Abstract
Urine 11-dehydro-thromboxane B2 (u11-dh-TxB2), 8-hydroxy-2’-deoxyguanosine (8-OHdG), and liver-type fatty acid binding protein levels (L-FABP) at the time of hospitalization were higher in coronavirus disease 2019 (COVID-19) patients with adverse events vs without events. Higher u11-dh-TxB2 and L-FABP levels were associated with longer hospitalization, more thrombotic events, and greater mortality, providing evidence for potential utility as early prognostic biomarkers for COVID-19.
Keywords: COVID-19, liver-type fatty acid binding protein, urinary 11-dehydro- thromboxane B2, urinary 8-hydroxy-2’-deoxyguanosine
Coronavirus disease 2019 (COVID-19), a global pandemic, can be associated with rapid and severe disease progression leading to death. Standard biomarkers of inflammation and hypercoagulability such as C-reactive protein, interleukin-6, procalcitonin, fibrinogen, and d-dimer are currently measured in blood samples as diagnostic and prognostic markers [1]. However, limited data exist on the use of urinary biomarkers and their relation to COVID-19 severity and outcomes. Early assessment of COVID-19 using urinary markers may facilitate rapid and effective intervention to prevent the progression of the disease to a severe state. Measurement of biomarkers in urine specimens may be advantageous compared with blood specimens due to the comparative ease of sample collection. Urine in some cases is also associated with improved analyte stability and reduced matrix-related assay interference.
METHODS
Patient Consent
We report a subanalysis of the evaluation of hemostasis in hospitalized COVID-19 patients (TARGET-COVID) study (https://www.clinicaltrials.gov; unique identifier: NCT04493307). The study was performed in accordance with standard ethical principles, and the design of the study was approved by the local institutional review board of LifeBridge Health. All patients provided written consent.
Patients and Laboratory Methods
We enrolled hospitalized patients who were diagnosed with COVID-19 by reverse transcription polymerase chain reaction assay (n = 123) and patients without COVID-19 but with pneumonia and an elevated D-dimer (COVID-19 negative, n = 18). Blood and urine samples were collected within 48 hours of hospital admission, and serial samples were collected on a subset of patients (n = 22) at day 3 and days 5–8 from the baseline. Standard blood biomarkers were determined in the central hospital laboratory (Sinai Hospital, Baltimore, MD, USA). Urinary 11-dehydro-thromboxane B2 (u11-dh-TxB2) levels were determined using an enzyme-linked immunoassay, and microalbumin levels were determined using the Dimension clinical chemistry system at Inflammatory Markers Laboratory (Wichita, KS, USA) [2]. Urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG) levels were measured by enzyme-linked immunoassay at CEDx Laboratory (Nashua, NH, USA), and liver-type fatty acid binding protein (L-FABP) levels were determined using a rapid, point-of-care lateral flow immunoassay (Timewell Medical, Tokyo, Japan) whose results were quantified using a CHR-631 Rapid Test Reader (Kaiwood Technology Co., Ltd, Tainan City, Taiwan) [3, 4]. In-hospital all-cause death, thrombotic events (venous thrombosis, pulmonary thromboembolism, myocardial infarction, and ischemic stroke), nonconvalescent plasma transfusion, and bleeding occurrences were recorded.
RESULTS
Sixty-seven percent of the patients were African American. There were no significant differences in age, sex, comorbidities, Sequential Organ Failure Assessment (SOFA) score, or levels of urine biomarkers between COVID-19-positive and -negative patients (P = ns for all measurements). Thirty-eight patients had an in-hospital clinical event including death (n = 21), pulmonary embolism (n = 6), stroke (n = 3), and myocardial infarction (n = 8). Compared with patients without events, patients with events were older (57 ± 19 vs 67 ± 14 years) and had a higher prevalence of diabetes (40% vs 53%), higher SOFA score (2.3 ± 1.7 vs 5.2 ± 4.3), more days of hospitalization (9 ± 6 vs 17 ± 20), and higher d-dimer (1.9 ± 2.7 fibrinogen equivalent units vs 4.5 ± 4.8 fibrinogen equivalent units), lactate dehydrogenase (402 ± 281 vs 540 ± 280), and procalcitonin levels (0.9 ± 3.5 ng/mL vs 3.6 ± 11.8 ng/mL; P < .05 for all).
