Abstract
Rosebush (Rosa “Radrazz”) plants are an excellent model to study light control of bud outgrowth since bud outgrowth only arises in the presence of light and never occurs in darkness. Recently, we demonstrated high levels of hydrogen peroxide (H2O2) present in the quiescent axillary buds strongly repress the outgrowth process. In light, the outgrowing process occurred after H2O2 scavenging through the promotion of Ascorbic acid–Glutathione (AsA–GSH)-dependent pathways and the continuous decrease in H2O2 production. Here we showed Respiratory Burst Oxidase Homologs expression decreased in buds during the outgrowth process in light. In continuous darkness, the same decrease was observed although H2O2 remained at high levels in axillary buds, as a consequence of the strong inhibition of AsA–GSH cycle and GSH synthesis preventing the outgrowth process. Cytokinin (CK) application can evoke bud outgrowth in light as well as in continuous darkness. Furthermore, CKs are the initial targets of light in the photocontrol process. We showed CK application to cultured buds in darkness decreases bud H2O2 to a level that is similar to that observed in light. Furthermore, this treatment restores GSH levels and engages bud burst. We treated plants with buthionine sulfoximine, an inhibitor of GSH synthesis, to solve the sequence of events involving H2O2/GSH metabolisms in the photocontrol process. This treatment prevented bud burst, even in the presence of CK, suggesting the sequence of actions starts with the positive CK effect on GSH that in turn stimulates H2O2 scavenging, resulting in initiation of bud outgrowth.
Light-induced bud outgrowth in rosebush results from cytokinin-mediated peroxide scavenging and glutathione metabolism stimulation.
Introduction
The aerial architecture of plants largely depends on the spatio-temporal comportment of the axillary buds, which can either remain quiescent or sprout to form new axis (Evers et al., 2011). The control of the bud outgrowth process relies on the integration of many internal plant factors (phytohormones, nutrients, redox status) that are themselves dependent upon environmental parameters (Considine and Foyer, 2014; Rameau et al., 2015; Le Moigne et al., 2018; Barbier et al., 2019a). Auxin and strigolactones prevent bud outgrowth (Thimann et al., 1933; Brewer et al., 2009) while cytokinins (CKs) stimulate it (Cline et al., 1997). CKs are well known to evoke bud outgrowth when applied to plants (Pillay and Railton, 1983; Kalousek et al., 2010; Dun et al., 2012) and have been recognized as a major actor of bud outgrowth. Indeed, CK synthesis is induced after apical dominance release, causing an increase of their quantity in bud and neighboring tissues, while the application of CK synthesis and signaling inhibitors (Lovastatin and LGR-991) both decreases bud outgrowth in rosebush (Roman et al., 2016).Involvement of reactive oxygen species (ROS), and notably of hydrogen peroxide (H2O2), in the branching process, was proposed to play a central role in bud outgrowth regulation (Sagi et al., 2004; Chen et al., 2016) that was recently confirmed in rosebush (Porcher et al., 2020). However, ROS contribution to bud outgrowth remains generally under considered in the recent literature. Wang and Faust (1988) firstly identified that highly oxidized compounds of quiescent apple tree buds decreased during outgrowth. Many studies in grapevine also reported changes in H2O2 metabolism after natural (chilling) or artificial (sodium azide or hydrogen cyanamide applications) induction of bud endodormancy release: a simultaneous inhibition of catalase activity occurred along with a transient increase in H2O2 (Pérez and Lira, 2005) followed by the strong induction of H2O2 scavenging activities (Or et al., 2000; Or, 2009; Sudawan et al., 2016). Although the mechanisms are different in eco or paradormancy, the activation of the Ascorbic acid–Glutathione (AsA–GSH)-dependent H2O2 scavenging pathway is constantly present during bud outgrowth, regardless of the dormancy type (Wang et al., 1991; Wang and Faust, 1994; Pérez and Noriega, 2018; Porcher et al., 2020). The negative action of H2O2 toward bud outgrowth was also evoked in actively growing tomato plants (Sagi et al., 2004; Chen et al., 2016): these authors showed that plants deficient for Respiratory Burst Oxidase Homologs (RBOHs) genes encoding NADPH oxidases (NOXs), an indirect H2O2 producer involved in plant development (Sagi and Fluhr, 2001; Foreman et al., 2003), displayed a highly branched phenotype while the application of H2O2 (5 mM) on rboh mutant plants restored the wild-type phenotype (Chen et al., 2016). Furthermore, H2O2 inhibited cell division in BY-2 menadione (a ROS inducer) treated tobacco (Nicotiana tabacum) cells (Reichheld et al., 1999), suggesting that H2O2 could prevent cell division in quiescent buds as recently confirmed in rosebush (Porcher et al., 2020).
Light has a major impact on the aerial branching process, allowing plants to adapt their development to environmental constraints (Wang and Faust, 1988; Leduc et al., 2014). As an example, the shade avoidance response, that occurred in low light intensity and low red/far-red ratio, favors the main axis growth and inhibits the ramification process, allowing the plant to rapidly escape unfavorable light conditions (Casal et al., 1986; Ballaré and Casal, 2000). In rosebush, branching also relies on light intensity and quality (Girault et al., 2008; Furet et al., 2014; Demotes-Mainard et al., 2016) in interaction with genotypes (Crespel et al., 2020). Interestingly, and in contrast to other species such as Arabidopsis, poplar, tomato, and grapevine, in Rosa “Radrazz” bud outgrowth is strictly light-dependent and does not occur in darkness (Girault et al., 2008; Signorelli et al., 2018). In dark condition, buds displayed a total inhibition of organogenesis that was concomitant with an inhibition of free hexose synthesis and a modification of the hormonal balance (Girault et al., 2008, 2010; Roman et al., 2016). Indeed, the absence of light inhibits both the synthesis and the transport of CK that normally arise in bud-bearing nodes in light (Roman et al., 2016). Moreover, CK applications to the apex or to the axillary bud itself were sufficient to rescue bud outgrowth in the absence of light. These results placed CK as an early and major actor in the photocontrol of rosebush bud outgrowth (Roman et al., 2016).
Some works pointed out an interaction between H2O2 and light intensity, while some others showed that light quality also affects H2O2 metabolism (Causin et al., 2009). For example, high light stress evoked a strong increase of H2O2 content (Mittler, 2002) and the concomitant activation of several enzymes such as catalase or peroxidases (APX, POD, and GPX; Caverzan et al., 2012) involved in H2O2 scavenging, along with an increase in GSH content (Müller-Moulé et al., 2003; Mullineaux and Rausch, 2005; Mullineaux et al., 2006). In contrast, the continuous reduction of light intensity unbalanced ROS metabolism after a drop in AsA content and H2O2-scavenging enzyme activities in Arabidopsis (Bartoli et al., 2006). The total absence of light also significantly affects the H2O2-scavenging metabolism, especially the AsA–GSH pathway. For example, huge decreases in AsA content and AsA recycling activity were observed in spinach post-harvested leaves kept in darkness (Toledo et al., 2003). Similarly, the observations of Noctor et al. (1997) consistently showed that GSH synthesis was light-dependent, explaining the low GSH level measured in poplar leaves in darkness.
We recently showed that quiescent buds display a high content in H2O2, that is systematically lowered during the outgrowth process, concomitantly with an increased H2O2 scavenging depending of AsA–GSH activity (Porcher et al., 2020). With regards to these results, we questioned the possible involvement of H2O2 metabolism in the photocontrol of bud outgrowth in rosebush. In particular, we wondered whether H2O2 metabolism in buds behaves similarly in the absence or presence of light and if so, whether H2O2 photo-regulation is linked to previously identified key actors, notably CK (Girault et al., 2008; Roman et al., 2016).
Here we show that the H2O2 decrease, that ordinarily occurs during bud outgrowth in light (Porcher et al., 2020) was totally prevented in darkness because of an almost total inhibition of AsA–GSH-dependent H2O2 scavenging. In contrast, RBOHs (NOX) appears to be not implicated in the bud outgrowth photocontrol. Furthermore, we established that when under dark conditions, bud outgrowth is triggered by an exogenous supply of CK (Roman et al., 2016), CK act in part through the induction of GSH synthesis and AsA–GSH scavenging pathways, causing, in turn, the decrease in internal bud H2O2 content observed during bud burst. Thus, our results confirm that CK is an early and key player in bud outgrowth photocontrol and demonstrate that, besides interactions with sugars and other hormones (Roman et al., 2016; Corot et al., 2017; Bertheloot et al., 2020), CK acts through the control of ROS homeostasis in the bud.
