Abstract
Background
There is currently a lack of information regarding ocular tropism and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Globally, the cumulative number of coronavirus disease 2019 (COVID-19) cases is increasing daily. Thus the potential for ocular transmission and manifestations of SARS-CoV-2 requires more investigation.
Methods
A systematic search of electronic databases for ocular transmission and manifestations of SARS-CoV-2 was performed. Pooled cross-sectional studies were used for conducting a meta-analysis to estimate the prevalence of ocular transmission of SARS-CoV-2 to the respiratory system and ocular manifestations (associated symptoms) of SARS-CoV-2.
Results
The highest prevalence of SARS-CoV-2-positive tears using reverse transcription polymerase chain reaction was found to be 7.5%. However, the highest prevalence of ocular conjunctivitis associated with SARS-CoV-2 was 32%. Thus, SARS-CoV-2 can evidently infect the eye, as revealed in the conjunctival secretions of COVID-19 patients.
Conclusion
The available data reflect the influence of the ocular structure on SARS-CoV-2. The analysis showed that ocular manifestation is an indication for SARS-CoV-2, particularly conjunctivitis. Moreover, there is no evidence that the ocular structure can be an additional path of transmission for SARS-CoV-2, however, it warrants further investigation.
Keywords: conjunctival swabs, conjunctivitis, coronavirus, COVID-19, nasopharyngeal swabs, ocular transmission, ocular manifestation, SARS-CoV-2, tears
Introduction
An overview of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome and the role of the replication process in pathogenicity
Coronaviruses are a large family of hundreds of viruses known to mainly affect the upper respiratory tract. They are spherical, enveloped particles with single-stranded RNA associated with a nucleoprotein within a capsid comprised of matrix proteins. They are considered to have the largest genome (26.4–31.7 kb) among all discovered viruses, with G + C contents ranging from 32% to 43%. The viral genome contains several features, including a unique N-terminal fragment found in the spike protein. Studies have revealed that SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) to permit it to enter and infect the host cells.1,2 It has been reported that there is >80% similarity between SARS-CoV-2 and SARS-CoV at the genome level. The similarity between SARS-CoV and SARS-CoV-2 may be due to its mechanism of binding to ACE2, which is present in various tissues, including the conjunctiva. In 2004, during the SARS-CoV outbreak, it was revealed that the virus could be transmitted through tears or the conjunctival sac.3 Since the SARS-CoV-2 outbreak, studies have concentrated on transmission and manifestation of the virus through the respiratory tract.4,5 The cornea and the conjunctiva may facilitate transmission of SARS-CoV-2, as they contain ACE2. In addition, the mucosa in the conjunctiva is linked to the respiratory tract, which raises the question of potential transmission through the eye, similar to SARS-CoV.
The ACE2 enzyme was detected on the human ocular surface using both western blot and immunohistochemical assay demonstrating staining of ACE2 in the conjunctiva, limbus and cornea, especially in the epithelial layer.6 However, the inner portion is still an uninvestigated area. These findings suggest an eye sensitivity to SARS-CoV-2 infection, raising concerns that the eye may act as a portal for or/and carrier of the disease. It is therefore vital to gain a greater understanding of this disease that is causing a significant burden globally.
COVID-19 presentation in the ocular system: what are the implications?
SARS-CoV-2 is primarily considered a respiratory disease, although its effect on other organs has been confirmed in several studies.7–9 The development of anomalies in different structures due to SARS-CoV-2 substantiates the broad physiological impact, which could be helpful as a diagnosis or/and prognosis tool for numerous pathologies.10 To date, neurological manifestations in patients with a positive expression of the disease have been confirmed in several studies, the first one being reported in China, where the outbreak started.11 These neurological manifestations included confusion, agitation, altered consciousness and acute cerebrovascular disease, among other indications of peripheral nervous system changes such as taste, smell and vision impairment.12 In this sense, numerous studies have established that common systemic diseases can affect the eye, including endocrine disorders, tuberculosis, human immunodeficiency syndrome and toxoplasmosis.13–16
The ocular system is considered privileged at many levels, immunologically and anatomically. Its uniqueness is a consequence of its physiology and its numerous transparent structures that are nourished by avascular aqueous humour and protected from outside pathogens by the tear film. Despite expression of the virus in tears, it has been suggested that the risk of ocular transmission of SARS-CoV2 is low.17 However, the positive expression of SARS-CoV-2 in tears and conjunctival secretions sheds light on the importance of the eye in the pathogenesis of this disease.18
It should be noted that the ocular system does not exclusively include the eye, but also the pathway from the optic nerve to the occipital lobe, as some critical SARS-CoV-2 patients have demonstrated anomalies observed through magnetic resonance imaging and in post-mortem brain biopsies.19
Methodology
Inclusion criteria
The analysis inclusion criterion was COVID-19-infected patients with ocular symptoms, to compare the results of diagnostic ocular tissue (conjunctival swab/tears) and nasopharyngeal swab or sputum samples in detecting SARS-CoV-2 using polymerase chain reaction (PCR).
