Abstract
Collagen of type I (Col I) and type II (Col II) are critical for cartilage and connective tissues in the human body, and several diseases may alter their properties. Assessing the identification and quantification of fibrillar collagen without biomarkers is a challenge. Advancements in non-invasive polarization-resolved second-harmonic generation (PSHG) microscopy have provided a method for the non-destructive investigation of collagen molecular level properties. Here we explored an alternative polarization modulated approach, dual-LC PSHG, that is based on two liquid crystal devices (Liquid crystal polarization rotators, LPRs) operating simultaneously with a laser scanning SHG microscope. We demonstrated that this more accessible technology allows the quick and accurate generation of any desired linear and circular polarization state without any mechanical parts. This study demonstrates that this method can aid in improving the ability to quantify the characteristics of both types of collagen, including pitch angle, anisotropy, and circular dichroism analysis. Using this approach, we estimated the effective pitch angle for Col I and Col II to be 49.7° and 51.6°, respectively. The effective peptide pitch angle for Col II gel was first estimated and is similar to the value obtained for Col I gel in the previous studies. Additionally, the difference of the anisotropy parameter of both collagen type gels was assessed to be 0.293, which reflects the different type molecular fibril assembly. Further, our work suggests a potential method for monitoring and differentiating different collagen types in biological tissues, especially cartilage or connective tissue.
1. Introduction
Collagen is ubiquitously found inside the human body and is the main structural protein in the extracellular matrix (ECM) of various tissues, such as skin, blood vessels, cartilage, and connective tissues [1–4]. Over the past decade, the investigation of the self-organisation processes of fibrous proteins has enabled and enhanced basic research in the field of biology and medicine, and it has helped in the better understanding of these mechanisms at the molecular level for observing the fibrous changes that occur in the microenvironment of tissues plagued by diseases. For example, collagen, the most abundant protein in the body, is highly altered in cases of cancer, corneal diseases, osteoarthritis, connective tissue diseases, autoimmune disorders, and cardiovascular diseases [5–9]. Since remodelling has been associated with poor prognosis in the connective tissue disease, we further suggest that quantitative structural analysis of the collagen assembly in the microenvironment will help improve the understanding of the etiology and classification of normal and diseased tissues such as osteogenesis imperfecta [10,11]. Collagen is divided into 28 various subtypes, with type I, II, and III making up 80–90% of all collagen in the human body [12]. It is well known that the basic structure of collagen fibrillars in tissue is tropocollagen, which is a triple helix molecule (∼1.5 nm in diameter and ∼300 nm in length) composed of three α-chain incorporations. For example, the most abundant collagen type I (Col I) and type II (Col II) exist as 2α1α2 (two a1(I) chains and one a2(I) chains) and 3α1 (three intertwining a1(II) chains) triple helices, respectively [13–15]. The identification and quantification of the structural organisation, assembly morphology, and amount of fibrillar collagen are important factors that affect the properties of those tissues and play an essential role in many diseases. This may lead to enhanced diagnostic and prognostic capabilities, and also to the development of more efficacious treatments. Additionally, collagen detection and analysis are relevant to fields such as tissue engineering, tissue characterisation, drug development and delivery, and biomechanics. Currently, there are several methods for the visualisation of collagen fibrillars or fibers in tissue, including stains, antibody detection, polarised microscopy, second-harmonic generation (SHG) imaging, atomic force microscopy, and scanning electron microscopy [16–21]. Owing to the highly routine and lower technical sample preparation application, SHG is widely used for imaging fibrillar collagen.
SHG is a label-free, coherent, second-order nonlinear investigation method, which has been successfully used for biological imaging. It was first reported in 1986, when Freund investigated the polarity of collagen fibers in rat tail tendon at a resolution of ∼50 μm [22]. Since SHG microscopy can be used for tissue imaging with rapid data acquisition at a high resolution, as reported by Mohler and Campagnola [23], SHG coupled with polarization analysis has great potential for tissue characterisation. In this way, the molecular and supramolecular structural details can be extracted, especially the nonlinear susceptibility tensor, the protein pitch angle and the dipole alignment angle of the collagen molecular level. In addition, it will allow the reconstruction of fiber orientation maps and the visualisation of the arrangement of the complex architecture of collagen fibrils because the SHG signal response radiates from individual collagen fibrils of approximately 50 nm in diameter and the coherent addition of them [24]. Therefore, polarization-resolved SHG has become a very popular tool for molecular scale information imaging based on polarised light–matter interaction. Furthermore, quantification and analysis methods of SHG signal responses have been developed in recent years. One approach is the textural analysis of SHG intensity [25], such as the spectral method (fast Fourier transform, FFT) [26] and statistical method (grey level co-occurrence matrices, GLCM) [27]. Another approach is based on the intensity and polarization of the generated SHG signal, such as linear polarization polarimetry [28], the stokes vector polarimetry method [10], degree of linear polarization [29] and circular dichroism [30]. Alternatively, Golaraei et al. combine two approaches (linear polarization and GLCM) to investigate alteration of collagen ultrastructure in tumor tissue [31]. Many studies have investigated tissue characterisation based on visualising collagen distribution as a biomarker using polarization-resolved second-harmonic generation (PSHG) microscopy. Researchers have increasingly been attempting to understand SHG-polarised excited matter interaction, specifically for measuring the difference of the SHG signals excited with left-handed circular polarization (LHCP) and right-handed circular polarizations (RHCP) [10, 30, 32–35]. Recently, Schanne-Klein et. al conducted a theoretical analysis on second-harmonic generation-circular dichroism (SHG-CD) signals for collagen chirality [36].
In general, polarization control is more important and more powerful in SHG measurements. In several studies, a motorised half-waveplate was placed in the infinity space as this after most of the distortion inducing elements. This requires an inordinately long time and mechanical noise is observed in the microscope, even if a distortion response model is used to calibrate the system. To overcome these limitations, in a previous study, we have shown the use of a liquid crystal modulator (LCM) to precisely control the linear (or circular) excitation angle in polarization-resolved SHG microscopy [37]. Although this approach has a faster response time than mechanical motors owing to its voltage-dependent control, it is necessary to the add a λ/4 plate following the LCM for the generation of the circular polarization state. Unfortunately, this lower polarization distortion control is restricted for modulating the excitation angle. Polarization state control of the excitation light has been repeatedly shown to play an important role in rapid measurements. Previous studies have used quick polarization control with lower distortion as a useful SHG microscopy tool for modulating the excitation angle of linear polarization; however, directionality has not been used for modulating the polarization state. Since polarization generator applications often involve the absence of mechanical parts, two liquid crystal retarders are preferred due to their optimal control over the state of polarization (SOP). As a result, fully polarized state presentation through two liquid crystal retarders and a quarter-wave plate setup can be achieved, as reported by Morales and Moreno [38]. Therefore, there have been efforts aiming at generating any arbitrary SOP using two voltage-controlled variable retarders in SHG measurement for biological applications.