Results
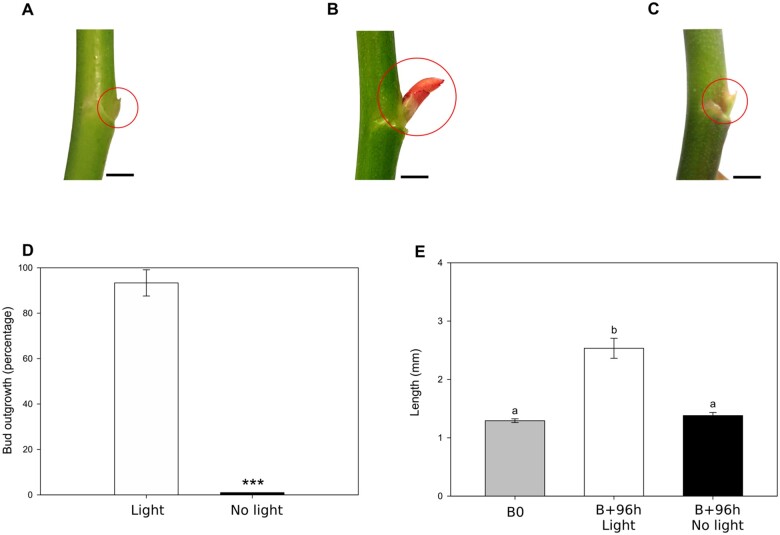
We used young rosebush plants at the Visible Floral Bud (VFB) stage (Figure 1;Supplemental Figure S1) when the median buds of the stem showed no signs of bud outgrowth (the protective scales only forming a slight bulge, Figure 2, A and E “gray bar”). As previously demonstrated in defoliated beheaded rosebush plant (Girault et al., 2008; Roman et al., 2016), light perception by the bud is required to promote its outgrowth. We replicated these observations using a non-defoliated experimental model: nearly all (93.3%) of plants exposed to white light (L:D 16 h:8 h) actually displayed median outgrowing buds (Figure 2, B and D) and showed a substantial elongation of the secondary axis 96 h after beheading (2.6 mm; Figure 2E “white bar”). In contrast, no bud outgrowth occurred in continuous darkness (Figure 2, C–E “black bars”). The expression level of several genes involved in bud outgrowth and known to be controlled by light (Girault et al., 2010; Barbier et al., 2015; Roman et al., 2016, 2017; Porcher et al., 2020) was also checked in this non-defoliated model. Expression of genes involved in cell division (RhPCNA, coding a facilitator of DNA polymerase II activity; RhCYCD3, coding a D-type cyclin involved in the transition from G1/S to S steps of the cell cycle), cell expansion (RhEXP, coding an expansin causing cell wall relaxation necessary for cell expansion), strigolactone signaling (RhMAX2, coding a F-box protein involved in strigolactone perception) or carbohydrate metabolism (RhVI, coding a vacuolar invertase involved in the hydrolysis of sucrose; RhNADSDH, coding a NAD-dependent sorbitol dehydrogenase, implicated in the conversion of sorbitol to fructose), were all modulated by light conditions and this validated the non-defoliated experimental model (Supplemental Figure S2).
Figure 1.
Experimental design. Rosa “Radrazz” was used as model plant for all experiments at the VFB stage. We numbered the different axillary buds from the basal (#1) to the apical one (#4), and set the beheading zone 1cm above the fourth bud. In-planta experiments were conducted to measure the oxidative metabolism in median buds (buds #2 and #3) in light (L:D 16:8) or continuous darkness and in the presence/absence of the synthetic CK (BAP). We collected samples to measure H2O2, GSH contents and key-genes expression levels at beheading, and at different time-points. The percentage of outgrowing buds and the secondary axis elongation were measured 96 h after beheading. We furthermore evaluated the effect of BAP (2 µM) and BSO (a GSH synthesis inhibitor; 1 mM) alone or in combination, by measuring the percentage of bud outgrowth and for 10 d the secondary axis elongation of in vitro cultivated axillary buds. The rosebush pictures presented here were obtained after digital extraction to illustrate the experimental design.
Figure 2.
Effects of light conditions on bud outgrowth. Typical aspect of buds at beheading time (B0) (A), 96 h later in light (L:D 16:8, B), and in continuous darkness (No light, C). The nodes (buds + stem fragments) in (A–C) were digitally extracted for comparison, scale bars = 2 mm. Percentage of bud outgrowth at 96 h after beheading in light or continuous darkness (D). Length of bud or of subsequent axis at beheading time (B0) or after 96 h in light or continuous darkness (E). Data are means of n = 3 biological independent replicates ±se. The asterisks indicates the significant difference (P <0.001) between light and darkness condition (Wilcoxon–Mann–Whitney nonparametric test). Letters indicate significant differences (P <0.05) after Kruskal–Wallis nonparametric ranks test followed by a two-by-two comparison matrix by Wilcoxon–Mann–Whitney nonparametric test.
Light conditions modulate H2O2 metabolism in bud
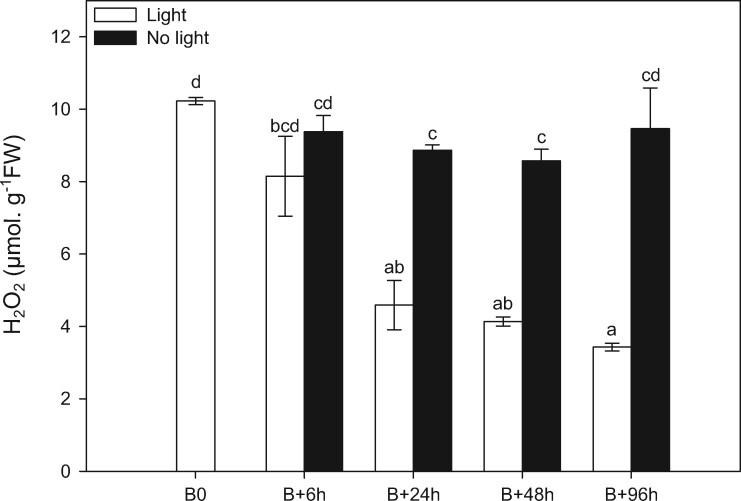
A previous study from our group established a correlation between H2O2 quantities in buds and the inhibition of the outgrowth process, and highlighted the importance of H2O2 scavenging to allow bud outgrowth (Porcher et al., 2020). Here we show that H2O2 content rapidly decreases after beheading in median buds in light. The amounts at the time of beheading (almost 10 µmol.g−1 FW) were statistically different from those measured 24 h (4.6 µmol.g−1 FW) and 96 h later (3.4 µmol.g−1 FW; Figure 3 “white bars”). At this latter time, the first leaf has protruded out of bud scales and bud is considered as burst. In contrast, H2O2 content remained at a high level after beheading in darkness (8–9 µmol.g−1 FW from 6 to 48 h), and even seemed to increase after 96 h, reaching a content similar to that measured in quiescent buds (9.5 µmol.g−1 FW; Figure 3 “black bars”).
Figure 3.
Effects of light conditions on bud H2O2 content. H2O2 contents measured in the median buds (Figure 1) at beheading and 6, 24, 48, and 96 h later in presence or absence of light. Data are means of three independent biological replicates ±se. Letters indicate significant differences (P <0.05) after Kruskal–Wallis nonparametric ranks test followed by a two-by-two comparison matrix by Wilcoxon–Mann–Whitney nonparametric test between all conditions of times and treatments.
The homeostasis of H2O2 in tissues results from the balance between production and scavenging. The RBOHs genes encode NOXs that were proven to be implicated in plant development through the production of superoxide anions, later converted to H2O2 in the apoplasm (Sagi and Fluhr, 2001). Here, we identified the 10 isoforms of rosebush RBOHs (Figure 4A) and evaluated their implication in light regulation of bud outgrowth. For this purpose, we measured their expression levels before and after beheading, in different tissues and light conditions. Some RBOHs (RBOHB1, -B2, -E1, -E2, -E3, and -HJ) were poorly expressed in buds (Figure 4B), nodes, and leaves (Supplemental Figure S3A). Some others (RBOH-D1, -D2, -D3, and -F) were highly expressed in buds and nodes, and RBOH-F was the sole isoform detected in leaves. RBOHDs and -F displayed a significant difference of expression between B0 and B + 96 h in buds (Figure 4B) while the expression was similar in the organs close to the bud. The expression of D1, -D3, and -F RBOH isoforms displayed 96 h after beheading a significant decrease (at least three-fold, Supplemental Figure S3B) compared to that observed at B0. These results were also observed in continuous darkness (Figure 4B), suggesting that the decrease of RBOHs expression after beheading could not account for the diminution of H2O2 content observed after beheading and its consequences on bud outgrowth.
Figure 4.
Identification of RhRBOHs isoforms in rosebush and effects of light conditions on transcript accumulations. A, Phylogenetic tree (ML algorithm) of protein sequences of RBOHs of A. thaliana, O. sativa, S. lycopersicum, and R. chinensis. The different RBOHs of Rosa were named after the Arabidopsis RBOHs’ classification (Sagi and Fluhr, 2006). B, Comparison of relative expressions of different RBOHs in the median buds, at beheading time (B0) and 96 h later (B + 96 h) in light or continuous darkness. The color backgrounds indicate the relative expressions of RhRBOHs in buds compared to the geometrical mean of the expression of the reference genes RhUBC and RhTCTP (n > 3 independent biological replicates). The significant differences between dormant (B0) and the other conditions are indicated using (*, **, and ***) as symbols for P-values (95%) inferior to 0.05, 0.01, and 0.001, respectively (Wilcoxon–Mann–Whitney nonparametric test).
Our previous data have highlighted changes occurring in H2O2 scavenging during bud outgrowth process (Porcher et al., 2020). Here we questioned the implication of light in the regulation of keys genes involved in H2O2 scavenging in buds after beheading. All the actors of AsA–GSH cycle but GR1 displayed minor expression changes (1.2- to 1.6-fold) during bud outgrowth in light while in darkness all of them displayed a strongly reduced expression as compared to light condition (−2.6 to −6.6-fold; Figure 5, A–C). GR1 encode a cytoplasmic GSH reductase that was largely induced in light from 6 to 96 h after beheading (1.6- to 4.4-fold; Figure 5D, “white bars”). The GR1 transcripts accumulation dropped from 3- to 22-fold in darkness from 6 to 96 h after beheading, respectively (Figure 5D, “black bars”). The expression of the plastidic GSH reductase kept nearly constant after beheading in light (Figure 5E, “white bars”), while it significantly decreased in darkness from 24 to 96 h after beheading (approximately five-fold; Figure 5E, “dark bars”). Aside from the AsA–GSH pathway, catalase is another major scavenging enzyme encoded in rosebush by a single gene (RhCAT; Porcher et al., 2020) that displays a lower affinity to H2O2 than ascorbate peroxidase. RhCAT transcript accumulation remained constant in median buds of light-grown plants after beheading (Figure 5F, “white bars) while it increased (two-fold) from 24 to 96 h in darkness after beheading (Figure 5F, “black bars”). This result showed that H2O2 scavenging systems were strongly altered in darkness, resulting in high level of H2O2 in buds (Figure 3).
Figure 5.
