Abstract
Background
Acute kidney injury (AKI) has been recognized as a significant risk factor for mortality among adults with severe acute respiratory syndrome coronavirus infection.
Aim
The aim of this study is to assess the prevalence and risk factors for AKI and mortality in children with coronavirus disease 2019 (COVID19) from a resource-limited setting.
Methods
Cross-sectional analysis of laboratory confirmed COVID19 children admitted from 1 March to 30 November 2020 in a tertiary care hospital in New Delhi, India was done. Clinical features and associated comorbidities of COVID19 were noted. Baseline serum creatinine (height-independent Hoste’s equation) and peak serum creatinine were used for staging of AKI by the 2012 Kidney Disease Improving Global Outcomes serum creatinine criteria. Univariate analysis and Kaplan–Meier survival analysis were used to compare the overall outcome in the AKI vs. the non-AKI group.
Results
A total of 64 810 children between 1 month and 18 years visited the hospital; 3412 were tested for suspected COVID19, 295 tested positive and 105 (54% boys) were hospitalized. Twenty-four hospitalized children (22.8%) developed AKI; 8 in Stage 1 (33.3%), 7 in Stage 2 (29.2%) and 9 in Stage 3 (37.5%) respectively. Overall, three patients received KRT. Highest reported mortality was (66.6%) in AKI Stage 3. Risk factors for AKI included associated sepsis (OR 95% CI, 1.22-9.43, p < 0.01), nephrotic syndrome (OR 95% CI, 1.13-115.5, p < 0.01), vasopressor support (OR 3.59, 95% CI, 1.37–9.40, p value< 0.007), shock at presentation (OR 2.98, 95% CI, 1.16–7.60, p value 0.01) and mechanical ventilation (OR 2.64, 95% CI, 1.04–6.71, p value< 0.03). Mortality (25.71%) was higher in the AKI group (OR 95% CI, 1.14-8.35, p < 0.023) with shock (OR 45.92; 95% CI, 3.44–612.0, p value <0.004) and ventilation (OR 46.24; 95% CI, 1.6–1333.0 p value< 0.02) as significant risk factors for mortality.
Conclusion
AKI is an important modifiable risk factor for mortality in children with COVID19 in a resource-limited setting. Our study supports the strengthening of kidney replacement therapy and its timely initiation to reduce the progression of AKI and thus mortality in children.
Keywords: acute kidney injury, coronavirus disease 19, sepsis, resource-limited setting
INTRODUCTION
The World Health Organization (WHO) declared the novel coronavirus disease outbreak as a pandemic in March 2020 due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Coronavirus disease 2019 (COVID19) has affected millions of adults across the world. The pediatric population also forms a small, but a significant cohort which suffered this impact. The multisystem involvement due to coronavirus has now been recognized beyond the respiratory complaints. A recent meta-analysis of 27 525 adult hospitalized patients with COVID19 showed a pooled prevalence of AKI as 28% and that of kidney replacement therapy (KRT) as 9%. The pooled prevalence of AKI among patients admitted to the ICU was 46% and 19% of these patients required KRT. Acute kidney injury (AKI) is a known complication in hospitalized sick children and an independent risk factor for mortality [1]. Initial reports from high-income countries (HICs) have reported a prevalence of AKI ranging from 29% to 44% among hospitalized sick children [2, 3]. Urine output was not used to define AKI in most studies during the pandemic, as the fluid balance was inaccurately recorded due to logistic reasons. Hence, it is possible that the actual prevalence of AKI might be even higher.
The mechanism of AKI due to SARS-CoV-2 is still evolving as new findings are being reported. In the podocytes (Bowman’s capsule), the SARS-CoV-2 causes an increased infiltration of lymphocytes and macrophages along with the membrane attack complex (C5b9 complex) deposition in the glomerular capillaries [4]. Neutrophils and macrophages accumulate in the distal convoluted tubules and renal interstitium, leading to the release of cytokines like interleukin 10 (IL-10), granulocyte colony stimulating factor (G-CSF) and tumor necrosis factor alpha (TNF-α) [5]. This initiates the cytokine storm causing acute tubular injury and necrotic damage of the proximal tubules [6].
In this study, the epidemiology of AKI in children with COVID19 and the outcome of patients in a resource-limited setting were investigated.
METHODS
We conducted the study in the Department of Pediatrics in a tertiary care teaching hospital in New Delhi, India from 1 March 2020 to 30 November 2020. We retrospectively reviewed the records of 105 COVID19 positive children with moderate/severe/critical illness admitted in our hospital. Patients presenting with respiratory illness secondary to COVID19 and/or COVID diagnosed in children with other illnesses and systemic involvement were included. The relevant data including the history, clinical examination, hospital course, investigations and treatment provided were retrieved. The primary objective was to estimate the prevalence of AKI as defined by the 2012 Kidney Disease Improving Global Outcomes (KDIGO) criteria using serum creatinine, in children with COVID19 [7]. Secondary objectives were to identify predictors for the development of AKI and mortality in the study population.
