The effect of space radiation on offspring was evaluated using mouse freeze-dried spermatozoa preserved on the Space Station.
Abstract
Space radiation may cause DNA damage to cells and concern for the inheritance of mutations in offspring after deep space exploration. However, there is no way to study the long-term effects of space radiation using biological materials. Here, we developed a method to evaluate the biological effect of space radiation and examined the reproductive potential of mouse freeze-dried spermatozoa stored on the International Space Station (ISS) for the longest period in biological research. The space radiation did not affect sperm DNA or fertility after preservation on ISS, and many genetically normal offspring were obtained without reducing the success rate compared to the ground-preserved control. The results of ground x-ray experiments showed that sperm can be stored for more than 200 years in space. These results suggest that the effect of deep space radiation on mammalian reproduction can be evaluated using spermatozoa, even without being monitored by astronauts in Gateway.
INTRODUCTION
NASA and several organizations are now beginning to plan manned missions to Mars. In the future, humans will probably live in other space habitats or planets for over many generations with animals such as dogs, cats, and livestock. However, interplanetary deep space is populated not only by microgravity but also by strong radiation such as galactic cosmic rays, which originate outside the solar system, and by solar particle events (accelerated emission of particles from the Sun into interplanetary space), which include high-charge, high-energy (HZE) particles, and it is difficult to shield it from a spacecraft (1–4).
Previous studies have shown that microgravity does not prevent the reproduction of several species, such as sea urchins, fish, amphibians, and birds (5–11). Although mammalian species cannot be compared with other species due to their viviparous and placental formation, some studies and our simulated microgravity experiments suggest that microgravity may not prevent mammalian reproduction in space (12–18).
In contrast, it was thought that space radiation would become a serious health issue, for example, a cancer risk, over long stay periods in future missions to Mars and other planets (1, 2, 19). The current NASA space radiation cancer risk model is built largely based on epidemiological data from the survivors of the Hiroshima and Nagasaki atomic bombings, not from real experiments on space (20). It is also known that radiation causes DNA damage to germ cells (21, 22). In particular, HZE particles can cause spatially clustered DNA double-strand breaks, which may not be easily repaired by cells (23). If these mutated germ cells were passed on to offspring and future generations, then they would have a more severe effect on the species. If radiation were continuously irradiated into the body of a species and several mutations accumulated in germ cells over a long period of time, then the species would become a different species. Therefore, it is very important to examine the effects of space radiation not only on living organisms but also on future generations before the “space age” arrives.
So far, many studies have been conducted on the ground using x-ray, γ-ray, or HZE ion exposure to cells. However, these ground experiments could not mimic space radiation completely because of the difference in radiation in space (1, 3). However, it was also impossible to use live animals or cells on the International Space Station (ISS) for longer periods of time. Live mammals, such as mice or rats, need constant maintenance to maintain comfortable conditions, but so far, the longest period of time that these animals have been maintained has only been 3 months (24). Live cells cultured in flasks also require frequent medium changes and passages, which is easier compared to taking care of animals on the ISS, but it is still impossible to maintain it for several years. Cryopreserved cells are still alive, but they have stopped metabolizing, and DNA damage might accumulate in them with a longer duration in space (25, 26). Thus, cryopreserved cells are a better medium to examine the effect of space radiation for a longer period of time than live animals or cells. However, somatic cells cannot be used to examine the inheritance of mutations in the next generation. On the other hand, mammalian oocytes, embryos, and spermatozoa can be used for reproduction experiments, but these cells require liquid nitrogen or ultradeep freezers for cryopreservation, which are not available to rockets or on the ISS. Therefore, because of the limitations of space experiments, only a few studies on mammalian reproduction in space could be performed so far (3).
In this study, we decided to use mouse freeze-dried spermatozoa (FD sperm) (27) for space radiation experiments because FD sperm can be preserved at room temperature for more than 1 year (28–30), which allows us to launch to the ISS without the need for a freezer in the rocket. The ampules for FD sperm are very light and small, which greatly reduced the cost of launching. Moreover, so far, this is the only method that has been used to examine the effect of space radiation on the next generation. We launched 12 male FD sperm samples to the ISS and confirmed that this project worked properly using first-returned samples 9 months later (31). The remaining samples were preserved on the ISS for 2 years and 9 months, and for 5 years and 10 months, which is the longest duration that samples have been preserved there in biological research (Fig. 1A) (32). Using these samples, we tried to evaluate the effect of space radiation on spermatozoa and examined whether long-term exposure to space radiation causes DNA damage to sperm and whether any accumulated mutations affect the next generation. This report is to study the one of most important questions on future space exploration: whether mammals, including humans, can reproduce in space or on other planets.
Fig. 1. Schematic diagram of the preparation of FD sperm and the type of experiments conducted.
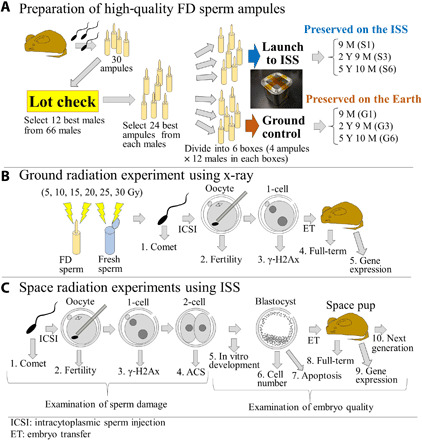
(A) Preparation of FD sperm. The 12 best males were selected from 66 males by the quality lot check of ampules, and the 24 best ampoules were selected from each male, which were then divided into six boxes. Three boxes were launched and preserved on the ISS, and the other three boxes were preserved on Earth. The first box was returned from the ISS 9 months later, the second box was returned 2 years and 9 months later, and the final box was returned 5 years and 10 months later. The ground-control boxes were moved from Japan Aerospace Exploration Agency (JAXA; Tsukuba) to our laboratory at the same time. (B) Tolerance of FD sperm against x-ray irradiation on ground. Fresh and FD sperm were irradiated up to 30 Gy, and then comet, fertilization potential, γ-H2AX, full-term development, and gene expression analyses were performed. (C) Examination of sperm damage and the quality of embryos after preservation on the ISS. To detect the DNA damage in the FD sperm, comet, γ-H2AX, and abnormal chromosome segregation (ACS) assays were performed at each stage. Cytoplasmic damage to FD sperm was detected by the potential of fertilization after the intracytoplasmic sperm injection (ICSI). To detect the quality of embryos, developmental potential in vitro, cell number of blastocysts, apoptosis rate, full-term development, and NGS analyses were performed. Photo credit: Sayaka Wakayama, University of Yamanashi.
RESULTS
Ground radiation experiment to evaluate FD sperm sensitivity
To demonstrate whether FD sperm can be used to evaluate space radiation on the ISS, the FD sperm of BDF1 (C57BL/6 x DBA/2) and C57BL/6 mice were exposed to up to 30 grays (Gy) of x-ray on the ground, and the sensitivity of the DNA and cytoplasm of FD sperm against radiation was examined (Fig. 1B). Fresh spermatozoa were used as controls. In the comet assays, the extent of DNA damage in sperm nuclei increased with increased doses of radiation in a dose-dependent manner (r = 0.407; Fig. 2A and table S1). A similar trend was observed in the C57BL/6 strain FD sperm (table S1). After fertilization, the extent of DNA damage in male pronuclei increased with increasing radiation doses, as determined by the γ-H2AX assays (r = 0.521; Fig. 2B and table S2). After the embryo transfer, although the success rate of offspring production decreased with increased radiation doses, healthy offspring were still obtained even after the 30-Gy treatment (Fig. 2C, fig. S1, and table S3), which was better than that of fresh spermatozoa. Although one of the pups had open eyes at the cesarean section, it had a normal karyotype (fig. S1). A similar observation had been made for spermatozoa in the B6 mouse strain, but their tolerance to radiation was lower than that of the BDF1 strain (fig. S1 and table S4).