Recently, Ajeti and Campagnola reported the investigation of the structure and modelling based on Col I and Col V gels for invasive breast carcinoma [39]. In a previous work, we investigated a quantitative approach for differentiating between Col I and Col III isoforms in gel matrix for the stromal models of ovarian cancer [30,40]. Chen et al. differentiated only Col I and Col II only in a series of tissue samples [41–44]. Owing to the challenges in improving the ability for understanding the difference between Col II fibers grown in vitro and native Col II fibers, herein, we report the production of a gel matrix by the self-assembly of pure Col II molecules. To the best of our knowledge, this is the first use of both liquid crystal polarization rotators (LPRs), which are in the linear and circular state, in quick modulation polarization excited SHG microscopy for quantitatively imaging fibrillar collagen in matrix, and for comparison with Col I. We note that the Dual-LC PSHG microscopy are suggested as a powerful tool for improving the visualisation of fibrillar collagen. PSHG images from both types of gel are quantitatively compared considering the peptide pitch angle, anisotropy parameters, anisotropy ratio, and SHG-CD analysis. Our investigation shows that the dual-LC PSHG microscopy has a simpler modality than the previous slower implementations mentioned above. It can be used for quantifying collagen fiber assembly characterisation in the microenvironment.
2. Materials and methods
2.1. Sample preparation
Col I solution, obtained from rat tail (354249, Corning, USA) and Col II solution, obtained from bovine articular cartilage (354257, Corning, USA), were used for preparing the fiber samples grown in vitro. Each series of comparisons of the SHG signatures from the different types of collagen were carried out with gels polymerized at the same time. To achieve the appropriate sample size, three experimental replicates were performed. A working collagen concentration of 1 mg/mL for both types was prepared in ice, with a total gel volume of 0.3 ml, diluted with a phosphate-buffered saline (PBS) solution. The pH of the collagen solution was neutralised using a solution of sterilised sodium hydroxide (NaOH). The collagen solution was slowly polymerised in a micro-slide 8-well culture chamber at 4 °C for 20 h. The gels with thickness of ∼1.5 mm were released from the bottom of the sides of the dish, and then fixed in 4% paraformaldehyde.
2.2. Experimental microscope setup
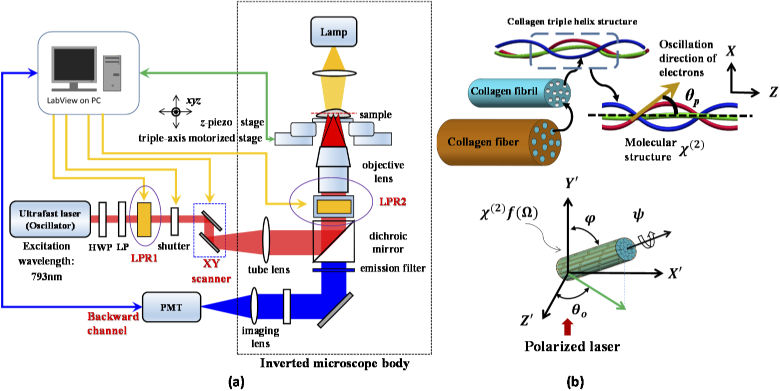
Figure 1 shows the basic system comprising a laser scanning unit mounted on an inverted microscope (Axiovert 200, Zeiss, Germany) and 3D geometric arrangement of a single collagen fiber with orientation angle relative to polarized light. The excitation source is a mode-locked titanium sapphire laser (Tsunami, Spectra-Physics, USA) with about 150 fs pulse width and a repetition rate of 80MHz. The laser power was controlled using a half-wave plate and a linear polariser. For polarization excitation control, we used the aforementioned high-quality circular and rotatable linear polarization, obtained using two LPRs (Meadowlark Optics, USA). The first liquid crystal polarization rotator (LPR1) was fixed outside the microscope body. To consider the infinity space of the microscope, a home-made module containing the second liquid crystal polarization rotator (LPR2) was placed between the dichroic mirror and the objective lens. Therefore, backward SHG was collected in the polarization control experiments in this study. This first use of both LPRs modulated for generating the purely linear, LHCP and RHCP for PSHG measurement. The pure linear polarization was rotated by applying different voltages to LPR2. This combination provides a faster and more precise approach for achieving any desired circular and linear polarization excitation, compared with the motion control approach. The FPGA module was designed to perform several tasks, including control of the galvanometer scanner and the z-axis piezoelectric stage for positioning the 3D focal spot, modulating the voltage for varying the rotation polarization direction, and processing of the single photon counting signals simultaneously. The real-time FPGA DAQ card based on our custom LabVIEW program can synchronously control the instrument through interfaces constructed in-house.
Fig. 1.
(a) Configuration of polarization-resolved optical system. (b) Graphical illustration shows the 3D geometric arrangement of a single collagen fiber with orientation angle relative to polarized light and shows hierarchical organization of collagen fibrils and molecular structure of the triple helices with tensor χ(2 (molecule) coordinate system. The Euler angles are set to Ω = (θ, φ, ψ), where ψ is the angle between the major axis of the distribution f() of dipoles moments within the point spread function (PSF) and its higher cylindrical symmetry axis. In golden yellow, helical pitch angle θp. The dimensions of the figure are nominal and do not correspond to reality.
Moreover, the above two liquid crystal devices were controlled electronically using this program, and it can rapidly produce any polarization state with a conservative response time of ∼30 ms for the rotation step sizes used here (e.g. retardation of 5 degrees). All imaging was performed with a fundamental laser wavelength of 793 nm with an average power of ∼10mW at the focal plane using 20X 0.7 NA objective lens (UPlanApo, Olympus). The resulting lateral and axial resolutions were ∼0.7 and 3μm, respectively. The bandpass filter was set at 370 nm to 410 nm for the SHG signal. The field of view size was 50 μm x 50 μm with a field of 256 by 256 pixels and 1 second exposure time. The optical sectioning images were recorded at the same depth (∼30μm distance between the bottom surface) due to minimum scrambling of optical scattering for comparisons of both types of collagen.
The polarization state with the rotated angle of the major axis could be described by four parameters of the Stokes vector are denoted as I, Q, U, and V (, , , and with the normalised total intensity, ) in the Cartesian coordinate system. More details are given in our previous paper [37] and by Morales et.al. [38]. Because of the initial linear polarization from the Ti:sapphire laser cavity and the use of the half-wave plate and Glan–Laser polariser for power control, we can assume the input polarization state with the major axis at 90° as the fully polarised initial condition. The Muller matrix of the LPR with retardation and an azimuthal angle of the fast axis can be expressed as [45, 46]
| (1) |
For our microscopy system, the polarization state was obtained at the optical sectioning plane. The excited light passes through both LPRs, and the relationship with both retardations was derived as
| (2) |
where and are retardations produced from LPR1 and LPR2 by the applied voltage, respectively. Note that and are 135° in our case.
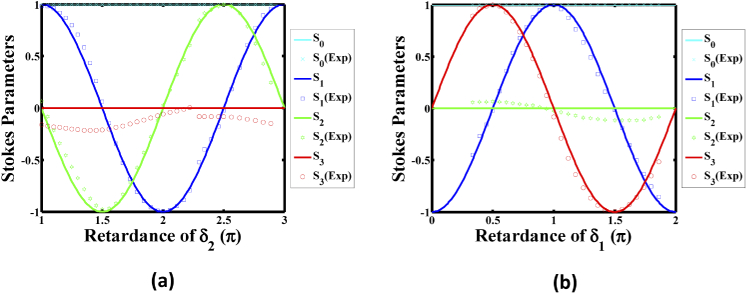
To achieve a full polarization conversion, it is required that each LPR changes retardance by at least 2π. Figure 2 demonstrates two simulation cases. For a linear polarization state, this allows the generation of any desired azimuthal angle, relative to the different retardations of with as zero, the continuous line of which is shown in Fig. 2(a). For another case, we considered the generated SOPs with the varying retardations of with as , the continuous line of which is shown in Fig. 2(b). The results indicated LHCP and RHCP occur at of and of , respectively. Hence, the combination of both LPRs provides a faster and more precise way to achieve any desired linear and circular polarization.
