Abstract
Background
Dorsal root ganglion field stimulation is an analgesic neuromodulation approach in use clinically, but its mechanism is unknown as there is no validated animal model for this purpose. The authors hypothesized that ganglion stimulation is effective in reducing pain-like behaviors in preclinical chronic pain models.
Methods
The authors provided ganglion stimulation or spinal cord stimulation to rats with traumatic neuropathy (tibial nerve injury), or osteoarthritis induced by intraarticular knee monosodium iodoacetate, or without injury (naïve). Analgesia was evaluated by testing a battery of pain-related reflexive, functional, and affective behaviors.
Results
In rats with nerve injury, multilevel L4 and L5 ganglion stimulation decreased hypersensitivity to noxious mechanical stimulation more (area under curve, −1,447 ± 423 min x % response; n = 12) than single level ganglion stimulation at L4 ([−960 ± 251 min x % response; n = 8; P = 0.012] vs. L4 and L5), and L5 ([−676 ± 295 min x % response; n = 8; P < 0.0001] vs. L4 and L5). Spontaneous pain-like behavior, evaluated by conditioned place preference, responded to single L4 (Pretest [−93 ± 65 s] vs. Test [87 ± 82 s]; P = 0.002; n = 9), L5 (Pretest [−57 ± 36 s] vs. Test [137 ± 73 s]; P = 0.001; n = 8), and multilevel L4 and L5 (Pretest: −81 ± 68 s vs. Test: 90 ± 76 s; P = 0.003; n = 8) ganglion stimulation. In rats with osteoarthritis, multilevel L3 and L4 ganglion stimulation reduced sensitivity to knee motion more (−156 ± 28 min x points; n = 8) than L3 ([−94 ± 19 min x points in knee bend test; n = 7; P = 0.002] vs. L3 and L4) or L4 ([−71 ± 22 min x points; n = 7; P < 0.0001] vs. L3 and L4). Conditioned place preference during osteoarthritis revealed analgesic effectiveness for ganglion stimulation when delivered at L3 (Pretest [−78 ± 77 s] vs. Test [68 ± 136 s]; P = 0.048; n = 9), L4 (Pretest [−96 ± 51 s] vs. Test [73 ± 111 s]; P = 0.004; n = 9), and L3 and L4 (Pretest [−69 ± 52 s; n = 7] vs. Test [55 ± 140 s]; P = 0.022; n = 7).
Conclusions
Dorsal root ganglion stimulation is effective in neuropathic and osteoarthritic preclinical rat pain models with peripheral pathologic origins, demonstrating effectiveness of ganglion stimulation in a placebo-free setting and justifying this model as a suitable platform for mechanistic studies.
Neuromodulation approaches, including spinal cord stimulation and dorsal root ganglion stimulation, are established methods for controlling chronic pain1 that may avoid the risks of addiction and overdose that accompany opioid use. Spinal cord stimulation is effective for a range of pain conditions, but relief may be incomplete1 and diminish over time. Dorsal root ganglion stimulation is achieved with electrodes placed adjacent to the ganglion in the intervertebral foramen, produces analgesia without anesthesia, and has proven effective for treating chronic neuropathic and nonneuropathic pain,2–4 advantages being that placement of ganglion stimulation electrodes may be more stable4 and anatomical targeting of the pain site may be more readily achieved, compared to spinal cord stimulation. Moreover, ganglion stimulation may provide relief for conditions in which spinal cord stimulation is ineffective, and in subjects for whom spinal cord stimulation has failed.5,6
The mechanism of ganglion stimulation analgesia has been only minimally explored,7 and functional studies will require a thoroughly validated animal model. We have initially described a rat model in which single level ganglion stimulation relieved neuropathic pain from tibial nerve injury.8 In order to have a suitable platform for mechanistic experiments, here we extend our observations to test if our model is a valid representation of clinical ganglion stimulation. As the mechanism by which ganglion stimulation provides analgesia may be through amplifying the natural impulse filtering at the sensory neuron T-junction,9 we also hypothesize that ganglion stimulation should be effective on all pain conditions with peripheral pathologic origins if the painful region is covered. First, we designed studies to address critical features of the model, including direct comparison to spinal cord stimulation, influence of sex, and effectiveness of ganglion stimulation measured by operant approaches (weight bearing and conditioned place preference). In clinical use, ganglion stimulation is usually applied at multiple ganglion levels to fully cover a painful region.6,10 Therefore, we also compared the effectiveness of single versus multiple levels of ganglion stimulation to identify if there is completeness of covering the painful region. Also, we have extended our neuropathic pain experiments to include examination of a somatic pain condition, specifically osteoarthritis, in which both inflammatory and neuropathic processes contribute to the development of chronic pain.11 Osteoarthritis is the most common variety of arthritis and its most common symptom is pain, which can become resistant to nonsteroidal antiinflammatory drugs, as well as steroids. Opioid medications are effective, but side effects and complications are common.12 So, osteoarthritis pain is an important potential target of ganglion stimulation. Intraarticular injection of the chondrocyte glycolytic inhibitor monoiodoacetate is a well-accepted model of osteoarthritis that replicates the histological findings of clinical osteoarthritis.13 Osteoarthritis can evolve into a chronic condition in which pain occurs even at rest and is resistant to nonsteroidal antiinflammatory drugs.14 We therefore chose this model to complement findings from the contrasting tibial nerve injury model of nerve trauma, and because of the frequency of osteoarthritis in the clinical chronic pain population.
Materials and Methods
Animals
Male and female Sprague–Dawley rats weighing 200 to 250 g were obtained from Taconic Farms Biosciences (USA), and were maintained and used according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (animal protocol AUA00454). Animals were housed in a pathogen-free facility, with two animals per ventilated cage, in a room maintained at 25 ± 1°C with 35 to 45% humidity, and a 12/12-h day/night cycle. Animals had free access to food and water, and bedding was aspen wood chips. At the termination of the study, euthanasia was performed by decapitation during deep isoflurane anesthesia.
Neuropathic Pain Model
Tibia nerve injury was performed as described in our previous report.8 Briefly, animals were anesthetized with 2% isoflurane/oxygen mixture, a 2-cm incision was made on the lateral mid-thigh of right leg, and the sciatic nerve was exposed at the point that it divides into its distal branches. At a distance of 5 mm distal to this branch point, the tibial nerve was ligated with 5.0 silk sutures and 2 to 3 mm of the nerve was removed distal to the ligation. Contact with the preserved sural and common peroneal nerves was avoided. Muscle and fascia were closed in layers, and skin was closed with staples. Sham tibial nerve injury control rats had only exposure of the nerves without further handling.
Monosodium iodoacetate–induced Osteoarthritis Pain Model
Intraarticular injection of monosodium iodoacetate induces articular cartilage loss, progressive subchondral bone lesions, and induces pain due to both peripheral and central mechanisms that responds to clinically relevant analgesics, indicating that monosodium iodoacetate-induced osteoarthritis is a suitable model for study on chronic osteoarthritis pain. Monosodium iodoacetate-induced osteoarthritis was performed as described in a previous report.15 Animals were anesthetized with 2% isoflurane/oxygen mixture, during which a single intraarticular injection of monosodium iodoacetate (catalogue no. I2512; Sigma, USA) through the infrapatellar ligament of the right knee was performed. Monosodium iodoacetate was dissolved in 0.9% sterile saline to a final concentration of 80 mg/ml and administered in a volume of 25 pl (containing 2 mg monosodium iodoacetate) using a 26-gauge needle. Sham osteoarthritis control animals were given a single intraarticular injection of 25 pl saline into the right knee. Evaluation of pain behaviors was performed 14 days after the intraarticular injection of monosodium iodoacetate or saline.
Dorsal Root Ganglion Stimulation Electrode implantation
Stimulation electrodes were prepared from two platinum-iridium wires (0.010 inch and 0.005 inch), as described in our previous report,8 where their implantation is also described. Briefly, rats were anesthetized with 2% isoflurane/oxygen while maintaining body temperature at 36.5°C. A dorsal paramedian incision was made to expose the external aspect of the intervertebral foramen at the required levels of ganglion stimulation, which included the fourth lumbar (L4), L5, or L3 spinal nerve, individually or in combination. This requires removal of the overhanging accessory process at the L3 and L4 levels. A probe with 0.4mm diameter was inserted into the intervertebral foramen dorsolateral to the ganglion, to create a passage into which the electrode was inserted in juxtaposition to the ganglion at that level. A stainless steel wire was used to fix the electrode to a screw inserted into the transverse process caudal to the foramen. The leads, which were contained in flexible plastic tubing for protection from excess flexion, were tunneled to the head, where the connection hub was secured to the skull with screws and dental cement.
Spinal Cord Stimulation Electrode Implantation
Spinal cord stimulation electrodes were made from two platinum–iridium wires (0.005 inch), each configured as circles with 1-mm diameter at their end and assembled to lie with their centers 2 mm apart. This was implanted as described in a previous report.16 Briefly, after a small central laminectomy of the T13 vertebra, a 1-mm diameter probe was inserted epidurally in the rostral direction to create a passage into which the electrode was inserted a distance of 10 mm to face the dorsal aspect of the spinal cord at T10–12 vertebral levels. The proximal end of the electrode was passed through a subcutaneous tunnel to the skull, where it was attached with screws and dental cement.