Fig. 2. Tolerance of FD sperm against x-ray irradiation.
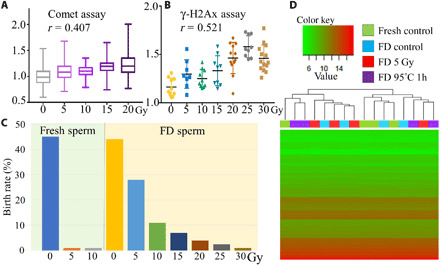
(A) The lengths of FD sperm DNA in the comet tails were standardized against the average lengths of the control sperm results for each irradiation level. (B) The brightness of each male pronucleus immunostaining with anti–γ-H2AX antibodies was plotted. (C) Production rate of offspring using x-ray–irradiated fresh or FD sperm derived from the BDF1 strain. (D) The results of the DNA microarray analysis of FD sperm derived from C57BL/6 sperm and oocytes shown as heatmaps. Four neonates derived from fresh spermatozoa (fresh control), intact FD sperm (FD control), 5 Gy–irradiated FD sperm (FD 5 Gy), and FD sperm treated at −95°C for 1 hour (FD –95°C 1h) were used for each heatmap.
The offspring looked normal and had normal fertility when mature (fig. S1), but we still analyzed the global gene expression profiles of offspring derived from the x-ray–irradiated FD sperm of C57BL/6 mice (5 Gy exposed) to detect whether there were any genotypic differences compared with offspring derived from fresh or untreated FD sperm. In addition, we also examined the pups derived from the high-temperature–treated (95°C for 1 hour) FD sperm at the same time because during deep space exploration, several accidents are likely to occur. The brains of four neonates in each treated group were examined. Although only a few genes showed differences between individual or treated groups, the global gene expression profile of all 16 offspring in all treatment groups showed extremely similar patterns (heatmapping, Fig. 2D; correlation coefficients are in table S5). These results and a previous study demonstrated that when sperm are freeze-dried, the nucleus acquires a strong tolerance against radiation compared to fresh sperm (33). The DNA damage or birth rate of FD sperm were related to the dose-dependent manner of radiation, which suggests that FD sperm can be used as an evaluation tool for deep space radiation for a longer period of time.
Preparation and collection of preserved space sperm samples
The FD sperm samples were prepared from 66 male individuals [BDF1, 24 males; BCF1 (C57BL/6 x C3H/He), 16 males; B6129F1–green fluorescent protein (GFP)-TG (C56BL/6-GFP x 129X1/Sv-GFP) males 10; and C57BL/6, 16 males] in 2012, and more than 30 glass ampules of FD sperm were prepared from each male. Then, one or two ampules were used to examine fertilization and full-term developmental potential after sperm injection [intracytoplasmic sperm injection (ICSI)] as a lot check. After this, we selected the best males (BDF1, 4 males; BCF1, 3 males; B6129F1-GFP-Tg, 2 males; and C57BL/6j, 3 males) on the basis of the success rate of offspring (Fig. 1A and tables S6 to S9). The second and third best male groups were prepared as backups for launch. The selected male ampules were divided into six boxes; three were launched to the ISS on 4 August 2013, and the other three were preserved on ground [Japan Aerospace Exploration Agency (JAXA), Tsukuba, Japan] in almost exactly the same condition without bringing them to the ISS.
The first box was returned to the ground on 19 May 2014 (9 months preservation: hereafter, we call this sample S1) and confirmed whether the experiment had been working properly or not. After examining this sample and comparing it with the ground-preserved control sample (hereafter, G1), we decided to move forward with this project (31). Then, the second box was returned to the ground on 11 May 2016 (2 years and 9 months preservation: hereafter, we call this sample S3 and ground control as G3). The last box was returned to the ground on 3 June 2019 (5 years and 10 months preservation), which makes it the longest space experiment in biological research. Here, we refer to this sample as S6 and ground control as G6. The ground-control boxes were also returned from JAXA to the University of Yamanashi at exactly the same time.
Total doses of space radiation
CR-39 plastic nuclear track detectors (PNTDs) and thermo luminescent dosimetry type MSO (TLD) in the Bio PADLES packages collected from the space-preserved cases could detect nuclear tracks and absorbed dose (34). As shown in fig. S2, each PADLES was maintained for 0.5 to 2.8 years, and a total of 12 PADLES packages (6 for flight and 6 for the ground control) were used to measure the entire period of this study. Figure S2 shows images of the etch pits that correspond to the nuclei tracks produced during space flight between 4 June 2017 and 16 May 2018. The background doses measured in the ground-control sperm sample case were subtracted from the net doses measured during space flight. From the results, the absorbed radiation dose rate was 0.41 mGy/day, or a dose-equivalent rate of 0.61 mSv/day (quality factor, 1.50), and the total absorbed dose was 869.8 mGy in water, or a total dose equivalent of 1302.9 mSv.
Detection of FD sperm damage
To detect the effect of space radiation on FD sperm, the comet assay, γ-H2Ax assay, and abnormal chromosome segregation (ACS) assay were used to assess the DNA damage in the sperm nucleus, and the ICSI assay was used to assess the cytoplasm/nucleoplasm damage in the FD sperm (Fig. 1C). To compare the results between the preservation period on the ISS and the ground-control samples more accurately, four ampules (S3, G3, S6, and G6) were rehydrated and used for experiments. Because these samples were very valuable and do not persist after adding water, we performed the ICSI in oocytes using up to nine micromanipulators set at the same time (fig. S3).
When we observed the morphology of the space sperm samples, they could not be distinguished from the ground-control samples, at least at the light microscopy level (Fig. 3, A and B). Some spermatozoa showed breakage between the head and tail or had fragmented tails, but this is typical for FD sperm (27, 31).
Fig. 3. Examination of sperm damage.
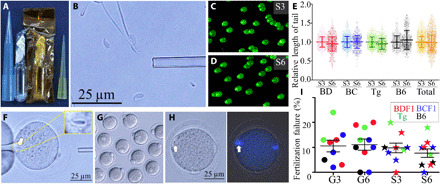
(A) Ampule of space-preserved FD sperm. (B) After rehydration, some of the sperm heads separated from the tails. (C and D) Comet DNA breakage assays of 2 years and 9 months (S3) and 5 years and 10 months (S6) space-preserved FD sperm. Two independent technicians measured the straight lengths from sperm heads (bright spot) to the edges of the faint light. (E) The lengths of DNA in the comet tails were standardized against the average lengths of the S3 FD sperm results for each mouse strain. (F) FD sperm were injected into fresh BDF1 oocytes. Arrows indicate injected sperm heads. (G) Six hours later, most of the oocytes were fertilized. (H) Some of the oocytes failed to fertilize after FD sperm injection, and those oocytes had pseudo-MII spindles derived from sperm nuclei [left, bright image; right, DNA under ultraviolet (UV) light]. (I) The rate of fertilization failure between preservation periods and between mouse strains. Key: mouse strains: BD, BDF1; BC, BCF1; Tg, B6129F1-GFP; B6, C57BL/6. Photo credits: (A, B, and F to H) Sayaka Wakayama and (C and D) Daiyu Ito, University of Yamanashi.
