Abstract
Background:
Practice guidelines recommend urine drug monitoring (UDM) at least annually in the setting of chronic opioid therapy as an objective assessment of substance use. However, empirical evidence on who gets tested and how often testing occurs is lacking.
Objectives:
This study investigates 10-year UDM trends in the United States based on 2 factors: (1) the duration of prescription opioid treatment, and (2) having an opioid use disorder (OUD) diagnosis and medications for opioid use disorder (MOUD) prescriptions.
Study Design:
Observational cross-sectional study.
Setting:
Research was conducted using administrative claims data from Optum’s deidentified Clinformatics Data Mart Database for the period 2007 to 2016. The dataset contained information on the plan enrollment and health care claims from 50 states and the District of Columbia.
Methods:
To examine trends in UDM based on the duration of prescription opioid treatment, persons receiving prescription opioid analgesics were categorized into 4 groups based on the number of days covered: (a) less than 90 days, (b) 90 to 179 days, (c) 180 to 269 days, and (d) at least 270 days. To examine trends based on an OUD diagnosis and MOUD prescriptions, persons diagnosed with OUD were identified and categorized based on the presence of MOUD prescriptions as follows: (a) OUD with buprenorphine (BPN) and naltrexone (NTX) in the same year; (b) OUD with BPN only; (c) OUD with NTX only; (d) OUD with chronic prescription opioid analgesics (≥ 90 days); (e) OUD without prescription opioid analgesics, BPN, or NTX; and (f) chronic prescription opioid analgesics (≥ 90 days) without an OUD diagnosis. For analysis, the percent receiving UDM was estimated per group per year. Then the data were restricted to those receiving at least one UDM to estimate the average number of UDM per person.
Results:
Data included an average of 364,485 persons per year receiving prescription opioid analgesics for chronic use, and 10,277 per year receiving an OUD diagnosis. Among those receiving prescription opioid analgesics, less than 50% received UDM. For those receiving at least one UDM, one additional UDM was performed per person as the duration of opioids increased by 90 days. Among persons with OUD, the percent receiving UDM was the highest for those receiving both BPN and NTX (87%), followed by those receiving BPN only (80%), chronic opioids (79%), NTX only (72%), and those not receiving any MOUD/opioids (54%).
Limitations:
Methadone dispensing for OUD treatments was not captured in administrative claims data.
Conclusions:
Although recommended for patients with chronic pain, UDM is provided less than half of the time for these patients. However, once patients received at least one UDM, they would continue to receive it on a fairly regular basis. Compared with those with chronic pain, persons diagnosed with OUD are more likely to receive UDM at a more frequent interval.
Keywords: Urine drug monitoring, urine drug testing, urine drug screening, chronic pain, opioid use disorder, prescription opioid analgesic, buprenorphine, naltrexone, medications for opioid use disorder
Urine drug monitoring (UDM) has become an important component of chronic pain management in patients receiving opioid analgesic prescriptions. Beyond its use in workplaces or criminal justice system settings, UDM in health care settings has been widely discussed and recommended by clinicians and health care agencies (1–6). Using UDM, potential illicit substance use or prescription drug misuse/diversion can be detected, as patients may not otherwise report it to their health care provider. The adherence monitoring of patients receiving opioid analgesic prescriptions is important for identifying individuals who might be at higher risk for drug overdose or opioid use disorder (OUD) and offering appropriate health care interventions that ultimately lead to better health outcomes (7,8).
Published in early 2016, the Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain stated that clinicians should consider UDM at least annually when prescribing opioids for chronic pain (i.e., pain persisting > 3 months) (3). In the following year, the American Society of Interventional Pain Physician (ASIPP) published guidelines for opioid prescribing for chronic noncancer pain, in which urine drug testing is recommended as a means to monitor adherence, abuse, and noncompliance (5). In 2018, an interdisciplinary group of clinicians developed consensus recommendations in which more frequent UDM (≥3 times per year) is suggested for persons at a higher risk of overdose (e.g., history of overdose, history of substance use disorder, high dosage opioid prescription, or concurrent benzodiazepine use) (4).
However, it has been reported that clinicians might not consistently provide UDM. In a few studies conducted within individual practices and one in the VA health care system, the percentage of patients receiving UDM if they receive chronic opioid treatment varied from 18% to 63% (9–11). Clinicians may not choose to provide UDM because they do not have sufficient time allocated to discuss the rationale with the patient (12). Some clinicians reported they are not proficient at interpreting and applying test results (12,13). Importantly, with the exception of these few small scale studies, information on who gets tested and how often testing occurs is lacking.
