Abstract
Introduction
Conventional imaging (CI) performs poorly to identify sites of disease in biochemically recurrent prostate cancer. 68Ga-PSMA-11 positron emission tomography/computed tomography (PET/CT) is most studied but has a very short half-life. This study reports the diagnostic performance of the novel prostate-specific membrane antigen (PSMA) radiotracer 18F-DCFPyL using real-life data and tumor board simulation to estimate the impact of 18F-DCFPyL PET on patient management.
Methods
Ninety-three 18F-DCFPyL PET/CT scans performed for patients previously treated for prostate cancer with a rising prostate-specific antigen (PSA) were retrospectively compared to contemporary CI and clinical imaging and PSA followups. A chart review was performed to document prior imaging, pathology results, serial serum PSA measurements, and other pertinent clinical data. Clinical utility of 18F-DCFPyL PET was measured using a simulated tumor board formed by three physicians with extensive prostate cancer experience deciding on management with and without knowledge of PET/CT results.
Results
At median PSA 2.27 (interquartile rage [IQR] 5.27], 82% of 18F-DCFPyL PET/CT demonstrated at least one site of disease: non-regional lymph nodes (37% of scans), regional lymph node metastases (28%), local recurrence (27%), and bone metastases (20%), with higher PET positivity at higher PSA. Compared to 18F-DCFPyL PET/CT, CI showed overall poor performance, with accuracy below 20% for all extent of disease. PET/CT changed management in 44% of cases. The most frequent scenario was a radical change from initiating androgen deprivation therapy (ADT) to stereotactic body radiotherapy (SBRT) of oligo-lesional disease. In univariate and multivariate analysis, no patient characteristic could predict change of management by PET/CT results.
Conclusions
18F-DCFPyL significantly outperforms CI in recurring prostate cancer and is likely to impact management.
Introduction
Mortality related to prostate cancer is high, nearing one death per four men diagnosed with the disease.1 Recurrences after definitive therapy and the presence of distant metastases are known drivers of lethality.2
Conventional imaging (CI), such as magnetic resonance imaging (MRI) or computed tomography (CT) of the abdomen and pelvis and whole-body bone scan (99mTc-MDP scintigraphy) shows poor diagnostic performance for the most common manifestations of prostate cancer outside the prostate bed, with roughly 40% sensitivity for lymph node metastases3 and numerous bone scan false-positives (including fractures, degenerative change, and a number of benign bone conditions). Moreover, CI in early hormone-sensitive biochemical recurrence, where prostate-specific antigen (PSA) levels are below 1 ng/L, is almost invariably negative, bringing into question this indication altogether. On the other hand, prostate-specific membrane antigen (PSMA) positron emission tomography (PET) finds and distinguishes local from metastatic recurrences. Additionally, PSMA PET provides whole-body evaluation of the disease, better estimation of volumes for targeted or salvage radiotherapy, and allows selection of patients for PSMA radioligand therapy (PSMA RLT).4–6
Among the many PSMA-targeting small molecule radioligands, 68Ga-PSMA-11 compound is most studied in the context of biochemical recurrence.7,8 However, the fluorine-18 labeled PSMA ligands, such as 18F-DCFPyL, have advantages over 68Ga-PSMA-11, including a longer half-life of 110 minutes (vs. 68 minutes), which allows for more flexible scheduling of patients and more delayed acquisitions — the latter facilitating tumor visualization.9 Furthermore, fluorine-18 cyclotron production is already broadly implemented and does not suffer from the logistical complexity and currently very limited production of the generator-produced gallium-68.
The current study is an analysis of data from prospectively enrolled prostate cancer patients in the setting of biochemical recurrence after at least one line of therapy. Our aim is to report the diagnostic performance of the novel PSMA radiotracer 18F-DCFPyL using real-life experience and clinical data in a large Canadian cohort, as well as an estimation of the impact of 18F-DCFPyL PET on patient management.
Methods
Study design
This study was approved by our institutional research ethics committee. Patients were consented for imaging and data use as part of a different (prospectively enrolling) clinical trial (NCT03459820). Ninety-three patients matching our criteria were imaged between July 2017 and October 2018 using 18F-DCFPyL according to our local PET/CT protocol: 9±1 mCi (333±37 MBq) of 18F-DCFPyL is injected intravenously. Approximately 60–90 minutes following 18F-DCFPyL injection, CT and PET images are consecutively acquired from the base of the skull to the toes on the hybrid PET/CT scanner (Discovery ST, General Electric Medical Systems, Waukesha, WI, U.S.). Furosemide 20 mg IV was injected at the time of radiotracer injection when not contraindicated. The selected cohort represent all comers with a clinical need for PSMA PET following any prior prostate cancer therapy. Inclusion criteria were pathology-proven prostate cancer treated with any therapy, rising PSA with negative or equivocal CI. Patients were excluded from analysis if followup data was not available.