Fig. 2.
Stokes parameters of the simulation (solid lines) and experimental (symbols) performance of the Dual-LC PSHG microscopy system with (a) keeping the retardance constant (zero), and (b) keeping the retardance constant (2π).
A commercial Stokes polarimeter (PAX1000 IR1, Thorlabs, USA) with an accuracy of ±0.25% was used to probe the performance of our measuring system by measuring the Stokes parameters of the SOP at focusing plane. Figure 2 shows the experimental performance of the PSHG system through the applied voltage (symbols), with thorough curve fitting corresponding to the retardance range of from 0 to 2π and to a phase delay range of from π to 3π, respectively. In comparing the simulation curves, it can be seen that the value of (red stars) in Fig. 2(a) and S2 (green stars) in Fig. 2(b) are not zero, and reached a maximum value of 0.21 and 0.11, respectively. The differences for this deviation are similar to a previous paper in the literature [37], suggesting the sources of distortion might be from non-perfect alignment of the scanning galvo mirror pair, Fabry-Perot interference effects, and temperature changes. We note that the practice polarization control for imaging, a look-up table (LUT) was created for LHCP, RHCP and linear polarization states for every 10° of rotation angle from 0–180°.
2.3. Peptide pitch angle and anisotropy analysis
Linear polarization excited image measurements were performed as previously described; images were taken every 10° through 180° of rotation for each optical section [37,40]. Here, the method was applied to different types of tissues. The SHG intensity is proportional to the square of the second-order polarization. The pixel-based SHG intensity polarization response and the maps of equivalent orientation angle were obtained by the pixel-based generic model, which can be expressed as [40]
| (3) |
where θ is the angle direction of the incident electric field E; is the equivalent orientation angle from the distribution function expansion; and r, p, and q are three numerical coefficients that depend on the molecular type and alignment state. These values agree with the experimental data. For the single-axis molecular model, this response can be fit to a single molecular axis model, yielding the α-helix pitch angle [38]
| (4) |
where N is the number density of the elemental dipoles; and the three parameters a, b, and c are numerical coefficients related to the non-vanishing matrix elements of χ(2). Herein, the peptide pitch angle is extracted by combining the pixel-based generic model with the single-axis molecular model in this study. Conventional SHG signal anisotropy was used to study the alignment of the dipole moments, only within the focusing volume. Its response was a function of the laser polarization, which is also related to the self-assembly condition of the collagen molecules. The anisotropy parameter of SHG signal can be obtained if the intensity parallel and perpendicular to the fiber axis I obtained to yield anisotropy parameter (b) in Eq. (5). Its relation with the SHG intensity can be expressed as
| (5) |
where and denote the parallel and perpendicular directions with fiber orientation, respectively. To quantify the tissular scaled orientation distribution of the collagen fibers/fibrils for different tissues, we report the morphological anisotropy analysis determined by the pixel-based equivalent orientation angle thorough the polarization-resolved SHG images. The orientation angle distribution was plotted and approximated with a normal Gaussian distribution curve for each optical section. The anisotropy ratio defined as the presented probability maximum difference (Pmax) over the angular dispersion width (Dwidth) of the fitted curve is given as
| (6) |
where Pmax and Dwidth are calculated by the maximum height and the full width at half maximum (FWHM) of the fitted curve, respectively.
2.4. Second-harmonic generation-circular dichroism
The nonlinear SHG-CD measurements were used to probe chirality of the collagen at the molecular and tissular scales for different tissues. In this paper, for quantifying the different SHG intensities in the tissue, the normalised SHG-CD response is reported and defined as [30,37]
| (7) |
where and are the integrated intensity of all pixels in the SHG images for LHCP and RHCP excitation, respectively. The absolute values were summed across the entire field of view, as the sign of the CD response will depend on fiber orientation.
2.5. Statistical analysis
For all measurements, there are three samples runs and each gel was imaged in three separate locations to provide the average and standard deviation of each parameter. The polarization data are expressed as the mean with standard error of the mean (SEM). A one-way analysis of variance (ANOVA) was used to test for statistical differences, and multiple comparisons were performed using Scheffe’s method. Statistical significance was set at p < 0.05.
3. Experimental results
3.1. Pixel-based effective pitch angle analysis
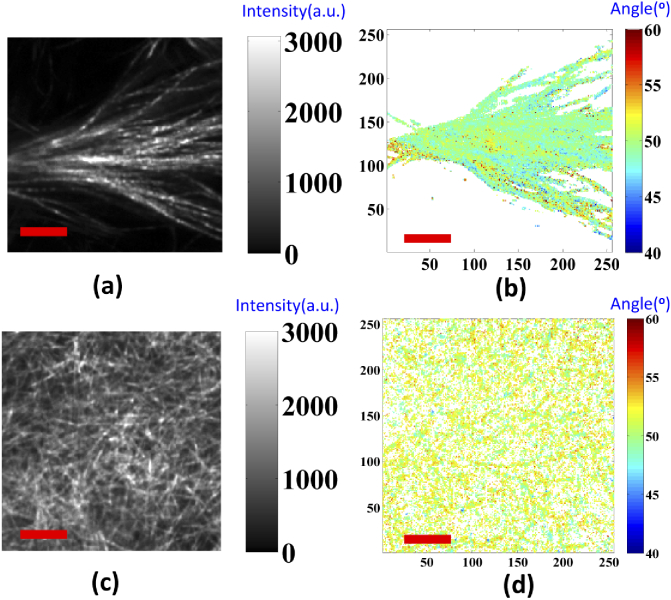
For the collagen gel matrix, we used a pixel-based linear polarization-resolved approach to evaluate the effective pitch angle for detecting the polarization images of collagen. The original images involved 18 optical cross-section images, with different linear polarization rotation angle excitations. The average SHG intensity images of the 100% Col I and Col II gels are shown in Figs. 3(a-b) and 3(c-d), respectively. In Figs. 3(b) and 3(d), the peptide pitch angle maps are obtained using the pixel-by-pixel calculation, based on the single axis of the molecular generic model, and the reconstructed polarization response data, which is expressed in Eqs. (3) and (4). In addition, an effective pitch angle was computed for both types of collagen using the data in Figs. 3(b) and 3(d). In Fig. 4, the represented peptide pitch angle of the 100% Col I gels was 49.7°, which is consistent with our previous findings [40]. For the Col II gels, the averaged extracted peptide pitch angle was 51.6°, as this Col II isoform of bovine has a higher pitch angle than Col I.
Fig. 3.
Representative SHG images and maps of pitch angles for the collagen gels matrix. (a-b) are Col I; (c-d) are Col II. Image size is 50 µm x 50 µm and scale bar (red line) is 10 µm.
Fig. 4.