One nasopharyngeal swab was collected as per the standard operating protocol for all patients at the time of admission. After aliquoting and RNA extraction, reverse transcription polymerase chain reaction or cartridge-based nucleic acid amplification test was performed. Patients who were subsequently diagnosed as COVID19 positive were isolated in the COVID ward of our hospital. Patients underwent basic blood investigations including a hemogram and metabolic profile at the time of admission and thereafter depending upon the clinical status. A bedside chest X-ray was done for patients with respiratory complaints. Creatinine estimation was done by enzymatic method using Erba Mannheim XL system packs (Made in Germany). As baseline serum creatinine (BSCr) and height was not available for all patients, we estimated the BSCr by the height-independent method using the Hoste’s equation [8]. We used the age-based eGFR normative values for children ≤2 years and eGFR = 120 ml/min/1.73 m2 for children >2 years [9]. The highest serum creatinine achieved during the hospital stay was noted. The stage of AKI was thus determined based on the change in the serum creatinine from the baseline. Urine output criteria were not used for diagnosing AKI, as the accurate measurement was not available due to logistic issues during the pandemic.
Patients presenting with different complications were managed as per protocol. Shock was managed as per the PALS guidelines [10]. Myocarditis was defined clinically based on ECG changes, chest radiograph and cardiac biomarkers including creatine kinase and troponin T [11]. Treatment included supportive therapy, management of congestive cardiac failure and immunomodulatory therapy. Pneumonia in children was diagnosed as per the updated WHO guidelines and treatment included respiratory support and intravenous antibiotics (Amoxyclav/Ceftriaxone) as per the hospital policy [12]. Azithromycin was considered for suspicion of atypical pneumonia. Sepsis in children was defined as per the updated 2020 Surviving Sepsis Campaign International Guidelines in Children and management included appropriate IV antibiotics as per the hospital policy [13]. Multisystem inflammatory syndrome in children (MIS-C) was diagnosed based on the Centres for Disease Control and Prevention criteria for cases enrolled after May 2020 and treated as per the standard protocol [14]. Anti-inflammatory measures with intravenous immunoglobulin as the first line therapy was given at a dose of 2 g/kg body weight by infusion with careful monitoring. In case of persistence of clinical or biochemical derangements, pulse therapy with methylprednisolone was given. Treatment for COVID19 was largely supportive and included routine oral supplementation of vitamin C, vitamin D and zinc to all patients. No child received remdesevir, hydroxychloroquine or any other specific drugs approved for adults in COVID19. The outcome of all patients as death, discharged or leave against medical advice was assessed till 15 December 2020. This study was approved by the Institutional Ethics Committee.
Statistical analysis
Data were entered on excel and analyzed on SPSS version 22. Median (inter-quartile range) and mean (standard deviation) were calculated for the quantitative data and proportions for the qualitative data. Test of normality was used to see the Gaussian pattern of data in different groups. Based on that parametric and non-parametric tests were applied wherever applicable. Mann–Whitney U-test for skewed data and unpaired t-test for normally distributed data was used to compare the difference in the means in unrelated data. Chi-square test and Fisher’s exact test was used to compare difference in proportions in various clinical findings between AKI vs. Non-AKI and survived vs. died. Kaplan–Meier survival analysis was done to estimate cumulative probability of survival between AKI and Non-AKI. Log-rank test was applied to see the difference in the cumulative probability of survival among the two groups. A p value <0.05 was considered significant. Binary logistic regressions were done to compute the adjusted odds ratio for the predictors of mortality among COVID19-positive patient. The omnibus test of model coefficients was highly significant (p = 0.000) indicating that the regression model was statistically significant. The goodness of fit of the logistic regression model was seen using the Hosmer and Lemeshow test (p = 0.799) which indicated a good fit. Cox and Snell R2 (0.562) and Negelkerke R2 (0.814) indicated a strong correlation between the dependent variable and the set of independent variables included. The explained variation in the dependent variables based on our model ranged from 55.5% to 81.0%. The overall prediction score was 92.9%.
