Abstract
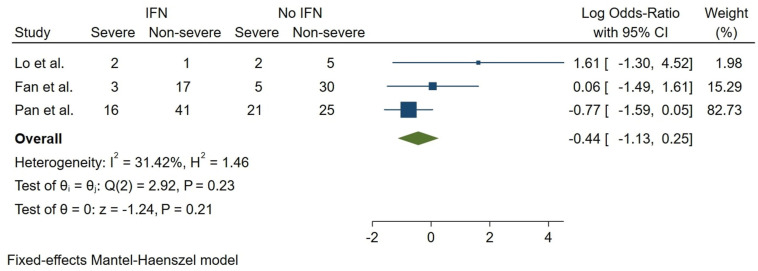
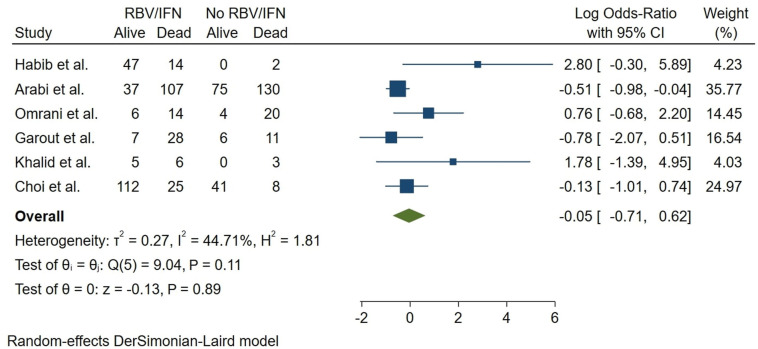
Concern regarding coronavirus (CoV) outbreaks has stayed relevant to global health in the last decades. Emerging COVID-19 infection, caused by the novel SARS-CoV2, is now a pandemic, bringing a substantial burden to human health. Interferon (IFN), combined with other antivirals and various treatments, has been used to treat and prevent MERS-CoV, SARS-CoV, and SARS-CoV2 infections. We aimed to assess the clinical efficacy of IFN-based treatments and combinational therapy with antivirals, corticosteroids, traditional medicine, and other treatments. Major healthcare databases and grey literature were investigated. A three-stage screening was utilized, and included studies were checked against the protocol eligibility criteria. Risk of bias assessment and data extraction were performed, followed by narrative data synthesis. Fifty-five distinct studies of SARS-CoV2, MERS-CoV, and SARS-CoV were spotted. Our narrative synthesis showed a possible benefit in the use of IFN. A good quality cohort showed lower CRP levels in Arbidol (ARB) + IFN group vs. IFN only group. Another study reported a significantly shorter chest X-ray (CXR) resolution in IFN-Alfacon-1 + corticosteroid group compared with the corticosteroid only group in SARS-CoV patients. In a COVID-19 trial, total adverse drug events (ADEs) were much lower in the Favipiravir (FPV) + IFN-α group compared with the LPV/RTV arm (P = 0.001). Also, nausea in patients receiving FPV + IFN-α regimen was significantly lower (P = 0.03). Quantitative analysis of mortality did not show a conclusive effect for IFN/RBV treatment in six moderately heterogeneous MERS-CoV studies (log OR = −0.05, 95% CI: (−0.71,0.62), I2 = 44.71%). A meta-analysis of three COVID-19 studies did not show a conclusive nor meaningful relation between receiving IFN and COVID-19 severity (log OR = −0.44, 95% CI: (−1.13,0.25), I2 = 31.42%). A lack of high-quality cohorts and controlled trials was observed. Evidence suggests the potential efficacy of several combination IFN therapies such as lower ADEs, quicker resolution of CXR, or a decrease in inflammatory cytokines; Still, these options must possibly be further explored before being recommended in public guidelines. For all major CoVs, our results may indicate a lack of a definitive effect of IFN treatment on mortality. We recommend such therapeutics be administered with extreme caution until further investigation uncovers high-quality evidence in favor of IFN or combination therapy with IFN.