Effects of light on transcript accumulations of genes involved in H2O2 scavenging. Relative transcript accumulations of ASCORBATE PEROXIDASE (RhAPX6) (A), MONODEHYDROASCORBIC ACID (RhMDHAR6) (B), DEHYDROASCORBIC ACID REDUCTASE (RhDHAR1) (C), CYTOPLASMIC GSH REDUCTASE (RhGR1) (D), PLASTIDIC GSH REDUCTASE (RhGR2) (E), CATALASE (RhCAT) (F), GLUTAMATE CYSTEIN SYNTHETASE (RhGSH1) (G) and GSH SYNTHETASE (RhGSH2) (H), in median buds at beheading time (B0) 6, 24, 48, and 96 h later in light (L:D 16:8) or continuous darkness. Data are means of three biological independent replicates ±SE of relative expressions compared to the control condition (beheading time, B0) after normalization using reference genes (RhUBC and RhTCTP). For each sub-figure, letters indicate significant differences (P <0.05) after Kruskal–Wallis nonparametric ranks test followed by a two-by-two comparison matrix by Wilcoxon–Mann–Whitney nonparametric test.
GSH, an important non-enzymatic H2O2 scavenger (Barnes et al., 2002), appears essential to engage the bud outgrowth mechanism (Takahashi et al., 2014; Porcher et al., 2020). Here, we measured the effect of light on the expression of two keys genes involved in GSH synthesis (GSH1 and GSH2). We also assayed the total, reduced, and oxidized GSH forms in median buds after beheading in different conditions (Table 1). The expressions of GSH1 and GSH2 both gradually increased in light after beheading to reach after 96 h a 5.4- and 3.8-fold increase, respectively (Figure 5, G and H, “white bars”) while the evolution was opposed in darkness where the two genes decreased to a minimum of expression 96 h after beheading (Figure 5, G and H, “black bars”). In comparison, GSH1 and GSH2 were, respectively, 12.8- and 13.4-fold less expressed in darkness 96 h after beheading in comparison to that measured in light (Figure 5, G and H).
Table 1.
Effect of lighting conditions and CK (BAP)/BSO supply on the contents of total, reduced GSH and oxidized GSH (GSSG).
| Total GSH (nmol.g-1 FW) | GSH (nmol.g-1 FW) | GSSG (nmol.g-1 FW) | ||||
|---|---|---|---|---|---|---|
| B0 | 119.76 (±4.55) | b | 79.54 (±5.88) | b | 20.11 (±1.91) | c |
| B + 24-h light | 252.77 (±22.18) | d | 215.88 (±16.62) | d | 18.44 (±3.56) | c |
| B + 24-h no light | 52.18 (±7.32) | a | 36.22 (±2.78) | a | 7.97 (±0.31) | a |
| B + 24-h no light + BAP | 228.14 (±36.82) | c | 177.83 (±23.08) | c | 25.15 (±6.86) | c |
| B + 96-h light | 253.18 (±7.45) | cd | 164.11 (±7.66) | c | 44.43 (±0.48) | d |
| B + 96-h no light | 56.99 (±5.88) | a | 37.72 (±3.87) | a | 9.63 (±3.40) | ab |
| B + 96-h no light + BAP | 448.46 (±50.71) | e | 425.74 (±53.36) | e | 11.36 (±1.60) | cb |
| T + 96-h light | 319.11 (±13.32) | 249.36 (±4.43) | 34.87 (±4.46) | |||
| T + 96-h light + BSO | 15.37 (±6.97) | *** | 13.46 (±6.96) | *** | 1.92 (±0.02) | *** |
| T + 96-h light + BAP + BSO | 10.01 (±1.46) | *** | 8.61 (±1.53) | *** | 1.39 (±0.07) | *** |
Upper part of this table displays the contents of total GSH, reduced GSH, and oxidized GSH (GSSG) in the median buds harvested from plants at beheading time (B0), and 24 and 96 h later “B” in light (L:D 16:8) or continuous darkness, in the presence/absence of BAP. Data are means ± se of three biological independent replicates. Significant differences (P-value <0.05) are indicated after Kruskal–Wallis nonparametric test. Roman, italic, and underlined letters, respectively, indicate total GSH and GSSG. The lower part of the table indicates the contents of GSH in excised buds cultivated in vitro in light for 96 h on the regular medium or in the presence of BAP or BSO, alone or in combination. Data are means ± SE of three biologically independent replicates. Significant differences (P-values <0.001) between these conditions and the “T + 96h light” control condition are indicated using asterisks (***Wilcoxon–Mann–Whitney nonparametric test).
Light also strongly impacted the GSH content of median buds (Table 1). In light, the content of total GSH was higher (2.1-fold) 24 h after beheading compared to B0, mainly because of an increase in the reduced GSH content while the oxidized form (GSSG) remained constant. If the total content of GSH remained essentially the same between 24 and 96 h, a substantial part (24%) of GSH was oxidized to GSSG (Table 1; Light conditions). In darkness, the total content of GSH decreased 4.5-fold compared to that measured in light, mainly because of a drop in GSH content and to a lesser extent to GSSG decreased (Table 1; no light). These results demonstrate that light is essential to drive the changes in bud oxidative metabolism that set the bud in a favorable physiological condition to outgrow. These changes do not occur in darkness, thus preventing bud burst.
CKs bypass dark inhibition of bud outgrowth through stimulation of H2O2 scavenging
We previously showed in rose that the inhibition of bud outgrowth in darkness can be released by an exogenous application of the synthetic CK 6-benzylaminopurine (BAP) directly on the bud or on the top of the beheaded stem (Roman et al., 2016). The dark-grown plants treated with BAP application on bud displayed after 96 h a total recovery of median bud outgrowth and secondary axis elongation (Figure 6, A and B, “striped bar”) as observed in light (Figure 2). As in defoliated and beheaded rose plants (Roman et al., 2016) CK treatment in nondefoliated beheaded rose plants stimulated the same genes (RhPCNA, RhCYCD3, RhEXP, and RhVI), and repressed the same genes preventing bud outgrowth (RhMAX2 and RhNADSDH; Supplemental Figures S4 and S5) while in the dark-untreated control condition, the evolution was opposite (Supplemental Figures S2, S4, and S5). BAP treatment induced a strong decrease of bud H2O2 content to reach after 96 h that observed in buds in light (3.8 µmol.g−1 FW; Figures 3 and 6C, “Striped bars”).
Figure 6.
Effects of CK treatment on bud outgrowth process in the absence of light. A, Percentage of bud outgrowth at 96 h after beheading in the absence of light condition in control or BAP treated (10 mM) plants. The nodes (buds + stem fragments) were digitally extracted for comparison. B, Length of bud or subsequent axis at beheading time (B0) or 96 h later in the absence of light in control or BAP treated (10 mM) plants. C, H2O2 contents measured in median buds at beheading time (B0) and 24 and 96 h later in continuous darkness in control or BAP treated (10 mM) plants. D, Evolution of gene expressions in median buds at 24 and 96 h of beheaded plants and CK application (10 mM BAP) in dark-grown plants. Changes in transcripts accumulation levels are indicated by the following color scheme: yellow/blue shades indicate induction/repression by BAP normalized to B0 and expressed as relative to untreated control plant. All RT-qPCR data used to build the heatmap are available as supplementary data (Supplemental Figure S5). All data are the means ± se of three independent biological replicates. For (A), the asterisks indicate significant difference (P <0.001) between light and no light condition (Wilcoxon–Mann–Whitney nonparametric test). For (B) and (C), letters indicate significant differences (P <0.05) after Kruskal–Wallis nonparametric ranks test followed by a two-by-two comparison matrix by Wilcoxon–Mann–Whitney nonparametric test between all conditions of times and treatments. For (D), asterisks indicate significant difference (*P <0.05; **P <0.01) between BAP treated condition and untreated control condition (Wilcoxon–Mann–Whitney nonparametric test).
While CK treatment appeared to have little effects on RBOHs expressions in the absence of light (Supplemental Figure S3C), it markedly stimulated the genes involved in H2O2 scavenging. Figure 6D shows the heatmap comparing gene expression in buds grown in darkness between control (untreated) and BAP-treated plants (CK). Treatment of dark-grown plants with BAP strongly increased (6.2-fold) the expression of APX6 gene 96 h after beheading (Figure 6D;Supplemental Figure S5A) in comparison to the untreated condition. Furthermore, CK application largely increased MDHAR1, GR1, and GR2 expression levels in comparison to untreated control condition 96 h after beheading (Figure 6D;Supplemental Figure S5, B, D, and E). Application of BAP to dark-grown plants had no effect on DHAR1 expression (Figure 6D;Supplemental Figure S5C). The increase in RhCAT expression in darkness was prevented by the application of BAP (Figure 6D;Supplemental Figure S5F) that even caused a transient decrease (2.4-fold) of its expression at 24 h before returning 72 h later to a level close to that observed at B0 (Supplemental Figure S5F). This result suggests that CK stimulates AsA–GSH-dependent H2O2 scavenging, an essential process for bud outgrowth (Porcher et al., 2020). In silico analysis of the promoter sequences of these different genes shows numerous sites for the binding of CK-associated transcription factors (Supplemental Figure S6) further enhancing the effect of CKs on the expression of the genes involved in H2O2 scavenging. Furthermore, the application of BAP on dark-grown plants evoked the expression of GSH1 and GSH2 (Figure 6D;Supplemental Figure S5, G and H) that were 5.1- to 8-fold higher, respectively, compared to that measured in untreated control conditions reaching levels to that measured in light (Supplemental Figure S5, G and H). CK promotion of GSH synthesis in darkness was also observed through the large increase of GSH quantity (Table 1; “darkness + BAP condition”). Indeed, 24 h after beheading, the quantity of total GSH increased in buds by five-fold and almost double 96 h after beheading compared to that observed in the untreated control condition (Table 1; Darkness + BAP conditions).
