What gets measured, gets improved. —Robert Sharma
Every critically ill patient requires care by a critical care pharmacist (CCP) for best possible outcomes. Indeed, these highly trained professionals generate benefit through direct patient care (eg, pharmacist-driven protocols, medication monitoring, etc), participation on the intensive care unit (ICU) interprofessional team (eg, pharmacotherapy recommendations, team education, etc), and leadership in the development and implementation of quality improvement initiatives.1 However, clinical CCP services are not provided for all ICU patients, and CCP staffing models often vary substantially across ICUs in a given hospital and among ICUs in the United States.2-4 In this narrative review, we use a gap analysis approach to define current levels of clinical CCP services, identify barriers to reaching an optimal level of these services, and propose strategies focused on expanding clinical CCP services and justifying those that currently exist.
Current critical care pharmacy clinical services.
The broad scope of beneficial activities performed by the CCP has been extensively reviewed and supported by a position statement from the American Society of Health-System Pharmacists (ASHP), the American College of Clinical Pharmacy (ACCP), and the Society of Critical Care Medicine (SCCM): the CCP is an essential member of the healthcare team for delivery of patient-centered care in the ICU.1
However, this recent position statement provides little guidance on how existing CCP resources can be optimized, how new CCP positions should be justified, and how the CCP, in their daily practice, can consistently reach the ICU practice goals the rest of the ICU interprofessional team, administrators, and patients expect from them.1 Indeed, while the ASHP/ACCP/SCCM statement considers establishing appropriate ICU pharmacist-to-patient ratios as “foundational,” it also states that “limited data are available to guide optimal [CCP] ratios” and “determinations regarding [CCP] coverage and service design should be based on patient acuity and complexity.” 1 A CCP-to–ICU patient ratio of 1:15 has been proposed as the optimal ratio for clinical CCP services. However, this ratio is not supported by rigorous research; ratios as low as 1:8 and as high as 1:30 have been proposed as being better.5-9
In the United States, 2,800 board-certified CCPs are currently available to provide care to the 100,000 ICU beds that are filled with 5 million patients per year. There is currently a substantial shortage of qualified CCPs to provide an optimal level of pharmacotherapeutic care to critically ill Americans.10-12 When surveyed, 27% of CCPs self-reported working at a CCP-to–ICU patient ratio of 1:18, and another 25% reported working at a ratio greater than 1:30.13 In a more recent survey of 441 US hospitals, a median ratio of 1:17 (interquartile range, 1:12 to 1:26) was reported.2 In this same survey, only 70.8% of institutions had direct clinical CCP services in the ICU, CCP coverage was limited on nights and weekends, and CCPs frequently had nondirect patient care responsibilities.2 Indeed, few institutions provide 24-7-365 clinical CCP coverage, despite the ever-increasing complexity of ICUs and calls for greater clinical CCP services during the current global pandemic.14 As a result, general practice pharmacists, without formal CCP training, may be placed in the ICU despite evidence suggesting that a lack of ICU experience may be associated with fewer clinically important interventions and fewer optimal-level clinical CCP activities.15-18
Research surrounding the current staffing challenges faced by critical care physicians, advanced practice providers, and nurses, the risk for clinician burnout that may result from this, and the impact on care quality is informative for CCP staff justification.19-25 Increased nursing workload as a result of reduced staffing is associated with greater patient mortality, particularly in those patients who are most seriously ill.25 These reports have resulted in calls for federal mandates to standardize ICU nurse-to-patient care ratios.26 Up to 60% of CCPs report experiencing burnout; increased roles and responsibilities are the most commonly reported factors.15,27-29 However, few studies exist regarding CCP staffing ratios and burnout. Survey results suggest that CCPs perceive higher CCP-to–ICU patient ratios to be associated with worse quality of care.13 This moral distress has previously been shown to be associated with clinician burnout.
Optimal CCP clinical services.
The first step to addressing the current CCP staffing gap is to more clearly define what is meant by “optimal” with respect to CCP clinical services in ICUs. We propose the optimal staffing model to be a function of identifying a CCP-to–ICU patient ratio that maximizes a composite of 3 key domains: patient outcomes, healthcare costs, and CCP welfare (Figure 1). Within this model, individual pharmacist time is appropriately allocated among ICU responsibilities, including direct patient care, quality improvement, teaching, and outside (the ICU) leadership and service activities. Clearly delineated relationships between ICU census, patient need for CCP services, and the CCP staffing time required to deliver these services would allow for institutions to develop staffing models that fit their unique needs. Globally, as our knowledge of CCP-to–ICU patient ratio individualization continues to evolve and CCP staffing models improve, reduced medication-associated harm, healthcare waste, and CCP burnout will ideally ensue.
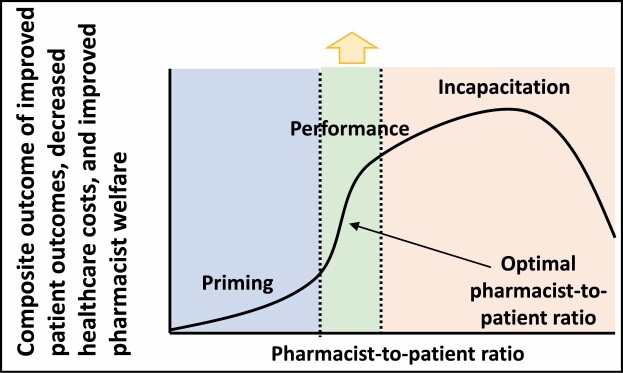
Figure 1.
Visualization of an optimal pharmacist staffing model. This theoretical construct incorporates the 3 stakeholders: institutions, pharmacists, and patients. Evaluation of how to optimize productivity or finding the “tipping point” before healthcare costs increase and return on investment is diminished is warranted. Performance sections indicate optimal time allocation for critical care pharmacist direct patient care and indirect patient care activities during a daily shift.
Gaps to achieving optimal critical care pharmacy clinical services.