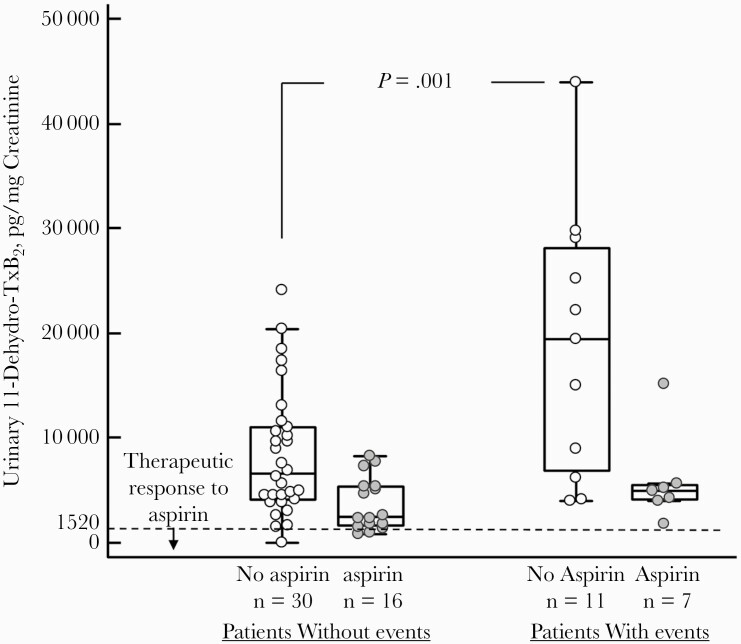
In our study, in patients who were on aspirin at the time of hospitalization, u11-dh-TxB2 levels were lower (P = .001) compared with patients not on aspirin, but only 17% of patients had u11-dh-TxB2 levels lower than the cutoff value for aspirin therapeutic response (<1520 pg/mg creatinine) [2]. At the time of hospitalization, u11-dh-TxB2 levels were higher in aspirin-treated patients with events vs without events (median, 7603 pg/mg creatinine; 95% CI, 7541–19 791 pg/mg creatinine; vs median, 4890 pg/mg creatinine; 95% CI, 5049–8290 pg/mg creatinine; P = .002) (Table 1) and in non-aspirin-treated patients with events vs without events (P = .001) (Figure 1). In addition, u11-dh-TxB2 levels were higher in patients with prolonged (≥10 days) hospitalization (P = .02), death (P = .004), and mechanical ventilation requirement (P < .001) (Table 1). Urinary 11-dh-TxB2 levels were increased at day 3 (P = .01) and returned to similar levels as baseline levels at days 5–8. In patients not treated with aspirin, there was a good correlation between u11-dh-TxB2 and d-dimer (area under the curve, 0.60; P < .001), but not between CRP or any other conventional markers tested.
Table 1.
