patients (HR for extubation: 0.53; 95% CI: 0.32, 0.90 for patients with a BMI of 30 to 39.99; and HR: 0.40; 95% CI: 0.19, 0.82 for those with a BMI of ≥40). Palaiodimos et al. (70) also found that severe obesity (i.e., BMI ≥35 compared with BMI = 25–34) was independently associated with higher in-hospital mortality (OR: 3.78; 95% CI: 1.45, 9.83) as well as a significant predictor for intubation (OR: 3.87; 95% CI: 1.47, 10.18).
Diabetes mellitus
Diabetes is a common comorbidity among COVID-19 patients and has been associated with poor outcomes in all included studies, with the exception of Cariou et al. (71) (see below). The frequency of diabetes among hospitalized patients was investigated in many studies, ranging from 3.8% in Iran (72), 5.5–35.7% in various studies from China (2, 73–81), 19.9% in the UK Biobank (82), and 33.8% in the United States (59).
Hyperglycemia in those with and without a history of diabetes may indicate a poor prognosis in COVID-19 (83). A study by Guo et al. (84) suggests that diabetes should be considered as a risk factor for a rapid progression and poor prognosis of COVID-19. The utility of diabetes screening after admission has been suggested by Wang et al. (85) who found high HbA1c concentration at admission to be associated with inflammation, hypercoagulability, and low blood oxygen saturation in COVID-19 patients. This severe inflammatory response was also reported by other studies (84, 86). The mechanism, although not completely understood, may be through metabolic derangement such as that leading to ketosis and ketoacidosis. A study found that ketosis and ketoacidosis disproportionately affected diabetic patients compared with those without diabetes (81).
Patients with diabetes are found to be more likely to develop severe or critical disease conditions with more complications, and had higher incidence rates of antibiotic therapy, noninvasive and invasive mechanical ventilation, and death (11.1% vs. 4.1%) (87). Chen et al. (88) found that diabetes and other factors such as increasing age, male sex, and hypertension delay viral clearance, thereby leading to a poor prognosis. These risk factors are similar to those found in other studies (89–91). COVID-19 patients with diabetes were more likely to develop severe or critical disease with more complications at presentation, and had higher incidence rates of antibiotic therapy, noninvasive and invasive mechanical ventilation, and death (11.1% vs. 4.1%) (92). In another study by Wu et al. (93), the prevalence of diabetes among those with COVID-19–related ARDS was significantly higher than in those without ARDS (difference: 13.9%; 95% CI: 3.6%, 24.2%). Bode et al. (94) found in patients with diabetes and/or hyperglycemia compared with those without these conditions, a longer median length of stay (LOS) in hospital (5.7 vs. 4.3 days, P < 0.001) and higher mortality rate (28.8% vs. 6.2%, P < 0.001). This mortality rate was similar to that found in another study (27.7%) (85). Shi et al. (95) found a higher proportion of ICU admission (17.6% vs. 7.8%, P < 0.01) and more fatal cases (20.3% vs. 10.5%, P < 0.017) were identified in COVID-19 patients with diabetes than in the matched patients. A study by Chang et al. (96) found that patients with diabetes were more likely to progress to severe disease compared with those without (OR: 64.1; 95% CI: 4.6, 895.5). The findings were similar to those of Huang et al. (97) (OR: 4.3; 95% CI: 1.1, 17.7). In Iran, Rastad et al. (98) found that diabetes alone or in association with other comorbidities was associated with increased risk of death [OR (95% CI): 1.69 (1.05, 2.74) and 1.62 (1.14, 2.30), respectively]. In a cohort of 28 diabetic patients, half required ICU admission (99).
A study by Li et al. (100) suggests that COVID-19 patients with newly diagnosed diabetes have a higher mortality risk of all-cause mortality (multivariable-adjusted HR: 9.42; 95% CI: 2.18, 40.7), but this was not statistically significant compared with patients with normal glucose (HR: 1.00), hyperglycemia (HR: 3.29; 95% CI: 0.65, 16.6), and known diabetes (HR: 4.63; 95% CI: 1.02, 21.0). Increased mortality for patients with diabetes and COVID-19 has been linked to older age (aOR: 1.09; 95% CI: 1.04, 1.15 per year increase), elevated C-reactive protein (CRP; aOR: 1.12; 95% CI: 1.00, 1.24), and insulin usage (aOR: 3.58; 95% CI: 1.37, 9.35) (101). The latter finding on insulin use is in contrast to findings by another study which showed that patients with hyperglycemia already treated with insulin infusion at admission had a lower risk of severe disease than patients without insulin infusion (102). Metformin use, however, was associated with better outcomes in diabetics compared with those not receiving it (103). These findings were complemented by Zhu et al. (104) who found that well-controlled blood glucose (glycemic variability within 3.9 to 10.0 mmol/L) was associated with markedly lower mortality compared with individuals with poorly controlled blood glucose (upper limit of glycemic variability exceeding 10.0 mmol/L; adjusted HR: 0.14) during hospitalization.
Only 1 study did not find diabetes to be associated with poor COVID-19 outcomes. Cariou et al. (71) found that diabetes, HbA1c, diabetic complications, and glucose-lowering therapies were not associated with disease severity (tracheal intubation for mechanical ventilation and/or death) within 7 d of admission.
Anemia
Landscape review
Anemia is a condition where an individual's hemoglobin concentration falls below the accepted lower threshold specific for their age, sex, and pregnancy status. Anemia remains highly prevalent worldwide, especially in low-income countries, and particularly in South Asia and sub-Saharan Africa. The most common cause of anemia worldwide is iron deficiency, which is caused by inadequate nutritional iron intake, impaired iron absorption, increased iron utilization (for example, during pregnancy or during rapid child growth), and blood losses (for example, menstrual blood losses, gastrointestinal bleeding, and blood donation). Anemia is thus most common in preschool children, women of reproductive age, and during pregnancy (105).
Beyond iron deficiency, there are many other causes of anemia. During inflammation, iron may be withheld from the plasma through elevated hepcidin concentrations (functional iron deficiency); coupled with impairments in erythropoiesis and reduced RBC survival, this can result in anemia of inflammation, which is common in patients with medical illnesses (such as cancer, infection, and autoimmune conditions) (106). Functional iron deficiency may also be an important component of the overall burden of anemia in low-income countries where exposure to endemic infections is intense.
Other acquired causes of anemia include hemolytic anemias. These include autoimmune hemolytic anemias, caused by autoimmune destruction of RBCs (usually provoked by viral infections, some bacterial infections, underlying lymphoproliferative disorders, and medications) (107). Other causes of hemolytic anemia include microangiopathic hemolysis (which can be due to many causes including congenital, infections, autoimmune conditions, cancer, pregnancy complications, and medications). Bone marrow failure (aplastic anemia, or replacement of the bone marrow by malignancy) can also cause anemia. In the tropics, a major cause of childhood anemia is malaria; malaria anemia has elements of hemolysis, marrow failure, and functional iron deficiency. Other important causes of anemia include genetic disorders of hemoglobin, including ɑ-thalassemia, B-thalassemia, and sickle cell disease.
Like all infections, acute viral infection can promote an innate immune response, elevation in hepcidin, and hence functional iron deficiency and anemia of inflammation. Viral infections can also cause bone marrow failure. For example, parvovirus B19 infection is frequently asymptomatic, or may cause a mild febrile illness with a rash (“slapped cheek disease”). However, in immunocompromised individuals and in individuals with chronic erythroid overactivity (e.g., hemolytic disease, sickle cell disease) it can cause cessation of erythropoiesis, resulting in a transient aplastic crisis with severe anemia. Parvovirus B19 during pregnancy can infect the fetus, causing failure of fetal erythropoiesis and severe fetal anemia, which can result in hydrops fetalis and fetal death (108).
Systematic review
From the PubMed and EMBASE database searches, after de-duplication, 407 articles were assessed at the title/abstract stage. Of those that mentioned anemia we only considered those addressing potential nutritional causes of anemia for formal data extraction, due to the scope of this review. However, several other types of anemia featured in the initial screen, which we briefly summarize here. For example, 2 articles described the management of pernicious anemia in the case of disrupted vitamin B-12 treatment (109, 110). Two case series have provided preliminary information on B-thalassemia major. A small series of 11 patients with B-thalassemia in Italy infected with COVID-19 all experienced mild to moderate disease and all survived, even despite the presence of comorbidities associated with iron overload (111). A nationwide study in Iran identified a lower incidence of diagnosed COVID-19 among patients with thalassemia compared with the general population (8.7 per 10,000 in the thalassemia population compared with 11.0 per 10,000 in the general population), although patients with thalassemia may have been sheltering in place. Patients with thalassemia experienced a higher mortality rate (26.6%) compared with the general population (6.3%); patients who did not survive had higher risks of comorbidities including diabetes, hypertension, and heart disease, although splenectomy was not a risk factor for mortality in this group (112). A case report identified combined autoimmune anemia (destruction of RBCs by autoantibodies) and thrombocytopenia (destruction of platelets by autoantibodies) (collectively termed “Evan's syndrome”) in a patient with COVID-19 (113). A case series from Belgian and French hospitals identified the onset of acquired warm and cold autoimmune hemolytic anemia associated with a positive direct antiglobulin test in 7 patients; 4 of the patients had a previous or new diagnosis of an indolent B-cell malignancy, and viral infection may have triggered the onset of hemolysis (114). These cases were each successfully treated using therapies including intravenous immunoglobulin, steroids, and even rituximab, and all patients across these case series survived. There have been further case reports describing the association between autoimmune hemolytic anemia and COVID-19 (115, 116).
Although hemoglobin measurement has not been included in the core-outcome dataset proposed by WHO (117), several studies suggest that anemia may be a clinical feature of COVID-19. For example, initial reports from Wuhan describing clinical features of COVID-19 pneumonia identified anemia in up to 50% of patients who mostly appeared to have severe disease (35). A subsequent report from Wuhan identified anemia in 15% of patients with COVID-19, with anemia more common among nonsurvivors (2). Similar hemoglobin concentrations have been reported in other COVID-19 cohorts (118) and several studies include anemia as a covariate in descriptive statistics. As in other medical conditions, anemia appears to be associated with poorer prognosis, perhaps as a biomarker for more severe inflammation (119, 120).
After the title and abstract review, 9 articles were taken to full screen. Six articles did not address nutritional causes of anemia. One paper by Cavezzi et al. (121) was a review on the possible pathophysiological pathways by which SARS-CoV-2 may cause both hemoglobin dysfunction and hypoxia (through hemolysis and forming complexes with heme) and tissue iron overload (through mimicking the action of hepcidin).
Ultimately, we found 2 eligible studies for formal inclusion. The first was a case report of a patient testing positive for COVID-19 alongside several comorbidities including severe iron-deficiency anemia (IDA) (122). He was successfully treated with antiviral treatment alongside recombinant human erythropoietin (rhEPO), leading the authors to propose further testing of the effectiveness of rhEPO in anemic COVID-19 patients. The second study was a retrospective analysis of 259 patients hospitalized with COVID-19 in Austria (123). The authors distinguished between those patients presenting with anemia of inflammation at admission and those with IDA. Compared with patients with no iron deficiency, having IDA was associated with a longer hospital stay but was not associated with increased mortality, risk of ICU admission, or of mechanical ventilator use. However, when considering purely anemic versus nonanemic patients, the anemic patients had a higher risk of death (OR: 3.73; 95% CI: 1.74, 8.00). Of these anemic patients, the majority (68.8%) had anemia of inflammation, which the authors describe could be linked to comorbidities, or to the advanced inflammation associated with COVID-19, or both. Collectively, these limited data indicate that anemia is an adverse prognostic indicator in severe COVID-19.
From the preprint server screen, of the 122 articles returned, 4 were taken to full-screen review and none were eligible.
Iron
Landscape review
Approximately 2% of human genes encode proteins that interact with iron, and ∼6.5% of enzymes depend on iron (124). Viruses co-opt host cellular processes to replicate, so it is unsurprising that viral replication utilizes proteins that are iron dependent (125), such as ribonucleotide reductase (the key enzyme involved in nucleotide biosynthesis). Consequently, viral pathogenesis could be influenced by cellular iron status. However, several features of host responses to viral infection could also be affected by iron—for example, macrophage polarization and lymphocyte proliferation, potentially influencing either disease susceptibility or course.
Iron deficiency is the most prevalent micronutrient deficiency worldwide, most prominently causing anemia. The major burden of iron deficiency is borne by young children and women of reproductive age—groups at lower risk of COVID-19 mortality (126)—and pregnant women [for whom patterns of COVID-19 hospitalization risk appear similar to the general population (127)] (128). Functional iron deficiency, where iron is present but sequestered and unavailable in circulation, occurs during many chronic conditions, including obesity (129), a known COVID-19 risk factor (126).
Effects of iron status on infection susceptibility are not fully defined, and likely vary according to age, setting (e.g., malarial or nonmalarial), and type of infection (130, 131), meaning that caution should be used in making extrapolations to viral infections in general and specifically to COVID-19. Iron deficiency protects against certain microbial infections including malaria (132), and iron supplementation exacerbates malaria risk in children in malaria-endemic areas in the absence of malaria-control measures (133, 134). Excess iron increases siderophilic bacterial infection risk (135), and elevated iron indices predict mortality during HIV-1 infection, even after adjustment for CD4 count and inflammation (136). Nonmalarial infections, including gastrointestinal and respiratory infections, are also reported in several trials of childhood iron supplementation (134). One large intervention trial in Pakistan reported increased signs of respiratory infection in children administered iron (137), although other smaller trials have reported contrasting effects of iron supplementation on incidence of RTIs in children (130, 138–140). However, high-quality evidence on interactions between iron status or interventions and specific respiratory viral infections in humans is lacking.
Although precedents from other human viral infections are limited, iron could, in principle, affect several aspects of the host–SARS-CoV-2 interaction, as follows:
As discussed above, viral replication, in general terms, co-opts several iron-dependent host cellular processes (125).
Impaired lung function and hypoxia are key features of severe COVID-19 disease, and iron deficiency exaggerates the pulmonary response to hypoxic stress (141, 142).
Iron concentrations may influence macrophage polarization and cytokine production (143), potentially influencing COVID-19–related inflammatory phenotypes.
In addition, a rare mutation of Transferrin Receptor gene(TFRC) (encoding the transferrin receptor) that disables cellular iron uptake causes severe combined immunodeficiency in children (144). Nutritional iron deficiency or pre-existing functional iron deficiency have also been linked to immune impairment (145). Moreover, during many infections, IL-6–mediated stimulation of the iron regulatory hormone hepcidin, as part of the hepatic acute-phase response, causes macrophage iron sequestration and acute reduction in serum iron concentration (131). Common respiratory infections and fevers are associated with hepcidin upregulation in African children (146). A key feature of COVID-19 severe/critical disease is excessive production of inflammatory cytokines, notably IL-6, and accordingly, raised hepcidin has been reported in hospitalized COVID-19 patients (147, 148). Consistent with involvement of hepcidin activity, extreme hypoferremia has been reported in several studies in severe COVID-19 patients, with serum iron concentration shown to be highly predictive of disease severity (147, 149–151). A further retrospective analysis (also described in the section on anemia) also reported perturbed markers of iron homeostasis in hospitalized COVID-19 patients, with functional iron deficiency classified in ∼80% of patients at admission (123). Whether or not this functional iron deficiency limits the development of the adaptive response [analogous to the effect of the TFRC mutation (144)] in the context of SARS-CoV-2 infection remains to be determined.
Systematic review
In addition to “iron,” our systematic search involved terms related to common biomarkers of iron status and iron handling, including “ferritin,” “transferrin,” “Tsat” (transferrin saturation), and “hepcidin.” The systematic screen of PubMed and EMBASE returned 110 papers after removing duplicates; 45 were taken to full-text screen, all of which were excluded as none examined the influence of iron deficiency or interventions on CoV susceptibility or disease course.