Validation
Positive lesions were classified based on their characteristics on 18F-DCFPyL PET/CT by experienced readers during clinical interpretation of the scan. When available, results were compared to contemporaneous CI, followup CI, followup 18F-DCFPyL, histopathology, or PSA response to radiation therapy (RT).
A chart review was performed to document prior imaging, pathology results, serial serum PSA measurements, and other pertinent clinical data.
Comparison with other imaging modality
Any CT, MRI, or bone scan performed within six months of the 18F-DCFPyL PET/CT study was included for comparison purposes. When available, the clinical report was used as the definitive result of the performed studies.
Because of the reported high specificity and high positive predictive value of 18F-DCFPyL PET, whenever CI identified a prostate cancer lesion that was not 18F-DCFPyL-avid in a patient for whom the remainder of the disease was otherwise 18F-DCFPyL-positive, this was considered a CI false-positive; 18F-DCFPyL PET findings in excess of CI were considered CI false-negative.
For local disease in the prostate or prostate bed, 18F-DCFPyL PET was compared to MRI. Regional and nonregional nodal disease were analyzed two ways: those with available CI had their 18F-DCFPyL PET results compared to the lymph nodes identified on the clinical report, on a region-based analysis; for those without CI, the findings on PET/CT were used as a surrogate, such that 18F-DCFPyL-positive lymph nodes were measured on the CT portion of the PET/CT and lymph node regions (regional pelvic chains and non-regional chains) were recorded as “equal or larger than 1 cm,” “smaller than 1 cm,” or both (simulated CI). All patients with at least one lymph node “equal or larger than 1 cm” were considered to have CT-detectable lymph nodes (true-positive). Patients with PSMA-positive lymph nodes smaller than 1 cm were considered CI false-negative.
Distant visceral or osseous metastases found on PET were compared to all other modalities available for the appropriate anatomical location. Bone metastases were considered as a whole, that is, globally positive or negative.
Accuracy was calculated as the sum of true-positives and true-negatives over the total of true-positives, true-negatives, false-positives, and false-negatives for CI and simulated CI per site of disease (local, regional, non-regional, bone, others) and using PET results as the reference standard.
Change of management
To account for the clinical utility of 18F-DCFPyL PET to guide management planning, a simulated tumor board was formed by three physicians with extensive prostate cancer experience: a urologist, a radiation oncologist, and a nuclear medicine physician. The tumor board was initially presented only with the clinical information, laboratory, and imaging findings prior to the 18F-DCFPyL PET. A decision of the best therapeutic recommendation was made by consensus. Then, 18F-DCFPyL PET results were revealed, and a new treatment plan was discussed and again approved by consensus. These discussions reflect our current intuitional clinical standard of care.
Statistical analysis
A univariate and multivariate analyses were performed to compare the distribution of population variables (age, PSA at time of PET, Gleason score, time since diagnosis, prior use of androgen deprivation therapy [ADT], current ADT, prior lines of therapy) and convention imaging results (overall, local recurrence, regional nodal disease, distant nodal disease, bone metastases, other visceral metastases) to the findings on 18F-DCFPyL PET. The p-values correlate to a Student t-test for numeric variables and to a Pearson’s Chi-squared test for categorical variables, using a level of significance α=0.05 (5%).
Results
Studied cohort
Patient characteristics can be found in Table 1. The mean PSA level at the time of PET imaging was 4.6 ng/L; median PSA was 2.27 (0.07–51.09, interquartile range [IQR] 5.27). Mean age was 70.4 years; median Gleason score was 7 (5–10, IQR 1). Forty-four patients were previously treated with ADT — either concomitant with initial therapy or in the context of biochemical recurrence; 49 were not. On average, 6.6 years elapsed since initial cancer diagnosis. Seventeen patients were on ADT at the time of PET scanning.
Table 1.
