Abstract
Introduction
Differentiation of renal cell carcinoma (RCC) from oncocytoma is a common diagnostic dilemma. A few studies have shown that 99mTc-sestamibi (MIBI) imaging has the potential to characterize indeterminate renal masses. This comparative study evaluated the utility of MIBI single-photon emission computed tomography-computed tomography (SPECT-CT) in the assessment and risk stratification of renal masses.
Methods
A total of 29 patients with 31 renal masses who had cross-sectional imaging and MIBI SPECT-CT were included. Lesions were categorized as either MIBI-positive or -negative on SPECT-CT. Individual lesion density ranged from 22–56 Hounsfield units (HU) on the non-contrast CT part of SPECT-CT. Quantitative relative MIBI uptake was calculated by measuring tumor to ipsilateral renal parenchymal uptake. The imaging results were correlated with histopathology.
Results
All oncocytic lesions, including seven oncocytomas and one hybrid oncocytic chromophobe tumor (100%), were positive on MIBI. One chromophobe RCC showed low-grade MIBI uptake. The remaining RCC subtypes, including 15 clear-cell, four papillary, two mixed clear-cell and papillary, and one chromophobe, were MIBI-negative. The quantitative relative tumor uptake showed statistically significant higher uptake in the low-risk/oncocytic lesions compared to RCCs.
Conclusions
This study demonstrates that MIBI SPECT-CT is valuable in the characterization of indeterminate renal masses. The combination of MIBI uptake on SPECT and lesion density on non-contrast CT can be used for risk stratification of renal masses. This technique may reduce the need for further imaging (multiphasic CT or magnetic resonance imaging), renal mass biopsy, or surgical resection of low-risk renal masses. Subsequently, more patients could be followed with active surveillance.
Introduction
The incidence of renal cell carcinoma (RCC) has almost doubled in Canada since 1970.1 This is likely related to the growing incidence of risk factors, such as hypertension and obesity, and higher detection of incidental solid renal masses on routine diagnostic imaging examinations.1,2
The most commonly encountered solid enhancing renal masses include RCC, oncocytoma, and fat-poor angiomyolipoma (AML).3 RCC has several typical and distinct histological subtypes; clear-cell is the most common (75%), followed by papillary (10%) and chromophobe (5%).3 Oncocytoma is a benign renal tumor that has significant imaging overlap with RCC.4 The classic central scar is typically found in oversized oncocytomas and is likely due to lesion growth, resulting in central infarction, hemorrhage, and necrosis.5 Additionally, a small percentage of lesions may have hybrid pathology, known as hybrid oncocytic chromophobe tumor (HOCT). As with oncocytomas, HOCT in previous studies showed no regional or metastatic progression during followup. 6
Due to the overlap in the imaging features between RCC and oncocytoma, many oncocytomas are surgically resected.4 Published literature demonstrates that currently, renal masses with features suspicious of malignancy maybe overtreated.7,8 A study by Frank et al, including 2770 resected renal masses, showed that 12.8% of the lesions were benign, and when further stratified based on size, 25% of lesions smaller than 3 cm were benign.3 Additionally, a recently published study with approximately 900 patients showed that 14% of patients who underwent partial nephrectomy had benign masses, with oncocytoma being the most common benign pathology.9
Currently, biopsy is the only reliable method to accurately differentiate between oncocytoma and RCC. Although biopsy has a diagnostic accuracy of 80–90%, 10–20% of cases remain indeterminate, and biopsy of small lesions remains challenging.10,11 In addition, some patients, particularly those who are young and healthy, are not willing to accept the risk of a false-negative biopsy result and may undergo surgery that is ultimately not necessary.12 As a result of the above factors, there is a need for a non-invasive method to differentiate RCC and oncocytoma and reduce overdiagnosis and overtreatment of renal tumors.
In 1996, 99mTc-sestamibi was first introduced for its potential in the diagnosis of oncocytoma.13 99mTc-sestamibi is a lipophilic, cationic molecule taken up by cells with a high concentration of mitochondria, such as myocardium, parathyroid adenoma, and breast cancer.14,15 Histologically, oncocytoma contains numerous mitochondria, as does chromophobe RCC. In the latter case, however, the mitochondria are typically abnormal.16 Clear-cell and papillary RCC have microvilli and lack mitochondria.16
There have been very few studies investigating the role of 99mTc-sestamibi single-photon emission computed tomography-computed tomography (MIBI SPECT-CT) in the assessment of renal masses.17–20 The purpose of this study was to evaluate this role further in the Canadian healthcare system, and also define the value of MIBI SPECT-CT in the risk stratification of renal masses.