RESULTS
Baseline characteristics
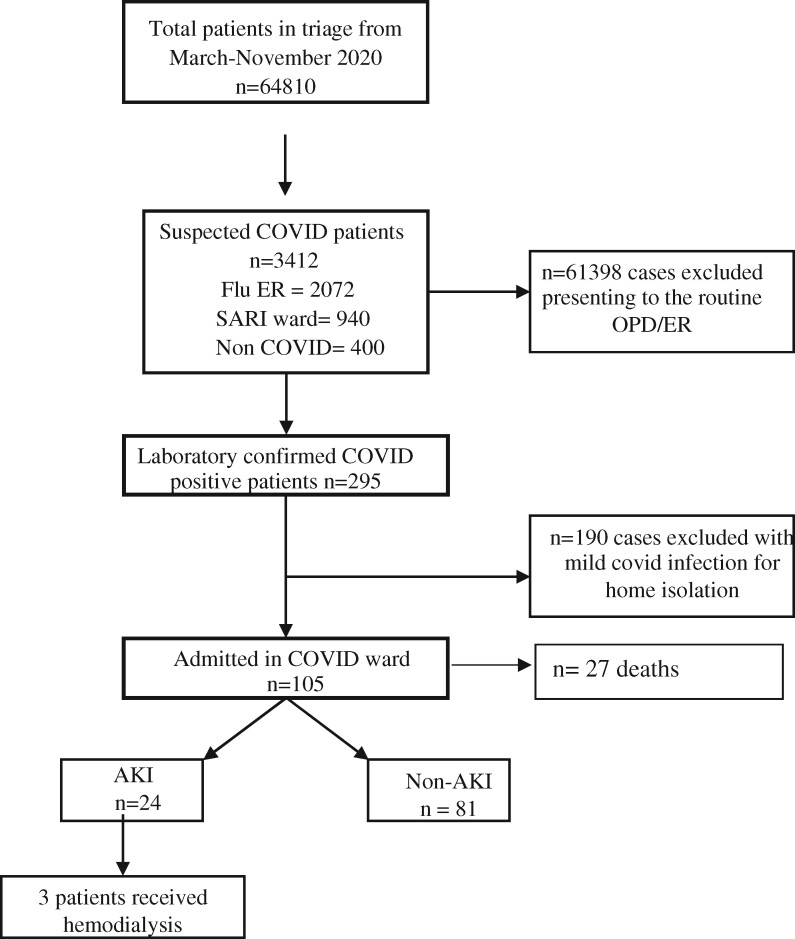
A total of 64 810 patients attended the hospital during the study period of which 3412 patients were suspected of infection. Two hundred and ninety-five patients were confirmed positive SARS CoV-2 infection and 105 (54% boys) hospitalized patients were included in the study (Fig. 1). None of the children had any preexisting renal developmental disorder. The median age was 6 years with majority (47.6%) in the 5–13-year group. The most common presenting clinical feature was fever (73.3%) followed by vomiting (37.1%). Diarrhea was also noted in 12 children (11.4%) with no significant association to AKI. Majority of children (76.2%) had an associated comorbidity at the time of presentation, of which 47 patients (44.8%) had sepsis followed by pneumonia (20%), tuberculosis (18%) and hematological disorders/malignancy (15%) (Table 1). The total leucocyte count (TLC) was significantly higher and the platelet count was lower in children with AKI. The difference in the peak serum creatinine among the AKI and non-AKI group was statistically significant (p value < 0.0001). Proteinuria (+3/+4 by dipstick) and gross hematuria was seen in 4.76% and 1.9% cases, respectively.
Fig. 1.
Flow chart of the study population. Laboratory confirmed patients with moderate/severe/critical illness were included in the study. Flu ER, flu emergency room; SARI ward, severe acute respiratory illness ward; OPD, outpatient department; AKI, acute kidney injury.
Table 1.
