Abstract
The global prevalence of nonalcoholic fatty liver disease (NAFLD) in children and adolescents is escalating and currently represents the most common chronic liver disease in the pediatric population. NAFLD is associated with high daily caloric intake and sedentary behavior, with excessive consumption of added sugar emerging as an important contributor to NAFLD risk in children. This is a particularly important factor for adolescents with obesity, who are the heaviest consumers of added sugar. Table sugar, or sucrose, is a disaccharide comprised of fructose and glucose, yet only fructose has been strongly linked to NAFLD pathogenesis largely due to the unique characteristics of its metabolism and detrimental effects on key metabolic pathways. To date, the relationship between excessive fructose intake and risk of NAFLD in children and adolescents remains incompletely understood, and it is not yet known whether fructose actually causes NAFLD or instead exacerbates hepatic fat accumulation and possible hepatocellular injury only within the context of cardiometabolic factors. The purpose of this review is to summarize recent studies linking fructose consumption with NAFLD in the pediatric population and integrate results from interventional studies of fructose restriction in children and adolescents on NAFLD and related metabolic markers. Given the overall positive impact of lifestyle modifications in the management of pediatric NAFLD, reduction of added sugar consumption may represent an important, early opportunity to mitigate or prevent NAFLD in high-risk children and adolescents.
Keywords: fructose, uric acid, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, hepatic steatosis, liver fibrosis
Pediatric NAFLD
Nonalcoholic fatty liver disease (NAFLD) is a chronic, progressive condition that arises from hepatic steatosis in the absence of other factors such as genetic or metabolic disorders, infections, use of steatogenic medications, or excessive alcohol consumption1. In the United States (US), NAFLD is the most common chronic liver disease in children2, 3, in whom the prevalence of NAFLD is broadly estimated at 13%2. Risk factors for NAFLD in children and adolescents include obesity and severe obesity4, increasing age, family history, and Latino ethnicity2, especially in those of Mexican ancestry5, 6. NAFLD encompasses a spectrum of histologically defined liver disease that includes steatosis and nonalcoholic steatohepatitis (NASH), characterized by steatosis, hepatic inflammation, and hepatocellular injury, and oftentimes accompanied by fibrosis. Some children develop a distinct pattern of injury, characterized by a periportal pattern of fibrosis, prevalent zone 1 inflammation, and an absence of hepatocyte ballooning7, that may be linked to a more severe phenotype8. In children and adolescents, NAFLD is often asymptomatic and is commonly detected as a result of abnormal liver enzymes or imaging9. Clinical screening recommendations do not differentiate the spectrum of NAFLD-related disorders, and liver biopsy is required for NASH diagnosis and fibrosis staging1.
Although the natural history of pediatric NAFLD is not yet thoroughly delineated, metabolic syndrome is a common feature at the time of diagnosis, with more than 80% of children exhibiting at least one component associated with metabolic syndrome, such as obesity, hypertension, dyslipidemia, or hyperglycemia10. Some of these components are also present in lean adolescents with suspected NAFLD, suggesting that metabolic derangement, even outside of the context of obesity, may be important in disease pathogenesis11. A recent prospective study in children with NAFLD receiving only standard-of-care lifestyle counseling reported that >30% of children developed NASH and/or experienced worsening fibrosis within two years12. Increasing obesity and higher baseline levels of ALT (alanine aminotransferase), AST (aspartate aminotransferase), GGT (gamma-glutamyl transferase), as well as total and LDL cholesterol (LDLC) were associated with progression to NASH, while progression in fibrosis was associated with white race, incident type 2 diabetes (T2D), and increasing ALT, GGT, and glycated hemoglobin (HbA1c). Any progression, either to NASH or increasing fibrosis, was associated with increasing age, higher baseline central adiposity, ALT, AST, GGT, and cholesterol levels (total and LDLC), and worsening values of ALT, HbA1c, and GGT. Not unexpectedly, incident T2D or T2D at any visit, was associated with significantly increased odds of progression to a more severe clinical phenotype. Some studies indicate that pediatric NAFLD is associated with poor long-term outcomes, including a greater risk of death and requirement for liver transplantation10 and greater liver-related morbidity and mortality in adulthood10. Therefore, pediatric NAFLD may represent an aggressive form of fatty liver disease13, 14 that is even more concerning than adult NAFLD15.
The high heritability estimates for NAFLD16 suggest that genetic factors contribute to NAFLD susceptibility1, 16. Variants in specific genes including patatin-like phospholipase domain containing 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), glucokinase regulatory protein (GCKR), and others have been associated with hepatic steatosis, fibrosis, or higher NAFLD activity score9, 17, 18. In Latino children and adolescents, who have the highest prevalence of NAFLD, the variant G allele at rs738409 in PNPLA3 is more common19, and those with a homozygous genotype have twice as much liver fat as those with the wildtype genotype20. Variation in PNPLA3 also appears to mediate effects of high dietary sugar intake on NAFLD susceptibility21. Joint effects among risk alleles in PNPLA3, TM6SF2, GCKR are associated with greater intrahepatic fat accumulation17 and explained 39%, 32%, and 15% of percent hepatic fat fraction variance in Black, White, and Latino children and adolescents with obesity, respectively22. However, a recent study reported that cardiometabolic risk factors explained more of the variation in hepatic fat fraction than genetic risk score in Latino adolescents who were overweight or obese (27.2% vs 4.3% [6.4% for PNPLA3 rs738409 alone]), while an opposite relationship was observed in lean adolescents23, suggesting that heritable effects are modulated, at least in part, by adiposity.
Dietary components, including saturated fat, cholesterol, and sucrose have been associated with NAFLD24, but recent observational studies, as well as intervention trials, point to the specific role that added sugar intake plays in the pathophysiology of NAFLD25. Given that adolescents consume the highest amount of added sugars relative to other age groups26, this single dietary component may represent an early and important target by which pediatric obesity27, 28, and more specifically, NAFLD risk can be attenuated in this population. The purpose of this review is to summarize the evidence linking high fructose consumption with pediatric NAFLD and review the effects of dietary fructose restriction on metabolic parameters associated with NAFLD in children and adolescents.