Objectives
In this study, we examined UDM trends in a commercially insured population of the United States based on 2 potential driving factors: the duration of prescription opioid analgesic use and the presence of an OUD diagnosis and medications for opioid use disorder (MOUD) prescriptions.
Setting
We used administrative claims data from Optum’s deidentified Clinformatics Data Mart Database. The study data included information on the plan enrollment and health care claims of a deidentified sample from 50 states and the District of Columbia for the period 2007 to 2016. Data use for this research was approved by the institutional review board.
Methods
Persons who were older than age 18 years and continuously enrolled for 12 months in a given year were considered for the analysis. Patients were identified as receiving UDM if at least one of the following Current Procedural Terminology (CPT) or Healthcare Common Procedure Coding System (HCPCS) codes were present in the medical claim in a given year: 80100-80104, 80300-80307, 80348, 80354, 80356, 80358, 80361-80365, 80367, 80368, 80372-80377, G0431, G0434, G0477-G0483, G0659, and H0049. These codes were selected based on the Centers for Medicare & Medicaid Services (CMS) regulations and the CPT code descriptions (14,15). During the study period, changes in billing codes occurred. For example, the CPT codes of 80100-80104 were commonly used until they were deleted and replaced with G0431 and G0434 in 2015. Then, those 2 codes were also deleted in the following year and replaced with other codes listed above.
As the first factor, we counted the number of days covered with prescription opioids for each patient using the proportion of days covered approach (16). When calculating the number of days covered, if a patient filled multiple prescriptions for different opioid analgesic agents on different days, each day was counted only once even if there was overlap. Prescription opioid analgesics were identified using the American Hospital Formulary Service (AHFS) Pharmacologic-Therapeutic Classification 28:08.08 (opiate agonists) and 28:08:12 (opiate partial agonists). Buprenorphine (BPN) and its combination products were excluded as it is more commonly used for the treatment of OUD. Approximately 0.3% (10-year total n = 53,693) of patients receiving prescription opioid analgesics were affected by this criterion. Patients receiving prescription opioid analgesics were categorized into 4 groups based on the number of days covered: (a) less than 90 days, (b) 90 to 179 days, (c) 180 to 269 days, and (d) at least 270 days. Patients’ age and gender were summarized for each category.
As the second factor, we examined persons with an OUD diagnosis and their prescription claims for MOUD and opioid analgesic medications. We identified OUD diagnoses using International Classification of Diseases (ICD)-9 of 304.0X, 304.7X, 305.5X, 965.5X, E850.0X, E935.0X, and ICD-10 category of F11. Of those diagnosed with OUD, patients filling BPN (including BPN/naloxone combination) and/or naltrexone (NTX) prescriptions on the same day or after the OUD diagnosis were identified as receiving MOUD. In our pharmacy claims dataset, methadone was not considered as MOUD because when used for OUD treatment, methadone claims would not appear in the pharmacy benefit as it can only be dispensed by specially licensed opioid treatment programs per federal requirements (17). Based on the presence of an OUD diagnosis and prescription data, persons were categorized into 6 mutually exclusive groups. These groups were identified in the following order without replacement: (a) OUD with BPN and NTX in the same year; (b) OUD with BPN; (c) OUD with NTX; (d) OUD with chronic prescription opioid analgesics (≥ 90 days); (e) OUD without prescription opioid analgesics, BPN, or NTX; and (f) chronic prescription opioids (≥ 90 days) without an OUD diagnosis. Patients’ age and gender were summarized for each category.
We restricted the sample to those receiving at least one UDM and estimated the average number of UDM per person. Occasionally, some persons received definitive testing within a few days after presumptive testing. In this case, 2 or more UDM billing codes could appear a few days apart. For our analysis, we evaluated the UDM occurrence on a monthly basis, and billing codes appearing in the same month were considered as part of a single monitoring event.
Results
An average of 5.6 million persons per year were older than age 18 years and continuously enrolled for the 12-month period in our dataset. Of those, 364,485 persons per year received prescription opioid analgesics for chronic use (≥ 90 days). These patients were 62 years old on average and 60% were women. In each year, nearly half of persons receiving chronic opioid analgesic prescriptions were in the 270 days or more category. A detailed breakdown of the sample size is presented in Table 1.
Table 1.