Baseline characteristics of the study population
| Baseline characteristics | COVID19 case, n = 105 | AKI, n = 24 (22.8%) | Non-AKI, n = 81 (77.2%) | p value |
|---|---|---|---|---|
| Age group (years) | ||||
| Infants 0–1 | 24 (22.9) | 2 (8.3) | 22 (27.2) | 0.91 |
| Toddlers 1–5 | 20 (19.0) | 7 (29.2) | 13 (16.1) | |
| Children 5–13 | 50 (47.6) | 14 (58.3) | 36 (44.4) | |
| Adolescents ≥13 | 11 (10.5) | 1 (4.2) | 10 (12.3) | |
| Median age (years) (IQR) | 6 (1.04–10) | 6.5 (3–9.5) | 5 (0.87–10.0) | 0.57 |
| Sex | ||||
| Male | 57 (54.3) | 13 (54.2) | 44 (54.3) | 0.98 |
| Female | 48 (45.7) | 11 (45.8) | 36 (45.7) | |
| All comorbidity | 80 (76.2) | 19 (79.2) | 61 (75.3) | 0.69 |
| Common comorbidities | ||||
| CNS | 10 (9.5) | 2 (8.3) | 8 (9.9) | 0.82 |
| Tuberculosis | 18 (17.1) | 1 (4.2) | 17 (21.0) | 0.05 |
| Hematological disorders/ malignancies | 15 (14.3) | 5 (20.8) | 10 (12.3) | 0.28 |
| Rheumatological | 5 (4.8) | 2 (8.3) | 3 (3.7) | 0.35 |
| Surgical conditions | 4 (3.8) | 1 (4.2) | 3 (3.7) | 0.91 |
| Liver abscess | 10 (9.5) | 2 (8.3) | 8 (9.9) | 0.82 |
| Bacterial pneumonia | 21 (20.0) | 5 (20.8) | 16 (19.8) | 0.90 |
| Severe acute malnutrition | 3 (2.9) | 1 (4.2) | 2 (2.5) | 0.66 |
| GI/hepatic disorders | 4 (3.8) | 1 (4.2) | 3 (3.7) | 0.91 |
| Sepsis | 47 (44.8) | 18 (75.0) | 38 (46.9) | 0.01 |
| Skin conditions | 2 (1.9) | 0 | 2 (2.5) | 0.437 |
| Storage disorder | 1 (1.0) | 0 | 1 (1.2) | 0.584 |
| Nephrotic syndrome | 4 (3.8) | 3 (12.5) | 1 (1.2) | 0.01 |
| Laboratory findings | ||||
| Hemoglobin (g/dl) | 9.5 ± 2.6 | 9.17 ± 2.67 | 9.60 ± 2.68 | 0.49 |
| Total leukocyte count | 17 267 ± 18 832 | 25 193 ± 31 496 | 14 918 ± 12 305 | 0.01 |
| Absolute neutrophilic count | 9076 ± 9009 | 11 404 ± 12 426 | 8333 ± 7579 | 0.16 |
| PMN (%) | 57.19 ± 22.9 | 56.38 ± 27.7 | 57.4 ± 32.4 | 0.84 |
| Lymphocyte (%) | 32.2 ± 20.8 | 30.5 ± 23.5 | 32.8 ± 20.1 | 0.65 |
| PMN/lymphocyte ratio (IQR) | 2.16 (1.12–5.1) | 2.56 (1.19–6.04) | 2.1 (0.98–4.81) | 0.57 |
| Platelet count | 1 296 000 ± 259 514 | 178 130 ± 166 839 | 282 623 ± 22 3068 | 0.04 |
| CRP | 86.48 ± 92.4 | 94.12 ± 98.0 | 80.0 ± 91.2 | 0.67 |
| Baseline serum creatinine (mg/dl), median IQR | 0.38 (0.33–0.48) | 0.37 (0.31–0.45) | 0.4 (0.33–0.48) | 0.21 |
| Peak serum creatinine (mg/dl), median IQR | 0.36 (0.25–0.60) | 0.95 (0.66–1.74) | 0.3 (0.22–0.40) | 0.001 |
| Duration of hospital stay <14 days | 78 (75.0) | 20 (83.3) | 58 (71.6) | 0.28 |
| Duration of hospital stay ≥14 days | 26 (25.0) | 4 (16.7) | 22 (27.2) |
Risk factors for AKI
Out of the 105 hospitalized patients, 24 children (22.8%) developed AKI; 8 children progressed to Stage 1 (33.3%), 7 to Stage 2 (29.2%) and 9 to AKI Stage 3 (37.5%). Three patients subsequently required hemodialysis. The presence of sepsis (OR 3.394, 95% CI, 1.22-9.43, p < 0.01) and patients with nephrotic syndrome (NS; OR 12.8, 95% CI, 1.13-115.5, p < 0.01) were significant risk factors for development of AKI. Risk factors associated with AKI included vasopressor support (OR 3.59, 95% CI, 1.37–9.40, p value< 0.007), shock at the time of presentation (OR 2.98, 95% CI, 1.16–7.60, p value 0.01) and the need for mechanical ventilation (OR 2.64, 95% CI, 1.04–6.71, p value< 0.03). Mortality was significantly higher in patients with AKI (OR 2.65,p < 0.01) (Table 2). A total of 70 patients (66.7%) were exposed to nephrotoxic medications (NMs; most commonly vancomycin, amikacin) during the hospital stay. The mean number of NMs used in the AKI group was 1.238 (SD 1.10) and in the non-AKI group was 1.245 (SD 1.11). A total of 20 (19.0%) children were diagnosed with MIS-C, of which 7 (29.2%) developed AKI.
Table 2.