GSH role in bud outgrowth cannot be overridden by CK
We demonstrated that CK treatment stimulates the expression of GSH synthesis genes and increases GSH contents in median buds of plants cultivated in darkness (Figure 6;Table 1). Previous results showed that buthionine sulfoximine (BSO), a powerful inhibitor of GSH synthesis, repressed bud outgrowth in gentian and rosebush (Takahashi et al., 2014; Porcher et al., 2020). Here the addition of BSO to the culture medium of isolated nodes bearing bud grown under light conditions strongly decreased GSH content after 96 h (Table 1), maintaining, in turn, H2O2 content at high level in buds (Figure 7D) and drastically reduced the percentage of outgrowing buds (from 94% in control condition to 6% BSO-treated plants; Figure 7, A and B), as well as the secondary axis elongation (Figure 7C). The addition of 2 µM of BAP in the culture medium stimulated the outgrowth of central and collateral buds in light (Figure 7A), and this corresponded with the decrease in H2O2 content in these buds to a similar level to that found in control conditions (no BSO and no BAP; Figure 7D). The simultaneous application of BAP and BSO inhibited bud outgrowth and secondary axis elongation very similarly to that observed with the application of BSO alone (Figure 7, A and B, “gray gridded bar”). GSH level did not increase and H2O2 level did not drop (Table 1; Figure 7D). In darkness, the application of CK to excised buds evoked their outgrowth (Supplemental Figure S7, A and B), as previously observed in planta (Figure 6A) and the application of BSO in the presence/absence of CK always kept the buds quiescent (Supplemental Figure S7, A and B). Consequently, overall growth of buds was negligible in darkness but occurred in the sole presence of CK (Supplemental Figure S7C). In darkness, H2O2 remains at a high level in the control (7.8 µmol.g-1 FW), and even higher under BSO and CK+BSO conditions (14.1 and 13.8 µmol.g-1 FW, respectively) while it becomes much lower in the presence of the CK alone (Supplemental Figure S7D) where bud outgrowth was observed. All these results indicate that CK failed to counteract the inhibitory effect of BSO on bud outgrowth regardless of the lighting conditions. These results also suggest that CK acts upstream of GSH synthesis pathways and highlights the critical role of GSH in the bud outgrowth photocontrol (Figure 7).
Figure 7.
Effect of the GSH synthesis inhibitor BSO on bud outgrowth in presence or absence of CK. A, Typical aspects of isolated nodes (stem fragment) bearing buds after 10 d of in vitro culture under light (L:D 16:8; 300 µE.m−2.s−1) in the control medium, supplemented with 2 µM of BAP, supplemented with 1 mM of BSO or supplemented with both 2 µM of BAP and 1 mM of BSO. The nodes cultivated in vitro (buds + stem fragments) were digitally extracted for comparison, scale = 3 mm. B, Percentage of bud outgrowth after 10 d of in vitro culture in the control medium (white bar), in the presence of BSO (white gridded bar), BAP (gray bar), and BAP + BSO (gray gridded bar). Data are means ± se of three independent replicates. Letters indicate the significant differences (P <0.05) after Kruskal–Wallis nonparametric test. C, Elongation of secondary axis of median buds during 9 d of in vitro culture in control medium (open circle), with 2 µM BAP (open triangle), with 1 mM BSO (dark circle) or with 2 µM BAP and 1 mM BSO (dark triangle). Significant differences between control conditions (without BSO) and BSO-treated conditions for each point are indicated by asterisks. Data are means of n > 3 independent biological replicates ± se, the significant at 95% or confidence range are indicated by (*, **, and ***) for P-values inferior at 0.05, 0.01, and 0.001, respectively (Wilcoxon–Mann–Whitney nonparametric test). D, H2O2 contents measured after 10 d of in vitro culture in the control medium (white bar), in the presence of BSO (white gridded bar), BAP (gray bar), and BAP + BSO (gray gridded bar). Data are means ± se of three independent replicates. Letters indicate the significant differences (P <0.05) after Kruskal–Wallis nonparametric ranks test followed by a two-by-two comparison matrix using the Wilcoxon–Mann–Whitney nonparametric tests.
Strong reduction of GSH level through BSO treatment impact expression of bud outgrowth marker genes
We studied the effect of BSO on bud outgrowth marker genes in buds cultivated in vitro under light condition. The expression of these genes in untreated control conditions was very similar to that observed in vivo in light (Figure 8;Supplemental Figure S2). However, the application of BSO drastically modified their expression: RhPCNA and RhCYCD3, both involved in cell division were highly repressed (5- and 42-fold, respectively) 96 h after culture initiation, in comparison to the untreated control condition (Figure 8, A and B, “gridded bars”). In the same way, the expansin gene RhEXP was induced in vitro during bud outgrowth in control condition (Figure 8C, “white bars”), but was strongly repressed in the presence of BSO (nearly five-fold) in comparison to the untreated control condition (Figure 8C, “gridded bars”). The same expression profile was observed for the vacuolar invertase gene (RhVI), that showed a strong increase of transcript levels in control condition (10.2-fold higher at 96 h; Figure 8D, “white bars”) that dropped in the presence of BSO (11.3-fold lower in comparison to the control at 96 h; Figure 8D, “gridded bars”). The RhMAX2 gene, that is involved in strigolactone signaling and known to be expressed in quiescent buds, was strongly repressed (20-fold) 24 h after the culture initiation. BSO caused a small decrease of its expression that remained, however, at a higher level compared to the untreated control (15.6- and 8.7-fold higher at 24 and 96 h, respectively; Figure 8E). Likewise, the RhNADSDH gene expression was also tremendously stimulated after BSO application (over 20-fold after 24 h in comparison to the untreated control; Figure 8F). These results confirm that dark-inhibition bud outgrowth is associated with GSH reduced level in bud (Figures 2, E and 7, B;Table 1).
Figure 8.
Effects of BSO treatment on transcript accumulations of marker genes involved in bud outgrowth mechanism. Transcript accumulations of genes PROLIFERATING CELL NUCLEAR ANTIGEN (RhPCNA) (A), CYCLIN D3 (RhCYCD3) (B), EXPANSIN (RhEXP) (C), VACUOLAR INVERTASE (RhVI) (D), MORE AXILLARY BRANCHING 2 (RhMAX2) (E), and NAD-DEPENDENT SORBITOL DEHYDROGENASE (RhNADSDH) (F) in median buds at the onset of in vitro culture (T0), and 24 and 96 h later in the presence (gridded bars) or absence (white bars) of 1 mM of BSO. Data are means of three biologically independent replicates ±se of relative expressions compared to the untreated control condition (culture set up time, T0) after normalization using reference genes (RhUBC and RhTCTP). For each sub-figure, letters indicate significant differences (P <0.05) after Kruskal–Wallis nonparametric ranks test followed by a two-by-two comparison matrix by Wilcoxon–Mann–Whitney nonparametric test.
Discussion
Branching is an important process in plant development that depends upon axillary bud outgrowth. Nitrogen nutrition, sugar, and phytohormone metabolisms have been shown to be important parameters for its regulation (Barbier et al., 2019a). NOXs encoded by RBOHs, through the indirect production of H2O2, have been shown to be important contributors to bud outgrowth regulation (Sagi et al., 2004; Chen et al., 2016). Recently, we demonstrated that H2O2 accumulates in quiescent buds, preventing their outgrowth and that its scavenging through the AsA–GSH pathways is necessary for buds to grow out (Porcher et al., 2020). Furthermore, bud outgrowth depends upon environmental factors, among which light appears as strictly required to engaged outgrowth process in rosebush, in contrast to many other plant species (Girault et al., 2008). This behavior in rosebush was validated in the nondefoliated experimental model where bud burst was observed for 96 h after beheading only in light, while it did not occur in darkness (Figure 2). Furthermore, the expression levels of several genes related to bud outgrowth in light in rosebush were in adequation with the phenotypic observations (Supplemental Figure S2). In the present work, we provide evidences showing that light drives H2O2 scavenging activity in bud, establishing a physiological state that is favorable for bud outgrowth. Consequently, the photocontrol of bud outgrowth appears to operate through the regulation of H2O2 level in bud.
Bud outgrowth photocontrol operates through H2O2 metabolism
High H2O2 levels were demonstrated to have an inhibitory effect on bud outgrowth (Wang and Faust, 1988; Chen et al., 2016; Porcher et al., 2020). Here, we showed that bud H2O2 content remains essentially constant at a high level in darkness, close to that found in quiescent buds (9–10 µmol.mg−1 FW; Figure 3), preventing bud outgrowth. In contrast, a marked decrease in H2O2 levels occurred in light, allowing the engagement of bud outgrowth process (Figures 2 and 3). Since H2O2 content results from a fine tuning between synthesis and scavenging pathways (Mhamdi and Van Breusegem, 2018), we addressed these two processes to understand light effect on H2O2 content and its consequences on bud outgrowth.
RBOHs genes are essential H2O2 producers that were implicated in plant branching (Sagi et al., 2004; Chen et al., 2016). Here, we found that 3 RBOHs genes out of 10 showed a change in their expression in buds during bud outgrowth (Figure 4A;Supplemental Figure S3): the accumulation of RBOH-D1, -D3, and -F mRNA strongly decreased (up to five-fold) 96 h after beheading in light as well as in darkness (Figure 4B). While these results are in adequation with the decrease of H2O2 content that occurred after beheading in light, they fail to explain constantly the high values in H2O2 content observed in the dark-grown plant (Figure 4B). Therefore, one could hypothesize that the regulation of RBOH expression is mainly the consequence of the apex suppression and the subsequent apical dominance release and, by extension, that of the decrease in auxin levels along the stem (Thimann and Skoog, 1934; Tanaka et al., 2006). The fact that auxin can induce the expression of RBOHs (Bartoli et al., 2013; Kim et al., 2019) strongly supports this hypothesis.
