Abstract
Background
SARS-CoV-2 entry in human cells depends on angiotensin-converting enzyme 2, which can be upregulated by inhibitors of the renin–angiotensin system (RAS). We aimed to test our hypothesis that discontinuation of chronic treatment with ACE-inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) mitigates the course o\f recent-onset COVID-19.
Methods
ACEI-COVID was a parallel group, randomised, controlled, open-label trial done at 35 centres in Austria and Germany. Patients aged 18 years and older were enrolled if they presented with recent symptomatic SARS-CoV-2 infection and were chronically treated with ACEIs or ARBs. Patients were randomly assigned 1:1 to discontinuation or continuation of RAS inhibition for 30 days. Primary outcome was the maximum sequential organ failure assessment (SOFA) score within 30 days, where death was scored with the maximum achievable SOFA score. Secondary endpoints were area under the death-adjusted SOFA score (AUCSOFA), mean SOFA score, admission to the intensive care unit, mechanical ventilation, and death. Analyses were done on a modified intention-to-treat basis. This trial is registered with ClinicalTrials.gov, NCT04353596.
Findings
Between April 20, 2020, and Jan 20, 2021, 204 patients (median age 75 years [IQR 66–80], 37% females) were randomly assigned to discontinue (n=104) or continue (n=100) RAS inhibition. Within 30 days, eight (8%) of 104 died in the discontinuation group and 12 (12%) of 100 patients died in the continuation group (p=0·42). There was no significant difference in the primary endpoint between the discontinuation and continuation group (median [IQR] maximum SOFA score 0·00 (0·00–2·00) vs 1·00 (0·00–3·00); p=0·12). Discontinuation was associated with a significantly lower AUCSOFA (0·00 [0·00–9·25] vs 3·50 [0·00–23·50]; p=0·040), mean SOFA score (0·00 [0·00–0·31] vs 0·12 [0·00–0·78]; p=0·040), and 30-day SOFA score (0·00 [10–90th percentile, 0·00–1·20] vs 0·00 [0·00–24·00]; p=0·023). At 30 days, 11 (11%) in the discontinuation group and 23 (23%) in the continuation group had signs of organ dysfunction (SOFA score ≥1) or were dead (p=0·017). There were no significant differences for mechanical ventilation (10 (10%) vs 8 (8%), p=0·87) and admission to intensive care unit (20 [19%] vs 18 [18%], p=0·96) between the discontinuation and continuation group.
Interpretation
Discontinuation of RAS-inhibition in COVID-19 had no significant effect on the maximum severity of COVID-19 but may lead to a faster and better recovery. The decision to continue or discontinue should be made on an individual basis, considering the risk profile, the indication for RAS inhibition, and the availability of alternative therapies and outpatient monitoring options.
Funding
Austrian Science Fund and German Center for Cardiovascular Research.
Introduction
The COVID-19 pandemic poses unprecedented challenges to health-care systems around the world. By the end of May, 2021, there were more than 169 million confirmed cases worldwide, with more than 3·5 million deaths.1 Mortality from COVID-19 varies widely among individuals,2 with older age and comorbities such as cardiovascular disease, diabetes mellitus, hypertension, chronic pulmonary diseases and obesity identified as major predisposing risk factors.3, 4, 5
SARS-CoV-2, the pathogen causing COVID-19, enters human cells via angiotensin-converting enzyme 2 (ACE2),6 which plays an important regulatory role in the renin–angiotensin system (RAS).7, 8 The broad expression of ACE2 in many organ tissues could provide an explanation for why COVID-19 is a systemic disease that can affect numerous organ systems besides the lungs, including the heart, gastrointestinal tract, or central nervous system.7, 9, 10 Experimental findings suggest that pharmacological RAS inhibition can increase ACE2 expression in organs including the heart,11, 12, 13 intestine,14 kidney,15 or urogenital tract,16 although conflicting studies exist.17 This raised great concerns with the onset of the pandemic,18, 19 based on the mechanistic considerations that increased ACE2 expression could adversely affect the progression of COVID-19 by increasing the availability of the target receptors of SARS-CoV-2.20 These concerns were reinforced by the observation that severe courses of COVID-19 occurred mainly in patients with comorbidities typically treated with ACE inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs).3, 4, 5 However, owing to its anti-inflammatory effect, increased ACE2 expression might also induce beneficial effects in COVID-19.21 Thus, the net effect on clinical outcome of COVID-19 by RAS inhibitor-induced ACE2 up-regulation remains unclear.
Research in context.
Evidence before this study
Mechanistic considerations suggested that previous treatment with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) might promote disease progression in COVID-19. Because ACEIs and ARBs are among the most commonly prescribed drugs worldwide, clarification of this issue through high-quality randomised trials is of paramount importance. We searched Pubmed on Feb 16, 2021, for articles, using the search terms “COVID-19”, “SARS-CoV-2”, “angiotensin-converting enzyme inhibitors”, and “angiotensin receptor blockers” and identified two randomised trials (the registry-based BRACE CORONA trial and the controlled REPLACE COVID trial), which showed no effect of discontinuing chronic ACEI–ARB-therapy in COVID-19. However, the net effect of discontinuing or continuing ACEI–ARB could be influenced by various factors, such as patients' baseline risk and age, genetic variability of the coronavirus receptor, and differences in health care systems. The results of the previous trials should therefore not be extrapolated to other populations and settings.
Added value of this study
In this, to our knowledge, first European, multicentre, randomised, controlled, open-label, trial of patients with recent-onset symptomatic SARS-CoV-2 infection enrolled between April, 2020, and January, 2021, discontinuation of chronic RAS inhibition did not significantly affect the maximum severity of disease within 30 days. However, in contrast to previous randomised trials we detected a significant effect on the course of COVID-19 with lower rates of organ dysfunction by secondary and explorative analysis during and at the end of the trial in patients with discontinued ACEI–ARB treatment. ACEI-COVID differs substantially from the two previous studies. It included significantly older patients (75 years [median], compared with 55 years [median] in BRACE CORONA and 62 years [mean] in REPLACE COVID). In BRACE CORONA only 15% of the patients were older than 70 years and in REPLACE only 16% of the patients were older than 75 years. The patients were exposed to the intervention earlier after symptom onset (4 days [median], compared with 8 days [median] in BRACE CORONA and 7 days [mean] in REPLACE COVID). The intervention was done for a full 30 days (compared with a median of 5 days in REPLACE COVID).
