Abstract
Immunotherapy that includes programmed cell death-1 (PD-1), programmed cell death- ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) inhibitors has revolutionized the therapeutic strategy in multiple malignancies. Although it has achieved significant breakthrough in advanced non-small cell lung cancer patients, immune-related adverse events (irAEs) including checkpoint inhibitor pneumonitis (CIP), are widely reported. As the particularly worrisome and potentially lethal form of irAEs, CIP should be attached more importance. Especially in non-small cell lung cancer (NSCLC) patients, the features of CIP may be more complicated on account of the overlapping respiratory signs compromised by primary tumor following immunotherapy. Herein, we included the previous relevant reports and comprehensively summarized the characteristics, diagnosis, and management of CIP. We also discussed the future direction of optimal steroid therapeutic schedule for patients with CIP in NSCLC based on the current evidence.
Keywords: immune checkpoint inhibitor, pneumonitis, non-small-cell lung cancer, diagnosis, management
Highlights
Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer presents complicated clinical and radiological manifestations.
The management of corticosteroids combined with immunosuppressive drugs is deemed to be effective for immune checkpoint inhibitor-associated pneumonitis.
Patients with immune checkpoint inhibitor-associated pneumonitis tend to suffer from a poor prognosis.
Introduction
Lung cancer has the greatest death rate, at 25%, of all types of cancer, with an estimated 135,720 deaths in the United States in 2020 (1). Non–small cell lung cancer (NSCLC) is the most common lung cancer subtype, and it comprises two major histological types: squamous cell carcinoma (SCC) and adenocarcinoma (AC) (2). Nearly 70% of patients with NSCLC are initially diagnosed at a locally advanced stage and suffer from a poor prognosis (2). The 5-year survival rate is less than 3% for patients with advanced NSCLC (3). Historically, the standard management recommended for patients with NSCLC who present with advanced-stage disease was chemotherapy regimens combined with radiotherapy (RT). However, the treatment provided generally modest responses, with an overall survival (OS) of approximately 12 to 18 months and a median progression-free survival (PFS) of just 4 to 8 months (4, 5).
Recently, immunotherapy that includes programmed cell death-1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitors, which enhance anti-tumor activity, has revolutionized the therapeutic strategy for multiple malignancies (6). PD-1, a type I transmembrane protein, exists inherently on activated T cells, B cells, natural killer cells, macrophages, dendritic cells, and monocytes. PD-L1 is highly expressed on both cancer cells and antigen-presenting cells (7). The interaction of these two molecules could promote self-tolerance and attenuate autoimmunity through T-cell exhaustion and reduced cytokine production (8). CTLA-4, a critical surface protein receptor and co-inhibitor, is typically located in stimulated CD4+/CD8+ T cells to dampen T-cell activity by binding CD80/CD86/CD28. Using the inhibitory mechanism checkpoint pathways or molecules, immune checkpoint inhibitors (ICIs) can tilt the immune equilibrium toward the beneficial promotion of tumor killing and the boosting of an immune attack (6, 9).
In advanced NSCLC, an increasing body of clinical studies suggests that the application of ICIs could achieve significant breakthroughs in PFS and OS (10–13). Therefore, the US Food and Drug Administration has rapidly incorporated ICIs into first-line therapies for advanced NSCLC (14). In the PACIFIC regimen, durvalumab (a PD-L1 inhibitor) has become the new standard of care after platinum-based chemoradiotherapy for unresectable stage III NSCLC in the United States, Europe, and Japan (15).
However, along with the killed tumor cells, virtually every organ system could be affected by ICIs (5). Immune-related adverse events (irAEs), such as cutaneous lesions, myocarditis, hepatitis, colitis, endocrinopathies, inflammatory arthritis, and pneumonitis, are widely reported (15, 16). The incidence of irAEs might be higher with combination ICI use, specific cancer types, and non-trial conditions (17, 18). Among all reported irAEs, checkpoint inhibitor pneumonitis (CIP) is particularly worrisome and potentially lethal (18–21). CIP may occur more often and have a faster onset in NSCLC than in other types of cancer (22). Since before ICI therapies, pulmonary function has been compromised by tumor location and size in patients with NSCLC. In addition, pre-existing lung comorbidities, such as chronic inflammatory respiratory diseases, interstitial fibrosis lung diseases, and radiation-induced pneumonitis (RIP), may cloud diagnostic accuracy because of the overlapping respiratory symptoms and signs (5, 6, 9, 14, 23). As a result, recognizing the unique clinical and imaging patterns of CIP is essential to facilitate expeditious diagnosis and optimized management principles.
Although previous studies have elucidated the incidence, potential mechanisms, diagnosis, risk factors, and management of CIP, they focused on variable focuses that were not comprehensive and deep enough (5, 6, 9, 14, 23, 24). This review offers a summary of cases or case series concerning CIP in NSCLC, and it aims to identify the characteristics of typical patients who develop CIP. We also comprehensively summarize the current knowledge and relevant studies of ICI-associated pneumonitis, and we discuss the future direction of evidence-based therapeutic schedules for patients with CIP in NSCLC.
Incidence and Onset of CIP
The definition of CIP is the occurrence of respiratory symptoms/signs related to a new emerging infiltration viewed on a chest X-ray but excluding new infections tested by sputum and/or bronchoalveolar lavage (BAL) (5). In different tumor types, the overall incidence of CIP varied from 3% to 5% for all grades and ranged from 0.8% to 1.0% for grade ≥ 3 CIP (5, 14, 25, 26). The overall fatality rate of CIP was 10% to 17%. In NSCLC, the incidence of CIP mainly originated from clinical trial and real-world data. In clinical trial data (10, 27–44), the incidence of CIP for all grades was approximately 2% to 38%, and incidence for grade ≥ 3 CIP was approximately 0.6% to 2.7%. In real-world data, the incidence of CIP in patients with NSCLC was 4.8% to 39.3% (18, 24, 27, 28, 45–52). The discrepancy between data from these two sources might be partly attributed to the increasing awareness of CIP in the medical community, which contributed to more frequent clinical detection and less stringent inclusion criteria for real-world studies compared with randomized trials.
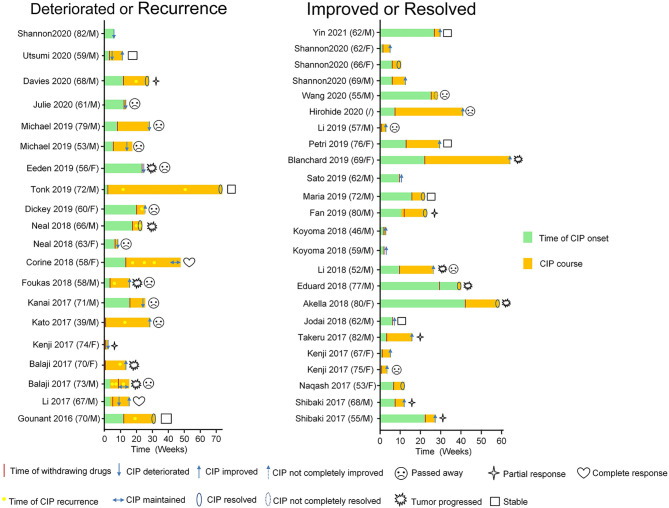
The median time to the onset of CIP was typically approximately 2.8 months, and the overall range spanned from 9 days to 19.2 months (18, 20, 53, 54). We included 44 occurrences of CIP in patients with NSCLC ( Figure 1 ; Table 1 ) by searching Pubmed and Web of Science from 2016 up to April 15th, 2020. We used the search terms “immune checkpoint inhibitors *” OR “immunotherapy *” AND “non-small cell lung cancer*” AND “pneumonitis*” with related terms including MeSH terms as well as keywords. All case reports were included. And we found that the mean time to CIP onset from the start of ICI therapy was approximately 10 weeks (2.5 months; Table 2 ). No difference was found in the median time from treatment to CIP onset between patients with improved/resolved CIP and deteriorated/maintained CIP (P=0.547) ( Table 2 ). The onset of CIP reportedly occurred as early as hours to days—or as late as several months—after the first ICI dose; however, more severe CIP grades usually had onset within the first 100 to 200 days of ICI therapy (87). The median time to CIP onset was not related to disease severity (88), and onset seemed to occur earlier for patients treated with combination ICIs (18). Of note, CIP might develop months after therapy termination, which suggests that continuous vigilance after drug discontinuation is necessary (54).
Figure 1.
Summary of checkpoint inhibitor pneumonitis patients with NSCLC, including deteriorated or recurrence (n = 20) and improved or resolved (n = 24) patients with more details in Table 1 .
Table 1.
Published case reports and case series of immune checkpoint inhibitor-associated pneumonitis.
| Author | Year | Patient | Country | Cancer Type | Histologic type | Genomic alterations (PD-1/PD-L1) (%) | Drug | Previous therapy | Time of onset | Grade of CIP | withdrew the drug | Time to withdrew the drug | Treatment | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD-1 inhibitors | PD-L1 inhibitors | CIP | CIP course(weeks) | Other iAEs | |||||||||||||
| Yin et al. (55) | 2021 | 62/M | China | NSCLC | AC | 55 | pembrolizumab | chemotherapy | After 27 weeks | 2 | Yes | After 27 weeks | prednisolone | Improved | 3 | / | |
| Shannon (9) | 2020 | 62/F | USA | NSCLC | AC | / | pembrolizumab | radiotherapy | After 11 days | 3 | Yes | After 11 days | solumedrol | Improved | / | / | |
| Shannon (9) | 2020 | 82/M | USA | NSCLC | unknown | / | nivolumab | / | After 6 weeks | 3 | Yes | After 6 weeks | / | Deteriorated | / | / | |
| Shannon (9) | 2020 | 66/F | USA | NSCLC | unknown | / | pembrolizumab | / | After 6 weeks | 2 | Yes | After 6 weeks | steroid | Resolved | / | / | |
| Shannon (9) | 2020 | 69/M | USA | NSCLC | unknown | / | nivolumab | / | After 6 weeks | 2 | Yes | After 6 weeks | steroid | Improved | / | / | |
| Davies et al. (56) | 2020 | 68/M | USA | NSCLC | AC | 1 | pembrolizumab | chemotherapy | After 12 weeks | 2 | Yes | After 12 weeks | prednisone; PPI; TMP/SMX | Recurrent(8w)-Resolved | 16 | / | |
| Utsumi et al. (57) | 2020 | 59/M | Japan | NSCLC | unknown | 1 | pembrolizumab | radiochemotherapy | After 3 weeks | 4 | Yes | After 3 weeks | methylprednisolone; prednisolone; tacrolimus; cyclophosphamide | Deteriorated(1w)-Improved | 8.4 | / | |
| Julie et al. (58) | 2020 | 61/M | USA | NSCLC | unknown | 1 | pembrolizumab | radiochemotherapy | After 12 weeks | 4 | Yes | After 12 weeks | antibiotics; high dose steroids | Deteriorated | / | NSTEMI and CHF exacerbation |
|
| Wang et al. (59) | 2020 | 55/M | China | NSCLC | AC | 60 | pembrolizumab | chemotherapy | After 24 weeks | 3 | Yes | After 24 weeks | methylprednisolone | Not completely resolved | 3 | / | |
| Hirohide et al. (60) | 2020 | / | Japan | NSCLC | AC | 55 | pembrolizumab | radiochemotherapy | After 7 weeks | 3 | Yes | After 7 weeks | methylprednisolone | Improved | 33 | grade 1 typical radiation pneumonitis | |
| Li et al. (61) | 2019 | 57/M | China | NSCLC | unknown | 60 | atezolizumab | concurrent radio-chemotherapy; bevacizumab | After 5 days | 3 | Yes | After 5 days | antibiotics; methylprednisolone | Improved | / | thrombocytopenia, and cardiac dysfunction | |
| Michael et al. (62) | 2019 | 79/M | Austria | NSCLC | both | 20 | nivolumab | radiochemotherapy | After 8 weeks | 3 | Yes | After 8 weeks | antibiotics; corticosteroids; TMP/SMX | Deteriorated | / | / | |
| Michael et al. (62) | 2019 | 53/M | Austria | NSCLC | AC | 70 | nivolumab | surgery; radiochemotherapy | After 6 weeks | 4 | Yes | After 6 weeks | antibiotic; corticosteroid mycophenolate mofetil; TMP/SMX; ganciclovir | Deteriorated (8W) | / | / | |
| Petri et al. (63) | 2019 | 76/F | USA | NSCLC | AC | / | pembrolizumab | chemotherapy | After 12 weeks | 4 | Yes | After 12 weeks | antibiotics; methylprednisolone; prednisone; immunoglobulin | Improved | 16 | / | |
| Eeden et al. (64) | 2019 | 56/F | USA | NSCLC | unknown | / | nivolumab | radiochemotherapy | About 6 months | 3 | Yes | About 6 months | antibiotics; corticosteroids; antituberculosis treatment | Deteriorated | / | grade 2 diarrhea | |
| Tonk et al. (65) | 2019 | 72/M | The Netherlands | NSCLC | unknown | / | durvalumab | radiochemotherapy | During infusion of the first cycle | 3 | Yes | During infusion of the first cycle | clemastin; dexamethasone and acetaminophen; prednisolone; mycophenolic acid | Recurrent after 12w,51w, finally maintained | 73.4 | / | |