Risk factors for AKI
| COVID19 case, n = 105 | AKI, n = 24 (22.8%) | Non-AKI, n = 81 (77.2%) | p value | Odds ratio | |
|---|---|---|---|---|---|
| Reason for admission,n (%) | |||||
| Hypoxia | 41 (39.0) | 8 (33.3) | 33 (40.7) | 0.51 | 0.727 |
| Pneumonia | 35 (33.3) | 7 (29.2) | 28 (34.6) | 0.62 | 0.779 |
| Shock | 36 (34.3) | 13 (52.2) | 23 (28.4) | 0.01 | 2.980 |
| Encephalitis | 16 (15.2) | 6 (25.0) | 10 (12.3) | 0.13 | 2.367 |
| Myocarditis | 9 (8.6) | 4 (16.7) | 5 (6.2) | 0.10 | 3.040 |
| Sepsis | 47 (44.8) | 18 (75.0) | 38 (46.9) | 0.01 | 3.394 |
| Nephrotic syndrome | 4 (3.8) | 3 (12.5) | 1 (1.2) | 0.01 | 12.8 |
| Exposure to nephrotoxic drugs | 70 (66.7) | 16 (66.7) | 54 (66.7) | 1.0 | 1.0 |
| Respiratory support, n (%) | |||||
| None | 52 (50.0) | 9 (37.5) | 43 (53.1) | ||
| Need for oxygenation | 53 (51.0) | 15 (62.5) | 38 (47.5) | 0.19 | 1.842 |
| Invasive | 36 (34.3) | 13 (54.2) | 25 (30.5) | 0.03 | 2.647 |
| Vasopressor support | 34 (33.3) | 13 (56.5) | 21 (25.6) | 0.007 | 3.590 |
| Mortality | 27 (25.7) | 10 (41.7) | 17 (20.9) | 0.01 | 2.650 |
| MIS-C | 20 (19.0) | 7 (29.2) | 13 (16.0) | 0.15 | 2.154 |
Predictors of mortality
The overall mortality in our study was 25.71% (27/105). Mortality was significantly higher in children developing AKI compared with the non-AKI patients (OR 3.088, 95% CI, 1.14-8.35, p < 0.023). Other predictors on univariate analysis were, hypoxia at admission (OR 3.843, p < 0.003), need for respiratory support (OR 33.01, p < 0.001) requirement of vasopressors (OR 56, p < 0.001), pneumonia (OR 3.684, p < 0.004), shock (OR 169, p < 0.001), encephalitis (OR 6.785, p < 0.001) and myocarditis (OR 13.4, p < 0.001) (Table 3). Binary logistic regression was done to compute the adjusted odds ratio for the predictors of mortality. Out of all, presence of shock (OR 45.92; 95% CI, 3.44–612.0, p value <0.004) and the need for ventilation (OR 46.24; 95% CI, 1.6–1333.0, p value< 0.02) were found to be statistically significant. On Kaplan–Meier survival analysis, the cumulative probability of survival was zero and 39% between AKI and non-AKI patients respectively after 25 days of follow up. The difference in curves was found to be statistically significant [p value< 0.03 (log-rank test)] (Fig. 2). Detailed analysis of characteristics of children with AKI Stage 3 showed mortality in 66.6% patients (Table 4). Overall KRT was done in three patients.
Table 3.
Predictors of mortality
| Predictors of mortality | Non-survivor (n = 27) | Survivor (n = 75) | p value | Odds ratio |
|---|---|---|---|---|
| Hypoxia at admission | 17 (63.0) | 23 (30.7) | 0.003 | 3.843 |
| Need for ventilation | 27 (100.0) | 11 (14.7) | 0.001 | – |
| Need for oxygen | 26 (96.3) | 27 (36.0) | 0.001 | 33.01 |
| Comorbidity | 19 (70.4) | 58 (77.3) | 0.407 | 0.696 |
| Shock | 26 (96.2) | 10 (13.3) | 0.001 | 169 |
| Pneumonia | 15 (55.6) | 19 (25.3) | 0.004 | 3.684 |
| Encephalitis | 10 (37.0) | 6 (8.0) | 0.001 | 6.785 |
| Myocarditis | 7 (25.9) | 2 (2.7) | 0.001 | 13.4 |
| AKI | 10 (37.0) | 12 (16.0) | 0.023 | 3.088 |
| Inotropes | 24 (88.9) | 9 (12.5) | 0.001 | 56.0 |
Fig. 2.