Dorsal Root Ganglion Stimulation and Spinal Cord Stimulation
Animals received dorsal root ganglion stimulation or spinal cord stimulation while awake and freely moving. In the clinic setting, the intensity used is that which can produce paresthesia,2,5,17 indicating that low-threshold mechanore- ceptors are activated, but at current levels that do not produce motor activity. Therefore, in these experiments on rats, we used a current at 80% of the motor threshold, similar to previous studies,16 and additionally tested the effectiveness of ganglion stimulation at 40, 60, and 98% of the motor threshold. The motor threshold was determined as the current at which any further increase resulted in perceptible hind limb movement for ganglion stimulation or back muscle shaking for spinal cord stimulation during stimulation at 2 Hz with a pulse width of 200 ps. This was established at each testing session. There were no differences in motor threshold between L3, L4, and L5 ganglia and spinal cord stimulation, both immediately after electrode implantation surgery and 14 days after the surgery, at which time, thresholds had increased uniformly in all groups (Supplemental Digital Content 1, http://links.lww.com/ALN/C385). Stimulation frequencies were 20 Hz for ganglion stimulation and 40 Hz for spinal cord stimulation, similar to levels used in previous animal studies and comparable to clinical settings used to obtain optimal analgesia.3,8,16
Behavioral Tests
Sensory testing of the plantar skin included eliciting reflexive behaviors induced at threshold intensity punctate mechanical stimulation (von Frey test), noxious mechanical stimulation (pin), dynamic nonnoxious mechanical stimulation (brush), cold stimulation (acetone), and heat stimulation (Hargreaves test). Use-dependent pain was measured using the static weight bearing test (“incapacitance” test), and the affective dimension of spontaneous pain with determined using conditioned place preference. Induced knee joint pain was tested by pressure application measurement and knee bend score. For animals with multiple behavioral tests on the same day, the presentation sequence was by the stress levels of those tests, specifically in the order of von Frey, brush, acetone, pin, Hargreaves, knee bend, and pressure application measurement. The investigator performing conditioned place preference, pressure application measurement, and the knee bend score was blinded on the test day as to the animal’s treatment group, and the investigator performing sensory behavior tests (von Frey, pin, acetone, brush heat, incapacitance) did not know whether animals in osteoarthritis study had received monosodium iodoacetate or normal saline injection into the knee. For ganglion stimulation and spinal cord stimulation treatments, the investigator was blinded as to administration of gabapentin (catalog no. PHR 1049; Sigma-Aldrich, USA) versus saline in experiments involving gabapentin, but the investigator was not blinded to the electrical stimulation treatment group as this was evident from induced motor activity during motor threshold testing for establishing the electrical stimulation current.
Threshold Punctate Mechanical Stimulation (von Frey)
The von Frey test was performed using calibrated monofilaments (Patterson Medical, USA). Briefly, beginning with the 2.8-g filament, the tip of filament was applied perpendicularly to the glabrous skin on the lateral third of the plantar aspect of the hind paw for 1 s, with just enough force to bend the fiber. If a paw removing response was observed, then the next weaker filament was applied, and if no response was observed, then the next stiffer fiber was applied, until a reversal occurred. After a reversal event, four more stimulations were performed following the same pattern. Each application is with intervals of at least 10 s between applications. The forces of the filaments before and after the reversal, and the four filaments applied after the reversal, were used to calculate the 50% withdrawal threshold. Rats not responding to any filament were assigned a score of 25 g.
In the absence of a hypersensitivity state, animals often default to the 25-g score. In some experiments, we wished to have a dynamic range that would allow identification of changes in sensitivity to mechanical stimulation. For these, we used von Frey fibers that we modified by attaching tungsten tips of 100-pm diameters with precisely squared ends to avoid points.18,19 This standardized the contact area, and testing in palmar glabrous skin in human subjects shows a liminal unpleasant sensation with a threshold of 9.5 ± 0.6 g (n = 3).
Noxious Punctate Mechanical Stimulation (Pin Test)
Pin test was performed using the point of a 22-gauge spinal anesthesia needle that was applied to the lateral third of the hind paw with enough force to indent the skin but not puncture it. This was repeated for five applications, with intervals of at least 10 s between applications; this set of applications was repeated after 1 min, for a total of 10 touches. Each application induced a behavior that was categorized as one of two types. The response typical of uninjured rats consisted of a very brief (less than 1 s) withdrawal, with immediate return of the foot to the cage floor. An alternate behavior that we termed a hyperalgesic response consisted of a complex event with sustained elevation of at least 1 s, variably combined with grooming that included licking and chewing of the paw, and with shaking of the limb.18 This hyperalgesic behavior is specifically associated with place avoidance,20 indicating that it represents an aversive experience. Hyperalgesia was quantified by tabulating the number of hyperalgesic responses as a percentage of total 10 touches.
Cold Stimulation (Acetone Test)
Sensitivity to cold was assessed using application of acetone, which was expelled through tubing to form a convex meniscus on the end of the tubing and was touched to the lateral plantar skin without contact of the tubing with the skin.21 The response was scored as positive if the paw was removed, and 3 repetitions were spaced at least 1min apart.
Dynamic Mechanical Stimulation (Brush Test)
A camel hair brush (4 mm wide) was applied to the lateral plantar skin of the hind paw by light stroking in the direction from heel to toe during a span of 2 s.18 The response was scored as either “positive” if the paw was removed, or “none” in the absence of movement. The test was applied three times to each paw, separated by intervals of at least 10 s.
Hargreaves Radiant Heat Stimulation
Animals were placed on temperature-regulated glass and exposed to a radiant heat source. Three determinations of withdrawal latency for each paw were separated by 1min.18
Weight-bearing (Incapacitance Test)
An incapacitance device (Columbus Instruments, USA) with a dual-channel weighing apparatus was used to measure asymmetry of weight-bearing as an indication of use-dependent pain.22 Animals were trained and gently restrained in a chamber with two hind paws placed on the weighing surfaces. The weight borne on each hind limb was measured.
Pressure Application Test
A pressure application measurement device (pressure application measurement; Ugo Basile, Italy) was used to give pressure stimuli directly to the knee. A calibrated force sensor is worn on the thumb of the experimenter. With the animal gently restrained, the pressure application measurement device was pressed against the medial and lateral aspects of the knee joint and the peak force at which a withdrawal response is initiated was measured.
Knee Bend Test
Pain induced by knee joint movement in the monosodium iodoacetate–induced osteoarthritis model was assessed by a knee bend test.23 While animals were gently restrained with one hand by holding the upper body, the experimenter flexed and extended the knee joint slowly for 2 to 3 s, then struggling movements and audible vocalizations induced by this maneuver were categorized according to the following scale: 0 points (no response to full range extension or flexion of the joint); 0.5 points (struggling in response to full flexion or extension); 1 point (struggle in response to medium range flexion or extension and also audible vocalizations to full range flexion or extension); and 2 points (audible vocalizations in response to medium range flexion or extension of the joint). The sum of the animal’s reactions to five flexions and extensions was recorded, such that the maximum value of the knee bend score is 20 points.
Conditioned Place Preference
This was performed as described in our previous report.8 A three-chamber conditioned place preference apparatus (Med Associates, USA) was used. On the preconditioning day, animals were allowed to explore both sides of chambers for 15 min and the time spent in each side was recorded. Animals that showed a preference for one chamber (greater than or equal to 67% of total time) were excluded from further study. In the immediately after 4 days, place conditioning was conducted using an unbiased procedure. Specifically, on each day animals received two 30 min sessions separated by 6 h in which either ganglion stimulation or sham ganglion stimulation without current (or in other experiments in which either gabapentin or saline) were administered. The chamber paired with ganglion stimulation or gabapen- tin was consistent on all 4 days for a given animal, but was randomly assigned for different animals. In the experiment in which we tested if gabapentin can occlude conditioned place preference induced by ganglion stimulation, gabapen- tin was intraperitoneally injected 60 min before conditioning those rats with ganglion stimulation and sham ganglion stimulation. Acquisition of conditioned place preference was tested on the day after the last conditioning session. At these final sessions, after each animal was placed in the central chamber, it was allowed to freely explore the chambers for 15 min, and the time spent on each side was recorded. A preference score was calculated as the total time spent in the chamber paired with ganglion stimulation or gabapen- tin, minus the total time spent in the other chamber paired with sham ganglion stimulation or saline. Each rat had only a single conditioned place preference test.
Protocol Design
In tibial nerve injury experiments, implantation of ganglion stimulation or spinal cord stimulation electrodes was performed after baseline (day 0) sensory testing. After 7 days or 14 days, during which no electrical stimulation was provided, sensory testing was performed again, after which tibial nerve injury was performed. For the experiment comparing ganglion stimulation in male and female rats, tibial nerve injury surgery was performed at the same time as electrode insertion. In monosodium iodoacetate experiments, the implantation of ganglion stimulation or spinal cord stimulation electrodes was performed 7 days after monosodium iodoacetate injection, at the time of the onset of monosodium iodoacetate–induced osteoarthritis. On days 7 to 11 after electrodes implantation surgery, ganglion stimulation was provided for 30 min. The ensemble of pain behavior tests (von Frey, brush, cold, pin test—all done within 5 min) were performed 15 min before ganglion stimulation or spinal cord stimulation, and again 15 min and 30 min after the initiation of ganglion stimulation or spinal cord stimulation (i.e., during stimulation), and again 15 min and 30 min after the end of ganglion stimulation or spinal cord stimulation. Knee pressure application measurement, knee bending, and incapacitance (weight-bearing asymmetry) tests were applied before ganglion stimulation, and only at 30 min after the initiation of ganglion stimulation, to avoid excessive stress on the animals due to multiple applications. Conditioned place preference testing was performed on days 17 through 25 after tibial nerve injury surgery or monosodium iodoacetate injection (at least 1d ay after pain behavior testing during electrical or sham stimulation). This consisted of 1 preconditioning test day, 4 days of conditioning (starting 2 days after the preconditioning test), and 1 test day. Animals without nerve injury (sham tibial nerve injury), but with dorsal root ganglion stimulation and animals with nerve injury or monosodium iodoacetate injection but without ganglion stimulation or spinal cord stimulation served as controls. All behavioral tests were performed between 9:00 am to 3:00 pm.
The sample size was based on our previous experience with this design.8 No statistical power calculation was conducted before the study. The primary outcomes of this study will be the effectiveness of ganglion stimulation on different pain models and genders. The secondary outcome will be whether there is a difference in effectiveness between ganglion stimulation and spinal cord stimulation.
