Abstract
Objective:
Migraine is three times more common in women. CGRP plays a critical role in migraine pathology and causes female-specific behavioral responses upon meningeal application. These effects are likely mediated through interactions of CGRP with signaling systems specific to females. Prolactin (PRL) levels have been correlated with migraine attacks. Here, we explore a potential interaction between CGRP and PRL in the meninges.
Methods:
Prolactin, CGRP, and receptor antagonists CGRP8–37 or Δ1–9-G129R-hPRL were administered onto the dura of rodents followed by behavioral testing. Immunohistochemistry was used to examine PRL, CGRP and Prolactin receptor (Prlr) expression within the dura. Electrophysiology on cultured and back-labeled trigeminal ganglia (TG) neurons was used to assess PRL-induced excitability. Finally, the effects of PRL on evoked CGRP release from ex vivo dura were measured.
Results:
We found that dural PRL produced sustained and long-lasting migraine-like behavior in cycling and ovariectomized female, but not male rodents. Prlr was expressed on dural afferent nerves in females with little-to-no presence in males. Consistent with this, PRL increased excitability only in female TG neurons innervating the dura and selectively sensitized CGRP release from female ex vivo dura. We demonstrate crosstalk between PRL and CGRP systems as CGRP8–37 decreases migraine-like responses to dural PRL. Reciprocally, Δ1–9-G129R-hPRL attenuates dural CGRP-induced migraine behaviors. Similarly, Prlr deletion from sensory neurons significantly reduced migraine-like responses to dural CGRP.
Interpretation:
This CGRP-PRL interaction in the meninges is a mechanism by which these peptides could produce female-selective responses and increase the prevalence of migraine in women.
Migraine is one of the most sexually dimorphic neurological disorders with prevalence in women at 2–3 times more than men. Among women ages 15–49, migraine is ranked as the most common cause of disability.1,2 While little is known about the underlying causes for the higher prevalence of migraine in females, the largest differences between sexes occurs after the onset of puberty and before menopause, many women have increased susceptibility to attacks during specific days of the cycle, and the frequency/severity of attacks can change across pregnancy, all of which strongly suggest that sex-specific hormone levels influence the pathology.1,3,4
Among the hormones potentially mediating the increased migraine prevalence in women, the pituitary-derived hormone prolactin (PRL) is among the least studied. PRL release from the pituitary of humans and rodents is regulated by gonadal hormones, but also by trauma and stress.5 Although migraine patients do not have higher basal serum PRL levels compared to controls,6 PRL has been shown to rise during migraine attacks in patients with microprolactinoma; importantly, PRL levels do not rise during tension-type-headaches.7 Further, some cases of migraine can be treated by bromocriptine, which blocks the release of PRL from the pituitary,8,9 a treatment typically given for hyperprolactinemia. It has been reported that women who have never experienced migraine, began reporting migraine attacks that correlate with being diagnosed with microprolactinomas.7 Moreover, patients with a history of migraine were found to develop chronic migraine that was correlated with increasing PRL levels.10 This suggests that the elevated PRL levels in hyper-prolactinemic patients increases the susceptibility to migraine.
Calcitonin gene-related peptide (CGRP) signaling plays a critical role in migraine pathology since plasma levels rise during attacks, intravenous administration can trigger attacks, and there are now multiple FDA-approved therapeutics that target CGRP or its receptor.11 Despite the established role for CGRP in migraine, the locations and mechanisms that mediate its actions remain unclear. We showed previously that dural application of CGRP caused female-specific behavioral responses in a preclinical migraine model,12 but the signaling system mediated the sexual dimorphism was not identified. The purpose of the present study was to (1) evaluate whether dural PRL signaling, particularly in sensory afferents, exhibits sexually-dimorphic effects that may contribute to sex/gender differences in migraine; and (2) determine whether there is potential crosstalk between PRL and CGRP signaling within the meninges that can explain the female-selective effects of CGRP in this tissue.
Methods
Animals
In this study, 12-to 14-week-old 260–300 g female and 300–350 g male Sprague-Dawley rats (Taconic; Rensselaer, NY, USA), 6- to 8-week-old female and male ICR mice (Envigo; Indianapolis, IN, USA), 6–8-week-old ovariectomized CD-1 mice (Charles River; Houston, TX, USA; ovariectomy surgeries were performed by Charles River before shipping) and 6- to 8-week-old Nav1.8crc/−/Prlrfl/fl mice on C57/BL6 background were used for behavioral experiments. The Prlrfl/fl line was generated by inserting lox sites for deletion of the 4th exon and causing a mRNA-eliminating frame shift as previously described.13 Ex-vivo experiments were conducted on 8–12-week-old female and male C57/BL6 mice (Jackson Laboratory; Bar Harbor, ME, USA). Primary cultures from Prlrcre/−/Rosa26LSL-tdTomato/+ animals were used for electrophysiology experiments. Additionally, these animals were used in immunohistochemistry experiments. Estrous phases were determined by vaginal gavage as described previously.14 Animals were housed with access to food and water ad libitum on a 12 hours light/dark cycle. Animals were allowed to acclimate to the facility for 72 hours prior to handling and habituation.
Rat Cannula Implantation and Drug Delivery
All rat dural injections were administered at a total volume of 10 μl via an implanted cannula. For the cannula implantation surgery, rats were initially anesthetized at 5% isoflurane via a nose cone; once animals no longer demonstrated a paw-pinch reflex, isoflurane was lowered to 2.0–3.5% for the entirety of the surgery. A longitudinal incision was made through the scalp down the midline to expose the skull. Two screws were inserted below bregma on either side of midline. Using a pin vise (Grainger Industries; Lake Forest, IL, USA) set to a length of 1 mm to leave the dura intact. A 1 mm burr hole was created using a stereotaxic frame at the coordinates to sit above the middle meningeal artery (8 mm anteroposterior, −2 mm mediolateral, 1 mm dorsoventral). A guide cannula (P1 Technologies; Roanoke, VA; C313G/SPC gauge 22) was then inserted into the burr hole and sealed using Vetbond (3M). Hygenic orthodontic resin (Coltene) was used to anchor the cannula to the screw and skull. After the resin hardened a dummy cannula (Pi Technologies 313DCSPC 0.014–0.36 mm fit 1 mm) was inserted into the guide cannula to prevent clogging. Following surgery, animals were given 8 mg/kg gentamicin and 0.25 mg meloxicam to prevent infection and for pain management, respectively. Animals were returned to their home cage and allowed to recover for approximately 7 days.
Mouse Dural Injections
Mouse dural injections were performed according to previously published methods.15 Briefly, mice were anesthetized under isoflurane for <2 minutes and injected with a modified internal-cannula (Invivol, part #8IC3131SPCXC, Internal Cannula, standard, 28 gauge). The inner portion of the cannula was adjusted with calipers to extend from 0.5 to 0.65 mm in length. This inner portion was used to inject a volume of 5 μl through the soft tissue at the intersection of the lambdoidal and sagittal sutures.
Mouse Von Frey Testing
Mice were handled for a single 5-minutes session at 24-hours prior to habituation to the behavior chambers. During each session of habituation animals were placed in 4 oz paper cups (Choice) for 2 h a day for three consecutive days as previously described.15 Habituation was done in the rooms where all further behavioral testing occurred to acclimate animals to the room and light conditions. Von Frey testing of the periorbital skin15 was used to assess baseline values following habituation prior to stress as well as mechanical hypersensitivity that resulted from drug treatments. Food was placed in the chamber for each animal for each day of testing. Baselined animals were defined as animals that exhibited a withdrawal threshold approximately 0.5–0.6 g. Filaments greater than 0.6 g were not used. This was set as the maximum gram weight in our initial development of this preclinical model.15 The majority of mice showed facial responses above the 0.6 g threshold. Mice with a baseline threshold lower than 0.5 g at the end of 4 days were excluded from experiments. Mechanical thresholds were determined by applying von Frey filaments to the periorbital region of the face (the midline of the forehead at the level of the eyes) in an ascending/descending manner starting from the 0.07 g filament. Briefly, if an animal did not respond, increasing filament forces were applied until the 0.6 g filament was reached or until a response was observed. If the animal responded to a specific filament, decreasing filament forces were applied until the 0.008 g filament was reached or until there were no responses. A response was defined as a mouse actually removing/swiping the filament away from its face during application. All animals were numbered and randomly allocated to experimental groups by drawing from pre-labeled paper slips. All experimenters were blinded to the treatment groups until the end of each experiment.
