Abstract
Introduction
Rates of testosterone therapy (TT) prescribing dropped dramatically following the U.S. Food and Drug Administration and Health Canada warning regarding potential cardiovascular morbidity in 2014. Since then, prescription rates appear to be increasing in the U.S., however, data on TT use in Canada is lacking. Current database studies suffer from incomplete prescription capture, lack of information on continued use, and confounding from concurrent population growth. Nova Scotia (NS) is a Canadian province with minimal population growth over the past decade. NS tracks every testosterone prescription and refill through their prescription monitoring program (NSPMP). All testosterone prescriptions must be written on triplicate forms, allowing for comprehensive tracking. The purpose of this study is to describe the long-term prescription trends of testosterone in a mid-sized Canadian province using a database that captures 100% of all TT prescriptions written and filled.
Methods
Data were extracted from the NSPMP database on all prescriptions and prescription refills of androgens for men over 18 years of age from 2007–2019. Population statistics were gained using publicly available data from Statistics Canada. Analysis of patterns on individual years and over time were examined for number of patients, prescriptions, and prescribers, as well as formulation.
Results
The male population of Nova Scotia remained relatively stable throughout the study period (2007: 455 064; 2019: 475 478; population increase of 4.3%). A total of 7883 men (1.7% of the male population) received a prescription for TT during the study period; 1673 men received only one prescription in the entire study period and 5446 men remained on TT for longer than six months. Of the 1730 men under 45 who were prescribed TT, 75% (n=1298) of them stayed on it for more than six months; 1856 men (24%) switched the type of testosterone they were on during the study period. The number of men receiving TT yearly increased by 98%, from 1235 in 2007 to 2448 in 2019. The number of men receiving TT plateaued in 2014, except for men under age 35, in whom it has steadily increased every year since 2007. Interestingly, primary care providers (PCPs) wrote 92% of all prescriptions, on average (interquartile range 90–93).
Conclusions
In a mid-sized Canadian province with stable population growth, prescriptions of testosterone increased until 2014, and then either stabilized or decreased. TT prescriptions in young men have continued to increase yearly. Injectable and gel-based formulations have increased in popularity over the past decade. Future efforts to educate prescribers, especially surrounding the effects on fertility in young men, should be largely focused on PCPs.
Introduction
Global testosterone therapy (TT) trends have been increasing over the past decade, with a change in prescription trends seen in 2014,1 likely in response to warnings by the U.S. Food and Drug Administration (FDA) and Health Canada suggesting an increased cardiovascular risk associated with TT.2,3
Understand and evaluating prescriber patterns of TT is difficult for many reasons. Many studies that evaluate prescription patterns of TT are confounded by concurrent population growth. Furthermore, many prescription databases do not capture all prescriptions made, as they rely on insurance claims, and thus will not capture prescriptions from men on Medicare, those who are uninsured, or who decide to pay privately.4 It is important to accurately capture prescription trends for many reasons: as a marker of disease prevalence; to focus educational efforts on patients and prescribers, and to track the impact of public health warnings.
Within the province of Nova Scotia (NS), Canada, testosterone is a controlled drug under the Controlled Drugs and Substances Act. Every prescription for TT is collected and stored in a database by the Nova Scotia Prescription Drug Monitoring Program (NSPMP), which was established in 2005.5 The population of NS has remained relatively stable; between 2007 and 2019 the population has increased by an average of 0.3% per year.6 This represents a unique opportunity where an entire population can be reliably captured and studied.
We aim to characterize the rates of testosterone prescription over time and to further characterize prescribing patterns within NS.
Methods
This study was approved by the Dalhousie University Institutional Review Board. Testosterone prescription data was collected from the NSPMP. All prescriptions of TT were collected on individuals 18 years and older from 2007–2019. Parameters collected included: age, gender, type of prescriber (stratified by primary care provider [PCP] and non-PCP), formulation, and dose. Data was collected on prescription refills and attrition.
The rates of patients with only one prescription, short duration (<6 months), and who switched formulations were reported. We calculated duration of use using the date of the first prescription fill and the end date based on the last prescription fill date, plus the total days’ supply for the last prescription. For injectable formulation, 100 mg was estimated to equal a one-week supply. A dropout was indicated if there was no new prescription 24 months following the last prescription.