Total number of persons and demographic characteristics by the duration of prescription opioids.
| Year | Enrolled for 12 Months (age > 18 years) | Duration of Prescription Opioids | Total Chronic Opioids (≥ 90 days) | |||
|---|---|---|---|---|---|---|
| < 90 days | 90–179 days | 180–269 days | ≥270 days | |||
| 2007 | 5,067,895 | 1,622,777 | 85,020 | 53,493 | 101,584 | 240,097 |
| 2008 | 5,395,390 | 1,650,254 | 89,976 | 55,470 | 120,763 | 266,209 |
| 2009 | 5,631,081 | 1,656,980 | 97,296 | 61,843 | 139,533 | 298,672 |
| 2010 | 5,627,286 | 1,625,806 | 105,403 | 68,352 | 159,217 | 332,972 |
| 2011 | 5,873,080 | 1,671,147 | 114,073 | 74,577 | 183,824 | 372,474 |
| 2012 | 5,955,673 | 1,673,791 | 120,292 | 80,416 | 205,656 | 406,364 |
| 2013 | 5,960,154 | 1,645,393 | 124,808 | 84,618 | 217,147 | 426,573 |
| 2014 | 5,345,676 | 1,436,341 | 114,637 | 78,762 | 201,688 | 395,087 |
| 2015 | 5,418,471 | 1,489,276 | 116,875 | 79,129 | 207,322 | 403,326 |
| 2016 | 5,571,260 | 1,650,445 | 139,490 | 97,072 | 266,511 | 503,073 |
| 10-year overall | ||||||
| Number of persons per year | 5,584,597 | 1,612,221 | 110,787 | 73,373 | 180,325 | 364,485 |
| Age, mean (SD) | 56 (18) | 51 (18) | 63 (15) | 63 (14) | 60 (14) | 62 (14) |
| Female, % | 56 | 56 | 61 | 62 | 59 | 60 |
Abbreviation: SD, standard deviation.
Among persons receiving prescription opioids, the percent receiving UDM steadily increased between 2007 and 2016 regardless of the duration of prescription opioid analgesic use (Fig. 1). The rate of increase was especially faster between 2015 and 2016, during which the slope of the graph increased across groups. When comparing between groups, the percent receiving UDM was the highest for those receiving opioid analgesics for at least 270 days as compared with those receiving opioid analgesics for relatively shorter durations. For example, 41% of the 270 days or more group received UDM in 2016, whereas 23% of the 180 to 269 days group, 15% of the 90 to 179 days group, and 3% of the less than 90 days group received UDM in the same year. Also, the rate of increase was faster for groups with a longer duration. The percent receiving UDM increased from 8% in 2007 to 41% in 2016 in the 270 days or more group (33% increase over 10 years), whereas it increased from 3% to 23% in the 180 to 269 days group (20% increase), 2% to 15% in the 90 to 179 days group (13% increase), and 0.4% to 3% in the less than 90 days group (2.6% increase). Among persons receiving at least one UDM, those in the more than 270 days group received UDM an average of 3 times in 2016. In the same year, those in the 180 to 269 days group received UDM twice, and those in the 90 to 179 days group and less than 90 days group received it once, on average (Table 2).
Fig. 1.
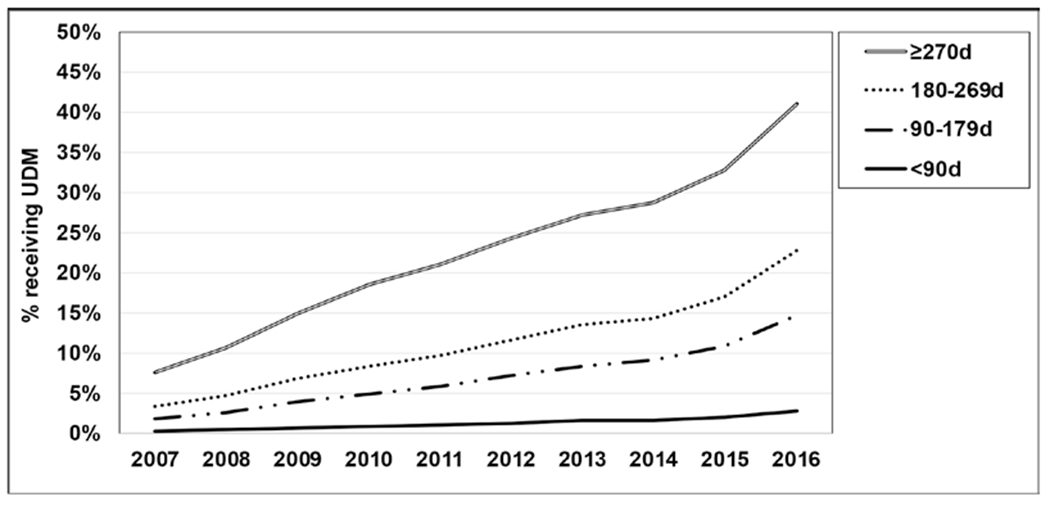
Percentage of persons receiving UDM by the duration of prescription opioids.