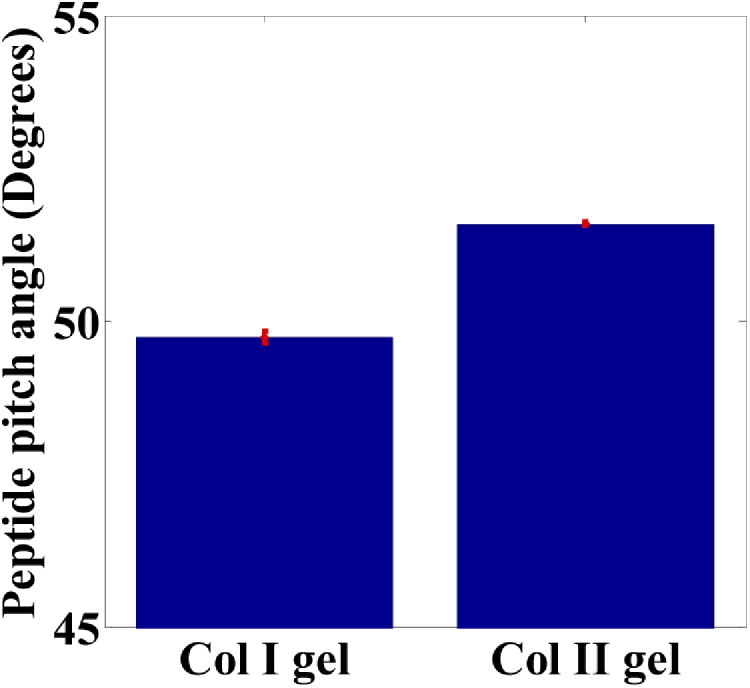
Effective peptide pitch angle for 100% Col I gel and 100% Col II gel. Error bars represent the standard error of the mean. The mean separation is determined at α = 0.001 level.
3.2. Morphological and molecular anisotropy analysis
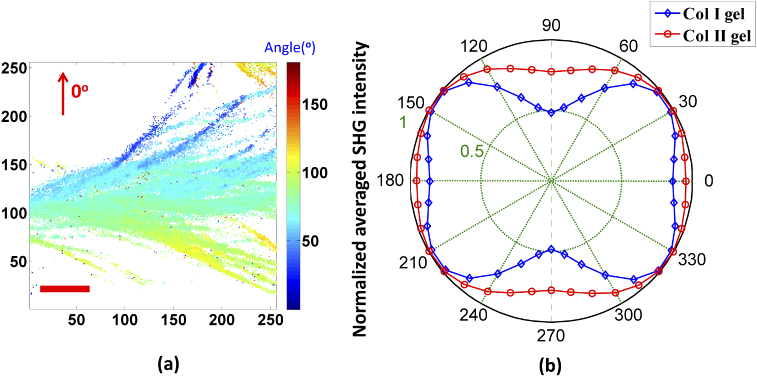
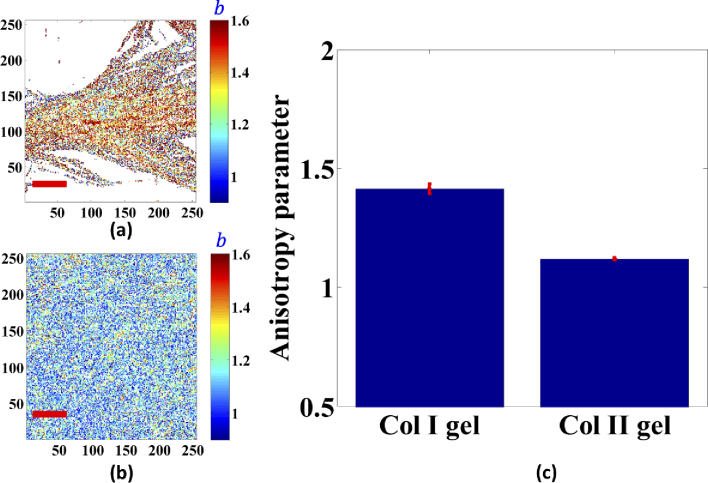
The representative map of pixel-based resolution was extracted by using the single axis of molecular generic model, which is shown in Fig. 5(a). Figure 5(b) statistically shows the polar dependence of the normalised SHG signal for both types of collagen gels. Note that the SHG polarization response was obtained referred to corresponding equivalent orientation angle of collagen fibers/fibrils for each pixel as the primary axis. For simplicity, we focus on describing the pixel-based anisotropic behaviour (anisotropy parameter) at 0° and 90° excitation, which correspond to directions that are parallel and perpendicular to the primary axis for the linear fiber, respectively. Figures 6(a) and 6(b) depict the representative resolved anisotropy parameter images of Col I and Col II gels. The statistical resulting values are shown in Fig. 6(c). The Col I gel has a significantly higher anisotropy parameter of 1.413 ± 0.021. For Col II gel, the obtained anisotropy parameter was 1.120 ± 0.004. These values are in good agreement with the values reported by Freund and Dong et al. for Col I and Col II of the tissue samples [22,42-45, 47], which are in the 1.2–2 range for Col I and in 1.1-1.3 for Col II.
Fig. 5.
(a) Representative orientation distribution of the collagen fibers/fibrils of Col I gel. Image size is 50 µm x 50 µm and scale bar (red line) is 10 µm. (b) Relation between the excitation angle of the linearly polarization light and the normalised averaged SHG intensity.
Fig. 6.
Resolved anisotropy parameter images of Col I gels (a), Col II gels (b), and the averaged anisotropy parameter (c). Error bars represent standard error of the mean. The mean separation is determined at α = 0.001. Image size is 50 µm x 50 µm and scale bar (red line) is 10 µm.
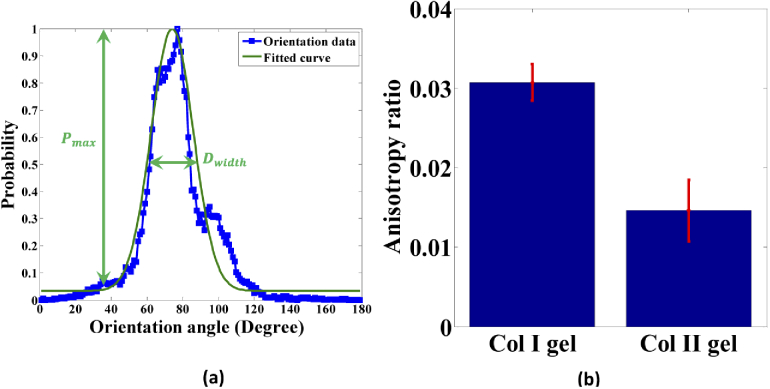
Owing to the quantitative estimation of the tissular scaled orientation distribution of the collagen fibers/fibrils in the gel matrix, the histogram of the orientation angle was normalised and fitted with a normal Gaussian curve, shown in Fig. 7(a). The morphological anisotropy is determined by the ratio of the probability maximum difference to the FWHM of the fitted curve. In Fig. 7(b), the mean anisotropy ratio values are 0.0307 ± 0.0023 and 0.0146 ± 0.0038 for Col I and Col II gels, respectively. Thus, we obtained Col II has a 52 percentage decrease in comparison with Col I. A high anisotropy ratio corresponds to a strong alignment orientation distribution of the collagen fibers, whereas a low anisotropy ratio indicates a more random orientation of fibers or fibrils in the gel matrix. Note that multiple dominant orientations may exist, which would increase the error of the normal fit. In comparison with the above molecular analysis approach, the coefficient of variation be calculated. We obtained that the values of 1.5% (Col I) and 0.4% (Col II) from Fig. 6(b) are all smaller than the values of 7.5% (Col I) and 26.0% (Col II) from Fig. 7(b). The results show the small variation of anisotropy parameter with the different the gel morphology or numbers of fibers and proof that is appropriated for quantitative molecular level.
