Abstract
Molecular oxygen, reactive oxygen species and free radicals derived from oxygen play important roles in a broad spectrum of physiological and pathological processes. The quantitative measurement of molecular oxygen in tissues by electron paramagnetic resonance (EPR) has great potential for understanding and diagnosing a number of diseases, and for developing and guiding therapies. This requires improvements in the free radical probe systems that sense and report molecular oxygen levels in vivo. We report on the encapsulation of existing free radical probes in lipophilic gel implants: an in-situ-oleogel and an emulgel, based only on well-known, safe excipients for the incorporation of lipophilic and hydrophilic radicals, respectively. The EPR signals of encapsulated radicals were not altered compared to dissolved radicals. The high solubility of oxygen in lipophilic solvents enhanced oxygen sensitivity. The gels extended the lifetime of the radicals in tissues from tens of minutes to many days, simplifying studies with extended series of measurements. The encapsulated radicals showed a good in vivo response to changes in oxygen supply and seem to circumvent concerns from toxicity of the radical probes. These gels simplify the development of new oxygen-sensitive free radical probes for EPR oximetry by making their in vivo stability, persistence and toxicity a function of the encapsulating gel and not a set of additional requirements for the free radical probe.
1. Introduction
The in vivo oxygen level in different tissues is a very important parameter in physiological and pathophysiological processes like wound and fracture healing, tumor growth and peripheral vascular diseases, and it is intimately involved in oxidative stress and the production of reactive oxygen species [1–5]. Knowledge of in vivo oxygen levels as these processes progress can help better understand these processes in order to improve and optimize pertinent therapies.
During the last decades significant efforts were put into the development of oxygen measurement techniques based on, e.g., PET (positron emission tomography), MRI (magnetic resonance imaging), the Clark electrode and EPR (electron paramagnetic resonance) [5,6]. Each method has advantages and drawbacks. EPR oximetry provides the possibility of direct and noninvasive measurement of the oxygen partial pressure (pO2) [1], but requires appropriate spin probes at the desired location. There are two different types of spin probes for EPR oximetry: particulate materials and soluble spin probes [6]. Particulate materials provide the possibility of repeated measurements, but they have low reproducibility between different preparations [7], are not biodegradable, and remain at the injection site indefinitely [1].
Soluble spin probes, on the other hand, are chemically well defined. Trityl radicals, in particular, are very attractive probes for EPR oximetry due to their very-narrow, single-line EPR signal and good oxygen sensitivity [7]. Soluble spin probes have short useable lifetimes in vivo because they redistribute throughout the organism, are degraded by reducing and/or oxidizing agents present in the organism, and some are eliminated rapidly from the body [8]. The chemical stability of trityl radicals in human blood at 37 °C varies from a few minutes to days, depending on their chemical structure [9]. The short biological half-lives, especially of lipophilic spin probes, make them unsuitable for measurements over an extended time period. The contact between tissues and the soluble probes also causes toxicity problems for the probes. The localization of a soluble probe in different microenvironments is a problem, too, because the physical properties of the microenvironment, such as viscosity or polarity, impacts the EPR linewidth, which is often used to measure pO2. Consequently, calibration of the oxygen response in the complex biological environment in vivo is problematic.
A good EPR probe must meet quite a number of different chemical, EPR, physical, biological, and toxicological requirements. It is not surprising that the ideal oxygen probe molecule has not been found although there are free radicals exhibiting some of the desired properties. Rather than search for a single-component probe that simultaneously has all the desired properties, we consider here multi-component systems, where each component provides a few of the desired properties. A rational approach is the encapsulation of radicals in particles, capsules or other structures. Several attempts to encapsulate radicals have been reported.
We described nanocapsules [10] for lipophilic radicals that have useful intravascular applications, e.g. the determination of physiological distribution processes or accumulation in certain organs or tissues like tumor tissue. However, the radical capacity of these capsules is low, rendering their in vivo use challenging. Lipophilic radicals have also been encapsulated in BSA microspheres [11] to improve the biocompatibility of lipophilic radicals like PTMTE which need to be dissolved in organic solvents. The half-life of these microspheres in vivo was relatively short. Nitroxide radicals also have been encapsulated in BSA microspheres which increased oxygen sensitivity and decreased the reduction rate in vivo, but unfortunately also decreased signal intensity [12]. Charlier et al. developed nanoemulsions based on fluorinated solvents loaded with lipophilic fluorinated trityl radicals. These emulsions showed very good oxygen sensitivity, but with a major loss of signal intensity in vivo due to increased linewidth and low radical capacity [13]. In addition to nano- and microparticles, a hydrogel based on poloxamer 407 was used in mice to shield hydrophilic radicals from the physiological environment [14]. The stability was greatly improved in anesthetized mice with only 7% loss in signal in 3 h. However, the gel was mechanically unstable in conscious mice and was rapidly degraded by motion.
Encapsulation allows the use of lipophilic radicals for in vivo EPR measurements, broadens the range of radicals that can be used, and can improve specific properties like chemical stability and in vivo lifetimes. The greater solubility of oxygen in lipophilic media greatly enhances the response to oxygen. Some properties of the encapsulating material are important because it is in contact with the organism, for example, biocompatibility, viscosity [10] and oxygen solubility [15], but there is a wide choice of available biocompatible materials for the construction of encapsulated EPR probes for oxygen measurement.
In this study we investigated representatives of two-different classes of lipophilic gels to encapsulate lipophilic and hydrophilic radicals: an in-situ-oleogel and an emulgel, respectively. To our knowledge, these types of lipophilic gels have not been used for local application of radicals and subsequent EPR measurements. We examined issues that are key for encapsulation to be a viable strategy for EPR measurement of oxygen: (1) Can encapsulation in lipophilic gels effectively protect the free radical probes from oxidizing and reducing physiological molecules and thus extend the lifetime of the probes in animals for measurements over several hours or days? (2) Will encapsulation reduce the toxicity of the probe molecules either by shielding the organism from the probe's toxic properties or by allowing a reduction in the required dose? (3) Will the encapsulated free radicals retain their sensitivity to oxygen?