Table 2.
Average number of UDM per person and standard deviation.
| Year | Duration of Prescription Opioids | |||
|---|---|---|---|---|
| < 90 days | 90–179 days | 180–269 days | ≥ 270 days | |
| 2007 | 1.0 (2.1) | 1.0 (1.7) | 1.0 (1.7) | 1.0 (1.8) |
| 2008 | 1.0 (1.9) | 1.0 (1.6) | 1.0 (1.6) | 1.0 (1.7) |
| 2009 | 1.0 (2.0) | 1.0 (1.6) | 1.0 (2.0) | 2.0 (2.0) |
| 2010 | 1.0 (2.0) | 1.0 (1.7) | 1.0 (1.7) | 2.0 (2.3) |
| 2011 | 1.0 (2.4) | 1.0 (2.2) | 1.0 (2.0) | 2.0 (2.4) |
| 2012 | 1.0 (3.0) | 1.0 (1.9) | 2.0 (2.4) | 2.0 (2.7) |
| 2013 | 1.0 (2.8) | 1.0 (2.0) | 2.0 (2.4) | 2.0 (2.9) |
| 2014 | 1.0 (3.3) | 1.0 (2.1) | 2.0 (2.3) | 2.0 (2.9) |
| 2015 | 1.0 (3.5) | 1.0 (2.2) | 2.0 (2.2) | 3.0 (2.9) |
| 2016 | 1.0 (3.3) | 1.0 (2.0) | 2.0 (2.5) | 3.0 (3.0) |
The numbers of persons with an OUD diagnosis based on different MOUD/opioid analgesic prescriptions are presented in Table 3. The number of persons diagnosed with OUD increased over time in all categories. In 2016, there were 225 receiving both BPN and NTX (a 7-fold increase from 2007), 7,135 receiving BPN (a 4-fold increase from 2007), 1,031 receiving NTX (a 13-fold increase from 2007), and 10,179 receiving chronic prescription opioid analgesics without BPN or NTX (an 11-fold increase from 2007). Also, 3,228 persons with OUD did not have a dispensing record of BPN, NTX, or opioids (a 6-fold increase from 2007). Overall, persons with an OUD diagnosis were age 44 years on average and 46% were women.
Table 3.
Total number of persons and demographic characteristics by the presence of OUD diagnosis and MOUD prescription.
| Year | With OUD Diagnosis (age > 18 years) | OUD & BPN+NTX | OUD & BPN | OUD & NTX | OUD & Chronic Opioids | OUD & No Opioids/MOUD | No OUD & Chronic Opioids |
|---|---|---|---|---|---|---|---|
| 2007 | 3,247 | 32 | 1,631 | 80 | 939 | 565 | 239,158 |
| 2008 | 4,231 | 33 | 2,293 | 124 | 1,243 | 538 | 264,966 |
| 2009 | 5,834 | 42 | 3,214 | 146 | 1,814 | 618 | 296,858 |
| 2010 | 6,921 | 70 | 3,721 | 243 | 2,189 | 698 | 330,783 |
| 2011 | 8,896 | 111 | 4,787 | 266 | 2,755 | 977 | 369,719 |
| 2012 | 11,251 | 150 | 5,993 | 332 | 3,587 | 1,189 | 402,777 |
| 2013 | 13,307 | 190 | 6,577 | 431 | 4,674 | 1,435 | 421,899 |
| 2014 | 12,876 | 150 | 5,786 | 472 | 4,930 | 1,538 | 390,157 |
| 2015 | 14,395 | 186 | 5,632 | 650 | 5,545 | 2,382 | 397,781 |
| 2016 | 21,798 | 225 | 7,135 | 1,031 | 10,179 | 3,228 | 492,894 |
| 10-year overall | |||||||
| Number of persons per year | 10,276 | 119 | 4,677 | 378 | 3,786 | 1,317 | 360,699 |
| Age, mean (SD) | 44 (18) | 32 (13) | 39 (13) | 37 (15) | 59 (14) | 42 (20) | 62 (14) |
| Female, % | 46 | 40 | 38 | 44 | 58 | 42 | 60 |
Abbreviation: SD, standard deviation.
