1. Introduction
The pervasive, detrimental consequences of trauma are well-recognized, with a history of adverse experiences implicated in increased risk for poorer physical and psychological health outcomes, including depression, anxiety, substance use, autoimmune diseases, risky sexual behavior, and sleep disturbances (Anda et al., 2006; Arata, Langhinrichsen-Rohling, Bowers, & O’Farrill-Swails, 2005; Chapman et al., 2004; Dube et al., 2009; Wells, Vanderlind, Selby, & Beevers, 2014). Not only is the impact extensive, the direct and indirect costs of trauma are substantial, both in terms of financial costs for individuals and society for psychiatric and medical services, and in quality of life costs (Kessler, 2000; Walker et al., 2003). Because traumatic experiences have a broad impact on functioning, identifying mechanisms that contribute to impairment is a critical endeavor, as this may reveal key psychopathology prevention and intervention targets.
One avenue of investigation that may reveal important intervention targets is the impact of trauma on learning processes, as learning impairments are common among individuals with trauma-related disorders (e.g., fear, safety, and instrumental/reinforcement learning, RL); Jovanovic et al., 2010; Jovanovic, Kazama, Bachevalier, & Davis, 2012; Harms, Bowen, Hanson, & Pollak, 2018). For example, adolescents with a history of early life stress exhibit poorer RL than controls in the context of both reward and punishment (Harms et al., 2018). Notably, the ability to efficiently learn and accurately predict outcomes is critical for functioning in several domains. For example, RL ability appears to be important for social functioning, as individual differences in associative learning predict social behavior (Heerey, 2011; Reeb-Sutherland, Levitt, & Fox, 2012). With regard to emotional functioning, overestimation of costs and likelihood of negative events is proposed to contribute to heightened threat perception (Berenbaum, 2010), which confers risk for the development of anxiety-related symptomology. In contrast with other anxiety-related disorders, which predict more specific deficits in threat learning and prediction, trauma history and PTSD also predict impairment in reward learning (Harms et al., 2018; Ross, Lenow, Kilts, & Cisler, 2018; May & Wisco, 2019), which supports approach behavior and is a potent risk factor for depressive symptomatology (Admon & Pizzagalli, 2015).
There have been relatively few investigations of the relation between trauma and specific, separable, dimensions of reinforcement-related learning. Though typically not considered, two distinct RL processes that are well-captured by computational models and are highly applicable to trauma-related research include model-free and model-based RL (Daw, Niv, & Dayan, 2005). Whereas model-free RL utilizes information acquired via trial and error and is primarily implemented within the ventral striatum (Beierholm, Anen, Quartz, & Bossaerts, 2011; Gläscher, Daw, Dayan, & O’Doherty, 2010), model-based RL involves the development of cognitive maps that contain possible outcomes (i.e., mappings of context-dependent conditions) to prospectively make predictions about actions and is primarily implemented via regions of the prefrontal cortex (PFC) and frontoparietal network (FPN), including dorsolateral prefrontal cortex (dlPFC), inferior frontal gyrus (IFG), and intraparietal sulcus (IPS; Beierholm et al., 2011; Gläscher et al., 2010; Daw, Gershman, Seymour, Dayan, & Dolan, 2011; Lee, Shimojo, & O’Doherty, 2014).
Available evidence indicates that trauma affects at least some aspects of model-free RL (e.g., Cisler et al., 2019). For example, Cisler et al. (2019) found that adolescent females with a history of assaultive trauma exhibited reduced model-free RL-derived negative prediction error encoding within the salience network during a RL tasks, which was not accounted for by PTSD. However, it is unclear whether a history of trauma impacts model-based RL processes, which would impact individuals’ ability to develop nuanced context-action-outcome representations. Suggestive of a relationship between trauma and model-based RL, a history of trauma predicts broad impairment in executive functioning (EF; DePrince et al., 2009; Gould et al., 2012; Mezzacappa, Kindlon, & Earls, 2001; Mueller et al., 2010; Navalta, Polcari, Webster, Boghossian, & Teicher, 2006; Spann et al., 2012), which is not fully accounted for by PTSD (e.g., DePrince et al., 2009; Navalta et al., 2006). EF subsumes a set of higher-order cognitive functions that activate brain regions/networks (e.g., FPN, lateral PFC, dlPFC, IFG; Alvarez & Emory, 2006; Collette & Van der Linden, 2002), which overlap with regions/networks that implement model-based RL (e.g., FPN, lateral PFC, dlPFC; Beierholm et al., 2011; Daw et al., 2011; Gläscher et al., 2010; Lee, et al., 2014). Indicative of functional overlap, better performance on tasks that measure cognitive control predicts greater use of model-based versus model-free strategies on RL tasks (Otto, Skatova, Madlon-Kay, & Daw, 2014).
The present study built on previous research by using computational modeling to investigate the impact of assaultive trauma on model-based RL among adolescent females. It was hypothesized that a history of assaultive trauma would predict 1) less use of model-based relative to model-free RL. Further, it was hypothesized that, 2) compared to adolescents without a history of assault, a history of assaultive trauma would predict reduced model-based encoding within the frontoparietal network (FPN), including dlPFC and IPS. The FPN was of primary interest given that regions of this network are most commonly activated across model-based RL studies, suggesting that these regions are particularly instrumental in the implementation of model-based RL (Gläscher et al., 2010; Daw et al., 2011). Based on previous research indicating that the impact of trauma on learning processes is not specific to PTSD (Cisler et al., 2019), it was anticipated that 3) the relationship between trauma and model-based RL would not be accounted for by PTSD diagnostic status. Finally, because there is evidence available that trauma types differentially affect some aspects of cognitive functioning (Gould et al., 2012; Majer et al., 2010), the unique impact of physical and sexual abuse on model-based RL was examined.
