Background:
The coronavirus disease (COVID-19) pandemic is currently a major challenge for health care systems around the world. For a time-sensitive emergency such as acute ischemic stroke (AIS), streamlined workflow times are essential to ensure good clinical outcomes.
Methods:
The aim of this single-center, retrospective, observational study was to describe changes in stroke workflow patterns and clinical care during the COVID-19 pandemic. Data from AIS patients undergoing emergent endovascular treatment (EVT) between 23 January and 8 April 2020 were retrospectively collected and compared with data from patients admitted during a similar period in 2019. The primary outcome was difference in time from symptom onset to recanalization. Secondary outcomes included workflow times, clinical management, discharge outcomes, and health-economic data.
Results:
In all, 21 AIS patients were admitted for emergent EVT during the 77-day study period, compared with 42 cases in 2019. Median time from symptom onset to recanalization was 132 minutes longer during the pandemic compared with the previous year (672 vs. 540 min, P=0.049). Patients admitted during the pandemic had a higher likelihood of endotracheal intubation (84.6% vs. 42.4%, P<0.05) and a higher incidence of delayed extubation after EVT (69.2% vs. 45.5%, P<0.05). National Institutes of Health Stroke Scale at hospital discharge was similar in the 2 cohorts, whereas neurointensive care unit stay was longer in patients admitted during the pandemic (10 vs. 7 days, P=0.013) and hospitalization costs were higher (123.9 vs. 95.2 thousand Chinese Yuan, P=0.052).
Conclusion:
Disruptions to medical services during the COVID-19 pandemic has particularly impacted AIS patients undergoing emergent EVT, resulting in increased workflow times. A structured and multidisciplinary protocol should be implemented to minimize treatment delays and maximize patient outcomes.
Key Words: acute ischemic stroke, endovascular treatment, workflow time, anesthesia, pandemic, COVID-19
A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first emerged in Wuhan, China in early December 2019. On March 11, 2020, due to the rapid spread of SARS-CoV-2, the World Health Organization declared a pandemic of coronavirus disease (COVID-19).1,2 As of October 2020, more than 37 million cases of COVID-19 and more than 1 million deaths had been reported globally.3 The COVID-19 pandemic is at different stages in different countries. It is unlikely to be a short-term pandemic, and a second wave of outbreaks may be inevitable.4
Patients with COVID-19 are at higher risk of ischemic stroke because of associated fibrinolysis, coagulopathy, and vascular endothelial dysfunction.5,6 The COVID-19 pandemic has generated a need to adjust treatment pathways to minimize risks of nosocomial transmission among health care staff and patients. As a consequence, established models of stroke care required substantial changes to minimize risk of health care provider infection without compromising quality of care.
We report a retrospective study of acute ischemic stroke (AIS) patients receiving endovascular treatment (EVT) during the COVID-19 pandemic. The primary outcome was the time from symptom onset to recanalization, and secondary outcomes included other workflow times, clinical management, discharge outcomes, and health-economic data.
METHODS
This retrospective observational study was approved by the ethics committee of Beijing Tiantan Hospital, Capital Medical University (KY 2020-036-02) on May 20, 2020. The requirement for informed consent was waived given the retrospective nature of the study. Patients were not subjected to investigational interventions as part of the study, and deidentified data were used.
Study Population
Data of AIS patients who underwent EVT at Beijing Tiantan Hospital, Capital Medical University between January 23, 2020 and April 8, 2020 were included in the study. This period coincided with the Chinese government lockdown in Wuhan and travel restrictions in most areas of China in an attempt to contain the spread of coronavirus. We also retrieved data of patients admitted during a similar 76-day period in the previous year (from February 3 to April 17, 2019) as a control group; these dates covered 1 day before and 76 days after the 2019 Spring Festival (Lunar New Year) as we wanted to avoid any effect of this holiday period. The data were retrieved by searching the “green pathway” in the electronic medical record system. The “green pathway” is a rapid access system for diagnosing and treating AIS patients at our institution; it was established early in the pandemic with multidisciplinary cooperation. The “green pathway” team members include those from neurointervention, neurosurgery, emergency care, radiology, anesthesiology, and the neurointensive care unit (NICU).