Study selection
SARS-CoV-2 ocular transmission and manifestation clinical studies published in original articles, preprint articles, case reports and letters were chosen for the meta-analysis conducted in this review.
Research criteria
To evaluate the possibility of bias pertaining to ocular manifestations and transmission, we critically assessed and evaluated the study objectives and design, sample size, selection criteria and outcomes measured for each study. As a first step, we searched all possible literature reviews based on the PubMed, Google Scholar and Web of Science search engines from January 2020 to August 2020 to ensure that there was no selection bias, and consulted an ophthalmologist as well as epidemiologist and statistician. The quality of each study was assessed using published checklists and scales.20,21 We used ‘COVID-19 ocular manifestation’, ‘COVID-19 ocular transmission’, ‘COVID-19 ocular findings’ and ‘COVID-19 conjunctivitis’ as keywords to search the literature. We selected 735 citations through the electronic databases. Then the unrelated titles were removed. A screening of abstracts was conducted for 139 citations. Full-text screening was performed for 33 articles. Finally, 16 published papers, including 8 original articles, 3 preprint articles, 3 case reports and 2 letters, fulfilled the inclusion criteria.
Analysis
We utilized the Cochrane Q-statistic to assess significant between-study statistical heterogeneity and estimated I2 to quantify the magnitude of between-study heterogeneity.22 The Q-statistic provides a p-value indicating the presence of heterogeneity and the I2 statistic provides information on the percentage of heterogeneity that exists. Moreover, to take into account the publication bias, we conducted a systematic review and meta-analysis to determine if any unpublished studies were missing from the initial systematic review, since large, statistically significant studies are more likely to be published, whereas smaller studies with no statistical significance have a lower chance of being published.23 Hence, we tried to include small as well as larger size studies.
Prevalence as well as its variance were computed for transmission and manifestation for each study. To assess the heterogeneity among studies, a meta-analysis was performed with a random effects model, using a generalised linear mixed model and the logit transformation (with continuity correction).24,25 Variance within and between studies was used to compute the variance of the final pooled prevalence. Heterogeneity was assessed by the inconsistency index (I2) and the Q test.26 Statistical heterogeneity was assumed for p-values ≤0.05 (type 1 error) with the Q test. To graphically visualise results, forest plots were used for the prevalence in each study and the combined estimated prevalence with their 95% confidence intervals (CIs). All statistical analyses were performed with R software version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical considerations
Almost all the selected studies obtained approval by their respective institutional ethics committee and all the participants were provided a consent form. Only one letter and two case studies did not state the ethical considerations.
Results
SARS-CoV-2 ocular transmission and manifestations were analysed independently. The purpose was to illustrate the possible transmission and manifestations of SARS-CoV-2 through the ocular conjunctival pathway.
Studies that collected and investigated laboratory conjunctival swabs for ocular transmission are discussed in Table 1. Eight studies with 451 patients were included in the SARS-CoV-2 ocular transmission analysis. The sampling technique to detect ocular transmission was nasopharyngeal and conjunctival swabs, which were then tested using reverse transcription polymerase chain reaction (RT-PCR). In total, 13 (2.8%) patients had positive tear results.