The H2O2 scavenging system predominantly regulate H2O2 levels in many plant developmental processes (Das and Roychoudhury, 2014). Interestingly, some H2O2 scavenging systems are sensitive to light quantity, notably after high light stress (Noctor et al., 2016). Here, we identify that light impacts transcript accumulation of several genes involved in the H2O2 detoxification processes: light evoked or left unchanged the expressions of genes encoding enzymes of the AsA–GSH cycle (APX, DHAR, MDHAR, and GR1&2), while darkness drastically reduced their expressions (Figure 5). These results suggest that this important scavenging pathway, involved in the fine tuning of H2O2 quantity, is dramatically affected by light. We previously demonstrated that gene expression is closely associated with ROS-related enzymatic activities during bud outgrowth in light condition (Porcher et al., 2020) and one could therefore reasonably hypothesize that it is also true in darkness. This has also been observed in poorly lit or unlit plants in which the content of AsA and AsA–GSH enzyme activity is low (Toledo et al., 2003; Bartoli et al., 2006). Therefore, photo-regulation of AsA–GSH cycle could account for the maintenance of high levels of H2O2 in buds in darkness (Figure 3). We also observed a strong light-dependent modification of GSH metabolism (Table 1), an important nonenzymatic H2O2 scavenging metabolite (Diaz-Vivancos et al., 2015). The total content of GSH increased in light after beheading and apical dominance release (Table 1); this rise was associated with the increase in the expressions of GSH1 and GSH2, both involved in GSH synthesis pathway (Figure 5) previously implicated in bud outgrowth mechanism (Wang and Faust, 1994; Takahashi et al., 2014; Porcher et al., 2020). The strong decrease of GSH content in buds of dark-grown plants (almost five-fold lower than that observed in light) could be explained by a drop in GSH synthesis (Figure 5). It could also account for the maintenance of high H2O2 content in the buds of dark-grown plants. This alteration in GSH synthesis in darkness have been previously observed in poplar (Noctor et al., 1997) and was explained by an inhibition of the transformation of γ-glutamylcysteine to GSH, that may result of insufficient glycine supply because of the absence of the photorespiration process in darkness (Buwalda et al., 1988, 1993; Noctor et al., 1997), or of an inhibition of GSH synthetase activity ensuing a chloroplast stroma acidification (Law and Halliwell, 1986). Here, we demonstrated that darkness also had a major effect in GSH synthesis during bud outgrowth, through on transcriptional regulation of GSH1 and GSH2 encoding, respectively, γ-glutamylcysteine synthetase and GSH synthetase (Figure 5).
In contrast to the lesser activity of the AsA–GSH scavenging pathway in darkness, we also demonstrated that the only gene coding for catalase in rosebush (Porcher et al., 2020) was not induced during bud outgrowth in light, while slightly increased in darkness (Figure 5F). Previous work has established that a compensation could occur between catalase and GSH scavenging systems: if one of them is impaired, the second takes over (Mhamdi et al., 2010a, 2010b). Here, our results suggest that such a mechanism takes place in darkness, as proposed in sweet potatoes exposed to dark (Chen et al., 2011). This could be the cause of the maintenance—and not the increase—of H2O2 levels in buds grown in darkness (Figure 3).
Taken together, these results emphasize the role of light in the activation of H2O2 scavenging through the GSH-dependent pathway to achieve the decrease in H2O2 content in buds, required to engage bud burst (Porcher et al., 2020). These results establish a formal and unequivocal relationship between the redox metabolism and the photocontrol of bud burst.
Are CKs the link between light and H2O2 metabolism in the photocontrol of bud burst?
CKs are well-known inducers of bud outgrowth (Sachs and Thimann, 1964). As a matter of fact, this process is associated with an increase in CK content in nodes (Tanaka et al., 2006; Roman et al., 2016), and the exogenous application of CK stimulates bud outgrowth in many plant species (Sachs and Thimann, 1964; Dun et al., 2012), notably rosebush (Figure 6). In darkness, Yoshida et al. (2011) and Roman et al. (2016) have noticed that CK synthesis and transport are strongly lowered. Furthermore, the exogenous application of CK in dark-grown plants stimulates organs formation in meristems (Yoshida et al., 2011) and induced bud outgrowth (Roman et al., 2016; Figure 6). This forced bud break after CK application is also associated with the upregulation of genes committed to bud outgrowth (Roman et al., 2016; Supplemental Figure S4). Here, we observed that CK application to dark-grown plants was sufficient to cause a decrease in H2O2 content that was similar to that observed in light (Figure 6, C and B; +96 h). The effect of CK toward H2O2 status was already reported after drought stress where high levels of CK prevented the accumulation of H2O2 in roots (Xu et al., 2016). We monitored marked changes in H2O2 scavenging between CK-treated and untreated control dark-grown plants (Figure 6D;Supplemental Figure S5; Table 1): CK treatment stimulated the expression of APX6, DHAR1, and GR1 (AsA–GSH cycle) in comparison to the untreated control darkness condition, but to a lesser extent to that observed in light (Figure 6D;Supplemental Figure S5). CK application also induced a strong increase in GSH content compared to untreated control in darkness (over 7.8-fold at 96 h) and became even higher to that observed in light at 96 h after beheading (Table 1). This increase could be in part explained by an increase in the expression of genes involved in GSH synthesis pathway after CK application (Figure 6D). Indeed, the analyses of the promoters of the different genes involved in the AsA–GSH cycle and GSH biosynthesis pathways revealed numerous putative CK-responsive cis-elements (Supplemental Figure S6; Supplemental Table S1), notably cis-elements of the ARR-B family (GARP-ARRB; Heyl and Schmülling, 2003; Bhargava et al., 2013), thus reinforcing the potential regulation of their expressions by CK (Figure 6D;Supplemental Figure S5). Similarly, H2O2 scavenging system was reported as the main pathway that limits H2O2 accumulation in roots after CK treatment (Xu et al., 2016). Likewise, it was also observed that GSH content increased during bud break in apple tree after the application of the synthetic CK thidiazuron (Wang et al., 1991).
Our results showed a strong relationship between CK and H2O2 scavenging during the process of bud outgrowth in rose, particularly through the GSH synthesis pathway. This could be the main plot of the redox-driven inhibition of bud outgrowth in darkness.
Perhaps, the role of CK in maintaining the dynamics and functioning of chloroplasts in darkness (Hudson et al., 2013; Cortleven and Schmülling, 2015) together with the partial dependence of GSH synthesis in chloroplasts (Reichheld et al., 2009) may explain in part this relationship.
GSH is one main relay for CK-dependent bud outgrowth
GSH synthesis has been shown to be essential to initiate the bud outgrowth process (Takahashi et al., 2014; Porcher et al., 2020). Using BSO as an inhibitor of γ-glutamylcysteine synthetase, we observed that the content in GSH decreased in bud (Table 1, lower part) along with a total repression of bud outgrowth in otherwise favorable conditions (Figure 7, A and B; Porcher et al., 2020). This repression of outgrowing process was associated with the maintenance of high levels of H2O2 in buds in the presence of BSO. The level of H2O2 that remains high along with the GSH content that decreases in buds after BSO application strongly reduced the expression of the genes favoring bud outgrowth, such as PCNA, CYCD3, EXP, and VI, while activating those preventing bud burst (MAX2 and NADSDH; Figure 8). Here, we demonstrated that CK stimulation of bud outgrowth operates through de novo GSH synthesis (Figure 7 andTable 1). Indeed, CK treatment is not able to evoke bud outgrowth in the presence of GSH synthesis inhibitors (Figure 7, A and B). Thus, CK treatment was not able to overcome BSO application with regards to GSH and H2O2 levels (Figure 7D andTable 1). Furthermore, not surprisingly the same conclusion arose from treatment performed in darkness (Supplemental Figure S7). These important results define, along with the effect of BSO, the sequence of events following the application of CK that operate upstream of GSH in bud outgrowth control. This observation agrees with former results designating CK as the initial target of light in the photocontrol of bud outgrowth in rosebush (Roman et al., 2016).
The scheme in Figure 9 presents a model for the involvement of oxidative metabolism in relation to CK in the bud outgrowth photocontrol. After the release of apical dominance in light, CKs are synthesized in the nodes and transported into the buds (Tanaka et al., 2006; Roman et al., 2016). Increasing CK levels in buds stimulate GSH synthesis and participate in the activation of AsA–GSH-dependent H2O2 scavenging, leading to a reduction of H2O2 content in buds (Porcher et al., 2020). The increase in the GSH pool leads to the decrease in H2O2 levels contributes to rise the locking point of the cell division and expansion processes in the bud, required for bud outgrowth. On the other hand, in darkness, CKs are not synthesized (Roman et al., 2016) and cannot induce the synthesis of GSH, which is instead catabolized. As a result, AsA–GSH-dependent H2O2 scavenging is not allowed, leading to the maintenance of high H2O2 levels in the bud, which prevents bud burst (Porcher et al., 2020).
Figure 9.
Regulation of redox metabolism by light during bud outgrowth process: a CK-dependent pathway. This synthetic scheme highlights the central role of redox metabolism in the bud outgrowth photocontrol process and its regulation by light and CK. In light (L:D 16:8), CK synthesis and transport in buds are promoted (Roman et al., 2016). Moreover, after beheading in light, the H2O2 content in buds decreased, while GSH pool increased (Porcher et al., 2020). Furthermore, CK stimulates GSH synthesis and contributed to the decrease of H2O2 contents, notably through the AsA–GSH scavenging pathways. In contrast, darkness prevents the H2O2 decrease that normally occurs in light and drastically reduces the GSH pool. In summary, the light stimulation of CK synthesis and transport increases the GSH content and activates the AsA–GSH scavenging system to initiate the decrease in H2O2 in bud that will, in turn, restore the cell division and expansion that are required to initiate the outgrowing process (Reichheld et al., 1999; Porcher et al., 2020). Green lines indicate stimulation, red lines inhibitions, and gray lines nonactive pathways.