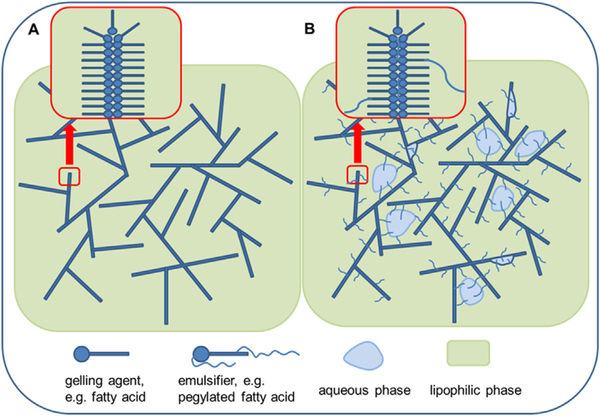
Oleogels, in general, consist of a lipophilic solvent in which a low-molecular weight gelling agent forms a three-dimensional lattice (Fig. 1 (A)) [16]. Lipophilic emulsion gels (“emulgels”) additionally contain an aqueous phase which is stabilized with an emulsifier (Fig. 1 (B)). The gels used in this study are based on isopropyl myristate, a lipophilic ester with low viscosity and high biocompatibility with 12-hydroxystearic acid as gelling agent. Lypophilic probes are localized in the lipophilic phase of the oleogels while hydrophilic probes are localized in the aqueous phase of the emulgels.
Fig. 1.
(A) Structure of an Oleogel. (B) Structure of a lipophilic Emulgel.
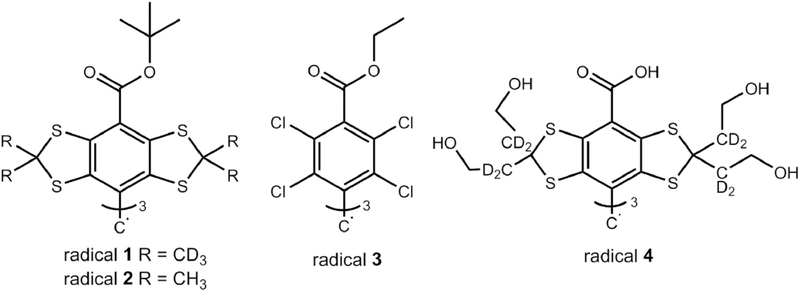
The first step was the development of tailored formulations. Then we compared the oxygen sensitivity in vitro of different trityl radicals (Fig. 2) in solution and encapsulated in the formulations. For the oleogel, we used different types of trityls: a chlorinated trityl (radical 3, also known as PTMTE) and the lipophilic tris-tert-butyl ester of the well-known “Finland trityl” with either deuterated or undeuterated methyl groups (radicals 1 and 2, tris [8-tert-butoxycarbonyl-2,2,6,6-tetra-methylbenzo[1,2-d:4,5-d']bis([1,3]dithiol)-4-yl]methyl radical), to investigate which radicals are suitable for oxygen measurement. For the emulgel we used the hydrophilic radical 4 (OXO71). We also report the first animal tests of the stability and the oxygen sensitivity of the formulations in vivo.
Fig. 2.
Chemical structures of the trityl radicals used for this study.
2. Results and discussion
2.1. Composition of the formulations
The formulations were based on our 2015 patent application “Injectable Depot Formulations for the Controlled Release of Active Ingredients” [17], which use only established, safe excipients that are approved for pharmaceutical and cosmetic formulations. Isopropyl myristate (IPM) was used as the lipophilic component for the oleogel because of its low viscosity (η = 5–6 mPa s at 20 °C; cf. medium-chain triglyceride with η = 25–33 mPa s at 20 °C) [10]. IPM is an important penetration enhancer in topical pharmaceutical formulations [18] that is well tolerated [19]. The low viscosity is important because high viscosity leads to EPR line broadening [20] and consequently to decreased sensitivity and specificity to oxygen. The fatty acid 12-hydroxystearic acid (12-HSA) is a common gelling agent for the preparation of organogels. It forms a super-saturated solution upon cooling below its gelation temperature and self-assembles into a three-dimensional lattice in organic solvents.
Following injection of the fluid-lipid formulation, the implant forms in the extracellular space at the site of the injection. N-Methylpyrrolidone (NMP) was added to keep it fluid until the gelation takes place through solvent exchange with the extracellular matrix. This strategy is already in commercial use; Eligard® (Tolmar Pharmaceuticals), a commercial in-situ forming biodegradable polymer implant, contains NMP.
To obtain an emulgel suitable for the incorporation of hydrophilic radicals, the oleogel formulation described above was modified to incorporate an aqueous phase. The emulsifier PEG-30-dipolyhydroxystearate (Cithrol® DPHS, Croda International), which is chemically similar to the gelling agent [21], was used to stably incorporate the aqueous phase. It improves the homogeneity of the formulation by incorporating the emulsifier into the lattice of the gelling agent. To ensure a homogeneous distribution of the aqueous phase inside the emulgel, it is better not to use an in-situ-gelation process because the lipophilic and aqueous phases may separate. In-situ gelation may therefore give an inhomogeneous distribution of the aqueous phase or improper gel formation in-vivo. The emulgel is a soft gel and has a yield point, but its shear thinning properties allow it to be injected with a 25G needle.
Different ratios of ingredients were tested to optimize the composition of the gel formulations. The addition of 10% NMP was necessary to keep the oleogel in a liquid state until injection. The emulgel could incorporate up to 70% aqueous phase, but 50% was chosen to reduce the effects of mechanical stress in vivo. Table 1 shows the exact composition of both formulations used for further in vitro and in vivo tests.
Table 1.