Statistical Analysis
All data including outliers are included. Statistical analyses were performed with Prism 8 (GraphPad Software, USA). To compare changes from baseline before simulation, responses to pin, brush, and cold were evaluated nonparametrically using two-way repeated measures ANOVA with post hoc Dunn test. Responses to von Frey and incapacitance tests were evaluated using repeated measures ANOVA, with post hoc comparisons using Dunnett test. The area under the curve (AUC) of behavior values during 30-min stimulation and 30 min after the stimulation was calculated with the value just before the initiation of ganglion stimulation as baseline from each animal and represented the comprehensive response to ganglion stimulation. AUC determinations from different groups were compared for difference using one-way ANOVA with post hoc comparison Tukey test. For ganglion stimulation, post hoc paired comparisons were performed for all possible treatment pairs among L4 ganglion stimulation, L5 ganglion stimulation, and simultaneous L4 and L5 ganglion stimulation (L4 and L5 ganglion stimulation), but spinal cord stimulation treatment was compared only to Sham spinal cord stimulation and L4 and L5 ganglion stimulation. Conditioned place preference scores were analyzed with paired t tests and one-way ANOVA with post hoc Tukey test was used for comparison between treatments. When ANOVA and t tests were used, quantile-quantile plots were used to test the assumption of normality. A two-tailed test of significance was use with P < 0.05. Data are reported as either mean ± SD for parametric data or as median ± interquartile range for nonparametric data. No responses to sham ganglion stimulation or spinal cord stimulation were found, so these data were combined into one Sham treatment group.
Results
Dorsal Root Ganglion Stimulation with Intensity at 80% of Motor Threshold Had Better Effectiveness than Lower Intensities
In our previous report, current at 80% of the motor threshold was effective in providing analgesia in rats after tibial nerve injury.16 Here, we tested the effectiveness of ganglion stimulation with different stimulating intensities in preventing reflex pain behaviors and spontaneous pain (conditioned place preference). Dorsal root ganglion stimulation at 100% motor threshold (i.e., the lowest current at which an induced movement that was detectable visually or by touching the hindlimb) produced arousal and raising and shaking of the hind paw, which disappeared when the intensity was reduced to subthreshold levels (98% motor threshold). Effectiveness of ganglion stimulation with currents at 40% motor threshold, 60% motor threshold, 80% motor threshold, and 98% motor threshold were compared. On the same rats with tibial nerve injury, ganglion stimulation with 40% motor threshold and 60% motor threshold produced less analgesic effects than that with 80% motor threshold (Supplemental Digital Content 2, http://links.lww.com/ALN/C386). At the same time, ganglion stimulation with 80% motor threshold (Pretest [−93 ± 65 s] vs. Test [87 ± 82 s]; P = 0.002; n = 9), but not 60% motor threshold (Pretest [−53 ± 51 s] vs. Test [−96 ± 117 s]; P = 0.430; n = 8) showed effect on conditioned place preference (Supplemental Digital Content 3, http://links.lww.com/ALN/C387). Mechanical failure of the external electrode connections caused two fewer data points in ganglion stimulation with 0% motor threshold and 98% motor threshold groups. On the basis of these findings, we used 80% motor threshold in the subsequent experiments.
Dorsal Root Ganglion Stimulation at L4 and L5 Together, but not Individually, Decreased Natural Sensitivity to Mechanical Stimulation and Heat Thresholds in Naïve Rats
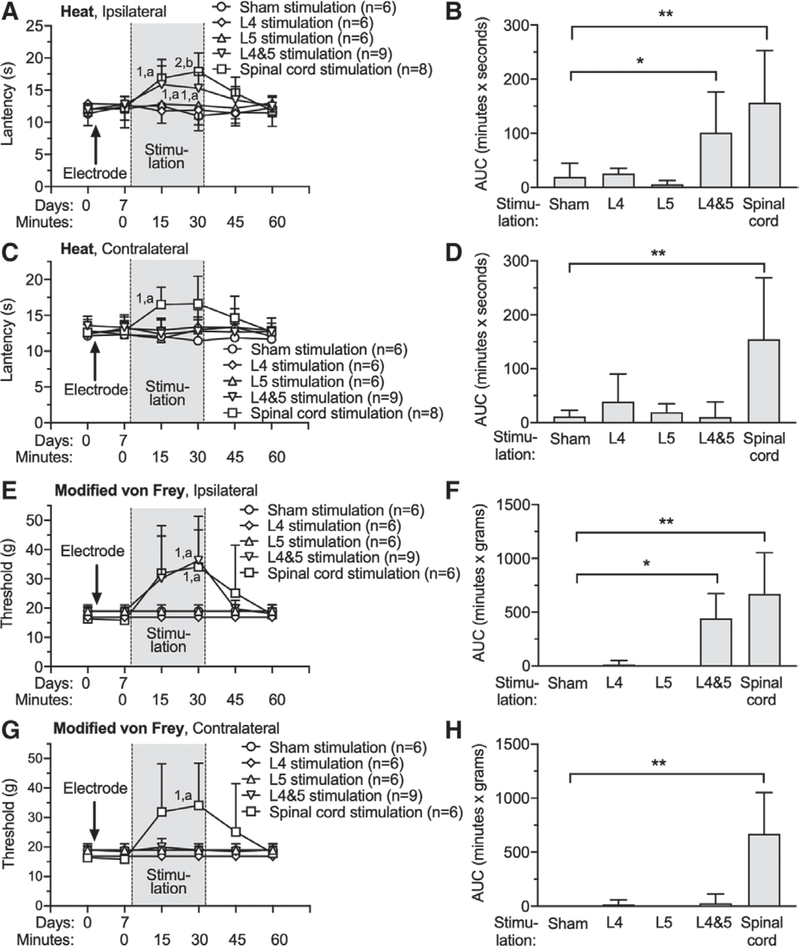
We first tested whether electrical stimulation at either the dorsal root ganglion or spinal cord alters normal sensory function by examining thresholds for withdrawal from heat and punctate mechanical stimulation, applied 7 days after electrode implantation. The design of this experiment used each animal for only one type of stimulation. Ganglion stimulation and spinal cord stimulation electrode implantation itself did not alter responses to sensory testing (fig. 1), which is consistent with our previous report.8 Spinal cord stimulation, as well as simultaneous L4 and L5 dorsal root ganglion stimulation applied ipsilateral with the tested foot, increased the latency for response to heat (fig. 1A), but such changes were not seen with single level ganglion stimulation. Comparison of treatment groups by calculating the AUC for heat latency normalized to the 0-min baseline showed increased latency by ipsilateral L4 and L5 ganglion stimulation ([101 ± 75 min x s; n = 9; P = 0.039] vs. Sham stimulation [20 ± 25 min x s; n = 6]) and by spinal cord stimulation ([157 ± 96 min x s; n = 8; P = 0.001] vs. Sham stimulation; fig. 1B). Sham treatment group consisted of animals with a spinal cord stimulation electrode or ganglion stimulation electrodes that were inserted but not activated. L4 or L5 dorsal root ganglion stimulation alone did not affect heat sensitivity (fig. 1, A and B). Whereas heat sensitivity was diminished bilaterally by spinal cord stimulation, single level and combined L4 and L5 ganglion stimulation applied contralateral to the tested foot had no effect (fig. 1, C and D). For testing mechanical sensitivity, normal rats typically fail to withdraw their paw upon plantar application of high-force von Frey fibers of the customary design because of their large cross-sectional areas. Since this makes a loss of sensitivity impossible to discern, we instead used modified fibers in which force is applied to the skin through standardized 100-pm diameter tips that were applied to the ends of the regular von Frey fibers.19 The threshold for response to mechanical stimulation with these modified von Frey fibers was decreased by ipsilateral L4 and L5 ganglion stimulation ([comparison by AUC, 444 ± 670 min x g; n = 9; P = 0.021] vs. Sham stimulation [0 ± 0 min x g; n = 6]) and by spinal cord stimulation ([comparison by AUC, 670 ± 383 min x g; n = 9; P = 0.002] vs. Sham stimulation), but ganglion stimulation at L4 or L5 individually did not affect mechanical sensitivity tested this way (fig. 1, E and F). Mechanical sensitivity was diminished bilaterally by spinal cord stimulation, but not by single level or L4 and L5 ganglion stimulation applied contralateral to the tested foot (fig. 1, G and H). There were no missing data in these experiments.
Fig. 1.
Effects of dorsal root ganglion stimulation and spinal cord stimulation on nociceptive sensation in normal rats. Left panels show the time course for latency to respond to radiant heat applied to the plantar skin on the right foot (ipsilateral to the dorsal root ganglion stimulation placement [A]) and left foot (C), and to noxious mechanical stimulation (modified von Frey, right foot [E], left foot [G]). Dorsal root ganglion stimulation or spinal cord stimulation electrodes were implanted immediately after the baseline behavioral tests at day 0. Dorsal root ganglion stimulation or spinal cord stimulation was given 30 min and behavioral responses were monitored for another 30 min. Right panels (B, D, F, H) show the average of the area under the curve calculated for each rat for the time period during and 30 min after the dorsal root ganglion stimulation or spinal cord stimulation, normalized to the baseline just before stimulation. Results are means ± SD. Here and in other time sequence data, each timepoint was compared to the 0 time baseline for that group and to the same timepoint for the control group, and a planned comparison design was used for these post hoc tests. *P < 0.05, **P < 0.01 by the Tukey test after one-way ANOVA; 1, P < 0.05 and 2, P < 0.01 compared to data immediately before dorsal root ganglion stimulation; a, P < 0.05 and b, P < 0.01 compared to sham treatment group by the Dunnett test after two-way repeated measures ANOVA with Greenhouse–Geisser correction; n, number of animals.