In the comet assay, the lengths of comet DNA tails were compared between S3 and S6 (Fig. 3, C to E, and table S10). Although there were significant differences between their preservation periods in space (total, 1.000 versus 0.985), it was very small. This result suggests that long-term exposure to space radiation on the ISS did not cause DNA damage to the FD sperm.
The rehydrated spermatozoa were injected into fresh oocytes (Fig. 3, F to I) to observe the fertilization potential of space-preserved spermatozoa. In normal fertilization, injected spermatozoa activate oocytes using several factors of the sperm head (35, 36). If spermatozoa fail to fertilize oocytes, then it suggests that their oocyte activation factors are lost from the head due to damage during preservation on the ISS. As shown in Fig. 3H, when fertilization failed after the ICSI, the FD sperm nuclei formed condensed chromosomes instead of pronuclear formation, which was caused by a defect in the activation potential of spermatozoa. When the rates of fertilization failure were compared between mouse strains, preservation periods (2 years and 9 months versus 5 years and 10 months), and preservation place (ground versus space), the rate varied from 0 to 24% in each individual male (Fig. 3I and table S11). When the average rate was compared between preservation periods, although the S6 group showed a slightly but significantly lower fertilization failure rate (8.4%) than the others (9.9 to 11.2%), the difference was not large. This rate was within the normal range when FD sperm were used (35, 36). These results suggest that space radiation did not affect the cytoplasmic factors of spermatozoa.
Next, we examined the DNA damage of male pronuclei derived from FD sperm in fertilized zygotes using immunostaining with the anti–γ-H2AX antibody, which is a marker of DNA damage sites (Fig. 4, A and B). Because it was difficult to count the number of foci inside the pronuclei, we measured the brightness of the whole male pronucleus, which was then subtracted from the brightness of the zygote cytoplasm. As shown in Fig. 4C and table S12, the brightness of the male pronucleus varied from 1.1 to 2.0 in each individual male. However, the average brightness was similar irrespective of mouse strain, preservation period, or preservation place. This result suggests that long-term exposure to space radiation on the ISS did not cause DNA damage to the FD sperm based on the amount of γ-H2AXof male pronuclei.
Fig. 4. Examination of sperm damage after fertilization.
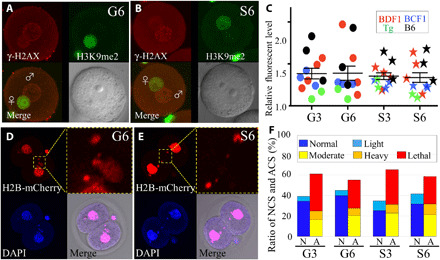
(A and B) Immunostaining of zygotes derived from G6 ground control (A) or S6 space sperm (B) by the anti–γ-H2AX antibodies. The foci of γ-H2AX signals show DNA double-strand breaks (top left, red). Female pronuclei were detected by H3K9me2 immunostaining (top right, green). Merged images (bottom left) and light microscopy image (bottom right). (C) The brightness of each male pronucleus was plotted. Nuclear staining of two-cell embryos derived from G6 ground control (D) and S6 space sperm (E). ACS was detected by H2B-mCherry mRNA injection (top left and its high magnification, red) and 4′,6-diamidino-2-phenylindole (DAPI; bottom left: blue) at the two-cell stage. The double-positive parts were judged as micronuclei using merged images (bottom right). (F) The ratio of normal chromosome segregation (NCS) and ACS. N, NCS; A, ACS. Light blue shows the rate of light ACS, but it was included in NCS. Photo credits: (A and B) Sayaka Wakayama and (D and E) Masatoshi Ooga, University of Yamanashi.
HZE particles can cause spatially clustered DNA double-strand breaks, which may not be easily repaired by the cells (23). Clustered and heavier DNA damage could not be detected by the comet or γ-H2AX assay but could be detected at the time of cell division as ACS (37). Therefore, we examined the incidence rate of ACS at the two-cell stage by staining histone H2B and DNA. In this study, we judged over a moderate level (fig. S4) of ACS because embryos with light level of ACS still have potential to reach full-term development (38). As shown in Fig. 4 (D to F), table S13, and movie S1, although the rate of normal chromosome segregation (NCS) was comparable in all groups, the rate of light abnormality (light blue in Fig. 4F) slightly increased when the FD sperm were preserved on the ISS (G3: 4.8% and G6: 5.1% versus S3: 9.6% and S6: 9.8%). This result suggests that the HZE space radiation particles may cause slightly heavier DNA damage to the FD sperm, but these damages were judged as “light,” which may not affect the rate of full-term development (38).
Detection of embryo abnormality
To observe the developmental potential of zygotes fertilized with space-preserved FD sperm, the embryos were cultured in vitro until they developed to the blastocyst stage 4.5 days after the ICSI. As shown in Fig. 5 (A and B), and in table S14, most of the fertilized embryos cleaved into two-cell embryos, then developmental rate was decreased with increased the culture period, and last, 28 to 34% of them developed into blastocysts, irrespective of the mouse strain, preservation period, and preservation place.
Fig. 5. Examination of embryo quality and full-term development.
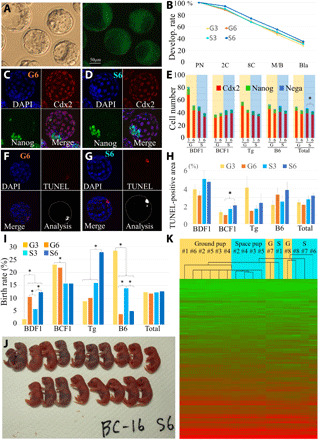
(A) Blastocysts derived from embryos fertilized with space-preserved spermatozoa of 129B6F1-Tg strain (left) and GFP expression under UV light (right). (B) Developmental rate of embryos up to blastocyst stage. PN, pronuclear stage; 2C, two-cell stage; 8C, eight-cell stage; M/B, morulae/blastocyst stage; Bla, blastocyst stage. Immunostaining of blastocysts fertilized with G6 ground control (C) and space sperm (D). The nuclei of embryos were detected by nuclear staining using DAPI (top left, blue). CDX2-positive cells [trophectoderm (TE)] are red (top right), Nanog-positive cells [inner cell mass (ICM)] are green (bottom left), and merged images (bottom right). The cell number of TE, ICM, and both negative Ne are shown in (E). The incidence of apoptosis in blastocysts derived from G6 ground control (F) or space sperm (G) were examined using the TUNEL assay. The foci of TUNEL signals show apoptosis cells (top right, red). Merged images (bottom left) and converted digital images using ImageJ software (bottom right). (H) The red area of each blastocyst was plotted. (I) Full-term development of embryos fertilized with G3 and G6 ground control and S3 and S6 space-preserved spermatozoa. Asterisks show significant differences (P < 0.05). (J) 19 pups were obtained from S6 space-preserved FD sperm from one experiment. Key for mouse strains: B6, C57BL/6N; BD, B6D2F1; BC, B6C3F1; Tg, 129B6F1 expressing GFP. (K) The results of the RNA sequencing (RNA-seq) analysis are shown as heatmaps. Photo credits: (A) Teruhiko Wakayama, (C and D) Kosuke Kazama, (F and G) Naoki Hirose, and (J) Sayaka Wakayama, University of Yamanashi.