Outcomes
The data collected included stroke characteristics, comorbidities, anesthesia management, hemodynamic variables, recanalization rates, NICU length of stay, and hospitalization costs. Clinical information and workflow time, including time from symptom onset to door (arrival at hospital), door to groin puncture, symptom onset to recanalization, procedure duration (from groin puncture to incision suture), and recanalization success (defined as modified treatment in cerebral infarction 2b to 3) were retrieved from the medical records. Pretreatment and discharge neurological status was evaluated using the National Institutes of Health Stroke Scale (NIHSS). Epidemiological information, including possible exposure to SARS-CoV-2, body temperature on admission, blood count, and pulmonary x-ray or computed tomography (CT) imaging, was collected from the COVID-19 screening data. Delayed extubation was defined as failure to extubate those patients that received general anesthesia (GA) at the end of the EVT procedure.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics version 24.0 software (IBM Corp., Armonk, NY). The Kolmogorov-Smirnov test was used to test data normality. Normally distributed continuous variables are reported as mean with SD and compared using 2-sample independent t tests. Non-normally distributed variables are reported as median with the interquartile range and compared using the Mann-Whitney U test. Categorical variables are summarized as number (n) and percentages (%) and compared using the Pearson chi-square test or Fisher exact test. A P-value <0.05 was used to indicate statistical significance.
RESULTS
Twenty-one AIS patients underwent EVT (15 males and 6 females, median age 64 y) during the pandemic study period, and 42 in the previous year (control). Baseline characteristics were similar between the 2 patient cohorts (pandemic vs. control). Comorbidities, including hypertension, atrial fibrillation, coronary artery disease, and diabetes mellitus, were also similar between the 2 groups, but a higher proportion of patients in the pandemic cohort had dyslipidemia (61.9% vs. 31%; P<0.05). Anticoagulant and antiplatelet therapy use before stroke onset and pre-EVT bridging with intravenous thrombolysis were similar between the 2 groups. Although there was a higher proportion of wake-up strokes during the pandemic, admission NIHSS scores were comparable (Table 1).
TABLE 1.
Baseline Characteristics
| During the Pandemic (n=21) | 2019 (n=42) | |
|---|---|---|
| Age (y) | 64 (54-72) | 65 (56-74) |
| Male | 15 (71.4) | 31 (73.8) |
| Height (cm) | 169 (161-175) | 170 (162-172) |
| Weight (kg) | 75 (65-88) | 70 (65-80) |
| BMI | 26.80 (23.3-29.3) | 25.2 (23.1-27.0) |
| Comorbidities | ||
| Hypertension | 17 (81.0) | 33 (78.6) |
| Atrial fibrillation | 9 (42.9) | 10 (23.8) |
| Coronary artery disease | 8 (38.1) | 8 (19.0) |
| Respiratory disease | 3 (14.3) | 3 (7.1) |
| Dyslipidemia* | 13 (61.9) | 13 (31.0) |
| Diabetes mellitus | 6 (28.6) | 9 (21.4) |
| History of smoking | 11 (52.4) | 24 (57.1) |
| History of alcohol consumption | 10 (47.6) | 21 (50.0) |
| Medicine history | ||
| Anticoagulant | 5 (23.8) | 8 (19.0) |
| Antiplatelet | 3 (14.3) | 9 (21.4) |
| Bridging IVT | 7 (33.3) | 15 (35.7) |
| Wake-up stroke* | 7 (33.3) | 3 (7.1) |
| Local patients (from Beijing area) | 14 (66.7) | 21 (50.0) |
| Pretreatment NIHSS | 12 (11-15) | 15 (12-18) |
Data presented as median (interquartile range) or number (%).
BMI indicates body mass index; IVT, intravenous thrombolysis; MRS, modified Ranking Scale; NIHSS, National Institutes of Health Stroke Scale.
Between-group P<0.05.
Despite similarities in the site of occlusion and successful recanalization rates between the two groups, there were clear differences in workflow times (Table 2). Median time from symptom onset to recanalization was 132 minutes longer during the pandemic compared with the previous year (672 vs. 540 min, P=0.049). Symptom onset to door time was 50 minutes longer, door to groin-puncture time 24 minutes longer, and procedure duration 14 minute longer during the pandemic; the differences in these time compared with those in 2019 were not significantly significant. NICU stay was longer during the pandemic (10 d vs. 7 d, P=0.013), and hospitalization costs were 28.7 thousand Chinese Yuan (CNY) more (123.9 thousand CNY vs. 95.2 thousand CNY, P=0.052). NIHSS scores at discharge were similar. One patient died during the pandemic due to cardiac causes, and one died from stroke in the 2019 cohort.