The percent receiving UDM increased between 2007 and 2016 among persons diagnosed with OUD, regardless of the presence of MOUD or opioid analgesic prescriptions (Fig. 2). When comparing between groups, in 2016, the percent receiving UDM was the highest among the persons receiving both BPN and NTX (87%), followed by persons receiving BPN (80%), chronic opioid analgesics (79%), NTX (72%), those not receiving BPN/NTX/opioid (54%), and those receiving chronic opioid analgesics without an OUD diagnosis (29%). Among persons receiving at least one UDM, those receiving BPN or chronic opioid analgesics received UDM 5 times in 2016, on average. In the same year, persons receiving BPN or NTX received UDM 4 times, and those receiving NTX received it 3 times, on average (Table 4).
Fig. 2.
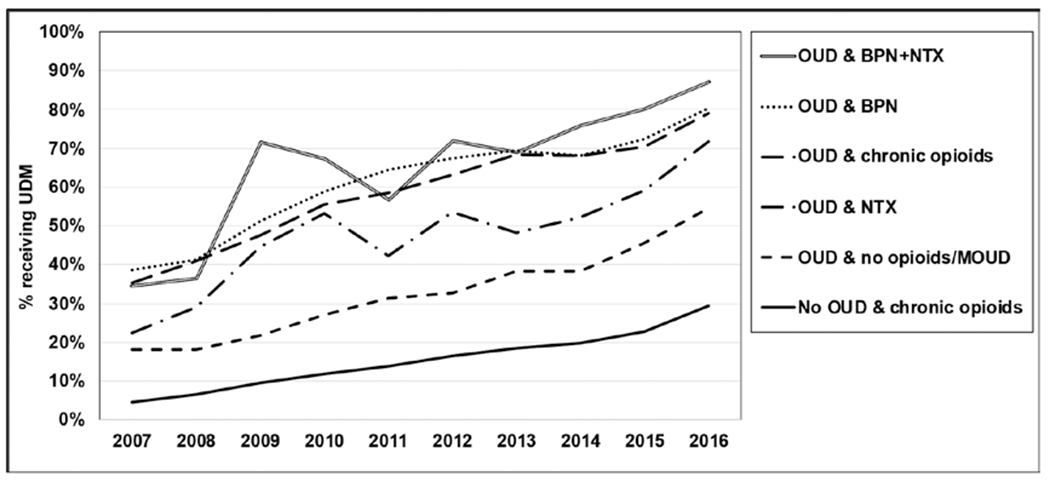
Percentage of persons receiving UDM by the presence of OUD diagnosis and MOUD prescription.
Table 4.
Average number of UDM per person and standard deviation.
| Year | OUD & BPN + NTX | OUD & BPN | OUD & NTX | OUD & Chronic Opioids | OUD & No Opioids/MOUD | No OUD & Chronic Opioids |
|---|---|---|---|---|---|---|
| 2007 | 2.0 (1.6) | 2.0 (2.2) | 3.0 (3.6) | 2.0 (3.5) | 2.0 (2.6) | 2.0 (7.2) |
| 2008 | 2.0 (2.1) | 2.0 (2.4) | 1.0 (1.4) | 3.0 (4.0) | 2.0 (2.3) | 2.0 (5.5) |
| 2009 | 2.0 (2.4) | 3.0 (2.8) | 2.0 (2.3) | 4.0 (5.0) | 2.0 (2.1) | 2.0 (4.7) |
| 2010 | 3.0 (2.4) | 3.0 (2.9) | 2.0 (2.2) | 3.0 (4.2) | 2.0 (2.2) | 2.0 (4.3) |
| 2011 | 2.0 (1.9) | 3.0 (3.0) | 2.0 (2.4) | 4.0 (5.0) | 2.0 (2.3) | 2.0 (4.1) |
| 2012 | 3.0 (2.4) | 4.0 (3.2) | 2.0 (2.1) | 4.0 (5.2) | 2.0 (2.5) | 2.0 (4.0) |
| 2013 | 4.0 (3.2) | 4.0 (3.4) | 2.0 (2.0) | 4.0 (5.3) | 2.0 (2.3) | 3.0 (3.9) |
| 2014 | 4.0 (2.7) | 4.0 (3.4) | 3.0 (2.6) | 4.0 (4.9) | 2.0 (2.4) | 3.0 (3.9) |
| 2015 | 4.0 (2.7) | 4.0 (3.4) | 3.0 (2.5) | 4.0 (5.0) | 2.0 (2.5) | 3.0 (3.8) |
| 2016 | 4.0 (3.0) | 5.0 (3.6) | 3.0 (2.7) | 5.0 (4.9) | 2.0 (2.7) | 3.0 (3.8) |
Discussion
This study examined the percentage of persons receiving UDM for the period 2007 to 2016 based on 2 potential driving factors: (1) duration of prescription opioid analgesics, and (2) presence of an OUD diagnosis and MOUD prescriptions. Our data suggest that persons receiving prescription opioid analgesics 270 or more days per year and persons with an OUD diagnosis are more likely to receive UDM. Among persons diagnosed with OUD, over 70% of those receiving BPN, NTX, or chronic opioid analgesics received UDM in 2016. Among persons receiving opioid analgesic prescriptions without an OUD diagnosis, the percentage of those receiving UDM increased as the duration of opioid analgesic prescriptions increased.