When the embryos developed into blastocysts, some were used to evaluate their quality on the basis of the cell number and allocation of inner cell mass (ICM) cells. The allocation of ICM cells did not show clear differences between the groups (Fig. 5, C and D). When counting the cell number of blastocysts, there were slight differences between mouse strains (Fig. 5E and table S15). Although the average number of ICM cells decreased significantly from S3 to S6 (6.6 cells versus 4.7 cells, P < 0.01), the difference was not large. The average cell number of trophectoderm (TE) decreased when the preservation period increased, but there were no significant differences between the groups.
In addition, we also examined the number of apoptotic cells in blastocysts, which is a marker for the quality of blastocysts (39). As shown in Fig. 5 (F to H) and table S16, when comparing the average brightness of apoptotic cell area in total, it slightly increased when the space-preserved sperm were used compared to the ground control, but this difference was not significant. This result suggests that long-term exposure to space radiation on the ISS did not affect the quality of blastocysts.
Full-term development and normality of offspring
The potential for full-term development is the strongest evidence of whether the long-term preserved space sperm was normal. Therefore, when the embryos reached the two-cell stage, some were transferred into recipient females and the birth rates between space and ground control, and between preservation periods, were compared. In this experiment, when B6 sperm were rehydrated, they were injected into B6 oocytes; otherwise, BDF1 oocytes were used for any strains of sperm. As shown in Fig. 5 (I and J) and table S17, we obtained many offspring from long-term space-preserved spermatozoa, irrespective of the mouse strain and preservation period. The birth rates varied in each strain, but the average of all 12 individual male sperm samples was almost the same, regardless of the preservation period and place (G3, 12.4%; G6, 12.1%; S3, 12.3%; and S6, 12.9%; there were no significant differences). The average body and placental weights of offspring were almost the same between G3 and S3, as well as G6 and S6 (fig. S5 and tables S18 and S19). When the placenta was compared histologically and the area of the thickest part of the placenta was compared as well, although S6 placenta were slightly larger than G6 placentae in the BDF1 mouse strain, there were no significant differences in placenta weight between G6 and S6 samples (fig. S5 and table S20). Some of the offspring, which were selected at random, grew to adulthood without exception. The sex ratio of randomly selected pups was slightly different between G6 and S6 pups [male rate of G6 was 0.74 (n = 33) and S6 was 1.62 (n = 55)].
When C57BL/6 offspring were obtained after 2 years and 9 months of preservation of B6 FD sperm in space with B6 fresh oocytes, five mice (two females and three males) were examined for their life span. As the results, the life span of the five mice ranged from 387 to 816 days (average, 604 days; table S21), which was lower than the general B6 life span (female, 866 days; male, 901 days; Mouse Phenome Database at the Jackson laboratory, Yuan2 life span).
The reproductive potential of randomly selected space pups was examined after they grew to adulthood (table S22) to examine whether they had normal reproductive potential and whether abnormality or recessive mutations would appear in the following generation. According to the results, all the examined pairs of space pup demonstrated normal reproduction potential and delivered the next generation 3 to 4 months later. Randomly selected offspring from the next generation were mated after growth to adulthood, and the third generation was obtained; all the offspring appeared normal (table S22 and fig. S6).
Next-generation sequencing analysis
To determine whether there were any nonphenotypic differences in the offspring derived from the space and ground-control sperm samples, we analyzed their global gene expression profiles using RNA sequencing (RNA-seq) and drew comparisons between G6 and S6. B6 offspring were derived from B6 FD sperm with fresh B6 oocytes, and their freshly collected brains from eight offspring of ground control and eight offspring of space sperm were examined (table S23). There was no difference in the gene expression profiles between all ground-control pups and all space pups (heatmapping, Fig. 5K; correlation coefficients are in table S24). Offspring of different sexes showed no differences in their gene expression profiles.
DISCUSSION
Space radiation includes various energetic particles, including solar wind, solar cosmic rays, and galactic cosmic rays, which cannot be reproduced on the ground (1–4). Therefore, to determine the effects of space radiation on animals and their offspring, we must use the ISS for long periods of time. Here, the longest biological preservation experiment using mouse spermatozoa was performed on the ISS, and specimens were exposed to real space radiation (32). As a result, we obtained many healthy “space pup” offspring from space-preserved spermatozoa with the same success rate as the ground controls, even after 5 years and 10 months of storage. These space pups did not show any differences compared to the ground-control pups, and their next generation also had no abnormalities.
In this study, we demonstrated that FD sperm had strong tolerance to radiation. Healthy offspring could be obtained even after the FD sperm samples were exposed to 30-Gy x-rays on the ground before fertilization. It is known that radiation induces DNA damage directly or indirectly through radicals generated in response to intracellular water molecules (40, 41). However, FD sperm does not contain any water inside the cytoplasm or nucleus; therefore, it was predicted that its tolerance against radiation would increase compared to fresh sperm (33). Although there are differences between DNA damage from x-rays and space radiation, it can roughly predict that FD sperm can be preserved on the ISS for over 200 years (30 Gy/0.41 mGy/365 = 201 years), even without any protection from space radiation. However, the ISS circles the Earth at an altitude of approximately 400 km, which is inside the Earth’s protective magnetic field (1). Interplanetary deep space is populated by densely ionizing particle radiation (4), which may cause more DNA damage to the cells compared to the ISS. In the near future, NASA will build a Lunar Orbital Platform-Gateway, which is a station that will orbit the Moon and provide an international cooperation platform for scientific experiments. However, astronauts will not stay in this Gateway; therefore, FD sperm will be appropriate for mammalian reproduction experiments on the Gateway because it can be preserved at room temperature for a longer period of time (30) without astronaut maintenance.
In the future, when the time comes to migrate to other planets, we will need to maintain the diversity of genetic resources, not only for humans but also for pets and domestic animals. For cost and safety reasons, it is likely that stored germ cells will be transported by spaceships rather than by living animals. Although it is necessary to investigate whether space radiation could have adverse effects on frozen oocytes/embryos, FD sperm or FD germ cells would be the optimal material for such studies due to their high tolerance.
Furthermore, genetic resources are an asset to humanity’s future. Although many genetic traits are not needed for survival, depending on the environmental context, it is necessary to preserve them as much as possible so that species can survive if unknown diseases are spread, or environmental changes occur, such as global warming. However, in the 2011 earthquake in Japan, an unexpectedly high tsunami that was over 40 m tall caused a nuclear reactor explosion, and the surrounding area was exposed to high levels of radiation (42). Thus, global and serious disasters will happen more than expected, and it is difficult to find a permanently safe place on Earth. To conserve genetic resources permanently even in such a situation, extraterrestrial preservation of germ cells is a new choice for our future. For this purpose, freeze-drying spermatozoa, oocytes, or embryos is a better solution than traditional cryopreservation. FD somatic cells may also be useful for this purpose when animal cloning techniques are used (43, 44). Although FD oocytes, embryos, and somatic cells have not yet succeeded in producing offspring, this will likely be achieved in the near future because FD sperm has already been successful (27). When we can demonstrate the reliability and integrity of space-preserved FD sperm or germ cells, in the far future, underground storage on the Moon, such as in lava tubes (45, 46), could be among the best places for prolonged or permanent preservation because of their very low temperatures, protection from space radiation by thick bedrock layers, and complete isolation from any disasters on Earth. In conclusion, these discoveries are essential and important for mankind to progress into the space age.
