Abstract
Coronaviruses (CoVs), which are enveloped, positive-sense RNA viruses, may cause infections in mammals and birds. Apart from the respiratory manifestations, CoVs are also responsible for infections of the gastrointestinal tract and nervous systems. Their propensity to recombine allows them to easily transmit and adapt to new hosts. The emergence of a new CoV in humans, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is attributed to a zoonotic origin, has provoked numerous studies to assess its pathogenicity for different animal species (pets, farm and wild animals). Available results indicate that numerous animal species are susceptible to infection with SARS-CoV-2. From April 2020, when the first SARS-CoV-2 infection in minks was reported in the Netherlands, to the end of January 2021, further outbreaks have been confirmed in Denmark, Italy, Spain, Sweden, the United States, Greece, France, Canada, Lithuania and Poland. It has also been established that human-to-minks and minks-to-human transmission may occur. The results obtained to date indicate that the virus was originally introduced into the minks population by humans, possibly at the start of the pandemic and had been circulating in the population for several weeks before detection. Recent data indicate that minks are highly susceptible to SARS-CoV-2 infection, but the route or routes of virus transmission between farms, other than by direct contact with infected humans, have not been identified. In minks, infection can occur in clinical and subclinical form, making it possibly difficult to detect. Therefore, minks could represent potentially dangerous, not always recognized, animal reservoir for SARS-CoV-2. The current data indicate that further studies on minks and other Mustelidae are needed to clarify whether they may be a potential reservoir for SARS-CoV-2, and if so, how and whether this can be prevented.
Keywords: COVID-19, Coronavirus, Mustelidae, Pathology, Zoonosis
Implications
This paper reviews the knowledge of severe acute respiratory syndrome coronavirus 2 infection in farmed minks. Recent data indicate that minks are highly susceptible to severe acute respiratory syndrome coronavirus 2 infection. Analysis of clinical and laboratory findings from infected farms indicates that infection in minks can occur in both clinical and subclinical forms, making it possibly difficult to detect. Thus, minks farms may potentially represent an unrecognized source of severe acute respiratory syndrome coronavirus 2. We emphasize the need for further research on minks and other Mustelidae to understand whether these species may be potential vectors and reservoirs of severe acute respiratory syndrome coronavirus 2 and, if so to prevent its establishment.
Introduction
Coronaviruses (CoVs) are enveloped positive-sense RNA viruses. They belong to the Coronaviridae family, order Nidovirales (Woo et al., 2006, Turlewicz-Podbielska and Pomorska-Mól, 2021). CoVs are pathogenic for mammals and birds, in which they cause infections manifested by a range of clinical signs (from respiratory, gastrointestinal and nervous systems) as well as subclinical infections (Herrewegh et al., 1998, Woo et al., 2006). To date, four genera of CoVs can be identified: Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus (Chen et al., 2020). Their propensity to recombine allows them to easily transmit and adapt to new hosts (Herrewegh et al., 1998, Woo et al., 2006, Turlewicz-Podbielska and Pomorska-Mól, 2021).
In December 2019, a new CoV, belonging to the genus Betacoronavirus, subgenus Sarbecovirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified to induce a global pandemic (Zhu et al., 2020, Malik et al., 2020). SARS-CoV-2 in humans causes a disease called COVID-19 (Zhu et al., 2020, Malik et al., 2020). The appearance of new Betacoronavirus in humans, which is attributed to a zoonotic origin, has provoked numerous studies to assess its pathogenicity for different animal species (pets, farm and wild animals) (Halfmann et al., 2020, Mykytyn et al., 2020, Richard et al., 2020, Sia et al., 2020, Shi et al., 2020, Zhao et al., 2020, Turlewicz-Podbielska and Pomorska-Mól, 2021). Due to the similarities of the SARS-CoV-2 to the SARS-CoV identified in 2003, it has been suspected from the beginning that the original outbreak was of zoonotic origin, possibly linked to a market in Wuhan, which sold a variety of animals including wild birds, poultry, fish, shellfish and other exotic species (Oude Munnink et al., 2021).
SARS-CoV-2 is another, after SARS-CoV and MERS-CoV, zoonotic CoV pathogenic to humans (Hemida et al., 2017, Dhama et al., 2020). Both previously discovered CoVs are thought to have been transmitted to humans via a mammalian intermediate host (Hemida et al., 2017). There is speculation that SARS-CoV-2 may also have emerged in the human population after crossing the species barrier, probably from a reservoir host (bats) via intermediate hosts (not yet confirmed, possibly pangolins or snakes) to humans (Malik et al., 2020). It has been shown that sarbecoviruses undergo common recombination and show significant genetic variation, however, SARS-CoV-2 is not a recombinant of any of the currently known sarbecoviruses (Boni et al., 2020). Its receptor-binding motif appears to be a feature derived from an ancestor shared with bat viruses. There is currently no indication that this is a trait acquired by recombination in the recent past (Boni et al., 2020).
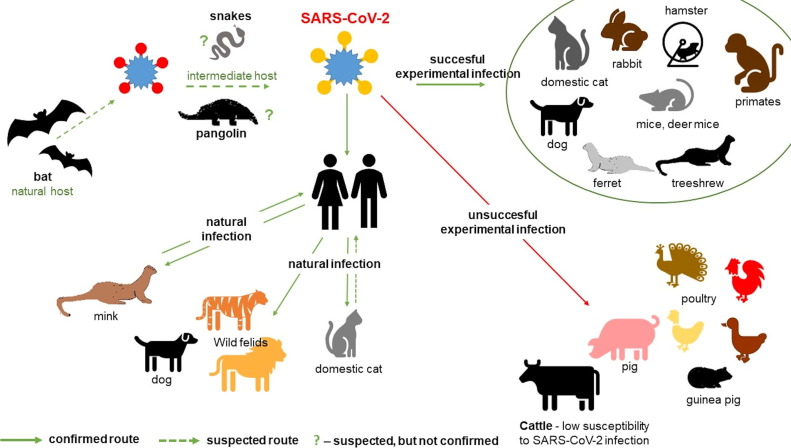
Closely related CoVs identified in bats and pangolins (Han, 2020, Lam et al., 2020, Zhou et al., 2020) share the highest sequence identity with novel SARS-CoV-2, but the most likely time of the divergence of SARS-CoV-2 from the closest related bat CoV is estimated to be around 1948–1982 (Boni et al., 2020), suggesting that the lineage that gave rise to SARS-CoV-2 circulated in the bat population undetected for years. (Boni et al., 2020). The most probable hypothesis for the genesis of SARS-CoV-2 is a more complicated viral pathway beginning with a bat, through another animal(s) ending with a human. The animal reservoir(s) of SARS-CoV-2 has not yet been definitively identified (Oude Munnink et al., 2021). Susceptibility of various animals to natural and/or experimental infection by SARS-CoV-2 with the possible role of a range of species in the transmission of SARS-CoV-2 is presented in Fig. 1 .
Fig. 1.
Susceptibility of various animals to natural and/or experimental infection by SARS-CoV-2 with possible role of a range of species in the transmission of SARS-CoV-2.
Like the previously identified sarbecovirus, SARS-CoV-2 binds to the host angiotensin 2 (ACE2) receptor. Based on the similarities of ACE2, a number of animal species have been selected for experimental infections: rhesus macaques (Munster et al., 2020), cats (Shi et al., 2020, Halfmann et al., 2020, Ruiz-Arrondo et al., 2021), dogs (Shi et al., 2020), ferrets (Richard et al., 2020), hamsters (Sia et al., 2020), tree shrews (Zhao et al., 2020), African green monkey (Woolsey et al., 2021), marmosets (Lu et al., 2020) rabbits (Mykytyn et al., 2020), fruit bats (Schlottau et al., 2020) and mice deer (Fagre et al., 2020). Experimental infections revealed that many species are susceptible to SARS-CoV-2, and that cats, tree shrews, mice deer, hamsters and ferrets can shed the virus. Other species, such as pigs and poultry, appear resistant (Schlottau et al., 2020, Shi et al., 2020, Suarez et al., 2020). Cattle show low susceptibility to SARS-CoV-2 infection and no intraspecies transmission to in-contact cattle has been observed (Ulrich et al., 2020). These findings indicate that the virus has a restricted host range.