| Blanchard and Bouchard (66) | 2019 | 69/F | Canada | NSCLC | SC | 40 | pembrolizumab | chemotherapy | After 21 weeks | 4 | Yes | After 21 weeks | methylprednisolone; bronchodilators; azithromycin | Not completely improved | 40.4 | / | |
| Dickey et al. (67) | 2019 | 60/F | Austria | NSCLC | SC | 75 | pembrolizumab | radiotherapy | After 15 weeks | 2 | Yes | After 15 weeks | antibiotics; methylprednisolone; prednisone | Recurrent(3W)-not completely improved | 3.4 | thrombotic thrombocytopenic purpura | |
| Sato et al. (68) | 2019 | 62/M | Japan | NSCLC | AC | 80 | pembrolizumab | / | After 9 weeks | 2 | Yes | After 9 weeks | dexamethasone | Improved | / | bowel perforation with acute diffuse peritonitis | |
| Maria et al. (69) | 2019 | 72/M | Greece | NSCLC | SC | / | nivolumab | radiochemotherapy | After 15 weeks | 2 | / | After 15 weeks | prednisolone | Resolved | 22 | grade 2 colitis; hypercalcemia | |
| Fan et al. (70) | 2019 | 80/M | China | NSCLC | SC | 50 | nivolumab | chemotherapy | After 10 weeks | 2 | Yes | After 12 weeks | prednisolone | Resolved | 12 | Febrile neutropenia | |
| Neal et al. (25), | 2018 | 66/M | USA | NSCLC | unknown | 70 | nivolumab | radiochemotherapy | After 18 weeks | 3 | Yes | After 18 weeks | methylprednisolone; prednisone; infliximab | Recurrent (2w, 4w) for 2 times finally resolved | 6.8 | / | |
| Neal et al. (25) | 2018 | 63/F | USA | NSCLC | unknown | 60 | pembrolizumab | radiotherapy | After 48 days | 4 | Yes | After 48 days | antibiotics; methylprednisolone; infliximab, cyclophosphamide | Deteriorated (2w) | 2 | / | |
| Corine et al. (71) | 2018 | 58/F | USA | NSCLC | unknown | 1 | nivolumab | radiochemotherapy; bevacizumab | After 14 weeks | 3 | Yes | After 14 weeks | antibiotics; prednisone | Recurrent for several times (6.4; 12.9; 17.1) and finally resolved | 38.6 | / | |
| Koyoma et al. (72) | 2018 | 46/M | Japan | NSCLC | unknown | / | nivolumab | chemotherapy; bevacizumab | After 2 weeks | 3 | / | After 2 weeks | methylprednisolone; prednisolone | Improved | 1 | / | |
| Koyoma et al. (72) | 2018 | 59/M | Japan | NSCLC | unknown | / | nivolumab | chemotherapy; erlotinib; bevacizumab | After 2 weeks | 3 | / | After 2 weeks | prednisolone | Deteriorated-Improved | / | / | |
| Foukas et al. (73) | 2018 | 58/M | USA | NSCLC | SC | / | nivolumab | radiochemotherapy | After 4 weeks | 3 | Yes | After 4 weeks | antibiotics; prednisolone; TMP/SMX | Recurrent after 4w and improved | 12 | / | |
| Li et al. (74) | 2018 | 52/M | China | NSCLC | unknown | 50 | pembrolizumab | radiochemotherapy | After 9 weeks | 2 | Yes | After 9 weeks | prednisolone | Not completely improved | 16 | / | |
| Eduard et al. (75) | 2018 | 77/M | Spain | NSCLC | AC | 85 | nivolumab | chemotherapy | After 36 weeks | 2 | Yes | After 28 weeks | antibiotics; methylprednisolone; TMP/SMX | Resolved | 3 | nephritis, hepatitis | |
| Akella et al. (76) | 2018 | 80/F | USA | NSCLC | unknown | / | nivolumab | chemotherapy | After 10 months | 2 | Yes | After 10 months | methylprednisolone | Resolved | 16.4 | / | |
| Jodai et al. (77) | 2018 | 62/M | Japan | NSCLC | AC | / | nivolumab | chemotherapy | After 6 weeks | 2 | Yes | After 6 weeks | antibiotics; prednisolone | Improved | / | / | |
| Li et al. (78) | 2017 | 67/M | USA | NSCLC | SC | 50 | nivolumab | radiochemotherapy | After 4 weeks | 3 | Yes | After 6 weeks | antibiotics; corticosteroid | Deteriorated(5w)-Improved | 13 | / | |
| Kanai et al. (79) | 2017 | 71/M | Japan | NSCLC | AC | / | nivolumab | chemotherapy | After 16 weeks | 3 | Yes | After 16 weeks | prednisolone; cyclosporine A; methylprednisolone; infliximab | Deteriorated (8W) | 10.4 | / | |
| Takeru et al. (80) | 2017 | 82/M | Japan | NSCLC | unknown | / | nivolumab | radiochemotherapy | After 3 weeks | 2 | / | / | methylprednisolone | Improved | 14.4 | radiation pneumonitis 2months after radiation; steroid | |
| Kato et al. (81) | 2017 | 39/M | Japan | NSCLC | unknown | / | nivolumab | radiochemotherapy | After 4 days | 2 | Yes | After 4 days | prednisone | Recurrent(12w)-improved | 28 | / | |
| Kenji et al. (82) | 2017 | 74/F | Japan | NSCLC | unknown | / | nivolumab | chemotherapy; bevacizumab | After 3 days | 3 | Yes | After 3 days | methylprednisolone; prednisolone | Deteriorated | 1.5 | / | |
| Kenji et al. (82) | 2017 | 67/F | Japan | NSCLC | unknown | / | nivolumab | radiochemotherapy; erlotinib; bevacizumab | After 1 week | 3 | Yes | After 1 week | betamethasone; methylprednisolone | Improved | 3.9 | / | |
| Kenji et al. (82) | 2017 | 75/F | Japan | NSCLC | unknown | / | nivolumab | radiochemotherapy | After 5 days | 3 | Yes | After 5 days | methylprednisolone; cyclophosphamide | Not completely improved | 2.7 | / | |
| Balaji et al. (83) | 2017 | 73/M | USA | NSCLC | unknown | / | nivolumab | chemotherapy | After 4 weeks | 2~4 | Yes | After 10 weeks | prednisone; bronchodilators; TMP/SMX | Recurrent (3weeks; 5weeks; 9 weeks) - maintained | 11.3 | / | |
| Balaji et al. (83) | 2017 | 70/F | USA | NSCLC | unknown | / | nivolumab | surgery; chemotherapy; ipilimumab (3 mg/kg) | After 3 days | 3 | Yes | After 3 days | prednisone | Recurrent(9W)-Improved | 13.3 | / | |
| Naqash et al. (84) | 2017 | 53/F | USA | NSCLC | AC | 0 | atezolizumab | concurrent radiochemotherapy | After 7 weeks | 2 | Yes | After 7 weeks | prednisone; tocilizumab | Resolved | 5.6 | arthritis | |
| Shibaki et al. (85) | 2017 | 68/M | Japan | NSCLC | SC | / | nivolumab | radiotherapy | After 8 weeks | 2 | Yes | After 8 weeks | prednisolone | Improved | 4 | ||
| Shibaki et al. (85) | 2017 | 55/M | Japan | NSCLC | unknown | / | nivolumab | radiotherapy | After 24 weeks | 2 | Yes | After 24 weeks | prednisolone | Improved | 4 | ||
| Gounant et al. (86) | 2016 | 70/M | USA | NSCLC | SC | 80 | nivolumab | chemotherapy; necitumumab (anti-EFGR monoclonal antibody) | After 12 weeks | 2 | Yes | After 12 weeks | prednisone | Recurrent 20w later-finally resolved | 23.4 | grade 2 hyperthyroidism | |
NSCLC, non-small cell lung cancer; TMP/SM, trimethoprim/sulfamethoxazole; PPI, proton pump inhibitors; NSTEMI, non–ST-segment elevation myocardial infarction; CHF, Congestive heart failure.
Table 2.
Baseline characteristics of the NSCLC cases with CIP according to the CIP outcome.
| CIP outcome Mean ± SD/N (%) | Total | Improved/Resolved | Deteriorated/Maintained | P-value |
|---|---|---|---|---|
| N | 44 | 34 | 10 | |
| Age | 65.23 ± 9.84 | 64.27 ± 9.83 | 68.40 ± 9.69 | 0.232 |
| Sex | 0.798 | |||
| Female | 14 (32.56%) | 11 (33.33%) | 3 (30.00%) | |
| Male | 29 (67.44%) | 22 (66.67%) | 7 (70.00%) | |
| Genomic alterations (%) | 45.90 ± 29.62 | 47.82 ± 29.60 | 37.75 ± 32.66 | 0.554 |
| Country | 0.195 | |||
| USA | 18 (40.91%) | 13 (38.24%) | 5 (50.00%) | |
| Japan | 14 (31.82%) | 12 (35.29%) | 2 (20.00%) | |
| China | 5 (11.36%) | 5 (14.71%) | 0 (0.00%) | |
| Austria | 3 (6.82%) | 1 (2.94%) | 2 (20.00%) | |
| Canada | 1 (2.27%) | 1 (2.94%) | 0 (0.00%) | |
| Greece | 1 (2.27%) | 1 (2.94%) | 0 (0.00%) | |
| The Netherlands | 1 (2.27%) | 0 (0.00%) | 1 (10.00%) | |
| Spain | 1 (2.27%) | 1 (2.94%) | 0 (0.00%) | |
| Grade of CIP | 0.002 | |||
| Grade 2 | 18 (40.91% | 18 (52.94%) | 0 (0.00%) | |
| Grade 3 | 19 (43.18%) | 13 (38.24%) | 6 (60.00%) | |
| Grade 4 | 7 (15.91%) | 3 (8.82%) | 4 (40.00%) | |
| Histologic type | 0.079 | |||
| AC | 12 (27.27%) | 10 (29.41%) | 2 (20.00%) | |
| SC | 8 (18.18%) | 8 (23.53%) | 0 (0.00%) | |
| Both | 1 (2.27%) | 0 (0.00%) | 1 (10.00%) | |
| Unknown | 23 (52.27%) | 16 (47.06%) | 7 (70.00%) | |
| ICIs | 0.548 | |||
| PD-1 inhibitors | 41 (93.18%) | 32 (94.12%) | 9 (90.00%) | |
| PD-L1 inhibitors | 3 (6.82%) | 2 (5.88%) | 1 (10.00%) | |
| Recurrence times | 0.325 | |||
| 0 | 34 (77.27%) | 26 (76.47%) | 8 (80.00%) | |
| 1 | 6 (13.64%) | 6 (17.65%) | 0 (0.00%) | |
| 2 | 2 (4.55%) | 1 (2.94%) | 1 (10.00%) | |
| 3 | 2 (4.55%) | 1 (2.94%) | 1 (10.00%) | |
| Dose of onset | 4.18 ± 3.80 | 4.26 ± 3.93 | 3.90 ± 3.51 | 0.793 |
| Time of onset | 10.14 ± 9.48 | 10.62 ± 10.12 | 8.53 ± 7.09 | 0.547 |
| Steroid initial dose(mg/d) | 425.29 ± 451.82 | 474.43 ± 475.24 | 196.00 ± 263.30 | 0.301 |
| Steroid initial dose groups(mg/d) | 0.222 | |||
| Low-dose <60 | 5 (29.41%) | 3 (21.43%) | 2 (66.67%) | |
| Intermediate-dose 60-500 | 6 (35.29%) | 5 (35.71%) | 1 (33.33%) | |
| High-dose 501-1000 | 6 (35.29%) | 6 (42.86%) | 0 (0.00%) | |
| Steroid initial dose(mg/kg/d) | 1.24 ± 0.58 | 1.15 ± 0.57 | 1.80 ± 0.28 | 0.149 |
| Steroid initial dose groups(mg/kg/d) | 0.177 | |||
| Low-dose <1 | 8 (53.33% | 8 (61.54%) | 0 (0.00%) | |
| Intermediate-dose1-2 | 6 (40.00%) | 4 (30.77%) | 2 (100.00%) | |
| High-dose >2 | 1 (6.67%) | 1 (7.69%) | 0 (0.00%) | |
| Steroid taper time | 10.46 ± 9.94 | 10.20 ± 10.13 | 12.00 ± 10.58 | 0.649 |
| Steroid course | 14.43 ± 15.14 | 13.46 ± 11.20 | 19.72 ± 30.35 | 0.404 |
| Antibiotics | 0.077 | |||
| No | 28 (63.64%) | 24 (70.59%) | 4 (40.00%) | |
| Yes | 16 (36.36%) | 10 (29.41%) | 6 (60.00%) | |
| Immunosuppressive drugs | 0.081 | |||
| No | 35 (79.55%) | 29 (85.29%) | 6 (60.00%) | |
| Yes | 9 (20.45%) | 5 (14.71%) | 4 (40.00%) | |
| OS | 0.001 | |||
| Alive | 20 (57.14%) | 19 (73.08%) | 1 (11.11%) | |
| Dead | 15 (42.86%) | 7 (26.92%) | 8 (88.89%) | |
| Survival weeks | 55.35 ± 46.26 | 61.44 ± 49.71 | 34.49 ± 23.88 | 0.168 |
| CIP Course (weeks) | 12.64 ± 14.20 | 11.66 ± 10.81 | 16.45 ± 23.93 | 0.402 |
| Clinical response | 0.027 | |||
| Complete response | 2 (5.71%) | 2 (7.69%) | 0 (0.00%) | |
| Partial response | 6 (17.14%) | 6 (23.08%) | 0 (0.00%) | |
| Tumor progressed | 5 (14.29%) | 5 (19.23%) | 0 (0.00%) | |
| Stable | 7 (20.00%) | 6 (23.08%) | 1 (11.11%) | |
| Unknown | 15 (42.86%) | 7 (26.92%) | 8 (88.89%) |
NSCLC, non-small cell lung cancer; CIP, checkpoint inhibitor pneumonitis; SC, squamous cell carcinoma; AC, adenocarcinoma; ICIs, immune checkpoint inhibitors; OS, overall survival.
Bold values: two-sided P-values less than 0.05 were considered to identify statistical significance.
Potential Mechanism of CIP
In animal models with deficiencies of PD-1 and CTLA-4, animals exhibited lung infiltration (89, 90), which could clarify questions about how CIP develops (91). The potential mechanisms driving ICI-related pneumonitis are outlined in the following sections.
Increased T-cell Activity Against Cross-Antigens
Enhanced and/or targeted T-cell activity against cross-antigens shared between tumor and normal tissues may result in irAEs (14, 91). Furthermore, cytotoxic antigen-directed T-cell responses may drive CIP pathogenesis. Significant lymphocytosis enriched with CD8+ T cells has been examined in the pulmonary tissues and BAL from patients with clinical typical CIP (92, 93). In NSCLC, Suresh et al. (94) noted that CD4+ T cells predominated in the BAL of patients with CIP. Notably, decreased expression of PD-1 and CTLA-4 and increased numbers of central memory T cells were observed within the regulatory T-cell population, which suggested that dysregulation of T cells may result from activation of pro-inflammatory immune subsets (alveolar T cells) and weakening of the anti-inflammatory regulatory T-cell phenotype.
In addition, Laubli et al. (95) conducted T-cell receptor sequencing on tumor-infiltrating lymphocytes and T cells infiltrating the inflammatory CIP lesions and found a notable overlap of T-cell repertoire in these sites but not in the secondary lymphoid organs or peripheral blood. Despite the indeterminant nature of antigen specificity, these data highlighted the cytotoxic effects of T cells on the instigation of CIP. Moreover, the predictive value of tumor-infiltrating lymphocytes has been illustrated in meta-analyses (96, 97). An elevated level of CD4+/CD8+ T-cell infiltration in the malignant cells showed superior outcomes in survival. However, an increasing number of FOXP3+ regulatory T cells, a subtype of CD4+ T cells with immunosuppressive actions, was associated with poor survival. These results have been reported from patients with ICI-related pneumonitis, and more evidence is needed from future studies to explore CIP mechanisms.