Composition of the formulations. Amounts are given in percent by mass (%, m/m).
| In-situ-Oleogel | Emulgel | ||
|---|---|---|---|
| IPM | 81.0 | IPM | 42.5 |
| 12-HSA | 9.0 | 12-HSA | 5.5 |
| NMP | 10.0 | Cithrol® DPHS | 2.0 |
| PBS 7.4 | 50.0 |
2.2. In vitro oxygen sensitivity of the radicals in solution and incorporated into the gels
The samples with fixed oxygen concentration were prepared by exposing them to gas mixtures with 0–20.9% oxygen partial pressure. Oxygen calibrations were performed with solutions of probes in PBS 7.4 or IPM. The data from the oleogel and emulgel samples were analyzed with these calibrations. The EPR signal-to-noise ratio at 300 MHz was too low for precise determination of the linewidths in air with 20.9% oxygen because of the broad EPR linewidths. But the oxygen partial pressures (pO2) in tissues range between 0% and 10% [5], so the highest oxygen level was omitted from the calibration curves because it is less relevant. The complete removal of oxygen from the IPM solutions was difficult. Even after several hours of deoxygenation with argon gas, the samples of radical 3 in IPM still showed a linewidth at what was supposed to be 0% oxygen that suggested some residual oxygen. The most plausible explanation seems to be residual oxygen, since only the 0% O2 measurements of radical 3 in the oleogel is an outlier in the calibration curves.
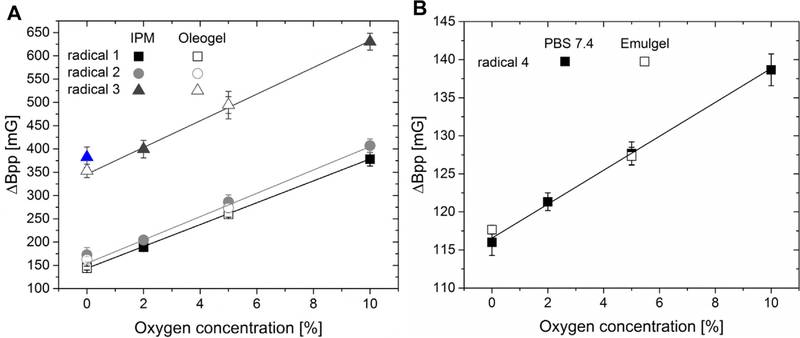
The oxygen sensitivity of the lipophilic radicals dissolved in IPM was about 25 mG/% O2, whereas the sensitivity of radical 4 dissolved in buffer was much lower at 2.2 mG/% O2 (Fig. 3). This can be explained by the lower solubility of oxygen in water compared to oils [22]. The oxygen solubility in water is about 0.6% (v/v) at 22 °C and 0.213 bar oxygen [23]. In IPM it is 2.9% (v/v) under the same conditions [10]. The higher oxygen sensitivity of the lipophilic radicals in IPM makes it easier to measure small changes in oxygen levels and reduces statistical errors in the determination of oxygen levels in comparison to measurements with hydrophilic radicals in the aqueous phase. However, the intense increase in linewidth accompanies a strong decrease in the spin-spin relaxation time T2, making the use of lipophilic radicals for pulsed EPR oximetry difficult [24]. Radicals 1 to 3 in IPM at 1 mM have weak EPR signal intensity at 300 MHz (data not shown). Pulsed EPR spectra were measurable up to 5% oxygen only for radical 1 and 2. The pulsed EPR signal of radical 3 was not useable above 0% oxygen.
Fig. 3.
(A) Oxygen calibration curves of the lipophilic radicals in IPM (radical 1 and 2: 1 mM; radical 3: 5 mM), compared with radical linewidths incorporated into oleogel. (B) Oxygen calibration curve of the hydrophilic radical 4 in PBS pH 7.4 (1 mM), compared with radical linewidths incorporated into the emulgel (n = 3). Linear regression was done with the data measured in IPM or PBS pH 7.4. The 0% oxygen data for radical 3 was omitted from the fitting as an outlier (see text).
The linewidths of chlorinated radical 3 are much broader than the linewidths of the tetrathia-radicals 1, 2 and 4, Fig. 3. As a result, the linewidth determination has reduced precision which appears as a higher standard deviation. Chlorinated trityls in general have broader peak-to-peak-linewidths than tetrathia-trityls because of unresolved hyperfine couplings of the unpaired electron with the chlorine atoms [25]. At 5 mM, radical 3 showed some broadening due to Heisenberg exchange (data not shown), but this concentration was used to obtain adequate signal intensity at higher oxygen levels. Higher concentrations of radicals 1 and 2 could not be used because of their limited solubility in IPM. The difference between the linewidths of radical 2 and its deuterated analog 1 was very small. Hence the less accessible deuterated radicals provided no benefit in these solutions. To evaluate whether incorporation of the radicals into gels influences the linewidths or the sensitivity, the gels were prepared and equilibrated (0%O2, 5%O2) as described in Sections 4.3 and 4.4. The linewidths of the radicals inside the gels are within the standard deviation of the linewidths measured in solution, Fig. 3. This means that the gels alter neither the linewidth nor the EPR sensitivity. Oxygen permeates the gels without any hindrance and the microviscosity of IPM or of PBS buffer does not increase in the gel state.
2.3. In vivo studies of the formulations
To determine the stability and oxygen sensitivity of the gels in vivo, a 0.2 ml aliquot of the radical-containing formulations was injected intramuscularly into the hind leg of female C3H mice, two mice for each radical-formulation combination. We followed the EPR signal in these mice over 3 weeks with measurements at day 0 (injection day), 2, 4, 7, 14 and 21. Between measurements the mice were housed in groups of five per cage with water and food ad libitum.
2.3.1. Stability and toxicity
After injection of the formulations, the mice showed no sign of pain or distress. Their behavior did not differ from untreated mice during 3 weeks. It is known that toxicity of trityl radicals increases with lipophilicity. The toxicity of the hydrophilic OXO63 – the nondeuterated analog of OXO71 - with an LD50 of 8 mmol/kg body weight (b.w.) is fairly low [26], while mice injected with an aqueous solution of Finland radical die shortly after application [27]. The 200 μl of oleogel injected contain 182 nmol of radical 1 or 2; or 910 nmol of radical 3. So only 7.3 μmol/kg (b.w.) of radical 1 or 2; or 36.4 μmol/kg b.w. of radical 3 were injected, which are relatively modest doses. The tert-butyl ester groups of radicals 1 and 2 easily hydrolyze in aqueous environments, producing relatively toxic Finland trityl radicals. Yet, in oleogel implants, radicals 1 and 2 persisted many days in the mice with no obvious effects. Any prolonged release of Finland trityl that took place did not produce the lethality seen with direct injection of Finland trityl for in vivo EPR.