SARS-CoV-2 has also been identified in naturally infected animals. SARS-CoV-2 RNA has been detected in dogs (Sit et al., 2020, Patterson et al., 2020) and cats (Sailleau et al., 2020, Shi et al., 2020, Segalés et al., 2020). Furthermore, SARS-CoV-2 has been found in tigers and lions at the New York Zoo (Gollakner and Capua, 2020). The antibodies specific to SARS-CoV-2 have been detected in cats in Italy, the Netherlands, China and Germany (Patterson et al., 2020, Zhang et al., 2020). SARS-CoV-2 has also been detected in American (Neovison vison) minks in several countries (Oude Munnink et al., 2021, Boklund et al., 2021b, Sharun et al., 2021).
At present, there is no direct reference to farmed minks and SARS-CoV-2 infection in the World Organisation for Animal Health (OIE) Terrestrial Animal Health Code, and the reporting of infection in minks by member countries is based on a definition of ‘emerging disease’ in animals (OIE, 2019). The OIE is now working to establish appropriate legislation to facilitate the control and eradication of this emerging disease in minks (OIE, 2019). Work is underway to enable the inclusion of minks in the World Animal Health Information Database and to encourage member countries to submit relevant data for this species, which will improve the monitoring of the epidemiological situation worldwide.
Occurrence of SARS-CoV-2 infections in minks
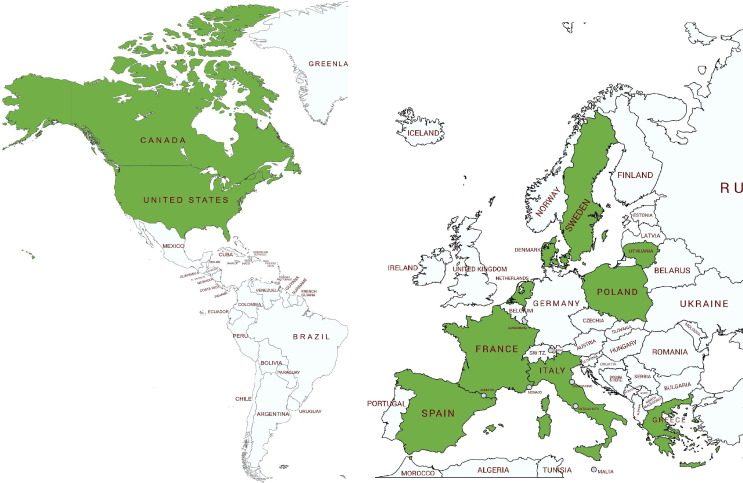
Since April 2020, when the first SARS-CoV-2 infection in minks was reported in the Netherlands, to date (February 2021) further outbreaks have been confirmed in Denmark, Italy, Spain, Sweden, the United States, Greece, France, Canada, Lithuania and Poland (Oreshkova et al., 2020, Molenaar et al., 2020, ProMED, 2020a, ProMED, 2020b, ProMED, 2020c, ProMED, 2020d, ProMED, 2020h, ProMED, 2020i, ProMED, 2021, Oude Munnink et al., 2021) (Fig. 2 ). It has also been established that human-to-minks and minks-to-human transmission may occur (Oude Munnink et al., 2021, Boklund et al., 2021b).
Fig. 2.
Countries with SARS-CoV-2 outbreaks in farmed minks (marked in green).
The Netherlands
The first outbreaks of SARS-CoV-2 were confirmed in April 2020 on two minks farms (Oude Munnink et al, 2021). Following the detection of SARS-CoV-2 on minks farms, in-depth analyses were initiated to identify potential transmission routes and to assess the environmental and occupational risks. Unfortunately, despite the introduction of rigorous biosecurity requirements, there have been further outbreaks of SARS-CoV-2 infection on minks farms at different time intervals.
In the response to SARS-CoV-2 infection on minks farms, a national zoonotic disease response system was launched. Already in May 2020, minks farmers, veterinarians and diagnostic laboratories were accordingly obliged to report all cases of signs that may indicate minks infection with SARS-CoV-2 or positive results obtained in laboratories to the Netherlands Food and Consumer Product Safety Authority and an extensive surveillance system was established (Netherlands Food and Consumer Product Safety Authority, 2020, Sikkema et al., 2020).
When interviewed as part of the epidemiological investigation on the first farm where minks infections were confirmed, four of the five farmworkers reported that they had developed respiratory signs prior to the outbreak in minks, but none of them had been tested for SARS-CoV-2. To date, detailed epidemiological findings from the first 16 outbreaks in the Netherlands minks farms have been published (Oude Munnink et al., 2021). A total of 97 individuals were tested by serological and/or reverse transcription–PCR (RT-PCR) tests as part of the investigation. 43 of 88 (49%) upper respiratory tract samples were positive by RT-PCR, while 38 of 75 (51%) serum samples were positive for SARS-CoV-2-specific antibodies. In total, 66 of 97 individuals (68%) were considered to be contacted with SARS-CoV-2 (Oude Munnink et al., 2021). Isolates obtained from 16 minks and one isolate from a farm worker have been sequenced (Oude Munnink et al., 2021).
A retrospective analysis on the second farm showed that one of its workers had been hospitalized on March 31 due to SARS-CoV-2. Samples from eight workers taken after the infection was identified in minks were negative in RT-PCR but positive in serological tests. The virus sequence obtained from the animals was different from that on the first positive farm (Oude Munnink et al., 2021). On another farm with SARS-CoV-2 infection in minks, all workers had initially tested negative for the presence of SARS-CoV-2 RNA, but 2–3 weeks later, five of the seven persons working or living on the farm tested positive for SARS-CoV-2 showing clinical signs of COVID-19 (Oude Munnink et al., 2021).
Sequence analysis of isolates from humans and minks, together with the initial negative test result and the subsequent appearance of COVID-19 signs in humans, indicates that workers were infected after the minks were infected. In addition, the epidemiological investigation showed likely further transmission of the virus to further persons not in contact with the minks, but only with the infected workers (Oude Munnink et al., 2021). Occurrence of zoonotic infection in humans was noted also on other farms (Oude Munnink et al., 2021).
Phylogenetic analysis of the SARS-CoV-2 minks genomes showed that sequences of isolates obtained from minks from 16 farms were grouped into five different clusters (Oude Munnink et al., 2021). Some of the farms from which isolates were included in a given cluster had the same owner; however, in most cases, a common factor could not be identified for the different farms and it was not possible to explain the presence in a given cluster on a given farm (Oude Munnink et al., 2021). Additionally, 18 sequences were generated from minks farmworkers or close contacts from few farms. In most cases, the human sequences were almost identical to those obtained from minks from the same farm (Oude Munnink et al., 2021). Several non-synonymous mutations were found among the minks sequences compared to the Wuhan reference sequence NC_045512.2 (Oude Munnink et al., 2021). However, no specific amino acid substitutions were found in samples from minks. It is worth noting that three of the five clusters had the 614G variant (clusters A, C, E) and two (B, D) had the original variant. To date, no significant differences have been found over the course of the disease in animals or humans caused by viruses grouped in different clusters (Oude Munnink et al., 2021).