Fig. 7.
Morphological anisotropy analysis. (a) Normalised histograms of orientation angle distribution with a normal Gaussian curve fitting corresponding to Fig. 5(a). (b) Anisotropy ratio values between Col I and Col II gels. The mean separation is determined at α = 0.05.
3.3. SHG circular dichroism analysis
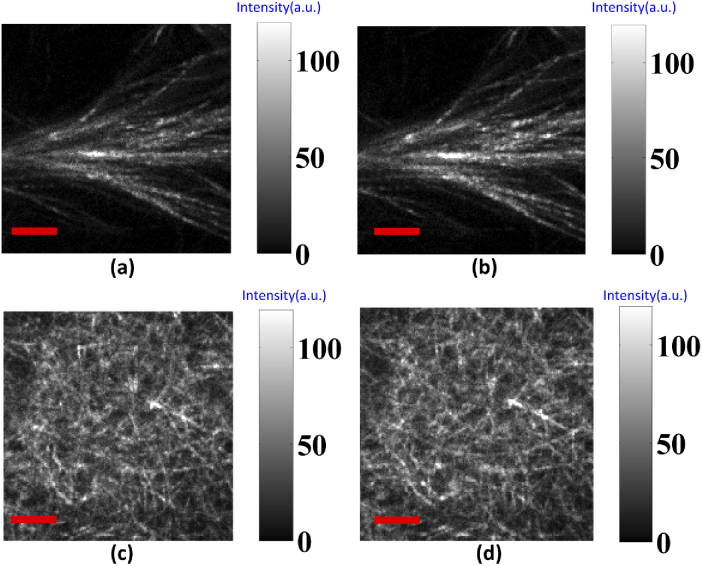
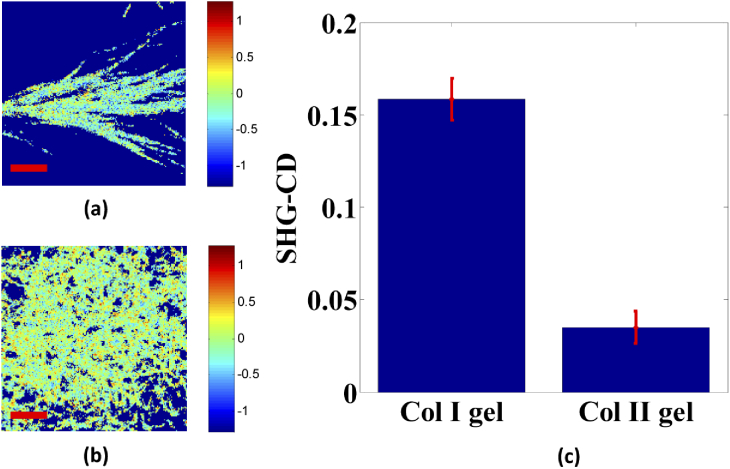
Next, the same samples at the same location were measured using LHCP and RHCP for exciting SHG signal; the original SHG images for both collagen gel types are shown in Fig. 8. Based on the SHG-CD method, representative pixel maps of SHG-CD for 100% Col I and Col II gel matrices are shown in Figs. 9(a) and 9(b), respectively. Additionally, averaged SHG-CD values for Col I and Col II gels were statistically calculated using the experimental data, obtained through the integrated intensity of all pixels in the SHG images for LHCP and RHCP excitation, yielding 0.159 and 0.035, respectively. Figure 9(c) shows the resulting experimental data. The mean SHG-CD response of purely Col II gel was small and significantly lower than that of Col I gel, respectively. This response is reflective of the net chirality within each pixel, and it would be expected that the rich presented Col I zone of articular cartilage (Col I) loses chirality, and the molecules align on the axis for the collagen fibril.
Fig. 8.
LHCP and RHCP SHG images for (a, b) Col I and (c, d) Col II gels, respectively. Image size is 50 µm x 50 µm and scale bar (red line) is 10 µm.
Fig. 9.
Results of SHG circular dichroism analysis. (a) and (b) the SHG-CD maps of Col I and Col II gels, respectively. Image size is 50 µm x 50 µm and scale bar (red line) is 10 µm. (c) Mean SHG-CD response. The mean separation is determined at α = 0.01.
4. Discussion
PSHG is a highly sensitive tool for visualising fibrillar collagen, which is a highly ordered molecule that exhibits intense second harmonic generation (SHG) with a high-contrast ratio of SHG circular dichroism (SHG-CD) at a macroscopic level. Based on this concept, recent PSHG instrumentation methods either modulate laser polarization through rotating wave plates or rotated laser linear polarization by applying voltage into a LPR, which is not sufficient for all SOP. The need for a more compact, rapid, and easily accessible applicable method led us toward using a two LPRs set-up, described in Section 2.2, for modulating all types pf polarization electronically without moving any part. Oldenbourg first used liquid crystals to demonstrate the proof of principle of universal compensation on a wide-field configuration in the PolScope in 1995 [48]. Eliceiri and Oldenbourg also investigated collagen organisation by using LC-PolScope in thin histology sections for measuring the orientation and alignment of collagen fibrillar [49].
Furthermore, Morales and Moreno proposed an optical system using two liquid crystal retarders and a quarter-wave plate to implement any arbitrary state of polarization with the phase-shift controlling of two voltage-controlled variable retarders [38]. Our proposed PSG consists of two liquid crystal polarization rotator devices with their fast axes oriented at the same angle (135°). This enables control of retardation values, producing the varying direction of the major axis by one device and the state of the polarised laser excitation by another. It can be implemented in any laser scanning microscope. Unlike other PSG systems based on two liquid crystals, our system is ideal for setup since this slight variation in configuration removes the requirement for any alignments of the optical axes of the other optical components. Hence, the time required for imaging is lower and the images have high resolution. Further, all images taken with different polarizations, including LHCP/RHCP and rotated laser linear polarization with any desired orientation angle, are perfectly registered. This is also supported by the imaging data and works well in Figs. 3–9, all of which show the expected behaviour for high polarization purity. We note that a small amount of polarization scrambling will occur at the NA used here (0.8), where this effect only becomes significant for NA>1 [37].
Using Col gels matrix samples, as the gold standard method, helped us verify that dual-LC PSHG microscopy can visualise images of Col I fibers with different excitation polarizations. In this study, we have combined the dual-LC PSHG approach with different quantitative analyses to visualise the maps of Col II fibrillar in gels. Collagen is an important component in the tissue of the meniscus, in which 60% collagen is type II and 40% collagen is type I. Both are major fibrillar collagen in many connective tissues of the knee meniscus, in which 90% collagen is type I, and of the articular cartilage, in which 90% collagen is type II [50]. The molecular distribution in tissues conveys important information on their state. For example, 90-95% of collagen in the hyaline articular cartilage, which is a cross-linked polymer with different ECM, is type II having a network form. This provides highly correlated tensile stiffness and strength but also helps in compression. Wherever Col I is found in articular cartilage, there are evidence of ineffective fibrocartilage repair tissue. This indicates damage or pathology and mechanical weakness, making it more permeable than healthy articular cartilage [51]. Hence, a morphological network form of collagen in tissue is also in good agreement with the collagen fibrillar distribution of our home-made self-assembled Col II gels from polarimetric images, which are shown in Figs. 3–9.