One advantage of encapsulation is that the radical remains where it is injected and does not immediately distribute through the entire animal as injected solutions do. The implanted oleogel contained roughly 1 mM radical for 1 and 2, or 5 mM of radical 3. To achieve these concentrations of radicals for a localized measurement with a probe that distributed uniformly through the entire mouse, doses of 0.9 mmol/kg for 1 or 2 would be needed, or 4.5 mmol/kg for radical 3, each day a measurement is made. Even this estimate is conservative because radicals are typically metabolized or localized in the bladder during measurements, requiring additional amounts. For instance, to maintain a steady state concentration of 1 mM OXO63 in blood, requires injection of a 0.75 mmol/kg b.w. bolus followed by 0.06 mmol/kg b.w./min infusion throughout the measurement [28].
The 200 μl emulgel implant contained only 100 nmol OXO71 or 4 μmol/kg b.w, again drastically reducing the total dose. Use of these encapsulated trityls is advantageous for localized EPR measurements. Much smaller amounts of radical are needed, which brings doses down well below toxic levels and conserves costly trityl radicals that can be difficult to obtain.
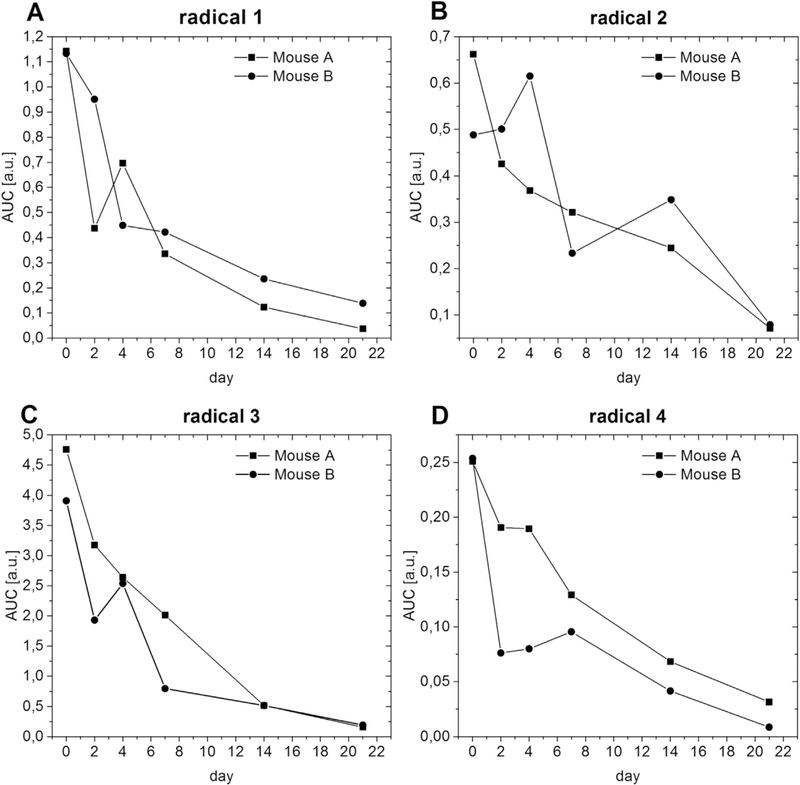
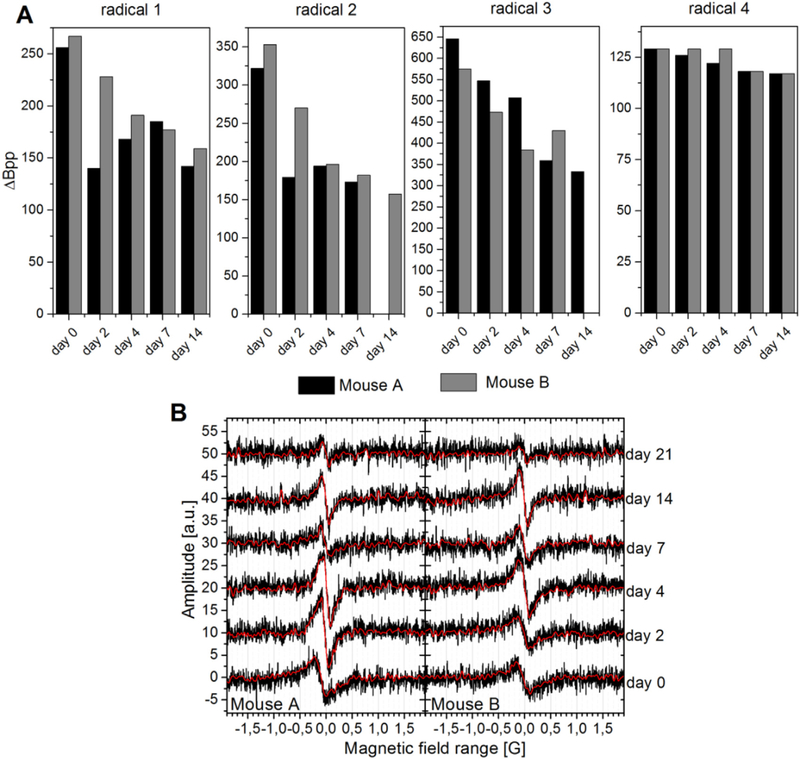
Fig. 4 shows the decrease of the in vivo EPR signal over time. Several experimental parameters, like the exact position of the mouse inside the resonator or the tuning of the system, affect the intensity of the EPR signal. Consequently, quantitative EPR measurements are very challenging, especially on animals over a longer period of time [29]. Nevertheless the signal intensity does give a broad indication of the in vivo stability of the formulations after i.m. injection. When radicals are injected without any protection, their EPR signals decay very fast due to redistribution in the body, metabolism, and excretion [14, 28, 30]. The tetrathia-trityls are rather unreactive towards different reducing agents and conditions. They are, for example, stable in the presence of ascorbic acid or cell lysates, which reduce chlorinated trityls rapidly [31,32]. This difference in stability of chlorinated versus tetrathia-trityls would suggest different in vivo half-lives, but regardless of radical type, EPR signals from the encapsulated trityls were measured up to three weeks. The signal intensity of each radical decreases quickly at first, but slows over time with a half-life of about a week, Fig. 4. This lifetime is considerably shorter than that of the gels themselves.