Fructose consumption and hepatic metabolism
Fructose and glucose, which combined form a molecule of sucrose, are metabolized by different mechanisms in humans29. Dietary glucose is predominantly metabolized by skeletal muscle to generate carbon dioxide, water, and adenosine triphosphate (ATP), and by adipose tissue to generate glycerol trisphosphate for triglyceride synthesis. Under normal physiological conditions, fructose is passively absorbed across the intestinal lumen, and then transported to the liver, where it is metabolized by ketohexokinase (KHK) to fructose-1-phosphate. Fructose-1-phosphate is subsequently metabolized to glucose, acetyl-CoA, fatty acids, lactate, and triglycerides, as shown in Figure 130. In contrast to the phosphorylation of glucose, fructose phosphorylation is rapid and is not regulated by feedback inhibition. Phosphorylation of fructose utilizes ATP, which depletes both ATP and intracellular phosphate, leading to the activation of adenosine monophosphate (AMP) deaminase, and the conversion of AMP to inosine triphosphate (IMP), and subsequently, to uric acid31. Uric acid has been linked to oxidative stress and mitochondrial dysfunction29. High levels of circulating uric acid are commonly observed in individuals with high fructose intake32, and uric acid levels increase in parallel with consumption of fructose33, 34. Because of the relationship between fructose consumption and circulating uric acid levels, uric acid is sometimes used as a biologic proxy for fructose intake35–38.
Fig 1. Hepatic metabolism of fructose.
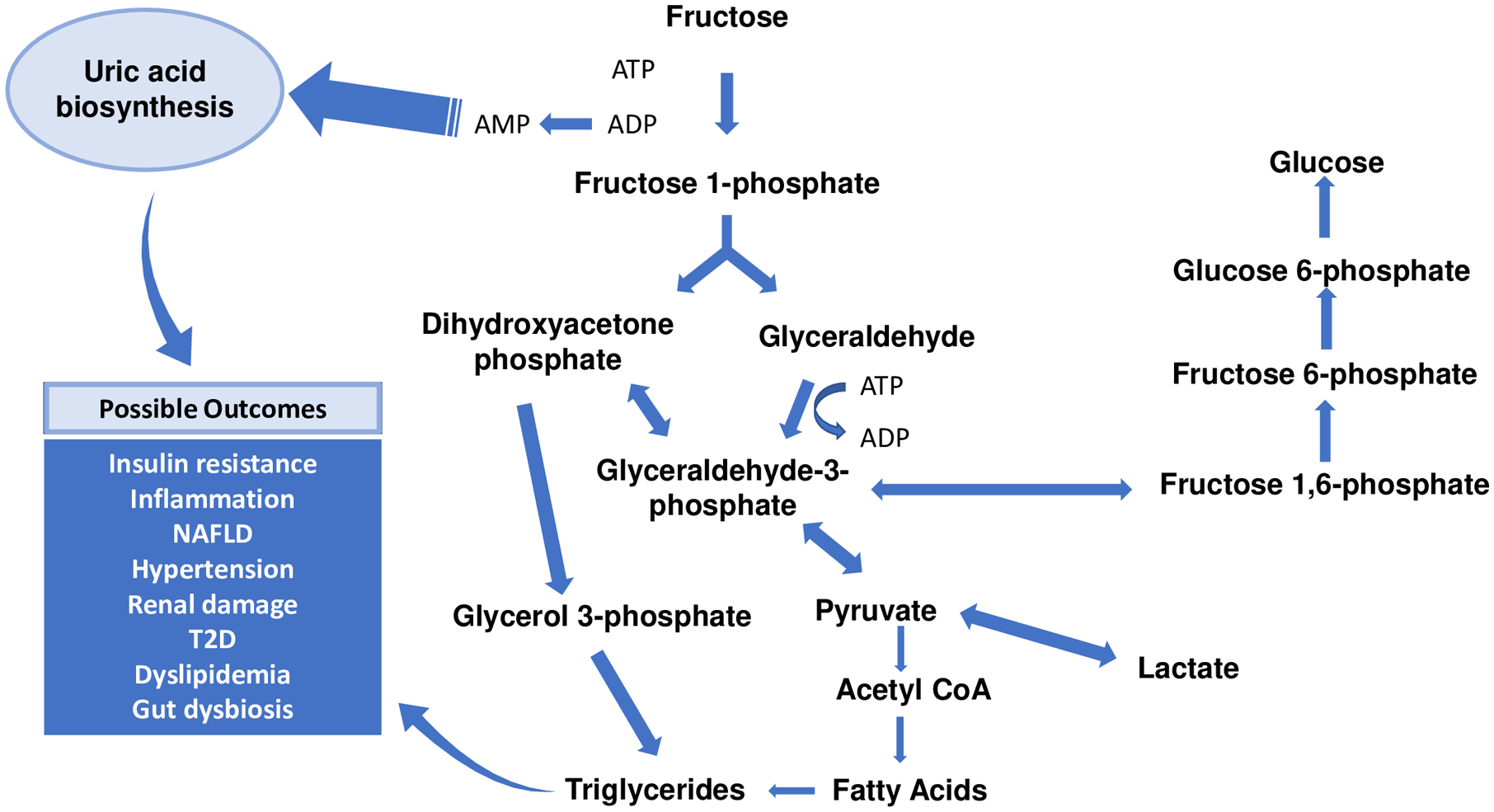
Following transport into liver cells, fructose is first rapidly metabolized by ketohexokinase to fructose-1-phosphate, which is then broken down by adolase B to dihydroxyacetone phosphate and glyceraldehyde. Fructose phosphorylation utilizes adenosine triphosphate (ATP); excessive amounts of incoming fructose result in ATP depletion from hepatic stores and generation of adenosine monophosphate (AMP), a precursor to uric acid. Both dihydroxyacetone and glyceraldehyde contribute to the synthesis of glyceraldehyde-3-phosphate, via the actions of glycerol-3-phosphate dehydrogenase and triokinase, respectively. Glycerol-3-phosphate serves as a precursor substrate for de novo lipogenesis (DNL). The large blue hashed arrows represent several enzymatic steps that have not been included in the figure. Increased rates of uric acid synthesis and DNL are known to contribute directly or indirectly to insulin resistance, hepatic and systemic inflammation, NAFLD, and other cardiometabolic diseases.