In previous studies by Chen et al., cartilage tissues were probed by P-SHG using the anisotropy parameter based on the second-order susceptibility tensor. Their results showed that anisotropy parameters are 1.4 and 1.14, corresponding to rat tendon and trachea cartilage, respectively [41,42]. Recently, Romijn et al. performed studies on the differentiation of type I and II collagen without fulfilled cylindrical symmetry, and the obtained anisotropy parameters were 1.33 and 1.36 for Col I in tendon and Col II in cartilage, respectively [43]. Legare et al. noted that the difference between the average of the forward and backward ratio was high, and the collagen of the foetal and adult meniscal connective tissue based on collagen II is also present in low amounts in foetuses, but increases with age [52]. The result confirms that an organised structure cannot be identified using this discriminatory approach alone. Also, despite having similar sequences of amino acids, this produces by the biochemical differences and assembly microenvironment with having variation sequences. Hence, we also suggest involving multiple SHG metrics. Table 1 summarises all the experimental information on Col I and II gels acquired using different quantitative analysis methods in our study, thus allowing for a thorough analysis. Moreover, the effective peptide pitch angle for Col II gel was first estimated to be 51.6° and is similar to the value obtained for Col I gel. The anisotropy parameter of 1.413 for Col I gels obtained in this study is in agreement with other values reported in the literature; the values vary between 1.13 and 1.7 [22,37,43,47]. For Col II gels, the value of 1.120 is similar to that reported by Su et al. (1.14) [41], Su et al. (1.26) [42], Kumar et al. (1.07-1.20) [44], but is lower than that reported by Romijn et al. (1.36) [43] for a tissue sample. This difference may be due to the difference in the mixture of the collagen types or incoherently oriented fibrils in a tissue sample. We note that the orientation of pixel-based fibers/fibrils obtained using our generic model does not directly fit with the single axis of the molecular model (assuming a strictly cylindrical symmetry) for the calculation of the anisotropy parameter.
Table 1. Results for Col I and Col II collagen gels using SHG polarization-resolved methods a .
| Material | Peptide pitch angle | Anisotropy parameter | Anisotropy ratio | SHG-CD |
|---|---|---|---|---|
| Col I gels | 49.7° (0.09) | 1.413 (0.021) | 0.0307 (0.0023) | 0.159 (0.011) |
| Col II gels | 51.6°(0.04) | 1.120 (0.004) | 0.0015 (0.0038) | 0.035 (0.009) |
Values in brackets represent standard mean measurement error.
For quantitative orientation distribution of the collagen fibers/fibrils at the tissular scale, effective anisotropy ratio values of 0.0307 and 0.0015 were first estimated for Col I and Col II gels, respectively. This result is not surprising, given the similar network form as the random orientation of the hyaline articular cartilage with a high percentage of Col II gel. However, this interesting result demonstrates firstly the gels through the incubation of pure isoforms of collagen molecules and to avoid there is overlap between the different collagen types in comparison with tissue samples.
Macroscopic chirality could dominate SHG-CD instead of molecular chirality, which was indeed the case in previous literature [37]. Additionally, the misfolded collagen resulted in a few fold lower SHG-CD response. Therefore, the SHG-CD approach is sensitive to the overall chirality of the collagen structural assembly. Compared with Col I gel, the significant decrease in the self-assembled Col II gel revealed similar conclusions for the anisotropy parameter and anisotropy parameter ratio, where it would decrease anisotropy or net chirality within the probed focal volume. Moreover, instead of using quantitative methods with rotated linear polarization, SHG-CD can potentially be used on thicker biological samples because circularly polarised light minimises the distortion of the polarization state compared to linear excited polarization [30]. We note that the SHG polarization method leads to significant polarization scrambling due to optical scattering in the thick collagenous tissue sample [53]. For better quantitative results for the thick tissue, optical clearing was utilized for these measurements to preserve the polarization response through the several-hundred-micron tissue thickness [54]. Comparing the forward emission detection schemes, the data acquired in this study using backward collection schemes could be useful for practical applications.
To date, although several studies have been performed on differentiated SHG signal of col I and Col II in tissue, the underlying changes in self-assembled isoforms in culture-based ECM have not been well explored. There is no published work where they are quantitatively compared using peptide pitch angle, anisotropy parameters, anisotropy ratio, and SHG-CD analysis using simpler modality Dual-LC PSHG microscopy. There remains great potential for visualising collagen in tissue, as well as looking for a way to distinguish between different collagen types, especially in cartilage or connective tissue.
5. Conclusion
To conclude, dual-LC PSHG microscopy provides a rapid and precise means of achieving any desired polarization state at the focus of the microscope. This approach is a new means of controlling the polarization state by using electrical voltage and can be used as a practical and viable alternative to previous implementation methods. Additionally, all the methods presented in this paper are promising tools for probing collagen fibrils even with different collagen types. The proposed approach allows not only visualising collagens but also performing a complete and exhaustive characterisation of collagen fibrils/fibers in a bio-sample at various hierarchical levels. It also provides different molecular assembly quantitative results, which can be significant for further studies on the structure of collagen at different scale levels. Hence, the combination of different image analysis methods may represent a powerful tool in the future to provide new insights on understanding the role of collagen in ECM structure and on the development of several cartilage or connective diseases.
Funding
Ministry of Science and Technology, Taiwan10.13039/501100004663 (MOST-108-2221-E-239-019-, MOST-109-2221-E-239-013-); Bio-ICT Funding of Kaohsiung Medical University10.13039/501100004694and National Yang Ming Chiao Tung University (NCTUKMU 108-BIO-04).
Disclosures
The authors declare no conflicts of interest.