Studies that collected and investigated conjunctival swabs for ocular manifestations are shown in Table 2. Ten studies included 1012 patients and 3 case reports had a total of 1015 patients. A total of 21 (2%) patients of the 1012 patients had ocular manifestations consistent with conjunctivitis.
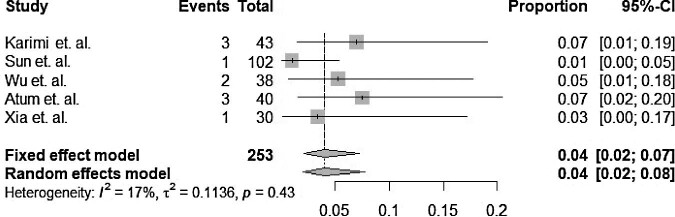
The forest plot is shown in Figure 1. The pooled prevalence for transmission was 4%. The fixed and random effects for transmission were the same, whereas the heterogeneity index (I2) for transmission was 17%, indicating that the heterogeneity between the studies was low (p=0.43). The differences between the results can be explained by the different sample sizes, the time of conjunctival swab collection, the severity of COVID-19, the lack of a control group and the number of samples collected.
Figure 1.
Forest plot showing the mean prevalence and 95% CIs of ocular transmission among confirmed COVID-19 patients.
Although the percentage of patients who tested positive for COVID-19 by conjunctival swabs was relatively low, the risk of transmission could not be eliminated. The American Academy of Ophthalmology has added new infection prevention strategies to the standard precautionary measures for ophthalmic testing, due to the close proximity of eye care professionals and their patients’ faces.41
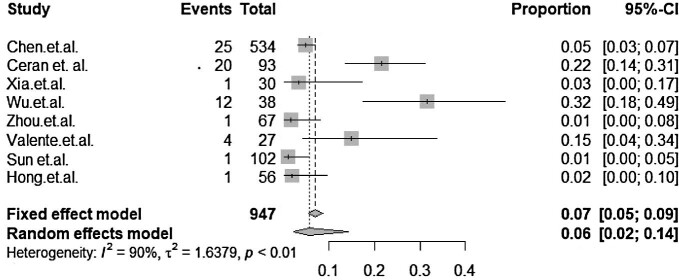
The forest plot for manifestations is shown in Figure 2. The pooled prevalence for manifestations was 7%. The fixed and random effects were 7% and 6%, respectively. The heterogeneity index in manifestation studies was 90%, giving the impression that substantial heterogeneity was reported across studies. The analysis determined strong heterogeneity between the results of the presented studies with p=0.00. This result reaffirms that the manifestation of conjunctivitis in COVID-19 patients cannot be neglected.
Figure 2.
Forest plot showing the mean prevalence and 95% CIs of ocular manifestations among confirmed COVID-19 patients.
From Tables 1 and 2, only the studies with positive nasopharyngeal swab or conjunctival swab results were included in the forest plot analysis in Figures 1 and 2. The studies with negative swabs for all the samples have no impact on the forest plot results.
Table 1.
Ocular transmission associated with COVID-19 studies
| Study | Age (years), | Sampling | ||||||
|---|---|---|---|---|---|---|---|---|
| no. | Author | Study title | Country | Design | N | mean±SD | technique | Findings |
| 1 | Zhou et al.27 | To investigate the possible transmission of SARS-CoV-2 through aerosol contact with the conjunctiva | China | Retrospective cohort | 67 | 35.7±10.6 | Nasopharyngeal and conjunctival swabs, RT-PCR | A total of 3 (4%) (1 case had positive PCR and 2 cases probable positive) |
| 2 | Deng et al.28 | To investigate the possible transmission of SARS-CoV-2 through the ocular conjunctival pathway | China | Cross-sectional | 114 | 61.4±16.7 | Nasopharyngeal swabs and conjunctival swabs, RT-PCR | No ocular complications or signs of ocular transmissible routes were reported |
| 3 | Seah et al.17 | To determine the possibility of transmission through tears by assessing for the presence of SARS-CoV-2 with viral isolation and quantitative RT-PCR analysis | Singapore | Prospective | 17 | Median 37 (range 20–75) | Collected by Schirmer test strip and analysed by RT-PCR | Risk of COVID-19 transmission through tears is low |
| 4 | Xia et al.29 | To assess the presence of novel coronavirus in tears and conjunctival secretions | China | Prospective | 30 | 54.50±14.17 | Sputum samples and conjunctival swabs, RT-PCR | All patients showed negative conjunctival swabs but one patient showed positive results |
| 5 | Karimi et al.30 | To assess the presence of SARS-CoV-2 in the tears of patients with COVID-19 | Iran | Prospective | 43 | 56±13 | Nasopharyngeal swabs and tear samples, RT-PCR | 3 (7%) patients had positive tear RT-PCR results |