No study has formally evaluated how the CCP-to–ICU patient ratio affects the number or quality of medication interventions, patient outcomes, healthcare costs, or pharmacist well-being. Indeed, the relationships that define the optimal CCP-to–ICU patient ratio, the quality of CCP care, the time required to deliver that care, and the resulting ICU patient–related outcomes remain poorly characterized, yet, a priori, these causative relationships are likely present. Among ICUs in the United Kingdom, research focused on pharmacist staffing resources, time spent by pharmacists in the ICU conducting direct patient activities, and the quality and impact of CCP interventions achieved support the hypothesis that the CCP-to–ICU patient ratio and quality of care are interrelated.16,18,30,31 However, the application of this research to US CCPs is limited given that pharmacist practice models are different, healthcare reimbursement is distinct, and CCP training is more structured in the United States. While many before-and-after studies have clearly shown that the addition of a CCP to the ICU interprofessional team improves patient outcomes, the intensity at which clinical CCP services should be delivered to optimize patient care outcomes remains unclear.32-39 In other words, while the presence of nearly any CCP service is better than the lack of a CCP presence altogether, the incremental relationship of workload to outcome is unknown. Key evidence gaps to address include the following:
If a CCP has fewer ICU patients for whom to provide clinical care, does their effectiveness improve?
If CCP clinical services are delivered on evenings and/or weekends, are ICU patient outcomes further optimized?
What factors most influence the optimal clinical CCP-to–ICU patient ratio that should be delivered (eg, severity of illness, number and/or type of medications)?
Are there additional CCP responsibilities with equally positive effects on patient outcomes that might influence this staffing ratio (eg, teaching, quality improvement, or medication order validation)?
Does a synergistic relationship exist between the delivery of direct patient care activities, indirect patient care responsibilities, service roles (eg, institutional committees, supervisor roles, national organizations), and education of students, trainees, and colleagues? Indeed, while it has been established that clinician-scientists fill a vital translational gap with regard to scientific discovery owing to the nature of the “practice-oriented” questions they can ask, this concept is less formally established for clinicians but may have similar ramifications with regard to quality improvement.40
These knowledge gaps prevent pharmacy administrators from being able to individualize CCP scheduling to a specific ICU patient ratio goal that will maximize return on investment. Importantly, a failure to be able to fully optimize the efficiency of this expensive resource may serve as a barrier to CCP service expansion and have the unintended consequence of dictating CCP practice models by available resources as opposed to clinically oriented patient outcomes guiding the need for new resource allocation. This approach to CCP modeling precludes allowing desired ICU clinical outcomes to drive, refine, and optimize the CCP model best suited to a particular ICU. A backwards-design approach may be more optimal; one such approach is depicted in Figure 2. Formalization of on- and off-service periods (as in many intensivist staffing models) may be beneficial to consider for CCPs. With many CCPs having multiple roles in the context of their primary direct ICU patient care role, a reasonable CCP-to-patient ratio would allow for both high-quality patient care and the completion of other nondirect care activities. Key knowledge gaps to optimizing CCP staff models and global resolution strategies are presented in Table 1.
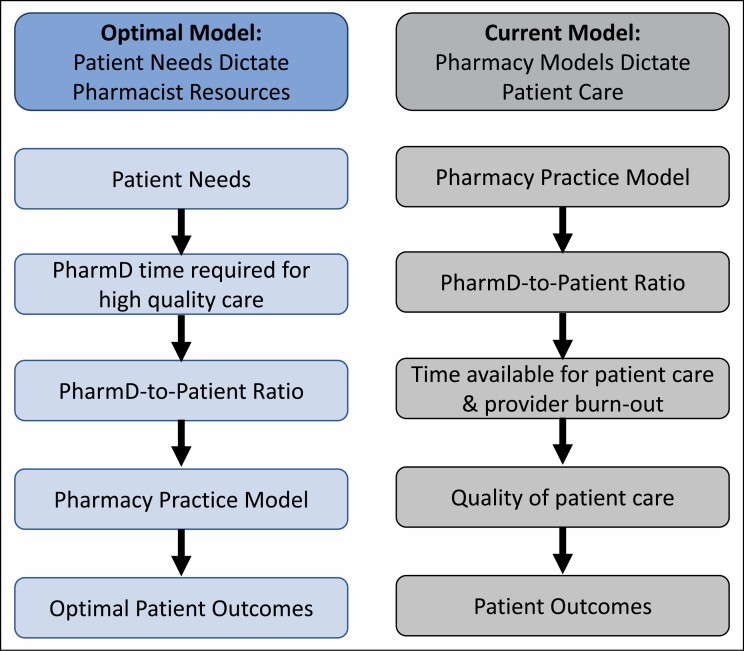
Figure 2.
Begin with the end in mind: evaluation of the ideal vs current model creation process. Following the dictum “begin with the end in mind,” pharmacy practice models would be dictated by clinically oriented patient needs; however, the ability to predict these needs with regard to critical care pharmacist resources is a vital knowledge gap.
Table 1.
