Introduction
Von Hippel-Lindau (VHL) syndrome is an autosomal dominant cancer syndrome associated with a germ-line alteration of the VHL gene located on the short arm of chromosome 3. Patients are at lifelong risk for developing various tumors, both benign and malignant, in multiple organ systems. Renal involvement can include cysts and solid tumors, primarily clear-cell renal-cell carcinoma.1 Management of these renal tumors requires a multidisciplinary approach, as the goal is to preserve functioning nephrons while preventing metastases. Our approach is to perform active surveillance until tumors reach 3 cm in size, then pursue active treatment. For most patients, surgical extirpation with partial nephrectomy is the preferred treatment, while ablative techniques such as cryoablation or radiofrequency ablation (RFA) are used selectively.2 Despite the technique chosen, close imaging surveillance follow-up is key to ensure adequate treatment and to monitor for local recurrent or de novo renal tumors.
Case Report
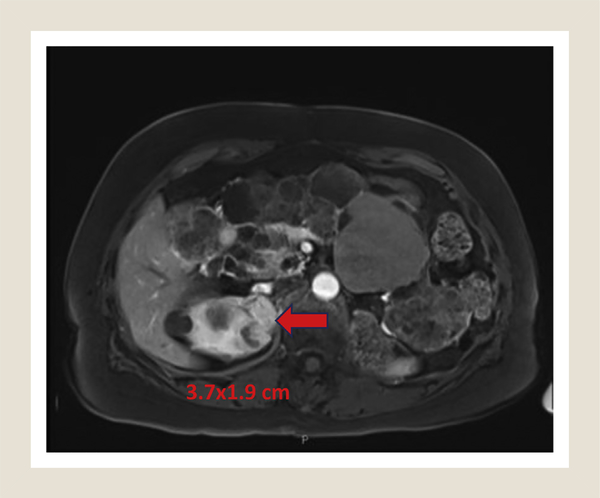
A 78-year-old woman has undergone surveillance at our institution of her von Hippel-Lindau disease. Her previous surgical history included a right radical nephrectomy in the 1980s at an outside institution and a laparoscopic right partial nephrectomy in 2008 for clear-cell renal cell carcinoma. She was followed by our standard imaging protocols for VHL and was noted to have 2 abutting tumors (total size, 3.5 cm) that had grown in her solitary kidney (Figure 1). Given her age and surgical history, the patient was counseled on options and elected percutaneous cryoablation of the lesion. She underwent a technically successful computed tomography—guided percutaneous cryoablation utilizing 3 cryoablation probes.
Figure 1.
Preoperative MRI Showing Upper Pole Renal Mass (Red Arrow). Appearance Was That of 2 Masses Abutting Each Other
Abbreviation: MRI = magnetic resonance imaging.
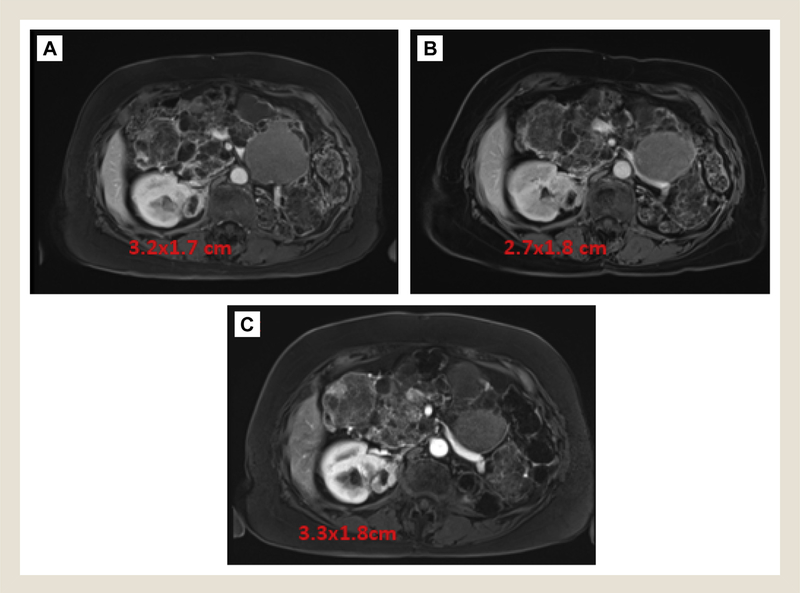
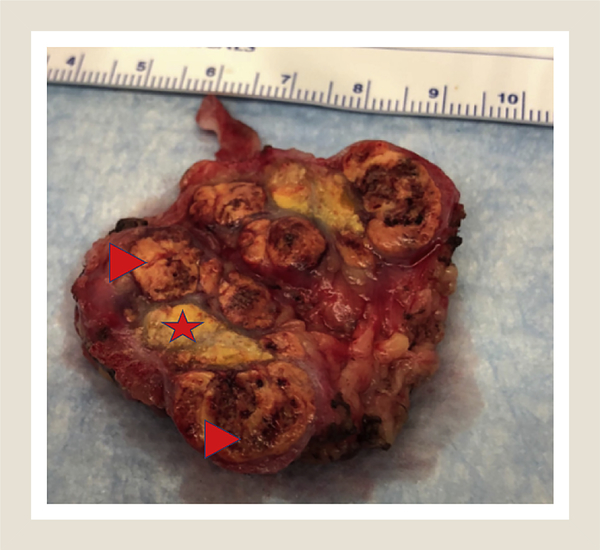
Imaging at 3 months after ablation revealed a decreasing size of the lesion but mild persistent enhancement of the larger of the 2 lesions. Follow-up imaging at 12 months (this patient underwent less frequent imaging, given our knowledge of her tumor biology and tumor kinetics) after ablation revealed continued decrease in size of lesions but persistent peripheral enhancement. At 18 months after ablation, imaging revealed continued enhancement and new growth (Figure 2). She subsequently elected surgical extirpation via uncomplicated open right partial nephrectomy; repeat cryoablation was deemed to be not feasible given the proximity to renal artery and previous treatment failure. Pathologic analysis of the specimen demonstrated an area of central area of necrosis consistent with a treatment effect of the cryoablation flanked by solid tumor consistent with Fuhrman grade 2 clear-cell renal-cell carcinoma (Figure 3).
Figure 2.
Surveillance MRI Images. MRI Images at (A) 3 Months, (B) 12 Months, and (C) 18 Months After Cryoablation. Mild Enhancement Persisted After Cryoablation, With Growth Noted on 18-Month Scan After Progressive Size Decrease Over 1 Year