In studies conducted by Oude Munnink et al. (2021), a high diversity of SARS-CoV-2 sequences was observed for some minks farms, which may indicate that the virus had been circulating on minks farms for some time or that multiple introductions of the virus had taken place (Candido et al., 2020, Oude Munnink et al., 2021). However, relatively high sequence variation was also observed in farms that were negative 1 week earlier, which may indicate a more rapid evolution of the virus in the minks population. The SARS-CoV-2 substitution rate in humans is estimated to be about 1.16 · 10-3 substitutions/location/year (about one mutation every 2 weeks) but minks live in high-density populations, which may favour virus transmission (Candido et al., 2020, Oude Munnink et al., 2021). The Dutch services have so far failed to identify common factors that could explain the spread of SARS-CoV-2 from farm to farm.
Denmark
Cases of SARS-CoV-2 infection in minks have also been reported in Denmark. In June, despite the animals not showing any symptoms, three of the first farms were culled (ProMED, 2020b, Larsen et al., 2021). On 4 November, it was decided to cull all minks in the country to prevent human infection. Most minks have been culled and most farms have closed, at least until the end of 2021 (Boklund et al., 2021a). The Act on the killing of and temporary ban on keeping minks (until 31 December 2021) took effect on 15 January 2021 (Boklund et al., 2021a).
In 2018, Denmark, as one of the largest producers in the world, had 1 500 farms that produced skins worth €1.1 billion. The first case of SARS-CoV-2 infection in minks on Danish farms was confirmed in June 2020 on a farm in North Jutland (ProMED, 2020b, OIE, 2019, Larsen et al., 2021). Samples were collected after some minks were reported to have respiratory symptoms. Positive results for SARS-CoV-2 were obtained from samples taken from animals and one worker (ProMED, 2020b). Finally, almost 11 000 animals from the farm were culled (ProMED, 2020b).
In order to be able to monitor the situation in Denmark, the Ministry of Environment and Food issued a decree with the aim of ensuring that the health of fur animals could be monitored in the context of SARS-CoV-2 infections. Through this decree, veterinary services can take samples from minks, as well as implement infection prevention measures (ProMED, 2020e). The regulations introduced required that any suspicion of SARS-CoV-2 infection in fur animals be notified to the competent services. A veterinarian should be called if a fur animal develops symptoms such as diarrhoea, vomiting, respiratory signs or an elevated rectal temperature. If a veterinarian suspects COVID-19 in a fur animal, they must also inform the relevant services and collect samples for laboratory testing (ProMED, 2020e). A total of 287 SARS-CoV-2 infected farms have been detected in Denmark (Boklund et al., 2021b). By November 2020, Denmark has reported 214 human infections with SARS-CoV-2 variants related to those found in minks (OIE, 2019, ProMED, 2020e, European Centre for Disease Prevention and Control, 2020, Boklund et al., 2021b).
The SARS-CoV-2 variants detected in Denmark belonged to at least five clusters, which may indicate several introductions of the virus into the farms and subsequent transmission from farm to farm (Boklund et al., 2021a). The mutation Y453F in the receptor‐binding domain (RBD) of the spike protein has been observed in many of the SARS‐CoV‐2 strains in Denmark, independently of the clustering (Boklund et al., 2021a; Oude Munnink et al., 2020). This mutation has also been observed in SARS‐CoV‐2 strains associated with minks, e.g. in The Netherlands, Greece and Sweden (Boklund et al., 2021a). However, it should be noted that the Y453F has also been observed in human cases not related to minks (Boklund et al., 2021a).
Each cluster contained minks-associated variants that were identified in both humans and farm animals in the same Danish region. One of the clusters (cluster 5), which mainly circulated in August and September 2020, is associated with a variant that showed four genetic changes: three substitutions and one deletion in the S protein (OIE, 2019, Mallapaty, 2020, ProMED, 2020e). To date however, it is not clear whether all the minks-associated mutations in human actually originated in minks. Given that the S protein contains the RBD and is a major target of the immune response, mutations in this area may affect pathogenicity to different human and animal species, virus seeding and spread and antigenic properties. Some researchers highlight that the Cluster-5 variant appears to be a ‘dead end’ in people, as it has not spread widely in the human population (Mallapaty, 2020). The majority of the people infected with this variant had worked on farms and were probably exposed to a high dose of the virus. Additionally, the variant has not been found in human samples since September 2020, despite the sequencing of an increasing number of isolates (Mallapaty, 2020). The emergence and spread of strains with increasing alterations in the functional domains of protein S could potentially affect the diagnostics and the effectiveness of immunoprophylaxis. Research is currently underway to help clarify the likelihood of these possible consequences (OIE, 2019, Mallapaty, 2020, ProMED, 2020e).
Since the decision announced on November 4th to cull all minks in Denmark, over 13 million minks have been culled. During the first cull period, approximately 25% of farms had been infected with SARS-CoV-2 (Boklund et al., 2021b). No new SARS‐CoV‐2 occurrences have been detected since 7 December 2020 (Boklund et al., 2021a). In December 2020, the Danish authorities introduced a temporary ban on minks farming (until the end of 2021). Despite the fact that the ban on minks farming in Denmark is temporary, and that the law introduced provides the possibility of a return to farming once the COVID-19 pandemic is brought under control, it seems unlikely that Danish minks farming will regain its full potential, given both the manner in which minks farming has been ended, the uncertain circumstances surrounding the course of the pandemic and the actions of animal rights activists.
Spain
Further reports of SARS-CoV-2 infection in minks come from Spain where CoV was found on a farm in the Aragon region (ProMED, 2020a, OIE, 2019). None of the animals on the farm showed any signs of disease and no elevated mortality was observed in the herd. Veterinary services were called by the farmer after his wife and several farmworkers tested positive for SARS-CoV-2 (ProMED 2020a). The first tests in minks, with negative results, were conducted 1 week after the positive results in workers have been confirmed (ProMED 2020a). A positive result in minks was confirmed after more than 3 weeks from first sampling. After another 2 weeks, more than 80% of the minks tested were positive. Although it could not be definitively established whether the infection was transmitted from humans to animals or from animals to human, the authorities decided to cull all minks on the farm (ProMED 2020a).
United States
In August 2020, United States Department of Agriculture (USDA) National Veterinary Services Laboratories (NVSL) announced the first confirmed cases of SARS-CoV-2 in minks on two farms in Utah. The affected farms also reported positive cases of COVID-19 in people who had contact with the minks (Animal and Plant Health Inspection Service, 2020, Cahan, 2020). In Utah, the first problems on a minks farm became apparent on August 6, when farmers informed the state USDA of a huge increase in mortality. Mortality continued to rise, prompting the authorities to take diagnostic measures. The results of pathological examinations showed the presence of inflammatory lesions in the lungs. The lesions were similar to those observed in minks in Europe in which SARS-CoV-2 infection had been confirmed (severe pneumonia, swollen, dark red, moist, not collapsed lung lobes) (Molenaar et al., 2020). The results of laboratory tests confirmed that the animals were infected with the SARS-CoV-2. Analysis of the situation on the farm has shown that minks, like humans, have a variable clinical course of the disease. Only some of them undergo the acute form of the disease, while in others, the disease is subclinical or with weak symptoms (Animal and Plant Health Inspection Service, 2020, Cahan, 2020).
In all cases confirmed to date in the United States, it is most likely that humans were the vector introducing the virus into the minks population; the reverse route of transmission has not yet been confirmed (Animal and Plant Health Inspection Service, 2020, Cahan, 2020). It is estimated that between August and mid-November, more than 15 000 minks died as a result of SARS-CoV-2 infection in the US. For the time being, the competent services and authorities in the states, where SARS-CoV-2 has been confirmed in minks, have stated that they do not plan to slaughter the animals and are continuously monitoring the situation (Animal and Plant Health Inspection Service, 2020, Cahan, 2020).