Patient characteristics
| Age (years) (n=93) | ||
| Minimum | 51 | |
| Mean | 70.4 | |
| Maximum | 87 | |
| PSA (ng/ml) at time of PET (n=93) | ||
| Minimum | 0.07 | |
| Median | 2.27 | |
| Mean | 4.57 | |
| Maximum | 51.09 | |
| Gleason score (n=73) | ||
| Minimum | 5 | |
| Median | 7 | |
| Maximum | 10 | |
| Prior therapy (n=93) | ||
| Radiotherapy | 25 (27%) | |
| Prostatectomy | 28 (30%) | |
| Prostatectomy + | 34 (37%) | |
| radiotherapy | ||
| Other | 7 (8%) | |
| Prior ADT (n=93) | ||
| Yes | 44 (47%) | |
| No | 49 (53%) | |
| Time elapsed between PET and initial therapy (years) (n=83) | ||
| Minimum | 0 | |
| Mean | 6.6 | |
| Maximum | 22 | |
| Median | 5 | |
|
| ||
| Available conventional imaging (within 6 months of PET) | Mean time between conventional imaging and DCFPyL PET (days) | |
|
| ||
| CT abdomen and pelvis | 32 (34%) | 55 |
| MRI pelvis | 9 (10%) | 99 |
| CT chest | 21 (23%) | 46 |
| Bone scan | 30 (32%) | 53 |
ADT: androgen deprivation therapy; CT: computed tomography; MRI: magnetic resonance imaging; PET: positron emission tomography; PSA: prostate-specific antigen.
PSMA PET and CI results
Of the 93 18F-DCFPyL PET scans, 76 (82%) demonstrated at least one site of disease. Non-regional lymph nodes were the most frequent finding (37% of scans), followed by regional lymph node metastases (28%), local recurrence (27%), and bone metastases (20%) (Table 2). The most frequent site of visceral metastases was the lungs (5% of scans). No liver metastases were detected in this series.
Table 2.
DCFPyL PET results
| Overall result | |
| Positive | 76 (82%) |
| Negative | 17 (18%) |
| Local disease | |
| Positive | 25 (27%) |
| Negative | 68 (73%) |
| Regional lymph nodes | |
| Positive | 26 (28%) |
| Negative | 67 (72%) |
| Non-regional lymph nodes | |
| Positive | 34 (37%) |
| Negative | 59 (63%) |
| Bone metastases | |
| Positive | 19 (20%) |
| Negative | 74 (80%) |
| Visceral metastases | |
| Positive | 7 (8%) |
| Negative | 86 (92%) |
PET: positron emission tomography.
When comparing initial curative-intent therapeutic strategies, 6/28 (21%) patients recurred in the prostate bed after radical prostatectomy (RP) compared with 13/25 (52%) patients whose primary treatment was RT. Combined prior RP and RT led to only 2/34 (6%) patients with local recurrence on PSMA PET. Patients whose initial therapy was high-intensity focused ultrasound (HIFU) (two patients) and cryotherapy (one patient), all recurred locally.
Accuracy of CI
Using PSMA PET as the gold standard, CI showed overall poor performance, with accuracy below 20% for all extent of disease (Table 3).
Table 3.
Composite accuracy of conventional imaging (n=36)
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Local disease | 30% | 100% | 100% | 79% | 8% |
| Regional lymph nodes | 36% | 96% | 80% | 77% | 14% |
| Non-regional lymph nodes | 22% | 83% | 57% | 52% | 19% |
| Bone metastases | 50% | 89% | 56% | 87% | 19% |
| Other distant metastases | 33% | 94% | 33% | 94% | 8% |
| Accuracy of low-dose CT (simulated CI) (n=57) | |||||
| Regional lymph nodes | 27% | 100% | 100% | 79% | 7% |
| Non-regional lymph nodes | 31% | 100% | 100% | 79% | 9% |
CI: conventional imaging; CT: computed tomography; NPV: negative predictive value; PPV: positive predictive value.
In 36 patients with available CT or MRI of the abdomen and pelvis, CI found 3/10 (30%) local recurrences, 4/11 (36%) regional lymph nodes, 7/21 (33%) non-regional lymph nodes. In 47 patients with a bone scan, MRI, or CT, bone or other distant metastases were found 6/13 (46%) times.
CI was falsely positive in 10 patients (11%) in various anatomical locations (one local node, three non-regional nodes, four in bones, two in lungs).