Implications of all the available evidence
Evidence from all randomised trials shows that temporary discontinuation of RAS inhibition at the onset of COVID-19 is safe and does not cause harm. The present study suggests that, regardless of the underlying mechanisms, older, vulnerable patients such as those enrolled in ACEI-COVID (relative to the younger patients of BRACE CORONA and REPLACE COVID) might benefit from temporary discontinuation of RAS inhibition in terms of better and faster recovery from COVID-19. The decision to continue or discontinue should be made on an individual basis, taking the risk profile, the indication for RAS inhibition, and the availability of alternative therapies and outpatient monitoring options into account.
ACEIs and ARBs are among the most commonly prescribed medications, with many millions of people treated worldwide. Widespread non-evidence-based and uncontrolled discontinuation of these medications could therefore have enormous consequences at the population level. Against this background, professional societies have uniformly warned against discontinuation of RAS inhibitors in the absence of clinical evidence.22, 23 Subsequent observational studies showed no association between RAS inhibitors and the risk of SARS-CoV-2 infection or the severity of COVID-19.24, 25 However, owing to their non-randomised design these studies cannot exclude important sources of bias and confounding.26 Therefore, there was a broad consensus that strictly controlled, randomised trials are urgently needed to definitively clarify whether ACEIs or ARBs are harmful or beneficial for patients with COVID-19.20, 26
With the registry-based BRACE CORONA trial27 and controlled REPLACE COVID trial28 two randomised trials have been published, which showed a neutral effect of discontinuing chronic ACEI–ARB-therapy in COVID-19. However, the net effect of discontinuing or continuing ACEI–ARB could be influenced by various factors, such as patients' baseline risk and age, genetic variability of the coronavirus receptor,29 and differences in health systems as well as the timing of the intervention. Therefore, the results of these trials should not be extrapolated to other populations and settings.
Here we report the results of ACEI-COVID, the first European randomised, controlled clinical trial testing the hypothesis that patients with recent symptomatic SARS-CoV-2 infection benefit from discontinuing chronic RAS inhibition.
Methods
Study design and participants
The Stopping ACE-inhibitors in Covid-19 (ACEI-COVID) trial was a prospective, parallel group, randomised, controlled, open-label study done at 35 centres, including 19 university clinics and 16 large referral hospitals, in Austria and Germany. The study was coordinated by the Medical University Innsbruck, Austria, and the LMU University Hospital Munich, Germany. The trial design was approved by the local ethics committees at all participating centres as well as by the legal authorities in Austria (Austrian Agency for Health and Food Safety) and Germany (Federal Institute for Drugs and Medical Devices).
Eligible patients were aged 18 years or older, had had a recent symptomatic SARS-CoV-2 infection confirmed by a positive RT-PCR test result within the last 5 days, were on chronic treatment with ACEIs or ARBs for any indication for treatment of arterial hypertension, diabetes, heart failure, or coronary artery disease for at least 1 month before study inclusion, and were in stable haemodynamic conditions (systolic arterial blood pressure <180 mm Hg). Individuals were excluded if they had had an acute coronary syndrome within the last 3 months, suffered from severe arterial hypertension requiring more than four antihypertensive drugs, were in New York Heart Association class III or IV owing to severe heart failure, had a left ventricular ejection fraction <30% or NTproBNP ≥600 pg/mL in combination with clinical signs of heart failure, and were unable to do ambulatory blood pressure monitoring. Individuals for whom substitution of ACEI or ARB with another class of drug was deemed impossible were also excluded. Admission to hospital was no requirement for study inclusion. A complete listing of all inclusion and exclusion criteria is shown in the appendix (p 1). All participants provided written informed consent before inclusion. The study was done in accordance with the Declaration of Helsinki and Good Clinical Practice principles. The protocol is available in the appendix.
Randomisation and masking
Participants were randomly assigned 1:1 to a strategy of discontinuation of ACEI or ARB therapy or continuation of therapy. If participants were randomly assigned to a discontinuation strategy, a substitution with an alternative substance class was at the discretion of the treating physician. We used blocked randomisation with a block size of 8. Allocation information was delivered to participating centres by means of a secure web-based system.
Investigators and treating physicians and patients were aware of the assigned treatment strategy. Cause-specific mortality adjudication was done by an independent endpoint adjudication committee, masked to the treatment strategy. For this purpose, the medical records were redacted at the appropriate positions without obscuring relevant information.
Procedures
After randomisation, the treating physicians were asked to follow the respective treatment strategy. Treating physicians could stop or initiate ACEI or ARB therapy at any time for clinical indications (eg, in case of sepsis, arterial hypotension, hyperkalaemia or renal insufficiency, uncontrolled hypertension). However, the treating physicians were encouraged to follow the assigned treatment strategy as far as possible.
Data were collected and managed by means of Askimed as a cloud-based web platform. Demographic, clinical, and laboratory data as well as medical treatment were collected from all participants at baseline. Clinical, laboratory, and treatment data as well as adverse events were updated daily during the index hospital stay. Participants discharged from hospital were contacted daily by telephone by the local study teams to obtain clinical and medical information. At discharge, participants were provided with a blood pressure monitor if they did not already own one. Outpatients were instructed to measure their blood pressure three times a day.
Outcomes
The primary outcome measure was the composite of the maximum sequential organ failure assessment (SOFA) score30 and death within 30 days. The score is calculated from six different components, each of which reflects the status of an organ system, including respiratory function, cardiovascular integrity, liver function, coagulation, renal function and neurological status. The score can range from 0 (best) to 24 (worst). In case of death, the patient was by definition assigned the maximum value of 24. Outpatients were assigned a value of 0 or 24 (in the case of death). The combination of score and death described here is denoted as death-adjusted SOFA score.
The mean and the area under the death-adjusted SOFA Score (AUCSOFA) were secondary endpoints. Other secondary endpoints included death, admission to the intensive care unit (ICU), mechanical intubation, non-invasive ventilation (continuous positive airway pressure ventilation; high flow ventilation), extracorporeal membrane oxygenation, renal replacement therapy, and hospitalisation. Secondary endpoints also included various biomarkers and quality of life. The analyses of these endpoints are not yet completed and are therefore not reported here. The composite of admission to ICU, mechanical ventilation, and death was a prespecified endpoint. Explorative not prespecified analyses included the SOFA score at day 30 and the number of deceased patients or with a SOFA score of 1 or more at 30 days. Througout the study, the number of patients in different SOFA categories was calcuated for both groups. Additionally, the integral of SOFA scores over days (patient x days) was calculated at the end of the study for both groups. Post-hoc, we also calculated the global rank score, which was used in the REPLACE COVID trial28 and ranks all patients across four hierarchies of clinical outcomes: days to death, followed by days on invasive mechanical ventilation or extracorporeal membrane oxygenation, followed by days on renal replacement therapy or vasopressor therapy, followed by the AUC of a modified SOFA score.28 In addition, we also used a modified version of the SOFA score, which was used in the REPLACE COVID trial28 and only integrates domains that are most likely to be affected by the RAS system (respiratory system, cardiovascular system, coagulation, and renal system), to exploratively recalculate primary and secondary endpoints.