| 6 | Sun et al.31 | To identify whether SARS-CoV-2 infected the ocular surface | China | Cross-sectional | 102 | 57.63±14.90 | Nasopharyngeal swabs and conjunctival swabs, RT-PCR | 1 (1.39%) patient showed positive RT-PCR results |
| 7 | Wu et al.32 | To investigate ocular manifestations and viral prevalence in the conjunctiva of patients with COVID-19 | China | Retrospective | 38 | Median 68 (interquartile range 53–76) | Nasopharyngeal swabs and conjunctival swabs, RT-PCR | 2 (5.26%) patients had positive RT-PCR results |
| 8 | Atum et al.4 | To identify SARS-CoV-2 RNA in conjunctival swabs from patients with confirmed SARS-CoV-2 infection | Turkey | Prospective | 40 | 41.38±23.72 | Nasopharyngeal, oropharyngeal and conjunctival swabs, RT-PCR | 3 (7.5%) patients had positive tear RT-PCR results |
Table 2.
Ocular manifestations associated with COVID-19 studies
| Study no. | Author | Study purpose | Country | Design | N | Age (years),mean±SD(range) | Patients with positive RT-PCR, n (%)/sampling technique | Findings |
|---|---|---|---|---|---|---|---|---|
| 9 | Chen et al.33 | To investigate the ocular manifestations and clinical characteristics of COVID-19 patients | China | Cross-sectional | 534 | Median 44 (range 28.5–54.2) | 342 (64.4)/nasopharyngeal swabs | A total of 25 (4.68%) presented with conjunctival congestion. A total of 3 (0.005%) had conjunctival congestion as the initial symptom. A total of 332 (62%) had eye–hand contact history |
| 10 | Ceran et al.34 | To describe the ocular manifestations and investigate the association between ocular involvement and clinical presentation and laboratory outcomes in COVID-19 patients | Turkey | Cross-sectional | 93 | 39.4±21.9 | 92 (99)/nasopharyngeal swabs, 1 (1.1)/endotracheal aspirate | A total of 20 patients (21.5%) had at least one ocular abnormality. A total of 2 (0.02%) had ocular manifestations consistent with conjunctivitis (hyperaemia, epiphora, increased secretion, chemosis, follicular conjunctivitis, episcleritis) |
| 3* | Seah et al.17 | To determine the possibility of transmission through tears by assessing for the presence of SARS-CoV-2 with viral isolation and quantitative RT-PCR analysis | Singapore | Prospective | 17 | N/A | 17 (100)/nasopharyngeal swabs and tear samples using the Schirmer test | None demonstrated ocular symptoms |
| 4* | Xia et al.29 | To assess the presence of novel coronavirus in tears and conjunctival secretions of SARS-CoV-2-infected patients | China | Prospective | 30 | 54.50±14.17 | 55 (91.6)/sputum samples (were taken twice), 1 (3)/tears and conjunctival secretions swab | One case (3%) had a positive SARS-CoV-2 result in the tears and conjunctival secretions samples. Notably, that was the only case presented with conjunctivitis |
| 7* | Wu et al.32 | To investigate ocular manifestations and viral prevalence in the conjunctiva of patients with COVID-19 | China | Retrospective | 38 | Median 68 (interquartile range 53–76) | 28 (73.7)/ nasopharyngeal swabs, 2 (5)/conjunctival swabs | A total of 12 cases (32%) had ocular manifestations consistent with conjunctivitis |
| 11 | Mungmung-puntipantip and Wiwanitki35 | To detect ocular manifestations in COVID-19 patients | Thailand | N/A | 48 | N/A | N/A (direct ophthalmoscopy and corneal scraping for suspected conjunctivitis case) | None demonstrated ocular symptoms |
| 1* | Zhou et al.27 | To investigate the possible transmission of 2019-nCoV through aerosol contact with the conjunctiva | China | Retrospective cohort | 67 | 35.7±10.6 | 63 (94)/nasopharyngeal swabs | One case (0.14%) of conjunctivitis as the initial symptom |
| 12 | Valente et al.36 | To evaluate ocular manifestations prevalence in the tears of children with COVID-19 | Italy | Prospective observational case series study | 27 | Range 1–216 months | 20 (74)/nasopharyngeal swabs, 3 (11)/conjunctival swabs | A total of 4 (15%) had ocular manifestations consistent with viral conjunctivitis |