Keywords: COVID-19, IFN, Interferon, MERS-CoV, Middle-east respiratory syndrome, SARS-CoV, SARS-CoV2, Severe acute respiratory syndrome
1. Introduction
Coronaviruses (CoVs) are single-stranded, positive-sense, RNA containing, and enveloped viruses responsible for several major global outbreaks (Poutanen, 2012; Raoult et al., 2020). Global epidemics of atypical pneumonia were first caused by SARS-CoV1 and MERS-CoV in 2002 and 2012, respectively (Al-Osail and Al-Wazzah, 2017; Huang, 2004), and continued to affect the globe with MERS-CoV reappearing in South Korea in 2015 (Ki, 2015). Recently, coronavirus disease 2019 (COVID-19), a disease caused by a novel variant of SARS-CoV known as SARS-CoV2, emerged in Wuhan, China (Cascella et al., 2020; Hanaei and Rezaei, 2020). While showing a lower mortality rate (2.3%) compared to MERS-CoV (9.5%) and SARS-CoV1 (34.4%), the COVID-19 pandemic has raised significant concern. The concern is partly due to the high spreading potential of SARS-CoV2, which influences and causes mortality in a significantly larger population (Petrosillo et al., 2020). The novel virus has an undetermined clinical presentation (Lotfi and Rezaei, 2020), as the recent evidence has suggested non-respiratory and asymptomatic presentations (Wang et al., 2020a). Hence, the diversity in the presentations and hurdles in detecting the virus (Basiri et al., 2020a) suggest the high importance of an effective onset-to-treat period regarding the treatment of COVID-19 patients (Saleki et al., 2020).
Numerous novel efforts have been carried out in the fields of drug discovery, vaccine development (Rahmani et al., 2021), and repurposing of previously suggested candidates for SARS- and MERS-CoV infections. Indeed, researchers have evaluated pharmacologic options, comprising combination interferon (IFN) therapy, traditional medicine, corticosteroid therapy, and antivirals such as ribavirin (RBV), lopinavir (LPV), ritonavir (RTV), oseltamivir, and Remdesivir (REM). However, to date, such efforts have not brought forth adequate success. Nevertheless, several protocols of past curatives are being used for COVID-19 patients due to a lack of effective treatments or alternatives when extreme adverse drug events (ADE) are indicated. The innate immune system comprises inflammasomes (Rasoulinejad et al., 2020), cytokines, and IFNs which help to clear viral disease and provide multi-system immunological protection (Kopitar-Jerala, 2017; Rostamtabar et al., 2021). It has been shown that SARS-CoV2 is sensitive to type I IFN therapy in human cell lines (Mantlo et al., 2020). A strong association between low type I IFN production and COVID-19 severity has been reported (Bastard et al., 2020; Bost et al., 2020; Zhang et al., 2020b). Nuclear factor-kappa light chain enhancer B (NF-kB) activation in the dendritic cells is crucial for large scale type I IFN production. In a study of COVID-19 by Meyts et al. patients with NF-kB1 or 2 mutations required hospitalization (Meyts et al., 2021), highlighting the functional role of IFNs. Administration with subcutaneous IFN β-1a has been shown to reduce morbidity in COVID-19 infected patients (Davoudi-Monfared et al., 2020). Lung infection in COVID-19 may evolve into systemic involvement. Also, IFNs specially IFN-α2b are capable of preventing lung abnormalities in such patients (Zhou et al., 2021). All of these statements emphasize the role of IFN therapy in severe acute CoVs disease. In addition to lungs, other organs like kidneys (Han and Ye, 2021), liver (Li and Xiao, 2020), and the brain (Baig et al., 2020; Saleki et al., 2020) are also involved. A major entry pathway for SARS-CoV2 is angiotensin-converting enzyme 2 (ACE2), which is present in multiple systems throughout the body. Research has shown IFNs can significantly alter ACE2 profile. ACE2 is regarded as an interferon-stimulated gene (ISG) (Ziegler et al., 2020). Thus, interferon-induced alteration in ACE2 production may be crucial for liability to COVID-19 or its corresponding adverse outcomes (Onabajo et al., 2020). Taken together, noteworthy for future research is that IFNs could play a crucial role in multi-organ involvement prevention of patients with COVID-19. The probable role of IFNs in the multi-organ involvement situation has been enlaced in Fig. 1 . Intriguingly, despite contradicting in vitro and in vivo studies and the absence of sufficient high-quality randomized controlled trials (RCTs) for the use of IFNs to treat SARS-CoV2, and that several studies indicate that it is not suggested for COVID-19 therapy, antivirals such as RBV have been commonly used in combination with IFN during epidemics (Arabi et al., 2020; Morra et al., 2018; Totura and Bavari, 2019). Also, combination therapies in RCTs have been undertaken for the novel CoV (e.g., NCT04276688). Surprisingly, current Chinese guidelines include IFNs as an alternative for combination therapy (WHO, 2020). Such efforts have led to rapidly increasing clinical data on IFN administration for COVID-19 cases. Notably, CoV outbreaks share remarkable similarities, and hence, investigating the experience with the previous spreading of SARS- and MERS-CoVs may assist in discovering an effective treatment or help determine if a candidate should be removed from treatment protocols (Omrani and Shalhoub, 2015). To our knowledge, there have not been any updated systematic reviews of the literature shedding light on the effectiveness of IFN therapy with the past outbreaks in mind. In the present systematic review and quantitative analysis of the evidence, we describe the characteristics of hospitalized cases with MERS-CoV, SARS-CoV1, and SARS-CoV2 patients and assess important treatment outcomes and ADEs of various combinational and non-combinational IFN treatments.