Key Knowledge Gaps to Critical Care Pharmacist Staffing Model Optimization and Strategies to Overcome Them
| Rationale | Potential Resolution Strategies |
|---|---|
| Mechanism(s) of how CCP activities improve patient outcomes are not clear | |
| • Unknown relationship of CCP activities to incremental improvements in patient outcomes (eg, renal dose adjustment in the ICU is counted via intervention tracking, but the effect on patient outcome is unknown) | • Increased research focus on measurement of ICU patient outcomes and evaluation of CCP impact in 2 distinct areas: direct patient care and indirect quality improvement activities37,41 |
| No standard/efficient method to characterize day-to-day CCP productivity | |
| • Cannot reliably predict the amount of CCP time spent per intervention, how many interventions a particular ICU patient will require, or whether an intervention with a potential clinical benefit (eg, preventing drug-drug interaction) will actually contribute to beneficial outcomes • Productivity tracking is dependent on the CCP’s efforts to individually record and appropriately classify each intervention.42-44 |
• Internal and external benchmarking across institutions • Investigating the relationship of CCP responsibilities to quality of interventions to predict what type of responsibilities are reasonable within a shift |
| Current methods to track CCP value are limited | |
| • Intervention tracking captures direct patient care activities but does not capture other indirect activities (eg, protocols, education) that likely prevent the need for direct “interventions.” • Value tracking supports the value of pharmacist services, but substantial limitations are present, notably the difference between cost avoidance and cost savings and low-quality data.45-48 The metric of “cost avoidance to pharmacist salary” may have more quickly diminishing ROI than using patient-centered outcomes (eg, length of stay). |
• Real-time documentation of CCP interventions that occurs concurrently with patient care and includes both direct and indirect interventions • While tracking the value of CCPs is essential for staffing justification, more robust foundational data and not “widget counting” are needed to lay the groundwork for more comprehensive and reliable evaluations of the holistic nature of clinical pharmacy services. |
| The potential for CCP workload to result in pharmacist burnout not considered | |
| • Practice models do not account for the high practice standards set by guidelines and may contribute to CCP BOS, which has higher prevalence across the whole critical care discipline and contributes to worse patient safety outcomes.29,49-52 | • Investigation of factors associated with CCP BOS (eg, lack of off-service time, vacation coverage, patient volume)50,53 • The link between CCP practice models, CCP-to–ICU patient ratios, ICU patient outcomes, and BOS requires exploration. |
| Limited ability to compare CCP practice models among institutions | |
| • No universal ICU pharmacy practice model can exist given the unique nature of each ICU and each institution (ie, there is no “one size fits all”) • Although scores like APACHE III and case-mix indices can provide general comparisons, the nature of the ICU patient population, specialty or focus area of the institution, geographic region, institution size, and other factors can lead to comparisons “between apples and oranges” when discussing the correct CCP-to–ICU patient ratio.54,55 |
• Development of pharmacy-specific metrics to “match” ICUs for more direct comparisons • Development of general standards regarding pharmacist-to–ICU patient ratio and staffing models (eg, evenings, weekends)4,56,57 |
| Lack of validated predictive metrics to define CCP resources | |
| • Tools have been derived to help predict workload for central pharmacy staffing but do not address the complexities of ICU patients and have significant limitations for objective measurement of pharmacy personnel allocation (Table 2).58-61 | • Development of a universally accepted and validated metric for productivity |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BOS, burnout syndrome; CCP, critical care pharmacist; ICU, intensive care unit; ROI, return on investment.
Strategies to bridge knowledge gaps regarding clinical CCP optimization.
Metrics-based approach. To effectively conduct a meaningful trial comparing clinical CCP practice models, outcome measures and covariates must be thoughtfully defined. A reliable metric able to define and incorporate the key domains and outcomes of CCP performance (eg, the institutional resources to hire and support CCPs and the influence of these pharmacists on patient outcome and medication-related healthcare costs) has a strong potential to nurture the development of robust CCP practice models. However, the tools and modes that have been developed to date each have notable limitations (Table 2).
Table 2.
Clinical Pharmacy Resource Prediction Tools
| Development | Limitations |
|---|---|
| pCATCH: uses 5 key components to identify areas of highest requirement for pharmacists | |
| • Developed at the University of North Carolina Medical Center to determine the number of CPSs by various medical services62 • The task force reached a consensus on 5 key components upon which to base CPS allocation. • After applying this methodology to each medical service, the service receives a score from 1 to 5, with 5 indicating the highest need for a CPS, at which time pharmacists were reallocated. |
• While a broad staffing model for a large academic medical center with an associated school of pharmacy, it is not specific to a critical care population. • Not linked to patient outcomes • Bases patient acuity on DRG • Limited external validity due to its design oriented toward a single academic medical center • Not specific to CCPs |
| PIS: resource‐based relative value intensity grouping system that utilizes pharmaceutical resource consumption data to allocate pharmacy personnel | |
| • Calculated by multiplying the number of patients with a specific DRG by a specific PIW, giving insight into pharmacy cost and patient acuity • PIW is calculated by comparing the median pharmacy cost for a given DRG with the median pharmacy cost for all DRGs to determine the “intensity” of pharmacy resource use in relation to other diagnoses. • Gives insight into medication expense at an institution and potentially patient acuity at that site63 |
• Assumes patient acuity is correlated with DRG, which has been shown to not always hold true64,65 • Has only been used to predict expenditure on resources and has not been shown to improve patient outcomes or determine optimal pharmacist-to-patient ratio • Not specific to CCPs |
| MRCI: measure of patient-level MRC through 3 key components: dosage form, dosage frequency, and additional medication directions66 | |
| • Developed from the MCI from 134 chronic obstructive pulmonary disorder patient regimens by using an expert panel of 5 researchers scoring 6 regimens to demonstrate construct- and content-related validity • Two researchers scored the same 6 regimens to determine interrater and test-retest reliability |
• Is a patient-oriented outpatient tool intended to screen for community pharmacist clinical services (vs those in the ICU) • Not intended to be related to patient acuity or patient outcomes67 |
Abbreviations: CCP, critical care pharmacist; CPS, clinical pharmacy specialist; DRG, diagnosis-related group; ICU, intensive care unit; MCI, medication complexity index; MRCI, medication regimen complexity index; pCATCH, census, patient acuity, teaching services, medication cost, and use of high-priority medications; PIS, pharmacy intensity score; PIW, pharmacy intensity weight.