Accuracy of the simulated CI
Using a 1 cm short-axis threshold on the CT of the PET/CT study for PSMA-detected regional and non-regional lymph nodes, results of positive nodal disease were populated for patients without available anatomical imaging (dedicated contrast-enhanced MRI or CT). Sensitivity and accuracy were 27% and 7% for regional lymph nodes and 31% and 9% for non-regional lymph nodes, respectively (Table 4). Because the detection criteria (positive 18F-DCFPyL PET) was used as the gold standard, specificity/positive predictive values are 100%, by definition.
Change of management
Of the 93 18F-DCFPyL PET/CT performed, 41 (44%) led to a change in management. The most frequent scenario was a radical change from initiating ADT to stereotactic body radiotherapy (SBRT) of oligo-lesional disease. Overall, PSMA PET resulted in therapy intensification in eight (9%) cases, reduced interventions in seven (8%) cases, and completely changed management in 26 (28%) cases. In other terms, the number needed to scan to change management in one was 2.3 patients.
Multivariate analysis
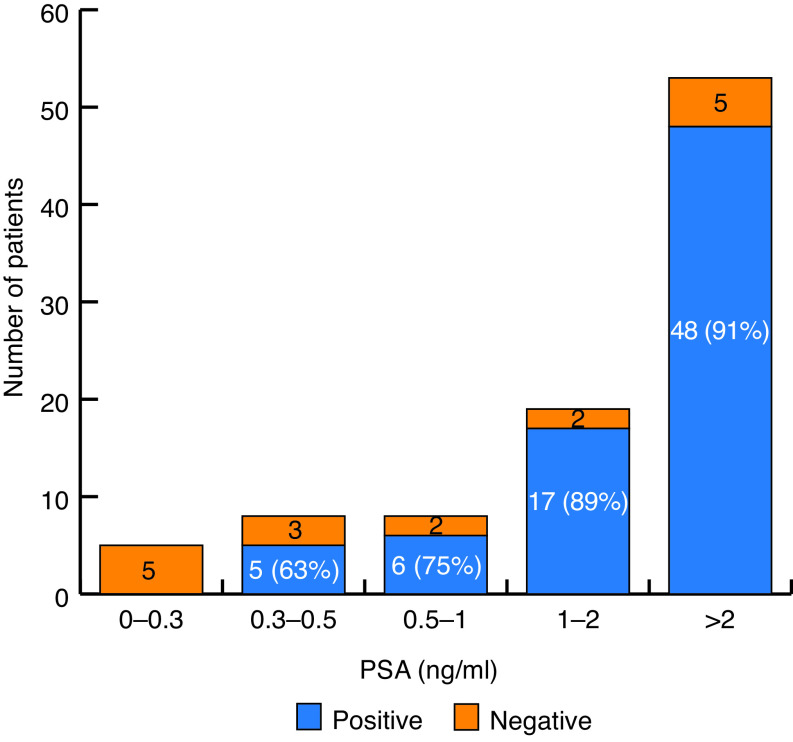
In the univariate analysis, PSA at time of PET was significantly positively associated with a positive overall PSMA PET (p=0.014), a finding that persisted after correcting for patients’ characteristics, with an odds ratio (OR) of 2.06 (1.02–4.17) (Fig. 1). No other factors, including Gleason score, time since diagnosis, prior ADT use, or prior means of therapy, could predict a positive scan.
Fig. 1.
18F-DCFPyL positivity per prostate-specific antigen (PSA) level.
However, when looking at specific sites of recurrence on PSMA PET, local recurrence varied significantly from one initial therapy modality to another. Compared to RT, RP and combination RT + RP both led to significantly fewer local recurrences (OR 0.12 [0.02–0.78] and OR 0.07 [0.01–0.59], respectively). Age and PSA at time of PET were both associated with increased risk of non-regional nodal recurrence whereas Gleason score, once corrected for other factors, was not. Of note, none of the above-mentioned characteristics were found to be related to other sites of recurrences (regional nodal disease, bones, and other visceral metastasis), nor predicted a change of management.
Discussion
Our overall detection rate of 82% with 18F-DCFPyL PET closely mirrors the rate of 85.5% in the current literature with 68Ga-PSMA-11, as reported by Grubmüller et al.8 Our clinical experience with both these radiotracers is also congruent with that result; we prefer the flexible, easy-to-use fluorine-18-labelled compound for day-to-day routine. Also, the injected activity of fluorine-18-labelled tracer is higher, resulting in higher picture quality and reduced image noise. It remains to be seen whether the improved image quality allows easier lesion identification and translates into higher interobserver reproducibility.