Safety outcome measures included severe hypertension (arterial blood pressure >180 mm Hg/120 mm Hg) and admission to hospital because of decompensated heart failure and causal relationship to stopping of ACEI–ARB therapy.
Statistical analysis
Continuous data are presented as median (IQR), and categorical data are summarised as numbers (frequencies; %). The two-sided Wilcoxon-Mann-Whitney rank sum test (WMW) and the χ2 tests (Chi) were used as appropriate. For 30-day analyses, missing values were handled by last value carried forward.
For the primary analysis, maximum death-adjusted SOFA scores across treatment groups were compared by a two-sided WMW test. We estimated that 83 participants per group are required to detect a difference of 3·0 (SD 1·5) to 2·3 (SD 1·2) in the maximum death-adjusted SOFA score at a two-sided 5% significance level with a power of 90% (NQuery, P(X>Y)=0·6449). Taking into account a drop-out rate of 20%, 208 patients were planned to be randomised. If the evaluation after 208 patients shows a trend for the superiority of a group with respect to the primary endpoint of the maximum death-adjusted SOFA score (p<0.1), the group size can be adjusted according to the Steering Committee and statistical advice. If the primary endpoint (maximum death-adjusted SOFA score) is met, the study will be extended to a total of 798 patients in order to hierachically test the composite of death, admission to ICU, and use of mechanical ventilation as coprimary endpoint.
Time-to-event outcomes were analysed by Cox proportional hazards models starting at time of enrolment. The effect of treatment on the SOFA score over time was estimated by means of (generalised) linear mixed-effects models for longitudinal data. All models were adjusted for time as linear-quadratic fixed effect and the random variability between patients as random effect. The effect of therapy on SOFA score and SOFA category (0 vs 1–24) over time was assessed by comparing the models with and without treatment by means of a likelihood ratio test (LRT). To test the effect of study centres on the primary endpoint we used mixed-effects linear regression models. Subgroup analyses were implemented by means of linear regression analyses and interaction testing between treatment and the variable of interest. Prespecified subgroup analyses included age (<75 years and ≥75 years) and ACEI therapy (as opposed to ARB therapy). All other subgroup analyses are explorative. Another prespecified subgroup analysis considered qSOFA greater than 2 or 2 and below. As no patient had a qSOFA greater than 1, a non-prespecified subgroup analysis of SOFA score 0 vs 1 and above is presented. A prespecified subgroup analysis regarding the indication of ACEI–ARB therapy was not done due to the small number of patients with indications other than arterial hypertension. A prespecified subgroup analysis regarding viral load could not be done as it was not systematically collected.
Analyses were done on a modified intention-to-treat basis in which patients who never received intervention per protocol or had no evidence of SARS-CoV-2 infection by means of a positive RT-PCR test ≤5 days were excluded. As sensitivity analysis, a per-protocol analysis was done with censoring patients at times of cross-over (ie, stopping ACEI–ARB treatment in the continuation group or starting ACEI–ARB treatment in the discontinuation group). Statistical analyses were done by means of the software R version 3.6.3 and the package lme4.31 The ACEI-COVID trial is registered with ClinicalTrials.gov, NCT04353596.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
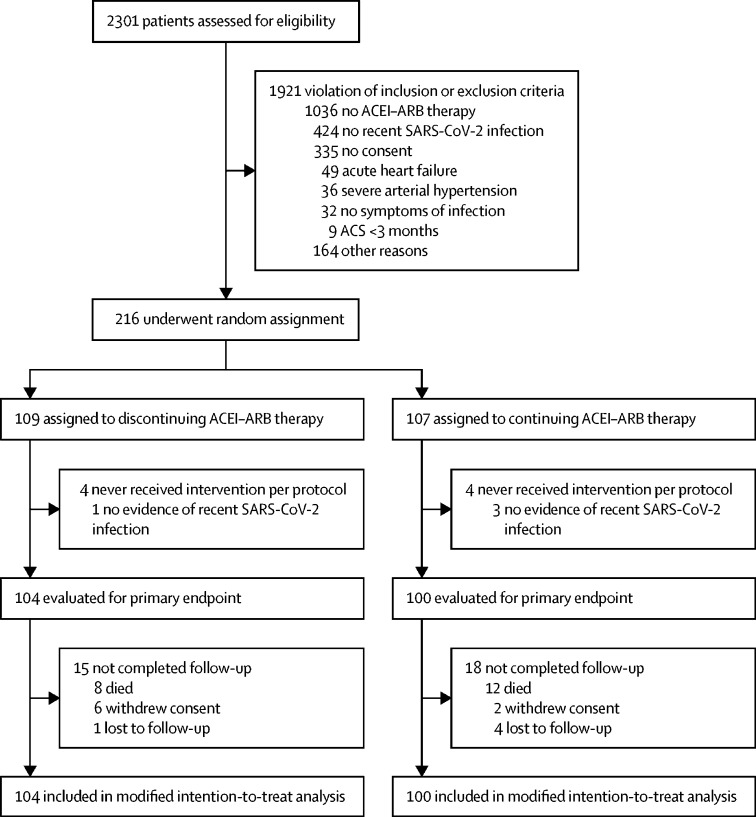
Between April 20, 2020, and Jan 20, 2021, 204 participants were randomly assigned to discontinuation (n=104) or continuation (n=100) of RAS inhibition (figure 1 ). Table 1 depicts the baseline characteristics of the study population. Median age was 75 years (IQR 66–80; mean 72 [SD 11] years), 75 (37%) of 204 patients were female, median body-mass index was 27·6 kg/m2 (25·4–30·5), 67 (33%) patients had diabetes, 45 (22%) had coronary artery disease, 18 (9%) had heart failure, and 199 (98%) had arterial hypertension. 115 patients (56%) had been treated with ACEIs and 89 patients (44%) had received ARBs at baseline. Baseline characteristics were well balanced between treatment groups (table 1) with the exception of chronic obstructive pulmonary disease (COPD) being more frequent in the continuation group. Six patients in the discontinuation group and eight patients in the continuation group received remdesivir for treatment of COVID-19 during the course of the study (p=0·65). The median time from onset of symptoms to randomisation was 4 days (2–7), the median time from positive test to randomisation was 2 days (1–4) and the median time from admission to randomisation was 1 day (0–3). In the discontinuation group, ten out of 104 patients (10%) were directly discharged from the emergency department. In the continuation group, five (5%) of 100 patients were discharged directly (p=0·24 for difference). Length of hospital stay was 10 days (6–15) in the discontinuation group and 11 days (7–19) in the continuation group (p=0·27). 17 patients (16%) in the discontinuation group and 21 (21%) patients in the continuation group crossed over during the study period for medical reasons. Medically unjustified crossovers occurred less frequently and occurred in six patients in the discontinuation group and one patient in the continuation group.