2. Materials and methods
2.1. Sample characteristics
The final sample of participants included in analyses consisted adolescent females between the ages of 11 and 17 with (n= 31) and without (n=29) histories of directly experienced physical or sexual assault (n = 17 with a current diagnosis of PTSD). See supplement for additional demographic characteristics and exclusion criteria. All participants overlap with the participants included in Cisler et al. (2019); whereas Cisler et al. (2019) focused on the impact of trauma on model-free RL, the present study focuses on model-based RL.
2.2. Clinical Interviews and Measures
The trauma assessment section of the National Survey of Adolescents (NSA) was used to assess for the presence or absence of a history of assaultive trauma (Kilpatrick et al., 2000; Kilpatrick et al., 2003). The Clinician-Administered PTSD Scale (CAPS), Child and Adolescent Version was used to assess symptoms of PTSD symptoms (Pynoos et al., 2015). Participants completed the short-form version of Childhood Trauma Questionnaire (CTQ), which is a self-reported measure that assesses for physical abuse, sexual abuse, emotional abuse, physical neglect, and emotional neglect (Bernstein et al., 2003; Bernstein & Fink, 2003). The CTQ can be used as a dimensional and/or categorical assessment of childhood maltreatment.
2.3. Three-Arm Bandit Task
Participants completed a social and neutral version of the three-arm bandit task . Because Cisler et al. (2019) found that the impact of assault severity on RL-related encoding was more robust in the social than neutral task, possibly reflecting differential engagement of learning processes, analyses focused on the social task (see Supplement for neutral task results). The social-, task consisted of 90 trials in which participants were presented with the faces of three individuals with a neutral expression (Supplemental Figure S1). Participants were asked to invest $10 in one of the individuals, each of whom had a different likelihood (80%, 50%, or 20%) of returning a reward of $20 (vs. $0). The probabilities of reward associated with each face switched every 30 trials (see Supplemental Figure S2). Each trial consisted of a decision, anticipation, and feedback phase. The primary trial phase of interest in the present study was the feedback phase, during which the outcome (reward vs. no reward) was delivered.
2.4. Computational Modeling
A risk-sensitive anti correlated Rescorla-Wagner (RW) model, identified as the bestfitting model in Cisler et al. (2019), was used to investigate model-free RL (Hauser, Iannaccone, Walitza, Brandeis, & Brem, 2015). The standard RW model updates parameters from trial to trial as follows: Vt+1,c=Vt,c + α × δt, where t is the current trial, Vt,c is the current associative strength, δt is the current prediction error (PE; outcomet – Vt,c), α is the learning rate, and c is the current selected option Additional RW model information is included in the Supplement.
For model-based RL, a latent state (LS) learning model was used (Cochran & Cisler, 2019). LS learning involves learning multiple associations between options and outcomes and inferring which association to use at each point in the task. A latent state is a set of associations that specify what a learner should expect from selecting any option and is conceptualized as a set of abstract task hypotheses. In aggregate, latent states describe multiple sets of hypotheses that define an entire task. To capture multiple associations, the LS model indexes associative strengths by integer l (the latent state), with current associative strength of option c for latent state l (Vt,c,l). The LS model also captures the degree to which individuals believe that current conditions reflect a given latent state (i.e., latent-state beliefs), denoted by pt,l
Beliefs and associative strengths are iteratively updated based on PEs specific to each latent state (see Supplemental Figure S3). Beliefs are updated according to an approximate Bayes rule based on PEs and measure the magnitude of changes in latent-state beliefs on a trial by trial basis (delta beliefs, dB). That is, dB on a trial reflects the degree to which individuals update internal models after observing the outcome on that trial, with larger updates reflecting greater changes in latent-state beliefs (i.e., beliefs about current task hypotheses). Behavior on the following trial is predicted by associative strengths weighted over latent states based on prior latent-state beliefs: e.g., for option c, Vt,c = ∑l Pl,t-l Vt,c,l. Individual differences in exploration and exploitation were captured by transforming associative strengths (Vt,c) using a softmax parameter. The following parameters derived from the LS model were used in the present study: trial-by-trial Vt,c, PE δt, and dB.
The RW and LS models were fit to each participant’s behavior using maximum likelihood estimation and resulting log likelihoods were used to calculate Akaike information criterion (AIC) to compare fit between the two models, with lower AIC values reflecting better fit. The difference between LS and RW model fit (delta AIC, dAIC) was calculated for each participant by subtracting RW AIC from LS AIC, with lower values reflecting the use of a strategy that is more consistent with LS than RW learning for the task.
The impact of trauma on learning strategy use was tested with a linear regression model: dAIC was regressed onto variables reflecting the contrast of controls versus assaultive trauma (controls = −1, trauma = 1), with age, IQ, and study site included as covariates. Separate linear models tested the impact of the CTQ assault-related subscales (Physical and Sexual Abuse) using identical covariates. To rule out the impact of PTSD, follow-up analyses included PTSD diagnostic status (controls and assaulted without PTSD = 0, PTSD = 1). Linear regressions were carried out with MATLAB (fitlm; The MathWorks, Inc., Natick, MA).