CI or its substitution by the low-dose CT of the PET/CT study both showed unacceptably low performance for clinical use in this patient cohort. Although specificity of CI for nodal disease was very high (100% for regional nodes), bone metastasis detection with Tc-99m-methylene diphosphonate whole-body bone scan showed a sensitivity of only 50%. The overall accuracy of CI was extremely low (8–19%), exposing the limit of CI in biochemical recurrence of prostate cancer when very low volume of disease is expected.
Our tumor board simulation provided insight into the potential of 18F-DCFPyL PET to both improve patient care and avoid futile therapy. Forty-four percent of scans led to a change in management, most often leading to an attempt at salvage SBRT for oligometastatic disease, for which our group has extensive experience and several ongoing clinical trials. A similar number of patients (8–9%) have seen their treatment intensified when more extensive disease was discovered and reduced when presumed disease sites were ruled out. Calais et al reported 53% change of management, a higher value that could be explained by differing inclusion criteria, technological differences between PET scanners used, and different distribution of serum PSA values (0.05–202 ng/mL) as compared to ours (0.07–51.09 ng/mL). Local clinical practice and availability of treatment options are also likely to play a role in explaining this difference.7 Rousseau et al10 also reported a higher impact on treatment (65.5%) and up to 87.3% change of management plans; more stringent patient selection with higher PSA values may again explain the difference. Nevertheless, the number needed to scan to change management is very low at 2.3 patients, making PSMA PET a powerful tool to advance quality of care, with the most frequent improvement being a transition from palliative systemic therapy to curative-intent, PSMA-guided, lesion-directed therapy. More studies with long-term followup will be needed to confirm whether this strategy improves overall survival (OS) and quality of life and decreases prostate cancer-related morbidity.
Based on the multivariate statistical analysis, comparison of patients’ characteristics and PET results revealed an expected positive correlation between the PSA level and the likelihood of finding a lesion on PSMA PET. This was already described in the initial PSMA PET literature and correlates well with our clinical experience.11 Interestingly, PSMA PET impacted change of management at PSA levels up to 19.48 ng/mL, the second highest value in this cohort. Therefore, this study cannot confirm a PSA threshold beyond which PSMA PET is no longer recommended.
Contrary to most cited literature on the initial treatment of prostate cancer, we found statistically significant variation in local recurrences among groups with different initial therapies. Whereas the ProtecT study12 reported same rates of disease progression among RP- and RT-treated patients, our study identified more local recurrences in RT-only-treated patients. The discrepancy likely arises from the technological superiority of PSMA PET, as the ProtecT study happened prior to the PSMA PET era and, as also pointed out by our comparison with CI, MRI and CT perform poorly for local recurrences (sensitivity 30% and accuracy 8%). This higher rate of CI-undetectable disease may not have impacted the ProtecT 10-year OS but could alter clinical management, especially when patients’ life expectancy exceeds 10 years.
Limitations
Using 18F-DCFPyL PET as the gold standard would miss the putative approximately 5–8% of cancers in which PSMA expression is weak. We partly mitigated this by assuming CI was false-positive only for PSMA-negative lesions in patients with at least one site of PSMA-avid disease. Intra-patient heterogeneity with PSMA-positive and PSMA-negative lesions has been reported in de-differentiation in very advanced, extensive metastatic disease13 and in liver metastases,14 both of which were not present in our cohort. Therefore, our most likely mistake would be a false-positive PSMA PET from another disease known to accumulate PSMA radioligands, such as renal cell carcinoma, certain lung cancers, or benign conditions (such as fibrous dysplasia, thyroid cancers, etc.).15 These phenomena are relatively rare, not identified in our cohort, and unlikely to significantly impact our results.
In retrospect, PSA doubling time and the knowledge of positive margins on initial surgical pathology were among the missing valuableGiven the inferiority of CI, patients with ultra-low PSA values could forego CI prior to 18F-DCFPyL PET/CT, resulting in fewer datapoints for comparison.16–19 For those, the simulated CI was used, yielding similar results.
The simulated tumor board used all available information for any given patient prior to the index 18F-DCFPyL PET/CT (including clinical notes, laboratory values, and prior PSMA and fluorocholine PET/CT reports), which best reflects the true standard of care at our institution. As such, physicians on the simulated tumor board could have inadvertently been unblinded to the identity of a patient they knew.