Conclusion
Our exploration of bud outgrowth photocontrol highlights the major contribution of AsA and GSH-dependant H2O2 scavenging systems in the branching process in rosebush. Induced in light and repressed in darkness, the H2O2-scavenging system dependent on GSH metabolism seems to totally condition the bud outgrowth process, as validated with the GSH synthesis inhibitor BSO. The CK-induction of GSH synthesis and recycling identified that CK act upstream of bud redox balance during the outgrowth process. These data provide new insights in the understanding of the branching process, making oxidative metabolism a major contributor to bud outgrowth process. However, its involvement in the photocontrol process should be investigated further, particularly to elucidate its interactions with other actors that were previously identified as key regulators, namely sugars (Girault et al., 2010; Rabot et al., 2014) and other phytohormones (Choubane et al., 2012; Djennane et al., 2014).
Materials and methods
Plant culture
Experiments were carried out on rosebush plants resulting from the vegetative propagation of a single clone as in previous works (Girault et al., 2008; Rabot et al., 2012; Barbier et al., 2015; Roman et al., 2016). Rooted cuttings were grown in a glasshouse for 7–9 weeks in 16-h light (L): 8-h darkness (D); L:D 16:8 at 25 (±5)°C in 0.5 L pots containing a substrate mix composed of Irish peat, perlite, and coconut fibers (50/40/10; v/v/v) amended with PgMix™(1 kg.m−3) and fertilized with nutrient solution (15N: 10P2O5: 30K20-Mg-Fe and trace elements, Yara, Nanterre, France, stabilized at pH 6.2). After 7–9 weeks when the plants had reached the VFB stage, they were transferred in a growth chamber for in vitro and in planta experiments (Figure 1).
In planta experiments
Plants at the VFB stage were beheaded by removing the uppermost part of the plant axis 1 cm above the fourth basal leaf-bearing five-leaflets using a razor blade and were immediately transferred to a growth chamber under controlled temperature (22:19°C) and fertilized through sub-irrigation with the same nutrient solution as used in the greenhouse. Some beheaded plants were grown in light condition (white light, 300–350 µE.m−2.s−1, L:D 16:8; Supplemental Figure S1A), while some others were covered with an opaque plastic shield (Supplemental Figure S1B) to mimic continuous darkness (0 µE) as described in Roman et al. (2016). These conditions are referred, respectively, as “light” or “darkness” in text and figures. A synthetic CK (BAP; Sigma, St. Louis, MO, USA ) was dissolved to the final concentration of 10 mM in 1% (w/v) DMSO, mixed in prewarmed (55°C) lanolin and directly applied (20 µL; Supplemental Figure S1C) on the studied median buds (buds #2 and #3); Figure 1) at the beheading time (B0; Figure 1). Plants treated with BAP were placed in continuous darkness. Bud outgrowth was measured for all treatments 96 h after beheading. Buds were considered as outgrown when a minimum of one new leaf protruded from the bud scales (Girault et al., 2008). Bud length was measured using a numeric caliper. Tissue sampling for molecular analysis was harvested at 0, 24, and 96 h after beheading, after the application of the diverse treatments. Median buds, along with the associated nodes and leaves were harvested separately and immediately frozen in liquid nitrogen and stored at −80°C. A minimum of three biological replicates (each comprising 20–40 plants) was grown and collected for each condition and each experiment, along with the corresponding untreated controls for each experiment.
In vitro experiments
Median nodes (1 mm of stem fragments bearing their correspondent axillary buds #2 or #3) were isolated from rosebush at the VFB stage and transferred in vitro on distinct solid media (Figure 1) based on a common basic composition: 4.4 g.L−1 of Murashige and Skoog salts (Duchefa Biochemie Haarlem, The Netherlands), 100 mM of sucrose and 8 g.L−1 of agarose (Sigma). This basic medium was used as it as a control condition or was supplemented before its solidification with either 2 µM of BAP (synthetic CK) or 1 mM of BSO; (a GSH synthesis inhibitor) or with both, to determine the capacity of buds to burst and produce a secondary axis in these conditions. Samples were placed in the same growth chamber and the same light condition as for in planta experiments for 10 d (from T0 to T + 10 d). Elongation of the secondary axis resulting from bud outgrowth was measured each day for 9 d using a numeric caliper (Figure 1). The axis length values represent the sum of lengths of the main and collateral buds (illustration on Figure 7B). Percentage of bud outgrowth was quantified after 10 d, according to Girault et al. (2008). Buds cultivated in vitro were harvested using the same scheme as described in the previous section in planta experiments and stored at −80°C for further analysis.
H2O2 quantification assay
A chemiluminescence technique described in Lu et al. (2009) was adapted to rosebush buds to determine their H2O2 content. Frozen bud tissues (20 mg) were ground in liquid nitrogen using a ball mill. The fine powder was homogenized in 500 µL of 5% trichloroacetic acid containing 5% (w/v) of insoluble polyvinylpolypyrrolidone and centrifuged at 13,000g for 10 min at room temperature. The supernatant (100 µL) was collected and diluted 100 times with 0.1 M sodium carbonate buffer (pH 10.2). Two separate aliquots of 40 µL were incubated at 30°C for 15 min in the presence/absence of 10 µL of 0.5 milliunits of catalase (Catalase from bovine liver, [Sigma]). H2O2 content was quantified in a white microplate (96 wells NUNC) by adding 10 µL of incubated samples with 250 µL of reaction mixture containing 65 µM of luminol and 10 µM of cobalt chloride in 0.1 M sodium carbonate buffer (pH 10.2) injected well by well. The luminescence was measured using a microplate reader (Fluostar OMEGA; BMG Labtech, Ortenberg, Germany) for 5 s after injection of the reaction mixture. H2O2 quantities in tissues samples were determined from a standard curve established from freshly prepared dilutions of commercial H2O2, corrected by subtracting the luminescence value of the sample containing only the catalase solution, and expressed as µmol of H2O2 per gram of fresh buds. The very low luminescence signal detected after mix injection in the presence of catalase demonstrated that the measured signal did not result from interfering compounds and readily reflected the H2O2 levels in bud tissues (Noctor et al., 2016).
GSH quantification assay
The reduced GSH and oxidized GSH (GSSG) were determined from 50 mg of bud tissues ground in frozen condition with a ball mill and homogenized in 500 µL of HCl (0.2 M). Samples were centrifuged at 15,000 g for 10 min at 4°C and 200 µL of supernatant were collected in new tubes and kept on ice. The extract was neutralized with 40 µL of 0.2 M phosphate buffer (pH 5.6) and 160 µL of 0.2 M NaOH to adjust pH to 4.5. Total and oxidized GSH were determined using the commercial protocol established for suspension cells (available at www.promega.com) and expressed as nmol−1.g−1 FW after taking into account the volume of the neutralization step. The reduced form of GSH was calculated by subtracting the amount of GSH oxidized form to the total amount of GSH with respect of molarity factor.
RBOHs gene identification and phylogenic analysis
RBOHs sequences were retrieved by similarity comparison of Rosa chinensis protein sequences (available on https://www.rosaceae.org) with those of Arabidopsis (Arabidopsis thaliana, https://www.arabidopsis.org), rice (Oryza sativa, rice.plantbiology.msu.edu), and tomato (Solanum lycopersicum; Sagi et al., 2004). Multiple sequence alignments were performed using ClustalW using standard parameters in MEGA X® software (Kumar et al., 2018). Phylogenetic analyses were performed using a maximum likelihood (ML) approach with Jones–Thornton–Taylor method (Jones et al., 1992). To construct a phylogenetic tree, a test of phylogeny was done using the Bootstrap method with 500 replications using MEGA X® software. Sequences of Rosa RBOHs genes were named after their homologs with the Arabidopsis gene annotation.
Primer design
Previous studies in Rosa “Radrazz” identified several genes contributing to the bud outgrowth process (Girault et al., 2010; Djennane et al., 2014; Roman et al., 2016; Hibrand Saint-Oyant et al., 2018; Wang et al., 2019; Porcher et al., 2020) and the designed primers (Supplemental Table S2) were reused after verification of primer sequences in the recent Rosa genome sequence available on http://www.rosaceae.org. We designed new primers for rosebush homologous genes of Arabidopsis NOXs (RBOHs), dehydroascorbic acid reductase (DHAR1), and mono-dehydroascorbic acid reductase (MDHAR6; Supplemental Table S3).
RNA extraction, Reverse Transcription-quantitative PCR (RT-qPCR)
Twenty milligrams of different frozen tissue (buds, nodes, and leaves) were ground in liquid nitrogen and used for total RNA isolation using the phenol/chloroform-free method developed by Barbier et al. (2019b) and adapted to rosebush tissues by increasing the volume of Sodium Dodecyl Sulfate (SDS) 10% from 60 to 100 µL in order to maximize the removal of compounds interfering with DNAse treatment step. The RNA quantities and qualities were checked using Nanodrop One (Thermo Scientific, Waltham, MA, USA) completed with total RNA migration on agarose gel to check their integrity. The conversion of mRNAs to cDNA was achieved from 300 ng of total RNA, using an iScript Reverse transcription supermix for RT-qPCR(Bio-Rad, Hercules, CA, USA). The RT-qPCR was carried out in a final volume of 15 µL with a mix containing 3 µL of 50 times diluted cDNA, 1-µL primer pairs (10 µM), 4 µL iQ SYBR Green supermix (Bio-rad) and 7 µL of ultrapure water, using the following amplification program: 95°C—1 min (initiating step), 40 cycles (5 s at 95°C and 30 s at 60°C) and terminated with a melting step stating an increase of 0.5°C every 2 s until 95°C. The fluorescence was detected using the CFX Connect real time PCR Detection system (Bio-rad). The abundance of transcripts was expressed relative to the control condition, as described by Pfaffl (2001). The reference genes used are UBIQUITIN CONJUGATING ENZYME E2 (RhUBC) and TRANSLATIONALLY CONTROLLED TUMOR PROTEIN (RhTCTP), previously described as reliable genes for normalization (Han et al., 2012) and previously used in rosebush (Hibrand Saint-Oyant et al., 2018; Wang et al., 2019).