Medication regimen complexity has been proposed as a quantifiable metric designed to connect all components of the optimal CCP practice model, including ICU patient outcomes, healthcare costs, pharmacist welfare, and pharmacist resources.3,68-75 The MRC-ICU scoring tool is the first validated, objective method to measure medication regimen complexity solely in critically ill adult patients.71,76 Adapted from methods used for the medication regimen complexity index (MRCI), the MRC-ICU scoring tool was developed and validated in a single-center study of medical ICU patients and demonstrated an appropriate construct, a convergent, discriminant, and internal validity.71 Subsequently, a prospective study in both medical and surgical ICU populations was conducted at an academic medical center and a community teaching hospital to confirm initial external validity.75 The MRC-ICU score has also been evaluated in a multicenter study of 4,052 patients and 28 institutions, and preliminary analysis from the published abstract showed promise, with each increase of 1 point in MRC-ICU demonstrating 2% increased risk of mortality following Cox regression.70 An abbreviated version of MRC-ICU, with 17 (vs 39) line items was then validated in an effort to simplify future information technology (IT) builds.68
To objectively quantify pharmacist activity, MRC-ICU at 24 hours has also been correlated with CCP interventions at 24 hours and cumulative interventions at discharge.74 Drug-drug interactions (DDIs) correlated with the MRC-ICU score at 24 hours.27 Notably, correlation with potential DDIs was an expected finding as the rate of DDIs is known to increase as the total number of medications increases, thus serving as another validity confirmation but also suggesting a potential clinical use to identify high-risk patients.77,78 For a construct such as medication regimen complexity to be effective, evaluation of the relationship with “real-time” events in which a CCP can intervene is necessary. In a multicenter, retrospective study, linear regression controlling for gender, age, and weight demonstrated that the MRC-ICU scoring tool has an association with fluid overload (as defined as a positive cumulative fluid balance that would be expected to produce weight gain of over 10% from baseline).73 This finding is promising as fluid overload is a common adverse drug event in ICU patients associated with poor outcomes but also has a documented role for CCP intervention and benefit.41,79,80
Potential roles for a metric like MRC-ICU include providing resource predictions from a hospital administrative perspective for clinical CCP position justification, real-time guidance to establish optimal CCP-to–ICU patient ratios, and clinician-oriented information for prioritization of those critically ill adults who are at greatest risk for unfavorable medication-related outcomes (eg, fluid overload). Ideally, by applying this tool in a wide variety of institution types and ICU settings without losing translatability, a common language to investigate and establish best pharmacy practice models can be established. A potential construct for applying such a metric may be where resources are objectively predicted by an algorithm and patient outcomes are evaluated as a result of this staffing model, allowing for future site-specific modifications. In such a construct, patient-specific data and a pharmacy-based metric would be fed into a predictive model that provides both predictions for patient outcomes (eg, mortality, fluid overload) and pharmacist resources per patient (eg, number of interventions, time per intervention, etc). At this juncture, institution-specific needs (eg, unit census, non–patient care responsibilities of the pharmacist) would be incorporated to identify a reasonable pharmacist-to-patient ratio and associated staffing model. This staffing model would then be evaluated by comparing actual patient outcomes and pharmacist activities against the tool’s original predictions to allow for further optimization (Figure 3).
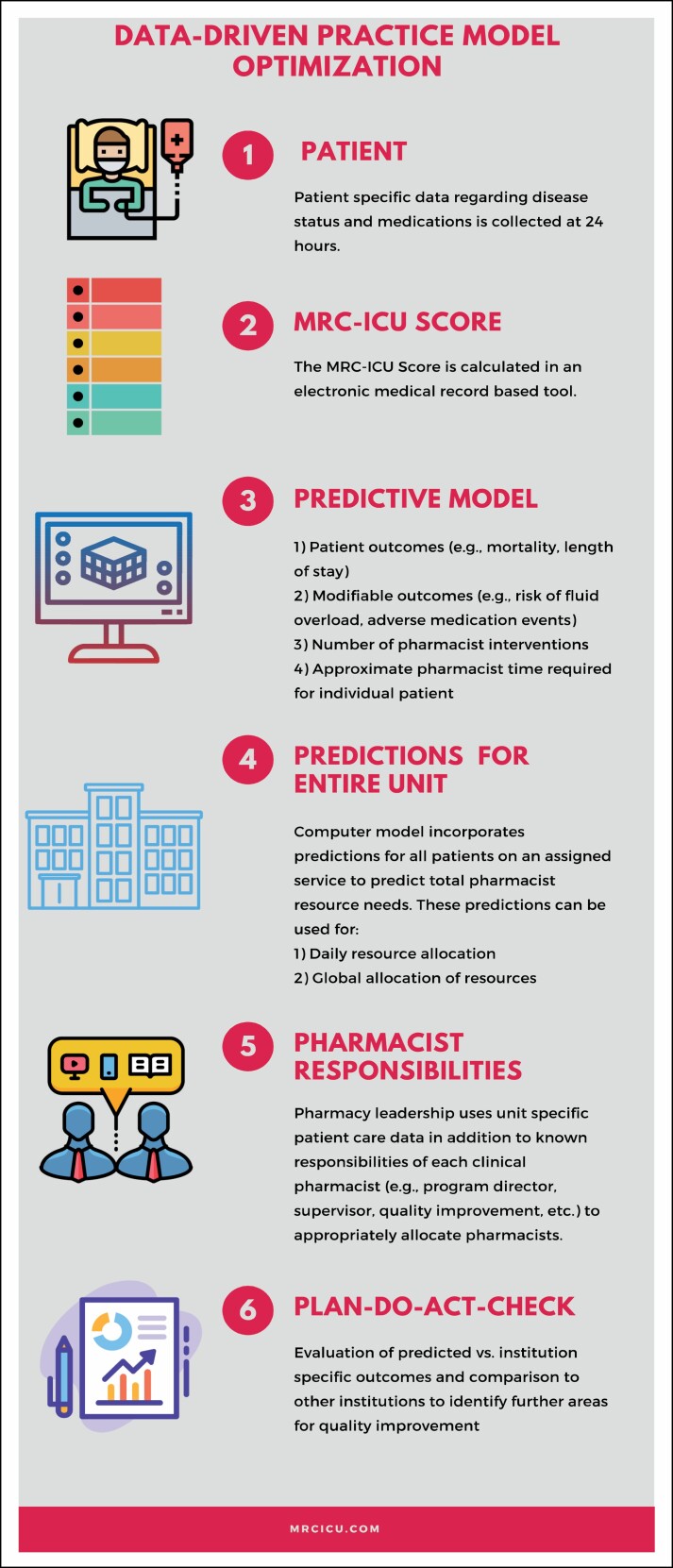
Figure 3.
Construct for metrics-driven staffing model optimization. The ability to compare predictions to actual outcomes through a metric has powerful ramifications for position justification and patient outcome optimization. This figure demonstrates a possible application cycle for such a metric.