MATERIALS AND METHODS
Animals
The following animals are previously described in (31). C56BL/6N, BDF1 (C57BL/6 × DBA/2), BCF1 (C57BL/6 × C3H/He), and 129B6F1 3-month-old male mice that carried the GFP gene (GFP-tg-129/Sv × GFP-tg-C57BL/6) were used to collect spermatozoa. The ICR, C57BL/6N, BDF1, and BCF1 strains of mice were purchased from Shizuoka Laboratory Animal Center (Hamamatsu, Japan). The 129B6F1 mice were bred in our mouse facility. The C57BL/6N and BDF1 strains of mice aged 8 to 10 weeks were used to produce oocytes. The surrogate pseudopregnant females that were used as embryo transfer recipients were ICR mice that mated with vasectomized males of the same strain. On the day of the experiments, or after the completion of all the experiments, the mice were euthanized via CO2 inhalation or cervical dislocation. All animal experiments conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Committee of Laboratory Animal Experimentation of the RIKEN Center for Developmental Biology and by the Institutional Committee of Laboratory Animal Experimentation of the University of Yamanashi.
Preparation of FD sperm
The following procedures are previously described in (27, 31). Both epididymides were collected from male mice and the ducts were cut with sharp scissors. Then, a few drops of the dense sperm mass were placed into a centrifuge tube with 2 ml of CZB medium (47) and were incubated for 30 min at 37°C in a 5% CO2 incubator. After measuring the sperm concentration, 50-μl aliquots of the sperm suspension were divided into 30 glass ampules. The ampules were frozen using liquid nitrogen and then freeze-dried (EYELA FDU-2200, Tokyo, Japan and LABCONCO Free Zone, Kansas, MO, USA). The lever of the freeze-drying machine was opened for at least 3 hours until the samples became completely dry. After drying, the ampules were sealed by melting their necks using a gas burner under vacuum conditions, as described (31, 35), and were kept in a −30°C freezer until further use.
For space preservation experiments, we prepared sperm samples from 65 male mice of four different strains and checked the quality of each lot by examining their fertility (see below) using one or two ampules from each mouse. From these results, we selected the 12 best mice: 4 from the BDF1 strain, 3 from the BCF1 strain, 3 from the C57BL/6 strain, and 2 from the 129B6F1 strain expressing GFP. Then, we selected the 24 best ampules from each male on the basis of stereomicroscopic observations. Each ampule was wrapped in polyimide film (Fig. 2A) and then divided into six groups and wrapped further into groups of four. All ampules from 12 male mice were placed into six small boxes (four ampules of each male × 12 male = 48 ampules in each box) (Fig. 1A). The three boxes were launched and preserved on the ISS (space sperm). The remaining three boxes were also moved to the launch site with the space sperm boxes but were preserved on Earth (Tsukuba, Japan) without being launched, as an exact control (ground control). The ground-control sperm samples were exposed to the same temperature changes at the same time and for the same duration as the space sperm samples.
Nine months later, the first box was returned to Earth, and we examined whether the experiment had been working properly or not by assessing whether the glass ampules had been damaged or not, for instance. Most of the glass ampules had not been damaged during the launch or return, and many healthy offspring had been obtained from the space-preserved FD sperm, which is why we had decided to move forward this project (31).
X-ray treatments
FD sperm were prepared as described above. Ampules of FD sperm (BDF1 and B6 strains) were irradiated with 200-kV x-rays at 3 Gy/min for a total of 5 to 30 Gy (TITAN-320, Shimadzu, Kyoto, Japan) at the National Institute of Radiological Science, Japan. After treatment, the ampules were kept in a −30°C freezer until use. For the controls, fresh epididymal spermatozoa were collected and cultured in H-CZB medium in 1.5-ml tubes, irradiated together with the FD ampules at the same time, and kept at 4°C until use. One or 2 days later, these irradiated fresh spermatozoa were microinjected into fresh oocytes.
Launch to the ISS and return to Earth
The sample cases, including the Bio PADLES radiation monitors (fig. S2), were launched aboard the H-II Transfer Vehicle KOUNOTORI 4 on 4 August 2013, at room temperature. After arrival at the ISS, astronauts stored them in a −95°C freezer on 10 August 2013. Two years and 9 months later (11 May 2016), and 5 years and 10 months later (3 June 2019), the second and third boxes were retrieved from the freezer and then returned to Earth on SpaceX-8 and SpaceX-17 at ambient temperature, respectively. Therefore, these space sperm samples were exposed to cosmic radiation for 1010 and 2129 days, respectively. At the same time, the ground-control sample boxes were returned to our laboratory.
Space radiation dosimetry
We used Bio PADLES (TLD/CR39) monitoring devices (Fukuvi Chemical Industry Co. Ltd., Fukui, Japan) to measure radiation dosages. These included CR-39 PNTDs. They were placed inside the cases of either the space or ground-control sperm samples. Because there were limitations on the monitoring period of Bio PADLES, the device was replaced periodically. We took pictures of the radiation-etched pits that reflected the tracks of the atomic nuclei that had accumulated during the space flight (fig. S2), measured the brightness of TLD that had increased with increased the energy of received radiation, and used these to calculate the total radiation dose (34).
Analysis and scoring of comet slides
The single-cell gel electrophoresis technique (designated as the comet assay) measures DNA damage, including double- and single-strand breaks (48). Thus, comet assays were used to detect sperm DNA damage and were performed according to the manufacturer’s protocol (Trevigen, MD, USA). Briefly, sperm specimens were collected from ampules immediately after opening and rehydrated in water. The 2 years and 9 months (S3) and 5 years and 10 months of space-preserved sperm (S6) from the same male mouse were mounted on four slides, and 100 to 300 sperm heads were analyzed following electrophoresis independently by two experimentalists. To standardize the results between the two experimentalists and between different mouse strains, the lengths of each DNA comet “tail” were divided by the mean length of the S3 results from each experimentalist or mouse strain.
ICSI, in vitro culture, and embryo transfer
To generate offspring from preserved spermatozoa, oocytes were collected from female BDF1 or C57BL/6N mice. Most experiments used BDF1 oocytes, irrespective of the male mouse strain. Only when next-generation sequencing (NGS) for genomic analysis and a normality test of the next generation were performed, C57BL/6 oocytes were used for C57BL/6 male mouse ICSI to generate inbred offspring. When the ampules were opened, we immediately added 50 μl of water to rehydrate the sperm samples and started ICSI using up to nine micromanipulators (fig. S3) as previously described (49, 50). When FD sperm preserved for 2 years and 9 months were examined, two ampules (S3 and G3) were used at same time. However when FD sperm preserved for 5 years and 10 months were examined, to perform a more accurate comparison of the results between four group (S3, G3, S6, and G6), four ampules of each were rehydrated and used for the experiments.