Single Level L4 or L5 Dorsal Root Ganglion Stimulation Decreased Neuropathy-induced Hypersensitivity, but not as Effectively as Combined L4 and L5 Dorsal Root Ganglion Stimulation
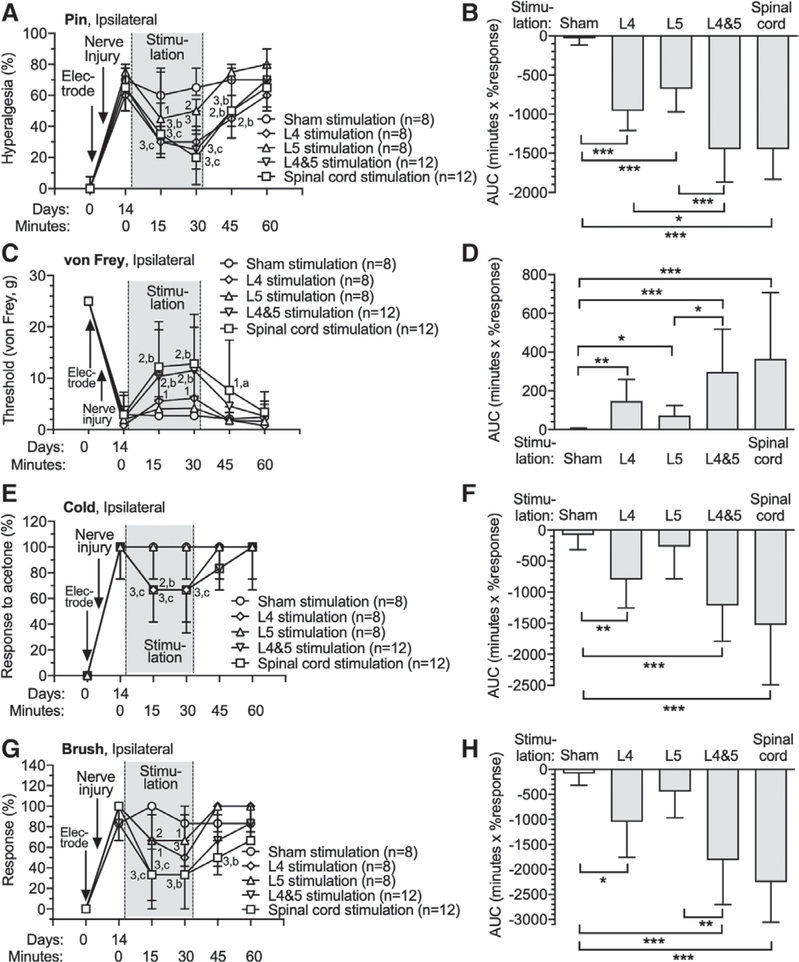
We next examined the effects of ganglion stimulation and spinal cord stimulation on the hypersensitivity that occurs after nerve injury. Animals first had implantation of either a spinal cord stimulation electrode, or a single ganglion stimulation electrode at L4 or L5, or a ganglion stimulation electrode at both L4 and L5. Each animal was used for only one type of stimulation. Tibial nerve injury was performed 7 days after electrode implantation, and 7 days after that, hypersensitivity was evident by elevated rates for hyperalgesic responses to noxious mechanical stimulation (pin), withdrawal from cold (acetone), and withdrawal from dynamic soft touch (brush), as well as lowered threshold for withdrawal from threshold mechanical stimulation, which used unmodified von Frey fibers as animals became sensitive to these after nerve injury (fig. 2). After this baseline testing session, testing was performed at 15-min and 30-min timepoints during stimulation. All forms of ganglion stimulation reversed hypersensitivity to all tested modalities at both timepoints after initiation of ganglion stimulation (fig. 2). Residual analgesia was evident 15 min after terminating stimulation, although this was variable. For instance, residual effects were evident for pin-induced hyperalgesia for all modalities except L5 ganglion stimulation, while no residual effects were proven for cold testing. Comparison between groups by AUC analysis showed analgesia from all treatments including L4 ([−960 ± 251 min X % response; n = 8; P < 0.0001] vs. Sham stimulation [−30 ± 85 min X % response; n = 8]), L5 ([−676 ± 295 min X % response; n = 8; P = 0.001] vs. Sham stimulation), and L4 and L5 ([−1447 ± 423 min X % response; n = 12; P < 0.0001] vs. Sham stimulation), i.e., a larger effect than the Sham group, and comparisons between treatments showed a greater effect for L4 and L5 dorsal root ganglion stimulation versus ganglion stimulation at individual levels (P = 0.012 vs. L4 and P < 0.0001 vs. L5; fig. 2B). Spinal cord stimulation generated similar analgesic effects ([−1447 ± 387 minutes X % response; n = 12; P < 0.0001] vs. Sham stimulation) as L4 and L5 ganglion stimulation, and also showed residual analgesia after stopping stimulation. There were no missing data in these experiments.
Fig. 2.
Effects of dorsal root ganglion stimulation and spinal cord stimulation on rats with tibial nerve injury. Dorsal root ganglion stimulation or spinal cord stimulation electrodes were implanted immediately after the baseline (day 0) behavioral determinations. Animals were assigned only to one treatment group and electrodes were only inserted at the sites at which active treatment was given. Tibial nerve injury surgeries were performed 7 days after the electrode implantation. Time course for effects are shown in the left panels, and area under curve analysis for group comparisons are shown in the right panels, for sensitivity to noxious mechanical stimuli (pin [A, B]), threshold mechanical stimuli (von Frey [C, D]), cold (E, F), and brush (G, H). The Sham treatment group consisted of animals with a spinal cord stimulation electrode or dorsal root ganglion stimulation electrode (at L4 and L5) that were inserted but not activated. Results in A, E, and G are median ± interquartile range. Results in B, C, D, F, and H are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 by the Tukey test after one-way ANOVA. 1, P < 0.05; 2, P < 0.01; and 3, P < 0.001 compared to data immediately before dorsal root ganglion stimulation; a, P < 0.05; b, P < 0.01; and c, P < 0.001 compared to Sham treatment group by the Dunnett test after two-way repeated measures ANOVA with Greenhouse–Geisser correction; n, number of animals.
In order to optimize the possibility of identifying differences between dorsal root ganglion stimulation at different levels or combined levels, an additional experiment was designed to compare these within the same animal, allowing repeated measures comparisons. Each animal had dorsal root ganglion stimulation electrodes implanted at both L4 and L5. Fourteen days later, sensory testing showed no changes induced by electrode placement (Supplemental Digital Content 4, http://links.lww.com/ALN/C388). Tibial nerve injury was then performed, which induced the expected hypersensitivity 7 days later (Supplemental Digital Content 4, http://links.lww.com/ALN/C388). At that time, sequential testing of each animal was initiated in which an initial sensory testing session was followed by testing during either L4 ganglion stimulation, L5 ganglion stimulation, L4 and L5 ganglion stimulation, or sham ganglion stimulation (no electrical current), which were applied to each rat in a random order with at least 1 day elapsed between testing. This experiment again revealed a greater effect for L4 and L5 ganglion stimulation versus stimulation at individual ganglion levels (L4 and L5 [−1447 ± 423 min x % response; n = 12; P = 0.014] vs. L4 [−895 ± 526 min x % response; n = 12; P = 0.002] vs. L5 [−607 ± 316 min x % response; n = 12]) for pin hyperalgesia, but no such differences were demonstrated for other tested modalities. There were no missing data in in these experiments.
Sex Had No Influence on Neuropathic Pain Treatment by Dorsal Root Ganglion Stimulation in Rats
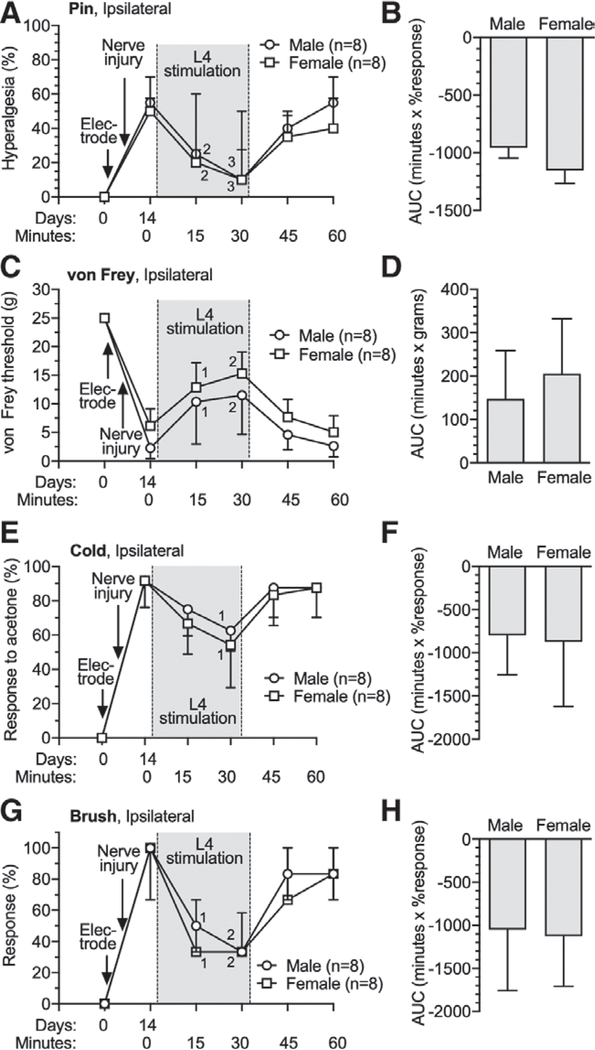
Sexual dimorphism in effectiveness of analgesics is recognized for multiple agents.24,25 To test if ganglion stimulation analgesia differs by sex, we tested effectiveness of L4 ganglion stimulation analgesia against neuropathic pain in male and female 14 days after ganglion electrode implantation and tibial nerve injury. This showed that ganglion stimulation was equally effective for male and female rats in reducing tibial nerve injury-induced hypersensitivity to pin, von Frey, brush, and cold testing at 15 min and 30 min after initiation of ganglion stimulation, compared to 0 min on day 14 in both male and female tibial nerve injury rats (fig. 3).
Fig. 3.
Effects of dorsal root ganglion stimulation on male and female rats with tibial nerve injury. Dorsal root ganglion stimulation or spinal cord stimulation electrodes were implanted immediately after the baseline (day 0) behavioral determinations. Time course for effects are shown in the left panels, and area under curve analysis for group comparisons are shown in the right panels, for sensitivity to noxious mechanical stimuli (pin [A, B]), threshold mechanical stimuli (von Frey [C, D]), cold (E, F), and brush (G, H). Results in A, E, and G are median ± interquartile range. Results in B, C, D, F and H are mean ± SD. 1, P < 0.05; 2, P < 0.01; and 3, P < 0.001 compared to data immediately before dorsal root ganglion stimulation by the Dunnett test after two-way repeated measures ANOVA with Greenhouse–Geisser correction; n, number of animals.