| 6* | Sun et al.31 | To identify whether SARS-CoV-2 infected the ocular surface | China | Cross-sectional | 102 | 57.63±14.90 | 72 (70.6)/nasopharyngeal swabs, 1 (0.98)/conjunctival swab | A total of 2 (0.019%) had ocular manifestations consistent with conjunctivitis. However, fragments of the SARS-CoV-2 were found in ocular secretions in one of these two patients |
| 13 | Hong et al.37 | To evaluate ocular symptoms and ocular tropism of SARS-CoV‐2 in patients with COVID-19 | China | NA | 56 | 48±12.1 | 1 (1.7)/conjunctival swab | A total of 15 (27%) had aggravated ocular symptoms, 2 of which had conjunctivitis. Only one of these two patients had a positive conjunctival swab |
| 14 | Colavita et al.38 | To present the early detection of infectious SARS-CoV-2 in ocular fluids from a patient with the first confirmed case of COVID-19 in Italy | Italy | Case report | 1 | 65 | Sputum sample and ocular swab | Positive ocular swab detected 3 d from SARS-CoV-2 symptoms onset to 9 days (severe conjunctivitis) |
| 15 | Cheema et al.39 | To present a case of COVID-19 disease with an initial medical presentation of keratoconjunctivitis, the first such reported case in North America | Canada | Case report | 1 | 29 | Nasopharyngeal swab and conjunctival swab | Keratoconjunctivitis. Weakly positive eye swab taken 4 d after conjunctivitis onset |
| 16 | Chen et al.40 | To report the ocular characteristics and the presence of viral RNA of severe SARS-CoV-2 in the conjunctival swab of a COVID-19 patient | China | Case report | 1 | 30 | Nasopharyngeal swab and conjunctival swab | The conjunctival swab was positive for COVID-19 on 14 and 17 d after onset |
*The same patients were analysed separately for transmission (Table1) and manifestations (Table 2).
Discussion
Transmission
A Chinese ophthalmologist was the first to warn against SARS-like pneumonia cases.42 He reported that he became infected after coming into contact with an asymptomatic SARS-CoV-2 glaucoma patient. This was followed by another specialist who complained of eye redness that then progressed to SARS-CoV-2.43 He stated that he was using an N95 mask but did not wear eye protection, which raised the question of the possibility of SARS-CoV-2 transmission through the eye. There were previous reports that SARS-CoV could spread through contact with the eyes.44,45 Additionally, SARS-CoV-2 has been detected in the conjunctival sac of confirmed patients with COVID-19, as shown in Table 1.
A report by Seah et al.17 (study 3 in Tables 1 and 2) investigated the possibility of transmission of SARS-CoV-2 via tears in 17 recruited patients. The tear samples were collected within 2 weeks of the onset of infection using the Schirmer test. The positive nasopharyngeal swab samples were compared with viral shedding in tears during the course of SARS-CoV2. The results showed no evidence that SARS-CoV-2 was shed in tears through the course of the disease. The authors stated some limitations for the study, including analysing the samples in different laboratories using two different assays and using only tear samples rather than conjunctival swabs. Furthermore, a study by Deng et al.28 (study 2 in Table 1) recruited 114 patients with ordinary, severe and intensive cases of SARS-CoV-2 at a hospital designated for receiving only critical COVID-19 cases. The patients were mainly elderly (67%; >60 y of age), 40% had chronic diseases and 18% had contact with a diagnosed COVID-19 patient. However, no ocular symptoms were observed. The conjunctival swabs collected and the tests results showed no evidence of viral nucleic acids in the conjunctival sac. According to Panoutsopoulos et al.,1 the possible transmission of SARS-CoV-2 from the eye to the respiratory system is through the link between the S protein and the heparan sulphate chains of heparan sulphate proteoglycans on the host cell. As a result, more binding occurs for the S protein to the ACE2 receptor of the host cell of the aqueous humour, followed by endocytosis of the viral particles. Further investigation is needed to determine the ACE2 distribution and activities on the ocular surfaces.