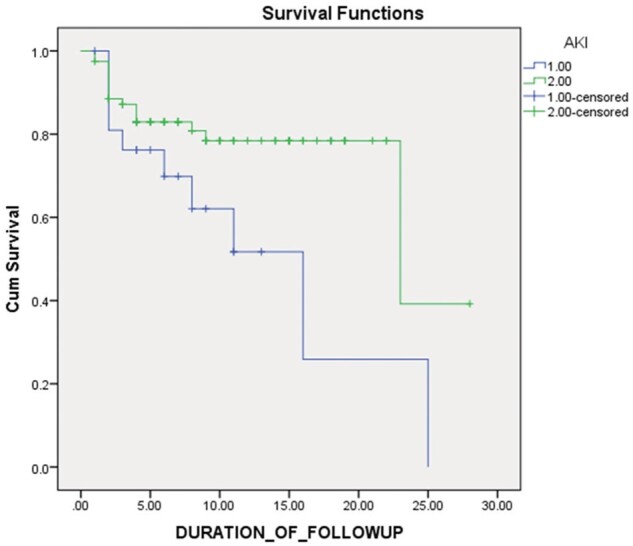
Kaplan–Meier survival analysis. The x axis depicts the duration of follow up in days and y axis depicts the cumulative survival. Cumulative probability of survival in AKI patients was zero and 39% among non-AKI patients after 25 days of follow up; log-rank p < 0.03.
Table 4.
Characteristics of COVID19 patients with AKI Stage 3
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| Age (months) | 18 | 132 | 156 | 120 | 24 | 144 | 9 | 36 | 132 |
| Sex | M | F | M | M | F | F | M | F | M |
| Hb (g/dl) | 10 | 8.3 | 5.7 | 11.5 | 6.2 | 6.9 | 7.4 | 5.1 | 6 |
| TLC (/mm3) | 2400 | 19 000 | 97 800 | 9400 | 20 700 | 42 000 | 16 700 | 118 000 | 750 |
| Platelet (/109/l) | 20 000 | 98 000 | 31 000 | 70 000 | 67 000 | 78 000 | 527 000 | 8000 | 6000 |
| BS. Cr (mg/dl) | 0.33 | 0.48 | 0.56 | 0.45 | 0.32 | 0.50 | 0.31 | 0.28 | 0.48 |
| PS. Cr (mg/dl) | 3.4 | 1.5 | 2.8 | 1.83 | 3.05 | 3.92 | 0.93 | 0.97 | 2.5 |
| Shock | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| Vasopressor | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| Ventilation | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes |
| Co-morbidity | Yesa | Yesb | Yesc | Yesd | Yese | Yesf | Yesg | Yesh | Yesi |
| MIS-C | No | Yes | Yes | Yes | No | No | No | No | Yes |
| Outcome | Death | Death | Death | Death | Death | Death | Disch | Disch | Disch |
Dich., discharge; MIS-C, multisystem inflammatory syndrome in children; BS. Cr, baseline serum creatinine; PS.Cr, peak serum creatinine; Hb, hemoglobin; TLC, total leukocyte count.
Severe acute malnutrition with sepsis.
Toxic shock syndrome.
B cell ALL/tumor lysis syndrome/Invasive pulmonary aspergillosis.
Severe dengue with shock/MODS.
Tubercular meningitis (Stage 3).
Liver abscess/pneumonia/sepsis/MODS.
Abdominal cystic mass/sepsis.
B cell ALL.
Severe aplastic anemia/hepatitis B/neutropenic enterocolitis/secondary HLH.
DISCUSSION
The initial focus of COVID19 was primarily on the respiratory complications. With more and more studies being reported from across the globe, AKI has been recognized as a common manifestation in children with COVID19. We found an incidence of 22.8% based only on the serum criteria (KDIGO) in our patients, with more than one third developing AKI Stage 3. AKI is a frequent complication occurring in critically ill non-COVID19 children. Our study revealed hemodynamic instability and mechanical ventilation as important risk factors for the development of AKI. The risk of mortality in the AKI group was also significantly higher compared with the non-AKI group. Therefore, AKI appears to be an important modifiable risk factor for mortality in COVID19 patients in a resource-limited setting.
While initial studies from China reported a lower incidence (7.09%), a recent systematic analysis on 30 639 hospitalized patients, across all age groups with COVID19 reported the pooled prevalence of AKI as 28% and that for KRT as 9%. This figure was higher (46% for AKI, 19% for KRT) in critically ill patients requiring ICU admission [15, 16]. Data from the pediatric population are rather limited and variable. Initial studies from China with 238 children reported the development of AKI in only critically ill patients (1.2%), all of who required continuous renal replacement therapy (CRRT) [17]. A study conducted on 52 hospitalized children from UK reported the prevalence of AKI as 29% (British Association of Pediatric Nephrology criteria for AKI) which was similar to our study [2]. A multicenter cross-sectional analysis on critically ill children from 41 centers with COVID19 reported nearly half (44%) of the children with AKI, however none required KRT [3]. In a multicenter study on 89 admitted children in a tertiary care hospital from the middle east, 21% developed AKI (32% in PICU admissions) [18].