Statistical analysis
Graphs and tables represent the means ± se of three biological-independent experiments, each resulting from at least three different technical replicates. The statistical significance was assayed using means comparison after Wilcoxon nonparametric test or a multi-comparison of groups after a Kruskal–Wallis nonparametric test. These different analyses were performed using the statistical software R (R Core Team, 2020). Graphics were drawn using Sigmaplot version 11.0 and figures were assembled using Inkscape version 0.92 software. All different statistical tests and figure annotations are described in the figure captions.
Accession numbers
RhAPX6 (RC6G0464200); RhCAT (RC7G0342400); RhCYCD3 (RC2G0200400); RhDHAR1 (RC7G0183000); RhEXP (RC2G0618000) RhGR1 (RC0G0077700); RhGR2 (RC6G0391800); RhGSH1 (RC7G0192300); RhGSH2 (RC3G0364000); RhMAX2 (RC2G0386000); RhMDHAR6 (RC1G0433100); RhNADSDH (RC1G0532900); RhPCNA (RC5G0585000); RhRBOHB1 (RC3G0349300); RhRBOHB2 (RC3G0349100); RhRBOHD1 (RC4G0330300); RhRBOHD2 (RC7G0236200); RhRBOHD3 (RC7G0106000); RhRBOHE1 (RC5G0105100); RhRBOHE2 (RC2G0174800); RhRBOHE3 (RC7G0059800) RhRBOHF (RC7G0180700); RhRBOHHJ (RC5G0534300); RhTCTP (RC4G0282100); RhUBC (RC7G0173600); RHVI (RC1G0516400).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Illustration of cultural conditions.
Supplemental Figure S2. Effects of light on transcript accumulation of marker genes for bud outgrowth mechanism.
Supplemental Figure S3. Evolution of RBOHs transcript abundances in different organs and impact of CK treatment.
Supplemental Figure S4. Effects of CK treatment on the expression of bud outgrowth marker genes in absence of light.
Supplemental Figure S5. Effects of light and CK treatments on transcript accumulations of genes involved in H2O2 scavenging.
Supplemental Figure S6. Position map of 5′-CK responsive cis-elements in the promoter sequences (1000pb) of Rosa genes involved in H2O2 scavenging pathways (RhAPX6, RhMDHAR6, RhDHAR1, RhGR1, RhGR2, and RhCAT) and GSH biosynthesis (RhGSH1 and RhGSH2).
Supplemental Figure S7. Effect of the GSH synthesis inhibitor BSO on bud outgrowth in presence or absence of CK in darkness condition.
Supplemental Table S1. CK-responsive cis-elements present in the 1,000 base pair promoter sequences of RhAPX6, RhMDHAR6, RhDHAR1, RhGR1, RhGR2, RhGSH1, RhGSH2, and RhCAT.
Supplemental Table S2. List of primer sequences already published in previous works in Rosebush.
Supplemental Table S3. List of primer sequences newly designed in rosebush.
Supplementary Material
Acknowledgments
We are grateful to Professor Eric Davies for manuscript English corrections and to the PHENOTIC platform of ImHorPhen core facility (IRHS, Angers, France) for its technical support.
Funding
This research was conducted within the framework of the regional program “Objectif Végétal, Research, Education and Innovation in Pays de la Loire,” supported by the French Pays de la Loire region, Angers Loire Métropole, and the European Regional Development Fund.
Conflict of interest statement. The authors declare that there is no conflict of interest.
A.P., A.V., J.L., and V.G. conceived and designed research. A.P. and A.L. performed the experiments. A.P. analyzed the data. A.P. and A.V. drafted the manuscript with the contribution of all the authors. A.P., A.V., J.L., V.G., and N.L. proofread the manuscript. All authors have read and validated the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Alain Vian (alain.vian@univ-angers.fr).
References
- Ballaré CL, Casal JJ (2000) Light signals perceived by crop and weed plants. Field Crop Res 67: 149–160 [Google Scholar]
- Barbier F, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA (2019a) An update on the signals controlling shoot branching. Trends Plant Sci 24: 220–236 [DOI] [PubMed] [Google Scholar]
- Barbier F, Péron T, Lecerf M, Perez-Garcia MDD, Barrière Q, Rolčík J, Boutet-Mercey S, Citerne S, Lemoine R, Porcheron B, et al. (2015) Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66: 2569–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier FF, Chabikwa TG, Ahsan MU, Cook SE, Powell R, Tanurdzic M, Beveridge CA (2019b) A phenol/chloroform-free method to extract nucleic acids from recalcitrant, woody tropical species for gene expression and sequencing. Plant Methods 15: 9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Zheng Y, Lyons T (2002) Plant resistance to ozone: the role of Ascorbate. Air Pollution and Plant Biotechnology. Springer, Tokyo, pp 235–252 [Google Scholar]
- Bartoli CG, Casalongué CA, Simontacchi M, Marquez-Garcia B, Foyer CH (2013) Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ Exp Bot 94: 73–88 [Google Scholar]
- Bartoli CG, Yu J, Gómez F, Fernández L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57: 1621–1631 [DOI] [PubMed] [Google Scholar]
- Bertheloot J, Barbier F, Boudon F, Perez-Garcia MD, Péron T, Citerne S, Dun E, Beveridge C, Godin C, Sakr S (2020) Sugar availability suppresses the auxin-induced strigolactone pathway to promote bud outgrowth. New Phytol 225: 866–879 [DOI] [PubMed] [Google Scholar]
- Bhargava A, Clabaugh I, To JP, Maxwell BB, Chiang YH, Schaller GE, Loraine A, Kieber JJ (2013) Identification of cytokinin-responsive genes using microarray meta-analysis and RNA-seq in Arabidopsis. Plant Physiol 162: 272–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda F, Kok J De, Kuiper PJC (1988) Cysteine, y-glutamyl-cysteine and glutathione contents of spinach leaves as affected by darkness and application of excess sulfur. Physiol Plant 74: 663–668 [Google Scholar]
- Buwalda F, De Kok LJ, Stulen I (1993) Effects of atmospheric H2S on Thiol composition of crop plants. J Plant Physiol 142: 281–285 [Google Scholar]
- Casal JJ, Sanchez RA, Deregibus VA (1986) The effect of plant density on tillering: the involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environ Exp Bot 26: 365–371 [Google Scholar]
- Causin HF, Roberts IN, Criado MV, Gallego SM, Pena LB, Ríos M del C, Barneix AJ (2009) Changes in hydrogen peroxide homeostasis and cytokinin levels contribute to the regulation of shade-induced senescence in wheat leaves. Plant Sci 177: 698–704 [Google Scholar]
- Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Afiyanti M, Huang GJ, Huang SS, Lin YH (2011) Characterization of a leaf-type catalase in sweet potato (Ipomoea batatas Lam. L). Bot Stud 52: 417–426 [Google Scholar]
- Chen XJ, Xia XJ, Guo X, Zhou YH, Shi K, Zhou J, Yu JQ (2016) Apoplastic H2O2 plays a critical role in axillary bud outgrowth by altering auxin and cytokinin homeostasis in tomato plants. New Phytol 211: 1266–1278 [DOI] [PubMed] [Google Scholar]
- Choubane D, Rabot A, Mortreau E, Legourrierec J, Péron T, Foucher F, Ahcène Y, Pelleschi-Travier S, Leduc N, Hamama L, et al. (2012) Photocontrol of bud burst involves gibberellin biosynthesis in Rosa sp. J Plant Physiol 169: 1271–1280 [DOI] [PubMed] [Google Scholar]
- Cline M, Wesse T, Iwamura H (1997) Cytokinin/auxin control of apical dominance in ipomoea nil. Plant Cell Physiol 38: 659–667 [Google Scholar]
- Considine MJ, Foyer CH (2014) Redox regulation of plant development. Antioxid Redox Signal 21: 1305–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corot A, Roman H, Douillet O, Autret H, Perez-Garcia MD, Citerne S, Bertheloot J, Sakr S, Leduc N, Demotes-Mainard S (2017) Cytokinins and abscisic acid act antagonistically in the regulation of the bud outgrowth pattern by light intensity. Front Plant Sci 8: 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortleven A, Schmülling T (2015) Regulation of chloroplast development and function by cytokinin. J Exp Bot 66: 4999–5013 [DOI] [PubMed] [Google Scholar]
- Crespel L, Le Bras C, Amoroso T, Unda Ulloa MG, Morel P, Sakr S (2020) Genotype × Light quality interaction on rose architecture. Agronomy 10: 1–16 [Google Scholar]
- Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2: 1–13 [Google Scholar]
- Demotes-Mainard S, Péron T, Corot A, Bertheloot J, Le Gourrierec J, Pelleschi-Travier S, Crespel L, Morel P, Huché-Thélier L, Boumaza R, et al. (2016) Plant responses to red and far-red lights, applications in horticulture. Environ Exp Bot 121: 4–21 [Google Scholar]
- Diaz-Vivancos P, De Simone A, Kiddle G, Foyer CH (2015) Glutathione - Linking cell proliferation to oxidative stress. Free Radic Biol Med 89: 1154–1164 [DOI] [PubMed] [Google Scholar]
- Djennane S, Hibrand-Saint Oyant L, Kawamura K, Lalanne D, Laffaire M, Thouroude T, Chalain S, Sakr S, Boumaza R, Foucher F, et al. (2014) Impacts of light and temperature on shoot branching gradient and expression of strigolactone synthesis and signalling genes in rose. Plant Cell Environ 37: 742–757 [DOI] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158: 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers JB, AR Van Der Krol, Vos J, Struik PC (2011) Understanding shoot branching by modelling form and function. Trends Plant Sci 16: 464–467 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Furet PM, Lothier J, Demotes-Mainard S, Travier S, Henry C, Guérin V, Vian A (2014) Light and nitrogen nutrition regulate apical control in Rosa hybrida L. J Plant Physiol 171: 7–13 [DOI] [PubMed] [Google Scholar]
- Girault T, Abidi F, Sigogne M, Pelleschi-Travier S, Boumaza R, Sakr S, Leduc N (2010) Sugars are under light control during bud burst in Rosa sp. Plant Cell Environ 33: 1339–1350 [DOI] [PubMed] [Google Scholar]
- Girault T, Bergougnoux V, Didier C, Viemont J-D, Leduc N (2008) Light controls shoot meristem organogenic activity and leaf primordia growth during bud burst in Rosa sp. Plant Cell Environ 31: 1534–1544 [DOI] [PubMed] [Google Scholar]