A further 10 distinct studies were identified through the preprint server screen; again, all were excluded for the same reasons. The combined screen of PubMed/EMBASE and preprint servers did identify 32 original studies or meta-analyses reporting effects of CoV infection on iron-related markers, most prominently the iron storage protein ferritin. However, in the context of typically extreme COVID-19–associated inflammation, serum ferritin is not useful as a marker of iron status, yet it does show relevance as an indicator of disease severity and could potentially reflect iron dysregulation besides inflammation (see Supplemental Material 4).
Vitamin A
Landscape review
Vitamin A has an established role in supporting immune function and protecting against viral infections. Evidence from animal studies shows clear effects of serum retinol concentration on mucosal immune function and intestinal lymphocyte action, and protection against viral infections of the respiratory and intestinal tracts (152–156).
The effectiveness of viral vaccines is compromised by low serum vitamin A through the suppression of IgG1 (155, 157) and inflammatory responses (156). Vitamin A also modulates other immune components through its action on dendritic and natural killer cells (158). It is essential in maintaining epithelial tissue integrity (159), which is severely damaged in viral infections such as measles (160). Recent systematic reviews conclude that vitamin A supplementation in children is associated with a reduction in all-cause mortality, and with reductions in the incidence of measles and diarrhea, but there is little evidence to support a beneficial effect on respiratory infections (161, 162).
Serious COVID-19 caused by SARS-CoV-2 infection has some manifestations similar to measles, including fever, cough, and pneumonia (although it is important to note that the severe lung pathology of COVID-19 has a distinct pathophysiology from other viral pneumonias) (163). People with underlying chronic diseases and impaired immunity are also at high risk for both COVID-19 (164, 165) and measles (166).
Vitamin A is recommended by the WHO as part of the standard treatment package for all children with acute measles (167). The COVID-19 pandemic has likely increased measles mortality—20 countries have suspended measles vaccination and vitamin A supplementation campaigns as health care workers focus attention on COVID-19, leading to a surge in measles infections and mortality, particularly in low-income settings such as the Democratic Republic of Congo where measles has killed >6500 children and is still spreading (168). Vitamin A is recommended mainly to reduce mortality (169) and risk of complications from pneumonia, croup, and ocular problems (170) by correcting the low or depleted retinol concentrations resulting from measles infection. The treatment regimen consists of the administration of high-dose vitamin A on 2 consecutive days. Children with evidence of deficiency (ocular symptoms) receive a repeated dose at 2 to 4 wk (167). A Cochrane systematic review of 8 trials (171) and another systematic review of 6 trials (172) showed no overall reduction in mortality with vitamin A treatment of measles. However, when stratified by vitamin A treatment dose, administering 2 doses (on consecutive days) reduced measles mortality significantly in both meta-analyses [RR: 0.38; 95% CI: 0.18, 0.81 (171) and RR: 0.21; 95% CI: 0.07, 0.66 (172)], and therefore forms the basis for the recommended regimen of vitamin A treatment of measles.
A recent nonrandomized study observed a reduction in mortality among 330 Ebola virus patients who received vitamin A supplementation compared with 94 patients who, due to supply problems, did not receive vitamin A (RR: 0.77; 95% CI: 0.59, 0.99) (173). This trial is limited by significant risk of confounding.
Systematic review
The systematic search of PubMed and EMBASE databases yielded 44 articles. After removal of duplicates (n = 5) and those not meeting inclusion criteria (n = 36), 3 systematic review articles were considered for full-text extraction to examine reference lists for potentially eligible articles. No papers were included from examining reference lists. Our preprint search on vitamin A and COVID-19 yielded 1 potential paper that did not meet the inclusion criteria.
Vitamin C
Landscape review
Vitamin C (ascorbic acid), synthesized by all mammals except for humans and guinea pigs, supports diverse aspects of immune function by strengthening epithelial barriers, enhancing the function of adaptive and innate immune cells, promoting cell migration to infection sites, and participating in macrophage microbial killing (174).
Unfortunately, vitamin C has a particularly checkered history in relation to viral infections. Double Nobel Laureate Linus Pauling blighted the end of his career by promoting mega-doses of vitamin C as a cure for common colds (175) and cancers (176) despite an absence of any robust evidence. Even today it is difficult to interpret the scientific and allied literature without encountering partisan opinions, and there remains a widespread popular view that vitamin C is effective. Pauling's favored mechanism of action was through its antioxidant effects. His belief in, and self-medication with, mega-doses of vitamin C runs contrary to the fact that there is a renal threshold leading to diminished retention and tissue saturation at oral intakes >200 mg/d (177, 178). Intravenous infusion of large doses of vitamin C can elevate leukocyte levels much further, but the putative mechanism of action against cancers (as yet unproven in humans) is proposed to be through its pro-oxidant effects of generating hydrogen peroxide at large doses (179). This is pertinent to the ongoing therapeutic trials in COVID-19 patients (Supplemental Table 3 and Supplemental Material 3).
With regard to the common cold, the most recent Cochrane review (180) summarized 24 trials with 10,708 participants and found no evidence in the general population that regular consumption of vitamin C at ≥200 mg/d reduced the incidence of colds (RR: 0.97; 95% CI: 0.94, 1.00). In contrast, 5 trials with 598 marathon runners, skiers, and soldiers on subarctic exercises yielded a combined RR of 0.48 (95% CI: 0.35, 0.64). The possibility that free radicals generated by extreme exercise are quenched by vitamin C provides a plausible explanation for this heterogeneity of results. Thirty-one trials covering 9745 episodes showed that taking regular vitamin C shortened the duration of symptoms in adults by 8% (95% CI: 3%, 12%) and in children by 14% (95% CI: 7%, 21%). Seven trials of therapeutic use of vitamin C administered at the start of an infection in 3249 episodes revealed no evidence of altered duration or severity. A single additional randomized controlled trial (RCT) in 1444 Korean soldiers has been published since the meta-analysis and reported a marginally significant reduction in incidence of colds among soldiers receiving 6000 mg vitamin C/d orally (RR: 0.80; 95% CI: 0.64, 0.99) (181).
A Cochrane meta-analysis of the potential effect of vitamin C on the prevention and treatment of pneumonia has been updated very recently (182). The results from 7 studies (5 RCTs and 2 quasi-RCTs) involving 2774 participants (children, adults, army personnel) receiving doses ranging from 125 to 2000 mg vitamin C/d were judged to provide very-low-quality evidence with respect to both prevention and treatment; hence, no conclusions can be securely drawn.
For critically ill patients, the prior evidence for efficacy of low- to moderate-dose vitamin C (alone or as a cocktail with other antioxidants) is weak. A recent systematic review and meta-analysis of 11 RCTs found no evidence of benefit for mortality (9 trials) or any secondary outcomes (183). There was a nonsignificant tendency towards mortality reduction in subgroup analysis confined to intravenous administration of high-dose vitamin C (183). The meta-analysis was dominated by a large and robust multicenter trial of 1223 ICU patients with half randomly assigned to antioxidants including 1500 mg enteral vitamin C/d (with or without glutamine), which reported no effect on survival (primary outcome) or on any secondary outcomes (184).
The evidence from prior trials of high-dose intravenous vitamin C in pneumonia and ARDS-type conditions is also of low quality and was either not summarized, summarized poorly, or in a biased manner in most trial registrations. One reason for the high interest in intravenous vitamin C can be traced to a single-center, uncontrolled, observational study of 94 sepsis patients that reported a 5-fold reduction in mortality when vitamin C and thiamin were combined with hydrocortisone (185). A follow-up multicenter RCT of the same regimen in sepsis patients [the Vitamin C, Hydrocortisone and Thiamine in Patients With Septic Shock (VITAMINS) trial] has very recently reported no benefit in any outcome (186). The Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure (CITRIS-ALI) trial in 7 US ICUs randomly assigned 167 patients with sepsis or ARDS to 200 mg ⸱ kg–1 ⸱ d–1 intravenous vitamin C or placebo for 4 d. There was no difference in the primary outcome of Sequential Organ Failure Assessment score or in the secondary outcomes of CRP or thrombomodulin (187). In un-prespecified exploratory analysis not adjusted for multiple testing there was some evidence of enhanced survival to 28 d.
Systematic review
From a total of 54 papers returned, 4 papers were identified for full screen. Most papers were commentaries or nonsystematic reviews. In no case were there any substantive new data on clinical outcomes. Two papers used a systems biology bioinformatic approach to explore mechanisms through which vitamin C might be active (188, 189).
The search of preprint servers yielded 13 relevant papers, all of which were accessed for full review; most were commentaries or editorials. Two systematic reviews concluded that the evidence that vitamin C was likely to benefit COVID-19 patients was weak or absent (190, 191).
Vitamin D
Landscape review
The widespread distribution of the vitamin D receptor (VDR) and vitamin D–metabolizing enzymes in cells and tissues, including those of the immune system, is evidence of a wide role for vitamin D in health. The role of vitamin D in the immune system has been reviewed recently (192, 193), including in relation to COVID-19 (194–197), and spans aspects of the immune system including the maintenance of barrier defenses, innate immune response, and an immunoregulatory role in antigen presentation and the adaptive immune responses (192, 198, 199). As part of the innate immune response, antimicrobial peptides play an important role in the first line of defense against infections, including in respiratory infections (200). Vitamin D is required for the production of antimicrobial peptides such as cathelicidins in macrophages and in the epithelial cells of the airways (199), and in an RCT vitamin D supplementation was shown to increase levels of antimicrobial activity in airway surface liquid (201). Vitamin D can also reduce the production of proinflammatory T-helper type 1 (Th1) cytokines (192, 194) that are implicated in the cytokine storm associated with more serious COVID-19 clinical outcomes, such as ARDS and multiple-organ failure (194, 202). The binding site for SARS-CoV-2 is ACE2 (203). Studies have shown that higher concentrations of ACE2 can reduce acute lung injury from infection and that vitamin D can modulate the expression of enzymes balancing the expression of ACE2 and ACE (reviewed in 204–206), providing a mechanism for a potential role for vitamin D in the prevention and progression of COVID-19. Plasma 25-hydroxyvitamin D [25(OH)D] concentration may decrease as part of the acute-phase response, so data from observational studies in acutely ill patients should be interpreted with a degree of caution (207–209).
Vitamin D deficiency (VDD) is prevalent across all continents, not only those at more extreme latitudes (210–213), and certain groups are at particular risk, including the elderly (especially those in care homes), ethnic minorities (living at higher latitudes), and the obese. There is a strong overlap between groups at risk of COVID-19 morbidity and VDD (ethnic minorities, obese, institutionalized elderly). Groups identified at higher risk of serious illness with COVID-19 (214) are also at risk for VDD, not only from low circulating 25(OH)D per se but also lower circulating vitamin D–binding protein (DBP) (e.g., in patients with renal or hepatic disease) (215).
Human data from both observational studies and intervention trials support a role for vitamin D in the prevention of respiratory infections. Meta-analyses of observational data have found associations between low vitamin D status and both risk of acute respiratory infection (216, 217) and severity of symptoms (217). A meta-analysis (218, 219) of individual participant data found a reduced risk of acute respiratory infection (aOR: 0.88; 95% CI: 0.81, 0.96), particularly in individuals receiving regular (weekly or daily) vitamin D supplementation and in those with baseline 25(OH)D <25 nmol/L (aOR: 0.30; 95% CI: 0.17, 0.53). More recent trials of respiratory infection prevention in children and adults have reported both a beneficial (220–222) and no effect (223–226) of vitamin D supplementation. The findings from a recently published large trial (n = 5110) in New Zealand found no effect of a bolus dose of vitamin D on the incidence of acute respiratory infection (227). The results of another large trial in 25,871 men (≥50 y) and women (≥55 y) of vitamin D and/or omega-3 fatty acids found no reduction in all-cause mortality, although results for respiratory conditions are yet to be published (228, 229).
Genetic polymorphisms within the genes for DBP, vitamin D–metabolizing enzymes, and the VDR may affect vitamin D transport, metabolism, and action. Polymorphisms within the DBP have a small effect on DBP and 25(OH)D concentration (230) and metabolism (231) as well as response to supplementation (232, 233). VDR polymorphisms may impact the risk and progression of disease, although results are mixed (234, 235). A recent meta-analysis in relation to enveloped-virus infection (a group that includes coronaviruses) found significant associations between certain VDR polymorphisms and susceptibility to respiratory syncytial virus (236).
Systematic review
From a total of 59 papers returned from PubMed and EMBASE searches, 9 were taken to full-text screen and 2 papers (205, 237) were identified for full screen. D'Avolio et al. (237) found that mean 25(OH)D concentration measured a median of 3 d after a COVID-19 polymerase chain reaction (PCR) test was lower in 27 PCR-positive patients compared with 80 PCR-negative patients (28 vs. 62 nmol/L; P = 0.004). In an ecological analysis, Ilie et al. (205) observed an inverse correlation between both COVID-19 case numbers and mortality figures against published population mean 25(OH)D concentrations (correlation coefficients: −0.44; P = 0.05 in both cases) across 20 European countries.
Screening of preprint servers revealed a total of 38 studies after exclusion of those previously identified from the PubMed/EMBASE search. Of these, 6 were taken to full review.
These 6 preprints described observational studies and investigated 25(OH)D concentration in COVID-19–positive cases. Three studies had <20 participants with both COVID-19 and vitamin D test results and no control group; 2 reports measured 25(OH)D concentration in hospital inpatients: Cuñat et al. (238) reported 13 of 17 ICU patients had 25(OH)D concentration <31 nmol/L, while Lau et al. (239) found that 11 of 13 ICU patients had 25(OH)D <75 nmol/L compared with 4 of 7 inpatients, although there was no significant difference in mean 25(OH)D concentration between groups. A third report from Indonesia in 10 hospitalized COVID-19–positive patients found that 9 of 10 had a 25(OH)D concentration <50 nmol/L and 4 of 10 had a concentration <25 nmol/L (240).
A larger Belgian study (241) described lower 25(OH)D concentrations and greater prevalence of VDD (defined as <50 nmol/L) in a group of hospitalized COVID-19 patients (n = 186) compared with a group of 2717 patients of similar age distribution sampled 1 y earlier (47 nmol/L and 54 nmol/L, P = 0.0016; 59% vs. 45%, P = 0.0005). However, when stratified by sex, the significant difference in 25(OH)D concentration and VDD only remained in males. In a study of 499 hospitalized patients or health care workers in the United States (Chicago) with a COVID-19 test result and vitamin D status measurement (in the past year) there was no difference between COVID-19–positive and –negative cases (P = 0.11) (242). An expanded analysis that sought to categorize the vitamin D status of an individual based on 1) their vitamin D status test result and 2) vitamin D treatment regimen in the previous 2 y found that participants who were predicted to be “vitamin D deficient” had an increased risk (relative risk: 1.77; P < 0.02) of testing positive for COVID-19 compared with participants with a predicted vitamin D status of “likely sufficient” (242). In a different approach, Hastie et al. (243) used baseline UK Biobank data from 348,598 participants collected 10 to 14 y ago, of whom 449 had a positive COVID-19 test in between March and April 2020. After inclusion of other factors such as season, ethnicity, and other health conditions there was no significant association between 25(OH)D and COVID-19 infection (OR: 1.00; 95% CI: 1.00, 1.01).
Two additional studies were identified from reference screening. A study from the Philippines found that, in 212 COVID-19 hospitalized patients, vitamin D status was associated with clinical outcomes, such that for each SD increase in 25(OH)D concentration, the odds of having a mild clinical outcome rather than a severe or critical outcome were 7.94 and 19.61, respectively (CI not reported) (244). A study of 780 COVID-19–positive hospitalized patients found that, after correction for age, sex, and comorbidity, the OR of death was 10.2 (P < 0.0001; 95% CI not reported) in cases with VDD (defined as <50 nmol/L) compared with “normal” vitamin D status (defined as 75 nmol/L) (245). However, this study has since been discredited (246).