Italy
In Italy, an outbreak on minks farm caused by SARS-CoV-2 was confirmed in August 2020. According to available information, at least two minks samples from one farm tested positive for SARS-CoV-2 genetic material (ProMED, 2020f, OIE, 2019). More detailed information on this outbreak and the epizootic situation in the country is currently not available.
Sweden
The first outbreak in Sweden was detected at the end of October 2020, with the first case of SARS-CoV-2 infection confirmed on a farm in the south-east of the country, in Blekinge County (ProMED, 2020g, OIE, 2019). Almost half of Sweden's minks farms are located in this area. The affected farm reared approximately 9 500 minks. The infection was detected by implementing a monitoring programme for SARS-CoV-2 infection in this species. The material from dead minks was tested by real-time PCR as part of a monitoring and surveillance programme implemented in Sweden. On 16.10.2020, one of the three minks was found to be weak positive after the examination of an oral and pharyngeal swab. The tissue samples from all animals were negative. 1 week later, samples from other five dead minks were positive. In parallel, a slight increase in mortality could be observed on the farm, but no other clinical signs of disease were identified (ProMED, 2020g). The source of infection has not yet been established, but both the farm owner and his father, who were tested for SARS-CoV-2 on 21 October, tested positive. Further analysis is currently underway to assess the similarities between the virus strains detected in samples taken from minks and humans. The veterinary services have implemented procedures, including movement restrictions and biosecurity measures, and do not intend to cull animals at this stage. Strict surveillance of minks farms has also been introduced (ProMED, 2020g).
Greece
Greece is the next country where minks infected with SARS-CoV-2 have been confirmed. The first outbreaks in minks were confirmed in November 2020 in Kozani and Kastoria regions (ProMED, 2020c). The Y453F mutation, observed in Denmark and The Netherlands, has been also confirmed in the virus found in samples from minks in Greece (Boklund et al., 2021a). Since SARS-CoV-2 infection was confirmed in the owner of one farm, local authority decided to test the remaining farmworkers. Finally, 2 500 minks on a farm in northern Kozani have been culled (ProMED, 2020c).
France
On 22 November, the French Ministry of Agriculture reported the first case of SARS-CoV-2 infection in minks in France (ProMED, 2020d, OIE, 2019). A minks infected with SARS-CoV-2 was found on a farm in the Eure-et-Loire region of western France, in a herd where 1 000 minks were being reared. It was decided to cull all minks from the farm (ProMED, 2020d). France started testing the minks in mid-November 2020. So far, tests have shown that the virus is circulating in only one of four farms registered in France (ProMED, 2020d).
Lithuania
Lithuania reported the first outbreak of COVID-19 in minks on 30 November 2020 (OIE, 2019, ProMED, 2020h). The presence of the new CoV was confirmed on a farm located in Jonava district. Increased minks mortality had been observed on the infected farm since 24 November. Samples from dead minks were collected as part of the passive monitoring related to the observed increase in mortality. A total of 169 dead minks were found from which 10 samples were randomly collected and tested positive for SARS-CoV-2 by PCR. A further 22 dead animals were sampled the following day and all tested positive for SARS-CoV-2 by PCR. The results of the epidemiological investigation carried out by the veterinary authorities do not exclude that the SARS-CoV-2 could have been transmitted to minks by a farm worker. The farm was closed, and any removal of animals, feed, or other animal products from the infected holding was prohibited. A decision was also made to cull infected minks. Unlike Denmark and Ireland, Lithuania has made no plans for a mass culling of minks (ProMED, 2020h).
Canada
The first notification of SARS-CoV-2 infection in Canadian minks was confirmed in December 2020 (ProMED, 2020i, OIE, 2019). The infected farm is located in Fraser Valley, British Columbia. The results of the epidemiological investigation indicate that human was the probable source of infection since COVID-19-positive minks farmworkers were diagnosed on 3 December 2020. The minks did not display clinical signs at that time. Minks sampled during the first days of December tested positive for SARS-CoV-2 by PCR. In the days following sampling, there was an increase in mortality (around 1%). From that time, few other outbreaks have been reported from Canada. The near complete genome sequences of SARS-CoV-2 were obtained for minks samples from the second minks farm (approx. 99.7% genome coverage). Sequence analysis identified at least three single nucleotide polymorphisms compared to the SARS-CoV-2 sequences from the first minks farm and other sequences reported in the Global Initiative on Sharing Avian Influenza Data. The three new minks sequences belong to SARS-CoV-2 lineage B.1.1.73, which is a lineage consisting primarily of North American sequences. The whole genome sequencing of the virus found in minks and humans at the beginning of the outbreak at the minks farm revealed that the people and animals were infected with an identical or nearly identical strain. The strain detected has been circulating in people in this region, indicating COVID-19 spread from people to animals and not the other way around (BC Centre for Disease Control, 2020).
Poland
In Poland, one of the world's top producers of minks fur, the first outbreak of SARS-CoV-2 in minks was confirmed on 30 January 2021, based on the results of laboratory tests carried out by national reference laboratory (ProMED, 2021, OIE, 2021, Domańska-Blicharz et al., 2021). The SARS-CoV-2 outbreak was detected on a holding with more than 5 800 of American minks at time of the outbreak, located in Pomeranian voivodeship. Samples (the nasopharyngeal swabs taken from 20 minks) were collected on January 27 during active monitoring, in accordance with applicable national regulations. Positive results were obtained from four animals. No clinical signs or increased mortality has been observed (ProMED, 2021, OIE, 2021). In the spike protein of SARS-CoV-2 detected in minks in Poland, four or five amino acid substitutions were identified, depending on the isolate tested. However, only one substitution (Y453F) is located in the RBD (Domańska-Blicharz et al., 2021). This location is found to be responsible for increased binding affinity to cellular receptors in minks (Andersen et al., 2020, Lauring and Hodcroft, 2021). In November 2020, the Polish Veterinary Authorities introduced official monitoring of minks farms (Domańska-Blicharz et al., 2021). 1 month later, the new legislation declaring SARS-CoV-2 infection in minks as a notifiable disease has been introduced (Domańska-Blicharz et al., 2021).
Course of infection in minks – case reports
In minks, the virus caused both clinical disease, mainly with respiratory signs and increased mortality, and subclinical infection (Oreshkova et al., 2020, Molenaar et al., 2020, Boklund et al., 2021b). The summary of clinical course observed in minks with SARS-CoV-2 infection is presented in Table 1 . So far, the course of the disease in four Dutch and 215 Danish minks farms has been described (Molenaar et al., 2020, Boklund et al., 2021b).
Table 1.
Clinical findings in farmed minks infected by SARS-CoV-2.
| Country | Date of first outbreak | Course of infection | Observed clinical symptoms |
References | ||
|---|---|---|---|---|---|---|
| Respiratory signs | Mortality | Other | ||||
| The Nederland | 26/04/2020 | Clinical | Yes | yes | no | Molenaar et al. (2020) |
| Denmark | 17/06/2020 | clinical and subclinical | in some herds | in some herds | no | Boklund et al. (2020) |
| Spain | 16/07/2020 | Subclinical | No | no | no | ProMED, 2020a, OIE, 2019 |
| US | 06/08/2020 | Clinical | Yes | yes | no | Cahan, 2020, Animal and Plant Health Inspection Service, 2020, OIE, 2019 |
| Italy | 30/10/2020 | Nd | nd | nd | nd | ProMED, 2020f, OIE, 2019 |
| Sweden | 29/10/2020 | Subclinical | no | slight | no | ProMED, 2020g, OIE, 2019 |
| Greece | 16/11/2020 | Subclinical | no | no | no | ProMED, 2020c, OIE, 2019 |
| France | 25/11/2020 | Subclinical | no | no | no | ProMED, 2020d, OIE, 2019 |
| Lithuania | 30/11/2020 | Clinical | no | yes | no | ProMED, 2020h, OIE, 2019 |
| Canada | 09/12/2020 | Subclinical | no | yes | no | ProMED, 2020i, OIE, 2019 |
| Poland | 31/01/2021 | Subclinical | no | no | no | ProMED, 2021, OIE, 2021 |
nd – no data.