Excessive and chronic consumption of fructose promotes hepatic de novo lipogenesis (DNL), impairs fatty acid oxidation, induces endoplasmic stress, and contributes to hepatic inflammation through the generation of both uric acid and gut-derived endotoxins37, 39–42. High dietary fructose intake has also been linked to altered gut microbiome composition, with a shift toward depletion of beneficial microbial species43. When consumed with a high fat diet, the addition of fructose but not glucose further impairs mitochondrial function in the liver44. Through impairment of these pathways, dietary fructose has been implicated in the indirect development of hepatic insulin resistance, and consequently, may be important in the pathogenesis of NAFLD (Fig 2)39.
Fig 2. Effects of excessive fructose consumption on biological pathways that contribute to increased risk of NAFLD development and progression in children and adolescents.
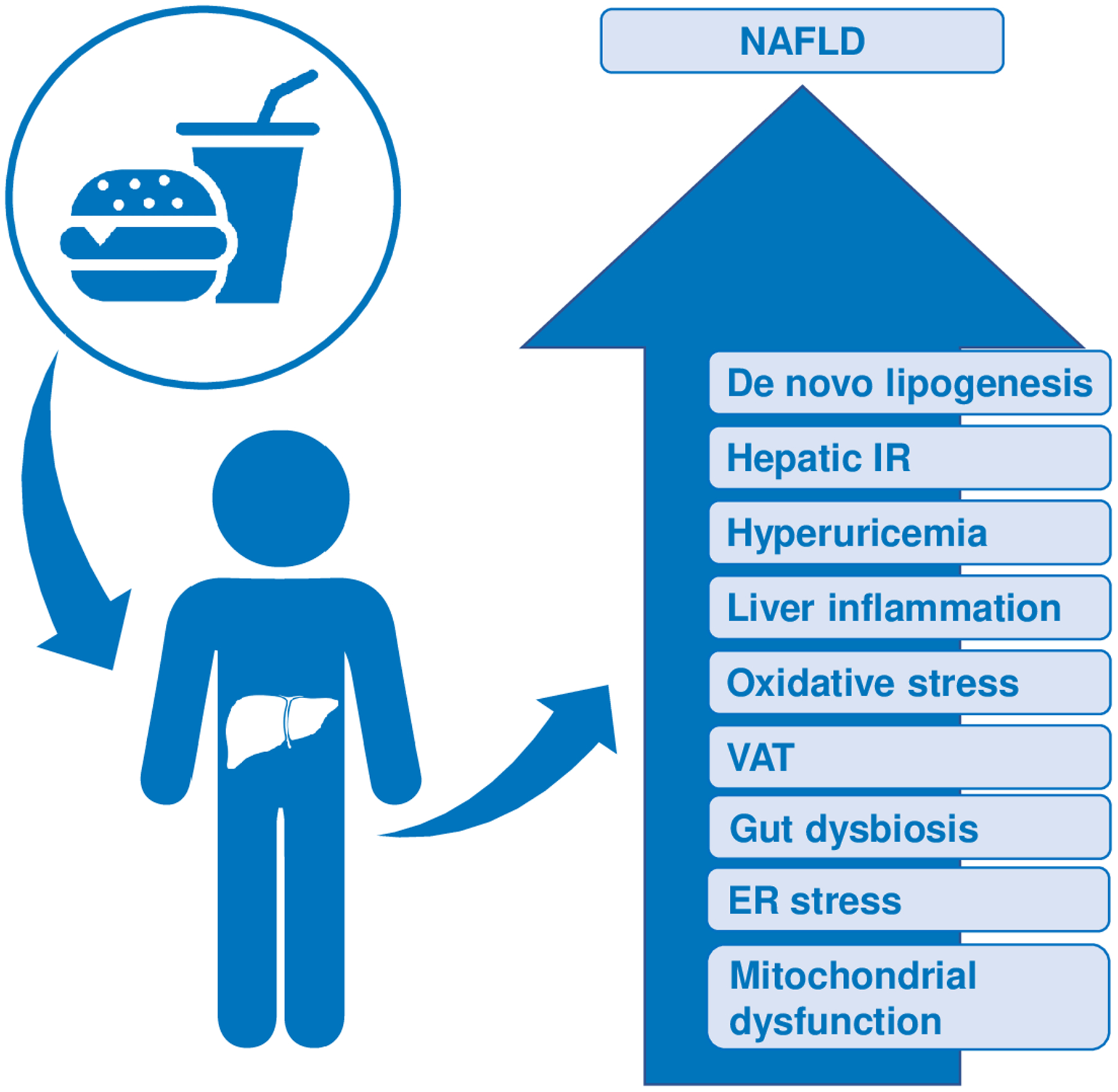
As discussed in the text, high intake of added sugar or fructose has been associated with increased DNL, hepatic insulin resistance (IR), hyperuricemia, hepatic inflammation, oxidative stress, and visceral adiposity. High fructose intake has also been associated with gut dysbiosis.
Association between fructose consumption and pediatric NAFLD
An overview of results from observational studies of fructose consumption in the pediatric population and fatty liver is shown in Table 1. One of the first studies characterized diet and physical activity patterns in children who were overweight or obese and had fatty liver, including some with histological evidence of NASH45. Three-day food records indicated low intakes of vitamin E, fiber and polyunsaturated fats, and high intakes of saturated fats and sugars, including sucrose and fructose, which represented 24% of daily carbohydrate calories. In these children, higher daily fructose intake was associated with insulin resistance, abdominal adiposity, low serum adiponectin concentrations, and elevated levels of GGT and tumor necrosis factor alpha. Multivariate analyses with fructose as a dependent variable identified significant positive interactions between GGT and body mass index (BMI) and GGT and male sex. Despite the significance of these findings, the sample size was limited, the study did not include a healthy comparison group, and the analyses did not control for potential confounders such as age, ethnicity, and sex. Further, a comparative analysis of fructose consumption in children with biopsy-proven NASH and children with less severe disease was not performed. An independent prospective, cross-sectional registry-based study subsequently sought to determine whether increased consumption of sugar-sweetened beverages was associated with worse pathologic features of NAFLD46. In this cohort of children enrolled in the multi-center NASH Clinical Research Network (NASH-CRN), NASH status was histologically determined and 39 children had no presence of NASH, 27 had borderline zone 3, 36 had borderline zone 1, and 47 had definite NASH. Self-reported sugar-sweetened beverage consumption, which was atypically low in this group, was not associated with histologic features of NAFLD. However, uric acid levels were significantly higher in children with definite NASH compared to those with milder forms of NAFLD. These findings suggest that 1) there may be sources of fructose intake other than sugar-sweetened beverages, or 2) self-reported instruments may not accurately depict actual intake of sugar-sweetened beverages.