2.5. Neuroimaging
Acquisition and preprocessing information are provided in the Supplement. Neuroimaging data was excluded from three participants due to head motion and malfunction with the head coil, yielding a sample of fifty-seven participants for neuroimaging analyses. All neuroimaging analyses focused on LS model parameters (see Behavioral Results section below). Independent Component Analysis.
An independent component analysis (ICA) identified spatially distributed neural networks of temporally coactivated voxels (Cisler et al., 2019). Thirty-five components were identified, 16 of which were functional networks Two functional networks were identified as a left and right FPN, which were of primary interest. In addition to the two FPNs, follow-up analyses were implemented with eight networks that have been identified within previous trauma or PTSD research (see Supplemental Figure S9). For within-subject analyses, participants’ time courses were regressed onto the design matrix using AFNI (3dREML; Cox, 1996), which included the anticipation, decision, and outcome phases of the task. The anticipation and decision phases were parametrically modulated by trial-by-trial associative strength (Vt,c) from the LS model, and the outcome phase was parametrically modulated by trial-by-trial PE (δt) and dB from the LS model (see Supplement for more information). The beta coefficients for the outcome phase modulated by dBs, which characterized model-based RL updates, were carried forward to second-level analyses.
Models tested the effect of trauma (using effect coded variables) on dB, controlling for age, IQ, and study site. Based on behavioral analyses where sexual abuse was uniquely associated with learning strategy (see below), CTQ Sexual Abuse severity scores were also entered as predictors of dB encoding. LMEs were implemented using MATLAB (fitlme; The MathWorks, Inc., Natick, MA). For the primary analyses, Bonferroni correction was applied for the two FPN networks (p < .025) and post-hoc analyses were corrected for eight networks (p < .006). Voxelwise analyses were also performed but, given that evidence indicates RL is implemented via widespread brain regions/neural networks (Cisler et al., 2019; Ross et al., 2018), were not of primary interest. See the Supplement for methods and results of voxelwise analyses.
3. Results
3.1. Behavioral Results
Performance, based on number of rewarded trials, was above chance (i.e., 33%; M = 58%, SD = 8%). Across participants, the LS model yielded a significantly lower AIC value than the RW model (128.6 vs. 133.8; t(59) = −4.33, p < .001), and the LS model provided a better fit for a greater proportion of participants than the RW model (n = 40 vs. n = 20, χ2(1) = 6.67, p = .010; see Figure 1A). As depicted in Figures 1B and 1C, trauma exposure neither predicted dAIC (t(55) = 1.89, p = .063), nor differential model fit (i.e., proportion of controls versus trauma-exposed adolescents whose behavior was better explained by the LS versus RW model: 72% vs. 61%, χ2(1) = .83, p = .361. Furthermore, CTQ Physical Abuse (PA) severity did not predict dAIC (CTQ PA: t(55) = 1.40, p = .168). By contrast, greater levels of CTQ Sexual Abuse (SA) predicted higher dAIC values (t(55) = 2.60, p = .012). While CTQ PA and CTQ SA were significantly correlated (r(58) = 0.60, p< .001), the effect of CTQ SA on dAIC held even after controlling for CTQ Physical Abuse (t(54)=2.16, p = .035) in a linear regression, whereas CTQ Physical Abuse remained a non-significant predictor (t(54) = −0.28, p = .777).
Figure 1.
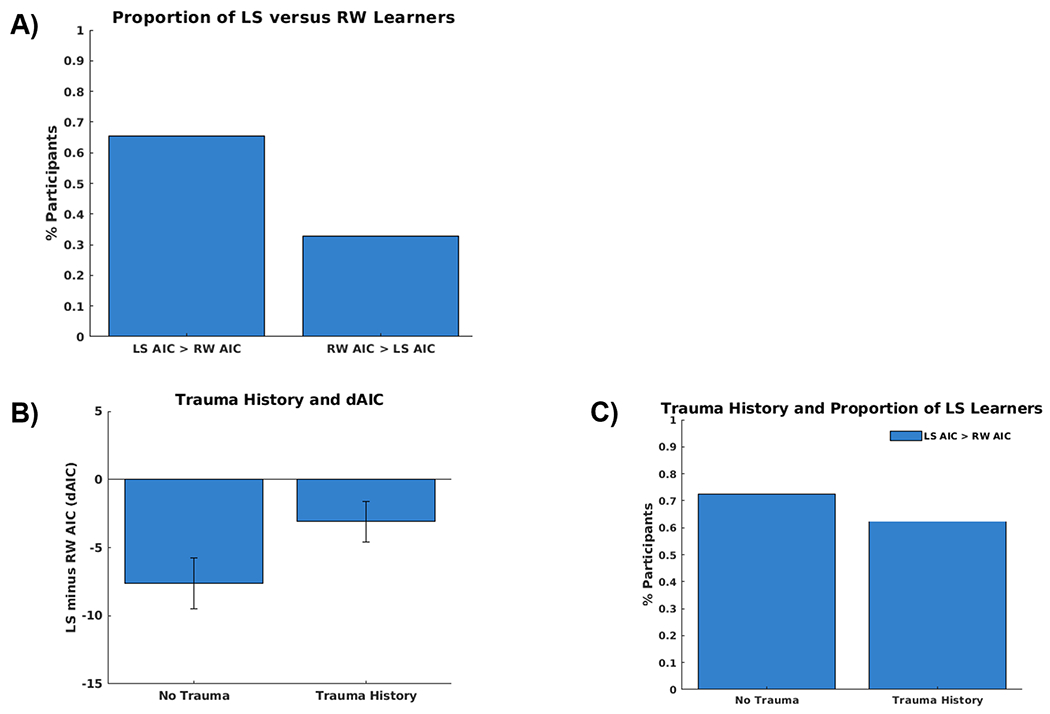
A) The behavior of a greater portion of participants is better captured by the latent state (67%) versus the risk-sensitive anti correlated Rescorla-Wagner (33%) model based on a comparison of each participants’ LS versus RW Akaike information criterion (AIC) values. B) The difference in LS and RW model fit (dAIC) did not differ between adolescents with versus without a history of trauma. C) The proportion of participants whose behavior was better captured by the LS versus RW model did not differ between adolescents with and without a history of trauma.