Molenaar et al. (2020) described the epidemiological situation on four minks farms in The Netherlands. The minks population on the farms ranged from 1 550 to 12 000 females. The farms had been positive for Aleutian minks disease but there were no significant clinical problems as a result. On two farms, increased mortality and symptoms from the respiratory tract were observed in some minks on 19 and 20 April 2020. Histopathological examination revealed interstitial pneumonia. Molecular testing (PCR) results for SARS-CoV-2 were positive. Investigation for influenza A virus and distemper virus gave negative results. No growth of bacterial pathogens was detected. On May 6 2020, the same results were obtained for next two farms. In contrast to the first three farms, on farm 4, no clinical symptoms were noted at the time the diagnostic tests were performed. Clinical signs appeared later on this farm (Molenaar et al., 2020).
On all farms, difficult breathing and watery or mucous discharge from the nose, with varying degrees of severity, were observed in minks. Some animals developed a loss of appetite after 1–2 days of such signs and were found dead after a further 24–48 h. Animals with moderate to severe clinical signs died within 2–3 days of the first symptoms appearing. At the peak of the infection, the morbidity was 2–3 times higher than the daily mortality. The average duration of the disease on each farm was 4 weeks, during which time a successive increase in mortality was observed (Molenaar et al., 2020). Most of the animals with clinical signs showed severe pneumonia, but good general body condition in postmortem examination. All lung lobes were swollen, dark red, moist, not collapsed. In the gastrointestinal tract, only small amounts or no digestive contents were observed. In some minks, bloody nasal discharge was found. Apart from diffuse reddish-brown foci in the lungs, observed in less than 10% of kits, no visible macroscopic changes were found in the offspring (Molenaar et al., 2020). Immunohistochemical studies confirmed the presence of SARS-CoV-2 antigen in the lungs in four of 11 adult minks and in one of five kits. In adult minks, viral antigens were also found in the trachea and nasal auricles (Molenaar et al., 2020). The study showed that all minks that were positive after examination of rectal swabs were also positive in throat swabs, but not all minks that were positive in throat swabs were positive in rectal swabs. CT values in pharyngeal swabs were always lower than in nasal swabs (taken from the same animal) indicating that in pharyngeal swabs, more virus was present. By examining throat swabs with a PCR test, the authors were able to detect SARS-CoV-2 even after the clinical form of the disease had disappeared. Moreover, positive results of throat swabs were observed even in animals in which pathological changes in the lungs were no longer detected (Molenaar et al., 2020).
Researchers from Denmark described prevalence and clinical data from 215 infected minks farms (Boklund et al., 2021b). According to the procedure implemented in this study, during the first visit on farm suspected of being infected with SARS-CoV-2, samples (throat swabs and blood) were collected from animals showing respiratory symptoms. If no symptoms were observed, samples were collected in a way that ensured monitoring of each sector of farm. During the second visit on farms, samples were taken randomly from alive and dead minks. Fur, air, water and feed samples were additionally taken on some farms. Samples from other animal species (cats, dogs, horses, dead birds found, dead rats found, flies) were also collected on several farms. Anti-SARS-CoV-2 antibodies and/or SARS-CoV-2 genetic material were analysed in collected samples (Boklund et al., 2021b).
A clinical course of infection was observed on about 70% of the farms. The remaining infected farms showed a subclinical course of disease. The most frequently observed signs included decreased feed intake, increased mortality and respiratory symptoms (Boklund et al., 2021b). Clinical signs were present in both young (juveniles) and adult minks. The most common clinical signs were nasal discharge (on 25% of farms), sneezing (on 23% of farms), other respiratory signs (on 31% of farms), depression (on 7% of farms) and diarrhoea (on 5% of infected farms). Decreased feed intake and increased mortality were recorded on 54% and 63% of infected farms, respectively. Increased mortality was observed for a period of approximately 10 days (Boklund et al., 2021b).
Studies have shown that the virus is rapidly spreading in minks. More than half of the farms had 100% SARS-CoV-2 prevalence at the first sampling and only 12% of the farms had SARS-CoV-2 prevalence lower than 50% (Boklund et al., 2021b).
100% prevalence was more frequently observed on farms with a clinical course of infection. On 69% of the farms, specific antibodies were detected in all animals sampled on the first visit. On the second visit, 100% seroprevalence was present in all the farms studied and the frequency of positive samples for the virus was much lower than at the first sampling (the time interval between the first and second visits varied from 2 to 17 days) (Boklund et al., 2021b). The presence of SARS-CoV-2 genetic material was confirmed in air samples, fur, flies and water. Feed tests were negative. In addition, infection was confirmed in four dogs from infected farms and in one cat (Boklund et al., 2021b). No virus was detected in samples taken from rabbits, chickens and horses from the same farms and from wild animals in the vicinity of infected farms (Boklund et al., 2021b). Flies collected from one out of two sampled farms were positive by real-time RT-PCR, but with high Ct values. A hazard analysis conducted by Boklund et al. (2021b) showed that farm size and minimum distance from the nearest detected infected farm were significantly correlated with the chance of being infected with SARS-CoV-2. The risk of infection increased with increasing farm size as well as with decreasing distance from the infected farm.
The current data indicate that a high prevalence of virus (up to 100%) and seroprevalence have been observed on farms with clinical signs. However, the prevalence and seroprevalence were also high on farms without a clinical manifestation of the disease (Boklund et al., 2021b, Hammer et al., 2021). Both findings suggest a very rapid spread of the virus within the farm or a delayed diagnosis of the disease in minks (Boklund et al., 2021b, Hammer et al., 2021, Oude Munnink et al., 2021). The rapid spread may indicate that SARS-CoV-2 has adapted to minks, increasing the transmission rate among minks (Hammer et al., 2021).
Conclusion
Animal experiments have shown that SARS-CoV-2 infection can occur in primates, cats, ferrets, hamsters, rabbits, mice deer and bats (Shi et al., 2020, Halfmann et al., 2020; Farge et al., 2020). In addition, SARS-CoV-2 has been detected in some animal species, e.g. cats, dogs and minks, which have been infected naturally (through contact with infected humans or animals) (Shi et al., 2020, Patterson et al., 2020, Segalés et al., 2020). The results suggest that SARS-CoV-2 was probably introduced into the minks population by humans and had been circulating unnoticed in the population of minks for some time before detection (Boklund et al., 2021b, Hammer et al., 2021, Oude Munnink et al., 2021). In the Netherlands, Denmark or Canada, despite increased biosecurity, surveillance, early warning and immediate culling of infected animals, transmission of SARS-CoV-2 between minks farms could not be stopped in many places.