Fig. 4.
Signal intensity (AUC, area under the curve of the integrated spectra) of the radicals encapsulated in oleogel or emulgel in-vivo. (A) – (B) Lipophilic radicals 1 to 3 in oleogel. (D) Hydrophilic radical 4 in emulgel.
Similar oleogels and emulgels are stable in vivo up to 3 months after subcutaneous injection [33]. Stability is limited by lipase-induced degradation of the lipid. In vitro exposure to phosphate buffer does not induce observable changes over several months. However, addition of lipase to the buffer induces oleogel erosion that depends on both gelator and lipase concentration [33]. The loss of the EPR signals seen in this study is more rapid than the degradation of subcutaneous gels with similar composition and is very likely because they are subjected to higher mechanical stress because of their i.m. injection. The fact that radicals with different redox properties and different stabilities in biological fluids have nearly identical lifetimes in these gels, indicates that their in vivo half-lives are determined primarily by degradation of the lipophilic formulations and that the gels provide the radicals consistent and effective protection from their surroundings. The incorporation of an aqueous phase into the emulgels does not influence the stability, which means that the gel type used here is useful for lipophilic and for hydrophilic radicals. The oleogel and emulgel reported here are apparently more stable in vivo under mechanical stress than the hydrogel [14] that was found to be displaced and degraded rapidly by motion in awake mice.
2.3.2. Oxygen measurements
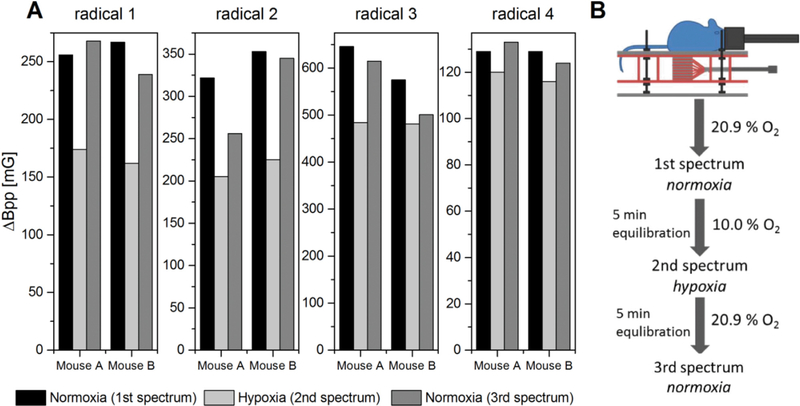
The oxygen sensitivity of the encapsulated radicals was checked in vivo under reduced oxygen supply, Fig. 5 (A). The linewidth of the signal clearly decreases under reduced oxygen supply for all radicals and gels. The linewidth broadens again when the breathing gas mixture returns to 20.9% oxygen. The pronounced difference in linewidth between normoxia and hypoxia clearly shows that the encapsulated probes do register different oxygen levels in vivo. As expected, the linewidth difference is much more pronounced for the lipophilic radicals than for hydrophilic radicals because of the higher partitioning of oxygen into the organic phase containing the lipophilic radicals.
Fig. 5.
(A) Linewidths of the encapsulated radicals in vivo under normoxia (20.9% O2) and hypoxia (10.0% O2) at day 0. (B) Procedure of hypoxia experiment.
The EPR linewidths measured under normoxia decreased from day 0 to day 21 in most of the mice, Fig. 6 (A). Furthermore, the apparent oxygen levels at day 0 are unusually high for muscle tissue. The oxygen partial pressure in muscles is reported to be about 3.8% [5], while the in vitro calibration curves suggest pO2 levels between 5% and 10% (see Supplementary information for data). These anomalies and the notable variations in response among the mice have several possible contributing factors. (a) The high pO2 levels may be a physiological response to tissue injury caused by injection of the gels, which diminishes as the injury heals. (b) Part of the gel may initially be in contact with blood vessels in the leg, so that part of the gel will report pO2 levels more characteristic of the vasculature. These levels would drop as normal blood flow is diverted to active tissue. (c) Aging of the gels can change the mobility (and linewidth) of the radicals and the mobility and solubility of oxygen. (d) The formation of fibrous capsules around implants is a very common physiological reaction after injection of nonself-material which can cause a diffusional barrier [34]. With time, the gel would be ‘walled off’ from the surrounding tissue and be unable to report tissue pO2 levels. (e) The apparent decline in pO2 levels may be an adaptation of the mice to repeated anesthesia, hypoxia, and handling during the three weeks of measurements. A detailed investigation of each of these factors goes far beyond the scope of this study, but fortunately, all can be addressed in future studies by changes in the composition of the gels or alterations in the implantation or EPR measurement protocols.
Fig. 6.
(A) Measured linewidths of all radicals under normoxia and (B) Spectra of radical 1 encapsulated in oleogel under normoxia at the different days after injection of the gel (black: raw data, red: filtered data).
Despite the various factors influencing the pO2 quantification, the experiment shows that probes encapsulated in lipophilic gels do register different pO2 levels in vivo during hypoxia and normoxia. This we consider sufficient proof that oleogels and emulgels show promise for encapsulation of oxygen-sensitive EPR probes, and are an attractive starting point to improved formulations for long-term, quantitative measurement of localized pO2 levels in vivo.