Based on prior research indicating that severe levels of abuse are particularly detrimental for functioning (Tomoda et al., 2009; van Harmelen et al., 2010) and on an inspection of the scatter plot of the relationship between dAIC and sexual abuse indicating that individuals with no and low-to-moderate sexual abuse history had comparable dAIC values (see Supplemental Figure S4), participants were grouped into CTQ SA categories following established cutoff scores (Bernstein et al., 2003; Bernstein & Fink, 2003). Post-hoc tests were utilized to assess for a non-linear association between model-based RL and SA. Participants with no (CTQ SA < 6) and low-to-moderate (CTQ SA ≥ 6 and < 13) SA scores (n = 49) were compared to participants with severe (CTQ SA ≥ 13) SA scores (n = 11; see Table 1 for demographics and clinical characteristics). To confirm that dAIC was elevated as a function of severe, but not low-to-moderate, SA, dAIC values for the low-to-moderate and severe SA groups were compared to those of adolescents with no history of SA. Whereas dAIC values were higher among adolescents with a history of severe relative to no SA (t(54) = 2.24, p = .029), they did not significantly differ between adolescents with a history of low-to-moderate relative to no SA (t(54) = −.77, p = .443).
Table 1.
Participants’ Demographic and Clinical Characteristics.
| No-to-Moderate Sexual Abuse History | Severe Sexual Abuse History | |
|---|---|---|
| n | 49 | 11 |
| Age, Mean (SD) | 15.2 (2.1) | 15.7 (1.2) |
| Race/Ethnicity (%) | ||
| White | 57 | 63 |
| Black/African American | 24 | 0 |
| Asian | 2 | 0 |
| Hispanic, Latina | 0 | 0 |
| Pacific Islander | 0 | 27 |
| Native American | 0 | 0 |
| Other | 12 | 10 |
| IQ, Mean (SD) | 110.1 (21.4) | 102.9 (20.7) |
| CAPS Total Severity, Mean (SD) | 15.1 (26.5) | 58.2 (25.9)a |
| CAPS Current # Symptoms | 2.2 (4.3) | 9.5 (4.7)a |
| Current PTSD Diagnosis (%) | 16 | 82a |
| Lifetime PTSD Diagnosis (%) | 24 | 91a |
| CTQ Total, Mean (SD) | 35.6 (12.5) | 62.4 (15.0)a |
| CTQ Physical Assault, Mean (SD) | 8.1 (3.5) | 14.0 (4.2)a |
| CTQ Sexual Assault, Mean (SD) | 5.6 (1.7) | 19.3 (4.3)a |
| CBCL Anxiety, Mean (SD) | 4.4 (4.7) | 10.0 (6.9)a |
| CBCL Depression, Mean (SD) | 3.0 (3.2) | 5.0 (2.8) |
| Psychiatric Medications (%) | 27 | 45a |
IQ=Intelligence Quotient, One-Word Receptive Vocabulary Test; CAPS=Clinician-Administered PTSD Scale, Child and Adolescent Version; CTQ=Childhood Trauma Questionnaire; CBCL=Child Behavior Checklist;
p<.05
Using the no and low-to-moderate SA versus severe SA groupings (no-to-moderate SA = −1, severe SA = 1), task performance was found to be poorer among those with severe SA than those with no-to-moderate SA (M = 60% vs. M = 52%, t(55) = −2.37, p = .022). As shown in Figure 2A, the LS model provided a better fit for a greater proportion of participants with no-to-moderate SA than those with severe SA (76% vs. 27%, χ2(1) = 9.41, p = .002). Additionally, Figure 2B shows that adolescents with a history of severe SA had higher dAIC values relative to adolescents with no-to-moderate SA (t(55) = 2.55, p = .014). Finally, the impact of severe versus no-to-moderate SA on dAIC held after controlling for a diagnosis of PTSD (t(54) = 2.15, p = .036).
Figure 2.