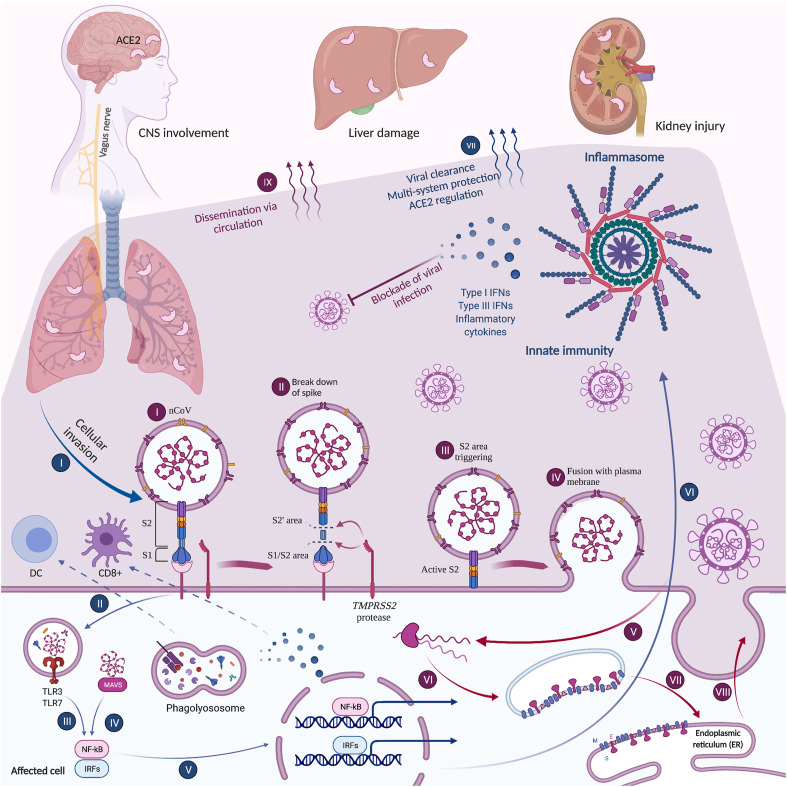
Fig. 1.
Role of IFNs and other innate immunity elements in multi-system CoV disease
Among innate elements, inflammasomes, ILs, and IFNs are of high importance (Rasoulinejad et al., 2020). Innate immunity helps to prevent the spread of viruses and affects ACE2 − a major entry path for SARS-CoV2. Therefore, impairments in these elements may contribute to severe clinical disease. COVID-19 similar to its ancestors, may utilize ACE2, which can be mediated by IFN secretion. IFN may inhibit the replication chain. Retrograde synaptic pathway through which SARS-CoV2 infects the central nervous system (CNS) also involves ACE2. Here, dissemination of COVID-19 and its replication have been illustrated (indigo, I-IX). Also, the activation of innate pathways that upregulate IFNs, inflammasome elements, and cytokines has been illustrated (blue, I-VII). Created with BioRender.com.
2. Materials and methods
The present systematic review has been conducted compatible with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements (Table S1). We designed the protocol to determine our scope, inclusion and exclusion criteria, and outcomes of evaluated studies. The protocol for the present study is provided in further detail in Supplementary Material.