Increased Level of Autoantibodies and Inflammatory Cytokines
Pre-existing autoantibodies potentially linked to the development of irAEs in NSCLC, such as anti–thyroid peroxidase antibodies, anti-thyroglobulin antibodies, antinuclear antibodies, anti–rheumatoid factor antibodies, have been explored in recent studies (98). Tahir et al. (99) performed a mass screening of autoantibodies in patients who underwent ICI therapy by using high-throughput serological analysis of recombinant cDNA expression (i.e. SEREX). They identified an elevated plasma level of anti-CD74 from two patients with CIP in a discovery cohort and subsequently verified a 1.34-fold increase from 10 patients with CIP in a confirmation cohort. Intriguingly, samples of viral-mediated interstitial pneumonitis have also displayed an overexpression of CD74 (100), presenting a pathogenic nidus for CIP development. However, the specific antibodies associated with CIP should be prioritized for exploration. In terms of inflammatory cytokines, case reports of severe CIP have identified some cytokines linked to the appearance of CIP. Interleukin-6 (IL-6), IL-17A, IL-35, C-reactive protein (CRP), procalcitonin (PCT), surfactant protein-D (SP-D), and Krebs von den Lungen-6 (KL-6) were reportedly more common in patients with NSCLC who developed CIP than in those without CIP (25, 52, 57, 82, 84). In particular, SP-D and KL-6 reflected alveolar epithelial cell injury. All these cytokines also broadly serve as biomarkers for adverse events caused by ICIs.
Enhanced Complement-Mediated Inflammation
The function of complement-mediated inflammation may be enhanced by the direct combination of anti–CTLA-4 with CTLA-4 located on benign tissues, including the pituitary gland (14, 91). This mechanism may explain why pituitary inflammation could be a specific irAE of anti–CTLA-4 antibodies (101). Although CIP is more frequently observed with PD-1/PD-L1 blockades than with CTLA-4 blockades (102), CIP has not yet become a symbolic irAE of anti–PD-1/PD-L1 antibodies. After a review of the relevant literature, we speculate that the major causes of CIP may be the first two mechanisms described before. Additional exploration is required to deepen our understanding of CIP in NSCLC.
Risk Factors of CIP
Current evidence from retrospective studies and case reports has identified many potential risk factors for ICI-related pneumonitis (6, 24, 53, 72, 103–105). These include baseline patient characteristics, disease features, and therapy management. Specific factors include age, sex, smoking status, previous lung disease, tumor histological type, PD-1 blockade, combination therapy, and prior RT.
Baseline Patient Characteristics
The influence of age on the response to immunotherapy has not been studied comprehensively or systematically. Cho et al. (28) found that patients who had CIP were often older than 70 years (54.5% of total population studied, P=0.025). However, other literature has suggested that older age would not adversely relate to rates of toxicities or therapeutic response to ICI therapies (106, 107). A retrospective study recruited 205 patients with NSCLC and reported a higher incidence of CIP in women than in men, though the difference was not significant (24). Similarly, in another study, former or current smokers developed CIP more often than nonsmokers (P=0.03) (108). The evidence must be verified, but it does offer a new direction for continued research (87).
Disease Features
Pre-existing pulmonary diseases, including interstitial lung disease (ILD), chronic obstructive pulmonary disease (COPD), asthma, pneumothorax, pleural effusion, and pulmonary fibrosis, have been closely associated with the development of CIP in patients with NSCLC (19, 27, 29, 49, 50). The incidence of ICI-related pneumonitis in patients with pre-existing ILD was approximately three times higher than in those without ILD (29% vs 10%, P=0.027) (49). Patients with asthma and COPD were more likely to develop CIP (2.3% higher incidence vs those without COPD) (27). Notably, Nicholas et al. (29) found increasing numbers of lymphocytes dominated by CD4+/CD8+ T cells and high PD-L1 expression in the lungs of patients with NSCLC who had COPD, which might suggest longer PFS in patients receiving ICIs without COPD. A case-control study that included patients with pneumothorax, pleural effusion, and pulmonary fibrosis found a high risk of CIP in these patients but noted a low mortality rate and a high remission rate in the same group after treatment with corticosteroids (104). With regard to the tumor type, subgroup analyses of previous research showed that patients with the SCC subtype of NSCLC experienced a greater occurrence of CIP, but a lower mortality rate, compared with those diagnosed with the AC subtype (5, 10, 11, 21, 24, 37, 38, 41).
Therapy Management
RT reportedly has a synergistic effect with immunotherapy (14, 23). Intriguingly, RT itself could induce radiation pneumonitis in more than 30% of patients (109). Even when the radiation pneumonitis resolves, patients may present with severe radiation recall pneumonitis after treatment with ICIs (60). The Keynote-001 trial (110) explored the clinical efficacy of PD-1 inhibitors in patients with NSCLC and found a higher incidence of any-grade CIP in patients who received RT before ICI therapy (pembrolizumab, 13%) compared with those who did not receive RT (1%, P<0.05). The timing of RT and ICI use must be studied and discussed in more detail, whether a shorter interval between the two treatments could increase mutual toxicity or not remains unclear. The PACIFIC trial (111) compared CIP rates according to the initial time to start durvalumab after chemoradiotherapy (within 14 days or between 14 and 56 days) and found that the earlier start time did not increase the risk of CIP. RT parameters that may influence the development of CIP have also been studied, dosimetric parameters of prior chest RT, courses, timing, and technique were not considered significant risk factors for CIP development (48).
Monotherapy and combination therapy with ICIs appear to have distinct incidences of CIP in NSCLC. With ICI monotherapy, use of PD-1/PD-L1 inhibitors instead of CTLA-4 inhibitors increased the risk of CIP development (64). A meta-analysis (87) that included 19 trials found that PD-1 blockade treatment was associated with a statistically significantly higher incidence of CIP than PD-L1 blockade (3.6% vs 1.3%, P=0.001). In addition, the analysis reported no significant difference in the incidence of CIP in patients who received pembrolizumab or nivolumab. However, Fukihara et al. (47) found that more patients treated with pembrolizumab than with nivolumab developed CIP (63% vs 37%, P=0.004). Moreover, the incidence of CIP in patients treated with combination therapy increased twofold to threefold compared with patients treated with monotherapy (30, 87). The need for antibiotics and immunosuppressive drugs (112) were also predominant risk factors for pulmonary infection after ICIs.
Manifestations of CIP
Clinical Manifestations
The main clinical symptoms of CIP are relatively nonspecific and usually are similar to certain forms of ILD (23). CIP is characterized by fever, cough, chest pain, shortness of breath, dyspnea, fatigue, or respiratory failure (104). Bloody sputum or hemoptysis, hypotension, tachycardia or palpitation, diarrhea, and joint pain are less common ( Supplemental Table 1 ). In our analysis, dyspnea accounted for the most significant symptom of CIP (63.64%), followed by cough (36.36%) and fever (25.00%). Rashes were also commonly reported. Crackles on thorax auscultation manifested only in more advanced-grade CIP (23, 86).
Imaging Manifestations
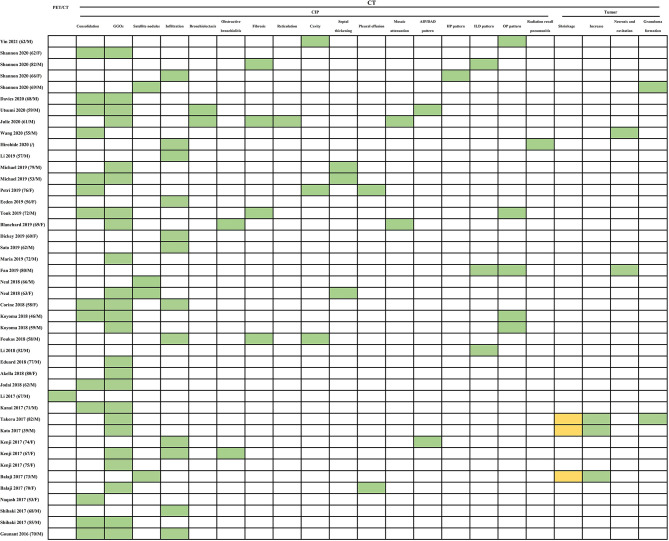
As awareness and experience with CIP increase among researchers, large-scale studies have categorized the various radiologic patterns. Acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS)/diffuse alveolar damage (DAD), cryptogenic organizing pneumonia (OP), ground-glass opacities (GGOs), nonspecific interstitial pneumonia, hypersensitivity pneumonitis (HP), bronchiolitis, radiation recall pneumonia, and an unclassified type have been recognized as subtypes of CIP according to imaging features in several studies concerning NSCLC (5, 6, 18, 20, 23, 88, 113). These different radiographic patterns of CIP could also be described as a spectrum of the pulmonary injury evolution process, from the acute stage (AIP/ARDS/DAD) to the organizing stage (OP) and fibrotic stage (nonspecific interstitial pneumonia) (5, 6). The GGOs and consolidation ( Figure 2 ) non-segmentally distributed in the dominant lung or bilaterally opposite the tumor, which have been considered typical computed tomography (CT) features in CIP of NSCLC (104, 113), represented 54.55% (24/44) and 31.82% (14/44), respectively, of the CT presentations in our analysis.
Figure 2.
Summarized results of radiological tests for diagnosis in published cases reports/case series. The radiological tests include PET/CT and CT findings. PET/CT, positron emission tomography/computed tomography; CT, computed tomography; GGOs, ground-glass opacities; AIP/DAD, Acute interstitial pneumonia (AIP)/diffuse alveolar damage (DAD); HP, hypersensitivity pneumonitis; ILD, interstitial lung disease; OP, organizing pneumonia. Abnormal: green grid; normal: orange grid; undone: white grid.
The OP pattern was the most common pattern for CIP in NSCLC (6, 113). The common manifestation of the OP pattern was bilateral peribronchovascular and subpleural GGOs, predominately in the middle to lower lung (113). Reversed atoll or halo sign, a circumferential consolidation surrounding an interior area of mosaic (ground-glass) attenuation, has been considered a relatively specific characteristic for OP in CIP (114). In addition, peribronchovascular pulmonary nodules smaller than 10 mm have been depicted in the OP pattern (113). However, the nodules could be mass-like with spiculated margins (115) or could be a peritumoral shadow (80, 104), reflecting obscure presentations of the tumor. This phenomenon has been regarded as pseudoprogression of malignancy (80, 104, 115). Two cases (80, 81) that we included presented with GGOs associated with an increase in tumor size (pseudoprogression). Pseudoprogression could be distinguished from CIP by evaluation of serum markers (carcinoembryonic antigen, cytokeratin fragment) (116) and by bronchoscopic narrow-band imaging and biopsy (117).
The nonspecific interstitial pneumonia pattern was the second most frequently reported pattern of CIP (113). It commonly manifests with GGOs and reticulation in the lower lobe of the lung (118). The specific finding was described as a subpleural sparing of the dependent and posterior lower lobe of the lung (115). Conversely, the HP pattern was a relatively uncommon radiologic abnormality of CIP. Centrilobular or diffuse GGOs with the predominance of mid-to upper-lobe location were the radiologic features of the HP pattern (113). This pattern can be distinguished from an HP pattern related to allergen exposure by obtaining definite patient histories about occupational and other exposures. The AIP/ARDS/DAD pattern exhibited the most severe extent of pulmonary involvement on imaging, presenting with diffuse or patchy GGOs or consolidation with involvement in the majority or all of the lung. This presentation often exhibits a “crazy-paving” pattern and interlobular septal thickening (115). Bronchiolitis has been found only in one retrospective cohort study and a few case reports (66, 86, 88, 118). Typically, it appears as a tree-in-bud pattern in the region of centrilobular nodularity. However, even bronchiolitis may be investigated as a distinct CIP pattern without infectious symptoms.
Radiation recall pneumonia is an inflammatory reaction that occurs in previously irradiated regions after exposure to some inciting agents; it manifests as consolidation and GGOs limited to the previously radiated area. Possible mechanisms of this type of pneumonia include stem cell function changes in the irradiated field triggered by hypersensitivity reactions to an idiosyncratic drug (119). Some case reports have presented radiation recall pneumonia in patients with NSCLC after treatment with ICIs (60, 85). Patients who receive RT and develop new pulmonary changes demarcated from the adjacent lung in the initial radiation field should be preferentially suspected of having radiation recall pneumonia.
Pathological Manifestations
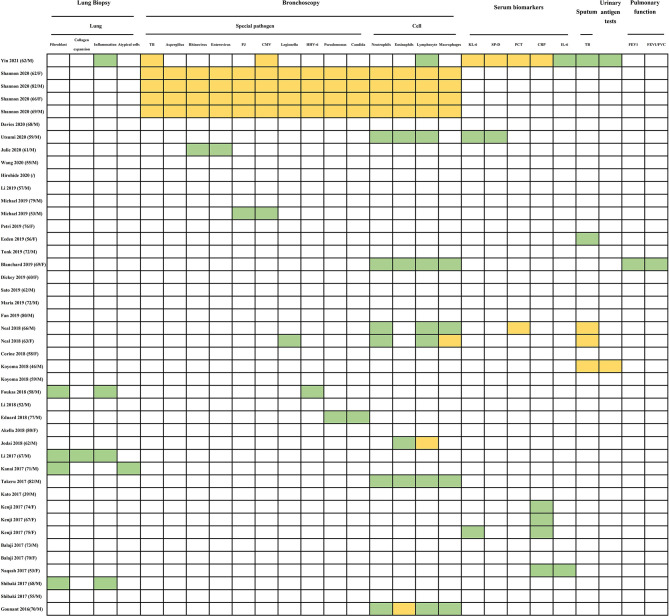
Not all patients with CIP will receive lung biopsy, especially in patients with ICI-related ILD. In our analysis, only 5 of 44 patients were considered for this examination ( Figure 3 ). Lung biopsies may increase the risk of acute deterioration in ILD and may not obtain definite histologic types if the harvested specimen is small. However, transbronchial lung biopsy could rule out alternative etiologies during the differential diagnosis. Literature reports have provided a limited pathological pattern of CIP, with a range of different presentations that includes OP, DAD, eosinophilic pneumonia, cellular interstitial pneumonitis, and nonspecific or granulomatous inflammation (6, 18, 88). The interstitial inflammatory infiltration might include elevated levels of eosinophil, poorly formed granulomas, and lymphocytes (18). The cases that we included specifically mentioned the pathological manifestation of alveolar parenchyma with fibroblast foci (four cases) (73, 78, 79, 85), mild collagen expansion of the alveolar septa (one case) (78), nonspecific chronic inflammation (four cases) (73, 78, 85), and atypical cells (one case) (79).
Figure 3.