TABLE 2.
Treatment, Workflow Times and Outcomes
| During the Pandemic (n=21) | Control (n=42) | |
|---|---|---|
| Mode of anesthesia | ||
| GA | 13 (61.9) | 33 (78.6) |
| MAC | 8 (38.1) | 9 (21.4) |
| Pretreatment intubation | 0 (0.0) | 1 (2.4) |
| Airway management* † | ||
| Intubation | 11/13 (84.6) | 14/33 (42.4) |
| LMA | 2/13 (15.3) | 19/33 (57.6) |
| Delayed extubation† | 9/13 (69.2) | 15/33 (45.5) |
| Conversion from MAC to GA | 1 (4.8) | 3 (7.1) |
| Site of occlusion | ||
| Anterior circulation | 17 (81.0) | 37 (88.1) |
| Posterior circulation | 4 (19.0) | 5 (11.9) |
| Workflow times (min) | ||
| Symptom onset to door | 260 (185-1039) | 210 (120-361) |
| Emergency door to groin puncture | 252 (120-276) | 228 (150-312) |
| Symptom onset to recanalization* | 672 (516-1796) | 540 (450-734) |
| Procedure duration | 121 (68-173) | 107 (77-170) |
| Recanalization (mTICI 2b-3) | 17 (81.0) | 32 (76.2) |
| Outcomes | ||
| NIHSS at discharge | 7 (3-11) | 7 (5-14) |
| In-hospital mortality | 1 (4.8) | 1 (2.4) |
| Health-economics | ||
| NICU length of stay (d)* | 10 (7-24) | 7 (4-13) |
| Hospitalization cost, (thousand CNY) | 123.9 (79.1-165.4) | 95.2 (66.0-124.2) |
Data presented as median (interquartile range) or number (%).
Thirteen cases during the pandemic and 33 cases in 2019 who received GA.
CNY indicates Chinese Yuan; GA, general anesthesia; LMA, laryngeal mask airway; MAC, monitored anesthesia care; mTICI, modified treatment in cerebral infarction; NICU, neurointensive care unit; NIHSS, National Institutes of Health Stroke Scale.
Between-group P<0.05.
There were no significant differences between the pandemic and 2019 cohorts in the incidence of pretreatment intubation, conversion from monitored anesthesia care to GA (Table 2), or in blood pressure before/during EVT and after recanalization (Fig. 1). Endotracheal intubation and mechanical ventilation were more common among patients receiving GA during the pandemic than in 2019 (84.6% vs. 42.4%). Moreover, of the patients receiving GA during the pandemic, 69.2% experienced delayed extubation compared with 45.5% in 2019 (P=0.146).
FIGURE 1.
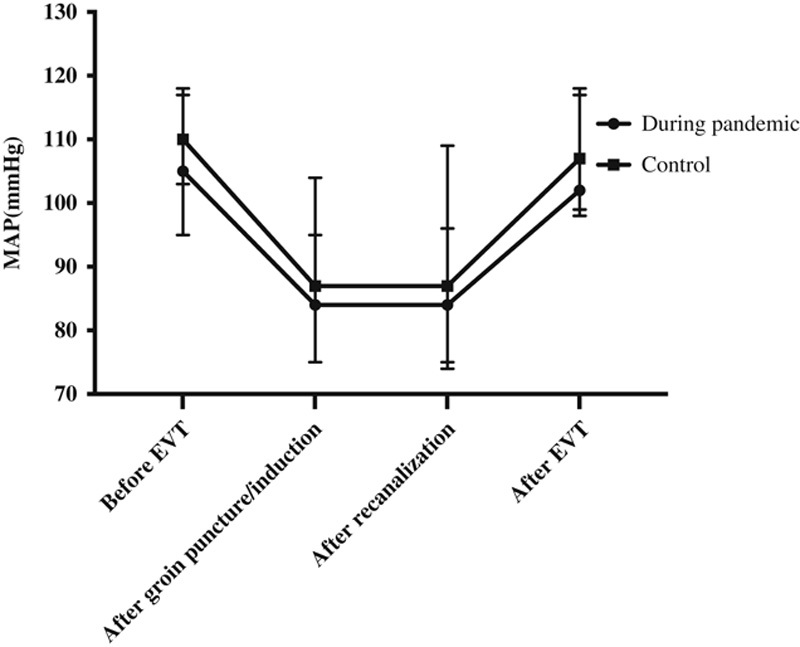
Intraoperative hemodynamics in the 2 groups. During the pandemic (2020), MAP before EVT, after groin puncture/induction, after recanalization and after EVT were 105 (95 to 117) mm Hg, 84 (75 to 104) mm Hg, 84 (75 to 109) mm Hg, and 102 (99 to 118) mm Hg, respectively. In the previous year (2019), MAP before EVT, after groin puncture/induction, after recanalization and after EVT were 110 (103 to 118) mm Hg, 87 (75 to 95) mm Hg, 87 (74 to 96) mm Hg and 107 (98 to 117) mm Hg, respectively. Data are expressed as median and interquartile range. EVT indicates endovascular treatment; MAP, mean arterial pressure.