A proof-of-concept model was developed using machine learning (ML) methods to validate the MRC-ICU score, and, despite a small dataset, the results suggest that prediction of patient outcomes can be improved with inclusion of medication regimen complexity data and the MRC-ICU score in comparison to the traditional predictor of Acute Physiology and Chronic Health Evaluation (APACHE) III.72 ML methods have shown promise in the domains of both critical care and pharmacy, but full application of a pharmacy-based metric for critical care pharmacy model optimization within a clinical decision support system (CDSS) requires rigorous prevalidation utilizing real-time scenarios.81,82 Indeed, use of artificial intelligence and ML predictive algorithms facilitates the interpretation and application of complex information to real-time data and may be the future of predicting CCP resource needs. ML tools have transformed healthcare data to predict, diagnose, and treat disease. Recently, CDSS improvements have begun to support complex decision-making with the assistance of IT, data mining, and ML methods. These methods have been developed and used to predict severe sepsis and septic shock, early warning of sepsis,83,84 unplanned transfer to the ICU,85 ICU discharge,86 acute injury risk,87 complications in the critical care setting,88 and hospital mortality.89 While prediction model interpretation provided by CDSS requires substantial testing and validation, including integration of expert knowledge, to improve its overall accuracy, application of CDSS utilizing pharmacy-based metrics has the potential to provide much-needed insight as an alternative analytic approach to handle complex interactions and may elucidate hidden patterns that generate clinically justified predictions.90,91
Quality improvement approach.
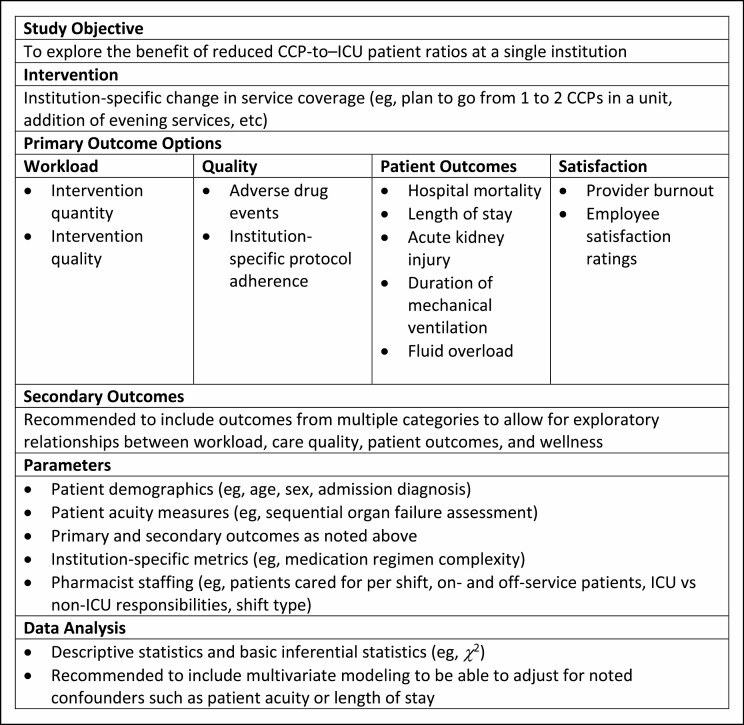
Institution-based evaluation and quality improvement efforts focused on potential clinical CCP staffing gaps are important. Available resources to justify additional CCP resources are reviewed elsewhere.3 In the only study of its kind, one institution employed a mixed methods approach to justify the addition of a second full-time CCP in a medical ICU.92 While the complexity of interventions (as defined by a perceived quality ranking) was not observed to increase, the absolute number of desirable and optimal clinical activities increased from baseline and a multiprofessional focus group of medical ICU team members qualitatively described the additional coverage as beneficial. Figure 4 provides recommendations for institution-based evaluation of current CCP services.
Figure 4.
A proposed quality improvement evaluation approach to critical care pharmacist ratios. ICU indicates intensive care unit.
Conclusion.
ICU workload for CCPs providing ICU clinical services has not been optimized, which exposes critically ill patients to worse outcomes and increased healthcare costs. Regardless of an institution’s current status, incremental improvements to optimize current and future resources are possible. Use of a quantifiable and externally validated metric that allows for cross-institution and cross–patient population evaluation of patient outcomes, healthcare costs, pharmacist welfare, and pharmacist resources has strong potential for optimizing CCP clinical services. While closing the gap between current and optimal CCP clinical services will require deliberate action and a coordinated effort, CCP-delivered care for every critically ill patient is a deeply worthwhile vision.
Disclosures
Dr. Newsome has received research funding through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award numbers UL1TR002378 and KL2TR002381; she consults for Ayma Therapeutics, Inc. Dr. Devlin has received research funding from the National Institute on Aging, National Heart, Lung, and Blood Institute, and the Canadian Institutes of Health Research; he is on the editorial board of Critical Care Medicine. The remaining authors have declared no potential conflicts of interest.