Rat Von Frey Testing
Rats were handled for a single 5-min session at 24 h prior to habituation to the behavior chambers. Rats were subject to 2-h sessions of habituation a day for 3 days. During each session of habituation animals were placed in testing chambers in the rooms where all further behavioral testing occurred to acclimate animals to the room and light conditions. Baselined rats were required to have a periorbital von Frey threshold of 8 g, animals were not tested above 8 g for comparison with previous experiments.12,16 If a rat did not meet this threshold by end of habituation they were not included in experiments. Rats’ withdrawal thresholds were assessed in the same ascending/descending manner as mice; however, testing began at 1 g with a maximum of 8 g, and minimum of 0.4 g.
Primary TG Neuron Cultures
Trigeminal ganglia (TG) neurons that innervate the dura mater were identified by dural injection (5 μl) of 1% WGA-488 (Applied Biosystems; Foster City, CA, USA) 48 hours prior to vaginal smear/TG neuronal culture generation. Vaginal smears were performed on mice from separate cages. Only mice in the estrous phase of the reproductive cycle were used for TG neuronal culture preparation and subsequent patch clamp recording. WT or reporter mice, including Prlrcre/−/Rosa26LSL-tdTomato/+, were used for patch clamp recording. For TG dissection, mice were deeply anaesthetized with isoflurane (0.3 ml in 1 L administered for 60–90 seconds) and sacrificed by cervical dislocation. The V1 area of the TG was quickly removed, and neurons were dissociated by treatment with a 1 mg/ml collagenase-dispase (Roche, Indianapolis, IN) solution. Cells were maintained in DMEM supplemented with 2% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin and no NGF. Experiments were performed within 6–24 hours after TG neuron plating.
Dural CGRP Release Assay
Mustard oil (MO: Fluka, St. Louis, MO, USA) stock (100%) was diluted in Hank’s buffer (HBSS) to 0.01% (1 mM). HBSS was pH adjusted to 6.9 using HEPES (20 mM). The entire mouse dura from two separate mice were combined and submerged into a single well and treated as an N of 1. Dura remained submerged for the entirety of release experiments. Experiments were carried out at 37°C. Dura tissues were washed once with HBSS and then soaked in HBSS for 30 min to equilibrate, the supernatant was collected for measurement of baseline CGRP release after 15 min in HBSS. Tissues were then exposed for 3 min to MO, pH 6.9 HBSS, MO + PRL or pH 6.9 HBSS + PRL (PRL was used at 1 μg/ml), then each solution was replaced with HBSS. Tissues were maintained for additional 15 min in HBSS. The total evoked CGRP release was measured by pooling the 3 min treatment exposure sample with a 15 min vehicle post-exposure sample. Dura biopsies were only used once and only exposed to one sequence of treatments. The CGRP radioimmunoassay was conducted as previously described17 with a primary antibody against CGRP (final dilution, 1:1 X 106; kindly donated by Dr. Michael J. Iadarola (NIDCR/NIH, Bethesda, MD, USA). CGRP release data were normalized by the weight of fresh dura biopsies, to avoid compromising tissue as it dries. Data are presented as percent release above baseline.
Electrophysiology
Recordings were made in whole-cell current clamp configuration at 22–24° C. Data were acquired and analyzed using an Axopatch 200B amplifier and pCLAMP 10.6 software (Molecular Devices, Sunnyvale, CA, USA). Recorded data were filtered at 5 kHz and sampled at 20 kHz. Borosilicate pipettes (Sutter, Novato, CA, USA) were polished to resistances of 2–3 MΩ. Access resistance (Rs) was compensated (40–80%) to maintain resistance <6–8 MΩ. Data were rejected when Rs changed >20% during recording, leak currents were >100pA, or input resistance was <300 MΩ. Standard external solution (SES) contained (in mM): 140 NaCl, 5 KC1, 2 CaCl2, 1 MgCl2, 10 D-glucose and 10 HEPES, pH 7.4. The standard pipette (internal) solution (SIS) contained (in mM): 140 KCl, 1 MgCl2, 1 CaCl2, 10 EGTA, 10 D-glucose, 10 HEPES, pH 7.3, 2.5 ATP and 0.2 GTP. Drugs were applied by a fast, pressure-driven and computer controlled 4-channel system (ValveLink8; AutoMate Scientific, San Francisco, CA, USA) with quartz application pipettes.
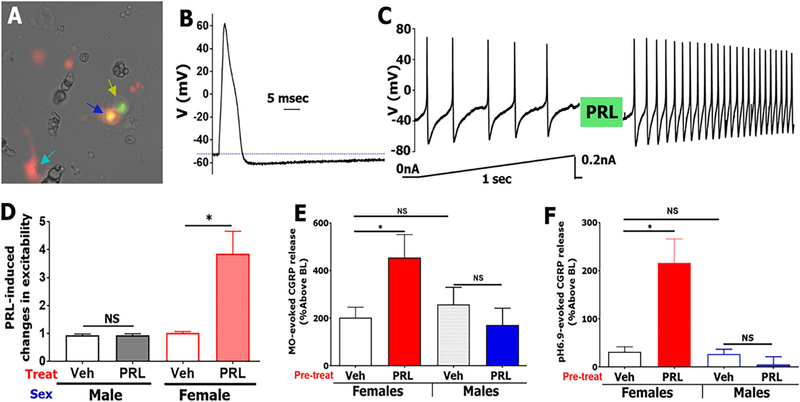
Small (<30 pF) WGA+/Prlr-cre+ TG neurons from Prlrcre/−/Rosa26LSL-tDTomato/+ reporter mice were randomly selected for recording (Fig 5A). To characterize modulation of these TG neurons excitation by vehicle (control) or PRL (200 ng/ml), the following sequence of recording protocols were applied: (1) a single AP in current clamp configuration was generated with a 0.5 ms and 1 nA current step to define the type of sensory neurons18 (Fig 5B); (2) a linear ramp from 0 to 0.2 nA for 1 second was applied to generate a control AP train (Fig 5C); (3) the patched neuron was treated for 2–5 min with vehicle or PRL; and then (4) the ramp as in step 2 was re-applied (Fig 5C). Data were accumulated from four independent mouse TG neuronal cultures for each sex. Each culture was generated from one male or estrous female mouse. Changes in neuronal excitability were calculated by dividing AP frequency generated by a current ramp after vehicle or drug-treatment to AP frequency produced by the ramp before treatment. Excitability was determined to be regulated by PRL when the PRL treatment produced statistically significant increase in AP frequency than vehicle-treatment (i.e. control).
FIGURE 5:
PRL selectively sensitizes Prlr-positive TG neurons innervating the dura of female mice. Prlr-cre+/WGA-488+ back-traced neurons from dura TG neuron (blue arrow) were selected for recording. Yellow arrow shows Prlr-cre-/WGA-488+ TG neuron. Sapphire arrow shows Prlr-cre+ non-neuronal cell. (A) Action potential (AP) from small-sized (25 pF) selected Prlr-cre +/WGA-488+ TG neuron. Stimulus waveform is 1,000 pA, 0.5 ms. (B) Train of APs in a selected Prlr-cre+/WGA-488+ TG neuron (same neurons as panels A and B) was stimulated with current ramp protocol shown below trace. The neuron was treated with exogenous PRL (200 ng/ml) for 5 min, and the same current ramp protocol was applied. Ratio of post-PRL AP frequency to before-PRL AP frequency reflects changes in excitability. (C) PRL-induced changes in excitability of Prlr-cre+/WGA-488+ TG neuron from females and males. Control is vehicle treatment between two current ramps, n = 7–14 (D) PRL (1 μg/ml)-induced sensitization of MO (0.01%)-evoked CGRP release from female and male dura biopsies, n = 4–6. (E) PRL (1 μg/ml)-induced sensitization of pH 6.9-evoked CGRP release from female and male dura biopsies, n = 4 (F) Statistics are 2-way ANOVA with variables as sex and treatment (NS, non-significant; *p < 0.05).