The present study aimed to assess the outcomes of IFN treatments or IFN combination therapies in hospitalized patients infected with MERS-CoV, SARS-CoV, and SARS-CoV2. Comparator therapies comprised placebo, sham therapy, and no intervention. Moreover, researches involving no comparator group were included. Outcome measures were selected according to our protocol. We assessed the efficacy of IFN therapies with or without combination with other pharmacotherapy options. As efficacy comprises numerous parameters, we took account many clinical outcomes, including mortality, discharge, CXR, hospital durations, inflammatory state, ADEs, and disease severity. Due to limited data and the emerging situation of the COVID-19 pandemic, both published and unpublished works were included. No restrictions were considered for the date of publication and language. Our classification for treatment regimens was in line with World Health Organization (WHO) Guidelines. For SARS-CoV, these groups included RBV, LPV/RTV (Kaletra), corticosteroids, IFN, convalescent plasma, and intravenous immunoglobulin (IVIG), which have been previously utilized in similar studies (Stockman et al., 2006). MERS-CoV treatments included IFNs, RBV, LPV/RTV, polyclonal anti-MERS-CoV human immunoglobulins, humanized murine anti-S monoclonal antibodies, nucleoside viral RNA polymerase inhibitors (e.g., REM), peptide inhibitors (e.g., HR2P-M2), and mycophenolate mofetil (MMF) (Organization, 2019). Moreover, possible SARS-CoV2 interventions according to WHO and Centers for Disease Control and Prevention (CDC) Guidelines comprised hydroxychloroquine, chloroquine, REM, oseltamivir, tocilizumab, LPV/RTV, IFN-β, convalescent plasma, IVIG, and corticosteroids (Organization, 2011). Treatments were selected if used in combination with IFN. We included human studies designed as randomized and non-randomized clinical trials, observational clinical studies (e.g., retrospective and prospective cohorts), case reports, and case series.
2.1. Search strategy and study selection
In May 2020, five reviewers (K.S., S.Y., E.H., M.B., M.G.) performed a systematic search. PubMed, Scopus, Cochrane's library, Web of Science (WoS), Global Index Medicus (WHO library), Google Scholar, and Scopus were searched for articles. An additional search was done for unpublished work (e.g., from BioRxiv, MedRxiv), and Reference lists were also screened (grey literature). Unpublished articles were checked, and updated with the published version of each, if available. For all articles, corrections and retractions were also checked. For Google Scholar, the following search strings were developed with the help of a skilled librarian: (“interferon” OR “IFN”) AND (“Middle East respiratory syndrome” OR “Middle Eastern Respiratory Syndrome” OR “MERS-CoV” OR “Severe Acute Respiratory Syndrome” OR “SARS-CoV” OR “COVID-19”) AND (“Patient” OR “Case” OR “Human”) AND (clinical OR case) -“in vitro” -review -“narrative review” -monkey -“rat model” -mouse -polymorphism, String #2 “Ribavirin and interferon” AND (“Middle East respiratory syndrome” OR “Middle Eastern respiratory syndrome” OR “MERS-CoV” OR “Severe Acute Respiratory Syndrome” OR “SARS-CoV”)), and String 3# (“Interferon Alfacon-1” AND “SARS-COV” OR “MERS-COV”) -monkey -“review article”. We used hyphen, “-”, to exclude phrases associated with preclinical research, as hyphen equals NOT operator in Google Scholar. All final records were imported into EndNote X9 software (Thomson Reuters, San Francisco, CA). Results were collected after duplicate removal by authors (K.S., S.Y., E.H., M.B, M.G.). A three-step screening was followed to determine eligible results by examining each title, abstract, and full-text. Five reviewers (K.S., S.Y., E.H., M.B, M.G.) screened records separately, and disagreements were solved by referring to a third author (A.S.). All included studies were updated until March 2021 (Fan et al., 2020; Fan et al., 2021; Zhang et al., 2020a; Zhou et al., 2020a). Further detail for the search strategy is provided in Supplementary Material.
2.2. Data collection
The following information was retrieved for each study: first author's name, year of publication, location, type of study, the period of data collection, personnel, setting, essential intervals (e.g., onset to treat period), number of patients, gender, disease severity, contact history, comorbidities, diagnostic methods, symptoms, drug information (e.g., name, dosage, duration, along with route and frequency of administration), and non-drug interventions. The extracted outcomes of interest were mortality, the number of discharged patients, inflammatory cytokines, ADEs, and chest imaging results.
Data from full-text of 12 eligible studies were extracted in piloted forms by two reviewers (K.S., S.Y., E.H., M.B, M.G.), independently. Consensus agreement in extracted form was accomplished through discussion with a third-author (A.S.). Table S2 is the table of data extraction.