Figure 1.
Trial profile
ACEI=angiotensin converting enzyme. ARB-angiotensin II receptor blocker.
Table 1.
Baseline characteristics
| Discontinuation group (n=104) | Continuation group (n=100) | ||
|---|---|---|---|
| Age, years | 74 (63–80) | 75 (69–80) | |
| Sex | |||
| Female | 39 (38%) | 36 (36%) | |
| Male | 65 (63%) | 64 (64%) | |
| Body-mass index, kg/m2 | 28·0 (25·4–31·3) | 27·4 (25·4–29·4) | |
| Medical history | |||
| Hypertension | 100 (96%) | 99 (99%) | |
| Current smoker | 6 (6%) | 9 (9%) | |
| Dyslipidaemia | 38 (37%) | 48 (48%) | |
| Diabetes | 30 (29%) | 37 (37%) | |
| Coronary artery disease | 22 (21%) | 23 (23%) | |
| Heart failure | 12 (12%) | 6 (6%) | |
| Atrial fibrillation | 18 (17%) | 17 (17%) | |
| Chronic obstructive pulmonary disease* | 10 (10%) | 22 (22%) | |
| Malignant disease | 7 (7%) | 13 (13%) | |
| Kidney disease | 16 (15%) | 21 (21%) | |
| History of stroke | 8 (8%) | 5 (5%) | |
| Angiotensin-converting enzyme inhibitor vs | 57 (55%) | 58 (58%) | |
| Angiotensin II receptor blocker | 47 (45%) | 42 (42%) | |
| Timeline | |||
| Days from test to randomisation | 2 (1–4) | 2 (1–4) | |
| Days from symptoms to randomisation | 4 (2–7) | 4 (2–7) | |
| Days from admission to randomisation | 1 (0–3) | 1 (0–3) | |
| Reported symptoms | |||
| Fever | 42 (40%) | 39 (39%) | |
| Cough | 53 (51%) | 54 (54%) | |
| Myalgia | 24 (23%) | 36 (36%) | |
| Fatigue | 66 (63%) | 65 (65%) | |
| Diarrhoea | 15 (14%) | 15 (15%) | |
| Vomiting | 9 (9%) | 3 (3%) | |
| Dyspnoea | 38 (37%) | 43 (43%) | |
| Vital parameters and SOFA | |||
| Heart rate, beats per min | 76 (68–83) | 76 (68–84) | |
| Respiratory rate, per min | 18 (16–22) | 18 (16–20) | |
| Body temperature, °C | 37·0 (36·4–37·7) | 37·0 (36·4–38·0) | |
| Systolic blood pressure, mm Hg | 130 (120–141) | 130 (120–140) | |
| Diastolic blood pressure, mm Hg | 77 (68–80) | 75 (70–80) | |
| Blood oxygen saturation, % | 94% (92–96) | 95% (93–96) | |
| SOFA score, median (IQR) | 0 (0–1) | 1 (0–1) | |
| SOFA score, mean (SD) | 0·7 (1·0) | 0·8 (1·0) | |
| Respiratory therapy | |||
| Need of oxygen substitution | 45 (43%) | 41 (41%) | |
| Oxygen mask therapy | 43 (41%) | 37 (37%) | |
| Non-invasive ventilation | 5 (5%) | 0 (0%) | |
| Radiological signs | |||
| Bipulmonal infiltrates on x-ray or CT | 36 (35%) | 38 (38%) | |
| Other opacities on x-ray or CT | 29 (28%) | 26 (26%) | |
| Laboratory findings | |||
| C-reactive protein, mg/dL | 5·1 (1·5–9·6) | 4·5 (1·6–8·3) | |
Data are median (IQR) or n (%); SOFA is also presented as mean (SD). SOFA=Sequential Organ Failure Assessment Score.
COPD was significantly different between the groups (p=0·025). Note that not all percentages add up to 100 owing to rounding up.