Immunohistochemistry (IHC)
Dura mater from perfusion-fixed female and male WT and Prlrcre/−/Rosa26LSL-tDTomato/+ mice were fixed again with 4% paraformaldehyde and cryoprotected with 30% sucrose in phosphate buffer. Anti-Prlr rabbit polyclonal (NSJ Bioreagents; San Diego, CA, USA; catalogue R31199; 1:200) (19,20); anti-CGRP rabbit polyclonal antibodies (Sigma; C8198; 1:300) (18,20); anti-PRL rabbit polyclonal antibodies (Bioss, Boston, MA, USA; cat: BS23763R; 1:200); and anti-CD31 rat monoclonal (Clone: MEC 13.3) antibodies (BD Pharmingen; cat: 553370; 1:400) were used for IHC. Sections were incubated with species appropriate Alexa Fluor secondary antibodies (1:200; Molecular Probes, Eugene, OR, USA). Images were acquired using a Nikon Eclipse 90i microscope (Melville, NY, USA) equipped with a C1 si laser scanning confocal imaging system. Images were processed with NIS-elements software (Nikon Instruments, Melville, NY, USA). Control IHC was performed on tissue sections processed as described but either lacking primary antibodies or lacking primary and secondary antibodies. IHC images were obtained from three to five independent tissue sections or whole tissues from three to four animals. Z-stack images were used for presentation and analysis.
Drugs
See Table 1 for information on drug sources, doses, and administration routes.
TABLE 1.
Drug Information
| Drug | Source | Dose | Administration route |
|---|---|---|---|
| Prolactin (human) | Vincent Goffin, INSERM | 5 μg, 0.5 μg | Dural |
| Δ1–9-G129R-hPRL | Vincent Goffin, INSERM | 5 μg | Dural |
| CGRP | Bachem | 1 pg | Dural |
| CGRP8–37 | Bachem | 100 ng | Dural |
Statistics
Data are presented as mean SEM. Data were analyzed among groups at each time point via two-way ANOVA and followed by Bonferroni post-test where appropriate. Prism (Graph-Pad Software) was also used for data analysis. Significance was set to p < 0.05 for all analyses. Experimenters were blinded to treatment groups. Allocation of animals to treatment groups was randomized by a "blinder" that drew animal numbers from a bag of paper slips.
Study Approval
All procedures were conducted with prior approval of the Institutional Animal Care and Use Committee at the University of Texas at Dallas and the University of Texas Health Science Center at San Antonio.
Results
Prolactin Causes Female-Specific Migraine-Like Behavioral Responses in Rodents
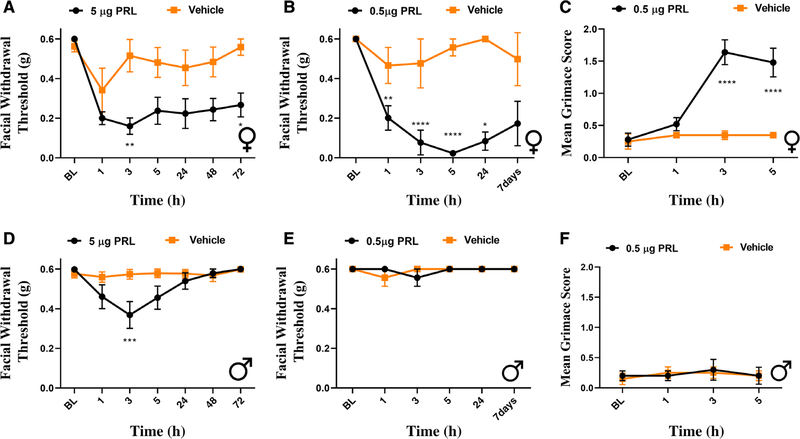
In order to investigate whether PRL signaling within the meninges causes differential behavioral responses in females and males, we used a preclinical migraine model in which stimuli are applied to the dura and cutaneous periorbital hypersensitivity and facial grimace are measured.15 Initially, 5 μg of PRL was applied to the dura of both female and male wild-type ICR mice. Significant facial hypersensitivity was observed in both female (Fig 1A) and male (Fig 1D) mice in response to PRL. However, this effect was prolonged in the female mice, lasting out to at least 72 h following application of PRL. In contrast, we observed a transient allodynia in males with significant facial hypersensitivity only at 3 h following PRL application. Next, since 5 μg PRL likely causes tissue concentrations that are higher than those found under physiological and pathological conditions,21,22 we applied a lower dose of PRL (0.5 μg) to the dura to determine whether this dose caused responses only in females. This lower PRL dose caused facial hypersensitivity in females (Fig 1B), lasting out to at least 7 days, while no effect was observed in males (Fig 1E). Additionally, only female mice exhibited grimace behaviors in response to this low dose PRL when compared with their respected controls (Fig 1C). These data demonstrate that dural application of PRL causes migraine-like responses that are female-specific. However, high, likely non-physiological dosages of PRL can cause transient and smaller-magnitude periorbital allodynia in males.
FIGURE 1:
Dural prolactin induces greater behavioral responses in female mice. Male and female mice had mechanical withdrawal thresholds assessed prior to dural injection of either 5 μg or 0.5 μg PRL. Following 5 μg PRL, both female (A) (n = 7 PRL, 6 vehicle) and male (D) (n = 7 PRL, 7 vehicle) mice exhibited facial hypersensitivity. Only females exhibited a significant hypersensitivity at low dose PRL (B) (n = 5 PRL, 4 vehicle). Animals that received 0.5 μg of PRL were additionally assessed for grimace prior to facial testing at each time point (C). Female mice that received this dose of PRL experienced significant grimace in comparison with respective controls, while male mice (n = 4 PRL, 4 vehicle) exhibited no significant grimace. Two-way ANOVA followed by Bonferroni multiple comparison analysis indicated significant differences between females that received PRL when compared with those that received vehicle. Data are represented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. See Table S1 for additional results of analysis.
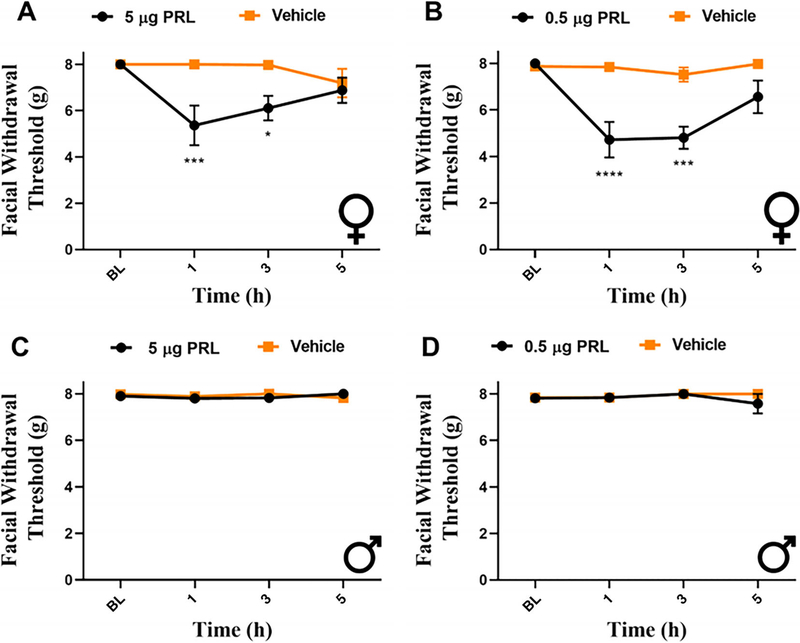
To rule out the possibility of a species-specific effect, PRL was also applied to the dura of Sprague-Dawley female and male rats. Unlike in mice, even the higher dose of PRL (5 μg) applied to the dura caused significant facial hypersensitivity only in females (Fig 2A, C). These responses were significant at 1 and 3 h post-injection, and animals returned to baseline by 5 h. The lower dose of PRL (0.5 μg; Fig 2B, D) was also female-specific, with hypersensitivity observed at 1 and 3 h following administration, and animals returned to baseline by 5 h. These findings demonstrate that dural application of PRL causes female-specific behavioral responses in both mice and rats.
FIGURE 2:
Dural prolactin causes female-specific behavioral responses in rats. Facial withdrawal thresholds were measured in female and male rats prior to and following dural injection of either 5 μg (A, C) or 0.5 μg dural PRL (B, D). Two-way ANOVA followed by Bonferroni multiple comparison analysis indicated significant differences between females that received 5 μg PRL (n = 8 PRL) when compared with those that received vehicle (n = 9). No significant responses were seen in males at this dose (n = 8 PRL, 7 vehicle). At the 0.5 μg PRL dose, female mice that received PRL (n = 8) demonstrated significant hypersensitivity when compared with controls (n = 7). No significant effect was found in males (n = 5 PRL, 5 vehicle). Data are represented as means ± SEM. *p < 0.05,***p < 0.001, ****p < 0.0001. See Table S1 for additional results of analysis.