Table 1.
Studies demonstrating association between fructose consumption and pediatric NAFLD
| Study population | Ethnicity | Study design | Main observations | Ref |
|---|---|---|---|---|
| 38 children (5–19 y) with fatty liver1 | Mixed | Retrospective |
|
45 |
| 149 children (6–17 y) with NAFLD or NASH2 | Mixed | Prospective, cross-sectional |
|
46 |
| 39 with UW, 424 with NW, 101 with OW, 38 with OB3 (start :14 y, end:17y) | Australian | Longitudinal, population-based |
|
47 |
| 55 with obesity and 30 matched NW controls (6–14 y)1 | Egyptian | Cross-sectional, case-control |
|
53 |
| 271 children with obesity and NAFLD or NASH (mean age 12.5 y)2 | Italian | Cross-sectional |
|
54 |
| 271 children with obesity and NAFLD or NASH (mean age 12.5 y)2 | Italian | Cross-sectional |
|
55 |
| 182 children with obesity and NAFLD or liver fibrosis (5–12 y)2 | Italian | Cross-sectional |
|
57 |
| 16 with OW with NAFLD, 73 with OW without NAFLD, 36 NW (5–9 y)1 | German | Cross-sectional |
|
58 |
Fatty liver determined by ultrasound;
histologically determined;
UW: underweight, NW: normal weight, OW: overweight, OB: obese, NAFLD at 17 years determined by ultrasound;
Using a prospective, population-based cohort of Australian adolescents, O’Sullivan et al47 investigated the relationship between fructose consumption at 14 years and the presence of NAFLD at 17 years. Fructose intake was extrapolated from a three-day food record from 592 adolescents (39 underweight, 424 normal weight, 101 overweight, and 28 with obesity). While fructose intake did not differ significantly between adolescents with or without NAFLD, for those with obesity and without NAFLD, there was a significantly lower fructose intake compared to the other groups. Adolescents who were in the higher half of fructose consumption and who also had obesity at age 14 were more likely to have NAFLD at 17 years compared to adolescents without obesity in the lower half of fructose consumers. This observation suggests that lower than usual fructose intake may be protective against the development of NAFLD in adolescents with obesity, as opposed to a high fructose intake causing an increased risk of NAFLD. For adolescents who had obesity at age 14 years, each gram of daily fructose consumed at this age was associated with a 9% increased risk of NAFLD at age 17 years. Multivariable logistic regression analysis showed that in adolescents with obesity, higher fructose intake was associated with increased odds of NAFLD. In this cohort, the relationship between fructose consumption and NAFLD risk appeared to be moderated by excess adiposity as adolescents with obesity who reported either low or high fructose consumption had a significantly greater risk of NAFLD compared to nonobese adolescents who had low fructose intake. The amount of fructose consumed, rather than the total amount of sugars, was likewise observed to have a greater impact on NAFLD risk in this cohort.
The potentially unique contribution of fructose to the development of NAFLD warrants strong consideration. While the majority of glucose enters the systemic circulation, fructose is primarily metabolized in the liver48, although the small intestine and kidney also metabolize fructose49–51. In addition, fructose, but not glucose, promotes bacterial overgrowth and increased intestinal permeability, resulting in movement of bacterial endotoxins into the portal plasma, which may contribute to hepatic inflammation. As noted, fructose phosphorylation is not rate-limiting, so excessive fructose intake can contribute to a high rate of triglyceride synthesis. Because fructose phosphorylation is also rapid, hepatic ATP can be rapidly depleted in the presence of high fructose levels. As obesity is known to increase lipid influx and DNL, ingestion of fructose in individuals with obesity may exceed the liver’s capacity to metabolize the increased lipid flux, consequently resulting in hepatic fat accumulation. Individuals with obesity may also be more generally susceptible to detrimental effects of fructose. For example, otherwise healthy individuals with overweight or obesity have reduced hepatic ATP stores compared to normal weight controls52, and this may confer a reduced ability to recover from hepatic ATP depletion resulting from excessive fructose exposure.
Other studies have reported association between fructose consumption and NAFLD severity in children. Increased fructose intake was associated with a progressive increase in NAFLD grade (5669.75 g/week (interquartile range [IQR] 8593.65) for grade 0, 8861.41 (4642.92) for grade 1, and 10,490.81 (4037.24) for grade 2) in Egyptian children with obesity53. In these children, the presence of NAFLD was also associated with higher fructose intake relative to healthy, age-, sex, and pubertal stage-matched controls who were not obese (171.27 g/week ± 80.52 vs 140.51 g/week ± 107.88; p=0.005). These results were corroborated by the observed association between levels of procollagen type III N-terminal peptide, a marker for steatohepatitis, and NAFLD, NAFLD grade, and intakes of fructose and total calories in this cohort.
Likewise, children and adolescents with obesity exhibiting histological evidence of NASH reported significantly higher fructose consumption compared with those without NASH54. Children and adolescents with NASH also had significantly higher rates of hyperuricemia and higher uric acid concentration than children without NASH. Logistic regression analysis showed that fructose consumption (OR 1.61, 95% CI: 1.25–1.86); p=0.001) and uric acid concentration (OR=2.49, 95% CI: 1.87–2.83; p=0.004) were independently associated with NASH. Fructose consumption was also associated with uric acid concentration in Italian children and adolescents with obesity (OR=2.02, 95% CI: 1.66–2.78; p=0.01). High dietary fructose consumption was associated with steatosis, inflammation, and fibrosis in both periportal and perivenous zones, even after adjustment for BMI, HOMA-IR (homeostatic assessment model for insulin resistance), triglyceride levels, and uric acid concentration55. Uric acid concentration was associated with steatosis and fibrosis in both zones, but with inflammation only in the periportal zone. Increasing evidence suggests that pediatric NAFLD is different than adult NAFLD and may involve greater periportal injury7, 56. Increased dietary fructose coupled with hyperuricemia might cause greater damage in the periportal zone, and therefore, it is possible that these two components may contribute to the different pattern of lobular disease observed in pediatric NAFLD. A follow-up study from this group reported that daily fructose consumption increased with severity of fibrosis and was associated with higher odds of high fibrosis degree57.