UDM is used as a tool to ensure the safe use of prescription opioids (3,5,18) and to promote patient concordance with OUD treatment (7,19). Per guidelines, UDM is recommended at least annually for patients receiving chronic opioid prescriptions (3–5). In real practice, our data suggest that UDM occurred less than half of the time for these patients. However, if patients received UDM, they appeared to receive it on a fairly regular basis. Among those receiving UDM, one additional UDM was performed per person as the duration of opioids increased by 90 days.
Despite the benefits of UDM, experts also caution that UDM results could be misinterpreted or false positive, and in which case, it could result in harming patients with stigmatization or inappropriate discontinuation of therapy (5,6,20). This could potentially explain the low rate of UDM (< 50%) observed in patients receiving chronic prescription opioid analgesics. The cost of having an inaccurate result could be substantial from the clinicians’ standpoint if it negatively affects the patient-provider relationship (13). As a response to this concern, in 2018, definitive testing was recommended to be used exclusively for the purpose of UDM as opposed to the conventional approach in which presumptive testing is first used to determine whether definitive testing is needed (4). This recommendation is based on the fact that definitive testing is more accurate and less likely to generate false-positive results. Although our article did not differentiate definitive testing from presumptive testing because the main objective was to examine the provision of UDM of any type, future investigations might need to consider the rate of definitive testing and the potential outcomes (i.e., risk mitigation).
Although it remained less than 50%, the percent receiving UDM steadily increased over time during the period 2007 to 2016 (Fig. 1). Interestingly, the rate of increase was particularly faster from 2015 to 2016 (33% to 41% in the ≥ 270 days group). This increase coincides with the publishing of the CDC Guideline for Prescribing Opioids for Chronic Pain in early 2016, in which UDM was recommended for persons receiving chronic prescription opioid analgesics. Although multiple factors likely affected the trend, the CDC guideline appears to be associated with the increased UDM in practice. It is possible that the effort to promote responsible prescribing of opioids was prevalent among health care providers at the time the CDC guideline was published, and it accelerated the adoption of UDM.
Our data reflect the increasing trend of OUD diagnosis, which is consistent with previously reported trends (21–23). In this study, it was observed that the number of persons diagnosed with OUD increased more than 6-fold, from 0.6 per 1,000 enrollees in 2007 to 3.9 in 2016 (these estimates were derived from Table 3). The rapid distribution of heroin and illicitly manufactured synthetic opioids, such as fentanyl, has been discussed as contributing factors to the trend (24). It is also possible that the enactment of the Affordable Care Act (ACA) in 2010 resulted in increased coverage for individuals with OUD and therefore promoted the identification of those patients (25). In fact, the increase in the number of enrollments after 2010 was also observed in our dataset (Table 1). Interestingly, as the number of persons diagnosed with OUD increased, the percent receiving UDM drastically increased. For example, 80% of patients with BPN received UDM in 2016, as compared with 38% in 2007 (Fig. 2). Also, UDM was generally more common for persons diagnosed with OUD as compared with those without the diagnosis, as at least half of them received UDM even if they did not receive BPN or NTX prescriptions. Patients diagnosed with OUD may be more receptive to UDM as a part of the treatment process and clinicians do not need to take extra time to discuss the rationale for testing. It is because patients with OUD are more likely to be knowledgeable about the risk of overdose and existing prevention strategies (26,27). However, persons taking prescription opioids for chronic pain who are not diagnosed with OUD are often less aware of opioid overdose risk factors and therefore engaging patients to UDM might be perceived as a barrier by clinicians as they fear about offending patients (12,13).