Recent data indicate that minks are highly susceptible to SARS-CoV-2 infection, but the route or routes of virus transmission between farms, other than by direct contact with infected humans, have not been identified. At this point, it is worth mentioning that SARS-CoV-2 transmission was observed between minks farms located close to each other. Epidemiological investigations to date have shown that a large proportion of minks on farms can become infected with SARS-CoV-2 within a few days, which may provide major virus exposure to farmworkers or veterinarians (Boklund et al., 2021b, Hammer et al., 2021, Oude Munnink et al., 2021). The scale of infection in minks on minks farms is worrying, mainly because of the ability of the virus to pass through a large population of highly susceptible animals, potentially allowing the emergence of new, dangerous mutations and/or the acquisition of new biological properties (Domańska-Blicharz et al., 2021).
The analysis of the clinical situation of the described cases in combination with the laboratory results obtained indicates that in minks, clinical and subclinical form of infection with SARS-CoV-2 can occur, making it potentially problematic to detect (Molenaar et al., 2020, Boklund et al., 2021b, Oude Munnink et al., 2021). Therefore, minks farms could represent a possibly dangerous, not always recognized, animal reservoir for SARS-CoV-2. To date, there is no evidence for the spread of the virus outside of farms, except by infected persons. However, the risk of virus transmission to persons working with infected minks as well as to their contacts cannot be excluded. Current results showed that contact persons were confirmed to be infected with strains present in minks which, together with other epidemiological circumstances, provides evidence of the transmission of SARS-CoV-2 from animals to humans within minks farms (Boklund et al., 2021b, Hammer et al., 2021, Oude Munnink et al., 2021).
Furthermore, the results obtained indicate that in animals with clinical signs of SARS-CoV-2 infection, molecular testing of pharyngeal swabs combined with histopathological examination of the lungs should be recommended for diagnostic purposes. For monitoring purposes, in animals without clinical disease manifestation, molecular testing of throat swabs from dead minks is recommended, even in the absence of postmortem lesions (Molenaar et al., 2020). The current data indicate that further studies on minks and other Mustelidae are needed to clarify whether they may be a potential reservoir for SARS-CoV-2 virus, and if so, how and whether this can be prevented.
Ethics approval
No ethics approval is necessary.
Data and model availability statement
Search results used for the present study are not deposited in an official data repository. Search results are available to reviewers and readers upon reasonable request.
Author ORCIDs
Author contributions
Conceptualisation; MPM
Writing – original draft; MPM
Writing – review & editing; MPM, JW, MG, MR
Collecting the literature data: MPM, MG, JW and MR
Funding acquisition & Supervision; MPM
Declaration of interest
None.
Acknowledgments
Acknowledgements
The authors acknowledge the efforts of the anonymous reviewers who contributed to improving the manuscript. The authors thank Wiktoria Mól for improving the use of English in the manuscript.
Financial support statement
The scientific activity of MPM was supported by the National Science Centre (DEC-2020/37/B/NZ7/00021) and statutory funding 506.514.05.00 of the Department of Preclinical Sciences and Infectious Diseases, Faculty of Veterinary Medicine and Animal Science, Poznan University of Life Sciences, Poland. This research was also supported by the Polish Ministry of Science and Higher Education's ‘Regional Initiative Excellence’ program 2019–2022, Project No. 005/RID/2018/19.
References
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nature Medicine. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animal and Plant Health Inspection Service, 2020. USDA Confirms SARS-CoV-2 in Mink in Utah. Retrieved on 15 December 2020, from https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2020/sa-08/sare-cov-2-mink.
- BC Centre for Disease Control, 2020. Genetic sequencing results completed for mink farm outbreak. Retrieved on 5 January 2021, from http://www.bccdc.ca/about/news-stories/news-releases/2020/genetic-sequencing-results-completed-for-mink-farm-outbreak.
- Boklund A., Gortazar C., Pasquali P., Roberts H., Nielsen S.S., Stahl K., Stegeman A., Baldinelli F., Broglia A., Van Der Stede Y., Adlhoch C., Alm E., Melidou A., Mirinaviciute G. Scientific Opinion on the monitoring of SARS-CoV-2 infection in mustelids. EFSA Journal. 2021;19:6459. doi: 10.2903/j.efsa.2021.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boklund A., Hammer A.S., Quaade M.L., Rasmussen T.B., Lohse L., Strandbygaard B., Jørgensen C.S., Olesen A.S., Hjerpe F.B., Petersen H.H., Jensen T.K. SARS-CoV-2 in Danish Mink Farms: Course of the Epidemic and a Descriptive Analysis of the Outbreaks in 2020. Animals. 2021;11:164. doi: 10.3390/ani11010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni M.F., Lemey P., Jiang X., Lam T.T.Y., Perry B.W., Castoe T.A., Rambaut A., Robertson D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nature Microbiology. 2020;5:1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- Cahan, E., 2020. COVID-19 hits U.S. mink farms after ripping through Europe. Posted in: Plants & Animals: Coronavirus. Retrieved on 15 December 2020, from 10.1126/science.abe3870. [DOI]
- Candido D.S., Claro I.M., de Jesus J.G., Souza W.M., Moreira F.R.R., Dellicoue S., Mellan T.A., du Plessis L., Pereira R.H.M., Sales F.C.S., Manuli E.R., Theze J., Almeida L., Menezes M.T., Voloch C.M., Fumagalli M.J., Coletti T.M., da Silva C.A.M., Ramundo M.S., Amorim M.R., Hoeltgebaum H.H., Mishra S., Gill M.S., Carvalho L.M., Buss L.F., Prete C.A., Jr, Asworth J., Nakaya H.I., Peixoto P.S., Brady O.J., Nicholls S.M., Tanuri A., Rossi A.D., Braga C.K.V., Gerber A.L., Guimaraes A.P., Gaburo N., Jr, Alencar C.S., Ferreira A.C.S., Lima C.X., Levi J.E., Granato C., Ferreira G.M., Francisco R.S., Jr, Granja F., Garcia M.T., Moretti M.L., Perroud M.W., Jr, Castineiras T.M.P.P., Lazari C.S., Hill S.C., de Souza Santos A.A., Simeoni C.L., Forato J., Sposito A.C., Schreiber A.Z., Santos M.N.N., de Sa C.Z., Souza R.P., Resende-Moreira L.C., Teixeira M.M., Hubner J., Leme P.A.F., Moreira R.G., Nogueira M.L., Ferguson N.M., Costa S.F., Modena J.L., Vasconcelos A.T.R., Bhatt S., Lemey P., Wu C.H., Rambaut A., Loman N.J., Aguiar R.S., Pybus O.G., Sabino E.C., Faria N.R. Brazil-UK Centre for Arbovirus Discovery, Diagnosis, Genomics and Epidemiology (CADDE) Genomic Network, Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369:1255–1260. doi: 10.1126/science.abd2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. Journal of Medical Virology. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., Singh K.P., Chaicumpa W., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Coronavirus disease 2019–COVID-19. Clinical Microbiology Reviews. 2020;33:e00028–20. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domańska-Blicharz K., Orłowska A., Smreczak M., Niemczuk K., Iwan E., Bomba A., Lisowska A., Opolska J., Trębas P., Potyrało P., Kawiak-Sadurska M., Rola J. Mink SARS-CoV-2 infection in Poland – short communication. Journal of Veterinary Research. 2021;65:1–5. doi: 10.2478/jvetres-2021-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control, 2020. Detection of new SARS-CoV-2 variants related to mink 12. Retrieved on 5 January 2021, from https://www.ecdc.europa.eu/sites/default/files/documents/RRA-SARS-CoV-2-in-mink-12-nov-2020.pdf.
- Fagre, A., Lewis, J., Eckley, M., Zhan, S., Rocha, S.M., Sexton, N.R., Burke, B., Geiss, B.J., Peersen, O., Kading, R., Rovnak, J., Ebel, G.D., Tjalkens, R.B., Aboellail, T., Schountz, T., 2020. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for reverse zoonosis to New World rodents. bioRxiv [Preprint] 2020.08.07.241810. https://doi.org/10.1101/2020.08.07.241810.