Population statistics were gained using publicly available data from Statistics Canada.6 Descriptive statistics were calculated for all collected data.
Results
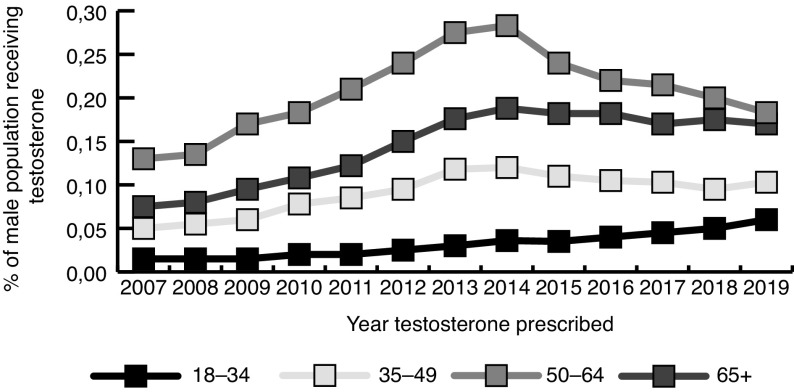
The male population of NS remained relatively stable throughout the study period (2007: 455 064; 2019: 475 478; population increase of 4.3%). A total of 7883 men (1.7% of the male population) received a prescription for TT during the study period; 1673 men received only one prescription in the entire study period and 5446 men remained on TT for longer than six months. Of the 1730 men under 45 years who were prescribed TT, 75% (n=1298) of them stayed on it for more than six months. Of the 6153 men 45 years and over who were prescribed TT, 67% (n=4148) stayed on it for more than six months. A total of 1856 men (24%) switched the type of testosterone they were on during the study period. Separated by decile, men aged 55–64 received the most prescriptions for TT (n=2404, 30%); 22% of men receiving TT were under age 45 years at the time of first prescription (Fig. 1).
Fig. 1.
Testosterone prescriptions as a percentage of the population, separated by age decile.
The number of men receiving TT yearly increased by 98%, from 1235 in 2007 to 2448 in 2019. Among all men, the number prescribed TT increased yearly from 2007 until 2014 (2863 men), at which point TT rates plateaued or decreased, depending on the age group. The only exception to this trend was in men aged 18–34, in whom TT rates have increased every year since 2007 (81 in 2007; 281 in 2019).
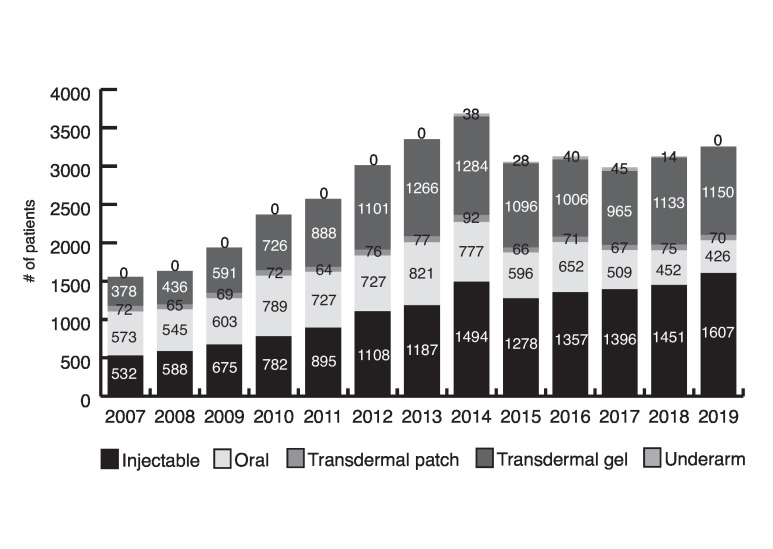
There has been a steady decrease in oral formulations prescribed since 2007 (36% in 2007; 13% in 2019), with an increase in injectable esters (34% to 49%) and transdermal gels (24% to 35%) (Fig. 2). All oral prescriptions were for testosterone undecanoate. Interestingly, PCPs wrote 92% of all prescriptions, on average (interquartile range [IQR] 90–93).
Fig. 2.