Pain Induced by Weight Bearing after Tibial Nerve Injury Was Relieved by Both Dorsal Root Ganglion Stimulation and Spinal Cord Stimulation
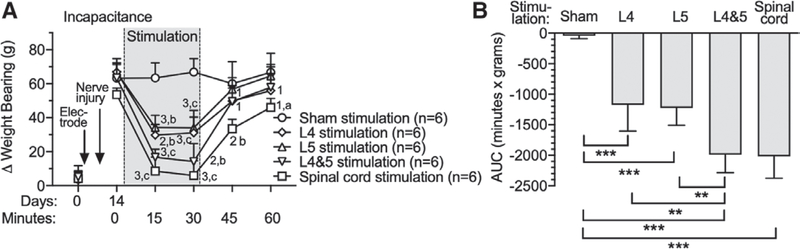
Static weight bearing asymmetry (incapacitance test) is designed to assess avoidance of pain induced by use of the limb for weight bearing in hindlimb pain models.26 Before tibial nerve injury surgery, rats bore weight on the hind paws equally (fig. 4), but after tibial nerve injury, less weight was borne by the injured leg (fig. 4A). AUC analysis (fig. 4B) showed reversal of the injury effect by both L4 dorsal root ganglion stimulation ([−1,173 ± 430 min x g; n = 6] vs. Sham stimulation [−43 ± 48 min x g; n = 6; P < 0.0001]) and L5 ganglion stimulation ([−1,225 ± 285 min x g; n = 6] vs. Sham stimulation [P < 0.0001]) compared to the Sham group, but greater improvement by L4 and L5 ganglion stimulation ([−1,992 ± 295 min x g; n = 6; P = 0.002] vs. L4, and P = 0.001 vs. L5) and spinal cord stimulation ([−2,018 ± 357 min x g; n = 6; P = 0.001] vs. L4, and P = 0.001 vs. L5) compared to single-level ganglion stimulation. There were no missing data in in these experiments.
Fig. 4.
Effects of dorsal root ganglion stimulation and spinal cord stimulation on incapacitance test in rats with tibial nerve injury. Time course for effects on weight-bearing asymmetry are shown in A, and area under curve analysis for group comparisons are shown in B. Results are mean ± SD. **P < 0.01; ***P < 0.001 by the Tukey test after one-way ANOVA. 1, P < 0.05; 2, P < 0.01; and 3, P < 0.001 compared to data immediately before dorsal root ganglion stimulation; a, P < 0.05; b, P < 0.01; and c, P < 0.001 compared to Sham treatment group by the Dunnett test after two-way repeated measures ANOVA with Greenhouse–Geisser correction; n, number of animals.
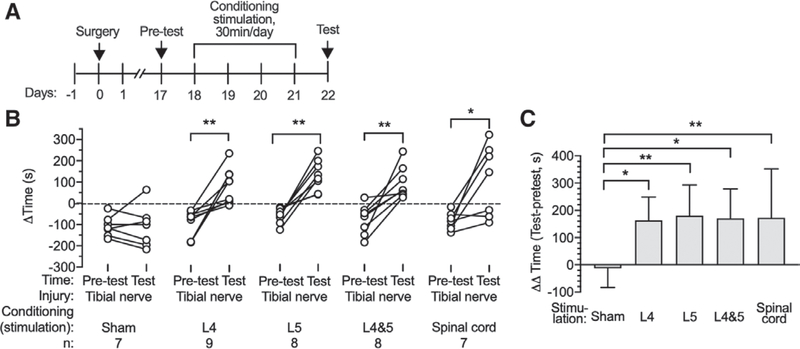
Spontaneous Pain-related Behavior after Tibial Nerve Injury Was Relieved by Dorsal Root Ganglion Stimulation and Spinal Cord Stimulation
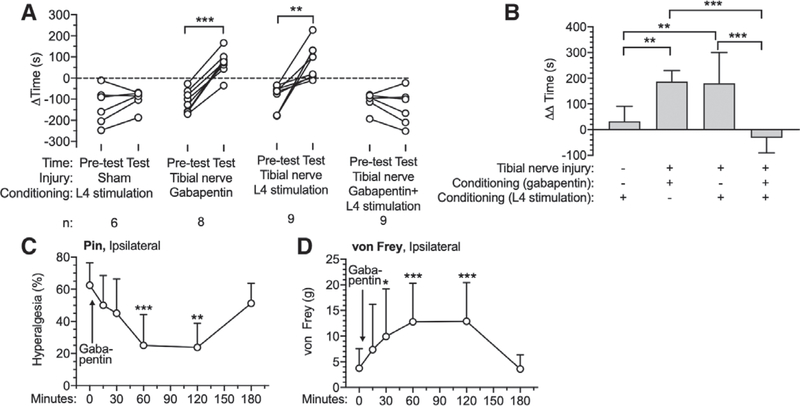
The ability of ganglion stimulation and spinal cord stimulation to relieve spontaneous pain and subsequent preference for the location at which it was provided was tested by the conditioned place preference test. After a 1-day preconditioning phase and 4 sequential days of conditioning, tibial nerve injury rats showed a strong preference for the chamber paired with L4 ganglion stimulation (Pretest [−93 ± 65 s] vs. Test [87 ± 82 s]; P = 0.002; n = 9), L5 ganglion stimulation (Pretest [−57 ± 36 s] vs. Test [137 ± 73 s]; P = 0.001; n = 8), L4 and L5 ganglion stimulation (Pretest [−81 ± 68 s] vs. Test [90 ± 76 s]; P = 0.003; n = 8), and spinal cord stimulation (Pretest [−87 ± 42 s] vs. Test [106 ± 168 s]; P = 0.020; n = 7), without any effect of Sham ganglion stimulation (fig. 5, A and B). This provides evidence of spontaneous pain after tibial nerve injury and its relief by these treatment modalities. We compared the effectiveness of the different treatments using the time differences (AA time) between the preference on test day and preconditioning day for each group (ganglion stimulation and spinal cord stimulation; fig. 5C). This showed comparable effect sizes for all ganglion stimulation (L4 [163 ± 85 s; n = 9; P = 0.017] vs. Sham stimulation [−12 ± 70 s; n = 7]; L5 [180 ± 113 s; n = 8; P = 0.006] vs. Sham stimulation; L4 and L5 [171 ± 108 s; n = 8; P = 0.012] vs. Sham stimulation) and spinal cord stimulation ([173 ± 179 s; n = 7; P = 0.006] vs. Sham stimulation) groups, compared to the Sham group. Since it is possible that ganglion stimulation induces conditioned place preference by positive effects other than pain relief, we next tested if ganglion stimulation can produce place preference in the absence of a painful condition, which revealed that animals with sham tibial nerve injury developed no place preference from conditioning with L4 dorsal root ganglion stimulation (Pretest [−132 ± 87 s] vs. Test [−100 ± 44 s]; P = 0.235; n = 6) (fig. 6A). The lack of place avoidance in this experiment (Sham tibial nerve injury with L4 ganglion stimulation in fig. 6A) also indicates that ganglion stimulation does not produce paresthesia at an aversive intensity. To specifically identify if pain relief is the means by which ganglion stimulation produces place preference after tibial nerve injury, we also tested if gabapentin, an established analgesic for treating neuropathic pain,27 can occlude ganglion stimulation-induced conditioned place preference. To optimize timing of the analgesic effect of gabapentin (100 mg/ kg, intraperitoneal injection), we measured the timing of its effects on hyperalgesia measured by the pin test and on mechanical hypersensitivity during the von Frey test (fig. 6, C and D). These both showed maximum effects at 1 h after injection, so gabapentin was given 1 h before the animal was put into ganglion stimulation-paired chamber or sham ganglion stimulation-paired chamber on conditioning days. We confirmed the analgesic effectiveness of gabapentin in our tibial nerve injury model by its ability to induce conditioning in tibial nerve injury animals (Pretest [−102 ± 54 s] vs. Test [85 ± 58 s]; P < 0.0001; n = 9) (fig. 6A). Gabapentin has also previously been shown to lack any positive conditioning effect itself.28,29 Here, when gabapentin was given to tibial nerve injury animals before their attempted conditioning with L4 ganglion stimulation, we found that ganglion stimulation no longer induced conditioned place preference (Pretest [−100 ± 44 s] vs. Test [132 ± 87 s]; P = 0.235; n = 6) (fig. 6, A and B), which demonstrates that the conditioning effect of ganglion stimulation is attributable to its analgesic effectiveness. There were no missing data in in these experiments.
Fig. 5.
Effects of dorsal root ganglion stimulation and spinal cord stimulation on Conditioned Place Preference test in rats with tibial nerve injury. (A) Sequence of events. (B) Preference at baseline (Pretest) and after treatment or Sham treatment, and the treatment effect normalized to the Pretest baseline. Results are mean ± SD. * P < 0.05; ** P < 0.01 by the paired t test (B) and the Tukey test after one-way ANOVA (C). n, number of animals.
Fig. 6.
Effects of dorsal root ganglion stimulation on Conditioned Place Preference test in rats, showing preference at baseline (Pretest) and after treatment (A), and the treatment effect normalized to the Pretest baseline (B). The time course for onset of analgesia by gabapentin (100 mg/kg intraperitoneally) in tibial nerve injury animals is shown for pin (C) and von Frey test (D; n = 8). Gabapentin was injected 60 min before conditioning those rats with dorsal root ganglion stimulation and Sham dorsal root ganglion stimulation. Results in A are median ± interquartile range. All other results are mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 by the paired t test (A), the Tukey test after one-way ANOVA (B), and the Dunnett test after one-way repeated-measures ANOVA (C). n, number of animals.