- Gollakner R., Capua I. Is COVID-19 the first pandemic that evolves into a panzootic? Veterinaria Italiana. 2020.Is;56:7–8. doi: 10.12834/VetIt.2246.12523.1. [DOI] [PubMed] [Google Scholar]
- Halfmann P.J., Hatta M., Chiba S., Maemura T., Fan S., Takeda M., Kinoshita N., Hattori S.I., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Kawaoka Y. Transmission of SARS-CoV-2 in Domestic Cats. The New England Journal of Medicine. 2020;383:592–594. doi: 10.1056/NEJMc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer A.S., Quaade M.L., Rasmussen T.B., Fonager J., Rasmussen M., Mundbjerg K., Lohse L., Strandbygaard B., Jørgensen C.S., Alfaro-Núñez A., Rosenstierne M.W., Boklund A., Halasa T., Fomsgaard A., Belsham G.J., Bøtner A. SARS-CoV-2 Transmission between Mink (Neovison vison) and Humans, Denmark. Emerging Infectious Diseases. 2021;27:547–551. doi: 10.3201/eid2702.203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G.Z. Pangolins Harbor SARS-CoV-2-Related Coronaviruses. Trends in Microbiology. 2020;28:515–517. doi: 10.1016/j.tim.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Chu D.K.W., Perera R.A.P.M., Ko R.L.W., So R.T.Y., Ng B.C.Y., Chan S.M.S., Chu S., Alnaeem A.A., Alhammadi M.A., Webby R.J., Poon L.L.M., Balasuriya U.B.R., Peiris M. Coronavirus infections in horses in Saudi Arabia and Oman. Transboundry and Emerging Diseases. 2017;64:2093–2103. doi: 10.1111/tbed.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A., Smeenk I., Horzinek M.C., Rottier P.J., de Groot R.J. Feline coronavirus type II strains 79–1683 and 79–1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. Journal of Virology. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T.Y., Jia N., Zhang Y.W., Shum M.H., Jiang J.F., Zhu H.C., Tong Y.G., Shi Y.X., Ni X.B., Liao Y.S., Li W.J., Jiang B.G., Wei W., Yuan T.T., Zheng K., Cui X.M., Li J., Pei G.Q., Qiang X., Cheung W.Y., Li L.F., Sun F.F., Qin S., Huang J.C., Leung G.M., Holmes E.C., Hu Y.L., Guan Y., Cao W.C. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Larsen, H.D., Fonager, J., Lomholt, F.K., Dalby, T., Benedetti, G., Kristensen, B., Urth, T.R., Rasmussen, M., Lassaunière, R., Rasmussen, T.B., Strandbygaard, B., Lohse, L., Chaine, M., Møller, K.L., Berthelsen, A.N., Nørgaard, S.K., Sönksen, U.W., Boklund, A.E., Hammer, A.S., Belsham, G.J., Krause, T.G., Mortensen, S., Bøtner, A., Fomsgaard, A., Mølbak, K., 2021. Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020. Euro Surveillance 26, pii=2100009. [DOI] [PMC free article] [PubMed]
- Lauring A.S., Hodcroft E.B. Genetic variants of SARS-CoV-2-what do they mean? Journal of the American Medical Association. 2021;325:529–531. doi: 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]
- Lu S., Zhao Y., Yu W., Yang Y., Gao J., Wang J., Kuang D., Yang M., Yang J., Ma C., Xu J., Qian X., Li H., Zhao S., Li J., Wang H., Long H., Zhou J., Luo F., Ding K., Wu D., Zhang Y., Dong Y., Liu Y., Zheng Y., Lin X., Jiao L., Zheng H., Dai Q., Sun Q., Hu Y., Ke C., Liu H., Peng X. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduction and Targeted Therapy. 2020;5:157. doi: 10.1038/s41392-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M., Tiwari R., Chaicumpa W. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. The Veterinary Quarterly. 2020;40:68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty, S., 2020. COVID mink analysis shows mutations are not dangerous — yet. Nature news. Retrieved on 15 December 2020, from https://doi.org/10.1038/d41586-020-03218-z. [DOI] [PubMed]
- Molenaar R.J., Vreman S.R., Hakze-van der Honing W., Zwart R., De Rond J., Weesendorp W., Smit L.A.M., Koopmans M., Bouwstra R., Stegeman A., Van der Poel W.H.M. Clinical and Pathological Findings in SARS-CoV-2 Disease Outbreaks in Farmed Mink (Neovison vison) Veterinary Pathology. 2020;57:653–657. doi: 10.1177/0300985820943535. [DOI] [PubMed] [Google Scholar]
- Munster V.J., Feldmann F., Williamson B.N., van Doremalen N., Pérez-Pérez L., Schulz J., Meade-White K., Okumura A., Callison J., Brumbaugh B., Avanzato V.A., Rosenke R., Hanley P.W., Saturday G., Scott D., Fischer E.R., de Wit E. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585:268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn, A.Z., Lamers, M.M., Okba, N.M.A., Breugem, T.I., Schipper, D., van den Doel, P.B., van Run, P., van Amerongen, G., de Waal, L., Koopmans, M.P.G., Stittelaar, K.J., van den Brand J.M.A., Haagmans, B.L., 2020. Susceptibility of rabbits to SARS-CoV-2 bioRxiv [Preprint] 2020.08.27.263988; https://doi.org/10.1101/2020.08.27.263988. [DOI] [PMC free article] [PubMed]
- Netherlands Food and Consumer Product Safety Authority, 2020. Bedrijfsmatig gehouden dieren en SARS-CoV-2 NVWA, Retrieved on 15 January 2021, from www.nvwa.nl/nieuws-en-media/actuele-onderwerpen/corona/g/bedrijfsmatig-gehouden-dieren-en-corona.
- OIE, 2019. COVID-19 Portal. Events in animals. Retrieved on 25 January 2020, form https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/events-in-animals/.
- OIE, 2021. SARS-CoV-2, Poland. Retrieved on 25 January 2020, form https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=38036.
- Oreshkova N., Molenaar R.J., Vreman S., Harders F., Oude Munnink B.B., Hakze-van der Honing R.W., Gerhards N., Tolsma P., Bouwstra R., Sikkema R.S., Tacken M.G., de Rooij M.M., Weesendorp E., Engelsma M.Y., Bruschke C.J., Smit L.A., Koopmans M., van der Poel W.H., Stegeman A. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance. 2020;25:2001005. doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., van der Spek A., Tolsma P., Rietveld A., Brouwer M., Bouwmeester-Vincken N., Harders F., Hakze-van der Honing R., Wegdam M.C.A., Bouwstra R.J., Geurtsvan K.C., van der Eijk A.A., Velkers F.C., Smit L.A.M., Stegeman A., van der Poel W.H.M., Koopmans M.P.G. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371:172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E.I., Elia G., Grassi A., Giordano A., Desario C., Medardo M., Smith S.L., Anderson E.R., Prince T., Patterson G.T., Lorusso E., Lucente M.S., Lanave G., Lauzi S., Bonfanti U., Stranieri A., Martella V., Solari Basano F., Barrs V.R., Radford A.D., Agrimi U., Hughes G.L., Paltrinieri S., Decaro N. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nature Communications. 2020;11:6231. doi: 10.1038/s41467-020-20097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ProMED, 2020a. PRO/AH/EDR> COVID-19 update (319): Spain (AR) animal, farmed mink, 1st rep. Retrieved on 25 January 2021, from https://promedmail.org/promed-post/?id=7584560.