Methods
A single-site, comparative study was conducted on 29 patients with 31 renal masses between December 2018 and March 2020. The study was approved by the institutional ethics committee. Patients who required further characterization of their renal mass prior to proceeding with treatment (based on the discretion of the treating urologist) were selected to undergo further imaging with MIBI SPECT-CT as part of standard of care.20 All patients provided consent to undergo the imaging investigations, renal mass biopsy, or surgical excision, and all care was funded by the Ontario Health Insurance Plan (OHIP). Data was subsequently collected and analyzed in a retrospective manner.
All patients had a CT scan and renal MIBI SPECT-CT scan. MIBI SPECT-CT imaging was performed 60–90 minutes after the administration of 30 mCi of MIBI on a 16-slice SPECT-CT camera (GE Discovery NM/CT 670). All MIBI SPECT-CT images were reviewed independently by an experienced nuclear medicine specialist and a dual radiology/nuclear medicine resident. A final diagnostic interpretation was made by consensus. The imaging physicians were not blinded to the patient record.
A visual approach was used in the evaluation of the renal masses, as has been previously described by Campbell et al.20 Six patterns of uptake were defined as follows: 0: no uptake; 1: uniformly high uptake; 2: variable tumor uptake with areas of high uptake; 3: peripheral uptake with central photopenia; 4: tumoral uptake but below the level of surrounding renal parenchyma; and 5: uptake in the endophytic portion of the lesion. Ultimately, MIBI scans were characterized as negative (0: no uptake) and positive (1–5: any pattern of uptake). Additionally, a quantitative relative tumor uptake was calculated by ratio of mean and maximum tumor uptake to ipsilateral renal parenchymal physiological uptake on SPECT (1.5 cm2 circular region of interest). The density of lesions was measured by placing a 1 cm2 circular region of interest within the mass on the non-contrast CT part of SPECT-CT.
The histopathology of the renal masses was determined through either image-guided percutaneous biopsy or surgical excision with partial or radical nephrectomy. Histopathology results were correlated with MIBI SPECT-CT imaging findings.
Statistical analysis was performed using Microsoft Excel, Version 16.36 (20041300). Descriptive data are presented as median, range, or percentage as appropriate. Univariate analysis was performed using Independent Sample t-test to compare means of continuous variables.
Results
Twenty-nine patients with 31 histopathologically confirmed lesions were included in the study. The information regarding patient demographics and baseline imaging characteristics of renal masses are detailed in Table 1. The results of the MIBI scans and corresponding histopathology are summarized in Table 2.
Table 1.
Demographics and characteristics of renal masses
| Characteristic | |
|---|---|
| Age (n=29) | |
| Median (years) | 59.9 |
| Range (years) | 30–83 |
| Gender (n=29) | n (%) |
| Female | 6 (20.7%) |
| Male | 23 (79.3%) |
| Renal mass size (n=31) | |
| Median (cm) | 2.9 |
| Range (cm) | 1.6–8 |
| <4 cm* | 26 (83.9%) |
| >4cm | 5 (16.1%) |
| Renal mass appearance | n (%) |
| Solid enhancing mass | 26 (83.9%) |
| Bosniak 4 cyst | 5 (16.1%) |
Lesions less than 4 cm are defined as small renal masses (SRMs).
Table 2.
MIBI SPECT-CT results are correlated with histopathology results
| Histopathology | Number of lesions | Positive MIBI scan | Negative MIBI scan |
|---|---|---|---|
| Oncocytoma | 7 | 7 (100%) | – |
| HOCT | 1 | 1 (100%) | – |
| Chromophobe RCC | 2 | 1 (50%) | 1 (50%) |
| Clear cell RCC | 15 | – | 15 (100%) |
| Papillary RCC | 4 | – | 4 (100%) |
| Mixed papillary and clear-cell RCC | 2 | – | 2 (100%) |
| Total | 31 | 9 | 22 |
Results are expressed as actual number (percentage of total number of lesions with specific histopathology). HOCT: hybrid oncocytic chromophobe tumor; MIBI: 99mTc-sestamibi; RCC: renal cell carcinoma; SPECT-CT: single-photon emission computed tomography-computed tomography.
The pattern of uptake in the nine MIBI positive lesions was as follows: three lesions showed uniformly high uptake; three had variable uptake with areas of high uptake; one had definite uptake but less than ipsilateral renal parenchyma; and two showed uptake in the endophytic portion of lesions. Lesion density ranged from 22–56 Hounsfield units (HU) on the non-contrast CT of the SPECT-CT scans. Selected images of an oncocytoma and an RCC are shown in Figs. 1 and 2.