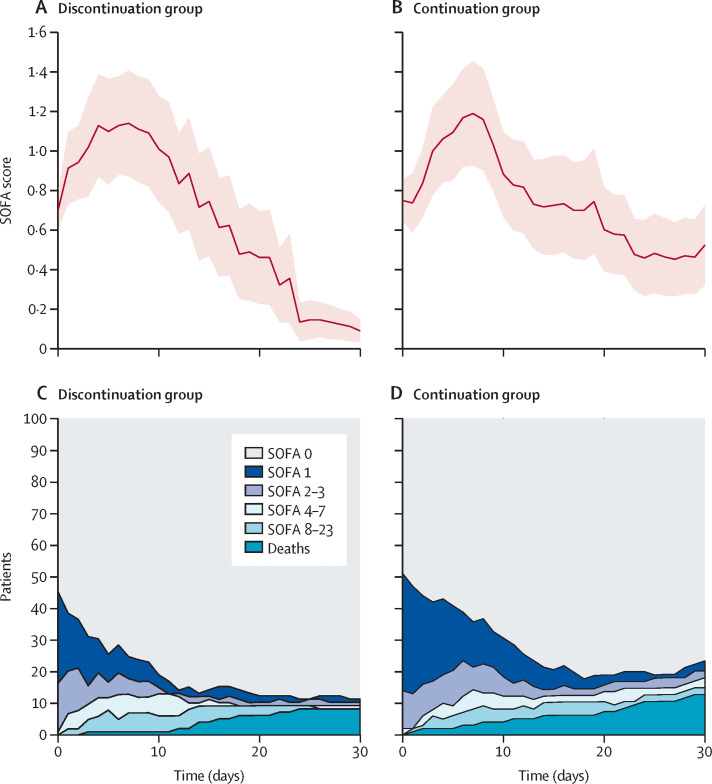
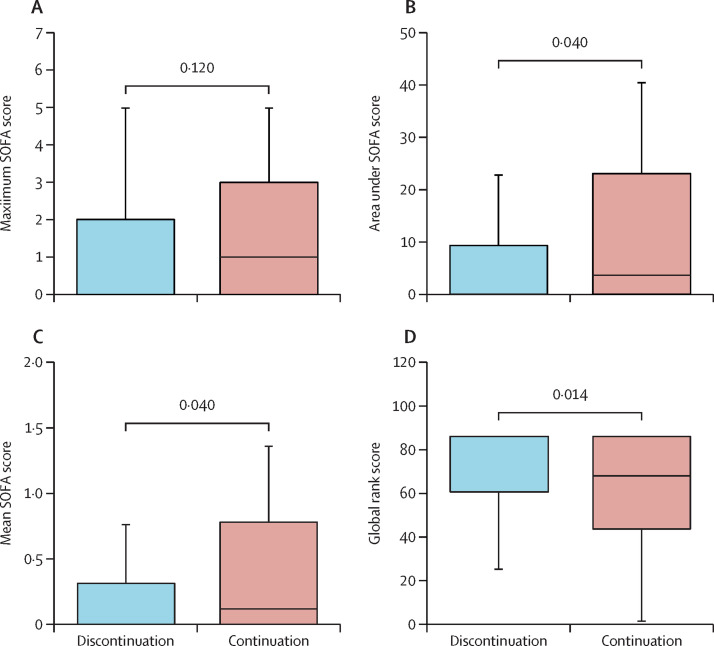
During the course of the study, eight patients (8%) in the discontinuation group and 12 patients (12%) in the continuation group died (pChi=0·42; table 2 ). Figure 2A and 2B show the SOFA score profiles throughout the study. In both groups, average SOFA scores initially increased within the first days after enrolment and subsequently decreased. The primary endpoint of our study, the maximum median (IQR) SOFA score, did not significantly differ between treatment groups (0·00 [0·00–2·00] vs 1·00 [0·00–3·00]; pWMW=0·12; figure 3A ; table 2). After the maximum, the average SOFA scores in the discontinuation group decreased more rapidly and reached lower levels than in the continuation group (figure 2A and 2B). Thus, integral measures AUCSOFA and mean SOFA were significantly lower in patients of the discontinuation group than in patients of the continuation group (0·00 [0·00–9·25] vs 3·50 [0·00–23·50]; pWMW=0·040, figure 3B, and 0·00 [0·00–0·31] vs 0·12 [0·00–0·78]; pWMW=0·040; figure 3C; table 2). SOFA scores at 30 days were also significantly lower in patients in the discontinuation group compared with patients in the continuation group (p=0·023; table 2).
Table 2.
Primary, secondary, and exploratory endpoints
| Discontinuation group (n=104) | Continuation group (n=100) | p value | |
|---|---|---|---|
| Primary endpoint | |||
| Maximum SOFA score | 0·00 (0·00–2·00) | 1·00 (0·00–3·00) | 0·12 |
| Secondary endpoints | |||
| Area under the SOFA score, days | 0·00 (0·00–9·25) | 3·50 (0·00–23·50) | 0·040 |
| Mean SOFA score | 0·00 (0·00–0·31) | 0·12 (0·00–0·78) | 0·040 |
| All-cause death | 8 (8%) | 12 (12%) | 0·42 |
| Admission to ICU | 20 (19%) | 18 (18%) | 0·96 |
| Mechanical ventilation | 10 (10%) | 8 (8%) | 0·87 |
| Non- invasive ventilation | 19 (18%) | 14 (14%) | 0·52 |
| Renal replacement therapy | 0 | 1 (1%) | 0·98 |
| Extracorporeal membrane oxygenation | 1 (1%) | 0 | 1·00 |
| Hospital admission, days | 10·00 (5·75–15·25) | 11·00 (6·75–19·00) | 0·27 |
| Exploratory endpoints | |||
| Composite of admission to ICU, mechanical ventilation, and death | 21 (20%) | 26 (26%) | 0·41 |
| SOFA score at 30 days | 0·00 (0·00–1·20)* | 0·00 (0·00–24·00)* | 0·023 |
| 30-day SOFA score ≥1 | 11 (11%) | 23 (23%) | 0·017 |
| SOFA 0 vs SOFA 1–24, patient days† | 2428 vs 428 (85% vs 15%) | 2112 vs 2729 (23% vs 77%) | 0·0031 |
| Global rank score‡ | 86·00 (60·75–86·00) | 68·50 (43·75–86·00) | 0·014 |
Data are median (IQR) or n (%). SOFA score included death and outpatient status as described in methods. SOFA=sequential organ failure assessment score. ICU=intensive care unit.
Median (10–90th percentile).
p value calculated from generalised linear mixed-effects model respecting structure of individual longitudinal data.
Global rank score as defined in the REPLACE-COVID trial.
Figure 2.
SOFA score profiles and distributions
SOFA score profiles in the discontinuation group (A) and continuation group (B). Red lines show mean values and red areas show SE of the mean. For this analysis, patients who died were censored at time of death. Distributions of SOFA scores over 30 days in the discontinuation group (C) and continuation group (D). A SOFA score of 24 was achieved only by death. Timepoint 0 denotes baseline in all graphs. SOFA=sequential organ failure assessment score.
Figure 3.
Box plots of maximum SOFA score (A), area under the death-adjusted SOFA score (B), mean SOFA score (C), and global rank score (D) in the discontinuation and continuation groups
The global rank score is a hierarchical clinical endpoint with higher values indicating better clinical outcomes.28 SOFA=sequential organ failure assessment score. The solid boxes indicate the 25th to 75th percentile, the horizontal line shows the median and the whiskers mark the 10th and 90th percentiles.
Figure 2 (C and D) illustrates the SOFA score categories in both treatment groups throughout the study. In line with the continuous assessment, SOFA score categories improved in both groups. The recovery in the continuation group was delayed. At 30 days, 11 (11%) of 104 patients in the discontinuation group as opposed to 23 (23%) of 100 in the continuation group were either dead or had a SOFA score ≥1 (pChi=0·017; figure 2C and D; table 2). A significant effect of treatment on both the mean SOFA score over time, as well as the SOFA category (0 vs 1–24) was confirmed by the mixed-effects regression models (pLRT=0·0019 and pLRT=0·0031, respectively; appendix p 2).