After the ICSI, the surviving oocytes were divided into four groups: one was used for the γ-H2AX assay at the one-cell stage, one was used for the ACS assay at the two-cell stage, one was used for in vitro culture up to 5 days, and the last one was used for embryo transfer. For in vitro culture, the fertilization rates of the surviving oocytes were examined 6 hours after ICSI and were cultured in 5% CO2 in air for 5 days in CZB medium at 37°C to examine their in vitro developmental potential to the blastocyst stage. When the embryos could develop into blastocysts, these embryos were used for the cell number and terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assays.
On the other hand, some other embryos at the two-cell stage were transferred into the oviducts of pseudopregnant ICR strain female mice at 0.5 days postcoitum (dpc), which had been mated with a vasectomized male the night before transfer (51). At 18.5 to 19.5 dpc, the offspring were delivered via cesarean section or naturally.
Immunostaining of zygotes
Ten hours after the ICSI, the zona pellucidae of surviving oocytes were removed using acetic tyrode solution, and then the naked oocytes were fixed for 30 min at 25°C in 4% (w/v) paraformaldehyde (PFA). The fixed oocytes were washed three times in phosphate-buffered saline (PBS)–polyvinyl alcohol (0.1 mg/ml; Sigma-Aldrich, St. Louis, MO) for 10 min and stored overnight at 4°C in PBS supplemented with 1% (w/v) bovine serum albumin (BSA/PBS; Sigma-Aldrich) and 0.1% (v/v) Triton X-100 (Nacalai Tesque Inc., Kyoto, Japan). The following procedures are previously described in (30). The primary antibodies used were an anti–phospho-H2AX (Ser139) rabbit polyclonal antibody (1:500; Millipore-Merck, Darmstadt, Germany) and an anti-histone H3 (dimethyl K9) mouse monoclonal antibody (1:500, Abcam, Cambridge, UK). The secondary antibodies used were Alexa Fluor 488–labeled goat anti-mouse immunoglobulin G (IgG; 1:500, Molecular Probes, Eugene, OR, USA) and Alexa Fluor 568–labeled goat anti-rabbit IgG (1:500 dilution; Molecular Probes). DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI; 2 μg/ml; Molecular Probes). The brightness of the whole male pronucleus was measured using ImageJ and was then subtracted from the brightness of the zygote cytoplasm.
Detection of ACS
Six hours after the ICSI, the surviving oocytes with 2PN were injected with histone H2B-mCherry mRNA to visualize their nuclei or micronuclei. The next day, two-cell stage embryos were fixed and then permeabilized with 4% PFA and 0.5% Triton X-100 for 15 min. These embryos were observed using confocal fluorescence microscopy (Olympus FV1000, Tokyo, Japan) in DAPI and 1% BSA–containing PBS. To reduce nonspecific misidentification, we only used mCherry and DAPI double-positive signals in the analysis. ACS was categorized into four groups: “small,” “medium,” “large,” and “multiple” (fig. S4, top); multiple meant that more than four micronuclei were observed regardless of the size. Light ACS was judged when only one micronucleus was detected (fig. S4, lower table). Moderate ACS was judged when two small, one to two medium, or one large micronucleus was detected. Heavy ACS was judged when three small, medium, or two to three large micronuclei were detected. Lethal ACS was judged when the embryos had multiple micronuclei. When two conditions co-occurred, the evaluation became more severe. For instance, when one medium micronucleus and two small micronuclei were observed in the embryo, the evaluation was judged as “heavy.” Because some of the light ACS embryos in this study could reach full-term development (38), we defined ACS as greater than or equal to “moderate” levels of abnormal segregation (fig. S4). To visualize the formation of ACS, some embryos that were injected with enhanced GFP–tubulin mRNA were subjected to live imaging with CV1000 (Yokogawa, Tokyo, Japan) (movie S1).
Examination of ICM and TE cell numbers in blastocysts using immunostaining
To evaluate the quality of the blastocysts from the space sperm samples, cell numbers were examined using immunofluorescence staining as previously described (31). The primary antibodies used were an anti-CDX2 mouse monoclonal antibody (1:500; MU392A-UC, BioGenex, San Ramon, CA, USA) to detect the TE cells and an anti-Nanog rabbit polyclonal antibody (1:500; ab80892, Abcam, Cambridge, UK) to detect the ICM cells. The secondary antibodies used were Alexa Fluor 568–labeled goat anti-mouse IgG (1:500; Molecular Probes Inc., Oregon, USA, A11004) and goat anti-rabbit IgG Cy5 (1:500; ab97077, Abcam, Cambridge, UK). DNA was stained with DAPI (2 μg/ml; Molecular Probes).
Examination of blastocyst apoptosis rates using the TUNEL assay
For the TUNEL assay, blastocysts were fixed with 4% PFA-PBS (Wako, Osaka, Japan) for 30 min at ambient temperature. After washing three times with PBS containing 3% BSA, embryos were permeabilized in 0.5% Triton X-100 for 15 min. The TUNEL analysis was performed using the ApopTag Red In Situ Apoptosis Detection Kit (S7165, Millipore-Merck, Darmstadt, Germany) following the manufacturer’s recommendations. After the TUNEL reaction, the embryos were washed three times, blocked in 3% BSA-PBS, and then immunostained for nuclei stained with DAPI. The TUNEL-positive area (red signal) of each blastocyst was calculated using the ImageJ software at the same time.
Gene expression analysis
At 18.5 dpc, fetuses were collected via cesarean section. All offspring were generated using B6 oocytes and B6 FD sperm or fresh spermatozoa as controls, and the brains were collected just after the cesarean section. These samples were frozen in LN2 immediately after collection and stored at −80°C until analysis.
In the x-ray experiment, four newborn pups in each treated group were examined using a microarray. Total RNA was prepared using RNeasy mini kits (Qiagen, Hilden, Germany). RNA labeling, hybridization, and scanning were performed using the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent SurePrint G3 Mouse GE 8X60K, v. 2 Microarrays v. 6.5, 2010; Agilent Technology Inc., Santa Clara, CA, USA). Raw data were extracted using the software provided by Agilent Feature Extraction Software (v. 11.0.1.1). The statistical significance levels of the expression data were determined from fold changes and the local pooled error test in which the null hypothesis was that there was no difference between the two groups being evaluated. The hierarchical cluster analysis was performed using complete linkage and Euclidean distance as a measure of similarity.
In the space experiment, the procedure previously described in (31). Briefly, eight pups (four female and four male) from ground control (#1 to #8) and eight pups (three female and five male) from space-preserved sperm (#1 to #8) were examined using RNA-seq. Total RNA was purified with an AllPrep DNA/RNA mini kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The library for RNA-seq studies was prepared using a Kapa Stranded RNA/mRNA-Seq kit (Illumina, San Diego, CA, USA). The RNA-seq library was sequenced for 36-base single-end RNAs using an Illumina GAIIx machine with a TruSeq SBS kit v5–GA kit (Illumina, San Diego, CA, USA). The resulting sequence data were mapped against the mouse reference genome sequence (GRCm38/mm10) using bowtie2 (52), followed by a calculation of the “reads per kilobase of exon per million mapped reads” value for each gene using the Bioconductor package “DEGseq” (53). The clustering analysis and principal components analysis were performed with “Cluster 3.0” (54).