- Han X, Lu M, Chen Y, Zhan Z, Cui Q, Wang Y (2012) Selection of reliable reference genes for gene expression studies using real-time PCR in tung tree during seed development. PLoS One 7: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyl A, Schmülling T (2003) Cytokinin signal perception and transduction. Curr Opin Plant Biol 6: 480–488 [DOI] [PubMed] [Google Scholar]
- Hibrand Saint-Oyant L, Ruttink T, Hamama L, Kirov I, Lakhwani D, Zhou NN, Bourke PM, Daccord N, Leus L, Schulz D, et al. (2018) A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat Plants 4: 473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson D, Guevara DR, Hand AJ, Xu Z, Hao L, Chen X, Zhu T, Bi YM, Rothstein SJ (2013) Rice cytokinin GATA transcription factor1 regulates chloroplast development and plant architecture. Plant Physiol 162: 132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton J (1992) The rapid generation of mutation data matrices from protein sequences. Oxford Univ Press 8: 275–282 [DOI] [PubMed] [Google Scholar]
- Kalousek P, Buchtová D, Balla J, Reinöhl V, Procházka S (2010) Cytokinins and polar transport of auxin in axillary pea buds. Acta Univ Agric Silvic Mendelianae Brun 58: 79–88 [Google Scholar]
- Kim EJ, Kim YJ, Hong WJ, Lee C, Jeon JS, Jung KH (2019) Genome-wide analysis of root hair preferred RBOH genes suggests that three RBOH genes are associated with auxin-mediated root hair development in rice. J Plant Biol 62: 229–238 [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MY, Halliwell B (1986) Purification and properties of glutathione synthetase from spinach (Spinacia oleracea) leaves. Plant Sci 43: 185–191 [Google Scholar]
- Leduc N, Roman H, Barbier F, Péron T, Huché-Thélier L, Lothier J, Demotes-Mainard S, Sakr S (2014) Light signaling in bud outgrowth and branching in plants. Plants 3: 223–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Song J, Campbell-Palmer L (2009) A modified chemiluminescence method for hydrogen peroxide determination in apple fruit tissues. Sci Hortic 120: 336–341 [Google Scholar]
- Mhamdi A, Van Breusegem F (2018) Reactive oxygen species in plant development. Development 145: dev164376. [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, Queval G, Han YY, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou JP, et al. (2010a) Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol 153: 1144–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010b) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61: 4197–4220 [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Le Moigne MA, Guérin V, Furet PM, Billard V, Lebrec A, Spíchal L, Roman H, Citerne S, Morvan-Bertrand A, Limami A, et al. (2018) Asparagine and sugars are both required to sustain secondary axis elongation after bud outgrowth in Rosa hybrida. J Plant Physiol 222: 17–27 [DOI] [PubMed] [Google Scholar]
- Müller-Moulé P, Havaux M, Niyogi KK (2003) Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol 133: 748–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Baker NR (2006) Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol 141: 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux PM, Rausch T (2005) Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth Res 86: 459–474 [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi AM, Jouanin L, Valadier Á, Roux Y, Foyer CH (1997) Light-dependent modulation of foliar glutathione synthesis and associated amino acid metabolism in poplar overexpressing c -glutamylcysteine synthetase. Planta 202: 357–369 [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39: 1140–1160 [DOI] [PubMed] [Google Scholar]
- Or E (2009) Grape bud dormancy release − the molecular aspect. Grapevine Molecular Physiology & Biotechnology. Springer Netherlands, Dordrecht, Netherlands, pp 1–29
- Or E, Vilozny I, Eyal Y, Ogrodovitch A (2000) The transduction of the signal for grape bud dormancy breaking induced by hydrogen cyanamide may involve the SNF-like protein kinase GDBRPK. Plant Mol Biol 43: 483–494 [DOI] [PubMed] [Google Scholar]
- Pérez FJ, Lira W (2005) Possible role of catalase in post-dormancy bud break in grapevines. J Plant Physiol 162: 301–308 [DOI] [PubMed] [Google Scholar]
- Pérez FJ, Noriega X (2018) Sprouting of paradormant and endodormant grapevine buds under conditions of forced growth: similarities and differences. Planta 248: 837–847 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT – PCR. Nucleic Acid Res 29: 16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay I, Railton ID (1983) Complete release of axillary buds from apical dominance in intact, light-grown seedlings of Pisum sativum L. following a single application of cytokinin. Plant Physiol 71: 972–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher A, Guérin V, Montrichard F, Lebrec A, Lothier J, Vian A (2020) Ascorbate glutathione-dependent H2O2 scavenging is an important process in axillary bud outgrowth in rosebush. Ann Bot 126: 1049–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Rabot A, Henry C, Baaziz K, Ben Mortreau E, Azri W, Lothier J, Hamama L, Boummaza R, Leduc N, Pelleschi-Travier S, et al. (2012) Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol 53: 1068–1082 [DOI] [PubMed] [Google Scholar]
- Rabot A, Portemer V, Péron T, Mortreau E, Leduc N, Hamama L, Coutos-Thévenot P, Atanassova R, Sakr S, Le Gourrierec J (2014) Interplay of sugar, light and gibberellins in expression of Rosa hybrida Vacuolar Invertase 1 regulation. Plant Cell Physiol 55: 1734–1748 [DOI] [PubMed] [Google Scholar]
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, Sakr S (2015) Multiple pathways regulate shoot branching. Front Plant Sci 5: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichheld J, Vernoux T, Lardon F, Montagu M, Van Inze D, Wilrijk B (1999) Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J 17: 647–656 [Google Scholar]
- Reichheld JP, Bashandy T, Siala W, Riondet C, Delorme V, Meyer A, Meyer Y (2009) Chapter 9 redundancy and crosstalk within the thioredoxin and glutathione pathways. A new development in plants. Adv Bot Res. 52: 253–276 [Google Scholar]
- Roman H, Girault T, Barbier F, Péron T, Brouard N, Pěnčík A, Novák O, Vian A, Sakr S, Lothier J, et al. (2016) Cytokinins are initial targets of light in the control of bud outgrowth. Plant Physiol 172: 489–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H, Girault T, Le Gourrierec J, Leduc N (2017) In silico analysis of 3 expansin gene promoters reveals 2 hubs controlling light and cytokinins response during bud outgrowth. Plant Signal Behav 12: e1284725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T, Thimann K V (1964) Release of lateral buds from apical dominance. Nature 201: 939–940 [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16: 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126: 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli S, Agudelo-Romero P, Meitha K, Foyer CH, Considine MJ (2018) Roles for light, energy, and oxygen in the fate of quiescent axillary buds. Plant Physiol 176: 1171–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudawan B, Chang C, Chao H, Ku MSB, Yen Y (2016) Hydrogen cyanamide breaks grapevine bud dormancy in the summer through transient activation of gene expression and accumulation of reactive oxygen and nitrogen species. BMC Plant Biol 16: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Imamura T, Konno N, Takeda T, Fujita K, Konishi T, Nishihara M, Uchimiya H (2014) The gentio-oligosaccharide gentiobiose functions in the modulation of bud dormancy in the herbaceous perennial gentiana. Plant Cell 26: 3949–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45: 1028–1036 [DOI] [PubMed] [Google Scholar]
- Thimann KV., Skoog F (1934) On the inhibition of bud development and other functions of growth substance in vicia faba. Proc R Soc B 114: 317–339 [Google Scholar]
- Thimann KV, Skoog F, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (1933) Studies on the growth hormone of plants: III. The inhibiting action of the growth substance on bud development. Proc Natl Acad Sci USA 19: 714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo MEA, Ueda Y, Imahori Y, Ayaki M (2003) L-ascorbic acid metabolism in spinach (Spinacia oleracea L.) during postharvest storage in light and dark. Postharvest Biol Technol 28: 47–57 [Google Scholar]
- Wang M, Ogé L, Voisine L, Perez-Garcia M-D, Jeauffre J, Hibrand Saint-Oyant L, Grappin P, Hamama L, Sakr S (2019) Posttranscriptional regulation of RhBRC1 (Rosa hybrida BRANCHED1) in response to sugars is mediated via its own 3′ untranslated region, with a potential role of RhPUF4 (Pumilio RNA-Binding Protein Family). Int J Mol Sci 20: 3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Faust M (1988) Metabolic activities during dormancy and blooming of deciduous fruit trees. Isr J Bot 37: 227–243 [Google Scholar]
- Wang SY, Faust M (1994) Changes in the antioxidant system associated with budbreak in “Anna” apple (Malus domestica Borkh.) buds. J Am Soc Hortic Sci 119: 735–741 [Google Scholar]
- Wang SY, Jiao HJ, Faust M (1991) Changes in ascorbate, glutathione, and related enzyme activities during thidiazuron‐induced bud break of apple. Physiol Plant 82: 231 [Google Scholar]
- Xu Y, Burgess P, Zhang X, Huang B (2016) Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J Exp Bot 67: 1979–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Mandel T, Kuhlemeier C (2011) Stem cell activation by light guides plant organogenesis. Genes Dev 25: 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.