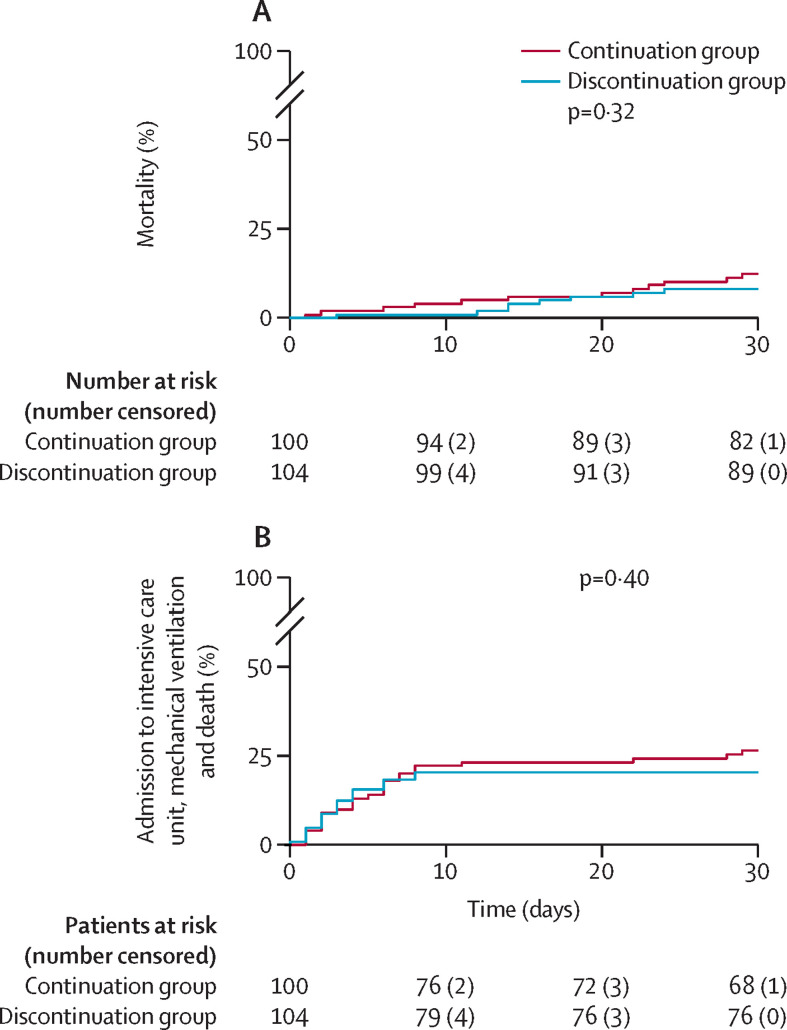
Figure 4A and B show cumulative event rates of clinical endpoints. There was no significant difference in the combined clinical endpoint of admission to ICU, mechanical ventilation and death, which was reached by 21 (20%) of 104 patients in the discontinuation group and 26 (26%) of 100 patients in the continuation group (p=0·41; table 2). This endpoint has to be considered exploratory, as the study was not extended due to the neutral results of the primary endpoint of the maximum death-adjusted SOFA score.
Figure 4.
Cumulative event rates of patients assigned to discontinuation (blue) or continuation (red) of ACEI–ARB therapy
Cumulative mortality rates (A). Cumulative event rates of the composite of admission to intensive care unit, mechanical ventilation and death (B).
Overall, the effect on the primary endpoint was consistent (ie, no effect on the primary outcome) across major predefined subgroups, including age, diabetes, RAS inhibition (ACEI vs ARB), baseline SOFA score, and renal insufficiency. However, there were significant interactions with male sex and presence of COPD, favouring discontinuation of ACEI–ARB therapy (appendix p 3).
There was no heterogeneity within treatment effects associated with the study centre. Similarly, prespecified per-protocol analysis censoring of patients at times of crossover did not affect the main results of the study. 30-day analyses were also not affected when patients who withdrew consent or were lost to follow-up were excluded.
In the discontinuation group, 58 (56%) of 104 patients received a replacement antihypertensive drug (appendix p 6). Calcium antagonists were used in most cases (90%). Systolic blood pressures greater than 180 mm Hg (as per safety endpoint) were reported in 14 patients, of which seven were in the discontinuation group. Arterial blood pressures throughout the study are depicted in the appendix (p 4) for both groups. There were no admissions to hospital owing to decompensated heart failure. 17 of the 20 deaths that occurred during the study were classified as COVID associated, whereas in three cases the cause of death was considered as unknown. The adverse events in both treatment groups are listed in the appendix (p 6).
As a further post-hoc exploratory analysis, we examined treatment effects on the global rank score, which served as the primary endpoint in the REPLACE COVID trial28 and ranks patients hierarchically by clinical outcomes. As shown in figure 3D, the global rank score was significantly better for patients in the discontinuation group than in the continuation group (median [IQR] 86 [60·75–86] vs 68·5 (43·75–86); pWMW=0·014). When we recalculated the primary endpoint of our study applying the modified version of the SOFA score as used in REPLACE COVID28, we also found a significant effect on the maximum SOFA score (appendix pp 6, 7).
Discussion
The primary analysis of our study indicates that discontinuation of RAS inhibition in patients with recent-onset COVID-19 had no significant effect on the maximum SOFA score within 30 days. In this sense, therefore, the outcome of our study should be considered neutral. Nevertheless, secondary and exploratory analyses strongly suggest that discontinuation of RAS inhibition has a favourable effect on disease progression in our study population for several reasons. First, we found striking differences in the temporal distributions of the SOFA scores between the two treatment groups. Second, patients assigned to discontinuation of RAS inhibition had a lower AUCSOFA, a lower mean SOFA score, and a lower 30-day SOFA score, indicating a significant treatment effect which was also confirmed by the mixed-effects regression models. Third, at 30 days, 11% in the discontinuation group but 23% in the continuation group were either dead or had a SOFA score of 1 or more. Finally, as further confirmation, discontinuation of RAS inhibition also favourably affected the global rank score, a hierarchical clinical endpoint used as the primary outcome measure in the REPLACE COVID trial.