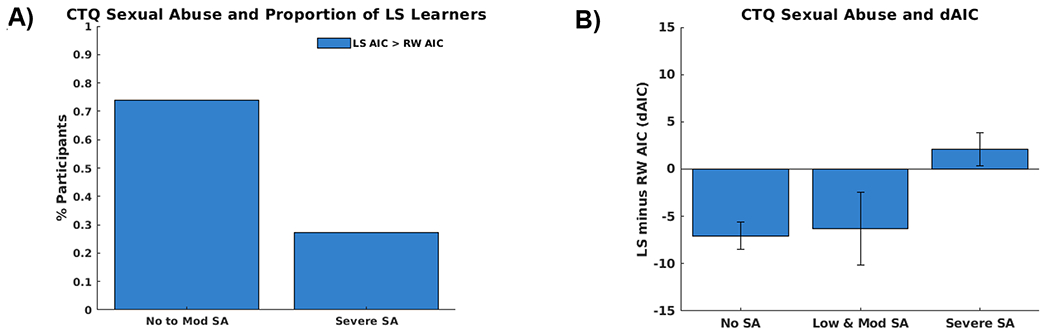
A) The behavior of a greater portion of participants with no-to-moderate (76%) versus severe (27%) Childhood Trauma Questionnaire (CTQ) Sexual Abuse is better captured by the latent state versus the risk-sensitive anticorrelated Rescorla-Wagner model based on a comparison of each participants’ LS versus RW Akaike information criterion (AIC) values. B) The difference in LS and RW AIC values (dAIC) is higher (higher dAIC values reflect poorer LS relative to RW model fit) among adolescents with severe versus no-to-moderate CTQ Sexual Abuse scores. (Note: the no and low-to-moderate groups are separated for visualization).
3.2. Independent Component Analysis
Prior to the ICA analyses, mean trial-by-trial dBs were calculated across participants to examine whether changes in the latent-state beliefs (dBs) followed changes in the task. Supporting the validity of the LS model, which purports that changes in task conditions lead to revisions in latent-state beliefs (i.e., the presence of different context/rules), the largest changes in dBs followed switches in task rules (every 30 trials), which prompted model updates of task representations (see Figure 3 A). There was no significant effect of assaultive trauma on left (t(49) = −1.29, p = .204) or right FPN (t(49) = −1.94, p = .059) activity relative to controls (see Supplemental Table S2 and Figure S7). There was a scalar relationship between SA and dB encoding in the left FPN (t(49) = −2.30, p = .026), which did not survive Bonferroni correction. In parallel with the behavioral analyses, when SA was coded based on severity, there was evidence of reduced dB encoding in the left FPN among adolescents with severe versus no-to-moderate SA (t(49) = −2.73, p = .009; see Figures 3B and 3C). The effect of severe versus no-to-moderate SA on left FPN was not accounted for by PTSD diagnosis (t(48) = 2.18, p = .034). Results of analyses with other networks for severe versus no-to-moderate SA are provided in the Supplement (see Supplemental Table S3 and Figure S8).
Figure 3.
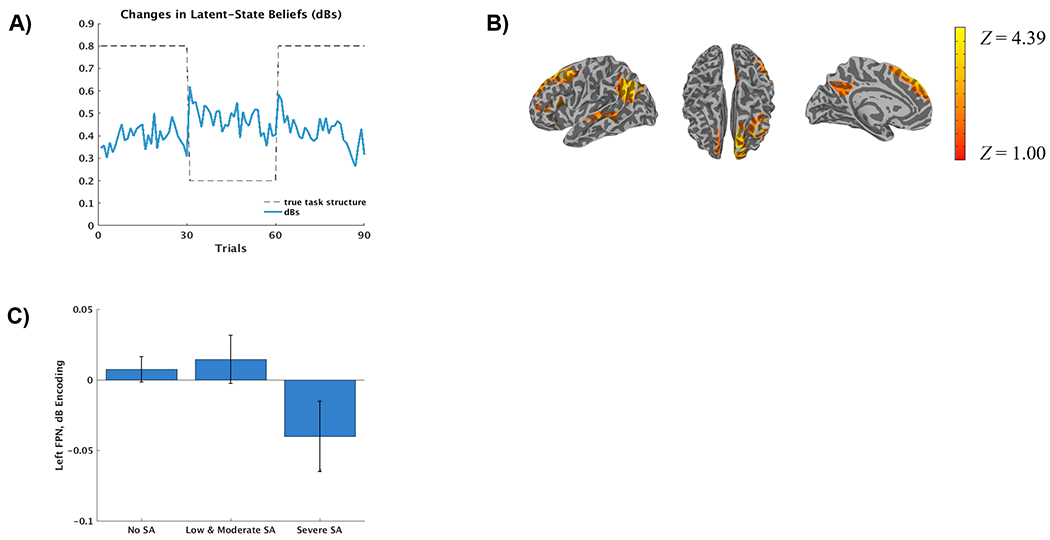
A) Trial by trial latent-state belief updates (delta beliefs) averaged across participants (blue line). The task’s reward structure, which changes every 30 trials, is represented by the dashed line (e.g., the likelihood of receiving a reward after selecting cue A is 80% during the first 30 trials, 20% during the second 30 trials and 80% during the last 30 trials). B) Spatial map of the left frontoparietal network (FPN) identified via independent component analysis (radiological convention). C) Left FPN encoding (regression coefficients from the first-level time-course modeling) decreases during changes in latent-state beliefs (delta beliefs) among adolescents with severe versus no-to-moderate CTQ Sexual Abuse scores. (Note: the no and low-to-moderate groups are separated for visualization).
3.3. Ruling Out Additional Confounds
Analyses that rule out the effects of additional confounds (psychotropic medication use, anxiety symptoms, depressive symptoms, lifetime PTSD) on results are included in the Supplement (see Supplemental Table S4). Only two results were no longer significant after accounting for covariates. Specifically, the impact of severe SA on performance, (t(54) = −1.86, p = .068), and dAIC values, (t(54) = 1.93, p = .059), became marginally non-significant after including anxiety symptoms and lifetime PTSD, respectively.