Relation of Urinary Biomarkers to Outcomes in Patients with COVID-19
| No. | Median (95% CI) | No. | Median (95% CI) | P Value | |
|---|---|---|---|---|---|
| Without Events | With Events | ||||
| L-FABP, ng/mL | 67 | 3.9 (3.4–8.6) | 24 | 9.9 (9.7–32.2) | <.001 |
| 11-dh-TxB2, pg/mg creatinine | 47 | 4890 (5049–8290) | 18 | 7603 (7541–19 791) | .002 |
| 8-OHdG, ng/mg creatinine | 37 | 64 (67–116) | 15 | 78 (78–227) | .04 |
| Microalbumin, mg/L | 36 | 22 (15–58) | 9 | 66 (24–283) | .47 |
| Creatinine, mg/dL | 95 | 0.8 (0.7–0.9) | 38 | 0.99 (0.8–1.4) | .45 |
| <10 d of Hospitalization | ≥10 d of Hospitalization | ||||
| Creatinine, mg/dL | 57 | 0.79 (0.76–0.99) | 54 | 0.91 (1.09–2.3) | .007 |
| 11-dh-TxB2, pg/mg creatinine | 35 | 4801 (3817–9196) | 30 | 8614 (7990–14 316) | .02 |
| L-FABP, ng/mL | 57 | 4.4 (3.8–10.4) | 34 | 6.2 (3.9–14.1) | .05 |
| No Death | Death | ||||
| 11-dh-TxB2, pg/mg creatininea | 48 | 5360 (5907–10 038) | 6 | 15 069 (1915–42 007) | .004 |
| L-FABP, ng/mL | 80 | 4.7 (3.7–5.3) | 11 | 10.6 (4.1–32.6) | .03 |
| No Mechanical Ventilation | Mechanical Ventilation | ||||
| 11-dh-TxB2, pg/mg creatinine | 56 | 5137 (4498–7512) | 9 | 20 121 (5364–41 015) | <.001 |
| L-FABP, ng/mL | 82 | 4.6 (3.6–5.2) | 9 | 5.4 (5.2–43.4) | .01 |
Abbreviations: 11-dh-TxB2, 11-dehydro-thromboxane B2; 8-OHdG, 8-hydroxy-2’-deoxyguanosine; COVID-19, coronavirus disease 2019; L-FABP, liver fatty acid binding protein.
aIncludes patients not on aspirin therapy.
Figure 1.
Urinary 11-dehydro-thromboxane B2 in aspirin- and non-aspirin-treated patients with and without events.
Similarly, L-FABP levels were higher in patients with events vs without events (median, 9.9 ng/mL; 95% CI, 9.7–32.2 ng/mL; vs median, 3.9 ng/mL; 95% CI, 3.4–8.6 ng/mL; P ≤ .001) and higher in patients with prolonged hospitalization (P = .05), death (P = .03), and mechanical ventilation requirement (P = .01) (Table 1). 8-OHdG levels were higher in patients with events vs without events (median, 78 ng/mg creatinine; 95% CI, 78–227 ng/mg creatinine; vs median, 64 ng/mg creatinine; 95% CI, 53–116 ng/mg creatinine; P = .04) (Table 1). Patients with L-FABP >10 ng/mL, an indicator of renal ischemia, compared with patients <10 ng/mL, had greater u11-dh-TxB2 levels (median, 13 070 pg/mg creatinine; 95% CI, 5122–19 488 pg/mg creatinine; vs median, 5203 pg/mg creatinine; 95% CI, 4264–7264 pg/mg creatinine; P = .02). Both 8-OHdG and L-FABP levels remained similar across all time points during hospitalization. Urinary microalbumin and urinary creatinine levels were numerically higher in patients with events (P = .47 and P = .45, respectively), whereas urinary creatinine levels were higher in patients with prolonged hospitalization (P = .007) (Table 1).
DISCUSSION
Our results suggest the prognostic utility of 11-dh-TxB2, L-FABP, and 8-OHdG measured in urine in patients with COVID-19 at the time of hospitalization. Urinary 11-dh-TxB2 is a marker of whole-body inflammation and TxA2 biosynthesis contributed by platelet, leukocytes, and endothelial cells [5]. The independent relation of u11-dh-TxB2 to adverse outcomes in patients with cardiovascular disease and diabetes treated with aspirin has been demonstrated in major clinical trials [6–8]. Among COVID-19 patients in our study, we found significantly elevated levels of u11-dh-TxB2 in patients with events compared with patients without events, and higher u11-dh-TxB2 levels were associated with prolonged hospitalization, death, and mechanical ventilation requirement. These observations highlight the potential prognostic utility of u11-dh-TxB2 in patients with COVID-19. This is in line with the observations of systemic cytokine storm, endothelial dysfunction, and elevated thrombotic risk in COVID-19. In addition, it is also interesting to note that aspirin therapy at the time of hospitalization in most patients was pharmacodynamically inadequate to provide a therapeutic response based on measurements of u11-dh-TxB2 [2].
