Abstract
Application of high concentrations of sodium ascorbate suppresses necrosis caused by the expression of recombinant proteins in Nicotiana benthamiana, resulting in an increase in protein accumulation.
Dear Editor,
The overexpression of recombinant proteins can sometimes result in the accumulation of unfolded or misfolded proteins, triggering leaf necrosis, and/or dehydration and subsequent degradation of recombinant proteins. To enhance the accumulation of active proteins, a fundamental and a simple treatment to overcome leaf necrosis is required. In this study, treatment of Nicotiana benthamiana plants with high concentrations (100 mM or more) of sodium ascorbate (AsA) via foliar spraying after agroinfiltration suppressed the necrosis of leaves expressing human Cul1 (hCul1) and F-box protein, Fbxw7. The suppression of necrosis accordingly enhanced the accumulation of recombinant proteins by three-fold or more. Treatment with 200 mM AsA enhanced the formation and accumulation of PMab-2, an anti-RAP (rat Aggrus/podoplanin [PDPN]) tag antibody with antigen-binding activity. Thus, spray application of high concentrations of AsA is simple and easily applicable in several kinds of facilities to effectively suppress leaf necrosis, leading to an increase in the accumulation of functional recombinant proteins.
Compared with traditional cell culture-based systems, whole plant-based systems for the production of recombinant proteins have advantages, including cost-effectiveness and production scalability (Buyel et al., 2017). Transient gene expression using a deconstructed viral vector is a promising technique for rapidly producing high amounts of recombinant proteins (Desai et al., 2010; Ma et al., 2013). However, some recombinant proteins, such as hepatitis B surface antigen and human growth hormone, causes necrosis and/or dehydration of N. benthamiana leaves (Gils et al., 2005; Huang et al., 2008) and there has been no way to prevent necrosis. To increase the yield of these recombinant proteins, a fundamental solution is required.
We constructed pBYR2HS-hCul1 and pBYR2HS-human F-box protein (hFbxw7; Supplemental Figure S1) and transfected those to N. benthamiana leaves by agroinfiltration. Leaves expressing hCul1 and hFbxw7 exhibited necrosis (Figure 1A; 0 mM). As necrosis appeared, expression of binding immunoglobulin protein (BiP) and H2O2 was induced (Supplemental Figure S2), suggesting that endoplasmic reticulum (ER) stress-triggered accumulation of H2O2 (Ozgur et al., 2014). Treatment with ER stress inhibitors had no appreciable improvement in plants (Supplemental Figure S3).
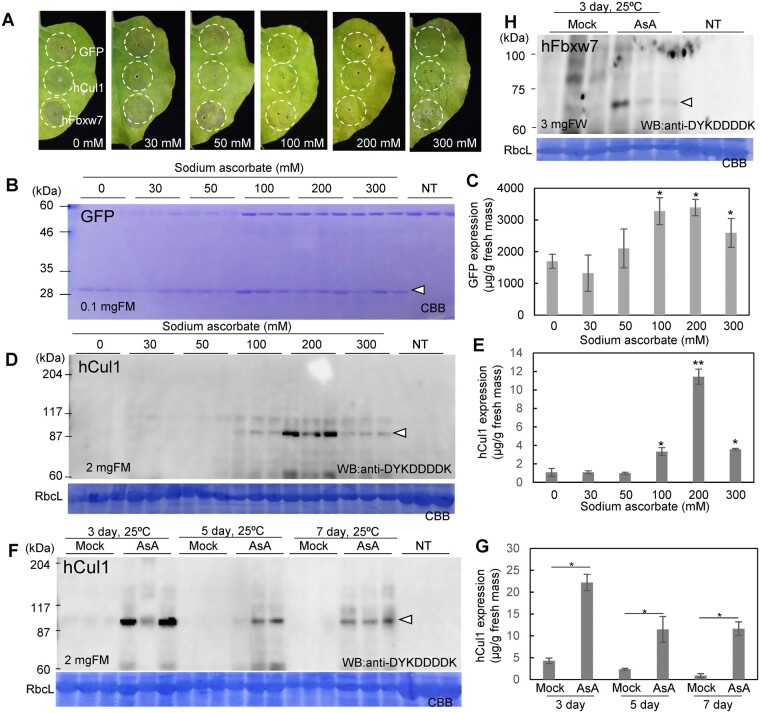
Figure 1.
Suppression of necrotic effects by foliar spray application of high concentration of AsA. A, Syringe agroinfiltration was performed to express GFP, hCul1, and hFbxw7 in the leaves of N. benthamiana, followed by foliar spray application of AsA at the indicated concentration at 2-d intervals. The plants were incubated at 20°C for 7 d. Soluble proteins from the indicated fresh mass (FM) of these leaves were separated by SDS-PAGE. GFP (B) or hCul1 (D) were detected by Coomassie Brilliant Blue (CBB) staining or anti-DYKDDDDK antibody. NT indicates nontransfected plants. The amount of GFP (C) or hCul1 (E) was determined from band intensities. N. benthamiana plants agroinfiltrated with different vectors were incubated at 25°C following the foliar spray application of 200 mM AsA, and soluble proteins were extracted from leaves at 3, 5, or 7 d after agroinfiltration. hCul1 (F) or hFbxw7 (H) were detected with anti-DYKDDDDK antibody. The amount of hCul1 (G) were determined from band intensities. Data represent the means ± sd (n = 3–4) and significance was determined using unpaired Student’s t tests (*P < 0.05) (C, E, G).