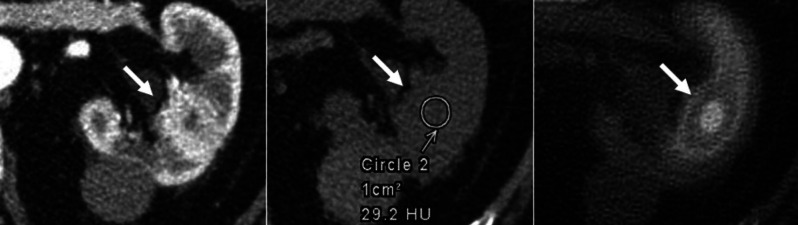
Fig. 1.
Selected axial contrast enhanced and non-contrast computed tomography (CT) and fused single-photon emission computed tomography (SPECT)-CT images demonstrate an enhancing mass with indeterminate density on non-contrast CT and increased 99mTc-sestamibi (MIBI) uptake on SPECT-CT. The biopsy was in keeping with an oncocytoma. Additionally, there is a hyperdense renal cyst posteriorly with density of more than 70 Hounsfield units (HU), most in keeping with a hemorrhagic cyst.
Fig. 2.
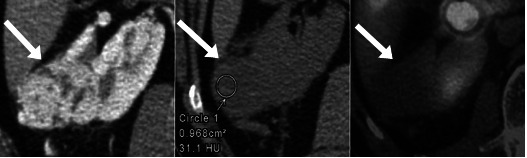
Selected axial contrast enhanced and non-contrast computed tomography (CT) and fused single-photon emission computed tomography (SPECT)-CT images demonstrate an enhancing mass with indeterminate density on non-contrast CT and no 99mTc-sestamibi (MIBI) uptake on SPECT-CT. The postoperative histopathology showed a clear-cell renal cell carcinoma.
The mean and maximum relative tumor uptake to ipsilateral renal parenchyma was calculated. The median of “mean relative tumor uptake” was 0.66 in oncocytic lesions and 0.27 in RCC lesions. The median of “maximum relative tumor uptake” was 0.76 and 0.33 in oncocytic and RCC lesions, respectively. The mean and maximum relative tumor uptake were statistically significantly higher in benign/oncocytic lesions compared to RCCs (p=0.016 and 0.012, respectively). It is noteworthy to clarify that lesions were not classified as MIBI-positive or -negative based on quantitative evaluation, and this quantitative relative tumor uptake was calculated to determine if a quantitative cutoff exists to categorize lesions as benign (oncocytic lesions) vs. malignant (RCC).
Of the five Bosniak IV lesions mentioned in Table 1, three were clear-cell RCCs, one was a chromophobe RCC, and one was an oncocytoma. One patient with Birt-Hogg-Dubé syndrome had seven bilateral renal masses that all showed high MIBI uptake. One of the lesions underwent biopsy and was confirmed to be an oncocytoma. The remaining lesions were not biopsied, as their imaging features were identical to the biopsied lesion. Only the biopsied lesion was included in the data analysis. One of the patients had an AML in the contralateral kidney with typical imaging characteristics on CT and MRI, which did not show uptake on MIBI scan but was not included in the analysis due to lack of histopathology confirmation.