References
- 1. Lat I, Paciullo C, Daley MJ, et al. Position paper on critical care pharmacy services: 2020 update. Crit Care Med. 2020;48(9):e813-e834. [DOI] [PubMed] [Google Scholar]
- 2. MacLaren R, Roberts RJ, Dzierba AL, et al. Characterizing critical care pharmacy services across the United States. Crit Care Explor. 2021;3(1):e0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erstad BL. Justification of the value of critical care pharmacists: still a work in progress? Am J Health-Syst Pharm. 2020;77(22):1906-1909. [DOI] [PubMed] [Google Scholar]
- 4. Murray B, Buckley MS, Newsome AS. Action plan for successful implementation of optimal ICU pharmacist activities: next steps for the critical care pharmacist position paper. Crit Care Med. 2021;49(2):e199-e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderegg SV, Wilkinson ST, Couldry RJ, et al. Effects of a hospitalwide pharmacy practice model change on readmission and return to emergency department rates. Am J Health-Syst Pharm. 2014;71(17):1469-1479. [DOI] [PubMed] [Google Scholar]
- 6. Faculty of Intensive Care Medicine, Intensive Care Society. Core Standards for Intensive Care Units. 1st ed. Published 2013. Accessed December 8, 2018. www.ficm.ac.uk/sites/default/files/Core%20Standards%20for%20ICUs%20Ed.1%20(2013).pdf [Google Scholar]
- 7. Gibson GA, Guinter JR, Vozniak JM, et al. Pharmacy practice model transformation from medication focus to patient centered care. Presentation at: ASHP Midyear Clinical Meeting; December 2017; Orlando, FL. [Google Scholar]
- 8. SHPA Committee of Specialty Practice in Clinical Pharmacy. SHPA standards of practice for clinical pharmacy. J Pharm Pract Res. 2005;35:122-146. [Google Scholar]
- 9. Horn E, Jacobi J. The critical care clinical pharmacist: evolution of an essential team member. Crit Care Med. 2006;34(3 suppl):S46-S51. [DOI] [PubMed] [Google Scholar]
- 10. Halpern NA, Goldman DA, Tan KS, et al. Trends in critical care beds and use among population groups and Medicare and Medicaid beneficiaries in the United States: 2000-2010. Crit Care Med. 2016;44(8):1490-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Hospital Association. Fast facts on U.S. hospitals, 2021. Accessed February 22, 2021. https://www.aha.org/statistics/fast-facts-us-hospitals
- 12. Board of Pharmacy Specialties. Find a board certified pharmacist. Accessed July 10, 2020. https://www.bpsweb.org/find-a-board-certified-pharmacist
- 13. Newsome A, Smith SE, Jones TW, et al. A survey of critical care pharmacists to patient ratios and practice characteristics in intensive care units. J Am Coll Clin Pharm. 2020;3:68-74. [Google Scholar]
- 14. Dopp AL, Hall KK, Gale B. Health care delivery and pharmacists during the COVID-19 pandemic. Patient Safety Network. Published June 29, 2020. Accessed February 22, 2021. https://psnet.ahrq.gov/perspective/health-care-delivery-and-pharmacists-during-covid-19-pandemic [Google Scholar]
- 15. Smith SE, Buckley MS, MacClaren R, et al. Examination of critical care pharmacist work activities and burnout. J Am Coll Clin Pharm. Published online February 5, 2021. doi: 10.1002/jac5.1408 [DOI] [Google Scholar]
- 16. Bourne RS, Shulman R, Jennings JK. Reducing medication errors in critical care patients: pharmacist key resources and relationship with medicines optimisation. Int J Pharm Pract. 2018;26(6):534-540. [DOI] [PubMed] [Google Scholar]
- 17. Jacobi J. Measuring the impact of a pharmacist in the intensive care unit—are all pharmacists created equal? J Crit Care. 2015;30(5):1127-1128. [DOI] [PubMed] [Google Scholar]
- 18. Rudall N, McKenzie C, Landa J, et al. PROTECTED-UK—clinical pharmacist interventions in the UK critical care unit: exploration of relationship between intervention, service characteristics and experience level. Int J Pharm Pract. 2017;25(4):311-319. [DOI] [PubMed] [Google Scholar]
- 19. Lilly CM, Oropello JM, Pastores SM, et al. Workforce, workload, and burnout in critical care organizations: survey results and research agenda. Crit Care Med. 2020;48(11):1565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pastores SM, Kvetan V, Coopersmith CM, et al. Workforce, workload, and burnout among intensivists and advanced practice providers: a narrative review. Crit Care Med. 2019;47(4):550-557. [DOI] [PubMed] [Google Scholar]
- 21. Clarke SP, Donaldson NE.In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 22. Lee A, Cheung YSL, Joynt GM, et al. Are high nurse workload/staffing ratios associated with decreased survival in critically ill patients? A cohort study. Ann Intensive Care. 2017;7(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burnham EL, Moss M, Geraci MW. The case for 24/7 in-house intensivist coverage. Am J Respir Crit Care Med. 2010;181(11):1159-1160. [DOI] [PubMed] [Google Scholar]
- 24. Griffiths P, Maruotti A, Recio Saucedo A, et al. Nurse staffing, nursing assistants and hospital mortality: retrospective longitudinal cohort study. BMJ Qual Saf. 2019;28(8):609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. West E, Barron DN, Harrison D, et al. Nurse staffing, medical staffing and mortality in intensive care: an observational study. Int J Nurs Stud. 2014;51(5):781-794. [DOI] [PubMed] [Google Scholar]
- 26. National Nurses United. National campaign for safe RN-to-patient staffing ratios. Accessed June 12, 2021.https://www.nationalnursesunited.org/ratios
- 27. Kang K, Absher R, Granko RP. Evaluation of burnout among hospital and health-system pharmacy technicians in North Carolina. Am J Health-Syst Pharm. 2020;77(24):2041-2042. [DOI] [PubMed] [Google Scholar]
- 28. Durham ME, Bush PW, Ball AM. Evidence of burnout in health-system pharmacists. Am J Health-Syst Pharm. 2018;75(23)(suppl 4):S93-S100. [DOI] [PubMed] [Google Scholar]
- 29. Jones GM, Roe NA, Louden L, et al. Factors associated with burnout among US hospital clinical pharmacy practitioners: results of a nationwide pilot survey. Hosp Pharm. 2017;52(11):742-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bourne RS, Whiting P, Brown LS, et al. Pharmacist independent prescribing in critical care: results of a national questionnaire to establish the 2014 UK position. Int J Pharm Pract. 2016;24(2):104-113. [DOI] [PubMed] [Google Scholar]