Dorsal Root Ganglion Stimulation and Spinal Cord Stimulation Relieved Mechanically Induced Knee Osteoarthritis Pain
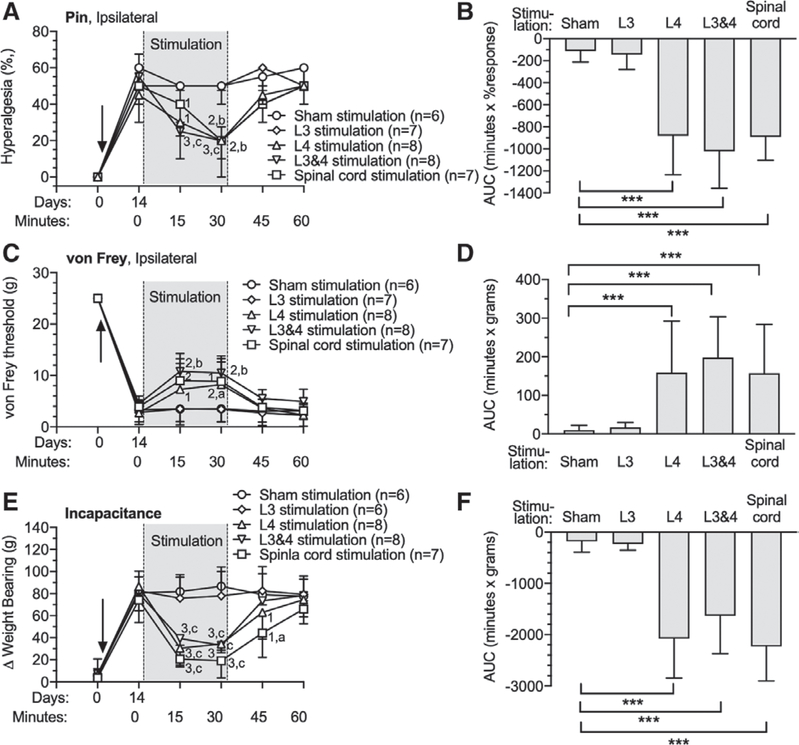
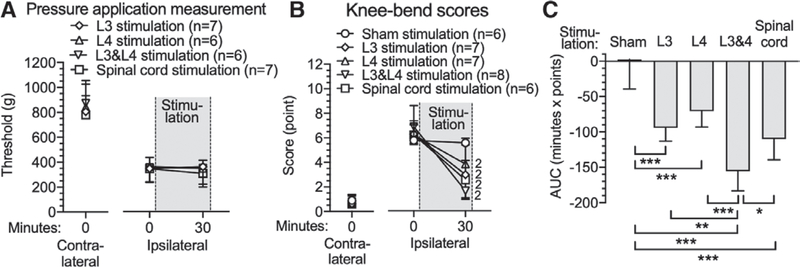
In order to expand our observations to include a different type of pain, we employed the rat monosodium iodoacetate model of osteoarthritis at the knee. Monosodium iodoacetate produces its peak sensory behavioral effects by 14 days after knee injection,30 at which time rats in our study showed weight bearing avoidance on the side of the injected knee (81 ± 15 g vs. 7 ± 14 g [Baseline at day 0]; P = 0.002), and secondary plantar hypersensitivity by Pin (57 ± 10% response vs. 2 ± 4% response [Baseline at day 0]; P = 0.03) and von Frey (3 ± 2 g vs. 25 ± 0 g [Baseline at day 0]; P = 0.002) tests (fig. 7). These changes were reversed by spinal cord stimulation, and by ganglion stimulation at the L4 level (in animals without an L3 electrode) and L3 and L4 combined ganglion stimulation, but not by ganglion stimulation at the L3 level (in animals without an L4 electrode) (fig. 7, A, C, and E). Comparisons by calculating the AUC demonstrated analgesic effects by L4 ganglion stimulation (Pin [−881 ± 354 min X % response; n = 8; P < 0.001] vs. Sham group; von Frey [159 ± 134 min X g; n = 8; P < 0.0001] vs. Sham group;Weight bearing [−2,080 ± 766 min X g; n = 8; P < 0.0001] vs. Sham group), L3 and L4 ganglion stimulation (Pin [−1,022 ± 335 min X % response; n = 8; P < 0.0001] vs. Sham group; von Frey [198 ± 106 min X g; n = 8; P < 0.0001] vs. Sham group; Weight bearing [−1,634 ± 736 min X g; n = 8; P < 0.0001] vs. Sham group), and spinal cord stimulation (Pin [−892 ± 211 min X % response; n = 7; P < 0.0001] vs. Sham group; von Frey [157 ± 127 min X g; n = 8; P < 0.0001] vs. Sham group; Weight bearing [−2,230 ± 670 min X g; n = 8; P < 0.0001] vs. Sham group) groups compared to the Sham group (Pin [−113 ± 101 min X % response; n = 6]; von Frey [10 ± 13 min X g; n = 8]; Weight bearing [−182 ± 210 min X g; n = 6]), whereas L3 ganglion stimulation (Pin [−144 ± 137 min X % response; n = 8]; von Frey [17 ± 13 min X g; n = 8];Weight bearing [−228 ± 124 min X g; n = 8]) was not different from Sham. No differences were found between L4 ganglion stimulation, L3 and L4 ganglion stimulation, and spinal cord stimulation groups. To directly test ganglion stimulation and spinal cord stimulation effects on the knee’s mechanical sensitivity, we used the knee pressure application measurement method, and pain behavior induced by bending the knee was scored. Monosodium iodoacetate decreased the threshold force to cause limb withdrawal for the monosodium iodoacetate–injected knee, but there was no change during 30-min stimulation by L3 ganglion stimulation, L4 ganglion stimulation, L3 and L4 ganglion stimulation, or spinal cord stimulation (fig. 8A). This negative result may reflect the relatively high intensity of stimulation necessary to reach the endpoint of audible vocalization. In contrast, the knee bend test measures pain induced by gradually increasing range of movement of the affected joint. This test showed that monosodium iodoacetate increased scores for the injected knee, and these elevated scores were reduced by L3 ganglion stimulation (Before stimulation [6 ± 1 points] vs. 30 min after [3 ± 1 points]; P = 0.009; n = 7), L4 ganglion stimulation (Before stimulation [6 ± 2 points] vs. 30 min after [4 ± 2 points]; P = 0.009; n = 7), L3 and L4 ganglion stimulation (Before stimulation [7 ± 1 points] vs. 30 min after [2 ± 1 points]; P = 0.007; n = 8; fig. 8B). The effect size of L3 and L4 ganglion stimulation (−156 ± 28 min X points; n = 8) was greater than L3 (−94 ± 19 min X points; n = 7; P = 0.002) or L4 (−71 ± 22 min X points; n = 7; P < 0.0001) ganglion stimulation alone, and also greater than spinal cord stimulation analgesia (−110 ± 30 min X points; n = 6; P = 0.044; fig. 8C). There were no missing data in these experiments.
Fig. 7.
Effects of dorsal root ganglion stimulation and spinal cord stimulation on rats with monosodium iodoacetate-induced osteoarthritis pain. The time course for effects are shown in the left panels, and area under curve analysis for group comparisons are shown in the right panels, for plantar sensitivity to noxious mechanical stimuli (pin [A, B]), plantar sensitivity to threshold mechanical stimuli (von Frey [C, D]), and weight-bearing asymmetry (E, F). Solid arrows in A, C, and Erepresent intraarticular injection of monosodium iodoacetate and stimulation electrodes implantation. Results in A are median ± interquartile range. Results in other panels are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 by the Tukey test after one-way ANOVA. 1, P < 0.05; 2, P < 0.01; and 3, P < 0.001 compared to data immediately before dorsal root ganglion stimulation; a, P < 0.05, b, P < 0.01, and c, P < 0.001 compared to Sham treatment group by the Dunnett test after two-way repeated measures ANOVA with Greenhouse–Geisser correction; n, number of animals.
Fig. 8.
Effects of dorsal root ganglion stimulation/spinal cord stimulation on rats with monosodium iodoacetate-induced osteoarthritis pain, showing responses ipsilateral and contralateral to the monosodium iodoacetate injection for the threshold for withdrawal from knee compression using a pressure application measurement device (A). Also shown are the scored response to knee bending (B), and group comparisons for area under curve. Results are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 by the Tukey test after one-way ANOVA; 2, P < 0.01 by the paired t test; n, number of animals.
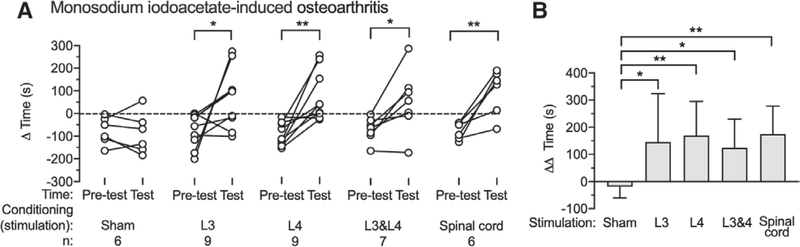
Dorsal Root Ganglion Stimulation and Spinal Cord Stimulation Relieved Spontaneous Knee Osteoarthritis Pain
Conditioned place preference testing was performed after 4 sequential days of conditioning (30 min paired with 20-Hz ganglion stimulation or 50-Hz spinal cord stimulation in one chamber at random), with each rat having only a single conditioned place preference test. No preference was detected in monosodium iodoacetate rats paired with sham ganglion stimulation or spinal cord stimulation (Pretest [−76 ± 60s s] vs. Test [−88 ± 90 s; P = 0.640; n = 6]), but rats showed a strong preference to the chamber paired with spinal cord stimulation and ganglion stimulation performed at either L3 (Pretest [−78 ± 77 s] vs. Test [68 ± 136 s; P = 0.048; n = 9]), L4 (Pretest [−96 ± 51 s] vs. Test [73 ± 111 s; P = 0.004; n = 9]), or L3 and L4 ganglion stimulation (Pretest [−69 ± 52 s] vs. Test [55 ± 140 s; P = 0.022; n = 7]; fig. 9A). Comparison of AA times between different treatment groups showed that ganglion stimulation at either L3 ([146 ± 178 s; P = 0.039] vs. Sham treatment [19 ± 42 s; n = 6]), L4 ([169 ± 126 s; n = 9; P = 0.004] vs. Sham stimulation), or L3 and L4 ([124 ± 106 s; n = 7; P = 0.038] vs. Sham stimulation), and spinal cord stimulation ([174 ± 103 s; n = 6; P = 0.003] vs. Sham stimulation) all had increased AA time compared to the Sham stimulation group, and there were no differences between L3, L4, and L3 and L4 dorsal root ganglion stimulation, and spinal cord stimulation groups (fig. 9B). There were no missing data in this part in these experiments.
Fig. 9.
Effects of dorsal root ganglion stimulation and spinal cord stimulation on Conditioned Place Preference test in rats with monosodium iodoacetate-induced osteoarthritis pain, showing preference at baseline (Pretest) and after treatment (A), and the treatment effect normalized to the Pretest baseline (B). Results are mean ± SD. *P < 0.05, **P < 0.01 by the paired t test (A) and the Tukey test after one-way ANOVA (B). n, number of animals.