In our study, only a fraction of persons diagnosed with OUD received prescriptions for BPN or NTX (38% in 2016). Instead, a majority of those with OUD received opioid analgesic prescriptions on a chronic basis without BPN/NTX or did not have a dispensing record of BPN, NTX, or opioid. A steep increase was observed in the number of persons receiving NTX between 2015 and 2016, which is potentially contributed to the CDC guidelines recommending NTX for the treatment of OUD (3). However, despite the increase, the number of persons receiving NTX took up less than 6% of those diagnosed with OUD in 2016. It is possible that these persons were not able to use third-party coverage for MOUD due to limited insurance coverage or the provider not accepting insurance for OUD treatment. If so, patients may pay for MOUD out-of-pocket or may not receive the treatment. In either case, access to OUD treatment for these patients is reduced as compared with those using third-party coverage for OUD treatment. This could result in a less frequent visit to the provider’s office, and they would be less likely to receive UDM. This trend could potentially explain our finding in which a higher percentage of persons received UDM if they received prescriptions for BPN, NTX, or opioids after being diagnosed with OUD, as compared with those not receiving these prescriptions while having OUD.
Limitations
Our study has several limitations. First, access to methadone treatment and other services including UDM for persons with OUD remains unexplained because methadone dispensing for OUD treatment is not captured in administrative claims data. Methadone has been used for OUD treatment for a long time, but it is frequently excluded from coverage (28–30). Also, unlike BPN or NTX, which is dispensed in retail pharmacies, methadone is subject to the separate federal requirement and it must be dispensed by licensed opioid treatment programs (17). Future studies with methadone utilization data may provide a more complete picture of patient care and OUD treatment. Second, our study data samples primarily consisted of individuals who were enrolled in a commercial health plan in the United States. Therefore the findings of this study may not be generalizable to other populations, such as individuals who are insured through a public third-party payer or those without health insurance. Third, this study investigated UDM trends based only on the duration of prescription opioid treatment and the presence of an OUD diagnosis and MOUD prescriptions. We focused on these factors because they are primarily considered in practice guidelines when making recommendations regarding UDM (3,5,31). However, it is highly likely that trends in UDM are also associated with other factors, such as types of prescription opioid analgesics prescribed, dosing amount, and whether the patient takes multiple opioids. Exploring these factors would further explain details of UDM trends.
Conclusions
To practice responsible prescribing and mitigate the risk of opioid overdose and OUD, UDM is recommended for patients receiving prescription opioid analgesics for chronic pain. Between 2007 and 2016, the percent receiving UDM steadily increased for these patients; however, despite the increasing trend, it remained less than 50% during the study period. However, UDM was more commonly provided to patients diagnosed with OUD. Also, if patients received UDM, they would receive it on a fairly regular basis (at least once in 90 days, on average). Our study suggests that interventions to help patients perceive UDM as a natural part of treatment could potentially improve its utilization.
Disclaimer:
This work was supported by the National Institute on Drug Abuse [R01 DA039928].
Footnotes
Conflict of interest: Each author certifies that he or she, or a member of his or her immediate family, has no commercial association (i.e., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted manuscript.