The signal-to-noise ratio of the spectra in vivo is relatively poor (Fig. 6 (B)) which has made the determination of the linewidth challenging. The signal was measurable even 14–21 days after injection, but it was not always possible to extract a reliable linewidth from this data. Fortunately, there is considerable room to increase the signal-to-noise ratio by: optimization of the measuring parameters; improvements in the hardware; use of, e.g., wavelet and denoising software [35]; and increasing the amount of radical in the formulations. For OXO63 it has been shown that no significant line broadening occurs up to a concentration of 3 mM [28]. The concentration of the tetrathiatrityls used in this study was well away from this threshold.
3. Conclusion
The results reported here show that encapsulation of spin probes is an attractive strategy for prolonging the lifetime of spin probes in vivo and for effectively reducing toxicity concerns in localized measurement of tissue pO2. The hypoxia measurements show that probes encapsulated in lipophilic gels can register oxygen levels in vivo, but the invasiveness of the gels used in this study is too high. To reduce the influence of the gels itself on the tissues, the volume to be injected must be reduced. This could be achieved, e.g., by increased radical concentrations, or by improvement of the in vivo gel stability for long-term measurements. Encapsulation can allow the use of costly, rare and/or toxic probes in much smaller amounts and permits extended or repeated measurements at the same location. In addition, encapsulation of probes, to some extent, separates the design of probes for measurement of pO2 levels from concerns about stability, lifetime, solubility, and toxicity. Those concerns are addressed better through the design of the encapsulating materials.
Implants and drug encapsulation are widely used and accepted means for prolonged delivery of drugs to treat and prevent a wide variety of diseases in animals and humans. We find they also have promise for delivery of molecular probes for prolonged, localized measurements of molecular oxygen. This would allow quantitative measurements of the most physiologically-important free radical, namely molecular oxygen, throughout the course of disease progression, treatment, and healing.
4. Experimental
4.1. Materials
12-Hydroxystearic acid was purchased from Rockleigh Industries Inc. (New Jersey, USA), isopropyl myristate (Pionier IPM) was purchased from Hansen & Rosenthal (Hamburg, Germany), anhydrous N-methyl-2-pyrrolidone was purchased from Alfa Aesar (Ward Hill, USA). Croda International Plc. (Snaith, UK) kindly provided Cithrol DPHS. Other materials used were Epoxy resin (Gorilla Glue, Inc., USA), PBS buffer pH 7.4 (BioWhittaker Reagents, Lonza Group AG, Basel, Switzerland). Gas mixtures were purchased from Roberts Oxygen Company Inc. (Rockville (MD), USA). Argon gas was purchased from Praxair Inc. (Danbury (CT), USA). The purity of argon gas was 99.995%. The water was of double distilled quality.
4.2. Synthesis
Radical 1 and 3 were synthesized as published earlier by us and other groups [10, 36, 37]. Radical 2 was synthesized using a modified literature approach [38]. Radical 4 was kindly provided by Nycomed Innovation AB (Malmoe, Sweden).
4.3. Gel preparation
Both gels were prepared based on injectable depot formulations for controlled release that were invented by us [17]. The preparation was done directly before use.
In-situ-oleogel.
12-Hydroxystearic acid (9%, m/m) was mixed with isopropyl myristate (81%, m/m) and N-methyl-2-pyrrolidone (10%, m/ m, Alfa Aesar). The mixture was heated to 75 °C until a homogeneous solution formed and then was vortexed for 10 s.
Emulgel.
12-Hydroxystearic acid (11%, m/m), Cithrol DPHS (4%, m/m, Croda International Plc.) and isopropyl myristate (85%, m/m) were mixed, heated to 75 °C and vortexed until a homogenous solution was formed. PBS buffer pH 7.4 was heated to 75 °C. The buffer was added in a ratio of 1:1 (m/m) to the lipophilic components. The mixture was vortexed for 5 s, then homogenized at 75 °C for 2 min (UltraTurrax T8). The formulation was filled into a 1 ml syringe before the gel forming temperature was reached.
4.4. Sample preparation for in-vitro oxygen calibration
Radical solutions.
Lipophilic radicals were dissolved in isopropyl myristate (cradical 1 = 1 mM, cradical 2 = 1 mM, cradical 3 = 5 mM). 0.5 ml of the solutions were equilibrated for 3 h in ampules with argon or the different gas mixtures to obtain samples with oxygen content between 0% and 10% (Table 2). After that the samples were sealed with epoxy resin.
Table 2.
Gas mixtures used for the preparation of samples with different oxygen content.
| Composition of gas mixtures | ||
|---|---|---|
| Oxygen (vol%) | Carbon dioxide (vol%) | Nitrogen (vol%) |
| 2 | 5 | 93 |
| 5 | 5 | 90 |
| 10 | 0 | 90 |
Radical 4 was dissolved in PBS buffer pH 7.4 (c = 1 mM). 0.1 ml of the solution were equilibrated for 45 min in Pyrex® vials with argon gas or with the gas mixtures shown in Table 2.
In-situ-oleogel and emulgel.
The gels were prepared as described in Section 4.3. For the in-situ-oleogels, radical solutions in isopropyl myristate (cradical 1 = 1 mM, cradical 2 = 1 mM, cradical 3 = 5 mM) were used. For the emulgel, radical 4 was dissolved in PBS buffer (c = 1 mM). 0.2 ml of each gel were injected in ampules filled with 0.4 ml water and were equilibrated for 3 h with argon gas or with the 5% oxygen gas mixture. All samples were prepared and stored under light protection.
4.5. Animal preparation
All in vivo experiments were carried out in compliance with the Guide for the care and use of laboratory animal resources (National Research Council, 1996) and were approved by the National Cancer Institute Animal Care and Use Committee (NCI-CCR-ACUC, Bethesda, MD, protocol nos. RBB-153, 155, and 159). Female C3H mice, about 15 weeks old and weighing about 25 g, were used for CW EPR experiments. The animals (Frederick Cancer Research Center, Animal Production, Frederick, MD) were housed in a climate and light controlled room and fed ad libitum.