Discussion
Electrical stimulation of segmental dorsal root ganglion neurons is a new clinical treatment for pain that has an uncertain mechanism of action. Development of a validated preclinical model can generate insights on the potential effectiveness of new clinical applications, and provide a platform for testing mechanistic hypotheses. We have identified analgesic effectiveness of ganglion stimulation in rat models of neuropathic pain and osteoarthritis pain, and have shown that treating two ganglia that innervate a painful area generates greater analgesia than single level stimulation, and that ganglion stimulation is equally effective in females and males. Conditioned place preference data demonstrate that ganglion stimulation reduces the negative affective aspects of spontaneous pain induced by both nerve injury and osteoarthritis. Together, these findings support the validity of this rat model of ganglion stimulation as a relevant replica of clinical use, making it suitable for exploring indications and mechanisms of ganglion stimulation. Additionally, this study confirms analgesic effectiveness of ganglion stimulation neuromodulation in a placebo-free preclinical setting, using tests that limit bias.
Our examination of the effectiveness of ganglion and spinal cord stimulation against normal nociception demonstrates that both forms of neuromodulation reduce responsiveness to heat and mechanical stimulation, although in the case of ganglion stimulation, analgesia was evident only if it was applied at both ganglia that provide the dominant sensory innervation to the plantar skin. There are no published observations testing effectiveness of ganglion stimulation against normal nociception in humans, although our findings suggest that such effects could be expected. For spinal cord stimulation, some animal studies demonstrate that spinal cord stimulation decreases responsiveness to noxious or threshold mechanical stimulation in uninjured areas,31,32 while others observed no changes.33,34 Human studies also suggest diminished cutaneous sensory function in the area of paresthesia induced by spinal cord stimulation,35,36 so reduced thresholds for mechanical and heat stimulation during spinal cord stimulation in this rat model are compatible with human observations.
In comparing multiple- versus single-level ganglion stimulation, our findings across various sensory modalities for both normal nociception and tibial nerve injury-induced neuropathic pain uniformly indicate greater suppression of hypersensitivity by stimulation at two adjacent ganglia compared to single-level stimulation. Overlap of the dermatomes for adjacent segmental levels is well established,37 so it is evident that both pathways for afferent fibers must be exposed to stimulation for shifting the threshold for response to these particular noxious stimuli (heat and punctate mechanical). In clinical application, electrodes are commonly implanted at multiple dorsal root ganglion levels, and success correlates with the number of ganglia treated,38,39 supporting the validity of our model.
The use of tests that evaluate an animal’s affective response to pain is a critical supplement to the more traditional evaluations of reflex behaviors. For instance, although we have shown that the hyperalgesia response reflects an aversive experience,20 no such evidence exists for the von Frey test, indicating that it may represent withdrawal that accompanies a nonpainful experience. The conditioned place preference paradigm quantifies the rat’s choice of location as an indication of analgesic effectiveness in treating spontaneous pain, and thereby permits measurement of the unpleasant, motivational aspect of the pain experience. After nerve injury, both spinal cord stimulation and ganglion stimulation conditioned a place preference, with comparable effectiveness for single- and multilevel ganglion stimulation. This difference from reflex-based tests, in which multilevel ganglion stimulation showed greater effectiveness, may reflect either a peak conditioning influence by even partial analgesia, or comparable analgesia for spontaneous pain when using single-level compared to multilevel ganglion stimulation. Like conditioned place preference, the rat’s choice of weight distribution between the injured and healthy leg reflects a decision influenced by avoiding use-dependent pain. For this test, there was less apparent benefit in two-level ganglion stimulation versus single-level than was seen with induced reflex behaviors. The relative sufficiency of single-level ganglion stimulation in this test and for conditioned place preference, compared to a lack of effectiveness for single-level dorsal ganglion stimulation in reflex-based pain testing, may indicate that ganglion stimulation has greater effectiveness in relieving affective influences of pain than in reducing nociceptive aspects.
We extended our examination of dorsal root ganglion stimulation to include a model of osteoarthritis, as this is a common chronic pain condition. There has been no study to examine if ganglion stimulation is effective against clinical osteoarthritis pain. We identified weightbearing avoidance and secondary plantar hypersensitivity in experimental osteoarthritis, as observed previously,40 attributed to dorsal horn sensitization and descending facilitation.15 The majority (63 to 88%41,42) of rat knee innervation passes through the L3 and L4 ganglia. However, L3 neurons minimally contribute to plantar innervation compared to L4 and L5,43,44 so the secondary hyperalgesia of the plantar skin may be insensitive to ganglion stimulation at L3, while stimulation at L4 was effective. It was unexpected, however, to find that only L4 dorsal root ganglion stimulation provided analgesia against mechanical stimulation of the knee by weightbearing, since this stimulus should engage activity in the L3 pathway. Also unexplained is failure by any treatment to lessen sensitivity to knee compression, although it is possible that the intensity of stimulation at the endpoint of withdrawal exceeds the analgesic effectiveness of neuromodulation. Proportionate contributions for L3 and L4 were, however, noted for pain induced by passive movement of the joint, and ganglion stimulation at each of these levels produced conditioned place preference, confirming effectiveness for spontaneous pain. This confirms previous observations that neuronal activity originating in the joint produces ongoing pain.14
Our study was not designed to directly examine possible mechanisms by which ganglion stimulation may produce analgesia, but some insights may be derived from the findings. Ganglion stimulation has no effect on sensory thresholds of the contralateral foot, supporting the view that analgesic effects arise from interactions with the local afferent neurons exposed to stimulation, rather than a generalized effect such as activation of descending analgesic pathways or a change in animal attentiveness. Bilateral effects of spinal cord stimulation are expected from its midline location, causing symmetric effects upon spinal cord pain processing. Analgesia did not follow insertion of the electrodes alone, indicating that mechanical damage was not responsible for sensory changes. Analgesia for hypersensitivity induced by ganglion stimulation has an onset within 15 min, suggesting that it is not attributable to reversal of the complex and diverse pathogenesis of these chronic pain states. Additionally, reduced sensitivity to cutaneous stimulation was also seen in rats without injury, which further supports a ganglion stimulation mechanism resembling a block-like state that engages normal neuronal processes sensitive to electrical stimulation. Responsiveness was reduced for diverse types of sensory stimuli, including thermal and mechanical stimulation, at both threshold level and high intensity, and for both cutaneous and deep somatic structures. This indicates that a broad range of sensory neuron subtypes are sensitive to ganglion stimulation, suggesting that blockade takes place not at their differentiated peripheral terminals where impulse trains are initiated by specific transduction processes, but rather that stimulation acts along the axon where the mechanism of impulse propagation is similar for all modality types. We have previously hypothesized that ganglion stimulation reduces transmission of impulse trains through the axonal T-junction by amplifying its natural low-pass filtering on a use-dependent basis.7,9,45 Our current observations are compatible with this explanation of ganglion stimulation as a locally acting blockade of action potential trains. As the process of T-junction filtering is sensitive to intracellular calcium accumulation and calcium/calmodulin-dependent protein kinase II activation,46,47 the residual period of relative analgesia we observed after termination of stimulation is also compatible with this hypothesis.
We previously observed that 2 weeks of ganglion stimulation is effective in blocking neurogenic inflammation of peripheral tissues in the setting of collagen-induced arthritis,48 and reduced tissue inflammation has been noted in one clinical report.17 Since inflammation of the dorsal root ganglion is evident in both the peripheral nerve injury49 and monosodium iodoacetate models,50 an alternative mechanism of ganglion stimulation analgesia might be blockade of neurogenic inflammation within the ganglion itself. However, clinical subjects report that pain returns promptly after discontinuation of a sustained period of successfully analgesic dorsal root ganglion stimulation,5 making reversal of pathogenic processes an unlikely analgesic mechanism. Future mechanistic experiments will test our hypothesis that ganglion stimulation acts locally by blocking transit of action potential trains through the sensory neuron T-junction. Refinement by identification of relevant parameters, such as stimulation intensity and comparative effectiveness of multiple stimulation levels, will also allow us to limit the number of animal groups in future mechanistic studies.
Supplementary Material
EDITOR’S PERSPECTIVE.
What We Already Know about This Topic
Dorsal root ganglion stimulation is a new approach to neuromodulation for the purpose of achieving pain relief
Neuromodulation research has been slowed by the lack of well characterized animal models
What This Article Tells Us That Is New
Using a rat model of osteoarthritis, stimulation of both the L3 and L4 dorsal root ganglia reduced nonreflexive knee motion scores and provided conditioned place preference more than sham stimulation
Sensitization from peripheral nerve injury responded to stimulation maximally when provided at two ganglia (L4 and L5) versus just one
Acknowledgments
Research Support
Supported by the National Institutes of Health grant No. R01NS103812 (Bethesda, Maryland; to Dr. Hogan).
Footnotes
Competing Interests
The authors declare no competing interests.
Information on purchasing reprints may be found at www.anesthesiology.org or on the masthead page at the beginning of this issue. Anesthesiology’s articles are made freely accessible to all readers, for personal use only, 6 months from the cover date of the issue.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are available in both the HTML and PDF versions of this article. Links to the digital files are provided in the HTML text of this article on the Journal’s Web site (www.anesthesiology.org).
Contributor Information
Guoliang Yu, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin
Ian Segel, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin
Zhiyong Zhang, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin Department of Colon and Rectal Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou City, Henan, China.
