A 50-year-old woman attended our emergency department with a 3-day history of severe back pain and a severe headache. 10 days earlier she had received the first dose of vaccine against SARS-CoV-2—ChAdOx1 nCoV-19 (AstraZeneca). The patient had no significant medical history—specifically no personal or family history of venous thromboembolism. She was not pregnant, she did not take an oral contraceptive, and did not have a hormone-eluting intrauterine device in situ.
On examination, the patient was afebrile, her peripheral oxygen saturation was 99% (with a 21% fraction of inspired oxygen), blood pressure was 130/80 mm Hg, heart rate was 80 beats per min, and body-mass index was 20 kg/m2. She had no obvious haematomas or petechial haemorrhages; she reported no chest pain, shortness of breath, swelling, redness, pallor, or cold in any limb.
Laboratory investigations showed a severe thrombocytopenia of 27 × 109 per L (normal 140–440). 3 days earlier it had been 163 × 109 per L. Serum D-dimer concentration was significantly elevated at greater than 33 mg/L (normal <0·5), fibrinogen concentration was 121 mg/dL (normal 210–400), coagulation factor XIII activity was 63% (normal >70%), and both prothrombin time and activated partial thromboplastin time (aPTT) were normal. Haemoglobin concentration was 10·8 g/dL (normal 12·0–15·3); microcytic–hypochromic red blood cell indices, a low ferritin concentration of 12 ng/mL (normal 22–112), and a soluble transferrin receptor concentration of 10·1 mg/L (normal 1·78–4·59) indicated an iron deficiency anaemia. No schistocytes were detected. A thrombophilia screen—including resistance to activated protein C, and protein C and protein S activity—was negative; no antibodies to platelet factor 4 were found and genetic testing for prothrombin mutations was negative.
Creatinine, electrolytes, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase concentrations were within normal range. SARS-CoV-2 antigen and RT-PCR were negative on a nasopharyngeal swab. Tests for hantaviruses, HIV, hepatitis B virus, hepatitis C virus, and antiphospholipid antibodies (cardiolipin, β2 glycoprotein, and lupus) were negative.
We undertook consecutive thrombelastography, which indicated a stable clot pattern—despite the low platelet count and low fibrinogen concentration, and excluded fibrinolysis—both primary and secondary.
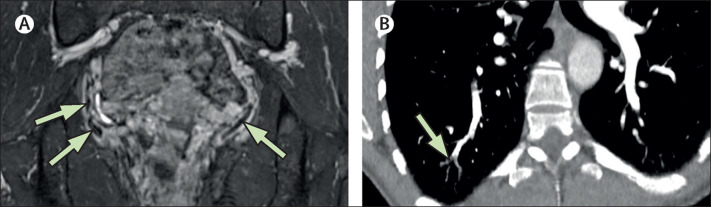
Contrast-enhanced MRI of the patient's abdomen and pelvis showed multifocal thrombus formations in the pelvic region—including bilateral thrombi in the parauterine venous plexus, a superficial vein of the left gluteus maximus muscle, and left presacral and lumbar veins (figure ; appendix).
Figure.
Vaccine-induced immune thrombotic thrombocytopenia
(A) Contrast-enhanced MRI shows bilateral hypointense thrombi in the parauterine venous plexus (arrows). (B) Contrast-enhanced CT pulmonary angiography shows a subsegmental embolus in the posterior–basal segment of the right lower lobe (arrows).
CT pulmonary angiography showed a subsegmental embolus in the posterior–basal right lower lobe (figure). CT cerebral venography and compression ultrasound of both legs showed no abnormalities—neither cerebral venous sinus thrombosis nor deep vein thrombosis.
We assumed the diagnosis was a vaccine-induced immune thrombotic thrombocytopenia and administered high-dose intravenous immunoglobulin (IVIG) followed by a second dose 24 h later, together with dexamethasone 40 mg orally for 4 days to avoid allergic reactions to IVIG. Simultaneously, anticoagulant therapy with intravenous argatroban—a direct thrombin inhibitor—was started at a dose of 2 μg per kg bodyweight per min; the dose was adjusted to obtain a steady state aPTT of between 1·5 to three times baseline.
The patient's condition rapidly improved; her pain subsided and her platelet count reached 91 × 109 per L 48 h after starting treatment. Additionally, concentrations of fibrinogen and coagulation factor XIII also steadily increased as D-dimer concentrations dropped (appendix). After 4 days, we switched argatroban to oral dabigatran 150 mg twice a day, and 6 days after her admission, the patient's platelets, fibrinogen, and coagulation factor XIII levels were within normal range (video).
Declaration of interests
We declare no competing interests.
Contributors
KG, TG, RBR, JS, AW, and MB were the physicians treating the patient. We were all involved in drafting, reviewing, and writing the final manuscript. Consent for publication was obtained from the patient.
Supplementary Materials
Vaccine-induced immune thrombotic thrombocytopenia
Treating vaccine-induced immune thrombotic thrombocytopenia
YouTube:https://youtu.be/mG1SpsdcACo
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vaccine-induced immune thrombotic thrombocytopenia
Treating vaccine-induced immune thrombotic thrombocytopenia
YouTube:https://youtu.be/mG1SpsdcACo