The radical containing formulations were injected under anesthesia intramuscularly into the right hind leg (0.2 ml/mouse) with a 25 G needle. The mice were anesthetized with isoflurane (1.5%) in breathing air while laying prone on a home built resonator coil with the right hind leg inside the resonator. The breathing rate (60 ± 10 per min) was monitored with a pressure transducer (SA Instruments, Inc., NY, USA). The body core temperature was measured with a rectal thermistor probe and was maintained (36 ± 1 °C) by a steady flow of warm air. For the normoxia experiments the mice inhaled breathing air with 20.9% oxygen. For the hypoxia experiments the oxygen concentration was reduced to 10.0%. The measurement was started after the mice inhaled this gas mixture for 5 min. After the hypoxia experiment the gas mixture was changed back to normoxia and another measurement was taken after 5 min.
4.6. CW EPR measurements
EPR measurements were made with a home built CW EPR spectrometer at 300 MHz using a 15 mm-parallel coil resonator. The following general settings were used: microwave power < 0.92 mW (lipophilic radicals), < 0.25 mW (radical 4); modulation frequency 13.5 kHz; sweep 2.0–4.24 G depending on the linewidth. Data acquisition was implemented with LabView (National Instruments, Austin, USA); each spectrum was measured with 1000 points, whereby each point is the sum of several measurements that were done in a time period of 0.2 s. The raw data was filtered with LabView using a Butterworth filter. The peak-to-peak-linewidth (ΔBpp) was determined from the filtered data using Matlab (The Mathworks, Inc, Natick, USA). For the determination of the oxygen sensitivity of each radical the peak-to-peak-linewidths of the in-vitro-samples were plotted as a function of the oxygen concentration and analyzed by linear regression. For the in vivo measurements the area-under-the-curve (AUC) was determined from the double integral of the first-derivative spectra using OriginPro2016G (OriginLab Corporation, Northampton, USA). The tissue oxygen concentrations were calculated from the regression functions of the in vitro data. The signal-to-noise ratio (SN ratio) was calculated as the ratio of the peak-to-peak amplitude of the filtered first-derivative spectrum to the standard deviation of the noise (raw data).
4.7. Pulsed EPR measurements
Measurements were made using a home-built 300 MHz pulsed EPR spectrometer with a 15 mm-parallel coil resonator. The following general settings were used: excitation pulse width, 70 ns; number of samples in FID, 592–1600; repetition time, 6–20 μs; receiver input range, 0.5; pulse power incident on resonator, 20 W; average trigger delay, 0.350 μs; averages, 4000–200,000. The FID data was analyzed using Matlab (The Mathworks, Inc, Natick, USA).
Supplementary Material
Acknowledgement
D.V.T, O.Yu. R, M.K.B and V.M.T. acknowledge the financial support from Ministry of Science and Education of the Russian Federation (grant 14.W03.31.0034, MEGAGRANT) and NIOC SB RAS budget project 0302-2016-0002.
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.freeradbiomed.2018.10.442.
References
- [1].Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, Sullivan D, Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy, Int. J. Radiat. Biol. 82 (2006) 699–757. [DOI] [PubMed] [Google Scholar]
- [2].Touyz RM, Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension. What is the Clinical Significance? Hypertension 44 (2004) 248–252. [DOI] [PubMed] [Google Scholar]
- [3].Sen CK, Wound healing essentials: let there be oxygen, Wound Rep. Regen. 17 (2009) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu C, Saless N, Wang X, Sinha A, Decker S, Kazakia G, Hou H, Williams B, Swartz HM, Hunt TK, Miclau T, Marcucio RS, The role of oxygen during fracture healing, Bone 52 (2013) 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C, Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia, J. Cell. Mol. Med. 15 (2011) 1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Springett R, Swartz HM, Measurements of oxygen in vivo: overview and perspectives on methods to measure oxygen within cells and tissues, Antioxid. Redox Signal. 9 (2007) 1295–1301. [DOI] [PubMed] [Google Scholar]
- [7].Ahmad R, Kuppusamy P, Theory, instrumentation, and applications of electron paramagnetic resonance oximetry, Chem. Rev. 110 (2010) 3212–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Serda M, Wu YK, Barth ED, Halpern HJ, Rawal VH, EPR imaging spin probe trityl radical OX063: a method for its isolation from animal effluent, redox chemistry of its quinone methide oxidation product, and in vivo application in a mouse, Chem. Res. Toxicol. 29 (2016) 2153–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ardenkjær-Larsen JH, Laursen I, Leunbach I, Ehnholm G, Wistrand LG, Petersson JS, Golman K, EPR and DNP properties of certain novel single electron contrast agents intended for oximetric imaging, J. Magn. Reson. 133 (1998) 1–12. [DOI] [PubMed] [Google Scholar]
- [10].Frank J, Elewa M, Said MM, El Shihawy HA, El-Sadek M, Muller D, Meister A, Hause G, Drescher S, Metz H, Imming P, Mader K, Synthesis, Characterization, and Nanoencapsulation of tetrathiatriarylmethyl and tetrachlorotriarylmethyl (Trityl) radical derivatives–a study to advance their applicability as in Vivo EPR oxygen sensors, J. Org. Chem. 80 (2015) 6754–6766. [DOI] [PubMed] [Google Scholar]