- 31. Shulman R, McKenzie CA, Landa J, et al. Pharmacist’s review and outcomes: treatment-enhancing contributions tallied, evaluated, and documented (PROTECTED-UK). J Crit Care. 2015;30(4):808-813. [DOI] [PubMed] [Google Scholar]
- 32. Preslaski CR, Lat I, MacLaren R, et al. Pharmacist contributions as members of the multidisciplinary ICU team. Chest. 2013;144(5):1687-1695. [DOI] [PubMed] [Google Scholar]
- 33. Kane SL, Weber RJ, Dasta JF. The impact of critical care pharmacists on enhancing patient outcomes. Intensive Care Med. 2003;29(5):691-698. [DOI] [PubMed] [Google Scholar]
- 34. Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008;36(2):427-433. [DOI] [PubMed] [Google Scholar]
- 35. MacLaren R, Bond CA, Martin SJ, et al. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36(12):3184-3189. [DOI] [PubMed] [Google Scholar]
- 36. Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282(3):267-270. [DOI] [PubMed] [Google Scholar]
- 37. Stollings JL, Foss JJ, Ely EW, et al. Pharmacist leadership in ICU quality improvement: coordinating spontaneous awakening and breathing trials. Ann Pharmacother. 2015;49(8):883-891. [DOI] [PubMed] [Google Scholar]
- 38. Leguelinel-Blache G, Nguyen TL, Louart B, et al. Impact of quality bundle enforcement by a critical care pharmacist on patient outcome and costs. Crit Care Med. 2018;46(2):199-207. [DOI] [PubMed] [Google Scholar]
- 39. Buckley MS, Park AS, Anderson CS, et al. Impact of a clinical pharmacist stress ulcer prophylaxis management program on inappropriate use in hospitalized patients. Am J Med. 2015;128(8):905-913. [DOI] [PubMed] [Google Scholar]
- 40. Fowler N, Ali R, Bannard-Smith J, et al. Critical care scientists: role, training and future directions. J Intensive Care Soc. 2021;22(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bissell BD, Laine ME, Thompson Bastin ML, et al. Impact of protocolized diuresis for de-resuscitation in the intensive care unit. Crit Care. 2020;24(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sayles TJ. Documentation of pharmacists’ interventions and associated cost savings. Am J Health-Syst Pharm. 2004;61(8):838,840. [DOI] [PubMed] [Google Scholar]
- 43. Pandya D. American Society of Health-System Pharmacists practice advancement initiative. [Google Scholar]
- 44. Kim Y, Schepers G. Pharmacist intervention documentation in US health care systems. Hosp Pharm. 2003;38(12):1141-1147. [Google Scholar]
- 45. Hammond DA, Flowers HJC, Meena N, et al. Cost avoidance associated with clinical pharmacist presence in a medical intensive care unit. J Am Coll Clin Pharm. 2019;2(6):610-615. [Google Scholar]
- 46. Haas CE, Vermeulen LC. Caution warranted when torturing data until they confess. J Am Coll Clin Pharm. 2019;2(6):606-607. [Google Scholar]
- 47. Hammond DA, Rech MA. Cautions heeded: a call to action for evaluating pharmacists’ direct and indirect patient care activities. J Am Coll Clin Pharm. 2020;3(2):546-547. [Google Scholar]
- 48. Vermeulen LC, Haas CE. Drs. Haas and Vermeulen reply to Drs. Hammond and Rech. J Am Coll Clin Pharm. 2020;3(2):548-549. [Google Scholar]
- 49. Poncet MC, Toullic P, Papazian L, et al. Burnout syndrome in critical care nursing staff. Am J Respir Crit Care Med. 2007;175(7):698-704. [DOI] [PubMed] [Google Scholar]
- 50. Bhatt M, Lizano D, Carlese A, et al. Severe burnout is common among critical care physician assistants. Crit Care Med. 2017;45(11):1900-1906. [DOI] [PubMed] [Google Scholar]
- 51. Embriaco N, Azoulay E, Barrau K, et al. High level of burnout in intensivists: prevalence and associated factors. Am J Respir Crit Care Med. 2007;175(7):686-692. [DOI] [PubMed] [Google Scholar]
- 52. Higuchi Y, Inagaki M, Koyama T, et al. A cross-sectional study of psychological distress, burnout, and the associated risk factors in hospital pharmacists in Japan. BMC Public Health. 2016;16:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ball AM, Schultheis J, Lee HJ, et al. Evidence of burnout in critical care pharmacists. Am J Health-Syst Pharm. 2020;77(10):790-796. [DOI] [PubMed] [Google Scholar]
- 54. Mendez CM, Harrington DW, Christenson P, et al. Impact of hospital variables on case mix index as a marker of disease severity. Popul Health Manag. 2014;17(1):28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Higgins TL. Quantifying risk and benchmarking performance in the adult intensive care unit. J Intensive Care Med. 2007;22(3):141-156. [DOI] [PubMed] [Google Scholar]
- 56. Banerjee R, Naessens JM, Seferian EG, et al. Economic implications of nighttime attending intensivist coverage in a medical intensive care unit. Crit Care Med. 2011;39(6):1257-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wallace DJ, Angus DC, Barnato AE, et al. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012;366(22):2093-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krogh P, Ernster J, Knoer S. Creating pharmacy staffing-to-demand models: predictive tools used at two institutions. Am J Health-Syst Pharm. 2012;69(18):1574-1580. [DOI] [PubMed] [Google Scholar]
- 59. Cooper SL, Zaske DE. Relationship between intensity of hospital services and pharmacy workload. Am J Hosp Pharm. 1987;44(10):2267-2271. [PubMed] [Google Scholar]
- 60. Lundgren LM, Daniels CE. Patient acuity indicators as predictors of pharmacy workload. Am J Hosp Pharm. 1986;43(10):2453-2459. [PubMed] [Google Scholar]
- 61. Day DL, Mason M, Reeme PD. Using a nursing-workload index to validate hospital pharmacy productivity. Am J Hosp Pharm. 1986;43(4):909-912. [PubMed] [Google Scholar]
- 62. Granko RP, Poppe LB, Savage SW, et al. Method to determine allocation of clinical pharmacist resources. Am J Health-Syst Pharm. 2012;69(16):1398-1404. [DOI] [PubMed] [Google Scholar]
- 63. Brink HL, Naseman RW, Porter K, et al. An evaluation of acuity adjustment metrics to track medication expense over time. Am J Health-Syst Pharm. 2015;72(24):2157-2165. [DOI] [PubMed] [Google Scholar]
- 64. Rough SS, McDaniel M, Rinehart JR. Effective use of workload and productivity monitoring tools in health-system pharmacy, part 2. Am J Health-Syst Pharm. 2010;67(5):380-388. [DOI] [PubMed] [Google Scholar]