3.4. Follow-up: Task Effects
The Supplement includes parallel analyses that sought to identify whether the impact of severe SA on model fit (dAIC) and left FPN dB encoding are evident in the non-social task. While most effects that emerged for the social task did not emerge for the non-social task, there were no significant CTQ SA (severity coded) x task interactions when both tasks were included in LMEs. This precludes any strong inferences regarding the specificity of results.
4. Discussion
The present study examined the impact of trauma on model-based RL. Whereas neither an overall effect of general assaultive trauma exposure nor a linear dose-dependent impact of general assaultive trauma exposure on model-based RL emerged, exposure to severe sexual abuse predicted impaired model-based RL. Computational modeling revealed that although more participants used a model-based than model-free approach during the social learning task, the majority of participants with a history of severe sexual abuse used a model-free strategy. Suggestive of model-free RL being a less effective strategy, task performance was generally poorer among adolescents with severe versus no-to-moderate sexual abuse. In addition to predicting the use of a strategy more consistent with model-free RL, severe sexual abuse predicted reduced FPN encoding of trial-by-trial updates in latent-state beliefs. As hypothesized, effects of sexual abuse were not accounted for by a diagnosis of PTSD. Rather than reflecting an epiphenomenon or outcome of PTSD, model-based RL deficits may reflect a trait-like impairment that results from abuse and precedes psychopathology onset (including PTSD). Results suggest that early sexual abuse adversely affects cognitive processes that support model-based RL of reward, especially in the context of social information. Broadly, attention toward facial stimuli has been found to be altered (e.g., enhanced) among individuals with a history of abuse (Gibb et al., 2009, van Harmelen et al., 2012), including adolescents with a history of sexual abuse specifically (Fang, Wang, & Gong, 2019; van Hoof et al., 2017). Not only is social information attention-capturing for individuals with a history of sexual abuse, individuals report experiencing greater distress within social situations than non-abused individuals (Feerick & Snow, 2005). Thus, although the facial stimuli included in the present study were of neutral valence, the social context may have contributed to difficulties utilizing model-based RL. However, although the neutral house task did not prompt more individuals to use a model-based relative to model-free RL strategy, results indicate that the impact of severe sexual abuse on model-based RL processes may not be specific to a social context.
One way that early sexual abuse may impair cognitive processes that implement model-based RL broadly is via disruption of the foundational FPN development. The anatomical structure and functioning of regions that comprise the FPN are particularly vulnerable to the effects of early stress (van der Kolk, 2003; Pechtel & Pizzagalli, 2011; Heim, Plotsky, & Nemeroff, 2004), given the protracted maturational time course of the PFC (Fuster, 2002). Relative to other forms of abuse, early sexual abuse may have particularly detrimental effects on cognitive and psychological processes. Indeed, sexual abuse predicts poorer outcomes beyond other forms of abuse in several domains, including language ability, memory performance, and risk for psychopathology (De Bellis, Woolley, & Hooper, 2013; Fergusson, Boden, & Horwood, 2008).
There may be broader implications of difficulties in model-based RL among youth with severe sexual abuse histories. Difficulty abstracting information and/or updating the cognitive maps that are used to predict outcomes as a result model-based RL deficits could lead to difficulties within complex and shifting social environments. General difficulties identifying contextual factors indicative of social or predatory threat could increase risk for revictimization, which is common among individuals with a history of trauma (Chu, 1992; Classen, Palesh, & Aggarwal, 2005; Widom, Czaja, & Dutton, 2008). More broadly, deficits in RL processes may contribute to inflexible, maladaptive behavior, such as withdrawal (related to underestimation of reward) and persistent avoidance (related to overestimation of threat), which are potent risk factors for the development of mental health disorders (Spinhoven, Drost, de Rooij, van Hemert, & Penninx, 2014). Finally, model-based RL deficits could have important implications for treatment outcomes for psychopathology that develops following abuse. Executive function impairment has been identified as a factor that predicts treatment responsivity (Mohlman & Gorman, 2005; Pimontel, Culang-Reinlieb, Morimoto, & Sneed, 2012), and, similarly, model-based RL impairment resulting from childhood sexual abuse may predict poorer treatment response, especially for treatments that rely upon EF and model-based RL processes (e.g., updating cognitive representations via cognitive therapy). In the future it will be important to further examine the impact of trauma, especially sexual trauma, on model-based RL, and the functional implications of model-based RL impairment.
There are several limitations of the present study. First, the study was limited to adolescent females, which impacts its generalizability to males and individuals of other ages. Another notable limitation was the small number of participants who were included in the severe sexual abuse group (n=11), as small samples can affect the reliability and generalizability of results and highlights the importance of replicating these effects with larger samples. Another limitation was the lack of cognitive assessment to rule out neurodevelopmental disorders, such as ADHD, that may have affected the use of latent-state learning. Regarding the CTQ, it should be noted that it does not assess important features of sexual abuse known to impact functioning, including the relation of the perpetrator to the victim and degree of force used (Ullman, 2007). While not a limitation per se, it is important that a developmental perspective be considered regarding the results, as structures that comprise the FPN are still developing during adolescence. Whereas adolescents exhibit greater model-based RL than children (who almost exclusively rely on model-free RL), model-based RL use is strongest among adults (Decker, Otto, Daw, & Hartley, 2016). Continued future research addressing these limitations will be important for continuing to define the impact of trauma on behavioral, computational, and neurodevelopmental outcomes.