References
- 1.Chen S. Y., Chen S. U., Wu H. Y., Lee W. J., Liao Y. H., Sun C. K., “In vivo virtual biopsy of human skin by using noninvasive higher harmonic generation microscopy,” IEEE J. Sel. Top. Quantum Electron. 16(3), 478–492 (2010). 10.1109/JSTQE.2009.2031987 [DOI] [Google Scholar]
- 2.Kwon G. P., Schroeder J. L., Amar M. J., Remaley A. T., Balaban R. S., “Contribution of macromolecular structure to the retention of low-density lipoprotein at arterial branch points,” Circulation 117(22), 2919–2927 (2008). 10.1161/CIRCULATIONAHA.107.754614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockbank K. G., MacLellan W. R., Xie J., Hamm-Alvarez S. F., Chen Z. Z., Schenke-Layland K., “Quantitative second harmonic generation imaging of cartilage damage,” Cell Tissue Banking 9(4), 299–307 (2008). 10.1007/s10561-008-9070-7 [DOI] [PubMed] [Google Scholar]
- 4.Bielajew B. J., Hu J. C., Athanasiou K. A., “Collagen: quantification, biomechanics and role of minor subtypes in cartilage,” Nat. Rev. Mater. 5(10), 730–747 (2020). 10.1038/s41578-020-0213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keikhosravi A., Bredfeldt J. S., Sagar A. K., Eliceiri K. W., “Second-harmonic generation imaging of cancer,” Methods Cell Biol. 123, 531–546 (2014). 10.1016/B978-0-12-420138-5.00028-8 [DOI] [PubMed] [Google Scholar]
- 6.Campagnola P., “Second harmonic generation imaging microscopy: applications to diseases diagnostics,” Anal. Chem. 83(9), 3224–3231 (2011). 10.1021/ac1032325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provenzano P. P., Inman D. R., Eliceiri K. W., Knittel J. G., Yan L., Rueden C. T., White J. G., Keely P. J., “Collagen density promotes mammary tumor initiation and progression,” BMC Med. 6(1), 11 (2008). 10.1186/1741-7015-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo W., Teng S. W., Tan H. Y., Kim K. H., Chen H. C., Lee H. S., Chen Y. F., So P. T. C., Dong C. Y., “Intact corneal stroma visualization of GFP mouse revealed by multiphoton imaging,” Microsc. Res. Tech. 69(12), 973–975 (2006). 10.1002/jemt.20373 [DOI] [PubMed] [Google Scholar]
- 9.Mansfield J. C., Mandalia V., Toms A., Winlove C. P., Brasselet S., “Collagen reorganization in cartilage under strain probed by polarization sensitive second harmonic generation microscopy,” J. R. Soc. Interface 16(150), 20180611 (2019). 10.1098/rsif.2018.0611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X. Y., Raggio C., Campagnola P. J., “Second-harmonic generation circular dichroism studies of osteogenesis imperfecta,” Opt. Lett. 37(18), 3837–3839 (2012). 10.1364/OL.37.003837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunziker E. B., Lippuner K., Keel M. J. B., Shintani N., “An educational review of cartilage repair: precepts and practice- myths and misconceptions -progress and prospects,” Osteoarthr. Cartil. 23(3), 334–350 (2015). 10.1016/j.joca.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 12.Sorushanova A., Delgado L. M., Wu Z., Shologu N., Kshirsagar A., Raghunath R., Mullen A. M., Bayon Y., Pandit A., Raghunath M., Zeugolis D. I., “The collagen suprafamily: from biosynthesis to advanced biomaterial development,” Adv. Mater. 31(1), 1801651 (2019). 10.1002/adma.201801651 [DOI] [PubMed] [Google Scholar]
- 13.Shoulders M. D., Raines R. T., “Collagen structure and stability,” Annu. Rev. Biochem. 78(1), 929–958 (2009). 10.1146/annurev.biochem.77.032207.120833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma U., Carrique L., Goff S. V. L., Mariano N., Georges R.-N., Delolme F., Koivunen P., Myllyharju J., Moali C., Aghajari N., Hulmes D. J. S., “Structural basis of homo- and heterotrimerization of collagen I,” Nat. Commun. 8(1), 14671 (2017). 10.1038/ncomms14671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An B., Abbonante V., Yigit S., Balduini A., Kaplan D. L., Brodsky B., “Definition of the native and denatured type II collagen binding site for fibronectin using a recombinant collagen system,” J. Biol. Chem. 289(8), 4941–4951 (2014). 10.1074/jbc.M113.530808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allon I., Vered M., Buchner A., Dayan D., “Stromal differences in salivary gland tumors of a common histopathogenesis but with different biological behavior: a study with picrosirius red and polarizing microscopy,” Acta Histochem. 108(4), 259–264 (2006). 10.1016/j.acthis.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Brisson B. K., Mauldin E. A., Lei W., Vogel L. K., Power A. M., Lo A., Dopkin D., Khanna C., Wells R. G., Puré E., Volk S. W., “Type III collagen directs stromal organization and limits metastasis in a murine model of breast cancer,” Am. J. Pathol. 185(5), 1471–1486 (2015). 10.1016/j.ajpath.2015.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drifka C. R., Loeffler A. G., Mathewson K., Mehta G., Keikhosravi A., Liu Y., Lemancik S., Ricke W. A., Weber S. M., Kao W. J., Eliceiri K. W., “Comparison of picrosirius red staining with second harmonic generation imaging for the quantification of clinically relevant collagen fiber features in histopathology samples,” J. Histochem. Cytochem. 64(9), 519–529 (2016). 10.1369/0022155416659249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Nadiarynkh O., Plotnikov S., Campagnola P. J., “Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure,” Nat. Protoc. 7(4), 654–669 (2012). 10.1038/nprot.2012.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plodinec M., Loparic M., Monnier C. A., Obermann E. C., Zanetti-Dallenbach R., Oertle P., Hyotyla J. T., Aebi U., Bentires-Alj M., Lim R. Y. H., Schoenenberger C.-A., “The nanomechanical signature of breast cancer,” Nat. Nanotechnol. 7(11), 757–765 (2012). 10.1038/nnano.2012.167 [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M., Furuya Y., Okabayashi T., Araki K., “Scanning electron microscopic study of the three dimensional structure of the collagen sheath surrounding cancer cells after single high-dose irradiation,” Med. Mol. Morphol. 39(2), 106–112 (2006). 10.1007/s00795-005-0308-1 [DOI] [PubMed] [Google Scholar]
- 22.Freund I., Deutsch M., Sprecher A., “Connective tissue polarity. Optical second-harmonic microscopy, crossed-beam summation, and small-angle scattering in rat-tail tendon,” Biophys. J. 50(4), 693–712 (1986). 10.1016/S0006-3495(86)83510-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campagnola P. J., Millard A. C., Terasaki M., Hoppe P. E., Malone C. J., Mohler W. A., “Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues,” Biophys. J. 82(1), 493–508 (2002). 10.1016/S0006-3495(02)75414-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams R. M., Zipfel W. M., Webb W. W., “Interpreting second-harmonic generation images of collagen I fibrils,” Biophys. J. 88(2), 1377–1386 (2005). 10.1529/biophysj.104.047308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kistenev Y. V., Vrazhnov D. A., Nikolaev V. V., Sandykova E. A., Krivova N. A., “Analysis of collagen spatial structure using multiphoton microscopy and machine learning methods,” Biochemistry (Moscow) 84(S1), 108–123 (2019). 10.1134/S0006297919140074 [DOI] [PubMed] [Google Scholar]
- 26.Lo W., Chen W. L., Hsueh C. M., Ghazaryan A. A., Chen S. J., Ma D. H. K., Dong C. Y., Tan H. Y., “Fast Fourier transform based analysis of second-harmonic generation image in keratoconic cornea,” Invest. Ophthalmol. Visual Sci. 53(7), 3051–3507 (2012). 10.1167/iovs.10-6697 [DOI] [PubMed] [Google Scholar]
- 27.Cicchi R., Kapsokalyvas D., Troiano M., Campolmi P., Morini C., Massi D., Cannarozzo G., Lotti T., Pavone F. S., “In vivo non-invasive monitoring of collagen remodelling by two-photon microscopy after micro-ablative fractional laser resurfacing,” J. Biophotonics 7(11-12), 914–925 (2014). 10.1002/jbio.201300124 [DOI] [PubMed] [Google Scholar]