In contrast to these findings, Nier et al58 did not observe significant differences in serum uric acid concentration between overweight children with and without NAFLD. However, fructose intake was significantly higher in children with NAFLD compared to those without, and these children consumed significantly more calories in the form of sugar-sweetened beverages than overweight children without NAFLD (208 ±35 kcal/day vs 132 ±10 kcal/day; p<0.05).
Effects of dietary fructose feeding or restriction on parameters of pediatric NAFLD
A number of studies have investigated the effects of fructose feeding or fructose restriction on NAFLD and related metabolic measures in children and adolescents (Table 2). One of the first studies used a two-day crossover feeding design to evaluate whether fructose- or glucose-sweetened beverage altered plasma lipids differentially in children with NAFLD compared to healthy counterparts59. In both healthy children and those with NAFLD, fructose-sweetened beverage consumption produced higher triglyceride incremental area under the curve (IAUC) compared to glucose-sweetened beverage; however, the response was greater in children with NAFLD. In all children, fasting plasma glucose levels were significantly higher under fructose feeding conditions compared to glucose feeding, and only fructose-sweetened beverages resulted in decreased postprandial and overnight levels of HDL-cholesterol. Interestingly, children with NAFLD responded to fructose beverages with an acute increase in plasma endotoxin levels, resulting in a higher IAUC at nine hours compared to healthy controls, an effect not observed with glucose beverage ingestion60. Although the amount of fructose/glucose used in this challenge was high, and the duration of the treatment period and sample size were limited, these results demonstrated that dietary fructose produced metabolic effects in healthy children and, to a greater extent, children with NAFLD.
Table 2.
Studies demonstrating effects of fructose feeding or restriction on pediatric NAFLD and/or related parameters
| Study population | Study design | Intervention | Main findings | Ref |
|---|---|---|---|---|
| 9 NAFLD/10 matched controls | 2-day randomized crossover feeding | Glucose (GB) or fructose beverage (FB)1 |
|
59 |
| 8 NAFLD/7 matched controls | 24-hour randomized crossover feeding | GB or FB1 |
|
60 |
| 16 NAFLD | 4-week randomized, controlled trial | GB or FB2 |
|
60 |
| 21 NAFLD | 4-week randomized, controlled, double-blinded trial | GB or FB2 |
|
61 |
| 9 NAFLD/6 controls with obesity/9 lean controls | Cross-sectional | Oral fructose challenge3 |
|
34 |
| 9 adolescents with NAFLD/13 lean controls | 3-, 6-month, cross-sectional, dietary intervention | FRAGILE diet4 |
|
62 |
| 41 children with obesity, 25 with high liver fat | 9-day randomized, controlled trial | Study-provided meals substituting starch for sugar5 |
|
63 |
| 13 NAFLD | 1-year nonrandomized | Nutritional counseling |
|
58 |
| 40 boys with NAFLD | 8-week randomized, clinical trial | Study-provided meals restricting sugar intake to <3% of daily calories or usual diet |
|
65 |
| 25 adolescents with obesity and NAFLD | 8-week randomized, clinical trial | Carbohydrate-restricted diet (CRD) vs fat-restricted diet (FRD) |
|
66 |
33% of total daily calories, isocaloric substitution;
33 g sugar, isocaloric;
1 g/kg based on ideal body weight, max 75g;
low fructose (<7% total energy intake), low glycemic index (45–55), and low glycemic load (<80);
sugar and fructose restricted to 10% and 4% of total energy intake
This group subsequently conducted a four-week randomized, controlled, double-blind beverage intervention study in overweight Hispanic adolescents with fatty liver, who were regular consumers of sweet beverages61. As in the first study, participants were provided either a fructose-only or glucose-only beverage. Children receiving the glucose beverage (i.e., fructose reduction) showed significant improvements in adipose insulin sensitivity and plasma levels of high sensitivity C-reactive protein, free fatty acids, and oxidized LDL (a measure of oxidative stress) at the end of the intervention. However, no significant changes in hepatic fat or in levels of ALT and AST were observed in either group.
The acute consumption of fructose during a 1g/kg oral challenge resulted in a differential metabolic effect in children with and without NAFLD34. Prior to fructose consumption, children with NAFLD exhibited higher mean serum uric acid levels compared to otherwise healthy children with obesity (7.5 ±1.4 vs 6.1 ± 1.6 mg/dL, P=0.04) or lean controls (7.5 ±1.4 vs 4.5 ±1.6 mg/dL; P=0.0003). These data may indicate a higher intake of dietary fructose35–37. Following the oral fructose challenge, serum uric acid levels, which were measured in 30-minute increments up to 360 minutes post-consumption, remained stable and were significantly higher in children with NAFLD at all time points compared with lean controls, and at 120 and 270 minutes compared to controls with obesity. The change in serum uric acid levels from baseline to peak was significantly higher in lean controls relative to children with NAFLD, and the AUC was also higher in the lean children. Participants with NAFLD had higher mean urinary uric acids concentrations at baseline and post-fructose administration compared to lean controls. Metabolically, children with NAFLD exhibited higher serum glucose levels in response to the fructose challenge compared with lean controls and a slower return to baseline levels compared to both groups of control individuals. Although serum fructose levels at baseline were similar among all groups, lean controls had higher fructose levels at 30 and 60 minutes compared to children with NAFLD and controls with obesity, and the change in fructose levels was significantly higher in lean controls compared to children with NAFLD. Baseline urine fructose was also similar among all groups; however, children with NAFLD had lower urinary fructose excretion following fructose ingestion relative to the control groups. A positive breath hydrogen test following fructose ingestion was observed in 67% of lean controls, 33% of controls with obesity, and 22% of NAFLD patients, and these results, combined with reduced urinary fructose excretion, suggest that children with NAFLD absorb fructose more efficiently34.