In plants, high levels of reactive oxygen species (ROS) can serve as a trigger for programmed cell death (van Breusegem and Dat, 2006). To remove ROS, a high concentration of AsA was applied to N. benthamiana plants expressing green fluorescent protein (GFP), hCul1, and hFbxw7. Leaves expressing hCul1 and hFbxw7 treated with 50 mM or less concentration of AsA via foliar spraying exhibited necrotic symptoms. In contrast, leaves treated with AsA at a concentration 100 mM or higher appeared healthy (Figure 1A). Consistently, accumulation of H2O2 was significantly reduced in leaves treated with 200 mM AsA (Supplemental Figure S2B).
To establish whether the alleviation of necrosis by AsA also resulted in an enhancement in the protein expression, soluble proteins were extracted from leaves and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) . Peak GFP expression (∼4 mg/g fresh mass [FM]) occurs 3 d postagroinfiltration (dpa), and thereafter declines (Yamamoto et al., 2018). At 7 dpa, only ∼2 mg/g FM of GFP protein accumulated in the leaves, whereas when AsA was applied at concentrations of at least 100 mM, ∼3 mg/g FM of GFP expression in these leaves (Figure 1, B and C). Similarly, in leaves expressing hCul1, no clear bands (˂1 μg/g FM) were observed when 50 mM or less AsA was treated, but notable protein expression (11.4 μg/g FM when applied at 200 mM AsA) was observed after treatment with AsA at concentrations of 100 mM or higher (Figure 1, D and E), indicating high concentrations of AsA have the effect of inhibiting protein degradation. Leaves were incubated at 25°C for 3, 5, and 7 d and 200 mM AsA was applied. A marked increase in the expression of hCul1 and hFbxw7 was observed in response to treatment with AsA, with peak hCul1 expression being attained at 3 dpa (Figure 1, F–H). These results indicate that the application of a high concentration of AsA can mitigate the necrosis and markedly enhances protein expression.
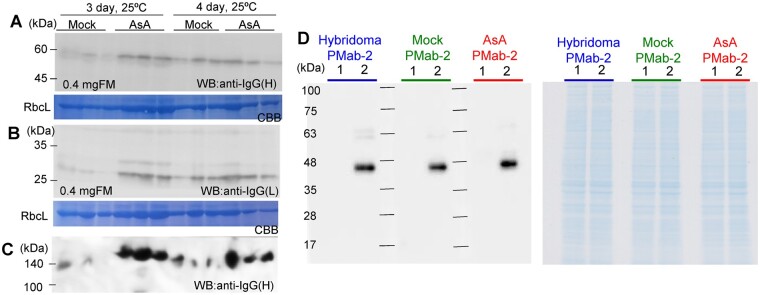
Approximately 0.3 mg/g FM of PMab-2, an antibody against the RAP epitope, which is in rat PDPN (Fujii et al., 2017), was accumulated in N. benthamiana without AsA treatment (Miura et al., 2020). In this study, 200 mM AsA was applied to N. benthamiana expressing the PMab-2 heavy chain (HC) and light chain (LC). The PMab-2 HC and LC were detected using anti-mouse IgG (H) and anti-mouse IgG (L) antibodies, respectively (Figure 2, A and B). The full tetrameric assembly of PMab-2 (2 HCs and 2 LCs) was detected by immunoblot analysis using native-PAGE (Figure 2C). Application of AsA increased the accumulation of PMab-2 HC and LC and enhanced the formation of tetrameric PMab-2. Without AsA application, the formation of antibodies was not stable and the peak of HC and LC production may be shifted. PMab-2 was purified from N. benthamiana. Fluorescence-activated cell sorting analysis indicated that AsA-treated plant-derived PMab-2 recognized Chinese hamster ovary (CHO)-K1 cell-expressed rat PDPN as did hybridoma-derived PMab-2 or plant-derived PMab-2. No substantial changes were observed in the CHO-K1 cells treated with PMab-2 (Supplemental Figure S4). Immunoblot analysis revealed that rat PDPN was similarly detected by PMab-2 derived from hybridoma cells and plant cells with or without AsA treatment (Figure 2D). These results indicate that the application of AsA did not affect the function of a recombinant antibody. No apparent phenotypic difference was observed when a high concentration of AsA was applied.
Figure 2.