Statistical analysis
The comet DNA breakage assay results were evaluated using the Wilcoxon-Mann-Whitney nonparametric test. The γ-H2AX assay was evaluated using the Student’s t test, and PN formation, in vitro development, and birth rate were evaluated using χ2 tests. Cell number and the TUNEL assay were evaluated using a one-way analysis of variance (ANOVA) followed by the Tukey-Kramer test. RNA-seq analyses were evaluated using the Wilcoxon-Mann-Whitney nonparametric test adjusted for a false discovery rate, and differentially expressed genes between the space and control pups were statistically analyzed using the DESeq2 package (55) with the raw sequence read count. P < 0.05 (birth rate and γ-H2AX staining) or P < 0.01 (others) were regarded as statistically significant.
Acknowledgments
We thank M. Shirakawa, Y. Fujimoto, M. Nakamura, and C. Yamaguchi for critical comments on the study. Funding: This work was partially funded by Naito Foundation for S.W., Takeda Science Foundation for T.W., and the Asada Science Foundation for T.W. Author contributions: S.W. and T.W. conceived and designed the study. R.E., A.H., E.H., N.H., D.I., R.I., H.K., K.K., S.Ka, S.Ki, Y.Ka, Y.Ki, H.N., I.O., M.O., H.Sa, H.Su, T.Sh, T.Su, M.N.T., M.U., R.W., S.W., T.W., L.Y., S.Y., and T.Y. performed experiments, analyzed the data, and interpreted the results. M.A., R.A., T.I., T.K., and A.N. analyzed data. T.W. wrote the manuscript. All authors read and edited the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Microarray and NGS data have been deposited in NCBI GEO database and DNA Data Bank of Japan Sequence Read Archive, respectively (accession numbers: GSE117740 and DRA011181, respectively).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/24/eabg5554/DC1
REFERENCES AND NOTES
- 1.Furukawa S., Nagamatsu A., Nenoi M., Fujimori A., Kakinuma S., Katsube T., Wang B., Tsuruoka C., Shirai T., Nakamura A. J., Sakaue-Sawano A., Miyawaki A., Harada H., Kobayashi M., Kobayashi J., Kunieda T., Funayama T., Suzuki M., Miyamoto T., Hidema J., Yoshida Y., Takahashi A., Space radiation biology for "living in space". Biomed. Res. Int. 2020, 4703286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edmondson E. F., Gatti D. M., Ray F. A., Garcia E. L., Fallgren C. M., Kamstock D. A., Weil M. M., Genomic mapping in outbred mice reveals overlap in genetic susceptibility for HZE ion- and γ-ray-induced tumors. Sci. Adv. 6, eaax5940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra B., Luderer U., Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 15, 713–730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeitlin C., Hassler D. M., Cucinotta F. A., Ehresmann B., Wimmer-Schweingruber R. F., Brinza D. E., Kang S., Weigle G., Böttcher S., Böhm E., Burmeister S., Guo J., Köhler J., Martin C., Posner A., Rafkin S., Reitz G., Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 340, 1080–1084 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Aimar C., Bautz A., Durand D., Membre H., Chardard D., Gualandris-Parisot L., Husson D., Dournon C., Microgravity and hypergravity effects on fertilization of the salamander Pleurodeles waltl (urodele amphibian). Biol. Reprod. 63, 551–558 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Ijiri K., Ten years after medaka fish mated and laid eggs in space and further preparation for the life-cycle experiment on ISS. Biol. Sci. Space. 18, 138–139 (2004). [PubMed] [Google Scholar]
- 7.Schatten H., Chakrabarti A., Taylor M., Sommer L., Levine H., Anderson K., Runco M., Kemp R., Effects of spaceflight conditions on fertilization and embryogenesis in the sea urchin Lytechinus pictus. Cell Biol. Int. 23, 407–415 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Serova L. V., Effect of weightlessness on the reproductive system of mammals. Kosm. Biol. Aviakosm. Med. 23, 11–16 (1989). [PubMed] [Google Scholar]
- 9.Souza K. A., Black S. D., Wassersug R. J., Amphibian development in the virtual absence of gravity. Proc. Natl. Acad. Sci. U.S.A. 92, 1975–1978 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tash J. S., Kim S., Schuber M., Seibt D., Kinsey W. H., Fertilization of sea urchin eggs and sperm motility are negatively impacted under low hypergravitational forces significant to space flight. Biol. Reprod. 65, 1224–1231 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Ubbels G. A., Berendsen W., Narraway J., Fertilization of frog eggs on a Sounding Rocket in space. Adv. Space Res. 9, 187–197 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Amann R. P., Deaver D. R., Zirkin B. R., Grills G. S., Sapp W. J., Veeramachaneni D. N., Clemens J. W., Banerjee S. D., Folmer J., Gruppi C. M., Effects of microgravity or simulated launch on testicular function in rats. J. Appl. Physiol. 73, S174–S185 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Fedorova N. L., Spermatogenesis of the dogs. Ugolyok and Veterok after their flight on board the satellite Kosmos 110. Kosm. Biol. Med. 1, 28–31 (1967). [Google Scholar]
- 14.Philpott D. E., Sapp W., Williams C., Stevenson J., Black S., Corbett R., Reduction of the spermatogonial population in rat testes flown on Space Lab-3. Physiologist 28, S211–S212 (1985). [PubMed] [Google Scholar]
- 15.Sapp W. J., Philpott D. E., Williams C. S., Kato K., Stevenson J., Vasquez M., Serova L. V., Effects of spaceflight on the spermatogonial population of rat seminiferous epithelium. FASEB J. 4, 101–104 (1990). [DOI] [PubMed] [Google Scholar]
- 16.Serova L. V., Denisova L. A., The effect of weightlessness on the reproductive function of mammals. Physiologist 25, S9–S12 (1982). [PubMed] [Google Scholar]
- 17.Zhang S., Zheng D., Wu Y., Lin W., Chen Z., Meng L., Liu J., Zhou Y., Simulated microgravity using a rotary culture system compromises the in vitro development of mouse preantral follicles. PLOS ONE 11, e0151062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakayama S., Kawahara Y., Li C., Yamagata K., Yuge L., Wakayama T., Detrimental effects of microgravity on mouse preimplantation development in vitro. PLOS ONE 4, e6753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sridharan D. M., Asaithamby A., Blattnig S. R., Costes S. V., Doetsch P. W., Dynan W. S., Hahnfeldt P., Hlatky L., Kidane Y., Kronenberg A., Naidu M. D., Peterson L. E., Plante I., Ponomarev A. L., Saha J., Snijders A. M., Srinivasan K., Tang J., Werner E., Pluth J. M., Evaluating biomarkers to model cancer risk post cosmic ray exposure. Life Sci. Space Res. 9, 19–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrington de Gonzalez A., Gilbert E., Curtis R., Inskip P., Kleinerman R., Morton L., Rajaraman P., Little M. P., Second solid cancers after radiation therapy: A systematic review of the epidemiologic studies of the radiation dose-response relationship. Int. J. Radiat. Oncol. Biol. Phys. 86, 224–233 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubrova Y. E., Plumb M., Gutierrez B., Boulton E., Jeffreys A. J., Transgenerational mutation by radiation. Nature 405, 37 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Barber R., Plumb M. A., Boulton E., Roux I., Dubrova Y. E., Elevated mutation rates in the germ line of first- and second-generation offspring of irradiated male mice. Proc. Natl. Acad. Sci. U.S.A. 99, 6877–6882 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sridharan D. M., Asaithamby A., Bailey S. M., Costes S. V., Doetsch P. W., Dynan W. S., Kronenberg A., Rithidech K. N., Saha J., Snijders A. M., Werner E., Wiese C., Cucinotta F. A., Pluth J. M., Understanding cancer development processes after HZE-particle exposure: Roles of ROS, DNA damage repair and inflammation. Radiat. Res. 183, 1–26 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Cancedda R., Liu Y., Ruggiu A., Tavella S., Biticchi R., Santucci D., Schwartz S., Ciparelli P., Falcetti G., Tenconi C., Cotronei V., Pignataro S., The Mice Drawer System (MDS) experiment and the space endurance record-breaking mice. PLOS ONE 7, e32243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi T., Takahashi A., Nagamatsu A., Omori K., Suzuki H., Shimazu T., Ishioka N., Detection of space radiation-induced double strand breaks as a track in cell nucleus. Biochem. Biophys. Res. Commun. 