Mager et al62 investigated the effects of six months of a low fructose, low glycemic index, and low glycemic load diet on nine children with NAFLD (four with NASH and five with hepatic steatosis) and 13 healthy, lean children. No significant differences in measures related to adiposity were observed in either group over the intervention. However, children with NAFLD showed significant reductions in the percentage of body fat and skin fold measures, as well as systolic blood pressure, percentage body fat, levels of ALT and apo-B100, and HOMA-IR at three and six months of the intervention. The strongest effects were seen in children with NASH, who also had the highest intakes of fructose at baseline. In contrast, no major changes in plasma biochemical markers or body composition were observed in lean children over the intervention, despite levels of fructose consumption comparable with those of children with NAFLD, suggesting that the context of adiposity may be necessary for the manifestation of metabolic consequences from high fructose consumption. However, these results also indicate that reductions of dietary fructose may be more important than weight loss, at least in the short term, for improving markers of liver dysfunction in children with NAFLD.
In a nine-day intervention of isocaloric substitution of starch for sugar, in which total sugar and fructose decreased from 28% to 10% and from 12% to 4% of total energy intake, respectively, significant reductions in liver fat (7.1% to 3.8%) and visceral adipose tissue (123 cm3 to 110 cm3) were observed in children with obesity who reported habitually high (>50g/day) sugar consumption63. Furthermore, insulin sensitivity, insulin clearance, and calculated insulin secretion significantly improved following intervention, and these effects remained significant even after adjusting for weight change. In addition, fractional DNL decreased significantly after nine days of fructose restriction and the DNL-AUC was significantly lower following the intervention (68.4 vs 29.7). DNL-AUC was not significantly different between individuals with high or lower liver fat at baseline, and decreased similarly in both groups. However, at the end of the study, DNL-AUC was significantly lower in the low liver fat group compared to the high liver fat group. Together, these findings suggest that fructose restriction exerts specific effects on liver fat that may be mediated by reduced DNL and are consistent with results from an independent study in which healthy adults showed higher DNL and liver fat during high fructose feeding compared to low fructose feeding64.
Similarly, adolescent boys with histologically diagnosed NAFLD administered a diet low in free sugars (i.e., 1% of daily calories) exhibited significantly greater improvements in hepatic steatosis after eight weeks compared to those eating a usual diet (~10% of daily calories supplied by free sugars)65. At the end of the dietary intervention, mean weight and mean levels of ALT, AST, GGT, and total cholesterol were significantly lower in the low sugar group compared to the usual diet group, although there were no significant differences in glucose, insulin, HOMA-IR, triglycerides, LDLC, or HDLC between the two groups. After adjusting for changes in weight, the reduction in hepatic fat fraction remained significant between the two groups. Despite significant reductions in hepatic fat, it is important to note that neither hepatic steatosis nor ALT were normalized following the relatively short 8-week intervention period. A recent study of adolescents with obesity and NAFLD randomized to either a carbohydrate- or fat-restricted diet observed significantly reduced hepatic fat fraction only in the carbohydrate-restricted group, although absolute changes in hepatic lipid content did not differ by diet66. Further, the carbohydrate-restricted group experienced significantly greater reductions in insulin resistance, abdominal fat mass, and body fat mass compared to the fat-restricted group.
Conclusions
NAFLD is a growing problem in the pediatric population. The studies summarized in this review provide compelling evidence that excessive fructose intake contributes to NAFLD pathogenesis in susceptible children and adolescents, and that restriction of fructose consumption may ameliorate the effects on hepatic steatosis and related metabolic and biochemical parameters. Furthermore, the consequences of excessive fructose intake and the benefits of fructose reduction may be independent of other cardiometabolic risk factors among children and adolescents at high risk for obesity-related liver disease. These findings are important, as high fructose is associated with more severe liver disease in adults67 and multiple studies have demonstrated that children and adolescents with NASH have higher fructose consumption compared to those with less severe fatty liver disease54, 62. Pediatric NASH patients have also been found to have higher uric acid levels and higher rates of hyperuricemia than those without NASH, and a strong association between uric acid concentration and histologic severity has been reported46, 54. Further, high dietary fructose intake and high uric acid levels may promote greater injury in the periportal zone and may contribute to the different pattern of lobular disease observed in pediatric NAFLD patients55. Additional studies to uncover both the short- and long-term effects of high fructose consumption, and possibly uric acid exposure, in children and adolescents are warranted to better understand NAFLD pathogenesis in this vulnerable population.
In conclusion, while there remains much to be learned about the effects of dietary fructose on the molecular mechanisms contributing to NAFLD pathogenesis, efforts to reduce global consumption of added sugars in the diet would most certainly yield a positive impact on overall health of children and adolescents. Dietary fructose restriction represents a particularly appealing target for health promotion and prevention of NAFLD due to its relative simplicity and focus on a single behavior. An emphasis on diet quality may also lead to improved dietary adherence over the long term. Given the overall positive impact of lifestyle modifications in the management of pediatric NAFLD, modulation of added sugar consumption may represent an important, early opportunity to mitigate or prevent NAFLD in this susceptible population.
Acknowledgments
Both authors were involved in the writing of the paper and had final approval of the submitted and published versions. This work was supported by grants from the National Institutes of Health, through the National Institute of Diabetes and Digestive and Kidney Disease (R01 DK107735, R01 DK120890, and R01 DK10757901).