2.3. Quality assessment
To assess the risk of bias, the following tools were used for each study design: Cochrane risk of bias tool for randomized clinical trials (Sterne et al., 2019), risk of bias in non-randomized studies of interventions (ROBINS-I) tool for non-randomized trials (Sterne et al., 2016), Newcastle-Ottawa Scale (NOS) for Cohort Studies (Penson et al., 2018), National Institute of Health (NIH) tool for case-series and descriptive cross-sectional studies (National Heart), and a recently suggested tool for case reports (Murad et al., 2018).
The studies were further assessed according to the U.S. Preventive Services Task Force scoring protocol, in which Level of Evidence (LOE) is determined as follows (Mohamed et al., 2020a):
Level I: Evidence acquired from a minimum of one properly designed RCT;
Level II-1: Evidence acquired from properly-designed controlled trials with no randomization;
Level II-2: Evidence acquired from a properly-designed cohort or case-control analytic research, preferably from more than one center or study group;
Level II-3: Evidence acquired from multiple time series, both with or without the intervention. Dramatic outcomes in uncontrolled trials may also be taken as such kind of evidence;
Level III: Opinions of validated authorities, in accordance with clinical experience, descriptive research, or reports of expert groups.
2.4. Data synthesis
The protocol details methods used for narrative and quantitative syntheses (Supplementary Material) (College Station).
2.5. Risk of bias across studies
The tool developed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group (www.gradeworkinggroup.org) was selected for evaluation of bias across studies eligible for meta-analysis. GRADE enables consistent evaluation of the certainty of evidence. It also allows recommendations based on high-quality observational studies. GRADE initially ranks the evidence-based on study design. Studies are then promoted or downgraded according to criteria, including the risk of bias, indirectness, and imprecision (GradePro, 2020).
3. Results
3.1. Study selection
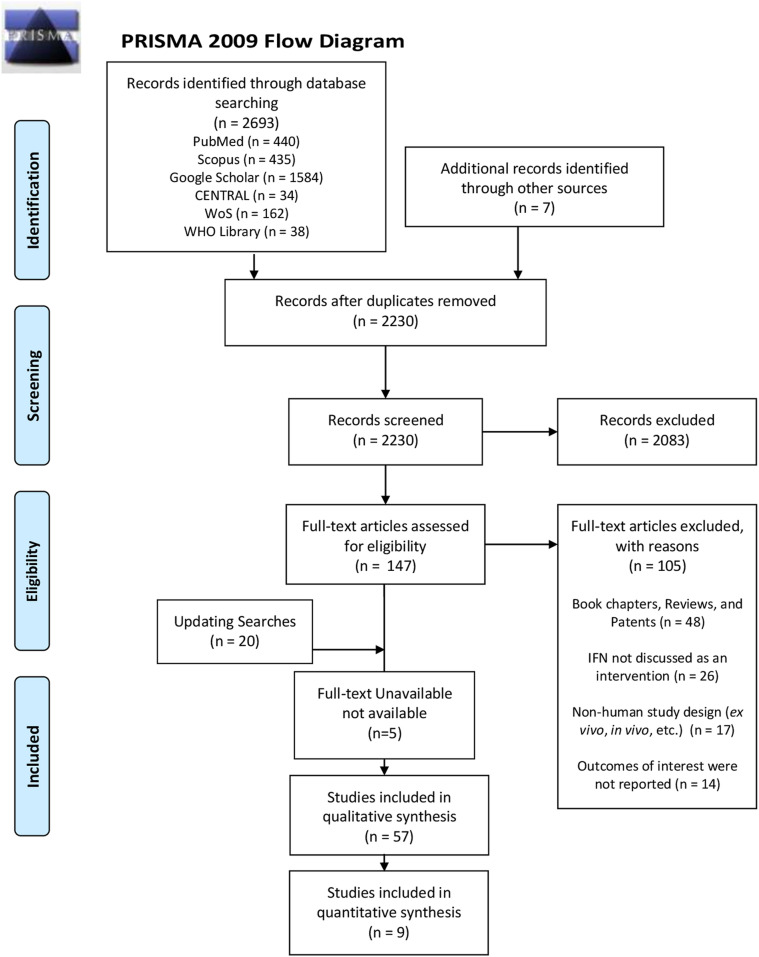
Our search strategy produced 2693 results from all six databases. Moreover, in addition to 42 initially included articles, our updated electronic search results identified 20 relevant results. An additional search yielded seven results. For five studies, full-text could not be obtained (Fig. 2 ) (Gao et al., 2003; Qing et al., 2005; Wu et al., 2003a, 2003b; Xu et al., 2008). Due to a lack of multilingual collaborators, we used online translators for foreign studies. All foreign articles that were sufficiently translatable via online translators were included (Rui et al., 2020; Xu et al., 2008).