Abbreviation: MRI = magnetic resonance imaging.
Figure 3.
Gross Picture of Bivalved Partial Nephrectomy Specimen. Note Central Dull Yellow Area Consistent With Necrosis/Treatment Effect of Cryoablation (Star) Flanked by Tan Masses Consisted of Fuhrman Grade 2 Clear-cell Renal-cell Carcinoma (Arrowheads)
Discussion
The reference-standard treatment for small renal masses remains minimally invasive partial nephrectomy.3,4 However, minimally invasive ablative therapies have become more widely used in patients with VHL in recent years.5 Few long-term data on the utilization of these in hereditary cancer syndromes are available, with most experience coming from patients without known hereditary predisposition to renal-cell carcinoma. In one such comparative study between partial nephrectomy and percutaneous ablation reported in 2015, equivalent outcomes of recurrence-free survival were demonstrated; a divergence in metastasis-free survival was noted when comparing partial nephrectomy and cryoablation with radiofrequency ablation.6 In a recent retrospective review comparing partial nephrectomy to percutaneous cryoablation for renal masses in patients with solitary kidneys, no difference was found between estimated glomerular filtration rate at 3 months, local recurrence, distant metastases, or cancer-specific mortality.7 Specifically in a population of VHL patients, a couple of small series have been reported mixed results, although these studies only utilizing RFA. One retrospective study of 11 patients and 48 treated tumors showed 8 (73%) of 11 had successful treatment, with an average of 2.6 treatment sessions required per patient. They noted an overall complication rate of 72.4% with a major complication rate of 6.9%; the study authors recommended against RFA in patients with VHL.8 A separate prospective cohort study in VHL patients undergoing RFA drew a different conclusion. Allasia et al9 performed RFA of 20 tumors in 9 patients with no complications. Three patients had either persistent or recurrent disease, which was successfully treated with repeat RFA. A similarly low complication rate and high success rate was seen in a cohort of VHL patients undergoing ablative treatment in the salvage setting.10 When treatment failures occur, they can be managed with repeat ablation or salvage surgery, as demonstrated in this case.11,12 RFA and cryoablation appear to be equivalent according to retrospective comparisons,13 but no direct head-to-head randomized controlled trial has been performed to determine if any difference in long-term outcomes is present according to treatment modality. Our preference is for cryoablation because the treatment-zone ice ball can be monitored real-time during treatment via ultrasound or computed tomography.14 Regardless of the technique chosen, an imaging follow-up protocol should be followed, typically starting at 1 month after treatment.15
Conclusion
While ablative techniques remain a viable treatment option with durable outcomes in appropriately selected VHL patients, this case demonstrates the need for close follow-up with routine imaging to determine adequate treatment and to monitor for local recurrence.