The results of our study complement those of two previous randomised trials that did not detect an effect of discontinuing RAS inhibition on outcomes. BRACE CORONA was a pragmatic, registry-based, randomised, open-label trial done in Brazil that impressively succeeded in recruiting 659 patients with recent COVID-19 and chronic ACEI–ARB treatment in just 3 months.27 The study found no effect of discontinuing RAS inhibition on the number of days alive or out of hospital. The study differs from ours in several key aspects. With a median age of 55 years, patients in BRACE CORONA were almost 20 years younger than patients enrolled in ACEI-COVID and suffered from substantially fewer comorbidities. As a consequence, 30-day mortality was much lower in BRACE CORONA compared with our study (2·7% vs 9·8%; pChi <0·001). It is conceivable that the effect of RAS inhibition is detectable only beyond a certain baseline risk threshold. Symptom duration before randomisation was longer than in our study (8 days [5–12] vs 4 days [2–7]). Hence, discontinuation in BRACE CORONA might have been too late to affect the course of COVID-19. Moreover, BRACE CORONA was not designed to capture organ status on a daily basis; hence, the effects described in our study might have been missed. REPLACE COVID, to our knowledge, the only other published randomised trial to examine the effect of discontinuing RAS inhibitors in COVID-19, included 152 patients from seven countries, 59% of whom were enrolled in the USA.28 The authors observed that discontinuing RAS inhibition had no effect on the global rank score, a non-parametric, ranked outcome which hierarchically included death, mechanical ventilation or extracorporeal membrane oxygenation, renal replacement therapy or vasopressor therapy, and area under a modified SOFA score.28 In terms of study design, REPLACE COVID is much more similar to our study than BRACE CORONA. Yet, there are important differences beyond a larger sample size in our trial: with a mean age of 62 years, patients were 10 years younger in REPLACE COVID compared with ACEI-COVID (mean of 72 years). Only 4% of the patients had a diagnosis of heart failure at baseline compared with 9% in our study (pChi=0·07). The actual intervention (ie, discontinuation or continuation of RAS inhibitors, was implemented later (mean of 7 days after symptom onset) and limited to the hospital stay only. In contrast, the intervention was maintained for 30 days in our study. When we applied the global rank score used in REPLACE COVID to our patient cohort in an exploratory post-hoc analysis, we detected a significant and favourable effect of discontinuing RAS inhibition. For their analyses, REPLACE COVID also used a modified SOFA score, which only considers those components that are most likely to be affected by the RAS system.28 When applied to our study, the modified version of the SOFA score instead of the original SOFA score also resulted in a significant effect on the primary endpoint (pWMW=0·045; appendix p 4), which underlines the consistency of our data. At this point, we can only speculate about the reasons for the different effects observed in the two studies, most importantly including differences in sample size, patient selection and characteristics, timing of infection, and duration of intervention (appendix p 8).
The mechanisms by which discontinuation of RAS inhibition might have affected disease progression in our study remain unclear. Experimental data showed that ACEI–ARB could increase ACE2 expression in the heart,11, 12, 13 intestine,14 kidney,15 or urogenital tract,16 although conflicting studies exist.17 Our findings therefore are consistent with the concept that discontinuation of RAS inhibition improves the course of COVID-19 by decreasing the expression of ACE2, the target receptor for SARS-CoV-2, in one or more organs. The fact that in our study effects were observed mainly in the later course of the disease and on different organ systems, could be consistent with these considerations. In addition, factors independent of ACE2 expression could be involved. These might include potentially beneficial effects on blood pressure regulation, particularly in older, clinically vulnerable patients who develop a systemic inflammatory response syndrome during the course of the disease, and potentially beneficial effects of calcium channel blockers, which were used as a replacement medication in 52 patients of the discontinuation group. The latter notion is based on evidence that endosomal calcium channels play important roles in cellular entry and uncoating of corona viruses,32 although evidence for a therapeutic effect of calcium channel blockers in COVID-19 is lacking.
Major professional societies22, 23 recommend continuation of RAS inhibition in COVID-19, balancing the presumed risks of uncontrolled discontinuation of cardiovascular drugs and absence of scientific evidence. With BRACE CORONA and REPLACE COVID, two randomised trials have shown no net effect of discontinuation. As our study did not meet its primary endpoint, it should also be considered neutral. Therefore, the secondary and exploratory results of our study showing a favourable effect of discontinuation of RAS inhibition on the course of COVID-19 are unlikely to change the general recommendations of professional societies. Nevertheless, these analyses suggest that under controlled conditions, in an older population with recent-onset symptomatic SARS-CoV-2 infection, temporary interruption of RAS inhibition might have beneficial net effects compared with BRACE CORONA and REPLACE COVID. At the same time, temporary discontinuation of RAS inhibition did not cause harm in any of the three randomised trials: in the combined populations of BRACE CORONA, REPLACE COVID, and our study, 27 (5%) of 515 patients in the discontinuation and 32 (6%) of 500 patients in the continuation groups died (p=0·43). Therefore, the results of our study might be important for patients in terms of individualised therapeutic decision-making. An individual decision should take into account factors such as the strength of indication for RAS inhibition, the availability of appropriate replacement medication, and the monitoring capability. In our study, we also found that male patients and those with obstructive lung disease might benefit from discontinuation of ACEI–ARB therapy. However, subgroup analyses of a non-significant endpoint should not be overinterpreted and be viewed with caution. Last but not least, a strategy of discontinuation should also ensure that temporarily halted RAS inhibition is reinitiated once infection is overcome.
The limitations of our study should be recognised. First, our study was not powered to evaluate the effect of intervention on clinical endpoints, which would have required a much larger sample size. The protocol of our study would have allowed the extension of the study population to 798 patients, powered to evaluate the coprimary endpoint of death, admission to ICU and use of mechanical ventilation. However, even with the involvement of our 35 study centres, it might be difficult to complete enrolment in a reasonable time frame. Nevertheless, the SOFA score is a standardised and well-established prognostic tool in critical care medicine that has been validated in numerous studies to predict clinical outcomes, including mortality.33, 34 Second, this was an open-label study. Therefore, inherent bias in the treatment of patients cannot be excluded. However, the design and conduct of a double-blind randomised trial in a pandemic setting would have been complex and time-consuming. Third, a small number of patients crossed over and stopped or started RAS inhibitors without clinical reasons. However, sensitivity analyses with censoring of these patients at the time of crossover showed no effect on outcomes. Fourth, the recording of blood pressure in outpatients was based on self-measurements, which carries a potential for self-reporting and measurement bias. Fifth, the distributions of the maximum SOFA score in our study population were much wider than assumed. Finally, when we designed the study, we had little information on COVID-19 progression and hence the outcome measures to best characterise the course of disease. Therefore, the primary endpoint of our study might not be optimally suited to detect effects of discontinuation on disease progression. These are likely to be more accurately captured by integral measures such as AUCSOFA and mean SOFA, or by analyses that assess the time course in a longitudinal approach.