References
- 1.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. February 2017;64(2):319–334. doi: 10.1097/MPG.0000000000001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. October 2006;118(4):1388–93. doi: 10.1542/peds.2006-1212 [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. November 2016;64(5):1577–1586. doi: 10.1002/hep.28785 [DOI] [PubMed] [Google Scholar]
- 4.Seth A, Orkin S, Yodoshi T, et al. Severe obesity is associated with liver disease severity in pediatric non-alcoholic fatty liver disease. Pediatric Obesity. 2020;15(2):e12581. doi: 10.1111/ijpo.12581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleischman MW, Budoff M, Zeb I, Li D, Foster T. NAFLD prevalence differs among hispanic subgroups: the Multi-Ethnic Study of Atherosclerosis. World J Gastroenterol. May 7 2014;20(17):4987–93. doi: 10.3748/wjg.v20.i17.4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallwitz ER, Daviglus ML, Allison MA, et al. Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/Latino individuals differs by heritage. Clin Gastroenterol Hepatol. March 2015;13(3):569–76. doi: 10.1016/j.cgh.2014.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. September 2005;42(3):641–9. doi: 10.1002/hep.20842 [DOI] [PubMed] [Google Scholar]
- 8.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, Network NCR. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. March 2011;53(3):810–20. doi: 10.1002/hep.24127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldner D, Lavine JE. Nonalcoholic Fatty Liver Disease in Children: Unique Considerations and Challenges. Gastroenterology. May 2020;158(7):1967–1983 e1. doi: 10.1053/j.gastro.2020.01.048 [DOI] [PubMed] [Google Scholar]
- 10.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. November 2009;58(11):1538–44. doi: 10.1136/gut.2008.171280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conjeevaram Selvakumar PK, Kabbany MN, Lopez R, Rayas MS, Lynch JL, Alkhouri N. Prevalence of Suspected Nonalcoholic Fatty Liver Disease in Lean Adolescents in the United States. J Pediatr Gastroenterol Nutr. July 2018;67(1):75–79. doi: 10.1097/MPG.0000000000001974 [DOI] [PubMed] [Google Scholar]
- 12.Xanthakos SA, Lavine JE, Yates KP, et al. Progression of Fatty Liver Disease in Children Receiving Standard of Care Lifestyle Advice. Gastroenterology. July 23 2020;doi: 10.1053/j.gastro.2020.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. September 2002;97(9):2460–2. doi: 10.1111/j.1572-0241.2002.06003.x [DOI] [PubMed] [Google Scholar]
- 14.Kohli R, Boyd T, Lake K, et al. Rapid progression of NASH in childhood. J Pediatr Gastroenterol Nutr. 2010;50(4):453–456. doi: 10.1097/MPG.0b013e3181a9387b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holterman A-XL, Guzman G, Fantuzzi G, et al. Nonalcoholic fatty liver disease in severely obese adolescent and adult patients. Obesity. 2013;21(3):591–597. doi: 10.1002/oby.20174 [DOI] [PubMed] [Google Scholar]
- 16.Schwimmer JB, Celedon MA, Lavine JE, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology. May 2009;136(5):1585–92. doi: 10.1053/j.gastro.2009.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goffredo M, Caprio S, Feldstein AE, et al. Role of TM6SF2 rs58542926 in the pathogenesis of nonalcoholic pediatric fatty liver disease: A multiethnic study. Hepatology. January 2016;63(1):117–25. doi: 10.1002/hep.28283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Hua W, Ji C, et al. Effect of the patatin-like phospholipase domain containing 3 gene (PNPLA3) I148M polymorphism on the risk and severity of nonalcoholic fatty liver disease and metabolic syndromes: A meta-analysis of paediatric and adolescent individuals. Pediatric Obesity. 2020;15(6):e12615. doi: 10.1111/ijpo.12615 [DOI] [PubMed] [Google Scholar]
- 19.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature genetics. December 2008;40(12):1461–5. doi: 10.1038/ng.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goran MI, Walker R, Le KA, et al. Effects of PNPLA3 on liver fat and metabolic profile in Hispanic children and adolescents. Diabetes. December 2010;59(12):3127–30. doi: 10.2337/db10-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis JN, Le KA, Walker RW, et al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr. December 2010;92(6):1522–7. doi: 10.3945/ajcn.2010.30185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santoro N, Zhang CK, Zhao H, et al. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. March 2012;55(3):781–9. doi: 10.1002/hep.24806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanislawski MA, Shaw J, Litkowski E, et al. Genetic Risk for Hepatic Fat among an Ethnically Diverse Cohort of Youth: The Exploring Perinatal Outcomes among Children Study. J Pediatr. May 2020;220:146–153 e2. doi: 10.1016/j.jpeds.2020.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parry SA, Hodson L. Influence of dietary macronutrients on liver fat accumulation and metabolism. J Investig Med. December 2017;65(8):1102–1115. doi: 10.1136/jim-2017-000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. July 9 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr. September 2011;94(3):726–34. doi: 10.3945/ajcn.111.018366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustig RH, Mulligan K, Noworolski SM, et al. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity (Silver Spring). February 2016;24(2):453–60. doi: 10.1002/oby.21371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebbeling CB, Feldman HA, Chomitz VR, et al. A Randomized Trial of Sugar-Sweetened Beverages and Adolescent Body Weight. New England Journal of Medicine. 2012;367(15):1407–1416. doi: 10.1056/NEJMoa1203388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol. May 2018;68(5):1063–1075. doi: 10.1016/j.jhep.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson RJ, Perez-Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. February 2009;30(1):96–116. doi: 10.1210/er.2008-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. October 2013;62(10):3307–15. doi: 10.2337/db12-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao X, Qi L, Qiao N, et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. August 2007;50(2):306–12. doi: 10.1161/HYPERTENSIONAHA.107.091041 [DOI] [PubMed] [Google Scholar]
- 33.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond). March 2010;34(3):454–61. doi: 10.1038/ijo.2009.259 [DOI] [PubMed] [Google Scholar]
- 34.Sullivan JS, Le MT, Pan Z, et al. Oral fructose absorption in obese children with non-alcoholic fatty liver disease. Pediatr Obes. June 2015;10(3):188–95. doi: 10.1111/ijpo.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox CL, Stanhope KL, Schwarz JM, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond). July 24 2012;9(1):68. doi: 10.1186/1743-7075-9-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin WT, Huang HL, Huang MC, et al. Effects on uric acid, body mass index and blood pressure in adolescents of consuming beverages sweetened with high-fructose corn syrup. Int J Obes (Lond). April 2013;37(4):532–9. doi: 10.1038/ijo.2012.121 [DOI] [PubMed] [Google Scholar]