In the pandemic cohort, all patients tested negative for COVID-19 despite an epidemiologic history of possible exposure to SARS-CoV-2; 9 patients did not receive nucleic acid tests at the beginning of the pandemic because of inadequate testing capabilities. All patients had a normal body temperature (<37.3°C) at admission to hospital, 8 (38.1%) had lymphopenia (lymphocytes <1.1×109/L), 10 (47.6%) had leukocytosis (leukocytes >9.5×109/L), and 8 (38.1%) had inflammatory infiltrates on pulmonary radiology. Nine (42.9%) patients in the pandemic cohort underwent intervention in the usual central facility before February 28, and the remaining 12 were treated in an isolated angiography suite dedicated for the treatment of AIS patients during the pandemic when this became available.
DISCUSSION
In this retrospective study, we analyzed 21 AIS patients who underwent EVT during the COVID-19 pandemic (2020) and compared them with patients admitted for EVT during a similar period in the previous year (2019). Patients admitted during the pandemic had a longer time between symptom onset and recanalization, longer NICU length of stay, and higher hospitalization costs.
A previous survey reported a 10% to 30% decrease in AIS cases worldwide and a 50% to 60% reduction in China during the pandemic7,8 similar to the 50% decrease in cases in our study. Reasons for the reduction in case numbers include fear of COVID-19 infection among patients and disruption to stroke care systems following pressure from the large number of patients with COVID-19. These reasons might also contribute to the delays in arrival at hospital after stroke onset during the pandemic. Suspension of stroke care in other centers also led to disruption of the “drip and ship” approach to stroke care, that is patients receiving early thrombolysis at the closest primary stroke center before transfer to a comprehensive stroke center; this might also have contributed to delays in patients’ arrival at hospital.9
A triage system was established for emergency treatment at our institution early in the pandemic.10 An isolated emergency room, radiological examination suite, and angiography suite were designated for AIS patients to avoid delays to treatment and minimize the risk of nosocomial infection.11 In the first 38 days of the pandemic outbreak, the nucleic acid test for COVID-19 was available only for patients where the clinical suspicion of COVID-19 was high. During this time, medical staff needed more time to don personal protection equipment before treatment because all patients were treated as possible risk of COVID-19 infection.12,13
The anesthesia and interventional management of AIS were similar in the 2 study cohorts, but more patients underwent endotracheal intubation during the pandemic. A possible reason for this is that patients with delayed arrival at hospital might present with worse neurological status and/or respiratory dysfunction. Moreover, it was impossible to completely rule out COVID-19 infection before EVT. Therefore, intubation and avoidance of extubation in angiography suite immediately at the end of EVT were preferred to reduce possible aerosol contamination of the operation suite and minimize the risk of nosocomial infection.14,15 Meticulous blood pressure management is essential in AIS patients, and blood pressure targets before and after reperfusion have been recommended.16,17 SARS-CoV-2 invades human respiratory epithelial cells mediated by its S-proteins and angiotensin-converting enzyme 2 (ACE2) receptors.18,19 The decreased expression of ACE2 in COVID-19 patients with hypertension may contribute to the failure of antihypertension therapy, potentially leading to posttreatment hyperperfusion or even intracranial hemorrhage. However, in our study patients admitted during the pandemic had similar perioperative hemodynamics compared with patients admitted in the previous year.
Discharge NIHSS scores and in-hospital mortality were similar in the pandemic and 2019 cohorts, although the small sample size may have prevented us from identifying outcome differences. Moreover, we did not examine long-term neurological outcomes. Although the reasons for the longer post-EVT NICU length of stay that we observed during the pandemic are unclear, it is possible that longer time from symptom onset to recanalization or longer intubation times were contributing factors.