L-FABP, expressed in proximal renal tubules, is excreted in urine following hypoxia and is a more accurate indicator of acute kidney injury than serum creatinine [9]. L-FABP may assist in predicting the severity of COVID-19 at an early stage [10]. Due to the high metabolic demand of the kidneys, combined with the sensitivity and specificity of urinary L-FABP for renal ischemia, it has been hypothesized that elevations of urinary L-FABP may be indicative of renal ischemia associated with COVID-19. In our study, urine L-FABP levels were higher in COVID-19 patients with events. These observations indicate that patients with more severe COVID-19 have evidence of renal ischemia and that acute renal injury may be, in part, responsible for the adverse outcomes observed in patients with COVID-19. 8-OHdG is a proposed biomarker of oxidative damage of deoxyribonucleic acid (Figure 2). It has been shown to be associated with cardiovascular disease and endothelial dysfunction in patients with diabetes [11–13]. Cytokine storm and a higher prevalence of diabetes among patients with events appear linked to the higher levels of 8-OHdG observed in these patients.
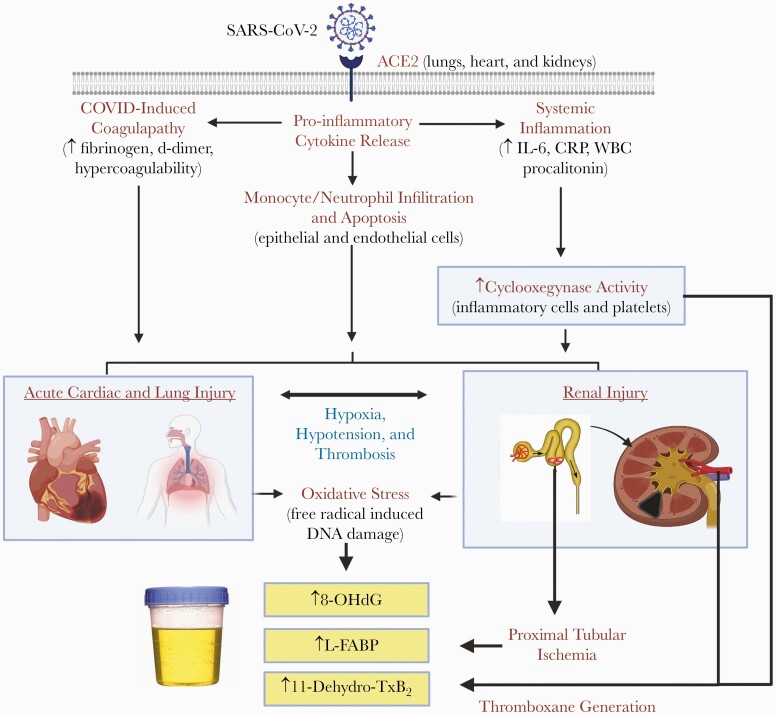
Figure 2.
Mechanistic pathways of urinary biomarkers and relation to coronavirus disease 2019 (COVID-19). COVID-19 is associated with a hypercoagulable state and marked systemic inflammation with resultant tissue ischemia and necrosis. Early elevation in markers of oxidative stress (8-OHdG), proximal glomerular tubular ischemia (L-FABP), and platelet activation/heightened inflammation (11-dehydro-TxB2) found in urine may facilitate assessment of risk.
These pilot data are hypothesis-generating, although this is the largest study to comprehensively study urine biomarkers in COVID-19. Nevertheless, the biomarkers we studied indicate that endothelial dysfunction and inflammation (11-dh-TxB2), renal hypoxia and apoptosis (L-FABP), and oxidative stress (8-OHdG) are associated with the severity of the COVID-19. Most importantly, this study provides the first evidence for early prognostic information on COVID-19 severity, including death, using a simple, noninvasive urine sample. Evidence from larger studies is required to confirm the clinical validity and utility of these noninvasive markers.
Acknowledgments
Financial support. This work was supported by Platelet and Thrombosis Research, LLC, Baltimore, MD, USA. 11-dehydro-thromboxane B2 kits were donated by Corgenix, Inc. (Broomfield, Colorado, USA).