Conclusions
18F-DCFPyL demonstrated similar detection rates to reported 68Ga-PSMA-11 in the literature, and significantly outperformed CI for biochemical recurrence of prostate cancer in our population. Moreover, our data seems to indicate higher local recurrence rates in patients treated with RT than with RP, something that may have been only discovered with the superior sensitivity of PSMA PET. This performance increment hints at a great potential to improve patients’ outcomes, with a high rate of change in management. The easier patient scheduling with fluorine-18 and greater availability are key advantages to broaden the use of PSMA PET as an inevitable step in prostate cancer care. Large, rigorous, randomized, controlled trials are needed to see if this superior diagnostic performance and high rate of change in management will translate into an OS advantage for patients.
Footnotes
See related commentary on page 179
Competing interests: Dr. Probst declares in-kind research support from Progenics Inc. The remaining authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Fradet Y, Klotz L, Trachtenberg J, et al. The burden of prostate cancer in Canada. Can Urol Assoc J. 2009;3:S92–S100. doi: 10.5489/cuaj.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner SP, Seale-Hawkins C, Carlton CE, Jr, et al. The risk of dying of prostate cancer in patients with clinically localized disease. J Urol. 1991;146:1040–5. doi: 10.1016/S0022-5347(17)37997-1. [DOI] [PubMed] [Google Scholar]
- 3.Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–95. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Eissa A, Elsherbiny A, Coelho RF, et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: A systematic review of the literature. Minerva Urol Nefrol. 2018;70:462–78. doi: 10.23736/S0393-2249.18.03081-3. [DOI] [PubMed] [Google Scholar]
- 5.Sathianathen NJ, Butaney M, Konety BR. The utility of PET-based imaging for prostate cancer biochemical recurrence: A systematic review and meta-analysis. World J Urol. 2019;37:1239–49. doi: 10.1007/s00345-018-2403-7. [DOI] [PubMed] [Google Scholar]
- 6.Walacides D, Meier A, Knöchelmann AC, et al. Comparison of (68) Ga-PSMA ligand PET/CT vs. conventional cross-sectional imaging for target volume delineation for metastasis-directed radiotherapy for metachronous lymph node metastases from prostate cancer. Strahlenther Onkol. 2019;195:420–9. doi: 10.1007/s00066-018-1417-9. [DOI] [PubMed] [Google Scholar]
- 7.Calais J, Fendler WP, Eiber M, et al. Impact of 68Ga-PSMA-11 PET/CT on the management of prostate cancer patients with biochemical recurrence. J Nucl Med. 2018;59:434–41. doi: 10.2967/jnumed.117.202945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grubmüller B, Baltzer P, D’Andrea D, et al. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy — diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging. 2018;45:235–42. doi: 10.1007/s00259-017-3858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chmuck S, Nordlohne S, von Klot CA, et al. Comparison of standard and delayed imaging to improve the detection rate of [(68)Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:960–8. doi: 10.1007/s00259-017-3669-5. [DOI] [PubMed] [Google Scholar]
- 10.Rousseau E, Wilson D, Lacroix-Poisson F, et al. A prospective study on 18F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate cancer. J Nucl Med. 2019;60:1587–93. doi: 10.2967/jnumed.119.226381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietlein F, Kobe C, Neubauer S, et al. PSA-stratified performance of 18F- and 68Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2017;58:947–52. doi: 10.2967/jnumed.116.185538. [DOI] [PubMed] [Google Scholar]
- 12.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 13.Beltran H, Tomlins S, Aparicio A, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–50. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Li B, Zhang P, et al. Clinical characteristics and prognostic factors of prostate cancer with liver metastases. Tumour Biol. 2014;35:595–601. doi: 10.1007/s13277-013-1083-6. [DOI] [PubMed] [Google Scholar]
- 15.Backhaus P, Noto B, Avramovic N, et al. Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur J Nucl Med Mol Imaging. 2018;45:860–77. doi: 10.1007/s00259-017-3922-y. [DOI] [PubMed] [Google Scholar]
- 16.Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin Radiol. 2008;63:387–95. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Shen G, Deng H, Hu S, et al. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A meta-analysis. Skeletal Radiol. 2014;43:1503–13. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- 18.Gabriele D, Collura D, Oderda M, et al. Is there still a role for computed tomography and bone scintigraphy in prostate cancer staging? An analysis from the EUREKA-1 database. World J Urol. 2016;34:517–23. doi: 10.1007/s00345-015-1669-2. [DOI] [PubMed] [Google Scholar]
- 19.Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: A systematic review and meta-analysis. Eur Uol. 2020;77:403–17. doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]