Summarized results of histological, laboratory and pulmonary function tests for diagnosis in published cases reports/case series. The histological test includes lung biopsy. The laboratory tests include BAL (special pathogen and cells) in bronchoscopy, serum, sputum, urinary antigen test. The pulmonary function tests include FEV1 and FEV1/FVC. TB, tuberculosis; PJ, pneumocystis jirovecii; CMV, cytomegalo-virus; HHV-6, human herpes virus 6; KL-6, krebs von den Lungen-6; SP-D, surfactant protein-D; PCT, procalcitonin; CRP, C-reactive protein; IL-6, interleukin-6; FEV1, forced expiratory volume in one second; FEV1/FVC, fractional volume change. Abnormal: green grid; normal: orange grid; undone: white grid.
Diagnosis of CIP
Because specific clinical or radiologic markers are absent, diagnosis of CIP is quite difficult. CIP is typically a diagnosis of exclusion, one that should rule out infection, tumor progression, and radiation-related pneumonitis (25). New emerging or the deterioration of respiratory symptoms—especially dry cough, dyspnea, and decreasing oxygen saturation—after ICI therapy for NSCLC require consideration of CIP (23). The diagnostic workup ( Figure 3 ) to identify an etiology should include tests for a source of infection (including nasal swab, sputum/urine culture, and blood culture); tests for special pathogens (fungus, tuberculosis spot test); chest radiography (high-resolution CT); and bronchoscopy with BAL (17, 18, 20). Lung biopsy is not mandatory, and both drugs and infectious history can occasionally help to interpret results. Utilization of diagnostic tests is related to the suspected pneumonitis grade (6).
Common differential diagnoses for CIP include pulmonary infections, pulmonary embolism, DAD, lung cancer with underlying progression, cancerous lymphangitis, pulmonary interstitial edema caused by heart failure, fulminant myocarditis (120), and RIP (5, 23). Opportunistic pulmonary infections, including tuberculosis (TB) pneumonia, aspergillosis, cytomegalovirus pneumonia (CMVP), and Pneumocystis jirovecii pneumonia (PJP), have been the foremost differential diagnoses for CIP in the NSCLC population (26, 121–125). Inthasot et al. (26) reported two cases of severe lung infections complicating the treatment of nivolumab for NSCLC and emphasized the importance of eliminating the possibility of opportunistic infections. Notably, ICIs could cause special pathogen infections in some patients through induction of CIP. We included several cases of patients who developed CIP during ICI therapy and consequently developed rhinovirus/enterovirus (58), CMVP (62), PJP (62), legionella (25), human herpesvirus 6 (HHV-6) (73), pseudomonas, or candida (75) infections ( Figure 3 ). Because ICIs activate tumor immunity by inhibiting PD-1/PD-L1/CTLA-4, they might also simultaneously inhibit immunity to infection. Although the infections we described here as differential diagnoses are not usually categorized as drug-induced pneumonias, we included this series of reports to exemplify challenges in differentiating intensified infection from drug-induced pneumonia. Since from a drug safety perspective, the infection did lead to a few deaths.
The CT manifestations of CIP in patients with pulmonary AC sometimes resembled those of interstitial pneumonitis (126), especially of the OP pattern. Ichikawa et al. (127) reported that 2% of patients (13/564) with resected pulmonary AC presented with an OP pattern. Kanai et al. (79) reported a case of coexisting CIP and tumor invasion, which complicated the diagnosis and management of the lung disease. Aggressive lung biopsy was recommended in that study to correctly diagnose CIP in patients with NSCLC that mimicked the OP pattern or existed the tumor invasion.
RIP, an early lung injury induced by radiation, is also a difficult differential diagnosis in CIP. The approximate onset (1 to 3 months), similar imaging features (GGOs and diffuse haziness), and shared pathological feature (lymphocytic alveolitis) increased the level of challenge in distinguishing CIP from RIP (128–130). However, a distinct lesion location may assist in finding the difference between the two. RIP mainly exists in the radioactive region, and CIP mostly occurs outside the RT fall-off dose or in the low-dose field (48). Interestingly, both CIP and RIP have the same first-line therapy (corticosteroids) (121, 128). Meanwhile, radionics has emerged as a new approach to predict CIP by automatically extracting radiologic features for synthesis analysis (131).
In summary, CIP requires a precise diagnosis, including grade assessment, and monitoring of CIP requires a multidisciplinary method. Such monitoring often involves infectious disease specialists, pathologists, radiologists, pulmonologists, and cardiologists (121).
Management of CIP
CIP is deemed a self-limiting disease. No prospective trials, to our knowledge, have evaluated the optimal therapeutic modality for CIP (5, 24). Current guidelines for CIP, therefore, recommended corticosteroids as the primary therapy approach (121, 132, 133). These decisions are based on the strength of case reports and clinical experience (5, 24). Different definitions of CIP grades are shown in Supplemental Table 2 (121, 133). Clinical improvement is usually observed after 48 to 72 hours of corticosteroid use, and patients without regression of CIP-related symptoms have been considered steroid refractory and treated with immunosuppressive agents (121, 133).
For patients with grade 1 CIP, clinical symptoms, imaging changes, and pulmonary function (diffusing capacity and spirometry) should be closely monitored for 3 to 4 weeks (122, 123, 134, 135). Tentatively stopping ICI treatment can be considered reasonable for mild cases of CIP (23). When the condition worsens, though, interruption of the ICI should be combined with initiation of low-dose steroids (0.5 to 1 mg/kg/d) (9, 136).
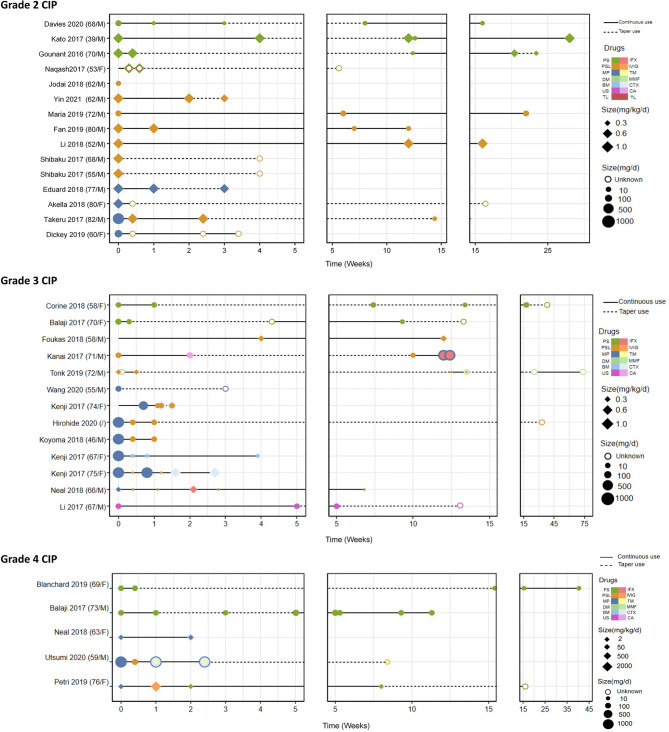
For patients with grade 2 CIP, withholding the ICIs and beginning intermediate-dose steroids (1 to 2 mg/kg/d) followed by a taper by 5 to 10 mg/week for 4 to 6 weeks have been proposed (133). In our analysis, we summarized the management characteristics stratifed by CIP grade ( Table 3 ) and listed every drug that every case used ( Figure 4 ). We converted the different steroid doses to methylprednisolone (MP) equivalents and divided these into three groups (low-dose, intermediate-dose, and high-dose groups) according to the initial equivalent administered at the beginning of the therapy. We also noticed that some cases did not describe the weight of patients, which led to two different specifications of steroid dose (mg/d and mg/kg/d). In patients with grade 2 CIP ( Table 3 ), 60% of patients were administered intermediate-dose steroids (60 to 500 mg/d). In other cases, 80% of patients with grade 2 CIP started with low-dose steroids (< 1 mg/kg/d). In addition, bronchoscopy and/or BAL plus initiation of empirical antibiotics when infection is suspected are recommended (14, 137). If clinical improvement does not happen after 2 to 7 days of monitoring, increasing the corticosteroid dose and adding immunosuppressive drugs should be considered (121, 138). Restarting ICI therapy may be considered when CIP is stable, has improved to grade ≤ 1, or has improved with 10 mg/d of prednisone (23). After re-initiation, physicians should evaluate clinical indicators every 3 days and perform chest imaging once a week to monitor for the flare and recurrence of CIP (9).
Table 3.
The characteristics related to management of CIP stratified by grade of CIP.
| Grade of CIP Mean ± SD/N (%) | Total | Grade 2 | Grade 3 | Grade 4 | P-value |
|---|---|---|---|---|---|
| N | 44 | 18 | 19 | 7 | |
| Steroid initial dose (mg/d) | 425.29 ± 451.82 | 280.40 ± 411.75 | 527.56 ± 469.29 | 360.00 ± 554.26 | 0.426 |
| Steroid initial dose groups (mg/d) | 0.379 | ||||
| Low-dose <60 | 5 (29.41%) | 1 (20.00%) | 2 (22.22%) | 2 (66.67%) | |
| Intermediate-dose 60-500 | 6 (35.29%) | 3 (60.00%) | 3 (33.33%) | 0 (0.00%) | |
| High-dose 501-1000 | 6 (35.29%) | 1 (20.00%) | 4 (44.44%) | 1 (33.33%) | |
| Steroid initial dose (mg/kg/d) | 1.24 ± 0.58 | 0.86 ± 0.10 | 2.00 ± 0.40 | 2.00 ± 0.00 | <0.001 |
| Steroid initial dose groups (mg/kg/d) | 0.007 | ||||
| Low-dose <1 | 8 (53.33%) | 8 (80.00%) | 0 (0.00%) | 0 (0.00%) | |
| Intermediate-dose 1-2 | 6 (40.00%) | 2 (20.00%) | 2 (66.67%) | 2 (100.00%) | |
| High-dose >2 | 1 (6.67%) | 0 (0.00%) | 1 (33.33%) | 0 (0.00%) | |
| Steroid taper time | 10.46 ± 9.94 | 7.20 ± 5.35 | 16.37 ± 14.60 | 8.25 ± 4.79 | 0.154 |
| Steroid course | 14.43 ± 15.14 | 12.23 ± 8.54 | 16.35 ± 20.75 | 15.62 ± 14.75 | 0.776 |
| Immunosuppressive drugs | 0.016 | ||||
| No | 35 (79.55%) | 17 (94.44%) | 15 (78.95%) | 3 (42.86%) | |
| Yes | 9 (20.45%) | 1 (5.56%) | 4 (21.05%) | 4 (57.14%) | |
| Antibiotics | 0.011 | ||||
| No | 28 (63.64%) | 14 (77.78%) | 13 (68.42%) | 1 (14.29%) | |
| Yes | 16 (36.36%) | 4 (22.22%) | 6 (31.58%) | 6 (85.71%) | |
| Recurrent times | 0.312 | ||||
| 0 | 34 (77.27% | 14 (77.78%) | 14 (73.68%) | 6 (85.71%) | |
| 1 | 6 (13.64%) | 4 (22.22%) | 2 (10.53%) | 0 (0.00%) | |
| 2 | 2 (4.55%) | 0 (0.00%) | 2 (10.53%) | 0 (0.00%) | |
| 3 | 2 (4.55%) | 0 (0.00%) | 1 (5.26%) | 1 (14.29%) | |
| CIP outcome | 0.003 | ||||
| Improved/Resolved | 34 (77.27%) | 18 (100.00%) | 13 (68.42%) | 3 (42.86%) | |
| Deteriorated/Maintained | 10 (22.73%) | 0 (0.00%) | 6 (31.58%) | 4 (57.14%) | |
| CIP course (weeks) | 12.64 ± 14.20 | 10.30 ± 7.90 | 14.85 ± 19.20 | 12.96 ± 13.09 | 0.673 |
| OS | 0.019 | ||||
| Alive | 20 (57.14%) | 12 (85.71%) | 5 (35.71%) | 3 (42.86%) | |
| Dead | 15 (42.86%) | 2 (14.29%) | 9 (64.29%) | 4 (57.14%) | |
| Survival time (weeks) | 55.35 ± 46.26 | 72.92 ± 58.13 | 41.00 ± 29.64 | 46.00 ± 37.33 | 0.198 |
| Clinical response | 0.018 | ||||
| Complete response | 2 (5.71%) | 0 (0.00%) | 2 (14.29%) | 0 (0.00%) | |
| Partial response | 6 (17.14%) | 6 (42.86%) | 0 (0.00%) | 0 (0.00%) | |
| Tumor progressed | 5 (14.29%) | 2 (14.29%) | 2 (14.29%) | 1 (14.29%) | |
| Stable | 7 (20.00%) | 4 (28.57%) | 1 (7.14%) | 2 (28.57%) | |
| Unknown | 15 (42.86%) | 2 (14.29%) | 9 (64.29%) | 4 (57.14%) |
CIP, checkpoint inhibitor pneumonitis; OS, overall survival.
Bold values: two-sided P-values less than 0.05 were considered to identify statistical significance.
Figure 4.
The steroid therapy including every drug that every case utilized and the definite continuous and taper time. PS, prednisone; PSL, prednisolone; MP, methylprednisolone; DM, dexamethasone; BM, betamethasone; US, unspecific; TL, tocilizumab; IFX, infliximab; IVIG, immunoglobulin; TM, tacrolimus; MMF, mycophenolate mofetil or mycophenolic acid; CTX, cyclophosphamide; CA, cyclosporine A.
For patients with grade 3 to 4 CIP, ICI therapy should be discontinued immediately and permanently. The initial doses of steroids (1 to 2 mg/kg/d and 2 to 4 mg/kg/d) were approved and included in guidelines by the American Society of Clinical Oncology guidelines and the European Society for Medical Oncology (15, 121), respectively. However, no clinical trials have identified optimal corticosteroid doses or durations; therefore, therapy duration has always been adjusted largely on the basis of response to steroid treatment. Our analysis showed that patients with grade 3 or 4 CIP most often received glucocorticoid pulse therapy (44% of patients with grade 3 and 33% of patients with grade 4; Table 3 ). Initial steroid dosages of 1 to 2 mg/kg/d were mostly used in patients with severe CIP ( Table 3 ), and this dosage was consistent with the recommendations of the American Society of Clinical Oncology. Institutionally, we continue at the initial dosage until patients improve or remain stable (usually 1 week), at which time corticosteroids can be very slowly tapered during at least 5 to 8 weeks (9). Our data showed that the mean duration of a steroid taper was nearly 10 weeks, and the longest duration was in patients with grade 3 CIP (mean ± standard deviation of 16.37 ± 14.60 weeks) ( Table 3 ). Additional immunosuppressants, including infliximab (IFX), mycophenolate mofetil, intravenous immunoglobulin, tacrolimus, ciclosporin (57, 79), and cyclophosphamide, should be considered when the symptoms do not regress after 48 to 72 hours of treatment with corticosteroids (6, 14, 23, 137). Empirical antibiotics may be used to prevent opportunistic infection (122, 139–141). Our data also showed that the rates of immunosuppressive drug use (grade 2: 5.56%, grade 3: 21.05%, grade 4: 57.14%, P=0.016) and antibiotic use (grade 2: 22.22%, grade 3: 31.58%, grade 4: 85.71%, P=0.011) gradually increased with increasing severity of CIP ( Table 3 ).