Fig. 2.
PRISMA flow diagram.
3.2. Study characteristics
Fifty-five distinct publications were included in line with our eligibility criteria. Classified by aetiology, there were 29 eligible clinical studies for SARS-CoV2 (Cai et al., 2020, Fernández‐Ruiz et al., 2020; Chen et al., 2020; Cheng et al., 2020; Du et al., 2020; Fan et al., 2020; ; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Pan et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhou et al., 2020b), 26 studies for MERS-CoV (24 distinct reports) (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2017, 2019; Cha et al., 2016; Choi et al., 2016, 2019; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2014, 2015, 2016; Kim et al., 2016, 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Rhee et al., 2016; Shalhoub et al., 2015, 2018; Sherbini et al., 2017), and seven studies for SARS-CoV1 from which two articles could be retrieved in full-text (Loutfy et al., 2003; Zhao et al., 2003). Three studies reported on a similar population of patients. There, they were merged (Arabi et al., 2017; Arabi et al., 2019, Shalhoub et al., 2018). More specifically, the report by Shalhoub et al. was based on a cohort of 32 cases derived from 330 cases previously described by Arabi et al. in 2017 in a conference paper (Arabi et al., 2017). The multi-center cohort by Arabi et al. (2019) is an extended version that includes 349 cases, most of whom were previously described in earlier publications. As a result, the three studies were merged according to the 2019 report by Arabi et al. (2019). A sum of 3122 cases, 1665 (53.3%) males and (46.7%) 1457 females, with either suspected or confirmed COVID-19, was explored in 29 distinct reports. The mean age for COVID-19 patients was 47.12 (n = 1046) for 12 studies (Chen et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020b; Jin et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Qiu et al., 2020; Sun et al., 2020; Wang et al., 2020a; Yu et al., 2020; Zhou et al., 2020b). After calculating the point estimate of the mean for the rest of the studies, which did not report study setting (Weir et al., 2018), the mean age for all COVID-19 cases reached 51.26 (n = 3122) (Cai et al., 2020; Chen et al., 2020; Cheng et al., 2020; Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Pan et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhou et al., 2020b). In 24 distinct MERS publications, 1196 patients, including 587 males, 269 females, and 340 whose gender was not reported, were investigated (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2019; Cha et al., 2016; Choi et al., 2016, 2019; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2014, 2015, 2016; Kim et al., 2016, 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Rhee et al., 2016; Shalhoub et al., 2015; Sherbini et al., 2017). The mean age for patients with MERS was 53.58 (n = 464) for 17 studies (Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Cha et al., 2016; Choi et al., 2019; Habib et al., 2019; Khalid et al., 2014, 2015; Kim et al., 2016, 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Rhee et al., 2016; Sherbini et al., 2017), and after estimation of missing mean age reached 53.33 (n = 1170) for all eligible MERS studies (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2019; Cha et al., 2016, Choi et al., 2016; Choi et al., 2016, 2019; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2014, 2015, 2016; Kim et al., 2016, 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Rhee et al., 2016; Shalhoub et al., 2015; Sherbini et al., 2017). Two studies did not report the age of 18 (Khalid et al., 2016) and 8 cases (Shalhoub et al., 2015), respectively. A SARS study reported a mean age of 28.6 (n = 190) for one study (Zhao et al., 2003). The overall mean of both studies was 30.39 (n = 212) after calculating the mean for the other study (Loutfy et al., 2003; Zhao et al., 2003).