Application of AsA increases the production of an antibody that retains binding activity. N. benthamiana leaves were infiltrated with a mixed preparation of Agrobacterium harboring pBYR2HS-PMab2H and pBYR2HS-PMab2L, after which, the leaves were treated with water (Mock) or 200 mM AsA via foliar spray application. At 3 or 4 d postinfiltration, leaves were harvested. Total protein extracts were separated and were transferred to PVDF membranes. The membranes were incubated with anti-mouse IgG(H) or anti-mouse IgG(L) to detect the HC (A) and LC (B) of PMab-2, respectively. C, Native PAGE was used to detect the tetrameric assembly of PMab-2 (two HCs and two LCs). D, Immunoblot analysis with PMab-2 to detect rat PDPN. Soluble proteins from CHO-K1 cells (1) or CHO-K1 expressing rat PDPN (2) were separated on SDS-PAGE. Rat PDPN was detected using PMab-2 derived from hybridoma cells (hybridoma) or N. benthamiana leaves treated without (Mock) or with 200 mM AsA. Total proteins were visualized by CBB staining.
In this study, ER retention signal was fused with genes of interest. Some proteins require late posttranslational modification, such as the formation of complex glycan. These modifications are typically performed downstream of the ER along secretory pathways (Faye et al., 2005). Thus, the production of modified proteins is likely to be enhanced by spray application of AsA.
Overall, our research demonstrated the application of high concentrations of AsA suppresses necrosis caused by the expression of foreign recombinant proteins in N. benthamiana, increasing in protein accumulation and AsA did not affect the antigen-binding activity of the recombinant antibody. The application of AsA by a foliar spray is simple, thus, it is easily applicable to several kinds of facilities.
Supplemental data
The following supplemental materials are available.
Supplemental Figure S1. Schematic representation of the T-DNA regions of the plasmids pBYR2HS-hCul1 and pBYR2HS-hFbxw7.
Supplemental Figure S2. BiP expression and H2O2 accumulation were induced by hCul or hFbxw7.
Supplemental Figure S3. Necrosis was observed in leaves treated with ER stress inhibitors.
Supplemental Figure S4. AsA-treated plant-derived antibody retained its binding activity.
Supplemental Methods. The methodology used in this study.
Funding
This work was supported by a JSPS Grant-in-Aid (19H04637 and 20K21302) and Program on Open Innovation Platform with Enterprise, Research Institute and Academia, Japan Science and Technology Agency (JST-OPERA, JPMJOP1851 to K.M.), a Cooperative Research Grant #2020 of the Plant Transgenic Design Initiative (PTraD) by the Gene Research Center, Tsukuba-Plant Innovation Research Center (T-PIRC), University of Tsukuba, and AMED (Grant numbers JP20am0101078 and JP20am0401013 to Y.K).
Conflict of interest statement. None declared.
Supplementary Material
K.M. designed the original concept and the project; S.N., M.K.K., F.T., H.Y., and K.M. performed the experiments; S.N., Y.K., and K.M. analyzed the data; S.N. and K.M. wrote the article. The authors have declared that no competing interests exist.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instruction of Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Kenji Miura (miura.kenji.ga@u.tsukuba.ac.jp).
References
- Buyel JF, Twyman RM, Fischer R (2017) Very-large-scale production of antibodies in plants: the biologization of manufacturing. Biotechnol Adv 35: 458–465 [DOI] [PubMed] [Google Scholar]
- Desai PN, Shrivastava N, Padh H (2010) Production of heterologous proteins in plants: strategies for optimal expression. Biotechnol Adv 28: 427–435 [DOI] [PubMed] [Google Scholar]
- Faye L, Boulaflous A, Benchabane M, Gomord V, Michaud D (2005) Protein modifications in the plant secretory pathway: current status and practical implications in molecular pharming. Vaccine 23: 1770–1778 [DOI] [PubMed] [Google Scholar]
- Fujii Y, Kaneko MK, Ogasawara S, Yamada S, Yanaka M, Nakamura T, Saidoh N, Yoshida K, Honma R, Kato Y (2017) Development of RAP tag, a novel tagging system for protein detection and purification. Monoclon Antib Immunodiagn Immunother 36: 68–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gils M, Kandzia R, Marillonnet S, Klimyuk V, Gleba Y (2005) High-yield production of authentic human growth hormone using a plant virus-based expression system. Plant Biotechnol J 3: 613–620 [DOI] [PubMed] [Google Scholar]
- Huang Z, LePore K, Elkin G, Thanavala Y, Mason HS (2008) High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol J 6: 202–209 [Google Scholar]
- Ma JK, Christou P, Chikwamba R, Haydon H, Paul M, Ferrer MP, Ramalingam S, Rech E, Rybicki E, Wigdorovitz A, et al. (2013) Realising the value of plant molecular pharming to benefit the poor in developing countries and emerging economies. Plant Biotechnol J 11: 1029–1033 [DOI] [PubMed] [Google Scholar]
- Miura K, Yoshida H, Nosaki S, Kaneko MK, Kato Y (2020) RAP tag and PMab-2 antibody: a tagging system for detecting and purifying proteins in plant cells. Front Plant Sci 11: 510444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgur R, Turkan I, Uzilday B, Sekmen AH (2014) Endoplasmic reticulum stress triggers ROS signalling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. J Exp Bot 65: 1377–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141: 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Hoshikawa K, Ezura K, Okazawa R, Fujita S, Takaoka M, Mason HS, Ezura H, Miura K (2018) Improvement of the transient expression system for production of recombinant proteins in plants. Sci Rep 8: 4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.