Manifestations
Several studies have reported conjunctivitis as the most common ocular manifestation presenting in COVID-19 patients.27,31–34,36–40 Apparently conjunctivitis manifested either concurrently with other SARS-CoV-2 clinical symptoms or even earlier. This may suggest that ocular complications could be one of the earliest signs of SARS-CoV-2 infection. For instance, Zhou et al.27 (study 1 in Table 1) determined that one patient presented with conjunctivitis as the first symptom of the disease, yet the conjunctival swab result was negative. Also, the case presented by Colavita et al.38 (study 14 in Table 2) reported the presence of bilateral conjunctivitis on admission day, which was also confirmed with a positive ocular swab, 4 d earlier than the presence of high fever. Furthermore, Cheema et al.39 (study 15 in Table 2) reported keratoconjunctivitis as the initial symptom presenting in the first COVID-19 patient in Canada. In contrast, Chen et al.40 (study 16 in Table 2) reported a COVID-19 patient with relatively late onset of a conjunctival congestion, on day 13 of illness confirmed by a positive conjunctival swab on day 14. According to the current literature, conjunctivitis has not been confirmed as an early symptom of SARS-CoV-2. However, since conjunctivitis had an early onset in several cases, it is appropriate to consider it as an early warning sign of SARS-CoV-2 infection, warranting close and careful observation of the patient. It is crucial to mention that other studies have reported the presence of conjunctivitis in COVID-19 patients, regardless of the stage of onset, including studies by Xia et al.29 (study 4 in Table 1) and Wu et al.32 (study 7 in Table 1).
Also, it seems that positive ocular swabs are not always consistent with the presence of conjunctivitis. As previously mentioned, Zhou et al.27 (study 1 in Table 1) found one case with early-onset conjunctivitis but a negative conjunctival swab of SARS-CoV-2. Similarly, Valente et al.36 (study 12 in Table 2) reported positive conjunctival swabs in three COVID-19 paediatric patients, but only one of them developed conjunctivitis. When swabs were repeated, the results were negative. Furthermore, the discrepancies that exist in regard to the presence of conjunctivitis can be interpreted according to COVID-19 case severity. Wu et al.32 (study 7 in Table 1) determined that the disease in patients who developed ocular symptoms was moderate in four patients, severe in two patients and critical in six patients. Additionally, Chen et al.33 (study 9 in Table 1) reported that 68% of those who experienced conjunctival congestion also had bilateral lung complications. These studies provided preliminary evidence that ocular complications, and in particular conjunctivitis, might be associated with severe COVID-19 cases. Moreover, some studies reported the presence of other ocular symptoms in confirmed COVID-19 patients. Chen et al.33 (study 9 in Table 1) reported that most ocular symptoms associated with SARS-CoV-2 were dry eye (20.97%), blurred vision (12.73%) and foreign body sensations (11.80%). Also, Ceran et al.34 (study 10 in Table 2) reported ocular manifestations in 21.5% of their study sample. Although the most common ocular symptoms differed between the two studies, they both reported similar symptoms such as dry eye, blurred vision, photophobia and itchiness. It is important for eye care providers to maintain close observation of the ocular symptoms of COVID-19 patients even after illness recovery. It is crucial to gain knowledge on ocular integrity after SARS-CoV-2 infection.
Conclusions
More investigations on the activities of ACE2 in the ocular surface are essential to identify its role in SARS-CoV-2 transmission from the eye to the respiratory system. Moreover, conjunctival swabs are crucial rather than tears samples to detect the possibility of SARS-CoV-2 transmission.