390, 485–488 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Yatagai F., Honma M., Takahashi A., Omori K., Suzuki H., Shimazu T., Seki M., Hashizume T., Ukai A., Sugasawa K., Abe T., Dohmae N., Enomoto S., Ohnishi T., Gordon A., Ishioka N., Frozen human cells can record radiation damage accumulated during space flight: Mutation induction and radioadaptation. Radiat. Environ. Biophys. 50, 125–134 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Wakayama T., Yanagimachi R., Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat. Biotechnol. 16, 639–641 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Kaneko T., Serikawa T., Long-term preservation of freeze-dried mouse spermatozoa. Cryobiology 64, 211–214 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Liu J., Lee G. Y., Lawitts J. A., Toner M., Biggers J. D., Live pups from evaporatively dried mouse sperm stored at ambient temperature for up to 2 years. PLOS ONE 9, e99809 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamada Y., Wakayama S., Shibasaki I., Ito D., Kamimura S., Ooga M., Wakayama T., Assessing the tolerance to room temperature and viability of freeze-dried mice spermatozoa over long-term storage at room temperature under vacuum. Sci. Rep. 8, 10602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakayama S., Kamada Y., Yamanaka K., Kohda T., Suzuki H., Shimazu T., Tada M. N., Osada I., Nagamatsu A., Kamimura S., Nagatomo H., Mizutani E., Ishino F., Yano S., Wakayama T., Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc. Natl. Acad. Sci. U.S.A. 114, 5988–5993 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planel H., Gaubin Y., Pianezzi B., Delpoux M., Bayonove J., Bès J. C., Heilmann C., Gasset G., Influence of a long duration exposure, 69 months, to the space flight factors in Artemia cysts, tobacco and rice seeds. Adv. Space Res. 14, 21–32 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Kusakabe H., Kamiguchi Y., Chromosomal integrity of freeze-dried mouse spermatozoa after 137Cs γ-ray irradiation. Mutat. Res. 556, 163–168 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Nagamatsu A., Murakami K., Kitajo K., Shimada K., Kumagai H., Tawara H., Area radiation monitoring on ISS Increments 17 to 22 using PADLES in the Japanese Experiment Module Kibo. Radiat. Meas. 59, 84–93 (2013). [Google Scholar]
- 35.Ito D., Wakayama S., Kamada Y., Shibasaki I., Kamimura S., Ooga M., Wakayama T., Effect of trehalose on the preservation of freeze-dried mice spermatozoa at room temperature. J. Reprod. Dev. 65, 353–359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakayama S., Ito D., Kamada Y., Yonemura S., Ooga M., Kishigami S., Wakayama T., Tolerance of the freeze-dried mouse sperm nucleus to temperatures ranging from −196 °C to 150 °C. Sci. Rep. 9, 5719 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamagata K., Suetsugu R., Wakayama T., Assessment of chromosomal integrity using a novel live-cell imaging technique in mouse embryos produced by intracytoplasmic sperm injection. Hum. Reprod. 24, 2490–2499 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Mashiko D., Ikeda Z., Yao T., Tokoro M., Fukunaga N., Asada Y., Yamagata K., Chromosome segregation error during early cleavage in mouse pre-implantation embryo does not necessarily cause developmental failure after blastocyst stage. Sci. Rep. 10, 854 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ono T., Li C., Mizutani E., Terashita Y., Yamagata K., Wakayama T., Inhibition of class IIb histone deacetylase significantly improves cloning efficiency in mice. Biol. Reprod. 83, 929–937 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Spitz D. R., Azzam E. I., Li J. J., Gius D., Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 23, 311–322 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Dayal D., Martin S. M., Limoli C. L., Spitz D. R., Hydrogen peroxide mediates the radiation-induced mutator phenotype in mammalian cells. Biochem. J. 413, 185–191 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nollet K. E., Komazawa T., Ohto H., Transfusion under triple threat: Lessons from Japan’s 2011 earthquake, tsunami, and nuclear crisis. Transfus. Apher. Sci. 55, 177–183 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Loi P., Matsukawa K., Ptak G., Clinton M., Fulka J. Jr., Nathan Y., Arav A., Freeze-dried somatic cells direct embryonic development after nuclear transfer. PLOS ONE 3, e2978 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ono T., Mizutani E., Li C., Wakayama T., Nuclear transfer preserves the nuclear genome of freeze-dried mouse cells. J. Reprod. Dev. 54, 486–491 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Coombs C. R., Hawke B. R., A search for intact lava tubes on the noon: Possible lunar base habitats. The second conference on lunar bases and space activities of the 21st century 1, 219–229 (1992). [Google Scholar]
- 46.Kaku T., Haruyama J., Miyake W., Kumamoto A., Ishiyama K., Nishibori T., Yamamoto K., Crites S. T., Michikami T., Yokota Y., Sood R., Melosh H. J., Chappaz L., Howell K. C., Detection of intact lava tubes at Marius Hills on the Moon by SELENE (Kaguya) Lunar Radar Sounder. Geophys. Res. Lett. 44, 10,155–10,161 (2017). [Google Scholar]
- 47.Chatot C. L., Lewis J. L., Torres I., Ziomek C. A., Development of 1-cell embryos from different strains of mice in CZB medium. Biol. Reprod. 42, 432–440 (1990). [DOI] [PubMed] [Google Scholar]
- 48.Haines G., Marples B., Daniel P., Morris I., DNA damage in human and mouse spermatozoa after in vitro-irradiation assessed by the comet assay. Adv. Exp. Med. Biol. 444, 79–91 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto Y., Hirose N., Kamimura S., Wakayama S., Ito J., Ooga M., Wakayama T., Production of mouse offspring from inactivated spermatozoa using horse PLCζ mRNA. J. Reprod. Dev. 66, 67–73 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura Y., Yanagimachi R., Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 52, 709–720 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Inoue R., Harada K., Wakayama S., Ooga M., Wakayama T., Improvement of a twice collection method of mouse oocytes by surgical operation. J. Reprod. Dev. 66, 427–433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langmead B., Trapnell C., Pop M., Salzberg S. L., Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L., Feng Z., Wang X., Wang X., Zhang X., DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 (2010). [DOI] [PubMed] [Google Scholar]
- 54.de Hoon M. J. L., Imoto S., Nolan J., Miyano S., Open source clustering software. Bioinformatics 20, 1453–1454 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/24/eabg5554/DC1