Moreover, it has been reported that nearly one-fourth to one-third of patients experience CIP flares or recurrence after rapid corticosteroid tapers and appear recalcitrant to corticosteroid treatment (5). CIP recurrence may occur early in patients with more severe grade (grade 3 or 4) initially and have occurred most often in patients whose therapeutic course was shorter than 5 weeks (71, 142). The lengths of steroid courses from our data varied from 1 week to 73.4 weeks, and the mean duration for grade ≥ 2 CIP was more than 10 weeks ( Tables 2 and 3 ). However, in patients whose steroid course was shorter than 5 weeks (25, 49, 55, 59, 67, 72, 75, 82), two patients (25, 67) experienced CIP recurrence. The highest CIP recurrence rate, 22.22%, occurred in patients with grade 2 CIP ( Table 3 ). In addition, the steroid courses were centrally distributed in the first 5 weeks ( Figure 4 ), which suggests that the changes to steroid dosages (in grade 2 CIP) and drugs (in grade 3 or 4 CIP) usually occurred in this window.
Current experience with immunosuppressive drugs to treat CIP is based mostly on extrapolation from data about their use to treat other irAEs, which lacked pathophysiological evidence (5). IFX and cyclophosphamide have been approved to treat ICI-related digestive toxicities, especially colitis (133, 143, 144). However, IFX could itself cause ILD and liver injury (145–147). In addition, it could weaken the ongoing anticancer immune activity initially launched by ICI treatment (25); this hypothesis is consistent with a prior study (18), which reported that half of patients with grade 3 CIP died despite receiving additional immunosuppressive drugs. As a second-line drug, mycophenolate mofetil remains controversial because of its suppressive effects on the T-cell response (148). IL-17 blockade reportedly relieved ICI-related gastrointestinal and skin irAEs (149). Current guidelines also recommend cyclophosphamide, mycophenolate mofetil intravenously (1 g twice daily), or IFX (5 mg/kg) as supportive care (121, 133, 135) for steroid-resistant patients with irAEs. Intravenous immunoglobulin was effective in ICI-mediated myasthenia gravis and did not blunt infection responses (150). Thus, intravenous immunoglobulin could become a logical choice for treating CIP in patients with suspected comorbid infections (24). Tocilizumab, an IL-6 inhibitor, has been used to treat rheumatologic irAEs (84). A case report showed that a patient with NSCLC and CIP experienced significant symptom relief after additional therapy with tocilizumab (151). However, whether tocilizumab should be included as an option in the second-line drugs to treat steroid-refractory patients with irAEs remains undetermined, because that approach lacks a comparison with other second-line drugs.
Prognosis of CIP
Most studies have found that patients with CIP, especially with lower-grade disease, could see symptoms improve or resolve if they received corticosteroid therapies (18, 152). Similarly, our data ( Table 3 ) demonstrated that patients with grade 2 CIP all experienced improvements in or resolution of CIP and had the highest OS (85.71%) versus patients with grade 3 or 4 CIP (OS of 35.71% or 42.86%, respectively, P=0.019). In addition, nearly half of patients with grade 2 CIP experienced a partial tumor response, whereas most patients with grade 3 or 4 CIP experienced tumor progression or maintenance. However, a single-center study (20) recently reported poor prognoses in patients with NSCLC who developed CIP. Suresh et al. (45) demonstrated that the ICIs did not significantly influence the short-term survival (disease control rate, overall response rate, or PFS) but did affect OS which decreased by 10 months in patients with CIP. Fukihara et al. (47) came to a similar conclusion regarding the decrease in OS. Patients with CIP (8.7 months) had a shorter OS after PD-1 blockade compared with those without CIP (23.0 months, P=0.015). We also evaluated the association between CIP and OS ( Figure 5 ), and we found that patients who experienced deteriorated or maintained CIP were significantly more likely to have a poor prognosis compared with patients who experienced improved or resolved CIP (P=0.006). One potential reason might be that patients with CIP were more likely to be forced to quit ICI therapy to avoid lethal respiratory failure. Moreover, as a result of deteriorating physical status, abrasive pulmonary symptoms, and prolonged steroid management for CIP, patients with CIP tended to reject—and their physicians were more likely to hesitate or delay commencement or continuation of—aggressive anti-tumor treatment.
Figure 5.
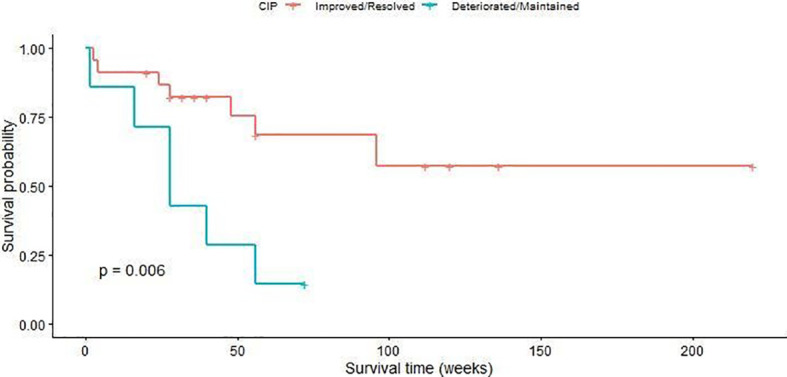
Overall survival curves of patients with checkpoint inhibitor pneumonitis.
Recurrent phenomena related to the management of CIP have been explored in patients with NSCLC who received ICI therapy. These phenomena included recurrent pneumonia after completion of a steroid taper with or without restarting immunotherapy. Reports of reusing ICI therapy mainly occurred in patients with grade 1 or 2 CIP initially, since patients identified with grade 3 or 4 CIP generally withdrew treatment permanently (47). The reported recurrence ratio after reusing immunotherapy varied from 17% to 30% (18, 55, 122). Our analysis ( Table 2 ) showed that the overall recurrence ratios with and without re-challenge ICI therapy were 6.82% (3/44) and 22.73% (10/44), respectively. Among three patients (65, 73, 81) who re-challenged ICI therapy after clinical regression of CIP, two experienced recurrence after restarting ICIs (65, 81), and one patient successfully improved by discontinuing immunotherapy and beginning treatment with antibiotics and steroids (73). Recurrent pneumonitis severity, location of involvement, and pattern might vary compared with the initial manifestation of CIP.
Predictive factors for CIP are still under investigation. Currently, the exploration of serum markers, cytokines/chemokines, and cellular biomarkers have interested clinicians (137). Increased carcinoembryonic antigen (CEA) serum levels reportedly relate to both tumor progression and the simultaneous regression of recurrent CIP (153), which represents an early association with both durable toxicity and durable response. In addition, a low level of serum albumin was an independent predictor of CIP in patients with NSCLC (odds ratio=0.381, 95% CI=0.179–0.808, P=0.012) (71). In solid tumors, other research found that elevated baseline lymphocyte levels were linked to irAEs (47). In patients with melanoma who experienced severe irAEs, peripheral blood samples were evaluated early during treatment, and 11 elevated cytokines were recruited in the validation group for the predictive model (154).
We also evaluated the relationship between the initial steroid dose and OS ( Supplement Figures 1 , 2 ). Unfortunately, no significant difference in OS was found among low-dose, intermediate-dose, and high-dose steroid groups. Some reasons might be that the sample size was small and the precise data about steroid doses were limited, so the optimal steroid dose for OS was not determined. Therefore, extensive multicenter studies, which have detailed management of steroid therapy, should be conducted in the future.
Post-CIP Evolution and Typical Sequela
The evolution of post-CIP patients is largely dependent on their CIP status. Patients with moderate or well-controlled CIP would have various subsequent treatment options including only supportive care, cytotoxic chemotherapy alone and ICIs rechallenge, based on the primary tumor response, irAEs evaluation, and patients’ willingness (155). Yamagata et al. (156) conducted a retrospective analysis concerning the NSCLC patients with CIP and reported the cancer therapy after CIP. They found that 34.6% of CIP patients decided to treat with cytotoxic chemotherapy, and 30.8% of CIP patients chose the best supportive care after CIP. The rechallenge of ICIs only applied on 3% of CIP patients. Actually, if the patients get complete or partial remission (CR or PR), the therapeutic strategies without ICIs could be considered for continued use (157). However, the options of rechallenge should be deliberated in the context of personalized consideration and multidisciplinary evaluation.
Patients with neurologic, cardiac, or any grade 4 irAEs are not recommended to continue or rechallenge ICIs (158). The evaluation of ICIs rechallenge mainly depends on risk-reward ratio (158). At present, there is no acknowledged guidance for re-challenging ICIs. Whether patients should resume ICI monotherapy after receiving doublet ICI therapy is still being investigated. A recent study recruited 80 patients with irAEs on doublet ICI therapy who subsequently reinstated ICIs as monotherapy, and the results indicated that the incidence of CIP (33%) was significantly higher than ophthalmic or gastrointestinal immune-related toxicity (159). However, in most instances, the ICI utilized for re-initiation in NSCLC could be the same ICIs used before, another PD-1/PD-L1 inhibitors, or the switching from PD-1 to PD-L1 inhibitors or the converse (160–164). Kitagawa et al. (157) included prior reports about ICIs rechallenge in NSCLC and analyzed its efficacy and safety. The results showed the generally lower overall response rate (ORR), disease control rate (DCR), and the median PFS presented in patients received the second ICI than in those received the first ICI among these studies. The greatest DCR (58.8%) and longest median PFS (4.0 months) during the second ICI treatment were showed in the 17 patients Kitagawa et al. (157) included. All these 17 patients switched the ICIs type when ICI rechallenge, of which 58.8% obtained PR or stable disease (SD) after switching ICIs administration. However, the efficacy of ICIs rechallenge is still controversial (165–167). Between two ICIs administration, shorter interval may exert better effects on outcome. Besides, the potential predictive factors of ICIs rechallenge outcome include early irAEs development, irAE therapy intensity, CIP phenotype, PD-1 inhibitors, and age more than 65 (155). As for the safety, the second attempt of ICIs could cause same irAEs or moderate new irAEs. Naidoo et al. (168) reported that 3 of 12 patients who reinstated ICI therapy developed CIP recurrence (initial CIP grade of 1 or 2), and 38 of 68 patients developed irAEs after re-treatment. Once patients experience recurrent CIP, the discontinued ICIs in time and the monotherapy of same steroid administrated before is universally acknowledged (19), while with a slower dose tapering and longer course (142).
Notably, there might exist durable anti-tumor activity after discontinuing ICIs therapy (44, 169, 170). This continuous treatment tendency could hold on until intolerable irAEs appearance, tumor progression or no more than 2 years. The correlation of tumor response and toxicity enhances the complexity of ICIs therapy and requires to be demonstrated further. Gauci et al. (170) found that the favorable predictive factors for prolonged response after stopping ICIs therapy included CR patients before discontinuation, with 13% increase of keeping disease stability compared to PR patients.
There are few reports about the sequela of CIP. The typical sequela might be the sustaining pulmonary interstitial fibrosis and poor pulmonary function caused by severe CIP (171, 172). Nintedanib, as an angiokinase blocker, has been reported to play significant role in progressive fibrosing interstitial lung disease, contributing to slow down the decline rate of forced vital capacity (FVC) (173) and further potentially strengthen the prevention of CIP (174).
Future Directions for CIP
Although quite a few researchers have intensively studied the characteristics of CIP in NSCLC, the studies with regard to the diagnosis, treatment and risk stratification require more exploration (175). First, timely and accurate diagnosis of CIP is necessary. The current biomarkers are based on the mechanism of irAEs. Among the various biomarkers, Isono et al. (176) recently found idiopathic interstitial pneumonias became the only risk factor of CIP in the multivariate Cox regression model. Therefore, the ability of these biomarkers to predict CIP should be investigated deeply.
Second, the management of CIP remains inconclusive. The optimal drug regimen of corticosteroid (taper and continuous time) for CIP and ICIs (onset) for post-CIP need more clinical studies with large sample size to evaluate. Currently, the corresponding two clinical protocols, NCT04036721 and NCT04169503, are ongoing and expected to present profound results.
Third, risk stratification for CIP contributes to precise treatment. CIP presents with different incidence and death rates in different histological types of NSCLC, which may be ascribed to the intrinsic features of tumor histological subtypes (19, 24). Thus, we need more research about the clinical, radiological, histological, and biological characteristics of CIP to determine whether specific subsets of patients should be treated prophylactically.
Statistics Analysis
We conducted the descriptive analyses to delineate the baseline characteristics and the intergroup differences in different CIP outcomes and CIP grade groups. Kruskal-Wallis test and chi-square or Fisher’s exact test were utilized to analyze continuous and categorical variables, respectively. The former variables were presented by means and standard deviations, and the latter variables were expressed as counts and proportions. The overall and CIP survival rate were estimated by Kaplan-Meier method with a log-rank test. The statistical software packages R and EmpowerStats (X&Y Solutions Inc., Boston, MA, USA) were utilized to conduct all the statistical analyses. Two-sided P-values less than 0.05 were considered to identify statistical significance.
Author Contributions
QZ and LT searched the literature and wrote the manuscript. YZ helped to collect literature and participated in discussions. LT and WH performed the statistics analysis. WL examined and verified the study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.663986/full#supplementary-material
Relationship between initial corticosteroids dose and checkpoint inhibitor pneumonitis outcome. Kaplan-Meier curves by initial corticosteroids dose (mg/d) (A), by initial corticosteroids dose (mg/kg/d) (B).