Vitamin E
Landscape review
Vitamin E is the collective term for 4 tocopherols and 4 tocotrienols (247). Human dietary requirements are based on α-tocopherol, but there is increasing evidence of biological functions for the related compounds, including in relation to immunity (248). Vegetable oils and nuts are rich sources of vitamin E and hence human deficiency is rare; thus, the interest in vitamin E and immunity is frequently related to the question of whether supplementary vitamin E might improve immunity in at-risk subgroups such as smokers or the elderly.
The main biological role of vitamin E is as an antioxidant that quenches oxidative cascades, especially of membrane PUFAs in which it is highly soluble and hence penetrant (247). Animal, human, and cell culture studies have examined the role of supplemental vitamin E on a wide range of innate and adaptive immune cells. Numerous possible mechanisms of action are postulated [maintenance of cell membrane integrity, increased (and decreased) cell proliferation, increased IL-2 and decreased IL-6 production, enhanced immunoglobulin production, etc.] but few confirmatory studies are available (247, 248).
Due to their dual and overlapping roles in antioxidant pathways there are close parallels between selenium and vitamin E with regard to immune function, roles that have been best studied in regard to viral infections. In the section on selenium, we describe the work by Beck and her team demonstrating that the virulence of Coxsackie B3 and influenza H3N2 viruses is enhanced in selenium-deficient hosts resulting from systematic viral mutations. Beck's team have used the same mouse protocol with vitamin E–deficient mice and demonstrated that the viral mutation and enhanced pathogenicity is recapitulated with either or both selenium and vitamin E deficiency (249–252), an effect that is enhanced in iron-loaded animals due to the increased oxidant stress.
The evidence for interactions between vitamin E status or supplementation and viral infections in humans is sparse and there are no available meta-analyses as a consequence. A recent (nonsystematic) review has tabulated summary outputs from 8 studies of human infections of which 5 relate to respiratory infections (247). Several of the studies involved post hoc subgroup analysis of smokers and hence have questionable validity and poor generalizability (253, 254). The best study was a 2 × 2 factorial design of multivitamin-mineral or vitamin E supplementation in free-living adults aged >60 y old (255). In 652 participants with 1024 respiratory infections there was no benefit of either regime in reducing incidence, and some evidence that vitamin E made the infections more serious (255).
Systematic review
From a total of 39 papers returned, 9 duplicates were removed and 30 titles and abstracts screened. Six review papers were considered for full-text screen and to check reference lists for possible papers. None had substantive novel relevant information.
The search of preprint servers yielded 4 papers, of which 2 were accessed for full review; these were both general reviews and lacked substantive new information in relation to coronaviruses or severe ARDS (190, 191).
PUFAs
Landscape review
Long-chain (LC) PUFAs are classified into 2 series (ω-3 or ω-6) according to the position of their double bonds. Both series have extensive immunomodulatory activity, with ω-3 PUFAs tending to be anti-inflammatory and ω-6 PUFAs tending to be proinflammatory. ω-3 Fatty acids are abundant in fish oils and ω-6 in vegetable oils. The ω-3 and ω-6 synthetic pathways compete for the same elongase, desaturase, and ω-oxidation enzymes, and hence the ratio of ω-3 to ω-6 series can be especially crucial. Comprehensive reviews of the immunomodulatory effects of PUFAs are available elsewhere (256–261).
In brief, LC PUFAs exert immunomodulatory effects through a number of generic mechanisms. EPA (ω-3) and arachidonic acid (AA, 20:4n−6; ω-6) are precursors of eicosanoids; AA generates inflammatory-type eicosanoids and EPA-derived eicosanoids tend to be anti-inflammatory (258, 260), a property that may be crucial to COVID-19 disease (see below) (257). When incorporated into cell membranes LC PUFAs can beneficially modulate the activity of T cells and other components of cellular immunity (260). They also modulate cytokine responses, with ω-3 fatty acids tending to enhance IL-10 and suppress IL-6 production as well as inhibiting NF-κB (260). More recently, PUFAs have been shown to play a crucial role in the production and action of specialized pro-resolution mediators (SPMs) that play a crucial role in ending the inflammatory cycle and thereby avoiding an excessive inflammatory response and cytokine storm. EPA and DHA (ω-3) are precursors for resolvins and DHA is the precursor for protectins and maresins (257).
Despite the wealth of biochemical evidence for key roles of ω-3 PUFAs in anti-inflammatory pathways, the evidence for clear roles in human health is less robust. Meta-analyses with a range of health outcomes have failed to provide evidence for efficacy and in those where efficacy seems secure it is usually only achieved at high doses.
There have been several meta-analyses of the effects of ω-3 fatty acids from fish oils in critically ill patients. Due to differences in selection criteria and outcome measures, the outcomes are varied. In 2018, Koekkoek et al. (262) reviewed 24 RCTs of fish-oil–containing EN involving 3574 patients. There was no significant benefit on the primary outcome of 28-d, ICU, or hospital mortality. However, fish-oil administration significantly reduced LOS in the ICU and duration of ventilation. In a pre-planned subgroup analysis there was a reduction in 28-d mortality (OR: 0.69; 95% CI: 0.54, 0.89), ICU LOS (−3.71 d; 95% CI: −5.40, −2.02 d), and duration of ventilation (−3.61 d; 95% CI: −5.91, −1.32 d) in patients with ARDS. In 2019, Langlois et al. (263) conducted a meta-analysis of the RCTs of ω-3 PUFA administration on gas exchange [ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2-to-FiO2)] and clinical outcomes in 12 trials involving 1280 ARDS patients. There was a significant early increase in PaO2-to-FiO2 that diminished but remained significant at days 4–7. There were nonsignificant trends towards reduced ICU LOS and duration of ventilation but not improvement in mortality, LOS in hospital, or infectious complications. Also, in 2019, Dushianthan et al. (264) meta-analyzed 10 RCTs of enteral ω-3 supplementation in a total of 1015 ARDS patients. There was no benefit on all-cause mortality (OR: 0.79; 95% CI: 0.59, 1.07) or any of the secondary outcomes. All of these meta-analyses encountered studies with high risk of bias and poor-quality evidence.
Notwithstanding rather weak evidence of benefit in critically ill patients including those requiring ventilation there have been calls for clinical trials of intravenous high-dose, fish-oil lipid emulsions (FOLEs) in hospitalized COVID-19 patients (265, 266). The first of these recommends use in patients at special risk of hyperinflammatory outcomes (e.g., the obese) (265). In the second call, Torrinhas et al. (266) emphasized the need to tailor dosage to body weight, recommending its use in all patients, and that it should be combined with aspirin. A very comprehensive summary of the putative benefits of high-dose fish oil has recently been published (257). Despite these calls for intravenous FOLE trials, none have yet been registered.
Systematic review
From a total of 37 papers returned, 5 were taken to full screen, and none yielded relevant information not already considered.
The search of preprint servers yielded 1 paper (267) that extensively reviews the role of inflammation and the cytokine storm in lung damage but cites no supportive evidence for a modulating role of PUFAs other than that already reviewed above.
Selenium
Landscape review
There is very strong evidence that selenium, through its role as a cofactor in the 2 key antioxidant pathways in humans (reduction of glutathione and thioredoxin), plays a key role in host–virus interactions. An excellent and comprehensive recent review is available that covers both the host and (putative) viral aspects of selenoprotein actions (268).
The selenium content of staple cereals is strongly determined by the selenium content of soils, which, prior to the use of selenium-enriched fertilizers or dietary supplements, caused regional disease outbreaks of which the iconic example is Keshan disease, a multifactorial syndrome whose etiology includes an interaction between selenium deficiency and Coxsackievirus B (see below) (269).
Selenium is incorporated into the 21st amino acid, selenocysteine [where it replaces the sulfur of cysteine (268)]. Gene mapping has identified 25 human selenoproteins, of which 5 are glutathione reductases and 3 are thioredoxin reductases critical to the regeneration of antioxidant potential (268). Activity of these enzymes is reduced in selenium deficiency. While acknowledging that host–viral interactions can be modulated by both pro-oxidant and antioxidant factors, it is clear that antioxidants are key players. In this respect, there are overlaps between the actions of selenium and vitamins C and E summarized elsewhere in this review.
The example of Keshan disease provides a fascinating example of human, viral, dietary, and environmental interactions with strong resonance with the emergence of SARS-CoV-2. Named after the Keshan region of China notable for selenium-deficient soils, Keshan is a serious multisystem disorder affecting children and women of reproductive age (250). A key feature is a congestive cardiomyopathy that has been linked to Coxsackievirus B and can be modeled in mice. Inspired by prior studies in China (270), Beck and colleagues passaged a benign variant of Coxsackievirus B3 through selenium-deficient and selenium-replete mice (271, 272). The viral genome mutated in the deficient mice undergoing 6 nucleotide changes (273), leading to myopathy and death (271, 272). Most critically, when the virus from the deficient mice was then passaged through healthy selenium-replete mice it retained its pathogenicity and caused the cardiomyopathy (271, 272). Although SARS-CoV-2 appears to be mutating slowly, these studies contain a general warning that circulating viruses may be more likely to mutate to highly pathogenic strains with pandemic potential in nutritionally deficient populations.
A meta-analysis of almost 2 million participants in 41 randomized trials has confirmed that selenium supplementation is highly protective against Keshan disease (OR: 0.14; 95% CI: 0.012, 0.016) (274). Programs of selenium enhancement in crops and direct supplementation of the population have largely eliminated Keshan disease from the Keshan district, although it remains prevalent in neighboring regions including Tibet and North Korea.
Beck and her team extended these studies to include the influenza A(H3N2) virus strain (249, 275). Using a similar experimental model, they showed viral stability in selenium-replete mice and high rates of mutation with downstream pathology in selenium-deficient mice (249, 275). As with Coxsackievirus, the mutated strains retained their pathogenicity when re-passaged through healthy well-nourished mice (275). Mechanisms by which selenium deficiency affect the host response to the virus were also described (276–278).
Prior non–COVID-19 trials have investigated the impact of selenium supplementation in critically ill patients in the ICU (for a range of conditions not including ARDS). No fewer than 9 meta-analyses have been performed with slightly different inclusion and grading criteria (279–287). These analyses mostly agree that intravenous sodium selenite might yield a significant improvement in short-term mortality (meta-analyzed ORs between 0.82 and 0.98), but in the latest Cochrane analysis the evidence was judged to be of very low quality due to potential for bias (280). There was no effect on longer-term (28 or 90 d) mortality. Surprisingly, in the light of the robust animal data, there have been almost no trials of selenium and influenza or other respiratory infections. A randomized trial in 25 geriatric centers in France reported a tendency toward slightly fewer respiratory infections in patients receiving zinc and selenium, and better responses to the A/Beijing/32/92(H3N2) component of a multivalent vaccine (288). A smaller study of a selenium-containing micronutrient supplement in English nursing homes found no effect on antibody titers after influenza vaccination (289). In a small randomized trial, Ivory et al. (290) reported no effect on mucosal influenza antibody responses to vaccination and both positive and negative effects on cellular immunity. Another small study reported that marginally deficient adults given selenium supplements had faster elimination of vaccine strains of poliovirus and fewer mutations in viral product extracted from feces (291).
Systematic review
From a total of 12 papers returned, 4 were taken to full-text screen and 2 papers were identified for full screen. One of these listed selenium as part of a COVID-19 treatment protocol but listed no results. Zhang and Liu (292) report a general systematic review of nutrition and coronaviruses but contained no new information not already summarized above.
The search of preprint servers yielded 4 papers, of which 2 were excluded. Of the remaining papers, 1 was a systematic review (293) and the other screened 12 organoselenium structural analogs of the antioxidant drug ebselen for inhibition of the SARS-CoV-2 papain-like protease critical to viral replication (294). Four possible drug targets were identified.
Zinc
Landscape review
Zinc is an essential trace element crucial for growth, development, and the maintenance of immune function (295). It is the second most abundant trace metal in the human body after iron, and an essential component of protein structure and function (295). The global prevalence of zinc deficiency is estimated to range from 17% to 20%, with the vast majority occurring in LMICs in Africa and Asia (296). Zinc deficiency is also common in subgroups of the population, including the elderly, vegans/vegetarians, and individuals with chronic disease such as liver cirrhosis or inflammatory bowel disease (295, 297, 298).
Zinc is required for a wide variety of immune functions (299) and those deficient in zinc, particularly children, are prone to increased diarrheal and respiratory infections. Zinc supplementation has been shown to significantly reduce the frequency and severity of both infections in children (300), although such findings are not universal [e.g., Howie et al. (301)], and a recent systematic review and meta-analysis found no evidence that adjunctive zinc treatment improves recovery from pneumonia in children in LMICs (302). Similar to vitamin C, zinc supplementation has also been suggested as a potential remedy for the treatment of the common cold (rhinovirus infection); a meta-analysis of 3 trials reporting on 199 patients supports a faster recovery time (303), although the small sample size (n = 199) of included studies warrants caution.
At the molecular level, zinc is an essential component of protein structure and function and is a structural constituent of ∼750 zinc-finger transcription factors, enabling gene transcription (295, 304). It is also a catalytic component of ∼2000 enzymes (305). The role of zinc homeostasis in antibacterial immune responses is well documented; binding and sequestering extracellular zinc (and calcium) can prevent bacterial and fungal overgrowth (306) while toxic endosomal zinc accumulation can inhibit intracellular Mycobacterium growth in macrophages (307). For viral infections, however, these mechanisms are less well described, although a number of new hypotheses are now being suggested (308).
The SARS-CoV-2 pandemic has resulted in a global search for suitable antiviral and immunomodulatory candidates. Attracting global attention at the start of the pandemic was the potential use of oral chloroquine (CQ) and hydroxychloroquine (HQ), prescription drugs normally used for the treatment of malaria. Emerging trial evidence, however, does not support the use of either CQ or HQ as a treatment option for the disease (309–311). Of relevance to the current review is the finding that CQ has characteristics of a zinc ionophore and specifically targets extracellular zinc to intracellular lysosomes (312). This has led to an interest in zinc as a potential target for antiviral therapies, most notably in combination with CQ/HQ in clinical trials for the prevention or treatment of SARS-CoV-2 (313).
Systematic review
From a total of 69 papers returned (after removal of 8 duplicates), 6 were taken to full-text screen. On full screen, 5 papers were rejected as ineligible and 1 review paper, although ineligible for this review as it included no new data presented, highlighted the potential synergistic action of zinc and CQ in patients with SARS-CoV-2 (314).
A review of preprint listings returned 10 potentially relevant papers. Five of these were duplicates (already identified via PubMed or EMBASE). Four were found to be review articles, with no novel data specific to COVID-19 disease susceptibility or progression. Only a single paper was eligible for inclusion, a retrospective observational study comparing hospital outcomes (New York) among patients who received HQ and azithromycin plus zinc versus HQ and azithromycin alone (315). Using data from 932 patients admitted over a 1-mo period (March–April 2020), the authors found that addition of zinc sulfate did not impact the length of hospitalization, duration of ventilation, or ICU duration. In univariate analyses, zinc sulfate increased the frequency of patients being discharged home, and decreased the need for ventilation, admission to the ICU, and mortality or transfer to hospice for patients who were never admitted to the ICU. After adjusting for the time at which zinc sulfate was added to the protocol, an increased frequency of being discharged home (OR: 1.53; 95% CI: 1.12, 2.09) and a reduction in mortality or transfer to hospice remained significant (OR: 0.449; 95% CI: 0.271, 0.744). These data provide initial in vivo evidence that zinc sulfate may play a role in therapeutic management of COVID-19.