Supplementary Material
References
- 1.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield CFL, Perry BD, … & Giles WH (2006). The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry and Clinical Neuroscience, 256, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arata CM, Langhinrichsen-Rohling J, Bowers D, & O’Farrill-Swails L (2005). Single versus multi-type maltreatment: An examination of the long-term effects of child abuse. Journal of Aggression, Maltreatment & Trauma, 11, 29–52. [Google Scholar]
- 3.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, & Anda RF (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82, 217–225. [DOI] [PubMed] [Google Scholar]
- 4.Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, & Croft JB (2009). Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine, 71, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells TT, Vanderlind WM, Selby EA, & Beevers CG (2014). Childhood abuse and vulnerability to depression: Cognitive scars in otherwise healthy young adults. Cognition & Emotion, 28, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC (2000). Posttraumatic stress disorder: The burden to the individual and to society. The Journal of Clinical Psychiatry, 61, 4–14. [PubMed] [Google Scholar]
- 7.Walker EA, Katon W, Russo J, Ciechanowski P, Newman E, & Wagner AW (2003). Health care costs associated with posttraumatic stress disorder symptoms in women. Archives of General Psychiatry, 60, 369–374. [DOI] [PubMed] [Google Scholar]
- 8.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, & Ressler KJ (2010). Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety, 27, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jovanovic T, Kazama A, Bachevalier J, & Davis M (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harms MB, Shannon Bowen KE, Hanson JL, & Pollak SD (2018). Instrumental learning and cognitive flexibility processes are impaired in children exposed to early life stress. Developmental Science, 21, el2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heerey EA (2014). Learning from social rewards predicts individual differences in self-reported social ability. Journal of Experimental Psychology: General, 143, 332–339. [DOI] [PubMed] [Google Scholar]
- 12.Reeb-Sutherland BC, Levitt P, & Fox NA (2012). The predictive nature of individual differences in early associative learning and emerging social behavior. PLoS One, 7, e30511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenbaum H (2010). An initiation—termination two-phase model of worrying. Clinical Psychology Review, 30, 962–975. [DOI] [PubMed] [Google Scholar]
- 14.Ross MC, Lenow JK, Kilts CD, & Cisler JM (2018). Altered neural encoding of prediction errors in assault-related posttraumatic stress disorder. Journal of Psychiatric Research, 103, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May CL, & Wisco BE (2019). Reward Processing and Decision-Making in Posttraumatic Stress Disorder. Behavior Therapy. [DOI] [PubMed] [Google Scholar]
- 16.Admon R, & Pizzagalli DA (2015). Dysfunctional reward processing in depression. Current Opinion in Psychology, 4, 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cisler JM, Esbensen K, Sellnow K, Ross M, Weaver S, Sartin-Tarm A, … & Kilts (2019). Differential roles of the salience network during prediction error encoding and facial emotion processing among female adolescent assault victims. Biological Psychiatry : Cognitive Neuroscience and Neuroimaging, 4, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daw ND, Niv Y, & Dayan P (2005). Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience, 8, 1704–1711. [DOI] [PubMed] [Google Scholar]
- 19.Beierholm UR, Anen C, Quartz S, & Bossaerts P (2011). Separate encoding of model-based and model-free valuations in the human brain. Neuroimage, 58, 955–962. [DOI] [PubMed] [Google Scholar]
- 20.Gláscher J, Daw N, Dayan P, & O′Doherty JP (2010). States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron, 66, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daw ND, Gershman SJ, Seymour B, Dayan P, & Dolan RJ (2011). Model-based influences on humans′ choices and striatal prediction errors. Neuron, 69, 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SW, Shimojo S, & O’Doherty JP (2014). Neural computations underlying arbitration between model-based and model-free learning. Neuron, 81, 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DePrince AP, Weinzierl KM, & Combs MD (2009). Executive function performance and trauma exposure in a community sample of children. Child Abuse & Neglect, 33, 353–361. [DOI] [PubMed] [Google Scholar]
- 24.Gould F, Clarke J, Heim C, Harvey PD, Majer M, & Nemeroff CB (2012). The effects of child abuse and neglect on cognitive functioning in adulthood. Journal of Psychiatric Research, 46, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mezzacappa E, Kindlon D, & Earls F (2001). Child abuse and performance task assessments of executive functions in boys. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 42, 1041–1048. [DOI] [PubMed] [Google Scholar]
- 26.Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, … & Ernst M (2010). Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia, 48, 3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navalta CP, Polcari A, Webster DM, Boghossian A, & Teicher MH (2006). Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. The Journal of Neuropsychiatry and Clinical Neurosciences, 18, 45–53. [DOI] [PubMed] [Google Scholar]
- 28.Spann MN, Mayes LC, Kalmar JH, Guiney J, Womer FY, Pittman B, … & Blumberg ΗP (2012). Childhood abuse and neglect and cognitive flexibility in adolescents. Child Neuropsychology, 18, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez JA & Emory E (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychology Review, 16, 17–42. [DOI] [PubMed] [Google Scholar]
- 30.Collette F, & Van der Linden M (2002). Brain imaging of the central executive component of working memory. Neuroscience & Biobehavioral Reviews, 26, 105–125. [DOI] [PubMed] [Google Scholar]