Relationship between initial corticosteroids dose and overall survival. Kaplan-Meier curves by initial corticosteroids dose (mg/d) (A), by initial corticosteroids dose (mg/kg/d) (B).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2. Bodor JN, Boumber Y, Borghaei H. Biomarkers for Immune Checkpoint Inhibition in non-Small Cell Lung Cancer (NSCLC). Cancer (2020) 126(2):260–70. 10.1002/cncr.32468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ozkaya S, Findik S, Dirican A, Atici AG. Long-Term Survival Rates of Patients With Stage IIIB and IV non-Small Cell Lung Cancer Treated With Cisplatin Plus Vinorelbine or Gemcitabine. Exp Ther Med (2012) 4(6):1035–8. 10.3892/etm.2012.714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38(7):706–+. 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suresh K, Naidoo J, Lin CT, Danoff S. Immune Checkpoint Immunotherapy for Non-Small Cell Lung Cancer: Benefits and Pulmonary Toxicities. Chest (2018) 154(6):1416–23. 10.1016/j.chest.2018.08.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naidoo J, Nishino M, Patel SP, Shankar B, Rekhtman N, Illei P, et al. Immune-Related Pneumonitis After Chemoradiotherapy and Subsequent Immune Checkpoint Blockade in Unresectable Stage III Non-Small-Cell Lung Cancer. Clin Lung Cancer (2020) 21(5):E435–44. 10.1016/j.cllc.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 7. Lin X, Lu X, Luo GS, Xiang H. Progress in PD-1/PD-L1 Pathway Inhibitors: From Biomacromolecules to Small Molecules. Eur J Medicinal Chem (2020) 186:111876. 10.1016/j.ejmech.2019.111876 [DOI] [PubMed] [Google Scholar]
- 8. Hayashi H, Nakagawa K. Combination Therapy With PD-1 or PD-L1 Inhibitors for Cancer. Int J Clin Oncol (2020) 25(5):818–30. 10.1007/s10147-019-01548-1 [DOI] [PubMed] [Google Scholar]
- 9. Shannon VR. Pneumonitis Associated With Immune Checkpoint Inhibitors Among Patients With non-Small Cell Lung Cancer. Curr Opin Pulm Med (2020) 26(4):326–40. 10.1097/MCP.0000000000000689 [DOI] [PubMed] [Google Scholar]
- 10. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. New Engl J Med (2015) 373(2):123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-Year Overall Survival for Patients With Advanced non-Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol (2019) 37(28):2518–+. 10.1200/JCO.19.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC. New Engl J Med (2018) 379(24):2342–50. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 13. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. New Engl J Med (2017) 377(20):1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 14. Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo H, et al. The Mechanism and Risk Factors for Immune Checkpoint Inhibitor Pneumonitis in non-Small Cell Lung Cancer Patients. Cancer Biol Med (2020) 17(3):599–611. 10.20892/j.issn.2095-3941.2020.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, et al. The Society for Immunotherapy of Cancer Consensus Statement on Immunotherapy for the Treatment of non-Small Cell Lung Cancer (NSCLC). J Immunother Cancer (2018) 6(1):75. 10.1186/s40425-018-0382-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hahn AW, Gill DM, Agarwal N, Maughan BL. PD-1 Checkpoint Inhibition: Toxicities and Management. Urol Oncol Seminars Original Invest (2017) 35(12):701–7. 10.1016/j.urolonc.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 17. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 Immune Checkpoint Antibodies. Ann Oncol (2015) 26(12):2375–91. 10.1093/annonc/mdv383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol (2017) 35(7):709–+. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi S. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer A Systematic Review and Meta-Analysis. JAMA Oncol (2016) 2(12):1607–16. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 20. Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res (2016) 22(24):6051–60. 10.1158/1078-0432.CCR-16-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishino M, Chambers ES, Chong CR, Ramaiya NH, Gray SW, Marcoux JP, et al. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol Res (2016) 4(4):289–93. 10.1158/2326-6066.CIR-15-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-Related Adverse Events Associated With Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front Pharmacol (2017) 8:730. 10.3389/fphar.2017.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Y, Shao C, Li S, Xu Y, Xu K, Zhang Y, et al. Programmed Cell Death 1 (PD-1)/PD-ligand 1(PD-L1) Inhibitors-Related Pneumonitis in Patients With Advanced Non-Small Cell Lung Cancer. Asia Pacif J Clin Oncol (2020). 10.1111/ajco.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suresh K, Khinh Ranh V, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thoracic Oncol (2018) 13(12):1930–9. 10.1016/j.jtho.2018.08.2035 [DOI] [PubMed] [Google Scholar]
- 25. Andruska N, Mahapatra L, Hebbard C, Patel P, Paul V. Severe Pneumonitis Refractory to Steroids Following anti-PD-1 Immunotherapy. BMJ Case Rep (2018) 2018:225937. 10.1136/bcr-2018-225937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inthasot V, Bruyneel M, Muylle I, Ninane V. Severe Pulmonary Infections Complicating Nivolumab Treatment for Lung Cancer: A Report of Two Cases. Acta Clin Belg (2020) 75(4):308–10. 10.1080/17843286.2019.1629078 [DOI] [PubMed] [Google Scholar]
- 27. Galant-Swafford J, Troesch A, Tran L, Weaver A, Doherty TA, Patel SP. Landscape of Immune-Related Pneumonitis in Cancer Patients With Asthma Being Treated With Immune Checkpoint Blockade. Oncology (2020) 98(2):123–30. 10.1159/000503566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, Incidence, and Risk Factors of Immune Checkpoint Inhibitor-Related Pneumonitis in Patients With Non-Small Cell Lung Cancer. Lung Cancer (2018) 125:150–6. 10.1016/j.lungcan.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 29. Mark NM, Kargl J, Busch SE, Yang GHY, Metz HE, Zhang H, et al. Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non-Small Cell Lung Cancer. Am J Respir Crit Care Med (2018) 197(3):325–36. 10.1164/rccm.201704-0795OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab Plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med (2018) 379(21):2040–51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 31. Ahn MJ, Yang J, Yu H, Saka H, Ramalingam S, Goto K, et al. Osimertinib Combined With Durvalumab in EGFR-mutant Non-Small Cell Lung Cancer: Results From the TATTON Phase Ib Trial. J Thoracic Oncol (2016) 11(4):S115–5. 10.1016/S1556-0864(16)30246-5 [DOI] [Google Scholar]
- 32. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab Plus Ipilimumab in Lung Cancer With a High Tumor Mutational Burden. N Engl J Med (2018) 378(22):2093–104. 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab Plus Ipilimumab as First-Line Treatment for Advanced Non-Small-Cell Lung Cancer (CheckMate 012): Results of an Open-Label, Phase 1, Multicohort Study. Lancet Oncol (2017) 18(1):31–41. 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol (2019) 37(12):992–1000. 10.1200/JCO.18.01042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang JC, Gadgeel SM, Sequist LV, Wu CL, Papadimitrakopoulou VA, Su WC, et al. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced Nsclc With Sensitizing EGFR Mutation. J Thorac Oncol (2019) 14(3):553–9. 10.1016/j.jtho.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 36. Yang JC, Shepherd FA, Kim DW, Lee GW, Lee JS, Chang GC, et al. Osimertinib Plus Durvalumab Versus Osimertinib Monotherapy in EGFR T790M-Positive NSCLC Following Previous EGFR TKI Therapy: CAURAL Brief Report. J Thorac Oncol (2019) 14(5):933–9. 10.1016/j.jtho.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 37. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab Plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med (2018) 378(22):2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 38. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med (2018) 378(24):2288–301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 39. Borghaei H, Langer CJ, Gadgeel S, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. 24-Month Overall Survival From KEYNOTE-021 Cohort G: Pemetrexed and Carboplatin With or Without Pembrolizumab as First-Line Therapy For Advanced Nonsquamous non-Small Cell Lung Cancer. J Thorac Oncol (2019) 14(1):124–9. 10.1016/j.jtho.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 40. Reck M, Socinski MA, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Primary PFS and Safety Analyses of a Randomized Phase III Study of Carboplatin Plus Paclitaxel Plus /- Bevacizumab, With or Without Atezolizumab in 1L non-Squamous Metastatic NSCLC (Impower150). J Thoracic Oncol (2018) 13(4):S77–8. 10.1016/S1556-0864(18)30409-X [DOI] [Google Scholar]
- 41. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2016) 375(19):1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 43. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389(10066):255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-positive, Advanced non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 45. Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, et al. Impact of Checkpoint Inhibitor Pneumonitis on Survival in NSCLC Patients Receiving Immune Checkpoint Immunotherapy. J Thorac Oncol (2019) 14(3):494–502. 10.1016/j.jtho.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 46. Cui P, Huang D, Wu Z, Tao H, Zhana S, Ma J, et al. Association of Immune-Related Pneumonitis With the Efficacy of PD-1/PD-L1 Inhibitors in non-Small Cell Lung Cancer. Ther Adv Med Oncol (2020) 12:175883592092203. 10.1177/1758835920922033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic Impact and Risk Factors of Immune-Related Pneumonitis in Patients With Non-Small-Cell Lung Cancer Who Received Programmed Death 1 Inhibitors. Clin Lung Cancer (2019) 20(6):442–50.e444. 10.1016/j.cllc.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 48. Voong KR, Hazell SZ, Fu W, Hu C, Lin CT, Ding K, et al. Relationship Between Prior Radiotherapy and Checkpoint-Inhibitor Pneumonitis in Patients With Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer (2019) 20(4):e470–9. 10.1016/j.cllc.2019.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shibaki R, Murakami S, Matsumoto Y, Yoshida T, Goto Y, Kanda S, et al. Association of Immune-Related Pneumonitis With the Presence of Preexisting Interstitial Lung Disease in Patients With non-Small Lung Cancer Receiving Anti-Programmed Cell Death 1 Antibody. Cancer Immunol Immunother (2020) 69(1):15–22. 10.1007/s00262-019-02431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-Associated Interstitial Pneumonitis in Nivolumab-Treated Patients With non-Small Cell Lung Cancer. JAMA Oncol (2018) 4(8):1112–5. 10.1001/jamaoncol.2017.4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y, et al. Pre-Existing Pulmonary Fibrosis is a Risk Factor for anti-PD-1-related Pneumonitis in Patients With non-Small Cell Lung Cancer: A Retrospective Analysis. Lung Cancer (2018) 125:212–7. 10.1016/j.lungcan.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 52. Wang YN, Lou DF, Li DY, Jiang W, Dong JY, Gao W, et al. Elevated Levels of IL-17A and IL-35 in Plasma and Bronchoalveolar Lavage Fluid are Associated With Checkpoint Inhibitor Pneumonitis in Patients With Non-Small Cell Lung Cancer. Oncol Lett (2020) 20(1):611–22. 10.3892/ol.2020.11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, et al. Risk Factors for Pneumonitis in Patients Treated With Anti-Programmed Death-1 Therapy: A Case-Control Study. Cancer Med (2018) 7(8):4115–20. 10.1002/cam4.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mandalà M, Merelli B, Indriolo A, Tondini C. Late-Occurring Toxicity Induced by an Immune Checkpoint Blockade in Adjuvant Treatment of a Stage III Melanoma Patient. Eur J Cancer (2018) 95:130–2. 10.1016/j.ejca.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 55. Yin B, Xiao J, Li J, Liu X, Wang J. Immune-Related Organizing Pneumonitis in non-Small Cell Lung Cancer Receiving PD-1 Inhibitor Treatment: A Case Report and Literature Review. J Cancer Res Ther (2020) 16(7):1555–9. 10.4103/jcrt.JCRT_971_20 [DOI] [PubMed] [Google Scholar]
- 56. Davies MJ, Chiang AC. Management of Pneumonitis and Neuropathy in Patients Receiving PD-1-Based Therapy for Non-Small-Cell Lung Cancer. JCO Oncol Pract (2020) 16(2_suppl):4s–9s. 10.1200/JOP.19.00676 [DOI] [PubMed] [Google Scholar]
- 57. Utsumi H, Araya J, Okuda K, Watanabe J, Takekoshi D, Fujita Y, et al. Successful Treatment of Steroid-Refractory Immune Checkpoint Inhibitor-Related Pneumonitis With Triple Combination Therapy: A Case Report. Cancer Immunol Immunother (2020) 69(10):2033–9. 10.1007/s00262-020-02600-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. LG JA, Sher A. Anti-PD-1-Related Exacerbation of Interstitial Lung Disease in a Patient With Non-Small Cell Lung Cancer: A Case Presentation and Review of the Literature. Cancer Invest (2020) 38(6):365–71. 10.1080/07357907.2020.1783677 [DOI] [PubMed] [Google Scholar]
- 59. Wang R, Li K, Pi J, Meng L, Zhu M. Cavitation and Fatal Hemoptysis After Immunotherapy for Advanced Lung Adenocarcinoma: A Case Report. Thorac Cancer (2020) 11(9):2727–30. 10.1111/1759-7714.13578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Itamura H, Ohguri T, Yahara K, Nakahara S, Kakinouchi S, Morisaki T, et al. Pembrolizumab-Induced Radiation Recall Pneumonitis After the Resolution of Typical Asymptomatic Radiation Pneumonitis. J UOEH (2020) 42(3):261–6. 10.7888/juoeh.42.261 [DOI] [PubMed] [Google Scholar]
- 61. Li J, Deng X, Wang B, Li W. Fatal Outcome of Atezolizumab in a Patient With Immune-Mediated Pneumonitis, Thrombocytopenia, and Cardiac Dysfunction: A case Report. Int J Clin Pharmacol Ther (2019) 57(12):607–11. 10.5414/CP203448 [DOI] [PubMed] [Google Scholar]