Antioxidants
Landscape review
During severe COVID-19, the SARS-CoV2 virus can trigger a strong host immune response. This can then result in the production of high concentrations of free radicals by both macrophages and neutrophils and the induction of severe oxidative stress (316). Oxidative stress causes protein and lipid oxidation, which then further activates and amplifies the immune response, creating a self-amplifying loop that can result in extensive tissue damage (317).
Oxidative stress is currently thought to be a major cause of the pathophysiology of severe COVID-19 infections and has previously been implicated as a mediator in ARDS (318). The level of oxidative stress may indeed determine the intensity of the organ damage seen during severe COVID-19 specifically to endothelial, pulmonary, cardiac, and immune cells (319). In addition, increased levels of oxidative stress pre-exist in individuals with comorbidities such as obesity, diabetes, and cardiovascular disease, and may play a role in increasing the risk of severe COVID-19 in these groups (320).
Antioxidants decrease oxidative stress and can be broadly divided into 4 groups: 1) endogenous antioxidants, which include molecules (e.g., glutathione, uric acid, and transferrin), vitamins (such as vitamins A, C, and E), and enzymatic cofactors (e.g., selenium and zinc) synthesized by the human body; 2) dietary antioxidant molecules and vitamins found in food (e.g., fruit, vegetables, green tea, olive oil, and red wine); 3) nutritional supplement antioxidants, which include supplements that contain increased doses of dietary antioxidants (e.g., vitamin C or quercetin tablets) and molecules from medicinal plants (e.g., molecules found in traditional Chinese medicine); and 4) synthetic molecules or drugs with known antioxidant activities (e.g., N-acetyl cysteine and metformin).
There is an abundance of epidemiological and in vitro evidence to suggest that concentrations of endogenous antioxidants and increased consumption of dietary antioxidants may decrease inflammation and oxidative stress (195), particularly in patients with cardiovascular disease (321). However, there is a lack of clinical evidence that consuming antioxidants from dietary sources or giving acute doses of naturally occurring antioxidants has direct long-term clinical benefits in the treatment of chronic conditions or acute viral infections (322). Some relevant evidence exists for the clinical utility of a synthetic antioxidant, N-acetyl cysteine, which is also an FDA-approved drug for the treatment of paracetamol toxicity. N-acetyl cysteine has been shown to have some modest benefit in ARDS (323) and there is limited evidence that it improves clinical outcomes in several viral diseases including HIV (324), hepatitis A (325), H1N1 influenza (326), dengue (327–329), and rotavirus infection (330).
Systematic review
From a total of 212 papers returned, 44 were taken to full-text screen. Nineteen papers were commentaries or nonsystematic reviews. In no case were there any new data related to antioxidants as a clinical therapy for COVID-19. Note that information on COVID-19 and vitamins A, C, and E as well as selenium and zinc has been reviewed in separate sections of this article and those papers were not included here.
The search of preprint servers yielded 6 relevant papers, all of which were accessed for full review. All were commentaries or editorials. None contained any new data on antioxidants as a treatment or preventative therapy for COVID-19.
Nutritional support
Landscape review
Evidence on best practice for nutritional support for patients with COVID-19 is currently lacking (331). In those infected, 80% have a mild condition (not requiring hospitalization), while 20% require inpatient care and 5% will require intensive care (332, 333). In the 80% with mild disease there is a growing body of evidence that the course of illness may take several weeks and, in some cases, many months for recovery and have multiple complications along the way (334).
In those admitted to hospital, nutritional support guidelines and advice are generally based on evidence drawn from treatment of viral pneumonia, sepsis, and ARDS. Specific evidence in relation to COVID-19 is not available as yet, but a pragmatic approach and “doing what we know, and doing it well” has been adopted in most settings. There is a huge wealth of literature on nutritional support in critically ill patients (335), which is beyond the scope of this review, but we will briefly discuss consensus on best practice.
Nutritional support during an acute illness has long been recognized as an important component to care (336). In an acute, severe illness there is a high risk of catabolism and the resulting malnutrition and sarcopenia can impact both on mortality and morbidity (335, 337).
Recommended nutritional support varies in mild, severe, and critical disease but there are overarching considerations that can be divided into patient factors, health care staffing factors, and system factors (338). Patient factors overlap in all disease states. There may be the need for special nutritional intervention in mild disease, especially in those with pre-existing conditions such as diabetes, heart failure, and other cardiac or chronic diseases. These may be exacerbated by an acute viral illness, especially if diarrhea, vomiting, or anorexia are present. A study from a rehabilitation center in Italy focused on patients once they were past the acute phase of their illness and found that 45% of COVID-19–infected patients were at risk of malnutrition (339). At the peak of cases, when health care systems have the potential to be overrun, the staffing shortages and other demands would make this easy to neglect to the detriment of the patients.
Patients with severe disease are usually admitted to the hospital. There is consensus that all patients admitted with COVID-19 should have their nutritional status assessed (340, 341). There are a number of important and practical considerations that affect nutritional care, as follows:
Risk of hypoxia on removal of oxygen delivery device (mask or noninvasive ventilation) to eat and drink.
Ability to remove oxygen delivery device independently to eat and drink.
Ease of access to food and drink.
Air leakage with noninvasive ventilation mask due to nasogastric (NG) tube.
These factors, along with isolation of COVID-19 patients in single rooms, limited visits by health care workers due to the need to conserve personal protective equipment (PPE) and reduce risk of transmission, and limited visits by family or friends, mean there is a real danger of malnutrition and dehydration (340).
A solution to this is the adoption of an early nutritional supplementation program as detailed in the pragmatic protocol by Caccialanza et al. (341). In this feeding protocol all patients are suggested to be screened at admission using a simplified nutritional risk score. Due to a high number of patients being unable to meet their nutritional needs on a normal diet, all patients would be started on whey proteins (20 g/d) and multivitamins, multiminerals, and trace elements supplements. Those at nutritional risk would then commence on 2–3 bottles of oral nutritional supplements and escalated to PN if they are unable to tolerate oral intake. Another solution adopted in the United Kingdom is the “Every Contact Counts” model, where patients are offered food and drink at every encounter with health professionals (340).
Both the consensus statement by nursing practitioners in China and the ESPEN expert statement agree on the following steps (46, 342):
Early screening for risk of malnutrition
Individualized nutritional plans
Oral nutritional supplements to be used
PN should be initiated within 3 d should EN not meet nutritional requirements
Ongoing monitoring of nutritional status
ESPEN provides the following additional details:
Aim for 30 kcal · kg–1 · d–1 to meet energy needs (may need to be adjusted in certain populations)
1 g protein · kg–1 · d–1 (may need to be adjusted in certain populations)
Fat-to-carbohydrate ratio in nonventilated patients of 30:70
Whichever approach is taken, prevention of inpatient malnutrition and its associated complications must be considered an essential component to clinical care and requires monitoring throughout the illness.
The ESPEN provides guidelines on nutritional support of patients admitted to the ICU and the documents specifying treatment in those with COVID-19 are thorough and comprehensive (46, 335). These guidelines, as well as the guidelines from the American Society for Enteral and Parenteral Nutrition, are based on evidence of feeding in critically ill patients and expert opinion on how that can be applied to COVID-19 (331).
COVID-19 patients in the ICU have a few special considerations relating to treatment and nutrition. For example, many patients required proning during the course of their ventilation and there is consensus in all guidelines on EN feeding being safe to continue as long as awareness of complications with NG tube placement and specified steps to minimize this were taken. Depending on the severity of lung injury and its availability, there can be many patients requiring extracorporeal membrane oxygenation and there was consensus in all guidelines that EN feeding can be started at trophic or hypocaloric levels. Use of PN differed, with American guidelines advocating early implementation and European and Chinese guidelines taking a more cautious case-by-case approach. Finally, nutritional support may well be needed post–ICU discharge with high rates of dysphagia being reported (339, 343).
Systematic review
Our systematic search yielded 17 papers for full review, none of which met the criteria for inclusion. Of the preprints, 15 were reviewed and none met the full criteria.
Discussion
As the pandemic continues to evolve at rapid pace, so does our understanding of the epidemiology and underlying mechanisms of the SARS-CoV-2 virus. However, despite the wealth of literature being published, the evidence directly linking nutritional status to the risk and progression of COVID-19 is still sparse. In Figure 2 we summarize the key themes emerging from our landscape and systematic reviews.
FIGURE 2.
A summary of potential ways that nutrition may influence susceptibility and severity of COVID-19. COVID-19, coronavirus disease 2019; Vit, vitamin.
Nutritional status has the potential to influence susceptibility to the risk of COVID-19 through its integral role in immune function. For example, above we have covered some of the ways that micronutrients support mucosal immune function (vitamin A), epithelial tissue integrity (vitamins A, C, and D), enhancing the function of certain adaptive and innate immune cells (vitamins A, C, D, and E; iron; zinc; and PUFAs), and potential pro-oxidant effects (vitamin C). Undernutrition, overweight, obesity, and type 2 diabetes are all associated with impaired immunity, through independent (although as yet not clearly defined) mechanisms as well as through the effects of concurrent micronutrient deficiencies. The various presentations of overnutrition have been the most frequently documented nutrition-related comorbidities among patients admitted to the hospital with COVID-19 to date. However, many markers of micronutrient deficiency are not routinely measured on hospital admission. Furthermore, at the time of writing, the pandemic is still penetrating LMICs, where the burden of undernutrition is higher. We therefore anticipate further evidence on the potential impact of undernutrition on COVID-19 susceptibility to be generated soon.
The influence of nutrition on immune function can also affect the progression of viral infections, with implications for the length, severity, and final outcomes of disease episodes. From our landscape reviews we only have limited insight from other viral diseases as to how nutritional supplementation may potentially influence outcomes. For example, although there is strong evidence of an association between vitamin A supplementation and reduced outbreaks of measles, there is insufficient evidence regarding the association with Ebola outcomes. For vitamin C, there is some positive, but inconsistent, evidence regarding supplementation and the prevention of pneumonia, but very limited evidence describing an effect of supplementation on overall mortality reduction. For vitamin D, we have mixed evidence describing the influence of supplementation on both the risk and severity of acute respiratory infections. For the minerals, we have documented evidence of an association between iron deficiency and increased risk of impaired lung function in hypoxic conditions and literature describing the association between zinc supplementation and reduction in diarrhea and respiratory infections. It is important to note, however, that not all evidence of nutritional supplementation points to positive, or null, outcomes. For example, we have described how there is some evidence linking vitamin E supplementation to the worsening of respiratory infections. Furthermore, some studies have found evidence of associations between iron supplementation or elevated iron status with increased risk of malaria, bacterial infections, HIV-1 progression, and certain respiratory infections.
However, when it comes to COVID-19 explicitly, our ability to draw conclusions between nutritional status and disease progression is limited by the current lack of high-quality data. We have documented some observational studies describing an association between lower vitamin D status and increased COVID-19 infection. We noted that a single observational study suggested that treatment with zinc sulfate showed signs of reduction in mortality and increased discharge from the hospital to home in patients treated with HQ and azithromycin. However, more recent findings from the Recovery trial find no beneficial effect of HQ in the absence of zinc (344, 345). We also summarized some observational studies that described how patients presenting with malnutrition on hospital admission (both under- and overnutrition) have increased risk of mortality from COVID-19. With studies on undernutrition in particular, it is not easy to distinguish between the effect of pre-existing undernutrition on immune function and increased disease severity and the subsequent nutritional impact of prolonged inflammatory states and intensive care admission through impaired appetite and dysregulated metabolism.
The literature has, however, highlighted some hypotheses regarding mechanisms through which nutrition could modulate disease severity and progression. Particularly relevant to COVID-19 is the role that antioxidants may play in reducing the impact of the cytokine storm during the acute phase of the infection. This has to be carefully balanced against not overly dampening the immune response during other phases of the illness, as described in detail in Iddir et al. (195). Of the micronutrients covered in our review, vitamins A, C, and E and certain dietary polyphenols have potentially important roles in quenching free radicals through their antioxidant properties, alongside zinc and selenium in their coenzyme roles. Synthetic antioxidants can be produced and are being tested for effectiveness in mitigating the damage from the cytokine storm, and it is not yet clear to what extent dietary components will play a synergistic role.
Micronutrients may help slow down processes vital for viral replication. For example, we have described how vitamin D may influence the expression of ACE2, implicated in SARS-CoV-2 binding. Animal studies have shown, tentatively, how deficiencies in selenium and vitamin E may increase viral replication as well as enhancing virulence and mutation rates.
To date, the role of nutritional support in the clinical management of severe COVID-19 cases is based on knowledge from successful protocols used in other viral infections and, more generally, in recovery from intensive care. There are, however, some new treatment regimens being tested. Treatments comprising combinations of various antioxidants are currently being investigated in the early stages of intervention trials. It is not possible to separate out the effects of individual micronutrients in these treatments. Higher doses of vitamins A, C, and D are also being trialed, some intravenously, but there is limited prior evidence to suggest they will be successful and many trials do not seem to take account of normal physiological thresholds. For the minerals, the potential role of iron chelation in reducing iron-induced lung toxicity is being considered. Zinc features mainly as an adjunct therapy alongside CQ and HQ interventions, although interest is growing in its potential as an intervention in its own right. Nutritional supplementation will require careful consideration of the extent to which the suggested micronutrients can be utilized, especially during acute inflammation and the related states of anemia of inflammation. It is likely that a period of stabilization to bring down inflammation will be essential before any positive effects from micronutrient supplementation can be seen (146, 346).
In this review we have focused on the direct relation between nutritional status and risk of infection and progression of COVID-19. This is an important but incomplete part of the vicious cycle of nutritional status, immune response, and infection. Beyond the scope of this review, but integral to the overall picture, are the impacts the pandemic has on livelihoods and health, which are inextricably linked to nutritional status and therefore overall morbidity and mortality. We know from the Ebola outbreak in West Africa during 2013–2016 that disruption to the health system brought about excess mortality equal to, if not greater than, direct deaths from the infection itself (347). The disruption from COVID-19 to food systems, the economy, and health infrastructure means that nutritional status of the most vulnerable will be enormously impacted. Headey et al. (8) summarized recent estimates from modeling, suggesting that an additional 140 million people are expected to fall into extreme poverty due to the pandemic in 2020 alone, with a doubling of people facing food insecurity (estimated at 265 million). An estimated 14.3% increase in wasting prevalence in children under 5 will equate to an additional 6.7 million children wasted compared with estimates without COVID-19 (8). Furthermore, the increase in numbers of people facing acute nutritional vulnerability will be compounded by the reduction in health services offered to the population during the pandemic. Roberton et al. (348) modeled scenarios estimating impacts of different levels of disruption to availability of health workers and supplies, and on demand and access to health services. Even in the best-case scenario they estimated the additional prevalence of acute malnutrition and reduced coverage of health services would result in an additional quarter of a million child deaths in the next 6 mo.
Many consortia have highlighted the urgency of tackling the immense impact of the pandemic on nutrition and health outlined above. Recommendations point both to nutrition-specific strategies, such as prevention and treatment of wasting, vitamin A supplementation, and breastfeeding support (349), and to nutrition-sensitive strategies, such as strengthening the food-supply chain, providing safety-net programs, implementing community-led sanitation initiatives, improving female empowerment, and ensuring access to health care (9).
Strengths and limitations of the review
Our review provides a synthesis of information to complement other existing comprehensive reviews (190, 195). However, to our knowledge, ours is the most detailed systematic search to date, bringing together 13 separate systematic reviews. Our inclusion of material from preprint servers and trial registries adds to the breadth of information we have been able to include.