Ovariectomy Does Not Prevent Responses to Dural PRL
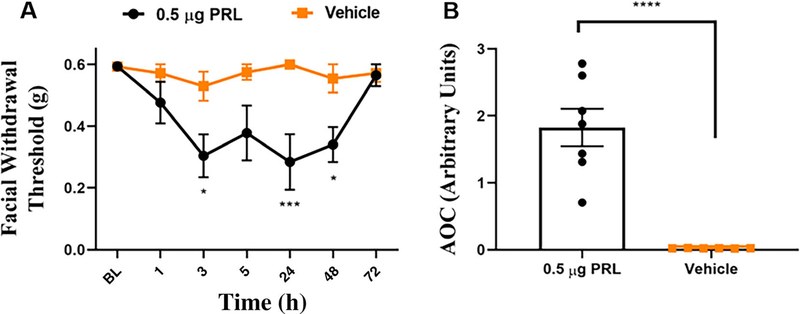
Given the role for ovarian-derived estrogen and progesterone in prolactin release and Prlr regulation, we asked whether disruption of the hypothalamic-pituitary-ovarian (HPO) axis would decrease the behavioral responses in females to levels similar to those observed in males. To address this, ovariectomized females were purchased 2 weeks following ovariectomy and received injection of either 0.5 μg PRL or vehicle onto the dura 3 weeks following surgery (Fig 3A, B). Ovariectomized females that received PRL demonstrated significant responses at 3, 24, and 48-h post injection compared with those that received vehicle. PRL-injected OVX females returned to baseline by 72 h, in contrast to intact females (Fig 1B). These data suggest that while there may be subtle differences in regulation of Prlr expression or signaling in the dura by HPO axis, PRL-induced migraine-like pain is largely independent of regulation by ovarian-derived hormones.
FIGURE 3:
Dural PRL induces behavioral responses in ovariectomized female mice. Ovariectomized female mice received dural injection of either 0.5 μg PRL (n = 8) or vehicle (n = 8), 3 weeks after surgery. Data are additionally represented as area over the curve (B). Two-way ANOVA followed by Bonferroni multiple comparison analysis indicated significant differences between OVX females that received PRL and those that received vehicle. Data are represented as means ± SEM. *p < 0.05, **p < 0.01„***p < 0.001, ****p < 0.0001. See Table S1 for additional results of analysis.
Expression of Prlr Is Higher in Female Dura Mater
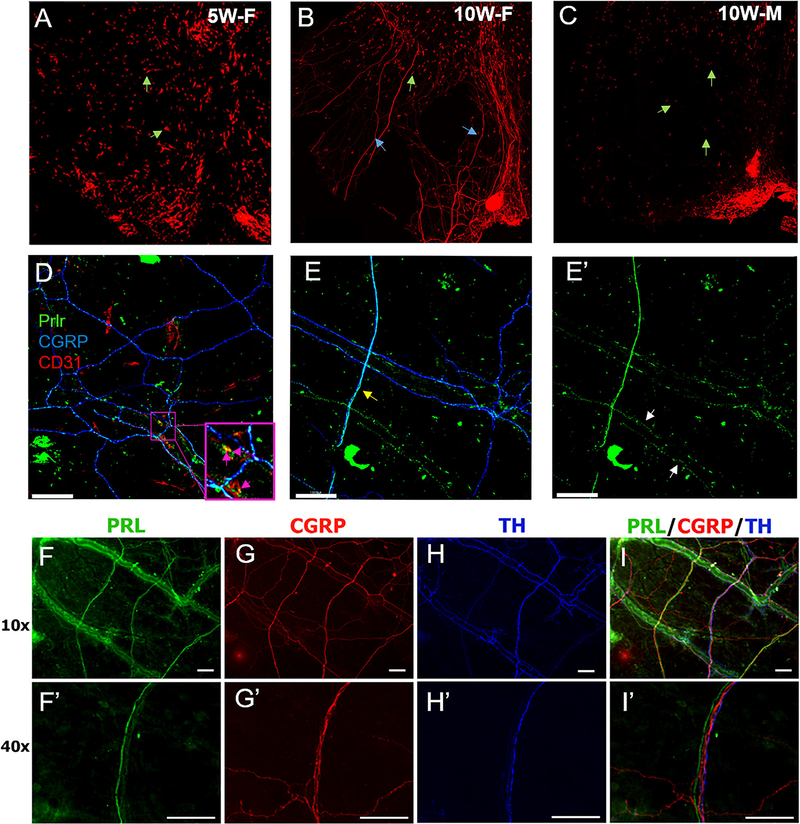
Given the ability of PRL to induce female-selective facial hypersensitivity, we investigated whether these sexually dimorphic effects could be due to differential expression of (Prlr) within this tissue. We took advantage of a mouse line where tdTomato is present in cells that express Prlr (Prlrcre/−/Rosa26LSL-tdTomato/+). In 5-week-old females (i.e. pre-puberty), Prlr expression appeared to be restricted to non-neuronal cells (Fig 4A). However, in 10-week-old reproductively mature females, Prlr expression was clearly evident on both resident dural cells as well as neuronal fibers that innervate the dura mater (Fig 4B). In 10-week-old males, tdTomato was observed almost entirely in non-neuronal cells, with only sparse expression on nerve fibers (Fig 4C).
FIGURE 4:
Expression of Prolactin and prolactin receptor in mouse dura. A 3 x 3 mm square area of dura mater from 5-week-old female (A), 10-week-old female (B) and 10-week-old male (C) Prlrcre/-/TdT mice. Green arrows mark Prlr-cre+non-neuronal cells. Blue arrows mark Prlr-cre+dural afferent fibers. Objective (10x, scale bars represent 120 μm). Dura mater from 10-week-old female Prlrcre/-/TdT mice labeled with anti-CGRP and anti-CD31 antibodies (D). Pink arrows show dural blood vessel co-expressing Prlr-cre+(Green) and CD31 + (Red) cells. Objective (20x, scale bars = 120 μm). Prlr-cre+dural afferent fibers express CGRP (Blue) and are indicated with yellow arrows (E). White arrows mark Prlr-cre+ expression on non CGRP expressing dural fibers (E'). Objective (20x). PRL (F, F'), CGRP (G, G'), and TH (H, H') expression in female mouse dura. Overlap of (F-H) shown in (I). For 40x images scale bars represent 100 μm.
Next, we identified Prlr+ cell types. Co-labeling for endothelial cells in blood vessels (CD31) and sensory fibers (CGRP) in female dura showed that Prlr is co-localized on both structures (Fig 4D, E’), as previously reported.2,23 Using immunohistochemistry for Prlr (instead of genetically-driven tdTomato expression), labeling was similarly found on both blood vessels and dural nerve fibers (Fig 4F, F’). These PRL-expressing fibers were found to run alongside CGRP expressing fibers (Fig 4G, G’) and fibers expressing Tyrosine hydroxylase (TH) (Fig 4I, I’). Labeling associated with blood vessels was not different between female and male dura while neuronal labeling appeared less extensive. These data suggest that PRL-induced female-selective migraine behavior is likely due to differential expression of Prlr on CGRP + nerve fibers in female and male dura.
PRL Has Female-Specific Effects on Dural Afferent Excitability and CGRP Release
Since the Prlr appeared to be more highly expressed on nerve endings in female dura, we tested whether there are selective actions of PRL on dural afferents using both in vitro patch-clamp electrophysiology of TG neurons innervating dura and evoked CGRP release from ex vivo dura tissue. First, TG neurons innervating the dura were identified by retrograde labeling using application of the tracer wheat-germ agglutinin 488 (WGA-488) to the dura. TG were dissected from females in estrous and male Prlrcre/−/Rosa26LSL-tdTomato/+ mice within 48 h after appliation of WGA-488 to the dura and neurons were cultured. Dural afferents were selected for recording in the cultures based on the presence of WGA-488, indicating retrograde tracing from the dura, and Prlr expression was determined by the presence of tdTomato (Fig 5A). Importantly, among the 40 culture dishes used from female mice in these experiments, there were 541 neurons that expressed both Prlr and WGA-488, and a majority of them were small-sized (<35 μm) neurons. Among 44 dishes from male mice, there were only 140 neurons expressing both Prlr and WGA-488, and approximately 90% of them were medium-sized (>35 μm) neurons. The predominant expression of Prlr in small-sized sensory neurons in females and medium-sized sensory neurons in males is consistent with that reported for dorsal root ganglia (DRG).20 A typical action potential (AP) shape from a small sized (<30 pF) female WGA-488+/tdTomato+ neuron is shown in Fig 4B, similar to those shown previously.18,24 Dural afferents were stimulated with 1 sec ramps from 0 to 200 pA. When PRL (200 ng/ml) was applied to these neurons after the first ramp protocol, the number of AP’s fired on a subsequent ramp protocol was significantly greater in female but not male neurons (Fig 5C, D). These data show that PRL application to dural afferent cell bodies selectively sensitizes female neurons.