Out of the 24 children developing AKI, 9 (37.5%) progressed to AKI Stage 3, of which 6 (66.6%) children expired and 3 were discharged. Among the six deceased patients, only one received KRT. KRT could not be done in the remaining, as three had severe thrombocytopenia with active bleeding, one patient had local site infection and one parent refused consent for PD. Overall three patients received KRT (hemodialysis) and two survived. We believe that early initiation peritoneal dialysis (PD) could have prevented progression of AKI, thus, improving the overall outcome. A recent study from a low middle-income country (LMIC) reported the need for KRT in 66% of critically ill children with AKI due to non-COVID19 sepsis [19]. Due to the limited availability of hemodialysis services and CRRT services for children in hospitals, PD can be a feasible option in children with COVID19 with severe AKI. Recent reports have shown successful application of PD in patients with COVID19 induced AKI, including children [20–22]. A prospective cohort study conducted on 50 children from LMIC also suggested that early initiation of PD in sepsis associated AKI, results in better outcome when compared with the standard indications [23]. The judicious use of fluids and intensive hemodynamic monitoring are imperative for prevention of AKI. The ‘cytokine storm’ induced by the SARS-CoV-2 is a pro inflammatory state with high level of circulating cytokines including IL-1, IL-6, TNF and interferon [24]. Animal models have shown the effective clearance of molecules including dialysate urea, IL-6 and TNF via PD fluid, thus reducing their systemic levels [25]. This concept may be extrapolated to humans. Thus, timely initiation of PD in patients with AKI may help in reducing the multisystem inflammatory response and systemic complications as seen in COVID19.
In our study, 44.8% of all the patients had associated sepsis at the time of presentation which increased the risk of development of AKI. An elaborate review recently, suggests the use of the term ‘COVID19 sepsis’ as most patients presenting with COVID illness also fulfill the criteria for sepsis, making the distinction between the two difficult [26]. This concept may be extended to AKI as well. This would help in streamlining the management strategies for both sepsis and COVID19 induced AKI. Hospitalized children with NS have a preexisting propensity to develop AKI and COVID19 appears to potentiate this risk. Even though we had a small number of patients with NS, the development of AKI in this high-risk cohort suggests the need for intensive monitoring.
A total of 66.7% of children received NM in our study. The mean number of NM in patients developing AKI was 1.23 (SD 1.105) and those without AKI was 1.24 (SD 1.11). Since their use was equitable in both the groups, it did not ascertain the risk for development of AKI in our study. However, as it is a modifiable risk factor for development of AKI, judicious use of NM is necessary.
The pathological findings on biopsy in patients with COVID19 have shown both glomerular and acute tubular injury. An observation from autopsy performed on 42 COVID19 patients confirmed the presence of acute tubular injury as one the mechanisms causing AKI in adults [27]. Another study on 14 patients with COVID19 suggested variable forms of glomerulopathy including collapsing type, minimal change disease, membranous glomerulopathy, crescentic transformation of lupus nephritis, anti-GBM nephritis and few cases of acute tubular injury as well [28]. Apolipoprotein L1 allele has also been identified as a high-risk genotype in these patients [29]. While most studies from the West suggest that the clustering of this high-risk allele is in the African descent, data from LMIC are still lacking [30]. Future research for identification of this genotype may help in prognostication of these cases for development of kidney injury.
This study was limited by the retrospective design, being from a single center and lack of utility of urine output monitoring and weight gain that could have resulted in missing out on cases of AKI. Nevertheless, it demonstrates that significant proportion of hospitalized children with COVID19 develops AKI and that it is challenging to manage severe AKI in resource-limited setting in this pandemic. After the recent introduction of the highly infectious mutant B.1.1.7 or SARS-CoV-2 VUI 202012/01 (Variant Under Investigation, year 2020, month 12, variant 01), there has been a resurgence in the pandemic. The 14 mutations that define this new mutant, increase the risk of transmission overall and also raise a possibility of affecting children more than they did previously, by the non-mutant virus [31]. Our report provides important insights of AKI in children with COVID19 and will help in better preparedness of KRT services in the COVID19 wards.
FUNDING
None.
Conflict of interest
None declared.
DATA AVAILABILITY
All data generated/analyzed during this study are included in this published article.