- 62. Schwarz M, Kocher F, Niedersuess-Beke D, Rudzki J, Hochmair M, Widmann G, et al. Immunosuppression for Immune Checkpoint-Related Toxicity can Cause Pneumocystis Jirovecii Pneumonia (PJP) in Non-small-cell Lung Cancer (NSCLC): A Report of 2 Cases. Clin Lung Cancer (2019) 20(3):e247–50. 10.1016/j.cllc.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 63. Petri CR, Patell R, Batalini F, Rangachari D, Hallowell RW. Severe Pulmonary Toxicity From Immune Checkpoint Inhibitor Treated Successfully With Intravenous Immunoglobulin: Case Report and Review of the Literature. Respir Med Case Rep (2019) 27:100834. 10.1016/j.rmcr.2019.100834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van Eeden R, Rapoport BL, Smit T, Anderson R. Tuberculosis Infection in a Patient Treated With Nivolumab for Non-small Cell Lung Cancer: Case Report and Literature Review. Front Oncol (2019) 9:659. 10.3389/fonc.2019.00659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tonk EHJ, van Lindert ASR, Verhoeff JJC, Suijkerbuijk KPM. Acute-Onset Pneumonitis While Administering the First Dose of Durvalumab. Case Rep Oncol (2019) 12(2):621–4. 10.1159/000502202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blanchard A, Bouchard N. Pembrolizumab-Induced Obstructive Bronchiolitis in a Patient With Stage IV non-Small-Cell Lung Cancer. Curr Oncol (2019) 26(4):e571–3. 10.3747/co.26.4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dickey MS, Raina AJ, Gilbar PJ, Wisniowski BL, Collins JT, Karki B, et al. Pembrolizumab-Induced Thrombotic Thrombocytopenic Purpura. J Oncol Pharm Pract (2020) 26(5):1237–40. 10.1177/1078155219887212 [DOI] [PubMed] [Google Scholar]
- 68. Sato S, Senmaru N, Ishido K, Saito T, Poudel S, Muto J, et al. Perforation of Small Intestinal Metastasis of Lung Adenocarcinoma Treated With Pembrolizumab: A Case Report. Surg Case Rep (2019) 5(1):166. 10.1186/s40792-019-0730-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Deligiorgi MV, Panayiotidis MI, Trafalis DT. Parathyroid Hormone Related Protein (PthrP)-mediated Hypercalcemia in Malignancy Associated With anti-PD-1 Immune Checkpoint Inhibitor Treatment and Related Inflammatory Reactions. Int Immunopharmacol (2019) 77:105942. 10.1016/j.intimp.2019.105942 [DOI] [PubMed] [Google Scholar]
- 70. Fan FS, Yang CF, Chang CL. Nivolumab Plus Carboplatin and Paclitaxel as the First-line Therapy for Advanced Squamous Cell Carcinoma of the Lung With Strong Programmed Death-ligand 1 Expression: A Case Report. Cureus (2019) 11(10):e5881. 10.7759/cureus.5881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Jong C, Peters BJM, Schramel F. Recurrent Episodes of Nivolumab-Induced Pneumonitis After Nivolumab Discontinuation and the Time Course of Carcinoembryonic Antigen Levels: A Case of a 58-Year-Old Woman With Non-Small Cell Lung Cancer. Chemotherapy (2018) 63(5):272–7. 10.1159/000494841 [DOI] [PubMed] [Google Scholar]
- 72. Koyama N, Iwase O, Nakashima E, Kishida K, Kondo T, Watanabe Y, et al. High Incidence and Early Onset of Nivolumab-Induced Pneumonitis: Four Case Reports and Literature Review. BMC Pulm Med (2018) 18(1):23. 10.1186/s12890-018-0592-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Foukas PG, Tsiodras S, Economopoulou P, Spathis A, Mademli M, Leventakos K, et al. Concomitant Human Herpes Virus 6 and Nivolumab-Related Pneumonitis: Potential Pathogenetic Insights. IDCases (2018) 11:101–3. 10.1016/j.idcr.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li D, He C, Xia Y, Du Y, Zhang J. Pembrolizumab Combined With Stereotactic Body Radiotherapy in a Patient With Human Immunodeficiency Virus and Advanced Non-Small Cell Lung Cancer: A Case Report. J Med Case Rep (2018) 12(1):104. 10.1186/s13256-018-1667-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Teixidor E, Sais E, Vásquez CA, Carbajal W, Hernández A, Sánchez G, et al. Immune-Related Adverse Events and Atypical Radiological Response With Checkpoint Inhibitor Immunotherapy in an Elderly Patient With High PD-L1 Expressing Lung Adenocarcinoma. Oncotarget (2018) 9(68):33043–9. 10.18632/oncotarget.25984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Akella P, Loganathan S, Jindal V, Akhtar J, Lal A. Anti PD-1 Immunotherapy Related Interstitial Lung Disease Presenting as Respiratory Failure - A Review With Case Series. Respir Med Case Rep (2019) 26:17–22. 10.1016/j.rmcr.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jodai T, Yoshida C, Sato R, Kakiuchi Y, Sato N, Iyama S, et al. A Potential Mechanism of the Onset of Acute Eosinophilic Pneumonia Triggered by an anti-PD-1 Immune Checkpoint Antibody in a Lung Cancer Patient. Immun Inflamm Dis (2019) 7(1):3–6. 10.1002/iid3.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li H, Ma W, Yoneda KY, Moore EH, Zhang Y, Pu LL, et al. Severe Nivolumab-Induced Pneumonitis Preceding Durable Clinical Remission in a Patient With Refractory, Metastatic Lung Squamous Cell Cancer: A Case Report. J Hematol Oncol (2017) 10(1):64. 10.1186/s13045-017-0433-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kanai O, Nakatani K, Fujita K, Okamura M, Mio T. Concurrence of Nivolumab-Induced Interstitial Lung Disease and Cancer Invasion. Respirol Case Rep (2017) 5(6):e00257. 10.1002/rcr2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kashiwada T, Saito Y, Minegishi Y, Kokuho N, Takahashi A, Takahashi S, et al. Granuloma-Forming Interstitial Pneumonia Induced by Nivolumab: A Possible Immune-Related Adverse Event of the Lung. Int Cancer Conf J (2017) 6(3):131–4. 10.1007/s13691-017-0291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kato R, Hayashi H, Tanizaki J, Tanaka K, Takeda M, Nakagawa K. Peritumoural Ground-Glass Opacity Associated With Tumour Pseudoprogression in a Patient With non-Small Cell Lung Cancer Treated With Nivolumab. ESMO Open (2017) 2(1):e000145. 10.1136/esmoopen-2016-000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nakahama K, Tamiya A, Taniguchi Y, Sasaki Y, Akira M, Atagi S. Severe Acute Interstitial Lung Disease After Nivolumab in Three Non-Small Cell Lung Cancer Patients With Imaging Findings of Airway Obstruction Adjacent to Lung Tumors. J Infect Chemother (2017) 23(12):826–9. 10.1016/j.jiac.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 83. Balaji A, Verde F, Suresh K, Naidoo J. Pneumonitis From Anti-PD-1/ PD-L1 Therapy. Oncol (Williston Park) (2017) 31(10):739–46, 754. [PubMed] [Google Scholar]
- 84. Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, Walker PR. Interleukin-6 as One of the Potential Mediators of Immune-Related Adverse Events in Non-Small Cell Lung Cancer Patients Treated With Immune Checkpoint Blockade: Evidence From a Case Report. Acta Oncol (2018) 57(5):705–8. 10.1080/0284186X.2017.1406668 [DOI] [PubMed] [Google Scholar]
- 85. Shibaki R, Akamatsu H, Fujimoto M, Koh Y, Yamamoto N. Nivolumab Induced Radiation Recall Pneumonitis After Two Years of Radiotherapy. Ann Oncol (2017) 28(6):1404–5. 10.1093/annonc/mdx115 [DOI] [PubMed] [Google Scholar]
- 86. Gounant V, Brosseau S, Naltet C, Opsomer MA, Antoine M, Danel C, et al. Nivolumab-Induced Organizing Pneumonitis in a Patient With Lung Sarcomatoid Carcinoma. Lung Cancer (2016) 99:162–5. 10.1016/j.lungcan.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 87. Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer. Chest (2017) 152(2):271–81. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 88. Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, et al. Immune-Checkpoint Inhibitors Associated With Interstitial Lung Disease in Cancer Patients. Eur Respir J (2017) 50(2):1700050. 10.1183/13993003.00050-2017 [DOI] [PubMed] [Google Scholar]
- 89. Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative Disorders With Early Lethality in Mice Deficient in Ctla-4. Science (1995) 270(5238):985–8. 10.1126/science.270.5238.985 [DOI] [PubMed] [Google Scholar]
- 90. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of Lupus-Like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity (1999) 11(2):141–51. 10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- 91. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378(2):158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 92. Shea M, Rangachari D, Hallowell RW, Hollie NI, Costa DB, VanderLaan PA. Radiologic and Autopsy Findings in a Case of Fatal Immune Checkpoint Inhibitor-Associated Pneumonitis. Cancer Treat Res Commun (2018) 15:17–20. 10.1016/j.ctarc.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 93. Wang GX, Kurra V, Gainor JF, Sullivan RJ, Flaherty KT, Lee SI, et al. Immune Checkpoint Inhibitor Cancer Therapy: Spectrum of Imaging Findings. Radiographics (2017) 37(7):2132–44. 10.1148/rg.2017170085 [DOI] [PubMed] [Google Scholar]
- 94. Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, et al. The Alveolar Immune Cell Landscape is Dysregulated in Checkpoint Inhibitor Pneumonitis. J Clin Invest (2019) 129(10):4305–15. 10.1172/JCI128654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Läubli H, Koelzer VH, Matter MS, Herzig P, Dolder Schlienger B, Wiese MN, et al. The T Cell Repertoire in Tumors Overlaps With Pulmonary Inflammatory Lesions in Patients Treated With Checkpoint Inhibitors. Oncoimmunology (2018) 7(2):e1386362. 10.1080/2162402X.2017.1386362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: A Meta-Analysis. Cell Physiol Biochem (2015) 37(4):1560–71. 10.1159/000438523 [DOI] [PubMed] [Google Scholar]
- 97. Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ, Xie CM, et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes for Clinical Therapeutic Research in Patients With Non-Small Cell Lung Cancer. Oncotarget (2016) 7(12):13765–81. 10.18632/oncotarget.7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced non-Small Cell Lung Cancer. JAMA Oncol (2019) 5(3):376–83. 10.1001/jamaoncol.2018.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H, et al. Autoimmune Antibodies Correlate With Immune Checkpoint Therapy-Induced Toxicities. Proc Natl Acad Sci U S A (2019) 116(44):22246–51. 10.1073/pnas.1908079116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. de Souza Costa VH, Jr., Baurakiades E, Viola Azevedo ML, Traiano G, Kowal Rosales J, Kunze Larsen KS, et al. Immunohistochemistry Analysis of Pulmonary Infiltrates in Necropsy Samples of Children With non-Pandemic Lethal Respiratory Infections (RSV; ADV; PIV1; PIV2; PIV3; FLU A; FLU B). J Clin Virol (2014) 61(2):211–5. 10.1016/j.jcv.2014.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, et al. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights Into Pathogenesis From an Autopsy Series. Am J Pathol (2016) 186(12):3225–35. 10.1016/j.ajpath.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Postow MA. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am Soc Clin Oncol Educ Book (2015) 2015:76–83. 10.14694/EdBook_AM.2015.35.76 [DOI] [PubMed] [Google Scholar]
- 103. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. Fda Approval Summary: Pembrolizumab for the Treatment of Patients With Metastatic non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist (2016) 21(5):643–50. 10.1634/theoncologist.2015-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kato T, Masuda N, Nakanishi Y, Takahashi M, Hida T, Sakai H, et al. Nivolumab-Induced Interstitial Lung Disease Analysis of Two Phase II Studies Patients With Recurrent or Advanced non-Small-Cell Lung Cancer. Lung Cancer (2017) 104:111–8. 10.1016/j.lungcan.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 105. Kanai O, Kim YH, Demura Y, Kanai M, Ito T, Fujita K, et al. Efficacy and Safety of Nivolumab in non-Small Cell Lung Cancer With Preexisting Interstitial Lung Disease. Thorac Cancer (2018) 9(7):847–55. 10.1111/1759-7714.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lichtenstein MRL, Nipp RD, Muzikansky A, Goodwin K, Anderson D, Newcomb RA, et al. Impact of Age on Outcomes With Immunotherapy in Patients With Non-Small Cell Lung Cancer. J Thorac Oncol (2019) 14(3):547–52. 10.1016/j.jtho.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 107. Ninomiya K, Oze I, Kato Y, Kubo T, Ichihara E, Rai K, et al. Influence of Age on the Efficacy of Immune Checkpoint Inhibitors in Advanced Cancers: A Systematic Review and Meta-Analysis. Acta Oncol (2020) 59(3):249–56. 10.1080/0284186X.2019.1695062 [DOI] [PubMed] [Google Scholar]
- 108. Voong KR, Hazell S, Hu C, Hayman J, Hales R, Marrone K, et al. Receipt of Chest Radiation and Immune-Related Pneumonitis in Patients With NSCLC Treated With Anti-PD-1/PD-L1. J Thoracic Oncol (2017) 12(11):S1837–7. 10.1016/j.jtho.2017.09.529 [DOI] [Google Scholar]
- 109. Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol (2017) 35(1):56–+. 10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous Radiotherapy and the Clinical Activity and Toxicity of Pembrolizumab in the Treatment of non-Small-Cell Lung Cancer: A Secondary Analysis of the KEYNOTE-001 Phase 1 Trial. Lancet Oncol (2017) 18(7):895–903. 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vansteenkiste J, Naidoo J, Faivre-Finn C, Ozguroglu M, Villegas A, Daniel D, et al. Pacific Subgroup Analysis: Pneumonitis in Stage Iii, Unresectable Nsclc Patients Treated With Durvalumab vs. Placebo After CRT. J Thoracic Oncol (2018) 13(10):S370–1. 10.1016/j.jtho.2018.08.350 [DOI] [Google Scholar]
- 112. Del Castillo M, Romero FA, Argüello E, Kyi C, Postow MA, Redelman-Sidi G. The Spectrum of Serious Infections Among Patients Receiving Immune Checkpoint Blockade for the Treatment of Melanoma. Clin Infect Dis (2016) 63(11):1490–3. 10.1093/cid/ciw539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Baba T, Sakai F, Kato T, Kusumoto M, Kenmotsu H, Sugiura H, et al. Radiologic Features of Pneumonitis Associated With Nivolumab in non-Small-Cell Lung Cancer and Malignant Melanoma. Future Oncol (2019) 15(16):1911–20. 10.2217/fon-2019-0102 [DOI] [PubMed] [Google Scholar]
- 114. Fragkou P, Souli M, Theochari M, Kontopoulou C, Loukides S, Koumarianou A. A Case of Organizing Pneumonia (OP) Associated With Pembrolizumab. Drug Target Insights (2016) 10:9–12. 10.4137/DTI.S31565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ferguson EC, Berkowitz EA. Lung CT: Part 2, The Interstitial Pneumonias–Clinical, Histologic, and CT Manifestations. AJR Am J Roentgenol (2012) 199(4):W464–76. 10.2214/AJR.10.7309 [DOI] [PubMed] [Google Scholar]
- 116. Dall'Olio FG, Brocchi S, Massucci M, Sperandi F, Melotti B, Ardizzoni A. Distinguishing Between Immune-Related Pneumonitis and Disease Progression in Advanced Non Small Cell Lung Cancer Treated With PD-1 Inhibitors: Can Serum Tumour Markers Have a Role? Eur J Cancer (2018) 95:127–9. 10.1016/j.ejca.2018.02.020 [DOI] [PubMed] [Google Scholar]