Next, we used freshly dissected dura from the mouse skull to measure CGRP release, which presumably originated from the sensory nerve endings innervating the dura. As mustard oil (MO) is known to stimulate sensory neurons and cause release of CGRP25 and also cause migraine-like behaviors when applied to the dura,15,26 we used this stimulus (0.01%) to determine whether PRL application to the dura would potentiate this response. Female dura treated with PRL (1 μg/ml) prior to MO exhibited an approximately 200% increase in CGRP release compared to females treated with MO and vehicle (Fig 5E; MO and vehicle causes a 200% increase in CGRP release over baseline). There was no significant difference in release of CGRP in males treated with vehicle or PRL, despite similar increase to females in CGRP release over baseline with MO alone. We also tested a pH 6.9-buffered solution, which activates acid-sensing ion channels (ASICs), a family of receptors that we have previously shown to play a role in afferent signaling from the dura.16,27,28 Unlike MO, the pH 6.9 solution caused little release of CGRP over baseline on its own (Fig 5F). However, the application of PRL (1 μg/ml) along with pH 6.9 led to a 200% increase in CGRP release over baseline, and at least 5-fold potentiation of responses. As with MO, the potentiating effect of PRL only occurred in females. These female-specific effects of PRL on dural afferents in vitro and on CGRP release from dura are consistent with the female-specific behavioral responses of dural PRL in Figs 1 and 2, as well as higher Prlr expression on sensory afferents in females shown in Fig 4.
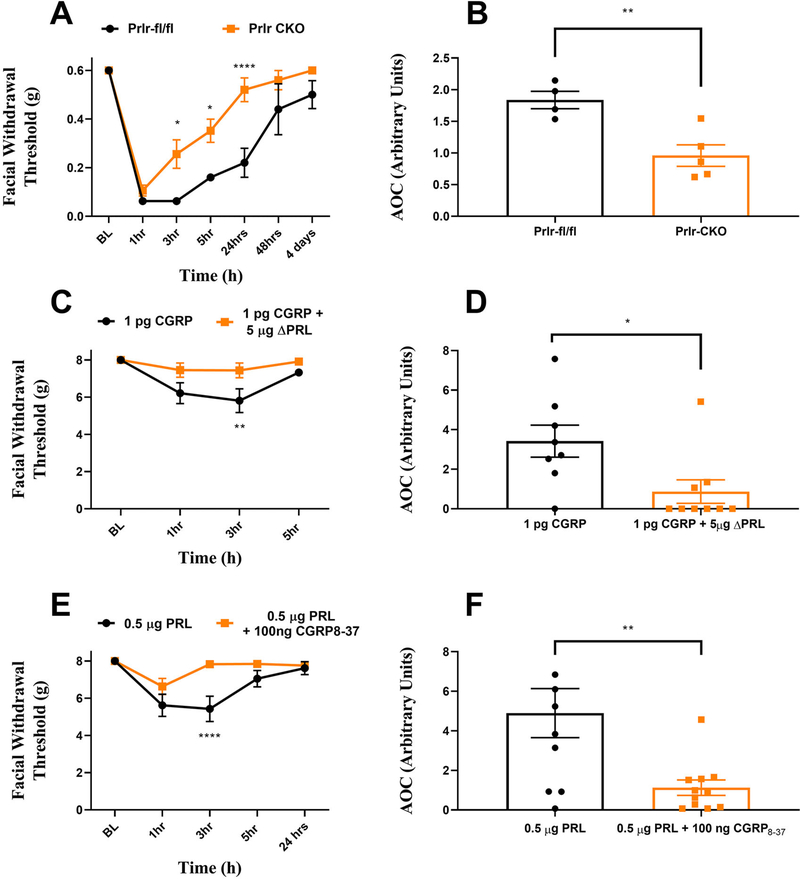
Loss of Prlr on Sensory Neurons or Block of Meningeal Prlr Attenuates Migraine-Like Behavioral Responses to Dural CGRP
The female-specific nature of the behaviors caused by dural application of PRL (Figs 1, 2) are remarkably similar to the female-specific responses to dural application of CGRP we showed previously.12 This led us to ask whether there might be a relationship between the signaling mechanisms engaged downstream of dural Prlr and CGRP receptor activation. We thus took advantage of a conditional knockout (CKO) mouse where the Prlr is selectively deleted from Nav1.8-positive sensory neurons, using a Navl.8-cre line crossed to a mouse line a floxed Prlr (Prlrfl/fl). We have previously characterized this mouse, showing the loss of Prlr expression in Nav1.8+ dorsal root ganglion neurons.23 Female Prlr CKO mice and their control littermates (Prlrfl/fl only) were administered 1 pg dural CGRP, a dose we showed previously to have female-specific effects in mice.12 While responses to dural CGRP in Prlr CKO mice were similar to their wild type counterparts at 1 h, the Prlr CKO mice showed significantly attenuated periorbital withdrawal thresholds at hours 3 and 5 post-injection as well as 24 h post injection (Fig 6A). These values are additionally represented as AOC (Fig 6B). In control mice, migraine-like responses to dural CGRP returned to baseline at 4 days post-injection. In contrast, in the Prlr CKO mice, return to baseline occurred significantly faster at 24 h post-injection. These critical findings suggest that the mechanism by which dural CGRP causes hypersensitivity is partially mediated via Prlr signaling in sensory neurons.
FIGURE 6:
Crosstalk between the CGRP and PRL signaling systems within the dura. Female prolactin receptor CKO mice (n = 5) and their control littermates (n = 4) had mechanical withdrawal thresholds tested prior to and following injection with CGRP (A, B). Female rats had baseline thresholds assessed and received dural injection of 1 pg CGRP (C, D) (n = 9) or 1 pg of CGRP co-injected with 5 μg of delta PRL (n = 9). Co-injection with delta prolactin significantly attenuated CGRP induced responses, as indicated via two-way ANOVA followed by Bonferroni post-hoc analysis. A separate cohort of female rats received injection of either 0.5 μg dural PRL (n = 10) or co-injection of 0.5 μg PRL with 100 ng CGRP8–37 (E, F) (n = 11). Co-injection with CGRP8–37 significantly reduced behavioral responses to dural PRL. Data are represented as means ± SEM. **p < 0.01,****p < 0.0001. See Table S1 for additional results of analysis.
To determine whether a similar Prlr-dependent mechanism of action of dural CGRP exists in rats, we tested whether the Prlr antagonist Δ1–9-G129R-hPRL (modified human PRL molecule that sterically inhibits Prlr activity in humans and rodents,29 hereafter referred to as ΔPRL) blocked the response to CGRP. In our previous study, dural administration of CGRP produced female-specific facial hypersensitivity in rats as well as mice.12 Here, female rats received either 1 pg CGRP or co-administration of 1 pg CGRP +5 μg ΔPRL, and migraine-like behaviors were tested as in Fig 2. Female rats responded to dural CGRP as shown previously, but these responses were significantly blocked at the 3-h time point by ΔPRL (Fig 6C). While we cannot precisely identify the location of the Prlr’s sites of actions in this experiment, the findings are nonetheless consistent with mice where Prlr was deleted from Nav1.8-expressing neurons. This suggests that one of the locations of Prlr in these rat experiments is on sensory fibers.
Given the data described above showing that Prlr contributes to the response to dural CGRP, we next asked whether the reverse might also be true, i.e. whether the CGRP receptor contributes to responses to dural PRL. Female rats received either 0.5 μg dural PRL or a co--injection of 0.5 μg PRL and 100 ng of the CGRP receptor antagonist CGRP8–37 Co-administration of PRL and CGRP8–37 significantly attenuated the response to dural PRL at the 3-h timepoint following injection (Fig 6E). These data demonstrate that not only does Prlr contribute to the CGRP response but the CGRP receptor also contributes to the PRL response. Thus, there is crosstalk between these two signaling systems in the mechanisms that are used to produce female-specific responses from the dura. Moreover, this crosstalk involves Prlr+ sensory neurons.