- 31.Otto AR, Skatova A, Madlon-Kay S, & Daw ND (2014). Cognitive control predicts use of model-based reinforcement learning. Journal of Cognitive Neuroscience, 27, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majer M, Nater UM, Lin JMS, Capuron L, & Reeves WC (2010). Association of childhood trauma with cognitive function in healthy adults: A pilot study. BMC Neurology, 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilpatrick DG, Aciemo R, Saunders B, Resnick HS, Best CL, & Schnurr PP (2000). Risk factors for adolescent substance abuse and dependence: data from a national sample. Journal of Consulting and Clinical Psychology, 68, 19–30. [DOI] [PubMed] [Google Scholar]
- 34.Kilpatrick DG, Ruggiero KJ, Aciemo R, Saunders BE, Resnick HS, & Best CL (2003). Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. Journal of Consulting and Clinical Psychology, 71, 692–700. [DOI] [PubMed] [Google Scholar]
- 35.Pynoos RS, Weathers FW, Steinberg AM, Marx BP, Layne CM, Kaloupek DG, et al. (2015). Clinician-Administered PTSD Scale for DSM-5: Child/Adolescent Version. White River Junction, VT: National Center for PTSD. [Google Scholar]
- 36.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, … & Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27, 169–190. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein DP & Fink L (1998). Childhood Trauma Questionnaire: A retrospective self-report manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- 38.Hauser TU, Iannaccone R, Walitza S, Brandeis D, & Brem S (2015). Cognitive flexibility in adolescence: neural and behavioral mechanisms of reward prediction error processing in adaptive decision-making during development. Neuroimage, 104, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cochran AL & Cisler JM (2019). A flexible and generalizable model of online latent-state learning. PLoS Computational Biology, 15, e1007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- 41.Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, & Teicher MH (2009). Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage, 47, T66–T71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, … & Elzinga BM (2010). Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry, 68, 832–838. [DOI] [PubMed] [Google Scholar]
- 43.Gibb BE, Schofield CA, & Coles ME (2009). Reported history of childhood abuse and young adults’ information-processing biases for facial displays of emotion. Child Maltreatment, 14, 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Harmelen AL, van Tol MJ, Demenescu LR, van der Wee NJ, Veltman DJ, Aleman A, … & Elzinga BM (2012). Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience, 8, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, Wang S, Liu J, & Gong J (2019). Early ERP components to emotional facial expressions in young adult victims of childhood maltreatment. Psychiatry Research, 275, 120–128. [DOI] [PubMed] [Google Scholar]
- 46.van Hoof MJ, van den Bulk BG, Rombouts SA, Rinne-Albers MA, van der Wee NJ, van IJzendoorn MH, & Vermeiren RR (2017). Emotional face processing in adolescents with childhood sexual abuse-related posttraumatic stress disorder, internalizing disorders and healthy controls. Psychiatry Research: Neuroimaging, 264, 52–59. [DOI] [PubMed] [Google Scholar]
- 47.Feerick MM, & Snow KL (2005). The relationships between childhood sexual abuse, social anxiety, and symptoms of posttraumatic stress disorder in women. Journal of Family Violence, 20, 409–419. [Google Scholar]
- 48.van der Kolk BA (2003). The neurobiology of childhood trauma and abuse. Child and Adolescent Psychiatric Clinics of North America, 12, 293–318. [DOI] [PubMed] [Google Scholar]
- 49.Pechtel P & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heim C, Plotsky PM, & Nemeroff CB (2004). Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology, 29, 641–648. [DOI] [PubMed] [Google Scholar]
- 51.Fuster JM (2002). Frontal lobe and cognitive development. Journal of Neurocytology, 31, 373–385. [DOI] [PubMed] [Google Scholar]
- 52.De Bellis MD, Woolley DP, & Hooper SR (2013). Neuropsychological findings in pediatric maltreatment: Relationship of PTSD, dissociative symptoms, and abuse/neglect indices to neurocognitive outcomes. Child Maltreatment, 18, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fergusson DM, Boden JM, & Horwood LJ (2008). Exposure to childhood sexual and physical abuse and adjustment in early adulthood. Child Abuse & Neglect, 32, 607–619. [DOI] [PubMed] [Google Scholar]
- 54.Chu JA (1992). The revictimization of adult women with histories of childhood abuse. The Journal of Psychotherapy Practice and Research, 1, 259–269. [PMC free article] [PubMed] [Google Scholar]
- 55.Classen CC, Palesh OG, & Aggarwal R (2005). Sexual revictimization: A review of the empirical literature. Trauma, Violence, & Abuse, 6, 103–129. [DOI] [PubMed] [Google Scholar]
- 56.Widom CS, Czaja SJ, & Dutton MA (2008). Childhood victimization and lifetime revictimization. Child Abuse & Neglect, 32, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spinhoven P, Drost J, de Rooij M, van Hemert AM, & Penninx BW (2014). A longitudinal study of experiential avoidance in emotional disorders. Behavior Therapy, 45, 840–850. [DOI] [PubMed] [Google Scholar]
- 58.Mohlman J, & Gorman JM (2005). The role of executive functioning in CBT: a pilot study with anxious older adults. Behaviour Research and Therapy, 43, 447–465. [DOI] [PubMed] [Google Scholar]
- 59.Pimontel MA, Culang-Reinlieb ME, Morimoto SS, & Sneed JR (2012). Executive dysfunction and treatment response in late-life depression. International Journal of Geriatric Psychiatry, 27, 893–899. [DOI] [PubMed] [Google Scholar]
- 60.Decker JH, Otto AR, Daw ND, & Hartley CA (2016). From creatures of habit to goal-directed learners: Tracking the developmental emergence of model-based reinforcement learning. Psychological Science, 27, 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ullman SE (2007). Relationship to perpetrator, disclosure, social reactions, and PTSD symptoms in child sexual abuse survivors. Journal of Child Sexual Abuse, 16, 19–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