Discussion
This single-center, comparative study is in alignment with previously published studies regarding the utility of MIBI SPECT-CT in the characterization of solid renal masses. In our study, 100% of oncocytomas (n=7), one HOCT (n=1), and one chromophobe RCC (n=1 of 2) were MIBI-positive. In the study by Gorin et al, 83.3% (5/6) of oncocytomas, 100% of HOCTs (n=2), and 50% of chromophobe RCCs (2/4) showed radiotracer uptake.17 Another study by Tzortzakakis et al evaluated 31 solid renal lesions and showed MIBI uptake in 91.6% of oncocytomas (11/12), 100% of HOCTs (n=3), one lipid-poor AML, and surprisingly one papillary RCC (1/3).18 Additionally, in a recently published study including 30 patients with solid solitary renal lesions, all oncocytomas (n=3), one AML, and one RCC (1/26) demonstrated radiotracer uptake.19
In our study, one chromophobe RCC had false-positive uptake and there were no false-negatives, resulting in a sensitivity of 100% and a specificity of 96% for MIBI in the detection of benign/oncocytic lesions vs. RCCs. A very recent meta-analysis showed sensitivity and specificity of 88% and 95%, respectively, for detecting benign vs. malignant renal lesions when HOCTs were characterized as benign.21 In our study, there were two chromophobe RCCs; one showed low-grade uptake (less than ipsilateral parenchyma), and the other showed no uptake (negative). As mentioned earlier, chromophobe RCCs do contain mitochondria similar to oncocytomas but typically the mitochondria are abnormal.16 This could account for the low-grade uptake in one case. Chromophobe RCC has a better prognosis and a lower risk of tumor progression, metastasis, and mortality compared to clear-cell and papillary RCCs.22
On quantitative analysis, the mean and maximum relative tumor uptake were found to be higher in the oncocytic lesions than in RCCs; the difference was statistically significant, although we did not find a quantitative cutoff to categorize lesions as benign vs. malignant. This technique was found to be more cumbersome and subject to sampling error, particularly in small lesions, where delineation of the region of interest becomes more difficult. Also, physiological uptake in normal adjacent renal parenchyma and nearby bowel loops may result in an inaccurate quantitative evaluation of uptake in the renal lesion. We found that qualitative/ visual assessment of MIBI uptake within the lesion is easier and more reliable in the classification of tumors.
Although the final result of the MIBI study is a simple binary designation (positive vs. negative), attention to different patterns of uptake is essential to avoid potential pitfalls in interpretation. Background knowledge and familiarity with the cases with heterogenous/non-uniform uptake of radiotracer is essential to limit lesion misclassification. Both oncocytoma and RCC can have cystic, hemorrhagic, or necrotic changes.4 These fluid-filled components demonstrate no MIBI uptake due to lack of cellularity, regardless of pathology. So, MIBI uptake should be evaluated in the solid component of lesions (contrast-enhancing component on CT, or the component with a density higher than 20 HU on non-contrast CT). Additionally, blooming artifact from physiological uptake in the adjacent normal renal parenchyma should be differentiated from uptake in the lesion, which may result in a false-positive characterization. This is potentially relevant in very small endophytic lesions and can result in an indeterminate scan.
A “completely characterized renal mass” is defined as a mass with imaging features diagnostic for a specific lesion, such as macroscopic fat in an AML. It may also refer to imaging features allowing a risk assessment and subsequent management recommendation, such as the Bosniak classification for renal cysts.23,24 Full characterization of solid renal masses by imaging studies has always been challenging, as there are no specific diagnostic imaging features for malignant lesions. As a result, there has been a need for a molecular imaging technique that can identify low-risk or indolent renal masses and subsequently assist with risk stratification of renal lesions.8 It appears that MIBI uptake is an imaging feature in low-risk renal lesions that aids in the diagnostic workup.
A large study by O’Connor et al reviewing the imaging features of renal masses on non-contrast CT, demonstrated that all lesions with a homogenous density <20 and >70 HU were benign and no further workup was needed.25 This is currently an established criterion and was added to American College of Radiology (ACR) appropriateness criteria. Non-contrast CT is an important part of the renal mass imaging protocol and can demonstrate fat that could be obscured after IV contrast administration.25 These features highlight the value of the non-contrast CT part of the MIBI SPECT-CT study.
In our study, all renal masses had a density of 20–70 HU, which is expected given the selection of lesions with solid components. Thus, all cases were in an indeterminate range of density for risk stratification (benign vs. malignant).
Currently, most “incompletely characterized renal masses” detected incidentally on cross-sectional imaging are referred for detailed evaluation with multiphasic CT or MRI. Presuming that MIBI becomes an established tool in the evaluation of incompletely characterized renal masses, then both the SPECT and CT components of scan have the potential to be used in the characterization and risk stratification of renal masses, as detailed in Fig. 3. As a result, the need for renal mass evaluation with multiphasic CT or costly MRI studies could potentially be curtailed.
Fig. 3.