The pandemic is evolving rapidly and new evidence has likely surfaced since our search dates. While the collation of 13 reviews in this article provided breadth, we were unable to ensure all searches took place exactly synchronously. We did not perform a risk-of-bias assessment of the included literature, and it is important to note that preprints are not peer-reviewed. Our inclusion criteria of literature written in English may have missed some pertinent information in other journals. We necessarily had to limit our scope to the most important nutrition-related conditions and micronutrients of interest. However, this is incomplete, and other potentially relevant areas of interest include the role of macronutrient intake, gut microbiota, dietary fiber, B vitamins, other minerals, phytochemicals, and carotenoids. These are covered in other narrative reviews (192, 195). Furthermore, we were unable to comprehensively cover all the additional factors that can influence the relation between nutrition, immunity, and disease progression. Interpretation of the included literature is necessarily restricted to the context of the original studies, and a wide range of factors (some measured, many not measured) preclude extrapolation to the wider population. Such considerations should include genetic polymorphisms and their frequency and impact in different populations, hemoglobinopathies, the environment (e.g., soil type, latitude), age, sex, access to health care, and other underlying economic and political factors determining nutritional vulnerability. Finally, there is always a degree of uncertainty and risk when extrapolating from 1 infection to another, especially when age profiles of the affected population vary. We find that much of the previous literature on micronutrient deficiencies and viral infection focus on the younger population, whereas SARS-CoV-2 is predominantly affecting older people.
Conclusions
Our review of the current literature highlights a range of mechanistic and observational evidence to highlight the role nutrition can play in the susceptibility to and progression of COVID-19. Prior knowledge of interactions between nutrition and other viral diseases can help inform hypotheses relevant to COVID-19. However, the literature taken from other viral diseases is far from consistent, and studies taken in isolation can be a source of rumors and ill-advised “quick fixes” surrounding COVID-19 prevention and cure. There is limited evidence to date that high-dose supplements of micronutrients will either prevent disease or speed up treatment. Attempting to ensure people have an adequate dietary intake is critical. However, we believe the focus should be on ways to promote a balanced diet and reduce the infective burden rather than reliance on high-dose supplementation, until more concrete evidence from clinical trials suggests otherwise. While the quantity of literature on these topics is increasing daily, this does not necessarily correspond to an increase in high-quality evidence. Reviews such as ours will continually need updating to allow for a balanced view of the available data in order to counter unjustified nutrition-related claims. To date, there is no evidence supporting adoption of novel nutritional therapies, although results of clinical trials are eagerly awaited. Given the known impacts of all forms of malnutrition on the immune system, public health strategies to reduce micronutrient deficiencies, undernutrition, and overnutrition remain of critical importance, drawing on the numerous lessons learned from other viral diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—AMP and PTJ: designed the research; PTJ, ZA, AEA, AB, CC, HD, MJ, KSJ, ZL, SEM, FM-B, HMN, S-RP, PS, MRT, and AMP: conducted the searches; PTJ, ZA, AEA, AB, CC, HD, MJ, KSJ, ZL, SEM, FM-B, HMN, BN, S-RP, PS, MJS, MRT, and AMP: wrote the manuscript; AMP and PTJ: had responsibility for final content; and all authors: read and approved the final manuscript.
Notes
ZA and PS are supported by the Wellcome Trust Our Planet Our Health Programme (FACE-Africa grant number: 216021/Z/19/Z). AB is supported by a Wellcome Clinical PhD Fellowship (ref 203905). MJ is supported by a Wellcome Trust grant (ref 216451/Z/19/Z). HD, AEA, and MRT are supported by the UK Medical Research Council (MRC Human Immunology Unit core funding to HD, award no. MC_UU_12010/10). SEM is supported by The Wellcome Trust (ref 220225/Z/20/Z) and the Medical Research Council (UK) (ref MR/P012019/1). AMP, CC, and MJS are jointly funded by the UK Medical Research Council and the Department for International Development (DFID) under the MRC/DFID Concordat agreement (MRC Program MC-A760-5QX00). KSJ is supported by the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (IS-BRC-1215- 20014). The NIHR Cambridge Biomedical Research Centre is a partnership between Cambridge University Hospitals National Health Service (NHS) Foundation Trust and the University of Cambridge, funded by the NIHR. All other authors received no specific funding for this work.
Author disclosures: The authors report no conflicts of interest. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Supplemental Materials 1–4 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: AA, arachidonic acid; ACE2, angiotensin-converting enzyme 2; aOR, adjusted OR; ARDS, acute respiratory distress syndrome; CoV, coronavirus; COVID-19, coronavirus disease 2019; CQ, chloroquine; CRP, C-reactive protein; DBP, vitamin D–binding protein; DM, diabetes mellitus; EN, enteral nutrition; ESPEN, European Society for Clinical Nutrition and Metabolism; FiO2, fractional inspired oxygen; FOLE, high-dose fish-oil lipid emulsion; HbA1c, glycated hemoglobin; HQ, hydroxychloroquine; ICU, intensive care unit; IDA, iron deficiency anemia; LC, long-chain; LMIC, low- and middle-income country; LOS, length of stay; MERS, Middle East respiratory syndrome; MNA-sf, Mini Nutrition Assessment - short form; MUST, Malnutrition Universal Screening Tool; NG, nasogastric; NRI, Nutrition Risk Index; PaO2, arterial oxygen partial pressure; PCR, polymerase chain reaction; PEM, protein-energy malnutrition; PN, parenteral nutrition; RCT, randomized controlled trial; rhEPO, recombinant human erythropoietin; RR, risk ratio; RTI, respiratory tract infection; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TFRC, transferrin receptor gene; VDD, vitamin D deficiency; VDR, vitamin D receptor; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Philip T James, Department of Population Health, London School of Hygiene & Tropical Medicine, London, United Kingdom.
Zakari Ali, Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene & Tropical Medicine, Fajara, The Gambia.
Andrew E Armitage, MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom.
Ana Bonell, Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene & Tropical Medicine, Fajara, The Gambia.
Carla Cerami, Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene & Tropical Medicine, Fajara, The Gambia.
Hal Drakesmith, MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom.
Modou Jobe, Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene & Tropical Medicine, Fajara, The Gambia.
Kerry S Jones, National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) Nutritional Biomarker Laboratory, MRC Epidemiology Unit, University of Cambridge, Cambridge, United Kingdom.
Zara Liew, Department of Population Health, London School of Hygiene & Tropical Medicine, London, United Kingdom.
Sophie E Moore, Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene & Tropical Medicine, Fajara, The Gambia; Department of Women and Children's Health, King's College London, London, United Kingdom.
Fernanda Morales-Berstein, Department of Population Health, London School of Hygiene & Tropical Medicine, London, United Kingdom.
Helen M Nabwera, Department of International Public Health, Liverpool School of Tropical Medicine, Liverpool, United Kingdom.
Behzad Nadjm, Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene & Tropical Medicine, Fajara, The Gambia.
Sant-Rayn Pasricha, Population Health and Immunity Division, Walter and Eliza Hall Institute of Medical Research, Parkville, Australia; Department of Medical Biology, The University of Melbourne, Parkville, Australia.
Pauline Scheelbeek, Department of Population Health, London School of Hygiene & Tropical Medicine, London, United Kingdom; Centre on Climate Change and Planetary Health, London School of Hygiene & Tropical Medicine, London, United Kingdom.
Matt J Silver, MRC Unit The Gambia at the London School of Hygiene & Tropical Medicine, London, United Kingdom.
Megan R Teh, MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom.
Andrew M Prentice, Medical Research Council (MRC) Unit The Gambia at the London School of Hygiene & Tropical Medicine, Fajara, The Gambia.
References
- 1. Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID-19 epidemiology based on current evidence. JCM. 2020;9:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu Xet al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet North Am Ed. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bousquet J, Anto JM, Iaccarino G, Czarlewski W, Haahtela T, Anto A, Akdis CA, Blain H, Canonica GW, Cardona Vet al. . Is diet partly responsible for differences in COVID-19 death rates between and within countries?. Clin Transl Allergy. 2020;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keusch GT. The history of nutrition: malnutrition, infection and immunity. J Nutr. 2003;133:336S–40S. [DOI] [PubMed] [Google Scholar]
- 6. Bhaskaram P. Micronutrient malnutrition, infection and immunity: an overview. Nutr Rev. 2002;60:S40–5. [DOI] [PubMed] [Google Scholar]
- 7. Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–77S. [DOI] [PubMed] [Google Scholar]
- 8. Headey D, Heidkamp R, Osendarp S, Ruel M, Scott N, Black R, Shekar M, Bouis H, Flory A, Haddad Let al. . Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet North Am Ed. 2020;396:519–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akseer N, Kandru G, Keats EC, Bhutta ZA. COVID-19 pandemic and mitigation strategies: implications for maternal and child health and nutrition. Am J Clin Nutr. 2020;112:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torero M. Without food, there can be no exit from the pandemic. Nature. 2020;580:588–9. [DOI] [PubMed] [Google Scholar]
- 11. International Panel of Experts on Sustainable Food Systems . COVID-19 and the crisis in food systems: symptoms, causes, and potential solutions. iPES FOOD; 2020, [Internet]. [Accessed 2020 Jun 6], Available at: http://www.ipes-food.org/_img/upload/files/COVID-19_CommuniqueEN.pdf. [Google Scholar]
- 12. Headey D, Ruel M. The COVID-19 nutrition crisis: what to expect and how to protect. IFPRI Blog Ser. 2020. [Internet]. [Accessed 2020 June 3]. Available from: https://www.ifpri.org/blog/covid-19-nutrition-crisis-what-expect-and-how-protect. [Google Scholar]
- 13. Pérez-Escamilla R, Cunningham K, Moran VH. COVID-19, food and nutrition insecurity and the wellbeing of children, pregnant and lactating women: a complex syndemic. Matern Child Nutr. 2020;16:e13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunn CG, Kenney E, Fleischhacker SE, Bleich SN. Feeding low-income children during the Covid-19 pandemic. N Engl J Med. 2020;382:e40. [DOI] [PubMed] [Google Scholar]
- 15. Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attinà A, Cinelli G, Leggeri C, Caparello G, Barrea L, Scerbo Fet al. . Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muscogiuri G, Barrea L, Savastano S, Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020;74:850–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kininmonth AR, Jamil N, Almatrouk N, Evans CEL. Quality assessment of nutrition coverage in the media: a 6-week survey of five popular UK newspapers. BMJ Open. 2017;7:e014633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rowe SB. Communicating science-based food and nutrition information. J Nutr. 2002;132:2481S–2S. [DOI] [PubMed] [Google Scholar]
- 19. Goldberg JP, Sliwa SA. Communicating actionable nutrition messages: challenges and opportunities. Proc Nutr Soc. 2011;70:26–37. [DOI] [PubMed] [Google Scholar]
- 20. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas Jet al. . Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waterlow JC. Protein energy malnutrition. London: Edward Arnold; 1992. [Google Scholar]
- 23. Global Nutrition Report Stakeholder Group . 2020 Global Nutrition Report: action on equity to end malnutrition. Bristol (UK): Development Initiatives; 2020. [Google Scholar]
- 24. WHO Multicentre Growth Reference Study . WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva (Switzerland): WHO; 2006. [Google Scholar]
- 25. Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nat Rev Dis Primers. 2017;3:17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schoenbuchner SM, Dolan C, Mwangome M, Hall A, Richard SA, Wells JC, Khara T, Sonko B, Prentice AM, Moore SE. The relationship between wasting and stunting: a retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. Am J Clin Nutr. 2019;110:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rytter MJH, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition—a systematic review. PLoS One. 2014;9:e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37:386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46:1582–8. [DOI] [PubMed] [Google Scholar]
- 30. Jones KD, Thitiri J, Ngari M, Berkley JA. Childhood malnutrition: toward an understanding of infections, inflammation, and antimicrobials. Food Nutr Bull. 2014;35:S64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013;76:296–302. [DOI] [PubMed] [Google Scholar]
- 32. Felder S, Lechtenboehmer C, Bally M, Fehr R, Deiss M, Faessler L, Kutz A, Steiner D, Rast AC, Laukemann Set al. . Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition. 2015;31:1385–93. [DOI] [PubMed] [Google Scholar]
- 33. Mehta S. Nutritional status and COVID-19: an opportunity for lasting change?. Clin Med. 2020;20:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats AJSet al. . GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Yet al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han MG, Cheon D-S, Zhang X, Saif LJ. Cross-protection against a human enteric coronavirus and a virulent bovine enteric coronavirus in gnotobiotic calves. J Virol. 2006;80:12350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papatsiros VG, Stylianaki I, Papakonstantinou G, Papaioannou N, Christodoulopoulos G. Case report of transmissible gastroenteritis coronavirus infection associated with small intestine and brain lesions in piglets. Viral Immunol. 2019;32:63–7. [DOI] [PubMed] [Google Scholar]
- 38. Baker SJ, Mathan M, Mathan VI, Jesudoss S, Swaminathan SP. Chronic enterocyte infection with coronavirus. Digest Dis Sci. 1982;27:1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edgeley GR, Davidson GP, Goodwin DA, Ringenbergs ML, Erlich J, Robb TA. Lactose malabsorption in Central Australian Aboriginal children hospitalized with acute enteritis. J Gastroenterol Hepatol. 1988;3:63–9. [Google Scholar]
- 40. Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158:1518–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Atkinson M, Yanney M, Stephenson T, Smyth A. Effective treatment strategies for paediatric community-acquired pneumonia. Expert Opin Pharmacother. 2007;8:1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tempia S, Walaza S, Moyes J, Cohen AL, von Mollendorf C, Treurnicht FK, Venter M, Pretorius M, Hellferscee O, Mtshali Set al. . Risk factors for influenza-associated severe acute respiratory illness hospitalization in South Africa, 2012–2015. Open Forum Infect Dis. 2017;4:ofw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reyes L, Arvelo W, Estevez A, Gray J, Moir JC, Gordillo B, Frenkel G, Ardón F, Moscoso F, Olsen SJet al. . Population-based surveillance for 2009 pandemic influenza A (H1N1) virus in Guatemala, 2010. Influenza Other Respi Viruses. 2010;4:129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, Seth S, Egan C, Hardwick HE, Halpin Set al. . Clinical characteristics of children and young people admitted to hospital with Covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, Shen B, Gong Z. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M, Singer P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li T, Zhang Y, Gong C, Wang J, Liu B, Shi L, Duan J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74:871–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu G, Zhang S, Mao Z, Wang W, Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020;74:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maccioni L, Weber S, Elgizouli M, Stoehlker AS, Geist I, Peter HH, Vach W, Nieters A. Obesity and risk of respiratory tract infections: results of an infection-diary based cohort study. BMC Public Health. 2018;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phung DT, Wang Z, Rutherford S, Huang C, Chu C. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev. 2013;14:839–57. [DOI] [PubMed] [Google Scholar]
- 51. Barber TM. COVID-19 and diabetes mellitus: implications for prognosis and clinical management. Expert Rev Endocrinol Metab. 2020;15:227–36. [DOI] [PubMed] [Google Scholar]
- 52. Leitner DR, Frühbeck G, Yumuk V, Schindler K, Micic D, Woodward E, Toplak H. Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies—EASO can lead the way. Obes Facts. 2017;10:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection. Circulation. 2020;142:4–6. [DOI] [PubMed] [Google Scholar]