- 117. Morikawa K, Kida H, Handa H, Inoue T, Miyazawa T, Mineshita M. Drastic Healing Process After Pembrolizumab Monotherapy in a Case of Advanced Squamous Cell Carcinoma With Severe Bronchial Stenosis Observed Over a Two-Year Period Using Continuous Bronchoscopy: A Case Report. Thorac Cancer (2020) 11(5):1339–43. 10.1111/1759-7714.13390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nishino M, Hatabu H, Hodi FS, Ramaiya NH. Drug-Related Pneumonitis in the Era of Precision Cancer Therapy. JCO Precis Oncol (2017) 1:1–12. 10.1200/PO.17.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Burris HA, 3rd, Hurtig J. Radiation Recall With Anticancer Agents. Oncologist (2010) 15(11):1227–37. 10.1634/theoncologist.2009-0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant Myocarditis With Combination Immune Checkpoint Blockade. N Engl J Med (2016) 375(18):1749–55. 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of Toxicities From Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2017) 28(suppl_4):iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 122. Rashdan S, Minna JD, Gerber DE. Diagnosis and Management of Pulmonary Toxicity Associated With Cancer Immunotherapy. Lancet Respir Med (2018) 6(6):472–8. 10.1016/S2213-2600(18)30172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Picchi H, Mateus C, Chouaid C, Besse B, Marabelle A, Michot JM, et al. Infectious Complications Associated With the Use of Immune Checkpoint Inhibitors in Oncology: Reactivation of Tuberculosis After Anti PD-1 Treatment. Clin Microbiol Infect (2018) 24(3):216–8. 10.1016/j.cmi.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 124. Fujita K, Terashima T, Mio T. Anti-PD1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J Thorac Oncol (2016) 11(12):2238–40. 10.1016/j.jtho.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 125. Reungwetwattana T, Adjei AA. Anti-PD-1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J Thorac Oncol (2016) 11(12):2048–50. 10.1016/j.jtho.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 126. Fujita K, Shim J, Nakatani K, Mio T. Patient With Lung Adenocarcinoma Manifesting as an Unusual Migratory Pulmonary Infiltration. BMJ Case Rep (2015) 2015:211771. 10.1136/bcr-2015-211771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ichikawa T, Hattori A, Suzuki K, Matsunaga T, Takamochi K, Oh S, et al. Clinicopathological Characteristics of Lung Cancer Mimicking Organizing Pneumonia on Computed Tomography-a Novel Radiological Entity of Pulmonary Malignancy. Jpn J Clin Oncol (2016) 46(7):681–6. 10.1093/jjco/hyw053 [DOI] [PubMed] [Google Scholar]
- 128. Sekine I, Sumi M, Ito Y, Nokihara H, Yamamoto N, Kunitoh H, et al. Retrospective Analysis of Steroid Therapy for Radiation-Induced Lung Injury in Lung Cancer Patients. Radiother Oncol (2006) 80(1):93–7. 10.1016/j.radonc.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 129. Chen Y, Williams J, Ding I, Hernady E, Liu W, Smudzin T, et al. Radiation Pneumonitis and Early Circulatory Cytokine Markers. Semin Radiat Oncol (2002) 12(1 Suppl 1):26–33. 10.1053/srao.2002.31360 [DOI] [PubMed] [Google Scholar]
- 130. Chuzi S, Tavora F, Cruz M, Costa R, Chae YK, Carneiro BA, et al. Clinical Features, Diagnostic Challenges, and Management Strategies in Checkpoint Inhibitor-Related Pneumonitis. Cancer Manag Res (2017) 9:207–13. 10.2147/CMAR.S136818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Colen RR, Fujii T, Bilen MA, Kotrotsou A, Abrol S, Hess KR, et al. Radiomics to Predict Immunotherapy-Induced Pneumonitis: Proof of Concept. Invest New Drugs (2018) 36(4):601–7. 10.1007/s10637-017-0524-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Nccn Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw (2020) 18(3):230–41. 10.6004/jnccn.2020.0012 [DOI] [PubMed] [Google Scholar]
- 133. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2018) 36(17):1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist (2016) 21(10):1230–40. 10.1634/theoncologist.2016-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, et al. Managing Toxicities Associated With Immune Checkpoint Inhibitors: Consensus Recommendations From the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5(1):95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Delaunay M, Prévot G, Collot S, Guilleminault L, Didier A, Mazières J. Management of Pulmonary Toxicity Associated With Immune Checkpoint Inhibitors. Eur Respir Rev (2019) 28(154):190012. 10.1183/16000617.0012-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kalisz KR, Ramaiya NH, Laukamp KR, Gupta A. Immune Checkpoint Inhibitor Therapy-Related Pneumonitis: Patterns and Management. Radiographics (2019) 39(7):1923–37. 10.1148/rg.2019190036 [DOI] [PubMed] [Google Scholar]
- 138. Gubens MA, Davies M. Nccn Guidelines Updates: New Immunotherapy Strategies for Improving Outcomes in Non-Small Cell Lung Cancer. J Natl Compr Canc Netw (2019) 17(5.5):574–8. 10.6004/jnccn.2019.5005 [DOI] [PubMed] [Google Scholar]
- 139. Matsumoto T, Fujita M, Hirano R, Sasaki T, Watanabe K. Risk Factors for Pneumocystis Pneumonia Onset in HIV-negative Patients Treated With High-Dose Systemic Corticosteroids. Infect Dis (Lond) (2019) 51(4):305–7. 10.1080/23744235.2018.1558368 [DOI] [PubMed] [Google Scholar]
- 140. Park JW, Curtis JR, Kim MJ, Lee H, Song YW, Lee EB. Pneumocystis Pneumonia in Patients With Rheumatic Diseases Receiving Prolonged, Non-High-Dose Steroids-Clinical Implication of Primary Prophylaxis Using Trimethoprim-Sulfamethoxazole. Arthritis Res Ther (2019) 21(1):207. 10.1186/s13075-019-1996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis Associated With Infliximab, a Tumor Necrosis Factor Alpha-Neutralizing Agent. N Engl J Med (2001) 345(15):1098–104. 10.1056/NEJMoa011110 [DOI] [PubMed] [Google Scholar]
- 142. Asher N, Marom EM, Ben-Betzalel G, Baruch EN, Steinberg-Silman Y, Schachter J, et al. Recurrent Pneumonitis in Patients With Melanoma Treated With Immune Checkpoint Inhibitors. Oncologist (2019) 24(5):640–7. 10.1634/theoncologist.2018-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in Patients With Cancer After Antibody Blockade of Cytotoxic T-lymphocyte-associated Antigen 4. J Clin Oncol (2006) 24(15):2283–9. 10.1200/JCO.2005.04.5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Dougan M. Checkpoint Blockade Toxicity and Immune Homeostasis in the Gastrointestinal Tract. Front Immunol (2017) 8:1547. 10.3389/fimmu.2017.01547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, Presentation, and Outcomes in Patients With Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterology (2013) 144(7):1419–25, 1425.e1411-1413; quiz e1419-1420. 10.1053/j.gastro.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 146. Ostör AJ, Chilvers ER, Somerville MF, Lim AY, Lane SE, Crisp AJ, et al. Pulmonary Complications of Infliximab Therapy in Patients With Rheumatoid Arthritis. J Rheumatol (2006) 33(3):622–8. [PubMed] [Google Scholar]
- 147. Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, et al. Interstitial Lung Disease Induced or Exacerbated by TNF-Targeted Therapies: Analysis of 122 Cases. Semin Arthritis Rheumatol (2011) 41(2):256–64. 10.1016/j.semarthrit.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 148. Martins F, Sykiotis GP, Maillard M, Fraga M, Ribi C, Kuntzer T, et al. New Therapeutic Perspectives to Manage Refractory Immune Checkpoint-Related Toxicities. Lancet Oncol (2019) 20(1):e54–64. 10.1016/S1470-2045(18)30828-3 [DOI] [PubMed] [Google Scholar]
- 149. Esfahani K, Miller WH., Jr. Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N Engl J Med (2017) 376(20):1989–91. 10.1056/NEJMc1703047 [DOI] [PubMed] [Google Scholar]
- 150. Makarious D, Horwood K, Coward JIG. Myasthenia Gravis: An Emerging Toxicity of Immune Checkpoint Inhibitors. Eur J Cancer (2017) 82:128–36. 10.1016/j.ejca.2017.05.041 [DOI] [PubMed] [Google Scholar]
- 151. Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, et al. Inflammatory Arthritis and Sicca Syndrome Induced by Nivolumab and Ipilimumab. Ann Rheum Dis (2017) 76(1):43–50. 10.1136/annrheumdis-2016-209595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, et al. Safety of Resuming anti-PD-1 in Patients With Immune-Related Adverse Events (irAEs) During Combined anti-CTLA-4 and anti-PD1 in Metastatic Melanoma. Ann Oncol (2018) 29(1):250–5. 10.1093/annonc/mdx642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. von Itzstein MS, Khan S, Gerber DE. Investigational Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Event Prediction and Diagnosis. Clin Chem (2020) 66(6):779–93. 10.1093/clinchem/hvaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships Between Lymphocyte Counts and Treatment-Related Toxicities and Clinical Responses in Patients With Solid Tumors Treated With PD-1 Checkpoint Inhibitors. Oncotarget (2017) 8(69):114268–80. 10.18632/oncotarget.23217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kartolo A, Holstead R, Khalid S, Emack J, Hopman W, Baetz T. Safety of Immunotherapy Rechallenge After Immune-Related Adverse Events in Patients With Advanced Cancer. J Immunother (2021) 44(1):41–8. 10.1097/CJI.0000000000000337 [DOI] [PubMed] [Google Scholar]
- 156. Yamagata A, Yokoyama T, Fukuda Y, Ishida T. Impact of Interstitial Lung Disease Associated With Immune Checkpoint Inhibitors on Prognosis in Patients With Non-Small-Cell Lung Cancer. Cancer Chemother Pharmacol (2021) 87(2):251–8. 10.1007/s00280-020-04205-x [DOI] [PubMed] [Google Scholar]
- 157. Kitagawa S, Hakozaki T, Kitadai R, Hosomi Y. Switching Administration of anti-PD-1 and anti-PD-L1 Antibodies as Immune Checkpoint Inhibitor Rechallenge in Individuals With Advanced non-Small Cell Lung Cancer: Case Series and Literature Review. Thorac Cancer (2020) 11(7):1927–33. 10.1111/1759-7714.13483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol (2019) 5(9):1310–7. 10.1001/jamaoncol.2019.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety and Efficacy of Re-treating With Immunotherapy After Immune-Related Adverse Events in Patients With NSCLC. Cancer Immunol Res (2018) 6(9):1093–9. 10.1158/2326-6066.CIR-17-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Fujita K, Uchida N, Kanai O, Okamura M, Nakatani K, Mio T. Retreatment With Pembrolizumab in Advanced non-Small Cell Lung Cancer Patients Previously Treated With Nivolumab: Emerging Reports of 12 Cases. Cancer Chemother Pharmacol (2018) 81(6):1105–9. 10.1007/s00280-018-3585-9 [DOI] [PubMed] [Google Scholar]
- 161. Niki M, Nakaya A, Kurata T, Yoshioka H, Kaneda T, Kibata K, et al. Immune Checkpoint Inhibitor Re-Challenge in Patients With Advanced non-Small Cell Lung Cancer. Oncotarget (2018) 9(64):32298–304. 10.18632/oncotarget.25949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Watanabe H, Kubo T, Ninomiya K, Kudo K, Minami D, Murakami E, et al. The Effect and Safety of Immune Checkpoint Inhibitor Rechallenge in Non-Small Cell Lung Cancer. Jpn J Clin Oncol (2019) 49(8):762–5. 10.1093/jjco/hyz066 [DOI] [PubMed] [Google Scholar]
- 163. Fujita K, Uchida N, Yamamoto Y, Kanai O, Okamura M, Nakatani K, et al. Retreatment With Anti-Pd-L1 Antibody in Advanced non-Small Cell Lung Cancer Previously Treated With Anti-PD-1 Antibodies. Anticancer Res (2019) 39(7):3917–21. 10.21873/anticanres.13543 [DOI] [PubMed] [Google Scholar]
- 164. Katayama Y, Shimamoto T, Yamada T, Takeda T, Yamada T, Shiotsu S, et al. Retrospective Efficacy Analysis of Immune Checkpoint Inhibitor Rechallenge in Patients With Non-Small Cell Lung Cancer. J Clin Med (2019) 9(1):102. 10.3390/jcm9010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Pardoll DM. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Toi Y, Sugawara S, Kawashima Y, Aiba T, Kawana S, Saito R, et al. Association of Immune-Related Adverse Events With Clinical Benefit in Patients With Advanced Non-Small-Cell Lung Cancer Treated With Nivolumab. Oncologist (2018) 23(11):1358–65. 10.1634/theoncologist.2017-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Dupont R, Bérard E, Puisset F, Comont T, Delord JP, Guimbaud R, et al. The Prognostic Impact of Immune-Related Adverse Events During anti-PD1 Treatment in Melanoma and non-Small-Cell Lung Cancer: A Real-Life Retrospective Study. Oncoimmunology (2020) 9(1):1682383. 10.1080/2162402X.2019.1682383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Naidoo J, Schindler K, Querfeld C, Busam K, Cunningham J, Page DB, et al. Autoimmune Bullous Skin Disorders With Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res (2016) 4(5):383–9. 10.1158/2326-6066.CIR-15-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol (2018) 36(17):1668–74. 10.1200/JCO.2017.75.6270 [DOI] [PubMed] [Google Scholar]
- 170. Gauci ML, Lanoy E, Champiat S, Caramella C, Ammari S, Aspeslagh S, et al. Long-Term Survival in Patients Responding to Anti-PD-1/PD-L1 Therapy and Disease Outcome Upon Treatment Discontinuation. Clin Cancer Res (2019) 25(3):946–56. 10.1158/1078-0432.CCR-18-0793 [DOI] [PubMed] [Google Scholar]
- 171. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med (2016) 194(3):265–75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 172. Nguyen M, Islam MR, Lim SW, Sahu A, Tamjid B. Pembrolizumab Induced Ocular Hypotony With Near Complete Vision Loss, Interstitial Pulmonary Fibrosis and Arthritis. Front Oncol (2019) 9:944. 10.3389/fonc.2019.00944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Fabre A, Nicholson AG. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med (2020) 382(8):780. 10.1056/NEJMc1917224 [DOI] [PubMed] [Google Scholar]
- 174. Reguera-Nuñez E, Xu P, Chow A, Man S, Hilberg F, Kerbel RS. Therapeutic Impact of Nintedanib With Paclitaxel and/or a PD-L1 Antibody in Preclinical Models of Orthotopic Primary or Metastatic Triple Negative Breast Cancer. J Exp Clin Cancer Res (2019) 38(1):16. 10.1186/s13046-018-0999-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Atchley WT, Alvarez C, Saxena-Beem S, Schwartz TA, Ishizawar RC, Patel KP, et al. Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health-Care Centers in North Carolina. Chest (2021) S0012-3692(21):00340–8. 10.1016/j.chest.2021.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Isono T, Kagiyama N, Takano K, Hosoda C, Nishida T, Kawate E, et al. Outcome and Risk Factor of Immune-Related Adverse Events and Pneumonitis in Patients With Advanced or Postoperative Recurrent non-Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. Thorac Cancer (2021) 12(2):153–64. 10.1111/1759-7714.13736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between initial corticosteroids dose and checkpoint inhibitor pneumonitis outcome. Kaplan-Meier curves by initial corticosteroids dose (mg/d) (A), by initial corticosteroids dose (mg/kg/d) (B).
Relationship between initial corticosteroids dose and overall survival. Kaplan-Meier curves by initial corticosteroids dose (mg/d) (A), by initial corticosteroids dose (mg/kg/d) (B).