Quinn H. Hogan, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin
Bin Pan, Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, Wisconsin
References
- 1.Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, Levy RM, Abejon D, Buchser E, Burton A, Buvanendran A, Candido K, Caraway D, Cousins M, DeJongste M, Diwan S, Eldabe S, Gatzinsky K, Foreman RD, Hayek S, Kim P, Kinfe T, Kloth D, Kumar K, Rizvi S, Lad SP, Liem L, Linderoth B, Mackey S, McDowell G, McRoberts P, Poree L, Prager J, Raso L, Rauck R, Russo M, Simpson B, Slavin K, Staats P, Stanton-Hicks M, Verrills P, Wellington J, Williams K, North R, Neuromodulation Appropriateness Consensus C: The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014; 17: 515–50; discussion 550 [DOI] [PubMed] [Google Scholar]
- 2.Liem L, Russo M, Huygen FJ, Van Buyten JP, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, Kramer J: One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation 2015; 18:41–8; discussion 48–9 [DOI] [PubMed] [Google Scholar]
- 3.Eldabe S, Burger K, Moser H, Klase D, Schu S,Wahlstedt A, Vanderick B, Francois E, Kramer J, Subbaroyan J: Dorsal root ganglion (DRG) stimulation in the treatment of phantom limb pain (PLP). Neuromodulation 2015; 18:610–6; discussion 616–7 [DOI] [PubMed] [Google Scholar]
- 4.Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Obray J, Scowcroft J, Golovac S, Kapural L, Paicius R, Kim C, Pope J, Yearwood T, Samuel S, McRoberts WP, Cassim H, Netherton M, Miller N, Schaufele M, Tavel E, Davis T, Davis K, Johnson L, Mekhail N: Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: A randomized comparative trial. Pain 2017; 158:669–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liem L, Russo M, Huygen FJ, Van Buyten JP, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, Kramer J: A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation 2013; 16:471–82; discussion 482 [DOI] [PubMed] [Google Scholar]
- 6.Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM: A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation 2013; 16:67–71; discussion 71–2 [DOI] [PubMed] [Google Scholar]
- 7.Koopmeiners AS, Mueller S, Kramer J, Hogan QH: Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation 2013; 16:304–11; discussion 310–1 [DOI] [PubMed] [Google Scholar]
- 8.Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH: Dorsal root ganglionic field stimulation relieves spontaneous and induced neuropathic pain in rats. J Pain 2016; 17:1349–58 [DOI] [PubMed] [Google Scholar]
- 9.Kent AR, Min X, Hogan QH, Kramer JM: Mechanisms of dorsal root ganglion stimulation in pain auppression: A computational modeling analysis. Neuromodulation 2018; 21:234–46 [DOI] [PubMed] [Google Scholar]
- 10.Zuidema X, Breel J, Wille F: Paresthesia mapping: A practical workup for successful implantation of the dorsal root ganglion stimulator in refractory groin pain. Neuromodulation 2014; 17:665–9; discussion 669 [DOI] [PubMed] [Google Scholar]
- 11.Krustev E, Rioux D, McDougall JJ: Mechanisms and mediators that drive arthritis pain. Curr Osteoporos Rep 2015; 13:216–24 [DOI] [PubMed] [Google Scholar]
- 12.Whittle SL, Richards BL, Buchbinder R: Opioid analgesics for rheumatoid arthritis pain. JAMA 2013; 309:485–6 [DOI] [PubMed] [Google Scholar]
- 13.Kalbhen DA: Chemical model of osteoarthritis—A pharmacological evaluation. J Rheumatol 1987; 14 Spec No:130–1 [PubMed] [Google Scholar]
- 14.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F: Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain 2012; 153:924–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havelin J, Imbert I, Cormier J, Allen J, Porreca F, King T: Central sensitization and neuropathic features of ongoing pain in a rat model of advanced osteoarthritis. J Pain 2016; 17:374–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shechter R,Yang F, Xu Q, Cheong YK, He SQ, Sdrulla A, Carteret AF, Wacnik PW, Dong X, Meyer RA, Raja SN, Guan Y: Conventional and kilohertz-frequency spinal cord stimulation produces intensity- and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology 2013; 119:422–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Buyten JP, Smet I, Liem L, Russo M, Huygen F: Stimulation of dorsal root ganglia for the management of complex regional pain syndrome: A prospective case series. Pain Pract 2015; 15:208–16 [DOI] [PubMed] [Google Scholar]
- 18.Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB: Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology 2004; 101:476–87 [DOI] [PubMed] [Google Scholar]
- 19.Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH: Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol 1999; 82:3347–58 [DOI] [PubMed] [Google Scholar]
- 20.Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH: Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain 2010; 11:280–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM: Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994; 59:369–76 [DOI] [PubMed] [Google Scholar]
- 22.Schott E, Berge OG, Angeby-Moller K, Hammarstrom G, Dalsgaard CJ, Brodin E: Weight bearing as an objective measure of arthritic pain in the rat. J Pharmacol Toxicol Methods 1994; 31:79–83 [DOI] [PubMed] [Google Scholar]
- 23.Ferreira-Gomes J, Adaes S, Castro-Lopes JM: Assessment of movement-evoked pain in osteoarthritis by the knee-bend and CatWalk tests: A clinically relevant study. J Pain 2008; 9:945–54 [DOI] [PubMed] [Google Scholar]
- 24.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ; Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP: Studying sex and gender differences in pain and analgesia: A consensus report. Pain 2007; 132 Suppl 1:S26–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craft RM: Sex differences in opioid analgesia: “From mouse to man”. Clin J Pain 2003; 19:175–86 [DOI] [PubMed] [Google Scholar]
- 26.Tétreault P, Dansereau MA, Doré-Savard L, Beaudet N, Sarret P: Weight bearing evaluation in inflammatory, neuropathic and cancer chronic pain in freely moving rats. Physiol Behav 2011; 104:495–502 [DOI] [PubMed] [Google Scholar]
- 27.Wiffen PJ, Derry S, Bell RF, Rice AS, Tolle TR, Phillips T, Moore RA: Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2017; 6:CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bannister K, Qu C, Navratilova E, Oyarzo J, Xie JY, King T, Dickenson AH, Porreca F: Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017; 158:2386–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griggs RB, Bardo MT, Taylor BK: Gabapentin alleviates affective pain after traumatic nerve injury. Neuroreport 2015; 26:522–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combe R, Bramwell S, Field MJ: The monosodium iodoacetate model of osteoarthritis: A model of chronic nociceptive pain in rats? Neurosci Lett 2004; 370:236–40 [DOI] [PubMed] [Google Scholar]
- 31.Qin C, Farber JP, Linderoth B, Shahid A, Foreman RD: Neuromodulation of thoracic intraspinal viscerore- ceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9:71–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyerson BA, Ren B, Herregodts P, Linderoth B: Spinal cord stimulation in animal models of mononeuropathy: Effects on the withdrawal response and the flexor reflex. Pain 1995; 61:229–43 [DOI] [PubMed] [Google Scholar]
- 33.Gong WY, Johanek LM, Sluka KA: A comparison of the effects of burst and tonic spinal cord stimulation on hyperalgesia and physical activity in an animal model of neuropathic pain. Anesth Analg 2016; 122:1178–85 [DOI] [PubMed] [Google Scholar]
- 34.Song Z, Viisanen H, Meyerson BA, Pertovaara A, Linderoth B: Efficacy of kilohertz-frequency and conventional spinal cord stimulation in rat models of different pain conditions. Neuromodulation 2014; 17:226–34; discussion 234—5 [DOI] [PubMed] [Google Scholar]
- 35.Nashold B, Somjen G, Friedman H: Paresthesias and EEG potentials evoked by stimulation of the dorsal funiculi in man. Exp Neurol 1972; 36:273–87 [DOI] [PubMed] [Google Scholar]
- 36.Lindblom U, Meyerson BA: Influence on touch, vibration and cutaneous pain of dorsal column stimulation in man. Pain 1975; 1:257–70 [DOI] [PubMed] [Google Scholar]
- 37.Foerster O: The dermatomes in man. Brain 1933; 56: 1–39 [Google Scholar]
- 38.Hunter CW, Sayed D, Lubenow T, Davis T, Carlson J, Rowe J, Justiz R, McJunkin T, Deer T, Mehta P, Falowski S, Kapural L, Pope J, Mekhail N: DRG FOCUS: A multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation 2019; 22:61–79 [DOI] [PubMed] [Google Scholar]
- 39.Morgalla MH, Bolat A, Fortunato M, Lepski G, Chander BS: Dorsal root ganglion stimulation used for the treatment of chronic neuropathic pain in the groin: A single-center study with long-term prospective results in 34 cases. Neuromodulation 2017; 20:753–60 [DOI] [PubMed] [Google Scholar]
- 40.Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, King T, Porreca F: Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett 2011; 493:72–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widenfalk B, Wiberg M: Origin of sympathetic and sensory innervation of the knee joint. A retrograde axonal tracing study in the rat. Anat Embryol (Berl) 1989; 180:317–23 [DOI] [PubMed] [Google Scholar]
- 42.Edoff K, Grenegard M, Hildebrand C: Retrograde tracing and neuropeptide immunohistochemistry of sensory neurones projecting to the cartilaginous distal femoral epiphysis of young rats. Cell Tissue Res 2000; 299:193–200 [DOI] [PubMed] [Google Scholar]
- 43.Takahashi Y, Nakajima Y, Sakamoto T: Dermatome mapping in the rat hindlimb by electrical stimulation of the spinal nerves. Neurosci Lett 1994; 168:85–8 [DOI] [PubMed] [Google Scholar]
- 44.Bajrovic F, Sketelj J: Extent of nociceptive dermatomes in adult rats is not primarily maintained by axonal competition. Exp Neurol 1998; 150:115–21 [DOI] [PubMed] [Google Scholar]
- 45.Gemes G, Koopmeiners A, Rigaud M, Lirk P, Sapunar D, Bangaru ML, Vilceanu D, Garrison SR, Ljubkovic M, Mueller SJ, Stucky CL, Hogan QH: Failure of action potential propagation in sensory neurons: Mechanisms and loss of afferent filtering in C-type units after painful nerve injury. J Physiol 2013; 591:1111–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gemes G, Oyster KD, Pan B, Wu HE, Bangaru ML, Tang Q, Hogan QH: Painful nerve injury increases plasma membrane Ca2+-ATPase activity in axoto- mized sensory neurons. Mol Pain 2012; 8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu H, Pan B, Weyer A, Wu HE, Meng J, Fischer G, Vilceanu D, Light AR, Stucky C, Rice FL, Hudmon A, Hogan Q: CaMKII controls whether touch is painful. J Neurosci 2015; 35:14086–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan B, Zhang Z, Chao D, Hogan QH: Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. Neuromodulation 2018; 21:247–53 [DOI] [PubMed] [Google Scholar]
- 49.Scholz J, Woolf CJ: The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci 2007; 10:1361–8 [DOI] [PubMed] [Google Scholar]
- 50.Im HJ, Kim JS, Li X, Kotwal N, Sumner DR, van Wijnen AJ, Davis FJ, Yan D, Levine B, Henry JL, Desevré J, Kroin JS: Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum 2010; 62:2995–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.