This study has several limitations. First, it was an observational study so we cannot exclude selection and information bias. Second, we only assessed early neurological outcome with the NIHSS score, and a longer-term follow-up is required to further evaluate the effect of the COVID-19 pandemic on AIS outcomes. Third, early outcomes and NICU stays may have been different between cohorts because of differences in vascular occlusion sites; posterior circulation occlusion patients may suffer severe respiratory dysfunction, unconsciousness, and other neurological disorders. Finally, the sample size of this study was small, which reduces its power and increases the margin of error.
CONCLUSION
AIS is a time-sensitive emergency. Disruptions to various aspects of medical services during the COVID-19 pandemic have particularly impacted patients undergoing emergent EVT and resulted in increased workflow times. A structured and multidisciplinary protocol should be implemented to minimize treatment delays and maximize patient outcomes. Our observation of a lack of impact on neurological outcomes of the prolonged symptom onset to recanalization times during the pandemic should be interpreted with caution given the limitations of this study. We recommend that all efforts should be made to facilitate early reperfusion while accounting for measures to minimize the risk of nosocomial infection.
Footnotes
This study was supported by the Beijing Municipal Science and Technology Commission (Z191100006619068) and Beijing Municipal Administration of Hospitals (DFL20180502). The study investigators did not receive compensation for participating in the study. No funding agencies had any role in the design or conduction of the study, study management, data collection, analysis, interpretation, decision to publish, or preparation of the manuscript.
S.L. and M.Z. contributed equally to the manuscript as co-first authors.
Y.P. and R.H. contributed equally to the manuscript.
R.H. is a member of the Editorial Board of Journal of Neurosurgical Anesthesiology. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Li Z, Wang Y, et al. Chinese Stroke Center Alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol. 2018;3:256–262 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Organization WH. Weekly Epidemiological and Operational updates-Coronavirus disease 2019 (COVID-19). 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201012-weekly-epi-update-9.pdf Accessed November 11, 2020.
- 4. Vaid S, McAdie A, Kremer R, et al. Risk of a second wave of Covid-19 infections: using artificial intelligence to investigate stringency of physical distancing policies in North America. Int Orthop. 2020;44:1581–1589 10.1007/s00264-020-04653-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Stroke Organization. The Global Impact of COVID-19 on Stroke—Survey Report from Prof. Marc Fischer, WSO President-Elect, May 04, 2020. Available at: https://www.world-stroke.org/news-and-blog/news/the-global-impact-of-covid-19-on-stroke-survey. Accessed November 11, 2020.
- 8. Zhao J, Li H, Kung D, et al. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;51:1996–2001 10.1161/STROKEAHA.120.030225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teo KC, Leung WCY, Wong YK, et al. Delays in stroke onset to hospital arrival time during COVID-19. Stroke. 2020;51:2228–2231. doi: 10.1161STROKEAHA.120.030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Chen Y, Li Z, et al. Providing uninterrupted care during COVID-19 pandemic: experience from Beijing Tiantan Hospital. Stroke Vasc Neurol. 2020;5:180–184 10.1136/svn-2020-000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma D, Rasmussen M, Han R, et al. Anesthetic management of endovascular treatment of acute ischemic stroke during COVID-19 pandemic: consensus statement from Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32:193–201 10.1097/ANA.0000000000000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu L, Wang D, Brainin M, et al. Approaches to global stroke care during the COVID-19 pandemic. Stroke Vasc Neurol. 2020;5:107–109. doi: 10.1136/svn-2020-000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leira EC, Russman AN, Biller J, et al. Preserving stroke care during the COVID-19 pandemic: potential issues and solutions. Neurology. 2020;95:124–133. doi: 10.1212/WNL.0000000000009713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zuo MZ, Huang YG, Ma WH, et al. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin Med Sci J. 2020;34:599–606 10.24920/003724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Li J, Zhou M, et al. Summary of 20 tracheal intubation by anesthesiologists for patients with severe COVID-19 pneumonia: retrospective case series. J Anesth. 2020;34:599–606 10.1007/s00540-020-02778-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saver JL, Altman H. Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke. 2012;43:1537–1541 10.1161/STROKEAHA.111.636928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]