- 28.Hristu R., Stanciu S. G., Tranca D. E., Stanciu G. A., “Improved quantification of collagen with polarization-resolved second harmonic generation microscopy,” J. Biophotonics 10(9), 1171–1179 (2017). 10.1002/jbio.201600197 [DOI] [PubMed] [Google Scholar]
- 29.Tokarz D., Cisek R., Golaraei A., Asa S. L., Barzda V., Wilson B. C., “Ultrastructural features of collagen in thyroid carcinoma tissue observed by polarization second harmonic generation microscopy,” Biomed. Opt. Express 6(9), 3475–3481 (2015). 10.1364/BOE.6.003475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell K. R., Campagnola P. J., “Wavelength-dependent second harmonic generation circular dichroism for differentiation of Col I and Col III isoforms in stromal models of ovarian cancer based on intrinsic chirality differences,” J. Phys. Chem. B 121(8), 1749–1757 (2017). 10.1021/acs.jpcb.6b06822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golaraei A., Mostaço-Guidolin L. B., Raja V., Navab R., Wang T., Sakashita S., Yasufuku K., Tsao M.-S., Wilson B. C., Barzda V., “Polarimetric second-harmonic generation microscopy of the hierarchical structure of collagen in stage I-III non-small cell lung carcinoma,” Biomed. Opt. Express 11(4), 1851–1863 (2020). 10.1364/BOE.387744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuo G.-Y., Chen M.-Y., Yeh C.-Y., Guo C.-L., Kao F.-J., “Fast determination of three-dimensional fibril orientation of type-I collagen via macroscopic chirality,” Appl. Phys. Lett. 110(9), 093902 (2017). 10.1063/1.4977502 [DOI] [Google Scholar]
- 33.Chen M.-Y., Huttunen M., Kan C.-W., Deka G., Lin Y.-Y., Ye C.-W., Wu M.-J., Liu H.-L., Chu S.-W., “Resonant nonlinear microscopy reveals changes in molecular level chirality,” Opt. Commun. 422, 56–63 (2018). 10.1016/j.optcom.2018.03.005 [DOI] [Google Scholar]
- 34.Golaraei A., Kontenis L., Mirsanaye K., Krouglov S., Akens M. K., Wilson B. C., Barzda V., “Complex susceptibilities and chiroptical effects of collagen measured with polarimetric second-harmonic generation microscopy,” Sci. Rep. 9(1), 12488 (2019). 10.1038/s41598-019-48636-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmeltz M., Teulon C., Latour G., Ghoubay D., Borderie V., Aimé C., Schanne-Klein M.-C., “Implementation of artifact-free circular dichroism SHG imaging of collagen,” Opt. Express 27(16), 22685–22699 (2019). 10.1364/OE.27.022685 [DOI] [PubMed] [Google Scholar]
- 36.Schmeltz M., Teulon C., Pinsard M., Hansen U., Alnawaiseh M., Ghoubay D., Borderie V., Mosser G., Aimé C., Légaré F., Latour G., Schanne-Klein M.-C., “Circular dichroism second-harmonic generation microscopy probes the polarity distribution of collagen fibrils,” Optica 7(11), 1469–1476 (2020). 10.1364/OPTICA.399246 [DOI] [Google Scholar]
- 37.Lien C.-H., Tilbury K., Chen S.-J., Campagnola P. J., “Precise, motion-free polarization control in second harmonic generation microscopy using a liquid crystal modulator in the infinity space,” Biomed. Opt. Express 4(10), 1991–2002 (2013). 10.1364/BOE.4.001991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales G. L., d M., Sánchez-López M., Moreno I., “Liquid-crystal polarization state generator,” Proc. SPIE 11351, 113511P (2020). 10.1117/12.2555697 [DOI] [Google Scholar]
- 39.Ajeti V., Suzanne O. N., Ponik M., Keely P. J., Eliceiri K. W., Campagnola P. J., “Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: implications for probing stromal alterations in human breast cancer,” Biomed. Opt. Express 2(8), 2307–2316 (2011). 10.1364/BOE.2.002307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilbury K., Lien C.-H., Chen S.-J., Campagnola P. J., “Differentiation of Col I and Col III isoforms in stromal models of ovarian cancer by analysis of second harmonic generation polarization and emission directionality,” Biophys. J. 106(2), 354–365 (2014). 10.1016/j.bpj.2013.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su P.-J., Chen W.-L., Li T.-H., Chou C.-K., Chen T.-H., Ho Y.-Y., Huang C.-H., Chang S.-J., Huang Y.-Y., Lee H.-S., Dong C.-Y., “The discrimination of type I and type II collagen and the label-free imaging of engineered cartilage tissue,” Biomaterials 31(36), 9415–9421 (2010). 10.1016/j.biomaterials.2010.08.055 [DOI] [PubMed] [Google Scholar]
- 42.Su P.-J., Chen W.-L., Chen Y.-F., Dong C.-Y., “Determination of collagen nanostructure from second-order susceptibility tensor analysis,” Biophys. J. 100(8), 2053–2062 (2011). 10.1016/j.bpj.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romijn E. I., Finnøy M A., Lilledahl B., “Analyzing the feasibility of discriminating between collagen types I and II using polarization-resolved second harmonic generation,” J. Biophotonics 12(1), e201800090 (2019). 10.1002/jbio.201800090 [DOI] [PubMed] [Google Scholar]
- 44.Kumar R., Grønhaug K. M., Romijn E. I., Finnøy A., Davies C. L., Drogset J. O., Lilledahl M. B., “The use of optical trap and microbeam to investigate the mechanical and transport characteristics of tunneling nanotubes in tumor spheroids,” J. Biophotonics 8(9), 730–739 (2015). 10.1002/jbio.201400086 [DOI] [PubMed] [Google Scholar]
- 45.Gil J. J., Bernabeu E., “Obtainment of the polarizing and retardation parameters of a non-depolarizing optical system from the polar decomposition of its Mueller matrix,” Optik 76(2), 67–71 (1987). [Google Scholar]
- 46.Bueno J. M., “Polarimetry using liquid-crystal variable retarders: theory and calibration,” J. Opt. A-Pure Appl. Op. 2(3), 216–222 (2000). 10.1088/1464-4258/2/3/308 [DOI] [Google Scholar]
- 47.Fuentes-Corona C. G., Licea-Rodriguez J., Younger R., Rangel-Rojo R., Potma E. O., Rocha-Mendoza I., “Second harmonic generation signal from type I collagen fibers grown in vitro,” Biomed. Opt. Express 10(12), 6449–6461 (2019). 10.1364/BOE.10.006449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oldenbourg R., “A new view on polarization microscopy,” Nature 381(6585), 811–812 (1996). 10.1038/381811a0 [DOI] [PubMed] [Google Scholar]
- 49.Keikhosravi A., Liu Y., Drifka C., Woo K. M., Verma A., Oldenbourg R., Eliceiri K. W., “Quantification of collagen organization in histopathology samples using liquid crystal based polarization microscopy,” Biomed. Opt. Express 8(9), 4243–4256 (2017). 10.1364/BOE.8.004243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eleswarapu S. V., Responte D. J., Athanasiou K. A., “Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint,” PLoS ONE 6(10), e26178 (2011). 10.1371/journal.pone.0026178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armiento A. R., Alini M., Stoddart M. J., “Articular fibrocartilage - Why does hyaline cartilage fail to repair?” Adv. Drug Delivery Rev. 146, 289–305 (2019). 10.1016/j.addr.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 52.Pinsard M., Laverty S., Richard H., Dubuc J., Schanne-Klein M.-C., Légaré F., “Maturation of the meniscal collagen structure revealed by polarization-resolved and directional second harmonic generation microscopy,” Sci. Rep. 9(1), 18448 (2019). 10.1038/s41598-019-54942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nadiarnykh O., Campagnola P. J., “Retention of polarization signatures in SHG microscopy of scattering tissues through optical clearing,” Opt. Express 17(7), 5794–5806 (2009). 10.1364/OE.17.005794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen X., Mao Z., Han Z., Tuchin V. V., Zhu D., “In vivo skin optical clearing by glycerol solutions: mechanism,” J. Biophotonics 3(1-2), 44–52 (2010). 10.1002/jbio.200910080 [DOI] [PubMed] [Google Scholar]