It is clear from the studies referred to above that discrepancies exists in regard to the presence of conjunctivitis, and one reason could be the severity of the cases. However, conjunctivitis is the most common ocular presentation in COVID-19 patients. Additionally, viral shedding in conjunctival swabs compared with nasopharyngeal swabs must be taken into consideration, as reported in several studies (studies 13 and 15 in Table 2). These findings suggest that the viral load in the ocular secretions might be less than in the nasopharyngeal area.
There is some evidence suggesting that ocular infections might be related to inadequate hygiene and hand–eye touching.31,33 Several studies have recommended the importance of wearing eye goggles or face shields to protect eyes from potential infection, especially for healthcare workers who work closely with infected patients.27,31,35,37–39
Acknowledgements
None.
Contributor Information
Ahmed Almazroa, Department of Imaging Research, King Abdullah International Medical Research Center, Riyadh, 11481, Saudi Arabia; King Saud bin Abdulaziz University for Health Sciences, 14611, Riyadh, Saudi Arabia.
Suhailah Alamri, Department of Imaging Research, King Abdullah International Medical Research Center, Riyadh, 11481, Saudi Arabia; King Saud bin Abdulaziz University for Health Sciences, 14611, Riyadh, Saudi Arabia.
Balsam Alabdulkader, Department of Optometry and Vision Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, 12372, Saudi Arabia.
Hanan Alkozi, Department of Imaging Research, King Abdullah International Medical Research Center, Riyadh, 11481, Saudi Arabia; King Saud bin Abdulaziz University for Health Sciences, 14611, Riyadh, Saudi Arabia.
Altaf Khan, Department of Biostatistics and Bioinformatics, King Abdullah International Medical Research center, Riyadh, 11481, Saudi Arabia; King Saud bin Abdulaziz University for Health Sciences, 14611, Riyadh, Saudi Arabia.
Walead Alghamdi, Optometry Department, College of Applied Medical Sciences, Qassim University, Qassim, 51452, Saudi Arabia; School of Optometry and Vision Science, University of New South Wales, Sydney, NSW 2033, Australia.
Authors’ contributions
AA conceived the study. AA, SA and BA designed the study protocol. AK, AA, SA, BA and HA carried out analysis and interpretation of the data. AA, SA, HA and BA drafted the manuscript. WA critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. AA and WA are guarantors of the paper.
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
Data availability
Any required links or identifiers for the data are present in the manuscript as described.
Conflict of interest
The authors declare no conflict of interest.
References
- 1. Panoutsopoulos AA. Conjunctivitis as a sentinel of SARS-COV-2 infection: a need of revision for mild symptoms. SN Compr Clin Med. 2020;2:859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar V. Understanding the complexities of SARS-COV2 infection and its immunology: a road to immune-based therapeutics. Int Immunopharmacol. 2020;88:106980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loon SC, Teoh SC, Oon LLet al. The severe acute respiratory syndrome coronavirus in tears. Br J Ophthalmol. 2004;88:861–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atum M, Boz AAE, Çakır Bet al. Evaluation of conjunctival swab PCR results in patients with SARS-CoV-2 infection. Ocul Immunol Inflamm. 2020;28(5):745–8. [DOI] [PubMed] [Google Scholar]
- 5. Lu R, Zhao X, Li Jet al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou L, Xu Z, Castiglione GMet al. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18(4):537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaya G, Kaya A, Saurat J-H.. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology (Basel). 2020;7(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Costa IBSdS, Bittar CS, Rizk SIet al. The heart and COVID-19: what cardiologists need to know. Arq Bras Cardiol. 2020;114(5):805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adapa S, Chenna A, Balla Met al. COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res. 2020;12(6):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paniz-Mondolfi A, Bryce C, Grimes Zet al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92(7):699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mao L, Jin H, Wang Met al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aghagoli G, Gallo Marin B, Katchur NJet al. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit Care. 2020;doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nwosu N. HIV/AIDS in ophthalmic patients: the Guinness Eye Centre Onitsha experience. Niger Postgrad Med J. 2008;15(1):24–7. [PubMed] [Google Scholar]
- 14. Kramer M, Lynn W, Lightman S.. HIV/AIDS and the eye. Hosp Med. 2003;64(7):421–4. [DOI] [PubMed] [Google Scholar]