- 54. Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, Kouretas D, Spandidos DA, Tsatsakis A. Obesity—a risk factor for increased COVID–19 prevalence, severity and lethality [review]. Mol Med Rep. 2020;22:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wösten-Van Asperen RM, Lutter R, Specht PA, Moll GN, Van Woensel JB, Van Der Loos CM, Van Goor H, Kamilic J, Florquin S, Bos AP. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225:618–27. [DOI] [PubMed] [Google Scholar]
- 57. Rao S, Lau A, So H-C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care. 2020;431:416. [DOI] [PubMed] [Google Scholar]
- 58. Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, He Q, Wang Z, Liu Y, Liu Let al. . Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;431:392. [DOI] [PubMed] [Google Scholar]
- 59. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SLet al. . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–59., doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, Mylonakis E. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28:1200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain Met al. . High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lemyze M, Courageux N, Maladobry T, Arumadura C, Pauquet P, Orfi A, Komorowski M, Mallat J, Granier M. Implications of obesity for the management of severe coronavirus disease 2019 pneumonia. Crit Care Med. 2020;48:e761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang F, Xiong Y, Wei Y, Hu Y, Wang F, Li G, Liu K, Du R, Wang C-Y, Zhu W. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. 2020;92:2536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Higham A, Singh D. Increased ACE2 expression in the bronchial epithelium of COPD patients who are overweight. Obesity. 2020;28(9):1586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity. 2020;28:1595–99., doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lokken EM, Walker CL, Delaney S, Kachikis A, Kretzer NM, Erickson A, Resnick R, Vanderhoeven J, Hwang JK, Barnhart Net al. . Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. Published online 19 May 2020. doi: 10.1016/j.ajog.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hajifathalian K, Kumar S, Newberry C, Shah S, Fortune B, Krisko T, Ortiz-Pujols S, Zhou XK, Dannenberg AJ, Kumar Ret al. . Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York City. Obesity. 2020;28:1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Busetto L, Bettini S, Fabris R, Serra R, Dal Pra C, Maffei P, Rossato M, Fioretto P, Vettor R. Obesity and COVID-19: an Italian snapshot. Obesity. 2020;28:1600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hur K, Price CPE, Gray EL, Gulati RK, Maksimoski M, Racette SD, Schneider AL, Khanwalkar AR. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, Amadou C, Arnault G, Baudoux F, Bauduceau Bet al. . Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nikpouraghdam M, Jalali Farahani A, Alishiri G, Heydari S, Ebrahimnia M, Samadinia H, Sepandi M, Jafari NJ, Izadi M, Qazvini Aet al. . Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in Iran: a single center study. J Clin Virol. 2020;127:104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zheng Y, Xiong C, Liu Y, Qian X, Tang Y, Liu L, Leung EL-H, Wang M. Epidemiological and clinical characteristics analysis of COVID-19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol Res. 2020;157:104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lian J, Jin X, Hao S, Cai H, Zhang S, Zheng L, Jia H, Hu J, Gao J, Zhang Yet al. . Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) outside Wuhan. Clin Infect Dis. 2020;71:740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guan W-J, Liang W, Zhao Y, Liang H-R, Chen Z, Li Y, Liu X, Chen R, Tang C-L, Wang Tet al. . Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Yet al. . Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen Q, Zheng Z, Zhang C, Zhang X, Wu H, Wang J, Wang S, Zheng C. Clinical characteristics of 145 patients with coronavirus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang J-J, Dong X, Cao Y-Y, Yuan Y-D, Yang Y-B, Yan Y-Q, Akdis CA, Gao Y-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41. [DOI] [PubMed] [Google Scholar]
- 79. Wang L, He W, Yu X, Hu D, Bao M, Liu H, Zhou J, Jiang H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu Xet al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo C-L, Kuchel GA, Melzer D. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. Published online 20 July 2020. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang Y, Li H, Zhang J, Cao Y, Zhao X, Yu N, Gao Y, Ma J, Zhang H, Zhang Jet al. . The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: a single-centre, retrospective, observational study in Wuhan. Diabetes Obes Metab. 2020;22:1443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du Ket al. . Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36:e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract. 2020;164:108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, Yu X, Dong K. Clinical characteristics and outcomes of patients with severe COVID-19 with diabetes. BMJ Open Diab Res Care. 2020;8:e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang Y, Cui Y, Shen M, Zhang J, Liu B, Dai M, Chen L, Han D, Fan Y, Zeng Yet al. . Association of diabetes mellitus with disease severity and prognosis in COVID-19: a retrospective cohort study. Diabetes Res Clin Pract. 2020;165:108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen X, Hu W, Ling J, Mo P, Zhang Y, Jiang Q, Ma Z, Cao Q, Deng L, Song Set al. . Hypertension and diabetes delay the viral clearance in COVID-19 patients. medRxiv [Preprint]. Published online January 2020. [Accessed 2020 Jun 4]. doi: 10.1101/2020.03.22.20040774. [Google Scholar]
- 89. Ebinger JE, Achamallah N, Ji H, Claggett BL, Sun N, Botting P, Nguyen T-T, Luong E, Kim EH, Park Eet al. . Pre-existing traits associated with Covid-19 illness severity. PLoS One. 2020;15:e0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ho FK, Celis-Morales CA, Gray SR, Katikireddi SV, Niedzwiedz CL, Hastie C, Lyall DM, Ferguson LD, Berry C, Mackay DFet al. . Modifiable and non-modifiable risk factors for COVID-19: results from UK Biobank, [Accessed 2020 Jun 17]. medRxiv [Preprint]. Published online May 2020. doi: 10.1101/2020.04.28.20083295, Available at:https://doi.org/10.1101/2020.04.28.20083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hu L, Chen S, Fu Y, Gao Z, Long H, Wang J-M, Ren H-W, Zuo Y, Li H, Wang Jet al. . Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71:2089–98., doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang Y, Cui Y, Shen M, Zhang J, Liu B, Dai M, Chen L, Han D, Fan Y, Zeng Yet al. . Comorbid diabetes mellitus was associated with poorer prognosis in patients with COVID-19: a retrospective cohort study, Available at: https://doi.org/10.1101/2020.03.24.20042358. medRxiv [Preprint]. Published online January 2020. doi: 10.1101/2020.03.24.20042358. [Accessed 2020 Jun 4]. [Google Scholar]
- 93. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du Cet al. . Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, Klonoff DC. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, Feng J, Yan S, Guan Y, Xu Det al. . Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43:1382–91. [DOI] [PubMed] [Google Scholar]
- 96. Chang MC, Park Y-K, Kim B-O, Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis. 2020;20:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Huang R, Zhu L, Xue L, Liu L, Yan X, Wang J, Zhang B, Xu T, Ji F, Zhao Yet al. . Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Negl Trop Dis. 2020;14:e0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rastad H, Karim H, Ejtahed H-S, Tajbakhsh R, Noorisepehr M, Babaei M, Azimzadeh M, Soleimani A, Inanloo SH, Shafiabadi Hassani Net al. . Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol Metab Syndr. 2020;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang F, Yang Y, Dong K, Yan Y, Zhang S, Ren H, Yu X, Shi X. Clinical characteristics of 28 patients with diabetes and COVID-19 in Wuhan, China. Endocr Pract. 2020;26:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, Qiu K, Zhang J, Zeng T, Chen Let al. . Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;221:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, Liu C, Xiong M, Deng A, Zhang Yet al. . Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–407. [DOI] [PubMed] [Google Scholar]
- 102. Sardu C, D'Onofrio N, Balestrieri ML, Barbieri M, Rizzo MR, Messina V, Maggi P, Coppola N, Paolisso G, Marfella R. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control?. Diabetes Care. 2020;43:1408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luo P, Qiu L, Liu Y, Liu X, Zheng J, Xue H, Liu W, Liu D, Li J. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang Wet al. . Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pasricha S-R, Drakesmith H, Black J, Hipgrave D, Biggs B-A. Control of iron deficiency anemia in low- and middle-income countries. Blood. 2013;121:2607–17. [DOI] [PubMed] [Google Scholar]
- 106. Ganz T. Anemia of inflammation. N Engl J Med. 2019;381:1148–57. [DOI] [PubMed] [Google Scholar]
- 107. Hill A, Hill QA. Autoimmune hemolytic anemia. Hematol Am Soc Hematol Educ Progr. 2018;2018:382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Landry ML. Parvovirus B19. Microbiol Spectr. 2016;4. doi: 10.1128/microbiolspec.DMIH2-0008-2015. [DOI] [PubMed] [Google Scholar]
- 109. Takhar A. Pernicious anaemia: switch to oral B12 supplementation to reduce risk of Covid-19 transmission. BMJ. 2020;369:m2383. [DOI] [PubMed] [Google Scholar]
- 110. Warren J. Pernicious anaemia: self-administration of hydroxocobalamin in the Covid-19 crisis. BMJ. 2020;369:m2380. [DOI] [PubMed] [Google Scholar]
- 111. Motta I, Migone De Amicis M, Pinto VM, Balocco M, Longo F, Bonetti F, Gianesin B, Graziadei G, Cappellini MD, De Franceschi Let al. . SARS-CoV-2 infection in beta thalassemia: preliminary data from the Italian experience. Am J Hematol. Published online 20 April 2020. doi: 10.1002/ajh.25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Karimi M, Haghpanah S, Azarkeivan A, Zahedi Z, Zarei T, Akhavan Tavakoli M, Bazrafshan A, Shirkavand A, De Sanctis V. Prevalence and mortality due to outbreak of novel coronavirus disease (COVID-19) in β-thalassemias: the nationwide Iranian experience. Br J Haematol. Published online 2 June 2020. doi: 10.1111/bjh.16911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li M, Nguyen CB, Yeung Z, Sanchez K, Rosen D, Bushan S. Evans syndrome in a patient with COVID-19. Br J Haematol. 2020;190:16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, Merabet F, Mekinian A, Braun T, Damaj Get al. . Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lopez C, Kim J, Pandey A, Huang T, DeLoughery TG. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190:31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Capes A, Bailly S, Hantson P, Gerard L, Laterre P-F. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol. 2020;99:1679–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192. Published online 12 June 2020. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSCet al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mei Y, Weinberg SE, Zhao L, Frink A, Qi C, Behdad A, Ji P. Risk stratification of hospitalized COVID-19 patients through comparative studies of laboratory results with influenza. EClinicalMedicine. 2020;26:100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mendy A, Apewokin S, Wells AA, Morrow AL. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID-19 patients. medRxiv [Preprint]. 2020. doi: 10.1101/2020.06.25.20137323. [Google Scholar]
- 121. Cavezzi A, Troiani E, Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects?. J Med Virol. 2020;92:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, Fritsche G, Wöll E, Weiss G. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. JCM. 2020;9:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Andreini C, Putignano V, Rosato A, Banci L. The human iron-proteome. Metallomics. 2018;10:1223–31. [DOI] [PubMed] [Google Scholar]
- 125. Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6:541–52. [DOI] [PubMed] [Google Scholar]
- 126. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby Pet al. . Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, O'Brien P, Quigley M, Brocklehurst P, Kurinczuk JJet al. . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. WHO . The global prevalence of anaemia in 2011. Geneva (Switzerland): WHO; 2015. [Google Scholar]
- 129. Zhao L, Zhang X, Shen Y, Fang X, Wang Y, Wang F. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. 2015;16:1081–93. [DOI] [PubMed] [Google Scholar]
- 130. Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S–35S. [DOI] [PubMed] [Google Scholar]
- 131. Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768–72. [DOI] [PubMed] [Google Scholar]
- 132. Gwamaka M, Kurtis JD, Sorensen BE, Holte S, Morrison R, Mutabingwa TK, Fried M, Duffy PE. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis. 2012;54:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Neuberger A, Okebe J, Yahav D, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2016;2:CD006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pasricha S-R, Armitage AE, Prentice AM, Drakesmith H. Reducing anaemia in low income countries: control of infection is essential. BMJ. 2018;362:k3165. [DOI] [PubMed] [Google Scholar]
- 135. Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. McDermid JM, van der Loeff MFS, Jaye A, Hennig BJ, Bates C, Todd J, Sirugo G, Hill AV, Whittle HC, Prentice AM. Mortality in HIV infection is independently predicted by host iron status and SLC11A1 and HP genotypes, with new evidence of a gene-nutrient interaction. Am J Clin Nutr. 2009;90:225–33. [DOI] [PubMed] [Google Scholar]
- 137. Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AK, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet. 2013;382:29–40. [DOI] [PubMed] [Google Scholar]
- 138. de Silva A, Atukorala S, Weerasinghe I, Ahluwalia N. Iron supplementation improves iron status and reduces morbidity in children with or without upper respiratory tract infections: a randomized controlled study in Colombo, Sri Lanka. Am J Clin Nutr. 2003;77:234–41. [DOI] [PubMed] [Google Scholar]
- 139. Jayaweera J, Reyes M, Joseph A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci Rep. 2019;9:12637. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140. Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75:126–32. [DOI] [PubMed] [Google Scholar]
- 141. Frise MC, Cheng H-Y, Nickol AH, Curtis MK, Pollard KA, Roberts DJ, Ratcliffe PJ, Dorrington KL, Robbins PA. Clinical iron deficiency disturbs normal human responses to hypoxia. J Clin Invest. 2016;126:2139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sonnweber T, Pizzini A, Tancevski I, Löffler-Ragg J, Weiss G. Anaemia, iron homeostasis and pulmonary hypertension: a review. Intern Emerg Med. 2020;15:573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cronin SJF, Woolf CJ, Weiss G, Penninger JM. The role of iron regulation in immunometabolism and immune-related disease. Front Mol Biosci. 2019;6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jabara HH, Boyden SE, Chou J, Ramesh N, Massaad MJ, Benson H, Bainter W, Fraulino D, Rahimov F, Sieff Cet al. . A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet. 2016;48:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Savy M, Edmond K, Fine PEM, Hall A, Hennig BJ, Moore SE, Mulholland K, Schaible U, Prentice AM. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr. 2009;139:2154S–218S. [DOI] [PubMed] [Google Scholar]
- 146. Prentice AM, Bah A, Jallow MW, Jallow AT, Sanyang S, Sise EA, Ceesay K, Danso E, Armitage AE, Pasricha S-Ret al. . Respiratory infections drive hepcidin-mediated blockade of iron absorption leading to iron deficiency anemia in African children. Sci Adv. 2019;5:eaav9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hippchen T, Altamura S, Muckenthaler MU, Merle U. Hypoferremia predicts hospitalization and oxygen demand in COVID-19 patients. medRxiv [Preprint]. Published online 26 June 2020. doi: 10.1101/2020.06.26.20140525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, Jia X, Wu M, Shi B, Xu Set al. . Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–40. [DOI] [PubMed] [Google Scholar]
- 149. Shah A, Frost JN, Aaron L, Donovan K, Drakesmith H, collaborators . Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit Care. 2020;24:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bolondi G, Russo E, Gamberini E, Circelli A, Meca MCC, Brogi E, Viola L, Bissoni L, Poletti V, Agnoletti V. Iron metabolism and lymphocyte characterisation during Covid-19 infection in ICU patients: an observational cohort study. World J Emerg Surg. 2020;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. 2020;7:faa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Langel SN, Paim FC, Alhamo MA, Lager KM, Vlasova AN, Saif LJ. Oral vitamin A supplementation of porcine epidemic diarrhea virus infected gilts enhances IgA and lactogenic immune protection of nursing piglets. Vet Res. 2019;50:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Chen X, Tu C, Qin T, Zhu L, Yin Y, Yang Q. Retinoic acid facilitates inactivated transmissible gastroenteritis virus induction of CD8+ T-cell migration to the porcine gut. Sci Rep. 2016;6:24152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. West CE, Sijtsma SR, Kouwenhoven B, Rombout J, van der Zijpp AJ. Epithelia-damaging virus infections affect vitamin A status in chickens. J Nutr. 1992;122: 333–9. [DOI] [PubMed] [Google Scholar]