In conclusion, discontinuation of RAS inhibition in patients with recent-onset COVID-19 had no significant effect on the maximum SOFA score over 30 days, the primary outcome measure of our study. However, secondary and exploratory analyses revealed that there might be a favourable net effect of discontinuation of RAS inhibition on organ functions as assessed by mean SOFA and area under the SOFA score, suggesting better and faster recovery of elderly high-risk patients with COVID-19. This effect remained evident until the end of the observation period. There was no evidence of harm from discontinuation of ACEI–ARB treatment. Further studies are needed to better understand the interaction between RAS inhibition and SARS-CoV-2 infection and to identify conditions under which RAS modification in COVID-19 might improve outcomes. Long-term follow-up of randomised patients is needed to evaluate whether RAS modification during the acute phase might have an effect on the long-term sequelae of SARS-CoV-2.
Data sharing
De-identified data collected during the conduct of the study can be made available for collaborative analyses on request after appropriate data sharing agreements have been concluded. Data will be made available after review and approval of research proposals by the principal investigators, to the extent permitted by existing local regulations and data sharing agreements. Proposals should be addressed to the corresponding authors.
Declaration of interests
AB received research funding from Pfizer and Medtronic as well as speaker honoraria from Bayer, Boehringer Ingelheim, Edwards, Medtronic and Novartis. KDR received a research grant from Daiichi-Sankyo Europe GmbH. UM received fees for participation in a data and safety monitoring board from Thermosome. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the Austrian Science Fund (FWF; Urgent funding SARS-CoV-2; KLI-900 to AB) and the German Center for Cardiovascular Research (DZHK; ACEI-COVID to SM and KDR). We thank Liane Fendt, Regina Gassler, and Nihad Muric and the team of the Competence Center for Clinical Trials at the Medical University of Innsbruck for their excellent support. We thank Florian Kronenberg and the ASKIMED team for data management. Finally, we thank Kristin Tessadri and Michael Toifl for coordination of the Austrian study centres and Monika Baylacher, Gabriele Baur, and Martina Schulz from the Ludwig-Maximilians-University of Munich for coordination of the German study centres.
Contributors
AB, SM, and KDR designed the study and analysed and interpreted the data. Data acquisition was done by all authors. UM, KDR, and NS contributed to the protocol and did the statistical analyses. LF, SS, and SE-A accessed and verified the data. AB and SM wrote the first draft with input from UM and submitted the final version for publication. AB, KDR, NS, UM, and SM had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors reviewed the final draft and agree with its content and conclusions.
Contributor Information
ACEI-COVID investigators:
Marcin Bantkowiak, Gabriele Baur, Monika Baylacher, Marcel Beaucamp, Manuel Berger, Lisa Besch, Stefan Brunner, Stephan Budweiser, Heiko Bugger, Raffaele Coletti, Uwe Dorwarth, Jozsef Egresits, Elodie Eiffener, Christian Faul, Armin Finkenstedt, Konstantinos Gatos, Nadine Gauchel, Frank Gindele, Wilhelm Grander, Markus Gunschl, Frank Hartig, Moritz Hecht, Tobias Heer, Lukas Heger, Marcus Hentrich, Lena Horvath, Dritan Keta, Stefan Kiechl, Rudolf Kirchmaier, Andreas Klein, Mathias Klemm, Ewald Kolesnik, Andreas König, Hans Christian Kossmann, Jana Kropacek, Lukas Lanser, Achim Lother, Anja Löw, Amir-Abbas Mahabadi, Stefan Malleier, Gert Mayer, Christoph Müller, Dirk Müller-Wieland, Bernhard Nagel, Hannes Neuwirt, Christoph Olivier, Thomas Raunegger, Martin Reindl, Sebastian Reinstadler, Lisa Riesinger, Michael Schäffner, Johannes Schier, Julia Schock, Peter Schönherr, Martina Schulz, Thomas Schütz, Johannes Schwarz, Johannes Siebermair, Marcus Siry, Anna Spaur, Wolfgang Sturm, Kristin Tessadri, Fabian Theurl, Markus Theurl, Liz Thommes, Christina Tiller, Michael Toifl, Matthias Totzeck, Hedda von zur Mühlen, Nadine Vonderlin, Reza Wakili, Clemens Wendtner, Felix Wenner, Daniela Wimmert-Roidl, and August Zabernigg
Supplementary Material
References
- 1.John Hopkins Coronavirus Resource Center COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. https://coronavirus.jhu.edu/map.html
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271. doi: 10.1016/j.cell.2020.02.052. 80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ocaranza MP, Godoy I, Jalil JE, et al. Enalapril attenuates downregulation of angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48:572–578. doi: 10.1161/01.HYP.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- 12.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 13.Nicin L, Abplanalp WT, Mellentin H, et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41:1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vuille-dit-Bille RN, Camargo SM, Emmenegger L, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 15.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 16.Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- 17.Wysocki J, Lores E, Ye M, Soler MJ, Batlle D. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: implications for COVID-19. J Am Soc Nephrol. 2020;31:1941–1943. doi: 10.1681/ASN.2020050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38:781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 20.Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between COVID-19 mortality and the renin-angiotensin system? A call for epidemiologic investigations. Clin Infect Dis. 2020;71:870–874. doi: 10.1093/cid/ciaa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16:305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Society of Cardiology Position statement of the ESC Council on hypertension on ACE-Inhibitors and angiotensin receptor blockers. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 23.American College of Cardiology HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. March 17, 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19
- 24.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarcho JA, Ingelfinger JR, Hamel MB, D'Agostino RB, Sr, Harrington DP. Inhibitors of the Renin-Angiotensin-Aldosterone System and Covid-19. N Engl J Med. 2020;382:2462–2464. doi: 10.1056/NEJMe2012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen JB, Hanff TC, William P, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 31.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67 [Google Scholar]
- 32.Zhao Z, Qin P, Huang YW. Lysosomal ion channels involved in cellular entry and uncoating of enveloped viruses: Implications for therapeutic strategies against SARS-CoV-2. Cell Calcium. 2021;94 doi: 10.1016/j.ceca.2021.102360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 34.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data collected during the conduct of the study can be made available for collaborative analyses on request after appropriate data sharing agreements have been concluded. Data will be made available after review and approval of research proposals by the principal investigators, to the extent permitted by existing local regulations and data sharing agreements. Proposals should be addressed to the corresponding authors.