- [11].Sostaric JZ, Pandian RP, Bratas A, Kuppusamy P, Encapsulation of a highly sensitive EPR active oxygen probe into sonochemically prepared microspheres, J. Phys. Chem. B 111 (2007) 3298–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu JK, Grinstaff MW, Jiang J, Suslick KS, Swartz HM, Wang W, In vivo measurement of oxygen concentration using sonochemically synthesized microspheres, Biophys. J. 67 (1994) 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Charlier N, Driesschaert B, Wauthoz N, Beghein N, Preat V, Amighi K, Marchand-Brynaert J, Gallez B, Nano-emulsions of fluorinated trityl radicals as sensors for EPR oximetry, J. Magn. Reson. 197 (2009) 176–180. [DOI] [PubMed] [Google Scholar]
- [14].Abbas K, Boutier-Pischon A, Auger F, Francon D, Almario A, Frapart YM, In vivo triarylmethyl radical stabilization through encapsulation in Pluronic F-127 hydrogel, J. Magn. Reson. 270 (2016) 147–156. [DOI] [PubMed] [Google Scholar]
- [15].Dang V, Wang J, Feng S, Buron C, Villamena FA, Wang PG, Kuppusamy P, Synthesis and characterization of a perchlorotriphenylmethyl (trityl) triester radical: a potential sensor for superoxide and oxygen in biological systems, Bioorg. Med. Chem. Lett. 17 (2007) 4062–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kempe S, Mäder K, In situ forming implants – an attractive formulation principle for parenteral depot formulations, J. Control. Release 161 (2012) 668–679. [DOI] [PubMed] [Google Scholar]
- [17].Mäder K, Windorf M, Kutza J, Injizierbare Depotformulierungen zur kontrollierten Wirkstofffreisetzung, (Halle-Wittenberg M-L-U, Ed.) A61K47/12 ed., Germany, 2015. [Google Scholar]
- [18].Lane ME, Skin penetration enhancers, Int. J. Pharm. 447 (2013) 12–21. [DOI] [PubMed] [Google Scholar]
- [19].Liebert MA, Final report on the safety assessment of myristyl myristate and isopropyl myristate, J. Am. Coll. Toxicol. 1 (1982) 55–80. [Google Scholar]
- [20].Eaton SS, Eaton GR, Berliner LJ, Biomedical EPR - Part A: Free Radicals, Metals, Medicine and Physiology, Springer, US, 2006. [Google Scholar]
- [21].Kutza J, Oleogele auf 12-Hydroxystearinsäurebasis als injizierbare Implantate zur kontrollierten Proteinfreisetzung, Institute of Pharmacy, Martin-Luther-Universität Halle-Wittenberg, Halle (Saale), Germany, 2014. [Google Scholar]
- [22].Battino R, Oxygen and Ozone. IUPAC Solubility Data Series 7 Pergamon Press, Oxford, UK, 1981. [Google Scholar]
- [23].Battino R, Rettich TR, Tominaga T, The solubility of oxygen and ozone in liquids, J. Phys. Chem. Ref. Data 12 (1983) 163–178. [Google Scholar]
- [24].Prisner T, Rohrer M, MacMillan F, Pulsed EPR spectroscopy: biological applications, Annu. Rev. Phys. Chem. 52 (2001) 279–313. [DOI] [PubMed] [Google Scholar]
- [25].Juliá L, Ballester M, Riera J, Castaner J, Ortin JL, Onrubia C, Inert carbon free radicals. 9. The first perchlorinated triarylmethyl and fluorenyl radicals with a heteroaromatic ring, and related compounds, J. Org. Chem. 53 (1988) 1267–1273. [Google Scholar]
- [26].Krumkacheva O, Bagryanskaya E, Trityl radicals as spin labels, Electron Paramagnetic Resonance, The Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 0WF, UK, 2017, pp. 35–60. [Google Scholar]
- [27].Howard Halpern, personal communication to V. M. T.
- [28].Matsumoto K, English S, Yoo J, Yamada K, Devasahayam N, Cook JA, Mitchell JB, Subramanian S, Krishna MC, Pharmacokinetics of a triarylmethyl-type paramagnetic spin probe used in EPR oximetry, Magn. Reson. Med. 52 (2004) 885–892. [DOI] [PubMed] [Google Scholar]
- [29].Nagye E, Quantitative EPR: some of the most difficult problems, Appl. Magn. Reson. 6 (1994) 259–285. [Google Scholar]
- [30].Bézière N, Decroos C, Mkhitaryan K, Kish E, Richard F, Bigot-Marchand S, Durand S, Cloppet F, Chauvet C, Corvol M-T, Rannou F. o., Xu-Li Y, Mansuy D, Peyrot F, Frapart Y-M, First Combined In Vivo X-ray tomography and high-resolution molecular electron paramagnetic resonance (EPR) imaging of the mouse knee joint taking into account the disappearance kinetics of the EPR probe, Mol. Imaging 11 (2012) 220–228. [PubMed] [Google Scholar]
- [31].Elewa M, Maltar-Strmecki N, Said MM, El Shihawy HA, El-Sadek M, Frank J, Drescher S, Drescher M, Mader K, Hinderberger D, Imming P, Synthesis and EPR-spectroscopic characterization of the perchlorotriarylmethyl tricarboxylic acid radical (PTMTC) and its 13C labelled analogue (13C-PTMTC), Phys. Chem. Chem. Phys. 19 (2017) 6688–6697. [DOI] [PubMed] [Google Scholar]
- [32].Jagtap AP, Krstic I, Kunjir NC, Hänsel R, Prisner TF, Sigurdsson ST, Sterically shielded spin labels for in-cell EPR spectroscopy: analysis of stability in reducing environment, Free Radic. Res. 49 (2015) 78–85. [DOI] [PubMed] [Google Scholar]
- [33].Windorf M, 12-Hydroxystearic Acid-based in Situ Forming Organogels: Development and Characterization, Institute of Pharmacy, Martin-Luther-University Halle-Wittenberg, Halle (Saale), Germany, 2017. [Google Scholar]
- [34].Wright JC, Burgess DJ, Long Acting Injections and Implants, Springer, New York, 2012. [Google Scholar]
- [35].Srivastava M, Anderson CL, Freed JH, A new wavelet denoising method for selecting decomposition levels and noise thresholds, IEEE Access 4 (2016) 3862–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bratasz A, Kulkarni AC, Kuppusamy P, A highly sensitive biocompatible spin probe for imaging of oxygen concentration in tissues, Biophys. J. 92 (2007) 2918–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu Y, Villamena FA, Sun J, Wang TY, Zweier JL, Esterified trityl radicals as intracellular oxygen probes, Free Radic. Biol. Med. 46 (2009) 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kuzhelev AA, Tormyshev VM, Rogozhnikova OY, Trukhin DV, Troitskaya TI, Strizhakov RK, Krumkacheva OA, Fedin MV, Bagryanskaya EG, Triarylmethyl radicals: epr study of 13C hyperfine coupling constants, Z. Phys. Chem. 231 (2017) 777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.