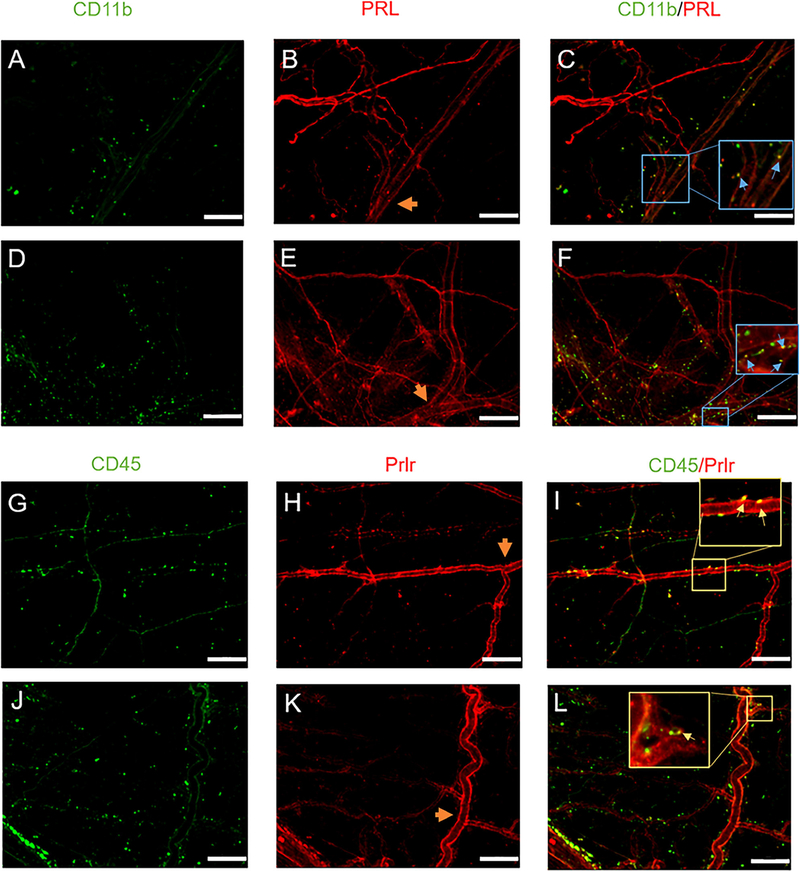
PRL and Prlr Expression on Multiple Cell Types Within the Dura
The effects observed in the Prlr CKO mice (Fig 6A) as well as with ΔPRL (Fig 6C) following dural CGRP suggest that endogenous PRL is involved in these behavioral responses which may be produced locally within the dura from extra-pituitary cells.30–32 To address this, dura from 10-week-old female mice were extracted and used for IHC. We observed PRL immunoreactivity in immune cells as marked by CD11b (Fig 7A–C; Blue arrows) indicating a potential role for PRL release from immune-like cell types of the dura. Ten-week-old males had similar expression patterns (Fig 7D–F), suggesting that differences in PRL signaling within the dura are likely due to differential receptor expression patterns (Fig 4A–C) and not due to differential expression of the ligand.
FIGURE 7:
Expression of prolactin and prolactin receptor in non-neuronal cells in the dura. Dura mater was removed from 3–5-month-old female (A-C) and male (D-F) mice. These dura were processed and stained for PRL expression and co-stained with CD11b. Images were taken at 20x. Scale bars represent 100 μm. Blood vessels are included as a potential source of prolactin release. Orange arrow heads indicate the middle meningeal artery (MMA) which served as a biological marker to ensure images were taken from the same region of animals. CD11b expression (A, D), PRL expression (B, E) and the overlay is shown in (C, F). Inserts in (C, F) highlight overlap of PRL and CD11b. Expression of Prlr in intact females. Prolactin receptor expression (H) was assessed in 3–5-month-*old female mice, dura was co-stained for CD45 (G) and overlap shown in (I). Dura of littermate Prlr conditional knockout animals, that have Prlr deleted from Nav1.8 sensory neurons, were also stained to assess the presence of Prlr (J-L). Inserts in (I, L) highlight overlap of Prlr and CD45.
To address whether other cell types within the dura may contribute to local PRL-dependent signaling following dural CGRP, we examined Prlr expression in relation to the immune cell marker CD45 in control females (Fig 7G–I) and Prlr CKO females (Fig 7J–L). These data show expression of Prlr on CD45-positive cells, and as expected, there was no influence of Prlr CKO on expression of Prlr within the CD45 cell population. This suggests that the responses to dural CGRP in the Prlr CKO mice shown in Fig 6 may involve Prlr activation of CD45-positive immune cells as part of this local dural signaling mechanism.
Discussion
While the mechanisms responsible for the increased prevalence of migraine in females are likely to be complex, the data shown here are the first to describe a female-specific signaling circuit within the meninges that may be an important component in the headache phase of attacks. This circuit is based on an interaction between PRL and CGRP, the latter a critical contributor to migraine, but one whose mechanisms in the disorder remain unclear. We show that exogenous application of PRL onto the dura of both rats and mice produces female-specific responses at the 0.5 μg dose. At the 5 μg dose, male mice demonstrated short lasting allodynia. The lower potency and reduced response in males are likely due to low neuronal expression of Prlr within the dura (Fig 4). Surprisingly, this same high dose (5 μg) of PRL in females (Fig 1A) resulted in less robust allodynia than the lower 0.5 μg dose (Fig 1B). This observation may be due to the higher concentrations of PRL causing Prlr internalization in females, as shown previously with Prlr on pancreatic β islet cells in rat.33 Regardless, both doses of dural PRL demonstrated facial hypersensitivity that was clearly more robust in females.
These female-specific responses are likely due to higher Prlr expression on sensory nerve endings in adult female dura compared to males (Fig 4A–C). Consistent with the behavioral data and female-specific expression of Prlr on nerve endings in the dura, we found a sensitizing effect of PRL on isolated female dura that led to increased CGRP release, while no effect was seen on male dura. This was similar in TG neurons, as electrophysiology on cultured TG cells back-labeled from the dura revealed that PRL only induced hyperexcitability in neurons from female mice. This may explain how PRL is able to potentiate CGRP release from female but not male dura. Regardless of the source of endogenous PRL release, whether from the pituitary or cells native to the dura, the pro-nociceptive actions of PRL are likely to be limited to females due to the mechanisms shown here. We also show bi-directional communication between the PRL and CGRP signaling systems as interventions targeted toward each receptor block responses to the opposing peptide. These data suggest that both PRL and CGRP play a more significant role in migraine in females and that these signaling systems are dependent on each other for their actions in the meninges.
These experiments also show that responses to dural PRL are maintained in ovariectomized females. This is not surprising, as ovariectomy only transiently alters endogenous PRL levels. Prolactin mRNA decreases following ovariectomy in female rats for the initial 2 weeks following surgery but returns to those of intact controls by week 3 and 4.34 Additionally, ovariectomy does not alter serum PRL in female rats at various stages of the reproductive cycle.35 In women that have undergone total abdominal hysterectomy with bilateral salpingo-oophorectomy, PRL levels returned to pre-surgery baseline levels by 6 weeks post-surgery36 and remain non-significantly altered 1 year after surgery.37 These data suggest continued Prlr expression after ovariectomy given the lack of changes in circulating levels of PRL caused by this surgery.