- 65. Rough SS, McDaniel M, Rinehart JR. Effective use of workload and productivity monitoring tools in health-system pharmacy, part 1. Am J Health-Syst Pharm. 2010;67(4):300-311. [DOI] [PubMed] [Google Scholar]
- 66. George J, Phun YT, Bailey MJ, et al. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369-1376. [DOI] [PubMed] [Google Scholar]
- 67. Alves-Conceicao V, Rocha KSS, Silva FVN, et al. Medication regimen complexity measured by MRCI: a systematic review to identify health outcomes. Ann Pharmacother. 2018;52(11):1117-1134. [DOI] [PubMed] [Google Scholar]
- 68. Newsome AS, Anderson D, Gwynn ME, et al. Characterization of changes in medication complexity using a modified scoring tool. Am J Health-Syst Pharm. 2019;76(suppl 4):S92-S95. [DOI] [PubMed] [Google Scholar]
- 69. Newsome AS, Jones TW, Smith SE. Pharmacists are associated with reduced mortality in critically ill patients: now what? Crit Care Med. 2019;47(12):e1036-e1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Newsome AS, Rech M, Hammond D, et al. 915: Pharm-crit: medication regimen complexity in the ICU (MRC-ICU) as a predictor of inpatient mortality. Crit Care Med. 2020;48(1):437. [Google Scholar]
- 71. Gwynn ME, Poisson MO, Waller JL, et al. Development and validation of a medication regimen complexity scoring tool for critically ill patients. Am J Health-Syst Pharm. 2019;76(suppl 2):S34-S40. [DOI] [PubMed] [Google Scholar]
- 72. Al-Mamun MA, Brothers T, Newsome AS. Development of machine learning models to validate a medication regimen complexity scoring tool for critically ill patients. Ann Pharmacother. 2021;55(4):421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Olney WJ, Chase A, Hannah SA, Smith SE, Newsome AS. Medication regimen complexity score as an indicator of fluid balance in critically ill patients. J Pharm Pract. Published online March 9, 2021. doi: 10.1177/0897190021999792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Newsome AS, Smith SE, Olney WJ, et al. Medication regimen complexity is associated with pharmacist interventions and drug-drug interactions: a use of the novel MRC-ICU scoring tool. J Am Coll Clin Pharm. 2019;3(1);47-56. [Google Scholar]
- 75. Newsome AS, Smith SE, Olney WJ, Jones TW. Multi-center validation of a novel medication regimen complexity scoring tool. Am J Health-Syst Pharm. 2020;77(6):474-478. [DOI] [PubMed] [Google Scholar]
- 76. Hirsch JD, Metz KR, Hosokawa PW, et al. Validation of a patient-level medication regimen complexity index as a possible tool to identify patients for medication therapy management intervention. Pharmacotherapy. 2014;34(8):826-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dookeeram D, Bidaisee S, Paul JF, et al. Polypharmacy and potential drug-drug interactions in emergency department patients in the Caribbean. Int J Clin Pharm. 2017;39(5):1119-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Corsonello A, Abbatecola AM, Fusco S, et al. The impact of drug interactions and polypharmacy on antimicrobial therapy in the elderly. Clin Microbiol Infect. 2015;21(1):20-26. [DOI] [PubMed] [Google Scholar]
- 79. Hawkins WA, Smith SE, Newsome AS, et al. Fluid stewardship during critical illness: a call to action. J Pharm Pract. 2020:33(6):863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Branan T, Smith SE, Newsome AS, et al. Association of hidden fluid administration with development of fluid overload reveals opportunities for targeted fluid minimization. SAGE Open Med. 2020;8:2050312120979464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nelson SD, Walsh CG, Olsen CA, et al. Demystifying artificial intelligence in pharmacy. Am J Health-Syst Pharm. Published online July 4, 2020. doi: 10.1093/ajhp/zxaa218 [DOI] [PubMed] [Google Scholar]
- 82. Gutierrez G. Artificial intelligence in the intensive care unit. Crit Care. 2020;24(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Churpek MM, Yuen TC, Winslow C, et al. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44(2):368-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ginestra JC, Giannini HM, Schweickert WD, et al. Clinician perception of a machine learning-based early warning system designed to predict severe sepsis and septic shock. Crit Care Med. 2019;47(11):1477-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wellner B, Grand J, Canzone E, et al. Predicting unplanned transfers to the intensive care unit: a machine learning approach leveraging diverse clinical elements. JMIR Med Inform. 2017;5(4):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McWilliams CJ, Lawson DJ, Santos-Rodriguez R, et al. Towards a decision support tool for intensive care discharge: machine learning algorithm development using electronic healthcare data from MIMIC-III and Bristol, UK. BMJ Open. 2019;9(3):e025925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Koyner JL, Carey KA, Edelson DP, et al. The development of a machine learning inpatient acute kidney injury prediction model. Crit Care Med. 2018;46(7):1070-1077. [DOI] [PubMed] [Google Scholar]
- 88. Meyer A, Zverinski D, Pfahringer B, et al. Machine learning for real-time prediction of complications in critical care: a retrospective study. Lancet Respir Med. 2018;6(12):905-914. [DOI] [PubMed] [Google Scholar]
- 89. Awad A, Bader-El-Den M, McNicholas J, et al. Predicting hospital mortality for intensive care unit patients: time-series analysis. Health Informatics J. 2020;26(2):1043-1059. [DOI] [PubMed] [Google Scholar]
- 90. Kim SY, Kim S, Cho J, et al. A deep learning model for real-time mortality prediction in critically ill children. Crit Care. 2019;23(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fagerstrom J, Bang M, Wilhelms D, et al. LiSep LSTM: a machine learning algorithm for early detection of septic shock. Sci Rep. 2019;9(1):15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McDaniel J, Bass L, Pate T, et al. Doubling pharmacist coverage in the intensive care unit: impact on the pharmacists’ clinical activities and team members’ satisfaction. Hosp Pharm. 2017;52(8):564-569. [DOI] [PMC free article] [PubMed] [Google Scholar]