References
- 1.Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc 2008; 83:66–76. [DOI] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration. Clinical drug testing in primary care. Technical Assistance Publication (TAP) 32. HHS Publication No. (SMA) 12-4668. Rockville, MD. www.samhsa.gov. Accessed 12/2/2019. [Google Scholar]
- 3.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016; 315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: Consensus recommendations. Pain Med 2018; 19:97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manchikanti L, Kaye AM, Knezevic NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2017; 20:S3–S92. [PubMed] [Google Scholar]
- 6.Gilbert JW, Wheeler GR, Mick GE, et al. Importance of urine drug testing in the treatment of chronic noncancer pain: Implications of recent Medicare policy changes in Kentucky. Pain Physician 2010; 13:167–186. [PubMed] [Google Scholar]
- 7.Manchikanti L, Manchukonda R, Damron KS, et al. Does adherence monitoring reduce controlled substance abuse in chronic pain patients? Pain Physician 2006; 9:57–60. [PubMed] [Google Scholar]
- 8.Pesce A, West C, Rosenthal M, et al. Illicit drug use in the pain patient population decreases with continued drug testing. Pain Physician 2011; 14:189–193. [PubMed] [Google Scholar]
- 9.Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med 2015; 16:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morasco BJ, Peters D, Krebs EE, et al. Predictors of urine drug testing for patients with chronic pain: Results from a national cohort of U.S. veterans. Subst Abus 2016; 37:82–87. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert JW, Wheeler GR, Mick GE, et al. Urine drug testing in the treatment of chronic noncancer pain in a Kentucky private neuroscience practice: The potential effect of Medicare benefit changes in Kentucky. Pain Physician 2010; 13:187–194. [PubMed] [Google Scholar]
- 12.Bair MJ, Krebs EE. Why is urine drug testing not used more often in practice? Pain Pract 2010; 10:493–496. [DOI] [PubMed] [Google Scholar]
- 13.Binswanger IA, Koester S, Mueller SR, et al. Overdose education and naloxone for patients prescribed opioids in primary care: A qualitative study of primary care staff. J Gen Intern Med 2015; 30:1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CMS. Proper coding for specimen validity testing billed in combination with urine drug testing. Available at: www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Fast-Facts/Proper-Coding-Specimen-Validity-Testing. Accessed November 20, 2019.
- 15.American Medical Association. CPT (Current Procedural Terminology). Available at: www.ama-assn.org/amaone/cpt-current-procedural-terminology. Accessed November 20, 2019.
- 16.National Committee for Quality Assurance. HEDIS measures and technical resources. NCQA. Available at: www.ncqa.org/hedis/measures/. 2019. Accessed November 20, 2019. [Google Scholar]
- 17.Substance Abuse and Mental Health Services Administration. Federal guidelines for opioid treatment programs. HHS Publication No. (SMA) PEP15-FEDGUIDEOTP. Rockville, MD. www.samhsa.gov. Accessed 12/2/2019. [Google Scholar]
- 18.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10:113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yee DA, Hughes MM, Guo AY, et al. Observation of improved adherence with frequent urine drug testing in patients with pain. J Opioid Manag 2014; 10:111–118. [DOI] [PubMed] [Google Scholar]
- 20.Levy S, Siqueira LM; Committee on Substance Abuse, et al. Testing for drugs of abuse in children and adolescents. Pediatrics 2014; 133e1798–e1807. [DOI] [PubMed] [Google Scholar]
- 21.Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid use disorder documented at delivery hospitalization-United States, 1999-2014. MMWR Morb Mortal Wkly Rep 2018; 67:845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins SS, Segura LE, Santaella-Tenorio J, et al. Prescription opioid use disorder and heroin use among 12-34 year-olds in the United States from 2002 to 2014. Addict Behav 2017; 65:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiner B, Westgate CL, Bernardy NC, et al. Trends in opioid use disorder diagnoses and medication treatment among veterans with posttraumatic stress disorder. J Dual Diagn 2017; 13:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman KA, Ponce Terashima J, McCarty D. Opioid use disorder and treatment: Challenges and opportunities. BMC Health Serv Res 2019; 19:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Health and Human Services. Patient protection and Affordable Care Act: Standards related to essential health benefits, actuarial value, and accreditation; final rule. 45 CFR Parts 147, 155, and 156. www.federalregister.gov. Accessed 08/01/2020. [PubMed]
- 26.Kerensky T, Walley AY. Opioid overdose prevention and naloxone rescue kits: What we know and what we don’t know. Addict Sci Clin Pract 2017; 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilder CM, Miller SC, Tiffany E, et al. Risk factors for opioid overdose and awareness of overdose risk among veterans prescribed chronic opioids for addiction or pain. J Addict Dis 2016; 35:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews C, Abraham A, Grogan CM, et al. Despite resources from the ACA, most states do little to help addiction treatment programs implement health care reform. Health Aff (Millwood) 2015; 34:828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hettema JE, Sorensen JL. Access to care for methadone maintenance patients in the United States. Int J Ment Health Addict 2009; 7:468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saloner B, Stoller KB, Barry CL. Medicaid coverage for methadone maintenance and use of opioid agonist therapy in specialty addiction treatment. Psychiatr Serv 2016; 67:676–679. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med 2017; 11:163–173. [DOI] [PubMed] [Google Scholar]