Our data are consistent with prior studies in models of postoperative and inflammatory pain, where PRL has been shown to contribute to nociception in a female-specific manner.20 These data support previous findings showing PRL increased TRPV1-, TRPM8-, and TRPA1mediated responses in sensory neurons from DRG in female mice, but not males.38 Moreover, female Prlr CKO mice, showed reduced behavioral responses to CFA, whereas males did not show significant deficits.38 While these findings were in the spinal system, PRL has been shown to sensitize TG neurons and increase capsaicinevoked CGRP release from TG neurons.39 In the current study, Prlr expression was not seen on dural fibers of 5-week female mice. During human adolescence the mean serum PRL levels in males and females are similar while adult PRL levels are significantly increased in females but not in males,40 which aligns with the findings here of differential receptor expression across age. In the approximately 10-week old animals used here, PRL causes a more robust allodynic response in females and induces excitability of TG neurons back-labeled from female dura. Thus, our data may fit with a general pattern for a role of PRL in nociceptive signaling in adult females but not males and may offer insight to the emergence of migraine following the onset of puberty.41,42
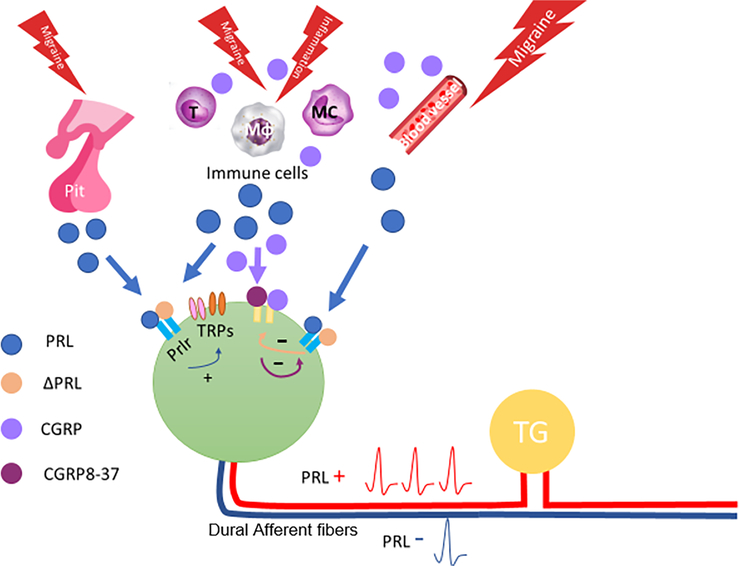
Importantly, this work demonstrates the apparent crosstalk between PRL and CGRP signaling in the meninges. Given the similar pattern of female-specific behavioral responses between PRL shown here and that of CGRP (shown previously12), it may not be surprising that the signaling systems interact. Prlr is known to be expressed on CGRP-positive sensory fibers23 and activation of these fibers by PRL (pituitary and/or extra-pituitary) likely promotes the release of CGRP (as shown in Fig 5). It is well known that CGRP is a potent vasodilator and its receptors are located on cells of the vasculature. CGRP may then act on these nearby blood vessels as well as immune cells,43,44 or other nerve fibers45,46 to promote a positive-feedback loop that potentiates local inflammatory or sensitizing conditions. Additionally, the CGRP receptor is expressed on dural mast cells46 and elevated CGRP may lead to mast cell degranulation resulting in increased PRL within the dura. Blocking CGRP receptors in the presence of PRL stimulation would disrupt this loop (Fig 6E).
CGRP application to the dura may act on blood vessels, immune cells, and/or other nerve fibers, causing PRL release from these structures (23,30,47,48; Fig 7) since PRL can also be supplied by sources outside of the pituitary (i.e. extra-pituitary PRL) to act via paracrine or autocrine mechanisms.31 Moreover, residential dural cells express mRNA for PRL49 and are immunoreactive for PRL protein expression. Endothelial and immune cells express Prlr and are capable of releasing PRL.50,51 Here we confirm that PRL is expressed on CD11b expressing cells within the dura of female and male mice; CD lib cell types are likely to be myeloid cells (macrophages, granulocytes, and mast cells) (Fig 7A–F), which are the predominant type of immune cells in dura.52 Additionally, we show that Prlr is expressed on CD45 expressing cells (Fig 7G–L). CD45 staining may further indicate mast cells, which have been found to present similarly in shape at dural blood vessels.53,54 PRL may then have one of two actions: (1) on Prlr expressed on nerve fibers, sensitizing afferent nociceptive signaling from the dura; (2) on Prlr expressed on immune cells in a cytokine-like manner causing further release of PRL and recruitment of more immune cells.55,56 Blocking Prlr may similarly disrupt this feedback loop (Fig 8). While there is some debate about the role of PRL in inflammation, PRL levels have been shown to increase as a result of peripheral inflammation57 and PRL can also induce inflammation,58 which may contribute to this potential feedback loop. Here we show that female mice that have Prlr deleted from Nav1.8-expressing fibers within the dura exhibited attenuated facial allodynia in response to previously reported effective doses of dural CGRP12 (Fig 6A). This is consistent with findings that CGRP co-injected with the Prlr antagonist Δ1–9-G129RhPRL blocked behavioral responses (Fig 6C), In either case, the mechanisms are female-specific, given the dimorphic expression/function of Prlr on dural afferents, as well as potential unidentified dimorphisms in CGRP receptor expression/function. While there could be dimorphic expression of PRL in cells native to the dura, we did not observe obvious differences in nerve fibers, blood vessels, or non-neuronal cells. This does not rule out the potential that PRL-expressing cell types are differentially recruited to the dura under specific conditions, but there does not appear to be baseline differences in PRL expression within the dura. These data show an interaction between the downstream mechanisms of PRL and CGRP within the dura that may have an important role in promoting meningeal afferent nociceptive signaling in females.
FIGURE 8:
Hypothesized intercellular interactions within the dura. The pituitary (Pit), endothelial cells on blood vessels, immune cells (macrophages (Mφ), mast cells (MC), T cells (T)), and sensory neurons can all serve as potential PRL release sites during migraine. PRL can sensitize dural afferents in a paracrine manner via TRP and other channels. CGRP released from dural afferents may interact with immune cells and blood vessels leading to further PRL release. CGRP and PRL receptor antagonists mitigate intercellular signaling, where CGRP8–37 may block release of PRL from dural cells and nerve fibers (indicated via purple arrow), and ΔPRL may block additional sensitization of dural afferents (yellow arrow). These combined actions lead to sensitized afferent signaling from the dura (represented by PRL+).
Finally, the data presented here demonstrate involvement of Prlr on sensory neurons in CGPR-PRL signaling within the dura. Moreover, our data suggest that communication between these signaling pathways could be an important female-selective mechanism contributing to the increased prevalence of migraine in females. Accordingly, targeting PRL signaling, whether through suppression of pituitary and/or extra-pituitary PRL release or development of a Prlr antagonist, could be novel therapeutic approach for migraine. Our data suggest that peripherally-restricted Prlr antagonists could be an option for the effects of PRL on the headache phase of attacks. While these therapeutic approaches may not be completely devoid of adverse effects, pharmacological reduction of pituitary PRL release is chronically prescribed to thousands of patients with hyperprolactinemia with minimal ill effects59 and would likely only be contraindicated in women who are pregnant or nursing, both conditions where extensive drug restrictions are common. Targeting PRL signaling for migraine could ultimately represent one of the first sex/gender-specific therapeutics for a neurological disorder and may pave the way for future approaches that would be designed to have efficacy only in females or males.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Nandita Ramkumar and Ajay Dave for technical support. This work was supported by National Institutes of Health grants NS072204 (GD) and NS104200 (GD and AA).
Abbreviations
- ASICs
acid-sensing ion channels
- CD31
Cluster of differentiation 31
- CGRP
Calcitonin Gene-Related Peptide
- CKO
Conditional Knockout
- DRG
Dorsal Root Ganglia
- HPO-axis
Hypothalamic-Pituitary-Ovarian axis
- PRL
Prolactin
- ΔPRL
Δ1–9-G129R-hPRL
- Prlr
Prolactin Receptor
- TG
Trigeminal Ganglia
- TH
Tyrosine Hydroxylase
Footnotes
Potential Conflicts of Interest
Nothing to report.
Additional supporting information can be found in the online version of this article.