- 37.Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. June 2013;57(6):2525–31. doi: 10.1002/hep.26299 [DOI] [PubMed] [Google Scholar]
- 38.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. January 15 2008;59(1):109–16. doi: 10.1002/art.23245 [DOI] [PubMed] [Google Scholar]
- 39.Softic S, Stanhope KL, Boucher J, et al. Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci. August 2020;57(5):308–322. doi: 10.1080/10408363.2019.1711360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russo E, Leoncini G, Esposito P, Garibotto G, Pontremoli R, Viazzi F. Fructose and Uric Acid: Major Mediators of Cardiovascular Disease Risk Starting at Pediatric Age. Int J Mol Sci. June 24 2020;21(12)doi: 10.3390/ijms21124479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. June 2008;138(6):1039–46. doi: 10.1093/jn/138.6.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. June 2007;85(6):1511–20. doi: 10.1093/ajcn/85.6.1511 [DOI] [PubMed] [Google Scholar]
- 43.Jones RB, Alderete TL, Kim JS, Millstein J, Gilliland FD, Goran MI. High intake of dietary fructose in overweight/obese teenagers associated with depletion of Eubacterium and Streptococcus in gut microbiome. Gut Microbes. 2019;10(6):712–719. doi: 10.1080/19490976.2019.1592420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Softic S, Meyer JG, Wang GX, et al. Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-translational Modifications of Mitochondrial Proteins. Cell Metab. October 1 2019;30(4):735–753.e4. doi: 10.1016/j.cmet.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mager DR, Patterson C, So S, Rogenstein CD, Wykes LJ, Roberts EA. Dietary and physical activity patterns in children with fatty liver. Eur J Clin Nutr. June 2010;64(6):628–35. doi: 10.1038/ejcn.2010.35 [DOI] [PubMed] [Google Scholar]
- 46.Vos MB, Colvin R, Belt P, et al. Correlation of vitamin E, uric acid, and diet composition with histologic features of pediatric NAFLD. J Pediatr Gastroenterol Nutr. January 2012;54(1):90–6. doi: 10.1097/MPG.0b013e318229da1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Sullivan TA, Oddy WH, Bremner AP, et al. Lower fructose intake may help protect against development of nonalcoholic fatty liver in adolescents with obesity. J Pediatr Gastroenterol Nutr. May 2014;58(5):624–31. doi: 10.1097/MPG.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 48.Bray GA. How bad is fructose? Am J Clin Nutr. October 2007;86(4):895–6. doi: 10.1093/ajcn/86.4.895 [DOI] [PubMed] [Google Scholar]
- 49.Bjorkman O, Felig P. Role of the kidney in the metabolism of fructose in 60-hour fasted humans. Diabetes. June 1982;31(6 Pt 1):516–20. doi: 10.2337/diab.31.6.516 [DOI] [PubMed] [Google Scholar]
- 50.Heinz F, Schlegel F, Krause PH. Enzymes of fructose metabolism in human small intestine mucosa. Enzyme. 1975;19(2):93–101. doi: 10.1159/000458978 [DOI] [PubMed] [Google Scholar]
- 51.Jang C, Hui S, Lu W, et al. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell metabolism. February 6 2018;27(2):351–361 e3. doi: 10.1016/j.cmet.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nair S, V PC, Arnold C, Diehl AM. Hepatic ATP reserve and efficiency of replenishing: comparison between obese and nonobese normal individuals. Am J Gastroenterol. February 2003;98(2):466–70. doi: 10.1111/j.1572-0241.2003.07221.x [DOI] [PubMed] [Google Scholar]
- 53.Hamza RT, Ahmed AY, Rezk DG, Hamed AI. Dietary fructose intake in obese children and adolescents: relation to procollagen type III N-terminal peptide (P3NP) and non-alcoholic fatty liver disease. J Pediatr Endocrinol Metab. December 1 2016;29(12):1345–1352. doi: 10.1515/jpem-2016-0015 [DOI] [PubMed] [Google Scholar]
- 54.Mosca A, Nobili V, De Vito R, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. May 2017;66(5):1031–1036. doi: 10.1016/j.jhep.2016.12.025 [DOI] [PubMed] [Google Scholar]
- 55.Nobili V, Mosca A, De Vito R, Raponi M, Scorletti E, Byrne CD. Liver zonation in children with non-alcoholic fatty liver disease: Associations with dietary fructose and uric acid concentrations. Liver Int. June 2018;38(6):1102–1109. doi: 10.1111/liv.13661 [DOI] [PubMed] [Google Scholar]
- 56.Carter-Kent C, Yerian LM, Brunt EM, et al. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. October 2009;50(4):1113–20. doi: 10.1002/hep.23133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosca A, De Cosmi V, Parazzini F, et al. The Role of Genetic Predisposition, Programing During Fetal Life, Family Conditions, and Post-natal Diet in the Development of Pediatric Fatty Liver Disease. J Pediatr. August 2019;211:72–77 e4. doi: 10.1016/j.jpeds.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 58.Nier A, Brandt A, Conzelmann IB, Ozel Y, Bergheim I. Non-Alcoholic Fatty Liver Disease in Overweight Children: Role of Fructose Intake and Dietary Pattern. Nutrients. September 19 2018;10(9)doi: 10.3390/nu10091329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin R, Le NA, Liu S, et al. Children with NAFLD are more sensitive to the adverse metabolic effects of fructose beverages than children without NAFLD. J Clin Endocrinol Metab. July 2012;97(7):E1088–98. doi: 10.1210/jc.2012-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin R, Willment A, Patel SS, et al. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int J Hepatol. 2014;2014:560620. doi: 10.1155/2014/560620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin R, Welsh JA, Le NA, et al. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients. August 8 2014;6(8):3187–201. doi: 10.3390/nu6083187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mager DR, Iniguez IR, Gilmour S, Yap J. The effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD). JPEN J Parenter Enteral Nutr. January 2015;39(1):73–84. doi: 10.1177/0148607113501201 [DOI] [PubMed] [Google Scholar]
- 63.Schwarz JM, Noworolski SM, Erkin-Cakmak A, et al. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children With Obesity. Gastroenterology. September 2017;153(3):743–752. doi: 10.1053/j.gastro.2017.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwarz JM, Noworolski SM, Wen MJ, et al. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J Clin Endocrinol Metab. June 2015;100(6):2434–42. doi: 10.1210/jc.2014-3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwimmer JB, Ugalde-Nicalo P, Welsh JA, et al. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. Jama. January 22 2019;321(3):256–265. doi: 10.1001/jama.2018.20579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goss AM, Dowla S, Pendergrass M, et al. Effects of a carbohydrate-restricted diet on hepatic lipid content in adolescents with non-alcoholic fatty liver disease: A pilot, randomized trial. Pediatr Obes. July 2020;15(7):e12630. doi: 10.1111/ijpo.12630 [DOI] [PubMed] [Google Scholar]
- 67.Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. June 2010;51(6):1961–71. doi: 10.1002/hep.23535 [DOI] [PMC free article] [PubMed] [Google Scholar]