- 155. Jee J, Hoet AE, Azevedo MP, Vlasova AN, Loerch SC, Pickworth CL, Hanson J, Saif LJ. Effects of dietary vitamin A content on antibody responses of feedlot calves inoculated intramuscularly with an inactivated bovine coronavirus vaccine. Am J Vet Res. 2013;74:1353–62. [DOI] [PubMed] [Google Scholar]
- 156. McGill JL, Kelly SM, Guerra-Maupome M, Winkley E, Henningson J, Narasimhan B, Sacco RE. Vitamin A deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves. Sci Rep. 2019;9:15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rahman MM, Mahalanabis D, Hossain S, Wahed MA, Alvarez JO, Siber GR, Thompson C, Santosham M, Fuchs GJ. Simultaneous vitamin A administration at routine immunization contact enhances antibody response to diphtheria vaccine in infants younger than six months. J Nutr. 1999;129:2192–5. [DOI] [PubMed] [Google Scholar]
- 158. Klebanoff CA, Spencer SP, Torabi-Parizi P, Grainger JR, Roychoudhuri R, Ji Y, Sukumar M, Muranski P, Scott CD, Hall JAet al. . Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med. 2013;210:1961–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42:753–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Coutsoudis A, Broughton M, Coovadia HM. Vitamin A supplementation reduces measles morbidity in young African children: a randomized, placebo-controlled, double-blind trial. Am J Clin Nutr. 1991;54:890–5. [DOI] [PubMed] [Google Scholar]
- 161. Tam E, Keats EC, Rind F, Das JK, Bhutta AZA. Micronutrient supplementation and fortification interventions on health and development outcomes among children under-five in low- and middle-income countries: a systematic review and meta-analysis. Nutrients. 2020;12:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev. 2017;3:CD008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Polak SB, Van Gool IC, Cohen D, von der Thüsen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Adhikari SP, Meng S, Wu Y-J, Mao Y-P, Ye R-X, Wang Q-Z, Sun C, Sylvia S, Rozelle S, Raat Het al. . Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JYet al. . Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Laksono BM, de Vries RD, McQuaid S, Duprex WP, de Swart RL. Measles virus host invasion and pathogenesis. Viruses. 2016;8:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. WHO . Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. Geneva (Switzerland): WHO; 2013. [PubMed] [Google Scholar]
- 168. Roberts L. Why measles deaths are surging—and coronavirus could make it worse. Nature2020;580:447–8. Published online 7 April 2020. [Accessed 2020 May 27], Available at: https://www.nature.com/articles/d41586-020-01011-6. [DOI] [PubMed] [Google Scholar]
- 169. Bester JC. Measles and measles vaccination: a review. JAMA Pediatr. 2016;170:1209–15. [DOI] [PubMed] [Google Scholar]
- 170. Alves Graber EM, Andrade FJ, Bost W, Gibbs MA. An update and review of measles for emergency physicians. J Emerg Med. 2020;58:610–5. [DOI] [PubMed] [Google Scholar]
- 171. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005;2005:CD001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sudfeld CR, Navar AM, Halsey NA. Effectiveness of measles vaccination and vitamin A treatment. Int J Epidemiol. 2010;39(Suppl 1):i48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Aluisio AR, Perera SM, Yam D, Garbern S, Peters JL, Abel L, Cho DK, Kennedy SB, Massaquoi M, Sahr Fet al. . Vitamin A supplementation was associated with reduced mortality in patients with Ebola virus disease during the West African outbreak. J Nutr. 2019;149:1757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci. 1971;68:2678–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc Natl Acad Sci. 1976;73:3685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5:66–74. [DOI] [PubMed] [Google Scholar]
- 178. Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140:533–7. [DOI] [PubMed] [Google Scholar]
- 179. Chen Q, Espey MG, Sun AY, Lee J-H, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GRet al. . Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci. 2007;104:8749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kim TK, Lim HR, Byun JS. Vitamin C supplementation reduces the odds of developing a common cold in Republic of Korea Army recruits: randomised controlled trial. BMJ Mil Heal. Published online 5 March 2020. doi: 10.1136/bmjmilitary-2019-001384. [DOI] [PubMed] [Google Scholar]
- 182. Padhani ZA, Moazzam Z, Ashraf A, Bilal H, Salam RA, Das JK, Bhutta ZA. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Langlois PL, Manzanares W, Adhikari NKJ, Lamontagne F, Stoppe C, Hill A, Heyland DK. Vitamin C administration to the critically ill: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2019;43:335–46. [DOI] [PubMed] [Google Scholar]
- 184. Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG; Canadian Critical Care Trials Group . A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–97. [DOI] [PubMed] [Google Scholar]
- 185. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151:1229–38. [DOI] [PubMed] [Google Scholar]
- 186. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, Deane AM, Shehabi Y, Hajjar LA, Oliveira Get al. . Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. 2020;323:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Fowler AA, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR, Natarajan R, Brophy DFet al. . Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322:1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Li R, Guo C, Li Y, Qin Z, Huang W. Therapeutic targets and signaling mechanisms of vitamin C activity against sepsis: a bioinformatics study. Brief Bioinform. Published online 11 May 2020. doi: 10.1093/bib/bbaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Chen L, Hu C, Hood M, Zhang X, Zhang L, Kan J, Du J. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: a perspective from system biology analysis. Nutrients. 2020;12:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr Clin Res Rev. 2020;14:367–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Rozga M, Cheng FW, Moloney L, Handu D. Effects of micronutrients or conditional amino acids on COVID-19-related outcomes: an evidence analysis center scoping review. J Acad Nutr Diet. Published online May 2020. doi: 10.1016/j.jand.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system—working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Sassi F, Tamone C, D'Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10:1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, Bohn T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12: 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lanham-New SA, Webb AR, Cashman KD, Buttriss JL, Fallowfield JL, Masud T, Hewison M, Mathers JC, Kiely M, Welch AAet al. . Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutr Prev Health. 2020;3:106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012;71:50–61. [DOI] [PubMed] [Google Scholar]
- 199. Greiller LC, Martineau RA. Modulation of the immune response to respiratory viruses by vitamin D. J Nutr. 2015;7:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci. 1998;95:9541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Vargas Buonfiglio LG, Cano M, Pezzulo AA, Vanegas Calderon OG, Zabner J, Gerke AK, Comellas AP. Effect of vitamin D(3) on the antimicrobial activity of human airway surface liquid: preliminary results of a randomised placebo-controlled double-blind trial. BMJ Open Resp Res. 2017;4:e000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche Aet al. . SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ghafouri-Fard S, Noroozi R, Omrani MD, Branicki W, Pośpiech E, Sayad A, Pyrc K, Łabaj PP, Vafaee R, Taheri Met al. . Angiotensin converting enzyme: a review on expression profile and its association with human disorders with special focus on SARS-CoV-2 infection. Vasc Pharmacol. 2020;130:106680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020:1–4.. Published online 06 May 2020. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Singh AK, Gupta R, Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr Clin Res Rev. 2020;14:283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Silva MC, Furlanetto TW. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr Res. 2015;35:91–6. [DOI] [PubMed] [Google Scholar]
- 208. Ghashut RA, Talwar D, Kinsella J, Duncan A, McMillan DC. The effect of the systemic inflammatory response on plasma vitamin 25(OH)D concentrations adjusted for albumin. PLoS One. 2014;9:e92614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Williams AM, Ladva CN, Leon JS, Lopman BA, Tangpricha V, Whitehead RD, Armitage AE, Wray K, Morovat A, Pasricha SRet al. . Changes in micronutrient and inflammation serum biomarker concentrations after a norovirus human challenge. Am J Clin Nutr. 2019;110: 1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. van Schoor N, Lips P. Global overview of vitamin D status. Endocrinol Metab Clin North Am. 2017;46:845–70. [DOI] [PubMed] [Google Scholar]
- 211. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180:P23–54. [DOI] [PubMed] [Google Scholar]
- 212. Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard Cet al. . Vitamin D deficiency in Europe: pandemic?. Am J Clin Nutr. 2016;103:1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Cashman KD, Sheehy T, O'Neill CM. Is vitamin D deficiency a public health concern for low middle income countries? A systematic literature review. Eur J Nutr. 2019;58:433–53. [DOI] [PubMed] [Google Scholar]
- 214. CDC . COVID-19. People with certain medical conditions. 2019.2020, [Accessed 2020 Jun 21]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html.
- 215. Bikle DD, Schwartz J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front Endocrinol. 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 2013;136:321–9. [DOI] [PubMed] [Google Scholar]
- 217. Pham H, Rahman A, Majidi A, Waterhouse M, Neale RE. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: a systematic review and meta-analysis. Int J Environ Res Public Health. 2019;16:3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AAet al. . Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall ECet al. . Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23:1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Arihiro S, Nakashima A, Matsuoka M, Suto S, Uchiyama K, Kato T, Mitobe J, Komoike N, Itagaki M, Miyakawa Yet al. . Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Loeb M, Dang AD, Thiem VD, Thanabalan V, Wang B, Nguyen NB, Tran HTM, Luong TM, Singh P, Smieja Met al. . Effect of vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: a randomized controlled trial. Influenza Other Respi Viruses. 2019;13:176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, Schwartz RS. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Hueniken K, Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe KE, Dai DWH, Laupacis A, Mamdani M, Mazzulli Tet al. . Effect of high-dose vitamin D supplementation on upper respiratory tract infection symptom severity in healthy children. Pediatr Infect Dis J. 2019;38:564–8. [DOI] [PubMed] [Google Scholar]
- 224. Shimizu Y, Ito Y, Yui K, Egawa K, Orimo H. Intake of 25-hydroxyvitamin D3 reduces duration and severity of upper respiratory tract infection: a randomized, double-blind, placebo-controlled, parallel group comparison study. J Nutr Health Aging. 2018;22:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Zhou J, Du J, Huang L, Wang Y, Shi Y, Lin H. Preventive effects of vitamin D on seasonal influenza A in infants: a multicenter, randomized, open, controlled clinical trial. Pediatr Infect Dis J. 2018;37:749–54. [DOI] [PubMed] [Google Scholar]
- 226. Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe K, Chen Y, Laupacis A, Mamdani M, Macarthur C, Hoch JSet al. . Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Scragg R. The Vitamin D Assessment (ViDA) Study—design and main findings. J Steroid Biochem Mol Biol. 2020;198:105562. [DOI] [PubMed] [Google Scholar]
- 228. Gold DR, Litonjua AA, Carey VJ, Manson JE, Buring JE, Lee I-M, Gordon D, Walter J, Friedenberg G, Hankinson JLet al. . Lung VITAL: rationale, design, and baseline characteristics of an ancillary study evaluating the effects of vitamin D and/or marine omega-3 fatty acid supplements on acute exacerbations of chronic respiratory disease, asthma control, pneumonia and lung function in adults. Contemp Clin Trials. 2016;47:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Manson JE, Bassuk SS, Buring JE. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J Steroid Biochem Mol Biol. 2020;198:105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Chun RF, Shieh A, Gottlieb C, Yacoubian V, Wang J, Hewison M, Adams JS. Vitamin D binding protein and the biological activity of vitamin D. Front Endocrinol. 2019;10:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Slow S, Pearson JP, Florkowski CM, Elder PA, Lewis JG, Kennedy MA, Murdoch DR. Effect of genetic factors on the response to vitamin D(3) supplementation in the VIDARIS randomized controlled trial. Nutrition. 2020;75–6:110761. [DOI] [PubMed] [Google Scholar]
- 233. Al-Daghri NM, Mohammed AK, Bukhari I, Rikli M, Abdi S, Ansari MGA, Sabico S, Hussain SD, Alenad A, Al-Saleh Yet al. . Efficacy of vitamin D supplementation according to vitamin D-binding protein polymorphisms. Nutrition. 2019;63–64:148–54. [DOI] [PubMed] [Google Scholar]
- 234. Jolliffe DA, Greiller CL, Mein CA, Hoti M, Bakhsoliani E, Telcian AG, Simpson A, Barnes NC, Curtin JA, Custovic Aet al. . Vitamin D receptor genotype influences risk of upper respiratory infection. Br J Nutr. 2018;120:891–900. [DOI] [PubMed] [Google Scholar]
- 235. Mansy W, Ibrahim NH, Al-Gawhary S, Alsubaie SS, Abouelkheir MM, Fatani A, Abd Al Reheem F, El Awady H, Zakaria EA. Vitamin D status and vitamin D receptor gene polymorphism in Saudi children with acute lower respiratory tract infection. Mol Biol Rep. 2019;46:1955–62. [DOI] [PubMed] [Google Scholar]
- 236. Laplana M, Royo JL, Fibla J. Vitamin D receptor polymorphisms and risk of enveloped virus infection: a meta-analysis. Gene. 2018;678:384–94. [DOI] [PubMed] [Google Scholar]
- 237. D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Cuñat T, Ojeda A, Calvo A. Vitamin D deficiency in critically ill patients diagnosed with COVID-19: are we doing enough? A retrospective analysis of 226 patients. Res Square. [Preprint]. Published online May 2020. doi: 10.21203/rs.3.rs-30390/v1, [Accessed 2020 Jun 7], Available at: https://www.researchsquare.com/article/rs-30390/v1. [Google Scholar]
- 239. Lau FH, Majumder R, Torabi R, Saeg F, Hoffman R, Cirillo JD, Greiffenstein P. Vitamin D insufficiency is prevalent in severe COVID-19. medRxiv [Preprint]. Published online May 2020. doi: 10.1101/2020.04.24.20075838, [Accessed 2020 May 22], Available at: https://doi.org/10.1101/2020.04.24.20075838. [Google Scholar]
- 240. Pinzon RT, Angela, Pradana AW. Vitamin D deficiency among patients with COVID-19: case series and recent literature review. Trop Med Health. 2020;48:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Vitamin D deficiency as risk factor for severe COVID-19: a convergence of two pandemics, [Accessed 2020 May 22]. Available at: https://doi.org/10.1101/2020.05.01.20079376. medRxiv [Preprint]. Published online May 2020. doi: 10.1101/2020.05.01.20079376. [Google Scholar]
- 242. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D deficiency and treatment with COVID-19 incidence. medRxiv [Preprint]. Published online May 2020. doi: 10.1101/2020.05.08.20095893, [Accessed 2020 May 22], Available at: https://doi.org/10.1101/2020.05.08.20095893. [Google Scholar]
- 243. Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, Jani BD, Welsh P, Mair FS, Gray SRet al. . Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr Clin Res Rev. 2020;14:561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Alipio MM. Vitamin D supplementation could possibly improve clinical outcomes of patients infected with coronavirus-2019 (COVID-19). SSRN Electron J, 2020. doi: 102139/ssrn3571484. Publication date 09 April 2020. [Google Scholar]
- 245. Raharusun P, Priambada S, Budiarti C, Agung E, Budi C. Patterns of COVID-19 mortality and vitamin D: an Indonesian study. SSRN Electron J. 2020. doi: 10.2139/ssrn.3585561, Publication date 26 April 2020. [Google Scholar]
- 246. Henrina J, Lim MA, Pranata R. COVID-19 and misinformation: how an infodemic fuelled the prominence of vitamin D. Br J Nutr. 2020;125:359–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Lee GY, Han SN. The role of vitamin E in immunity. Nutrients. 2018;10:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA, Beck MA. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001;15:1846–8. [PubMed] [Google Scholar]
- 250. Beck MA, Handy J, Levander OA. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Beck MA, Kolbeck PC, Rohr LH, Shi Q, Morris VC, Levander OA. Vitamin E deficiency intensifies the myocardial injury of coxsackievirus B3 infection of mice. J Nutr. 1994;124:345–58. [DOI] [PubMed] [Google Scholar]