References
- 1.Steiner TJ, Stovner LJ, Vos T, et al. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain 2018;19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reuwer AQ, Nowak-Sliwinska P, Mans LA, et al. Functional consequences of prolactin signalling in endothelial cells: a potential link with angiogenesis in pathophysiology? J Cell Mol Med 2012;16: 2035–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology 1994;44:S17–S23. [PubMed] [Google Scholar]
- 4.Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American migraine prevalence and prevention (AMPP) study. Headache 2013; 53:1278–1299. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev 2008;29:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guldiken S, Guldiken B, Demir M, et al. Soluble CD40 ligand and prolactin levels in migraine patients during interictal period. J Headache Pain 2011;12:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosco D, Belfiore A, Fava A, et al. Relationship between high prolactin levels and migraine attacks in patients with microprolactinoma. J Headache Pain 2008;9:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman N, Voron SC, Hershman JM. Resolution of migraine following bromocriptine treatment of a prolactinoma (pituitary microadenoma). Headache 1995;35:430–431. [DOI] [PubMed] [Google Scholar]
- 9.Gordon ML, Lipton RB, Brown SL, van Praag HM. The neuroendocrine challenge paradigm in headache research. Cephalalgia 1995; 15:292–296. [DOI] [PubMed] [Google Scholar]
- 10.Cavestro C, Rosatello A, Marino MP, et al. High prolactin levels as a worsening factor for migraine. J Headache Pain 2006;7:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edvinsson L, Goadsby PJ. Discovery of CGRP in relation to migraine. Cephalalgia 2019;39:331–332. [DOI] [PubMed] [Google Scholar]
- 12.Avona A, Burgos-Vega C, Burton MD, et al. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J Neurosci 2019;39:4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RS, Kokay IC, Phillipps HR, et al. Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus. J Neurosci 2016;36:9173–9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci 2009;48:A–4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgos-Vega CC, Quigley LD, Trevisan Dos Santos G, et al. Noninvasive dural stimulation in mice: a novel preclinical model of migraine. Cephalalgia 2018;1:333102418779557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgos-Vega CC, Quigley LD, Avona A, et al. Dural stimulation in rats causes brain-derived neurotrophic factor-dependent priming to subthreshold stimuli including a migraine trigger. Pain 2016;157: 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 2008;135:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil MJ, Hovhannisyan AH, Akopian AN. Characteristics of sensory neuronal groups in CGRP-cre-ER reporter mice: comparison to Nav1.8-cre, TRPV1-cre and TRPV1-GFP mouse lines. PLoS One 2018;13:e0198601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buteau H, Pezet A, Ferrag F, et al. N-glycosylation of the prolactin receptor is not required for activation of gene transcription but is crucial for its cell surface targeting. Mol Endocrinol 1998;12: 544–555. [DOI] [PubMed] [Google Scholar]
- 20.Patil M, Hovhannisyan AH, Wangzhou A, et al. Prolactin receptor expression in mouse dorsal root ganglia neuronal subtypes is sex-dependent. J Neuroendocrinol 2019;31:e12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutia R, Kim AJ, Mosharov E, et al. Regulation of prolactin in mice with altered hypothalamic melanocortin activity. Peptides 2012;37: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil MJ, Green DP, Henry MA, Akopian AN. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience 2013;253:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patil M, Belugin S, Mecklenburg J, et al. Prolactin regulates pain responses via a female-selective nociceptor-specific mechanism. iScience 2019;20:449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy PW, Lawson SN. Differing action potential shapes in rat dorsal root ganglion neurones related to their substance P and calcitonin gene-related peptide immunoreactivity. J Comp Neurol 1997; 388:541–549. [PubMed] [Google Scholar]
- 25.Grant AD, Pinter E, Salmon AM, Brain SD. An examination of neurogenic mechanisms involved in mustard oil-induced inflammation in the mouse. Eur J Pharmacol 2005;507:273–280. [DOI] [PubMed] [Google Scholar]
- 26.Edelmayer RM, Le LN, Yan J, et al. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain 2012;153: 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 2006;29:578–586. [DOI] [PubMed] [Google Scholar]
- 28.Yan J, Wei X, Bischoff C, et al. pH-evoked dural afferent signaling is mediated by ASIC3 and is sensitized by mast cell mediators. Headache 2013;53:1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernichtein S, Kayser C, Dillner K, et al. Development of pure prolactin receptor antagonists. J Biol Chem 2003;278:35988–35999. [DOI] [PubMed] [Google Scholar]
- 30.Donoso AO, Banzan AM. Release of prolactin and LH and histaminecontaining cells in brain. J Neural Transm 1979;44:327–332. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev 1996;17:639–669. [DOI] [PubMed] [Google Scholar]
- 32.Marano RJ, Ben-Jonathan N. Minireview: extrapituitary prolactin: an update on the distribution, regulation, and functions. Mol Endocrinol 2014;28:622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brelje TC, Stout LE, Bhagroo NV, Sorenson RL. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of langerhans. Endocrinology 2004;145:4162–4175. [DOI] [PubMed] [Google Scholar]
- 34.Abbot SD, Docherty K, Clayton RN. Regulation of LH subunit mRNA levels by gonadal hormones in female rats. J Mol Endocrinol 1988;1: 49–60. [DOI] [PubMed] [Google Scholar]
- 35.Szawka RE, Rodovalho GV, Helena CV, et al. Prolactin secretory surge during estrus coincides with increased dopamine activity in the hypothalamus and preoptic area and is not altered by ovariectomy on proestrus. Brain Res Bull 2007;73:127–134. [DOI] [PubMed] [Google Scholar]
- 36.Aravantinos D, Tzingounis V. Changes in plasma prolactin levels after gynecologic surgery. J Gynecol Obstet Biol Reprod 1983;12: 461–463. [PubMed] [Google Scholar]
- 37.Castelo-Branco C, Martinez de Osaba MJ, Vanrezc JA, et al. Effects of oophorectomy and hormone replacement therapy on pituitarygonadal function. Maturitas 1993;17:101–111. [DOI] [PubMed] [Google Scholar]
- 38.Patil MJ, Ruparel SB, Henry MA, Akopian AN. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: contribution of prolactin receptor to inflammatory pain. Am J Physiol Endocrinol Metab 2013;305:E1154–E1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diogenes A, Patwardhan AM, Jeske NA, et al. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci 2006;26: 8126–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guyda HJ, Friesen HG. Serum prolactin levels in humans from birth to adult life. Pediatr Res 1973;7:534–540. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 2015;14:65–80. [DOI] [PubMed] [Google Scholar]
- 42.Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45:S3–S13. [DOI] [PubMed] [Google Scholar]
- 43.Baliu-Pique M, Jusek G, Holzmann B. Neuroimmunological communication via CGRP promotes the development of a regulatory phenotype in TLR4-stimulated macrophages. Eur J Immunol 2014;44: 3708–3716. [DOI] [PubMed] [Google Scholar]
- 44.Assas BM, Pennock JI, Miyan JA. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front Neurosci 2014;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain 2013;14:1289–1303. [DOI] [PubMed] [Google Scholar]
- 46.Lennerz JK, Ruhle V, Ceppa EP, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol 2008;507:1277–1299. [DOI] [PubMed] [Google Scholar]
- 47.Matera L, Muccioli G, Cesano A, et al. Prolactin receptors on large granular lymphocytes: dual regulation by cyclosporin A. Brain Behav Immun 1988;2:1–10. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez C, Rosas-Hernandez H, Jurado-Manzano B, et al. The prolactin family hormones regulate vascular tone through NO and prostacyclin production in isolated rat aortic rings. Acta Pharmacol Sin 2015;36:572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clapp C, Lopez-Gomez FJ, Nava G, et al. Expression of prolactin mRNA and of prolactin-like proteins in endothelial cells: evidence for autocrine effects. J Endocrinol 1998;158:137–144. [DOI] [PubMed] [Google Scholar]
- 50.Galsgaard ED, Rasmussen BB, Folkesson CG, et al. Re-evaluation of the prolactin receptor expression in human breast cancer. J Endocrinol 2009;201:115–128. [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Meyer K, Friedl A. STAT5 and prolactin participate in a positive autocrine feedback loop that promotes angiogenesis. J Biol Chem 2013;288:21184–21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mcllvried LA, Cruz JA, Borghesi LA, Gold MS. Sex-, stress-, and sympathetic post-ganglionic-dependent changes in identity and proportions of immune cells in the dura. Cephalalgia 2017;37: 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arac A, Grimbaldeston MA, Galli SJ, et al. Meningeal mast cells as key effectors of stroke pathology. Front Cell Neurosci 2019; 13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nurkhametova D, Kudryavtsev I, Guseinikova V, et al. Activation of P2X7 receptors in peritoneal and meningeal mast cells detected by uptake of organic dyes: possible purinergic triggers of Neuroinflammation in meninges. Front Cell Neurosci 2019;13:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brand JM, Frohn C, Cziupka K, et al. Prolactin triggers pro-inflammatory immune responses in peripheral immune cells. Eur Cytokine Netw 2004; 15:99–104. [PubMed] [Google Scholar]
- 56.Dill R, Walker AM. Role of prolactin in promotion of immune cell migration into the mammary gland. J Mammary Gland Biol Neoplasia 2017;22:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scotland PE, Patil M, Belugin S, et al. Endogenous prolactin generated during peripheral inflammation contributes to thermal hyperalgesia. Eur J Neurosci 2011;34:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu W, Sun M, Zhang HP, et al. Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut 2014;63:1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koivisto MB, Eschricht F, Urhausen C, et al. Effects of short-term hyper- and Hypoprolactinaemia on hormones of the pituitary, gonad and -thyroid Axis and on semen quality in male beagles. Reprod Domest Anim 2009;44:320–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.