All studies used nucleic acid real-time polymerase chain reaction (RT-PCR) test to detect the presence of CoVs in respiratory (e.g., nasopharyngeal, throat, upper respiratory swab) or urinary specimen (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015, Cha et al., 2016; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2017; Arabi et al., 2019; Cai et al., 2020; Chen et al., 2020; Cheng et al., 2020, Choi et al., 2019; Choi et al., 2016, Du et al., 2020; Fan et al., 2020 Fernández-Ruiz et al., 2020; Garout et al., 2018; Habib et al., 2019; Huang et al., 2020a; Huang et al., 2020b; ; Hung et al., 2020, Jian-ya, 2020; Jiang et al., 2020 Jin et al., 2020; Khalid et al., 2016; Khalid et al., 2015; Khalid et al., 2014; Kim et al., 2017; Kim et al., 2016; Lee et al., 2017; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Pan et al., 2020; Qiu et al., 2020; Rhee et al., 2016; Rui et al., 2020; Shalhoub et al., 2018; Shalhoub et al., 2015; Sherbini et al., 2017; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhou et al., 2020b), except for SARS-CoV1-infected patients who were included according to clinical inclusion criteria and IgG testing (Loutfy et al., 2003; Zhao et al., 2003). A both positive and clear contact history with suspected or confirmed CoV cases or travelling to epidemic areas was reported in 20 (Cai et al., 2020; Chen et al., 2020; Du et al., 2020; Fan et al., 2020; Huang et al., 2020a; Huang et al., 2020b; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Liao et al., 2020; Liu et al., 2020a; Lo et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; Wan et al., 2020; Wang et al., 2020a; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020), 16 (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Cha et al., 2016; Choi et al., 2016, 2019; Garout et al., 2018; Khalid et al., 2014, 2015; Kim et al., 2016, 2017; Malik et al., 2016; Oh et al., 2015; Rhee et al., 2016; Shalhoub et al., 2015; Sherbini et al., 2017), and 2 (Loutfy et al., 2003; Zhao et al., 2003) studies for COVID-19, MERS, and SARS infections, respectively. Moreover, a descriptive study divided COVID-19 patients into cases with “clear” and “unclear” contact history but did not determine whether the “clear” cases had a positive or negative contact history with a SARS-CoV2 patient or a high prevalence area (Pan et al., 2020).
At least one patient was treated with IFN in each selected study. Type of IFN and its combined treatments varied between studies. Additionally, IFN types in all CoV studies included pegylated or recombinant IFN-α2a, IFN-α2b, IFN-β1b, and IFN Alfacon-1 administered via inhalation, subcutaneous (SC) injection, or nebulization (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2017; Arabi et al., 2019; Cai et al., 2020; Cha et al., 2016; Chen et al., 2020; Cheng et al., 2020; Choi et al., 2019; Choi et al., 2016; Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Garout et al., 2018; Habib et al., 2019; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Khalid et al., 2016; Khalid et al., 2015; Khalid et al., 2014; Kim et al., 2017; Kim et al., 2016; Lee et al., 2017; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Loutfy et al., 2003; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Pan et al., 2020; Qiu et al., 2020; Rhee et al., 2016; Rui et al., 2020; Shalhoub et al., 2018; Shalhoub et al., 2015; Sherbini et al., 2017; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020b, Wang et al., 2020c; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhao et al., 2003; Zhou et al., 2020b). Non-IFN pharmacological treatments comprised antivirals such as Umifenovir, also called Arbidol (ARB), (Cheng et al., 2020; Du et al., 2020; Fan et al., 2020; Huang et al., 2020b; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Liu et al., 2020a; Wang et al., 2020a; Wang et al., 2020b; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhou et al., 2020b), REM (Wang et al., 2020c), Oseltamivir (Du et al., 2020; Fan et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Liu et al., 2020b; Sun et al., 2020; Yu et al., 2020), Ganciclovir (Chen et al., 2020; Cheng et al., 2020; Yu et al., 2020), LPV and RTV (or Kaletra (LPV/RTV)) (Arabi et al., 2017; Cai et al., 2020; Chen et al., 2020; Cheng et al., 2020; Choi et al., 2019; Choi et al., 2016; Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020a; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Kim et al., 2017; Kim et al., 2016; Liu et al., 2020a; Lo et al., 2020; Pan et al., 2020; Qiu et al., 2020; Rhee et al., 2016; Rui et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yuan et al., 2020), FPV (Yuan et al., 2020), and RBV (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2017; Arabi et al., 2019; Cha et al., 2016; Chen et al., 2020; Cheng et al., 2020; Choi et al., 2019; Choi et al., 2016; Du et al., 2020; Fan et al., 2020; Garout et al., 