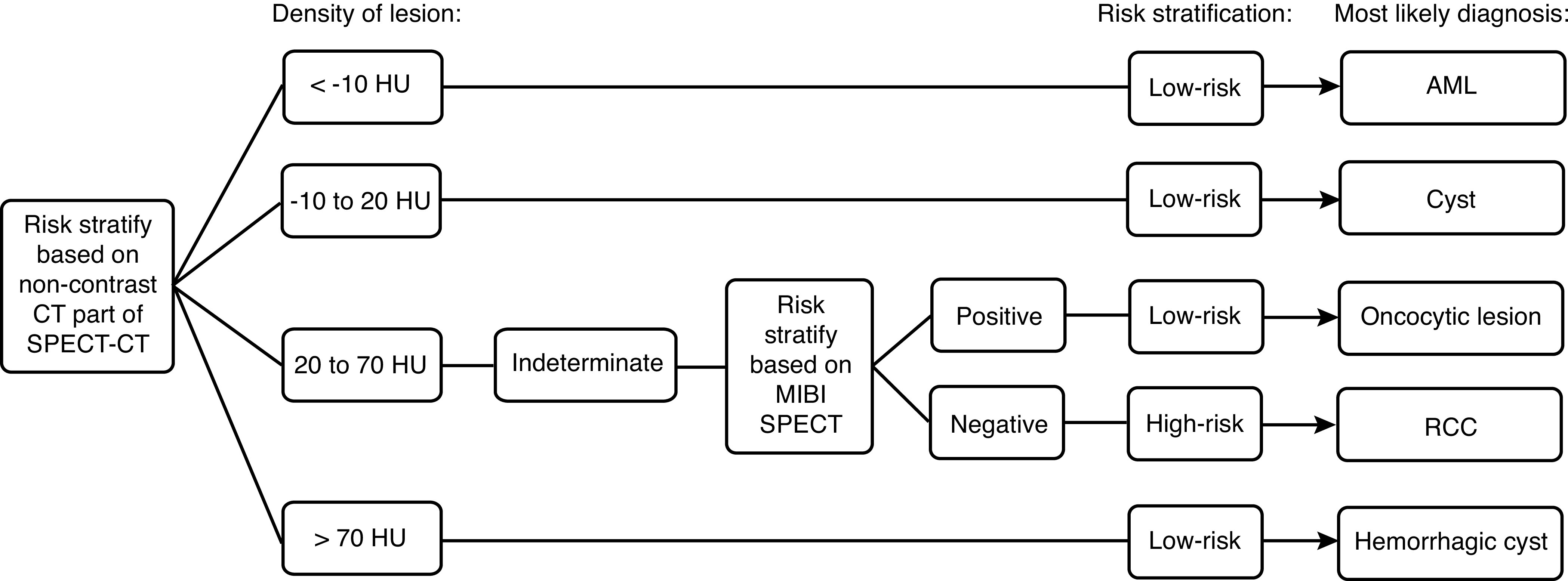
Risk stratification of renal masses by 99mTc-sestamibi (MIBI) single-photon emission computed tomography-computed tomography (SPECT-CT). AML: angiomyolipoma; HU: Hounsfield units; RCC: renal cell carcinoma.
Limitations of this study are the small sample size and limited number of less frequent pathologies, such as HOCT and chromophobe RCCs. Also, this study was neither prospective nor blinded.
Conclusions
Our results demonstrate that MIBI SPECT-CT improves the characterization and risk stratification of renal masses with the potential to replace or reduce the need for invasive biopsy in differentiating benign from malignant renal lesions. Also, MIBI SPECT-CT scan can help to avoid unnecessary renal mass resection and increase the number of patients offered active surveillance as the primary management for their renal mass. Further studies are required to confirm that positive MIBI is a diagnostic imaging feature for low-risk renal lesions.