REFERENCES
- 1. Kaddourah A, Basu RK, Bagshaw SM, AWARE Investigators, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 2017;376:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart DJ, Hartley JC, Johnson M, et al. enal dysfunction in hospitalized children with COVID-19. Lancet Child Adolesc Health 2020;4:e28–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjornstad EC, Krallman KA, Askenazi D, SPARC Investigators, et al. Preliminary assessment of acute kidney injury in critically ill children associated with SARS-CoV-2 infection: a multicenter cross-sectional analysis. Clin J Am Soc Nephrol 2021;16:446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diao B, Chenhui W, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MedRxiv 2020. doi: 10.1101/2020.03.04.20031120 [DOI] [PMC free article] [PubMed]
- 5. Su H, Yang M, Wan C, Yi LX, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020;98: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ronco C, Reis T.. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol 2020;16:308–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. KDIGO AKI Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [Google Scholar]
- 8. Hoste L, Dubourg L, Selistre L, et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant 2014;29:1082–9. [DOI] [PubMed] [Google Scholar]
- 9. Hessey E, Ali R, Dorais M, et al. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr Nephrol 2017;32:1953–62. [DOI] [PubMed] [Google Scholar]
- 10. de Caen AR, Berg MD, Chameides L, et al. Pediatric advanced life support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132:S526–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tunuguntla H, Jeewa A, Denfield SW.. Acute myocarditis and pericarditis in children. Pediatr Rev 2019;40:14–25. [DOI] [PubMed] [Google Scholar]
- 12. Revised WHO classification and treatment of childhood pneumonia at health facilities. WHO, 2014. Avialable from https://wwww.ncbi.nlm.nih.gov/books/NBK264162/
- 13. Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med 2020;46:10–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Multisystem Inflammatory Syndrome in Children (MIS-C) associated with coronavirus disease COVID19. CDC, 2019. https://emergency.cdc.gov/han/2020/han00432.asp (Last accessed on 21 December 2020)
- 15. Silver SA, Beaubien-Souligny W, Shah PS, et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Med 2021;3:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng S, Wang HY, Sun X, et al. Early versus late acute kidney injury among patients with COVID-19 - a multicenter study from Wuhan, China. Nephrol Dial Transplant 2020;35:2095–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Chen X, Tang F, et al. Be aware of acute kidney injury in critically ill children with COVID-19. Pediatr Nephrol 2021;36:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kari A, Shalaby M, Albanna S, et al. Acute kidney injury in children with COVID-19.05 October 2020, Preprint (version1)available at Research Square [ 10.21203/rs.3.rs-67804/v1] [DOI]
- 19. Choudhary P, Kumar V, Saha A, et al. Peritoneal dialysis in critically ill children in resource-limited setting: a prospective cohort study. Perit Dial Int 2021;41:209–16. [DOI] [PubMed] [Google Scholar]
- 20. Shankaranarayanan D, Neupane SP, Varma E, et al. Peritoneal dialysis for acute kidney injury during the COVID-19 pandemic in New York City. Kidney Int Rep 2020;5:1532–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimonov D, Srivatana V.. Peritoneal dialysis for acute kidney injury during the COVID-19 pandemic. Clin J Am Soc Nephrol 2020;15:1829–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCulloch M, Abugrain K, Mosalakatane T, et al. Peritoneal dialysis for treatment of acute kidney injury in a case of paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. Perit Dial Int 2020;40:515–7. [DOI] [PubMed] [Google Scholar]
- 23. Tomar A, Kumar V, Saha A.. Peritoneal dialysis in children with sepsis associated AKI (SA-AKI): an experience in a low to middle income country. Paediatr Int Child Health 2021;17:1–8. [DOI] [PubMed] [Google Scholar]
- 24. Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol 2020;11:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Altmann C, Ahuja N, Kiekhaefer CM, et al. Early peritoneal dialysis reduces lung inflammation in mice with ischemic acute kidney injury. Kidney Int 2017;92:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kellum JA, Nadim MK, Forni LG.. Sepsis-associated acute kidney injury: is COVID-19 different? Kidney Int 2020;98:1370–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santoriello D, Khairallah P, Bomback AS, et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 2020;31:2158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kudose S, Batal I, Santoriello D, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 2020;31:1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shetty AA, Tawhari I, Safar-Boueri L, et al. COVID-19-associated glomerular disease. J Am Soc Nephrol 2021;32:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reidy KJ, Hjorten R, Parekh RS.. Genetic risk of APOL1 and kidney disease in children and young adults of African ancestry. Curr Opin Pediatr 2018;30:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahase E. COVID-19: what have we learnt about the new variant in the UK? BMJ 2020;23:371m4944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated/analyzed during this study are included in this published article.