Testosterone formulations prescribed, stratified by year.
Discussion
We present comprehensive data on testosterone prescribing trends among a stable Canadian population of men over 12 years. This database captures all testosterone prescriptions filled in the province of NS.
This study reveals several interesting findings. First, nearly a quarter of men received only a single testosterone prescription in the study period, and another quarter switched to another testosterone formulation at least once after their initial prescription. Much less testosterone is prescribed in NS compared to published U.S. data (0.6% vs. 3.2% of the population in 2013, for example).4 Whether this represents underuse of TT/lack of awareness of androgen deficiency, or instead is a result of overuse of testosterone in the U.S. is difficult to know. Furthermore, the vast majority of prescribers in NS are PCPs — much more than the 60% PCP prescriber rate reported in U.S. data.7 Because of this, education efforts in NS regarding risks and benefits of TT should be largely focused among this prescriber group. These data reveal a relatively young cohort of men taking TT (50% of TT users are under 55 years). Healthcare professionals and their patients are often unaware that exogenous testosterone will result in infertility.8 Some men will never recover spermatogenesis after cessation of testosterone, and in those that do, it may take up to two years to recover sperm in the ejaculate.9,10 These data highlight the importance of fertility counselling in these men prior to starting on TT.
Limitations
This study has limitations. First, TT tracking ends with the prescription being filled, and compliance cannot be tracked. Our calculation of duration of therapy may be inaccurate, as it is calculated based on standard dose estimates. Finally, we have no information on the diagnostic evaluation prior to prescription. Regardless, the manner in which TT is tracked in NS allows for one of the more robust measures of testosterone use available in the literature.
Conclusions
Approximately 0.5% of the male population of a mid-sized Canadian province is prescribed testosterone on an annual basis, of which most prescribers are PCPs. Prescription trends in NS mirror published prescription trends. With more young men using TT, and the cardiovascular impacts of TT remaining uncertain, it is important to continue to track TT prescription trends. Targeted education regarding the risks and benefits of TT should be focused primarily on PCPs. Future consideration should be given to establishing a national database that is linked to health outcomes.
Acknowledgements
The authors would like to acknowledge the indispensable efforts of Kristin Crabtree and Lisa Green at the Nova Scotia Prescription Monitoring Program, without whom we would not have been able to compile such exhaustive and detailed data.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Handelsman DJ. Global trends in testosterone prescribing, 2000–2011: Expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199:548–51. doi: 10.5694/mja13.10111. [DOI] [PubMed] [Google Scholar]
- 2.Research C for DE and Drug Safety and Availability – FDA Drug Safety Communication: FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. [Accessed July 18, 2020]. Available at: https://wayback.archive-it.org/7993/20170112031612/http://www.fda.gov/Drugs/DrugSafety/ucm383904.htm.
- 3.Government of Canada. Information Update – Possible cardiovascular problems associated with testosterone products. [Accessed July 18, 2020]. Available at: https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2014/40587a-eng.php.
- 4.Baillargeon J, Kuo Y-F, Westra JR, et al. Testosterone prescribing in the United States, 2002–2016. JAMA. 2018;320:200–2. doi: 10.1001/jama.2018.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NSPMP. 2020. [Accessed July 18, 2020]. Available at: http://www.nspmp.ca/
- 6.Statistics Canada. Table 17.10-0009-01. Population estimates, Quarterly [Google Scholar]
- 7.Garnick MB. Testosterone replacement therapy faces FDA scrutiny. JAMA. 2015;313:563–4. doi: 10.1001/jama.2014.17334. [DOI] [PubMed] [Google Scholar]
- 8.Crosnoe LE, Grober E, Ohl D, et al. Exogenous testosterone: A preventable cause of male infertility. Transl Androl Urol. 2013;2:106–13. doi: 10.3978/j.issn.2223-4683.2013.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenker EP, Dupree JM, Langille GM, et al. The use of HCG-based combination therapy for recovery of spermatogenesis after testosterone use. J Sex Med. 2015;12:1334–7. doi: 10.1111/jsm.12890. [DOI] [PubMed] [Google Scholar]
- 10.Liu PY, Swerdloff RS, Anawalt BD, et al. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab. 2008;93:1774–83. doi: 10.1210/jc.2007-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]