- 15. Kamboj A, Lause M, Kumar P.. Ophthalmic manifestations of endocrine disorders—endocrinology and the eye. Transl Pediat. 2017;6(4):286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butler NJ, Furtado JM, Winthrop KLet al. Ocular toxoplasmosis II: clinical features, pathology and management. Clin Exp Ophthalmol. 2013;41(1):95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seah IYJ, Anderson DE, Kang AEZet al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127(7):977–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Güemes-Villahoz N, Burgos-Blasco B, Arribi-Vilela Aet al. SARS-CoV-2 RNA detection in tears and conjunctival secretions of COVID-19 patients with conjunctivitis. J Infect. 2020;81(3):452–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kremer S, Lersy F, de Sèze Jet al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297(2):E242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Mental Health. 1998;1:37–9. [Google Scholar]
- 21. Loney PL, Chambers LW, Bennett KJet al. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19(4):170–9. [PubMed] [Google Scholar]
- 22. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez Fet al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Davey-Smith G, Altman DG. Systematic reviews in health care: meta-analysis in context. New York: John Wiley & Sons; 2008. [Google Scholar]
- 24. Borenstein M, Hedges LV, Higgins JPet al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 25. Stijnen T, Hamza TH, Ozdemir P.. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29(29):3046–67. [DOI] [PubMed] [Google Scholar]
- 26. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. [Google Scholar]
- 27. Zhou Y, Zeng Y, Tong Yet al. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. medRxiv. 2020; 10.1101/2020.02.11.20021956. [DOI] [Google Scholar]
- 28. Deng C, Yang Y, Chen Het al. Ocular detection of SARS-CoV-2 in 114 cases of COVID-19 pneumonia in Wuhan, China: an observational study. SSRN 2020; http://dx.doi.org/10.2139/ssrn.3543587. [Google Scholar]
- 29. Xia J, Tong J, Liu Met al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karimi S, Arabi A, Shahraki Tet al. Detection of severe acute respiratory syndrome coronavirus-2 in the tears of patients with coronavirus disease 2019. Eye (Lond). 2020;34(7):1220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun X, Zhang X, Chen Xet al. The infection evidence of SARS-CoV-2 in ocular surface: a single-center cross-sectional study. Ocul Surf. 2020;18(3):360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu P, Duan F, Luo Cet al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen L, Deng C, Chen Xet al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol. 2020;98(8):e951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bostanci Ceran B, Ozates S.. Ocular manifestations of coronavirus disease 2019. Graefes Arch Clin Exp Ophthalmol. 2020;258(9):1959–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mungmungpuntipantip R, Wiwanitkit V.. Ocular manifestation, eye protection, and COVID-19. Graefes Arch Clin Exp Ophthalmol. 2020;258(6):1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Valente P, Iarossi G, Federici Met al. Ocular manifestations and viral shedding in tears of pediatric patients with coronavirus disease 2019: a preliminary report. J AAPOS. 2020;24(4):212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hong N, Yu W, Xia Jet al. Evaluation of ocular symptoms and tropism of SARS-COV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 2020;98(5):e649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colavita F, Lapa D, Carletti Fet al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020;173(3):242–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheema M, Aghazadeh H, Nazarali Set al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol. 2020;55(4):e125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen L, Liu M, Zhang Zet al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020;104(6):748–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chodosh J, Holland GN, Yeh S. Important coronavirus updates for ophthalmologists. Available from:https://www.aao.org/headline/alert-important-coronavirus-context [accessed 15 May 2020]. [Google Scholar]
- 42. Lee KJ. Coronavirus kills Chinese whistleblower ophthalmologist. Available from: https://www.aao.org/headline/coronavirus-kills-chinese-whistleblower-ophthalmol [accessed 28 May 2020]. [Google Scholar]
- 43. Lu C-W, Liu X-F, Jia Z-F.. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belser JA, Rota PA, Tumpey TM.. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013;77(1):144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peiris J, Guan Y, Yuen K.. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any required links or identifiers for the data are present in the manuscript as described.