Footnotes
Competing interests: Dr. Romsa has particpiared in clinical trials supported by Endocyte and Progenics. Dr. Pautler has been a paid procter in robotic surgery for Minogue Medical. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.De P, Otterstatter MC, Semenciw R, et al. Trends in incidence, mortality, and survival for kidney cancer in Canada, 1986–2007. Cancer Causes Control. 2014;25:1271–81. doi: 10.1007/s10552-014-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner RM, 2nd, Morgan TM, Jacobs BL. Epidemiology of the small renal mass and the treatment disconnect phenomenon. Urol Clin North Am. 2017;44:147–54. doi: 10.1016/j.ucl.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: An analysis of pathological features related to tumor size. J Urol. 2003;170:2217–20. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 4.Ishigami K, Jones AR, Dahmoush L, et al. Imaging spectrum of renal oncocytomas: A pictorial review with pathologic correlation. Insights Imaging. 2015;6:53–64. doi: 10.1007/s13244-014-0373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo S, Cho JY. Imaging findings of common benign renal tumors in the era of small renal masses: Differential diagnosis from small renal cell carcinoma: Current status and future perspectives. Korean J Radiol. 2015;16:99–113. doi: 10.3348/kjr.2015.16.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginzburg S, Uzzo R, Al-Saleem T, et al. Coexisting hybrid malignancy in a solitary sporadic solid benign renal mass: Implications for treating patients following renal biopsy. J Urol. 2014;191:296–300. doi: 10.1016/j.juro.2013.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewett MA, Zuniga A. Renal tumor natural history: The rationale and role for active surveillance. Urol Clin North Am. 2008;35:627–34. doi: 10.1016/j.ucl.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Esserman LJ, Thompson IM, Jr, Reid B. Overdiagnosis and overtreatment in cancer: An opportunity for improvement. JAMA. 2013;310:797–8. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 9.Bauman TM, Potretzke AM, Wright AJ, et al. Partial nephrectomy for presumed renal cell carcinoma: Incidence, predictors, and perioperative outcomes of benign lesions. J Endourol. 2017;31:412–7. doi: 10.1089/end.2016.0667. [DOI] [PubMed] [Google Scholar]
- 10.Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, non-diagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60:578–84. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Richard PO, Jewett MA, Bhatt JR, et al. Renal tumor biopsy for small renal masses: A single-center, 13-year experience. Eur Urol. 2015;68:1007–13. doi: 10.1016/j.eururo.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–9. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 13.Gormley TS, Van Every MJ, Moreno AJ. Renal oncocytoma: Preoperative diagnosis using technetium 99m sestamibi imaging. Urology. 1996;48:33–9. doi: 10.1016/S0090-4295(96)00095-7. [DOI] [PubMed] [Google Scholar]
- 14.Maffioli L, Steens J, Pauwels E, et al. Applications of 99mTc-sestamibi in oncology. Tumori. 1996;82:12–21. doi: 10.1177/030089169608200103. [DOI] [PubMed] [Google Scholar]
- 15.Scopinaro F, Schillaci O, Scarpini M, et al. Technetium-99m sestamibi: An indicator of breast cancer invasiveness. Eur J Nucl Med. 1994;21:984–7. doi: 10.1007/BF00238124. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan B, Truong LD. Renal epithelial neoplasms: The diagnostic implications of electron microscopic study in 55 cases. Hum Pathol. 2002;33:68–79. doi: 10.1053/hupa.2002.30210. [DOI] [PubMed] [Google Scholar]
- 17.Gorin MA, Rowe SP, Baras AS, et al. Prospective evaluation of (99m)Tc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. Eur Urol. 2016;69:413–6. doi: 10.1016/j.eururo.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 18.Tzortzakakis A, Gustafsson O, Karlsson M, et al. Visual evaluation and differentiation of renal oncocytomas from renal cell carcinomas by means of (99m)Tc-sestamibi SPECT/CT. EJNMMI Res. 2017;7:29. doi: 10.1186/s13550-017-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Cheng C, Dong A, et al. Prospective evaluation of 99mTc-MIBI SPECT/CT for the diagnosis of solid renal tumors. J Nucl Med. 2019;60:1572. [Google Scholar]
- 20.Campbell SP, Tzortzakakis A, Javadi MS, et al. (99m)Tc-sestamibi SPECT/CT for the characterization of renal masses: A pictorial guide. Br J Radiol. 2018;91:20170526. doi: 10.1259/bjr.20170526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson MP, Katlariwala P, Murad MH, et al. Diagnostic accuracy of 99mTc-sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: A systematic review and meta-analysis. Abdom Radiol (NY) 2020;45:2532–41. doi: 10.1007/s00261-020-02469-8. [DOI] [PubMed] [Google Scholar]
- 22.Volpe A, Novara G, Antonelli A, et al. Chromophobe renal cell carcinoma (RCC): Oncological outcomes and prognostic factors in a large, multicenter series. BJU Int. 2012;110:76–83. doi: 10.1111/j.1464-410X.2011.10690.x. [DOI] [PubMed] [Google Scholar]
- 23.Israel GM, Bosniak MA. How I do it: Evaluating renal masses. Radiology. 2005;236:441–50. doi: 10.1148/radiol.2362040218. [DOI] [PubMed] [Google Scholar]
- 24.Silverman SG, Israel GM, Trinh QD. Incompletely characterized incidental renal masses: Emerging data support conservative management. Radiology. 2015;275:28–42. doi: 10.1148/radiol.14141144. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor SD, Pickhardt PJ, Kim DH, et al. Incidental finding of renal masses at unenhanced CT: Prevalence and analysis of features for guiding management. AJR Am J Roentgenol. 2011;197:139–45. doi: 10.2214/AJR.10.5920. [DOI] [PubMed] [Google Scholar]