- 252. Levander OA, Ager AL, Beck MA. Vitamin E and selenium: contrasting and interacting nutritional determinants of host resistance to parasitic and viral infections. Proc Nutr Soc. 1995;54:475–87. [DOI] [PubMed] [Google Scholar]
- 253. Hemilä H, Virtamo J, Albanes D, Kaprio J. Vitamin E and beta-carotene supplementation and hospital-treated pneumonia incidence in male smokers. Chest. 2004;125:557–65. [DOI] [PubMed] [Google Scholar]
- 254. Hemilä H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging. 2016;11:1379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons. JAMA. 2002;288:715. [DOI] [PubMed] [Google Scholar]
- 256. Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007. [DOI] [PubMed] [Google Scholar]
- 257. Torrinhas RS, Calder PC, Lemos GO, Waitzberg DL. Parenteral fish oil: an adjuvant pharmacotherapy for coronavirus disease 2019?. Nutrition. 2021;81:110900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Calder PC. n-3 Fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc. 2013;72:326–36. [DOI] [PubMed] [Google Scholar]
- 259. Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fat Acids. 2007;77:15. [DOI] [PubMed] [Google Scholar]
- 260. Wu D, Lewis ED, Pae M, Meydani SN. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol. 2019;9:3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. [DOI] [PubMed] [Google Scholar]
- 262. Koekkoek W, Panteleon V, van Zanten AR. Current evidence on ω-3 fatty acids in enteral nutrition in the critically ill: a systematic review and meta-analysis. Nutrition. 2019;59:56–68. [DOI] [PubMed] [Google Scholar]
- 263. Langlois PL, D'Aragon F, Hardy G, Manzanares W. Omega-3 polyunsaturated fatty acids in critically ill patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Nutrition. 2019;61:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Dushianthan A, Cusack R, Burgess VA, Grocott MPW, Calder PC. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst Rev. 2019;1:CD012041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Bistrian BR. Parenteral fish-oil emulsions in critically ill COVID-19 emulsions. J Parenter Enter Nutr. 2020;44:1168–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Torrinhas RS, Calder PC, Waitzberg DL. Response to Bistrian BR. Parenteral fish-oil emulsions in critically ill COVID-19 emulsions. J Parenter Enter Nutr. 2020;44:1169–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Messina G, Polito R, Monda V, Cipolloni L, Di Nunno N, Di Mizio G, Murabito P, Carotenuto M, Messina A, Pisanelli Det al. . Functional role of dietary intervention to improve the outcome of COVID-19: a hypothesis of work. Int J Mol Sci. 2020;21:3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133:1463S. [DOI] [PubMed] [Google Scholar]
- 270. Bai J. The combined effect of selenium deficiency and viral infection on the myocardium of mice (preliminary study). Acta Acad Med Sin. 1980;2:29–31. [PubMed] [Google Scholar]
- 271. Beck MA, Kolbeck PC, Rohr LH, Shi Q, Morris VC, Levander OA. Benign human enterovirus becomes virulent in selenium-deficient mice. J Med Virol. 1994;43:166–70. [DOI] [PubMed] [Google Scholar]
- 272. Beck MA, Kolbeck PC, Shi Q, Rohr LH, Morris VC, Levander OA. Increased virulence of a human enterovirus (coxsackievirus B3) in selenium-deficient mice. J Infect Dis. 1994;170:351–7. [DOI] [PubMed] [Google Scholar]
- 273. Beck MA, Shi Q, Morris VC, Levander OA. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433–6. [DOI] [PubMed] [Google Scholar]
- 274. Zhou H, Wang T, Li Q, Li D. Prevention of Keshan disease by selenium supplementation: a systematic review and meta-analysis. Biol Trace Elem Res. 2018;186:98–105. [DOI] [PubMed] [Google Scholar]
- 275. Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15:1481–3. [PubMed] [Google Scholar]
- 276. Jaspers I, Zhang W, Brighton LE, Carson JL, Styblo M, Beck MA. Selenium deficiency alters epithelial cell morphology and responses to influenza. Free Radic Biol Med. 2007;42:1826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Sheridan PA, Zhong N, Carlson BA, Perella CM, Hatfield DL, Beck MA. Decreased selenoprotein expression alters the immune response during influenza virus infection in mice. J Nutr. 2007;137:1466–71. [DOI] [PubMed] [Google Scholar]
- 278. Stýblo M, Walton FS, Harmon AW, Sheridan PA, Beck MA. Activation of superoxide dismutase in selenium-deficient mice infected with influenza virus. J Trace Elem Med Biol. 2007;21:52–62. [DOI] [PubMed] [Google Scholar]
- 279. Alhazzani W, Jacobi J, Sindi A, Hartog C, Reinhart K, Kokkoris S, Gerlach H, Andrews P, Drabek T, Manzanares Wet al. . The effect of selenium therapy on mortality in patients with sepsis syndrome. Crit Care Med. 2013;41:1555–64. [DOI] [PubMed] [Google Scholar]
- 280. Allingstrup M, Afshari A. Selenium supplementation for critically ill adults. Cochrane Database Syst Rev. 2015;(7):CD003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Huang T-S, Shyu Y-C, Chen H-Y, Lin L-M, Lo C-Y, Yuan S-S, Chen P-J. Effect of parenteral selenium supplementation in critically ill patients: a systematic review and meta-analysis. PLoS One. 2013;8:e54431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Kong Z, Wang F, Ji S, Deng X, Xia Z. Selenium supplementation for sepsis: a meta-analysis of randomized controlled trials. Am J Emerg Med. 2013;31:1170–5. [DOI] [PubMed] [Google Scholar]
- 283. Landucci F, Mancinelli P, De Gaudio AR, Virgili G. Selenium supplementation in critically ill patients: a systematic review and meta-analysis. J Crit Care. 2014;29:150–6. [DOI] [PubMed] [Google Scholar]
- 284. Li S, Tang T, Guo P, Zou Q, Ao X, Hu L, Tan L. A meta-analysis of randomized controlled trials: efficacy of selenium treatment for sepsis. Medicine. 2019;98:e14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Manzanares W, Dhaliwal R, Jiang X, Murch L, Heyland DK. Antioxidant micronutrients in the critically ill: a systematic review and meta-analysis. Crit Care. 2012;16:R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Manzanares W, Lemieux M, Elke G, Langlois PL, Bloos F, Heyland DK. High-dose intravenous selenium does not improve clinical outcomes in the critically ill: a systematic review and meta-analysis. Crit Care. 2016;20:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhao Y, Yang M, Mao Z, Yuan R, Wang L, Hu X, Zhou F, Kang H. The clinical outcomes of selenium supplementation on critically ill patients: a meta-analysis of randomized controlled trials. Medicine. 2019;98:e15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Girodon F, Galan P, Monget A-L, Boutron-Ruault M-C, Brunet-Lecomte P, Preziosi P, Arnaud J, Manuguerra J-C, Hercberg S. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients. Arch Intern Med. 1999;159:748. [DOI] [PubMed] [Google Scholar]
- 289. Allsup SJ, Shenkin A, Gosney MA, Taylor S, Taylor W, Hammond M, Zambon MC. Can a short period of micronutrient supplementation in older institutionalized people improve response to influenza vaccine? A randomized, controlled trial. J Am Geriatr Soc. 2004;52:20–4. [DOI] [PubMed] [Google Scholar]
- 290. Ivory K, Prieto E, Spinks C, Armah CN, Goldson AJ, Dainty JR, Nicoletti C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin Nutr. 2017;36:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Broome CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80:154–62. [DOI] [PubMed] [Google Scholar]
- 292. Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Weglarz-Tomczak E, Tomczak JM, Giurg M, Burda-Grabowska M, Brul S. Discovery of potent inhibitors of PLproCoV2 by screening libraries of selenium-containing compounds. bioRxiv [Preprint]. 2020. doi: 10.1101/2020.05.20.107052. [Google Scholar]
- 295. Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7:e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Himoto T, Masaki T. Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease. Nutrients. 2018;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Siva S, Rubin DT, Gulotta G, Wroblewski K, Pekow J. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Overbeck S, Rink L, Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch Immunol Ther Exp (Warsz). 2008;56:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Aggarwal R, Sentz J, Miller MA. Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a meta-analysis. Pediatrics. 2007;119:1120–30. [DOI] [PubMed] [Google Scholar]
- 301. Howie S, Bottomley C, Chimah O, Ideh R, Ebruke B, Okomo U, Onyeama C, Donkor S, Rodrigues O, Tapgun Met al. . Zinc as an adjunct therapy in the management of severe pneumonia among Gambian children: randomized controlled trial. J Glob Health. 2018;8:010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Brown N, Kukka AJ, Mårtensson A. Efficacy of zinc as adjunctive pneumonia treatment in children aged 2 to 60 months in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Paediatr Open. 2020;4:e000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Hemilä H, Fitzgerald JT, Petrus EJ, Prasad A. Zinc acetate lozenges may improve the recovery rate of common cold patients: an individual patient data meta-analysis. Open Forum Infect Dis. 2017;4:059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR, Weirauch MT. The human transcription factors. Cell. 2018;172:650–65. [DOI] [PubMed] [Google Scholar]
- 305. Andreini C, Bertini I. A bioinformatics view of zinc enzymes. J Inorg Biochem. 2012;111:150–6. [DOI] [PubMed] [Google Scholar]
- 306. Becker KW, Skaar EP. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev. 2014;38:1235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GMet al. . Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Mayor-Ibarguren A, Busca-Arenzana C, Robles-Marhuenda Á. A hypothesis for the possible role of zinc in the immunological pathways related to COVID-19 infection. Front Immunol. 2020;11:1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, Williams DA, Okafor EC, Pullen MF, Nicol MRet al. . Hydroxychloroquine in nonhospitalized adults with early COVID-19. Ann Intern Med. Published online 16 July 2020. doi: 10.7326/m20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa Tet al. . Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. Published online 23 July 2020. doi: 10.1056/nejmoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi Met al. . A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding W-Q. Chloroquine is a zinc ionophore. PLoS One. 2014;9:e109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Derwand R, Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19?. Med Hypotheses. 2020;142:109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Shittu MO, Afolami OI. Improving the efficacy of chloroquine and hydroxychloroquine against SARS-CoV-2 may require zinc additives—a better synergy for future COVID-19 clinical trials. Le Infez Med. 2020;28:192–7. [PubMed] [Google Scholar]
- 315. Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Morgan MJ, Liu Z-G. Reactive oxygen species in TNFα-induced signaling and cell death. Mol Cells. 2010;30:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Salzano S, Checconi P, Hanschmann E-M, Lillig CH, Bowler LD, Chan P, Vaudry D, Mengozzi M, Coppo L, Sacre Set al. . Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci. 2014;111:12157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Lenz A-G, Jorens PG, Meyer B, De Backer W, Van Overveld F, Bossaert L, Maier KL. Oxidatively modified proteins in bronchoalveolar lavage fluid of patients with ARDS and patients at-risk for ARDS. Eur Respir J. 1999;13:169. [DOI] [PubMed] [Google Scholar]
- 319. Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Grandl G, Wolfrum C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol. 2018;40:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, Helmstädter J, Kröller-Schön S, Münzel Tet al. . Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019;2019:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Schmidt HHHW, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, Cuadrado A. Antioxidants in translational medicine. Antioxid Redox Signal. 2015;23:1130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Zhang Y, Ding S, Li C, Wang Y, Chen Z, Wang Z. Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: a meta-analysis. Exp Ther Med. 2017;14:2863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Åkerlund B, Jarstrand C, Lindeke B, Sönnerborg A, Åkerblad A-C, Rasool O. Effect of N-acetylcysteine (NAC) treatment on HIV-1 infection: a double-blind placebo-controlled trial. Eur J Clin Pharmacol. 1996;50:457–61. [DOI] [PubMed] [Google Scholar]
- 325. Sotelo N, de los Ángeles Durazo M, Gonzalez A, Dhanakotti N. Early treatment with N-acetylcysteine in children with acute liver failure secondary to hepatitis A. Ann Hepatol. 2009;8:353–8. [PubMed] [Google Scholar]
- 326. De Flora S, Grassi C, Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J. 1997;10:1535–41. [DOI] [PubMed] [Google Scholar]
- 327. Abeysekera R, Illangasekera U, Jayalath T, Sandeepana A, Kularatne S. Successful use of intravenous N-acetylcysteine in dengue haemorrhagic fever with acute liver failure. Ceylon Med J. 2013;57:166. [DOI] [PubMed] [Google Scholar]
- 328. Kumarasena RS, Mananjala Senanayake S, Sivaraman K, de Silva AP, Dassanayake AS, Premaratna R, Wijesiriwardena B, de Silva HJ. Intravenous N-acetylcysteine in dengue-associated acute liver failure. Hepatol Int. 2010;4:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Senanayake M, Jayamanne M, Kankananarachchi I. N-acetylcysteine in children with acute liver failure complicating dengue viral infection. Ceylon Med J. 2013;58:80. [DOI] [PubMed] [Google Scholar]
- 330. Guerrero CA, Torres DP, García LL, Guerrero RA, Acosta O. N-acetylcysteine treatment of rotavirus-associated diarrhea in children. Pharmacotherapy. 2014;34:e333–40. [DOI] [PubMed] [Google Scholar]
- 331. Martindale R, Patel JJ, Taylor B, Arabi YM, Warren M, McClave SA. Nutrition therapy in critically ill patients with coronavirus disease 2019. J Parenter Enter Nutr. 2020;44:1174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. WHO, Bruce A, Liang W. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020, World Health Organization, Geneva. [Google Scholar]
- 334. Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: post-viral syndrome post COVID-19. Med Hypotheses. 2020;144:110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard Cet al. . ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. [DOI] [PubMed] [Google Scholar]
- 336. Romano L, Bilotta F, Dauri M, Macheda S, Pujia A, De Santis GL, Tarsitano MG, Merra G, Di Renzo L, Esposito Eet al. . Short report—medical nutrition therapy for critically ill patients with COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4035–9. [DOI] [PubMed] [Google Scholar]
- 337. Cena H, Maffoni S, Braschi V, Brazzo S, Pallavicini C, Vietti I, Portale S, Corradi E. Position paper of the Italian Association of Medical Specialists in Dietetics and Clinical Nutrition (ANSISA) on nutritional management of patients with COVID-19 disease. Med J Nutrition Metab. 2020;13:113–7. [Google Scholar]
- 338. Naja F, Hamadeh R. Nutrition amid the COVID-19 pandemic: a multi-level framework for action. Eur J Clin Nutr. 2020;74:1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Brugliera L, Spina A, Castellazzi P, Cimino P, Arcuri P, Negro A, Houdayer E, Alemanno F, Giordani A, Mortini Pet al. . Nutritional management of COVID-19 patients in a rehabilitation unit. Eur J Clin Nutr. 2020;74:860–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Anderson L. Providing nutritional support for the patient with COVID-19. Br J Nurs. 2020;29:458–9. [DOI] [PubMed] [Google Scholar]
- 341. Caccialanza R, Laviano A, Lobascio F, Montagna E, Bruno R, Ludovisi S, Corsico AG, Di Sabatino A, Belliato M, Calvi Met al. . Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Wang H, Zeng T, Wu X, Sun H. Holistic care for patients with severe coronavirus disease 2019: an expert consensus. Int J Nurs Sci. 2020;7:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Frajkova Z, Tedla M, Tedlova E, Suchankova M, Geneid A. Postintubation dysphagia during COVID-19 outbreak—contemporary review. Dysphagia. 2020;35:549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon Bet al. . Hydroxychloroquine for COVID-19—preliminary report. Effect of hydroxychloroquine in hospitalized patients. medRxiv [Preprint]. Published online 15 July 2020. doi: 10.1101/2020.07.15.20151852, [Accessed 2020 Jul 28], Available at: https://doi.org/10.1101/2020.07.15.20151852. [Google Scholar]
- 345. The RECOVERY Collaborative Group . Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. James P, Friis H, Woodd S, Rehman AM, PrayGod G, Kelly P, Koethe JR, Filteau S. Minimal impact of an iron-fortified lipid-based nutrient supplement on Hb and iron status: a randomised controlled trial in malnourished HIV-positive African adults starting antiretroviral therapy. Br J Nutr. 2015;114:387. [DOI] [PubMed] [Google Scholar]
- 347. Sochas L, Channon AA, Nam S. Counting indirect crisis-related deaths in the context of a low-resilience health system: the case of maternal and neonatal health during the Ebola epidemic in Sierra Leone. Health Policy Plan. 2017;32:iii32–9. [DOI] [PubMed] [Google Scholar]
- 348. Roberton T, Carter ED, Chou VB, Stegmuller AR, Jackson BD, Tam Y, Sawadogo-Lewis T, Walker N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Heal. 2020;8:e901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Fore HH, Dongyu Q, Beasley DM, Ghebreyesus TA. Child malnutrition and COVID-19: the time to act is now. Lancet. 2020;396:517–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.