- ProMED, 2020b. PRO/AH/EDR> COVID-19 update (266): Denmark (ND) animal, farmed mink, 1st rep. Retrieved on 25 January 2021, from https://promedmail.org/promed-post/?id=20200617.7479510.
- ProMED, 2020c. PRO/AH/EDR> COVID-19 update (490): animal, Greece (EM) mink, 1st report, OIE, assessment. Retrieved on 25 January 2021, from https://promedmail.org/promed-post/?id=20201115.7944705.
- ProMED, 2020d. PRO/AH/EDR> COVID-19 update (503): animal, France, mink, 1st rep. Retrieved on 25 January 2021, from https://promedmail.org/promed-post/?id=20201123.7965554.
- ProMED, 2020e. PRO/AH/EDR> COVID-19 update (284): Denmark (ND) animal, farmed mink, spread, dog. Retrieved on 5 January 2021, from https://promedmail.org/promed-post/?id=7506728.
- ProMED, 2020f. PRO/AH/EDR> COVID-19 update (458): animal, Italy, mink, RFI. Retrieved on 5 January 2021, from https://promedmail.org/promed-post/?id=7897986.
- ProMED, 2020g. PRO/AH/EDR> COVID-19 update (461): animal, Sweden, mink, 1st rep, RFI. Retrieved on 5 January 2021, from https://promedmail.org/promed-post/?id=20201030.7903582.
- ProMED, 2020h. PRO/AH/EDR> COVID-19 update (510): animal, mink, Lithuania, Poland, 1st reports, France, OIE. Retrieved on 5 January 2021, from https://promedmail.org/promed-posts/.
- ProMED, 2020i. PRO/AH/EDR> COVID-19 update (531): animal, Canada (BC) mink, OIE. Retrieved on 5 January 2021, from https://promedmail.org/promed-post/?id=8008864.
- ProMED, 2021. PRO/AH/EDR> COVID-19 update (47): Poland (PM) animal, mink, OIE. Retrieved on 4 February 2021, from https://promedmail.org/promed-post/?id=8162830.
- Richard M., Kok A., De Meulder D., Bestebroer T.M., Lamers M.M., Okba N.M.A., Fentener, Van Vlissingen M., Rockx B., Haagmans B.L., Koopmans M.P.G., Fouchier R.A.M., Herfst S. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nature Communications. 2020;11:3496. doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Arrondo I., Portillo A., Palomar A.M., Santibáñez S., Santibáñez P., Cervera C., Oteo J.A. Detection of SARS-CoV-2 in pets living with COVID-19 owners diagnosed during the COVID-19 lockdown in Spain: A case of an asymptomatic cat with SARS-CoV-2 in Europe. Transboundary and Emerging Diseases. 2021;68:973–976. doi: 10.1111/tbed.13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailleau C., Dumarest M., Vanhomwegen J., Delaplace M., Caro V., Kwasiborski A., Hourdel V., Chevaillier P., Barbarino A., Comtet L., Pourquier P., Klonjkowski B., Manuguerra J.C., Zientara S., Le Poder S. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transboundry Emerging Diseases. 2020;67:2324–2328. doi: 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottau K., Rissmann M., Graaf A., Schön J., Sehl J., Wylezich C., Höper D., Mettenleiter T.C., Balkema-Buschmann A., Harder T., Grund C., Hoffmann D., Breithaupt A., Beer M. Experimental transmission studies of SARS-CoV-2 in fruit bats, ferrets, pigs and chickens. Lancet Microbe. 2020;1:e218–e225. doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalés J., Puig M., Rodon J., Avila-Nieto C., Carrillo J., Cantero G., Terrón M.T., Cruz S., Parera M., Noguera-Julián M., Izquierdo-Useros N., Guallar V., Vidal E., Valencia A., Blanco I., Blanco J., Clotet B., Vergara-Alert J. Detection of SARS-CoV2 in a cat owned by a COVID-19-affected patient in Spain. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:24790–24793. doi: 10.1073/pnas.2010817117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharun K., Tiwari R., Natesan S., Dhama K. SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the One Health approach during the ongoing COVID-19 pandemic. The Veterinary Quarterly. 2021;41:50–60. doi: 10.1080/01652176.2020.1867776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., Zhao Y., Liu P., Liang L., Cui P., Wang J., Zhang X., Guan Y., Tan W., Wu G., Chen H., Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia S.F., Yan L.-M., Chin A.W.H., Fung K., Choy K.T., Wong A.Y.L., Kaewpreedee P., Perera R.A.P.M., Poon L.L.M., Nicholls J.M., Peiris M., Yen H.L. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema R.S., Pas S.D., Nieuwenhuijse D.F., O’Toole A., Verweij J.J., van der Linden A., Chestakova I., Schapendonk C., Pronk M., Lexmond P., Bestebroer T., Overmars R.J., van Nieuwkoop S., van den Bijllaardt W., Bentvelsen R.G., van Rijen M.M.L., Muiting A.G., van Oudheusden A.J.G., Diederen B.M., Bergmans A.M.C., van der Eijk A., Molenkamp R., Rambaut A., Timen A., Kluytmans J.A.J.W., Oudo Munnink B.B., Kluytmans van den Bergh M.F.Q., Koopmans M.P.G. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: A cross-sectional study. The Lancet Infectious Diseases. 2020;20:1273–1280. doi: 10.1016/S1473-3099(20)30527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit T.H.C., Brackman C.J., Ip S.M., Tam K.W.S., Law P.Y.T., To E.M.W., Yu V.Y.T., Sims L.D., Tsang D.N.C., Chu D.K.W., Perera R.A.P.M., Poon L.L.M., Peiris M. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez D.L., Pantin-Jackwood M.J., Swayne D.E., Lee S.A., Deblois S.M., Spackman E. Lack of susceptibility of poultry to SARS-CoV-2 and MERS-CoV. Emerging Infectious Diseases. 2020;26:3074–3076. doi: 10.3201/eid2612.202989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlewicz-Podbielska H., Pomorska-Mól M. Porcine coronaviruses: overview of the state of the art. Virolologica Sinica. 2021 doi: 10.1007/s12250-021-00364-0. Published online by Springer Nature 15 March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich L., Wernike K., Hoffmann D., Mettenleiter T.C., Beer M. Experimental Infection of Cattle with SARS-CoV-2. Emerging Infectious Diseases. 2020;26:2979–2981. doi: 10.3201/eid2612.203799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Yip C.C., Tsoi H., Chan K., Yuen K. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. Journal of Virology. 2006;80:7136–7145. doi: 10.1128/JVI.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey C., Borisevich V., Prasad A.N., Agans K.N., Deer D.J., Dobias N.S., Heymann J.C., Foster S.L., Levine C.B., Medina L., Melody K., Geisbert J.B., Fenton K.A., Geisbert T.W., Cross R.W. Establishment of an African green monkey model for COVID19 and protection against re-infection. Nature Immunology. 2021;22:86–98. doi: 10.1038/s41590-020-00835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang J., Kuang D., Xu J., Yang M., Ma C., Zhao S., Li J., Long H., Ding K., Gao J., Liu J., Wang H., Li H., Yang Y., Yu W., Yang J., Zheng Y., Wu D., Lu S., Liu H., Peng X. Susceptibility of tree shrew to SARS-CoV-2 infection. Scientific Reports. 2020;10:16007. doi: 10.1038/s41598-020-72563-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang H., Huang K., Yang Y., Hui X., Gao J., He X., Li C., Gong W., Zhang Y., Peng C., Gao X., Chen H., Zou Z., Shi Z., Jin M. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerging Microbes and Infections. 2020;9:2013–2019. doi: 10.1080/22221751.2020.1817796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China. The New England Journal of Medicine. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]