Clinical Practice Points.
Management of hereditary cancer syndromes requires a multidisciplinary approach to provide the best patient outcomes.
The long-term oncologic utility of ablative techniques in hereditary associated renal-cell carcinoma has not been established.
Close imaging follow-up is essential after ablative treatment of renal tumors.
According to the clinical scenario, ablative treatment failure can be treated with repeat ablation or salvage surgery.
Acknowledgment
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure
The authors have stated that they have no conflict of interest.
References
- 1.Lonser RR, Glenn GM, Walther M, et al. Von Hippel-Lindau disease. Lancet 2003; 361:2059–67. [DOI] [PubMed] [Google Scholar]
- 2.Shuch B, Singer EA, Bratslavsky G. The surgical approach to multifocal renal cancers: hereditary syndromes, ipsilateral multifocality, and bilateral tumors. Urol Clin North Am 2012; 39:133–48. [DOI] [PubMed] [Google Scholar]
- 3.Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol 2017; 198:520–9. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Albiges L, Bensalah K, et al. EAU guidelines on renal cell carcinoma, 2018. In: European Association of Urology Guidelines. Arnhem, Netherlands: European Association of Urology Guidelines Office; 2018. [Google Scholar]
- 5.Joly D, Méjean A, Corréas JM, et al. Progress in nephron sparing therapy for renal cell carcinoma and von Hippel-Lindau disease. J Urol 2011; 185:2056–60. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2015; 67:252–9. [DOI] [PubMed] [Google Scholar]
- 7.Bhindi B, Mason RJ, Haddad MM, et al. Outcomes after cryoablation versus partial nephrectomy for sporadic renal tumors in a solitary kidney: a propensity score analysis. Eur Urol 2018; 73:254–9. [DOI] [PubMed] [Google Scholar]
- 8.Park BK, Kim CK. Percutaneous radio frequency ablation of renal tumors in patients with von Hippel-Lindau disease: preliminary results. J Urol 2010; 183: 1703–7. [DOI] [PubMed] [Google Scholar]
- 9.Allasia M, Soria F, Battaglia A, et al. Radiofrequency ablation for renal cancer in von Hippel-Lindau syndrome patients: a prospective cohort analysis. Clin Genitourin Cancer 2018; 16:28–34. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Autorino R, Remer EM, et al. Probe ablation as salvage therapy for renal tumors in von Hippel-Lindau patients: the Cleveland Clinic experience with 3 years follow-up. Urol Oncol 2013; 31:686–92. [DOI] [PubMed] [Google Scholar]
- 11.Karam JA, Wood CG, Compton ZR, et al. Salvage surgery after energy ablation for renal masses. BJU Int 2015; 115:74–80. [DOI] [PubMed] [Google Scholar]
- 12.Okhunov Z, Chamberlin J, Moreira DM, et al. Salvage percutaneous cryoablation for locally recurrent renal-cell carcinoma after primary cryoablation. J Endourol 2016; 30:632–7. [DOI] [PubMed] [Google Scholar]
- 13.Pirasteh A, Snyder L, Boncher N, Passalacqua M, Rosenblum D, Prologo JD. Cryoablation vs. radiofrequency ablation for small renal masses. Acad Radiol 2011; 18:97–100. [DOI] [PubMed] [Google Scholar]
- 14.Maria T, Georgiades C. Percutaneous cryoablation for renal cell carcinoma. J Kidney Cancer VHL 2015; 2:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iannuccilli JD, Grand DJ, Dupuy DE, Mayo-Smith WW. Percutaneous ablation for small renal masses-imaging follow-up. Semin Intervent Radiol 2014; 31: 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]