Potential conflicts of interest. Dr. Gurbel reports grants and personal fees from Bayer HealthCare LLC, Otitopic Inc., Amgen, Janssen, and US WorldMeds LLC; grants from Instrumentation Laboratory, Haemonetics, Medicure Inc., Idorsia Pharmaceuticals, and Hikari Dx; and personal fees from UpToDate; Dr. Gurbel is a relator and expert witness in litigation involving clopidogrel; in addition, Dr. Gurbel has 2 patents, Detection of Restenosis Risk in Patients Issued and Assessment of Cardiac Health and Thrombotic Risk in a Patient. Dr. Tantry reports receiving honoraria from UptoDate and Aggredyne. Gordon Ens and Malina Traianova are employees of Inflammatory Markers Laboratory. The other authors report no disclosures. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Udaya Tantry, Kevin Bliden, Malina Traianova, and Jeffrey Dahlen performed experiments. Naval Walia, Christophe Jerjian, Alastair Cho, Kevin Bliden, and Abira Usman enrolled patients and collected patient information. Alastair Cho, Gordon Ens, Kevin Bliden, and Udaya Tantry performed the data analysis. Udaya S Tantry Kevin Bliden and Paul Gurbel wrote the manuscript. Paul A Gurbel supervised the study. All authors contributed to the final manuscript.
Clinical trial registration. Clinical Trials.gov NCT# 04493307.
References
- 1. Elshazli RM, Toraih EA, Elgaml A, et al. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: a meta-analysis of 6320 patients. PLoS One 2020; 15:e0238160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gurbel PA, Bliden KP, Tantry US. Defining platelet response to acetylsalicylic acid: the relation between inhibition of serum thromboxane B2 and agonist-induced platelet aggregation. J Thromb Thrombolysis 2021; 51:260–4. [DOI] [PubMed] [Google Scholar]
- 3. Endo K, Miyashita Y, Sasaki H, et al. Probucol and atorvastatin decrease urinary 8-hydroxy-2’-deoxyguanosine in patients with diabetes and hypercholesterolemia. J Atheroscler Thromb 2006; 13:68–75. [DOI] [PubMed] [Google Scholar]
- 4. Katagiri D, Doi K, Honda K, et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg 2012; 93:577–83. [DOI] [PubMed] [Google Scholar]
- 5. Tantry US, Mahla E, Gurbel PA. Aspirin resistance. Prog Cardiovasc Dis 2009; 52:141–52. [DOI] [PubMed] [Google Scholar]
- 6. Eikelboom JW, Hirsh J, Weitz JI, et al. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 2002; 105:1650–5. [DOI] [PubMed] [Google Scholar]
- 7. Eikelboom JW, Hankey GJ, Thom J, et al. ; Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) Investigators . Incomplete inhibition of thromboxane biosynthesis by acetylsalicylic acid: determinants and effect on cardiovascular risk. Circulation 2008; 118:1705–12. [DOI] [PubMed] [Google Scholar]
- 8. Rocca B, Buck G, Petrucci G, et al. he ASCEND Study Collaborative Group. Thromboxane metabolite excretion is associated with serious vascular events in diabetes mellitus: a sub-study of the ASCEND trial. Eur Heart J 2020; 41(Suppl 2):ehaa946. 2926. [Google Scholar]
- 9. Doi K, Negishi K, Ishizu T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med 2011; 39:2464–9. [DOI] [PubMed] [Google Scholar]
- 10. Katagiri D, Ishikane M, Asai Y, et al. Evaluation of coronavirus disease 2019 severity using urine biomarkers. Crit Care Explor 2020; 2:e0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Minno A, Turnu L, Porro B, et al. 8-hydroxy-2-deoxyguanosine levels and cardiovascular disease: a systematic review and meta-analysis of the literature. Antioxid Redox Signal 2016; 24:548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009; 27:120–39. [DOI] [PubMed] [Google Scholar]
- 13. Bigagli E, Lodovici M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid Med Cell Longev 2019; 2019:5953685. [DOI] [PMC free article] [PubMed] [Google Scholar]