2018; Habib et al., 2019; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Khalid et al., 2016; Khalid et al., 2015; Khalid et al., 2014; Kim et al., 2017; Kim et al., 2016; Lee et al., 2017; Liu et al., 2020b; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Rhee et al., 2016; Shalhoub et al., 2018; Shalhoub et al., 2015; Sherbini et al., 2017; Yuan et al., 2020; Zhao et al., 2003). Administered curatives also included IVIG (Al-Qaseer, 2015; Chen et al., 2020; Choi et al., 2016; Du et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020b; Jian-ya, 2020; Jiang et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Loutfy et al., 2003; Pan et al., 2020; Rui et al., 2020; Shalhoub et al., 2018; Sun et al., 2020; Xiao-Wei et al., 2020), thymopentin (Jian-ya, 2020), corticosteroids (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2017; Arabi et al., 2019; Chen et al., 2020; Cheng et al., 2020; Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; ; Huang et al., 2020b; Hung et al., 2020 Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Khalid et al., 2016; Khalid et al., 2015; Khalid et al., 2014; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Loutfy et al., 2003; Oh et al., 2015; Omrani et al., 2014; Pan et al., 2020; Rhee et al., 2016; Rui et al., 2020; Shalhoub et al., 2018; Sherbini et al., 2017; Sun et al., 2020; Wan et al., 2020; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhao et al., 2003), antibiotics such as imipenem, meropenem, cilastatin, quinolones, cephalosporins, and macrolides (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq et al., 2014; Cha et al., 2016; Choi et al., 2016; Du et al., 2020; Fan et al., 2020; Huang et al., 2020a; Hung et al., 2020; Jiang et al., 2020; Jin et al., 2020; Khalid et al., 2014, 2015, 2016; Kim et al., 2017; Lee et al., 2017; Lo et al., 2020; Loutfy et al., 2003; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Pan et al., 2020; Rhee et al., 2016; Sherbini et al., 2017; Sun et al., 2020; Wan et al., 2020; Wang et al., 2020b, 2020c; Yu et al., 2020; Zhao et al., 2003; Zhou et al., 2020b), albumin (Jian-ya, 2020; Liu et al., 2020a), and traditional Chinese medicine (TCM) (Huang et al., 2020a; Jian-ya, 2020; Sun et al., 2020; Wan et al., 2020; Wang et al., 2020a). Non-drug interventions included ventilation (both invasive and non-invasive) (Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq et al., 2014; Arabi et al., 2017; Arabi et al., 2019; Cha et al., 2016; Chen et al., 2020; Cheng et al., 2020; Choi et al., 2016; Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Habib et al., 2019; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Khalid et al., 2016; Khalid et al., 2015; Khalid et al., 2014; Kim et al., 2017; Lee et al., 2017; Liao et al., 2020; Liu et al., 2020b; Loutfy et al., 2003; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Rhee et al., 2016; Shalhoub et al., 2018; Shalhoub et al., 2015; Sherbini et al., 2017; Sun et al., 2020; Wan et al., 2020; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Zhao et al., 2003), continuous renal replacement therapy (CRRT) (Arabi et al., 2017, 2019; Khalid et al., 2014; Omrani et al., 2014), hemodialysis (Choi et al., 2016), Continuous Positive Airway Pressure (CPAP) (Fernández-Ruiz et al., 2020; Zhao et al., 2003), nutrition therapy (Jian-ya, 2020), extracorporeal membrane oxygenation (ECMO) (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Alfaraj et al., 2019; Arabi et al., 2017, 2019; Chen et al., 2020; Choi et al., 2016; Fan et al., 2020; Garout et al., 2018; Khalid et al., 2014; Omrani et al., 2014; Rhee et al., 2016; Shalhoub et al., 2018; Wan et al., 2020; Wang et al., 2020b, 2020c), electrolyte correction (Cai et al., 2020), oral or IV rehydration (Cai et al., 2020), prone positioning (Arabi et al., 2017; Khalid et al., 2014; Omrani et al., 2014; Shalhoub et al., 2018), blood transfusion (Al-Qaseer, 2015; Arabi et al., 2017; Omrani et al., 2014; Shalhoub et al., 2018), NO therapy (Arabi et al., 2019; Shalhoub et al., 2018), tracheostomy (Cha et al., 2016; Khalid et al., 2016; Lee et al., 2017; Shalhoub et al., 2018), intubation (Al-Hameed et al., 2016; Loutfy et al., 2003), and oxygen therapy (e.g., via nasal cannula) (Al-Hameed et al., 2016; Cai et al., 2020; Cha et al., 2016; Fernández-Ruiz et al., 2020; Huang et al., 2020b; Hung et al., 2020; Khalid et al., 2015; Liu et al., 2020a; Lo et al., 2020; Qiu et al., 2020; Rhee et al., 2016; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Yu et al., 2020). Several studies reported patients’ initial symptoms on admission, including fever, cough, shortness of breath, sputum or phlegm production, runny nose, nose obstruction, sore throat, myalgia, headache, dizziness, asthenia, and GI symptoms (e.g., diarrhea, nausea, and vomiting) (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Cha et al., 2016; Chen et al., 2020; Cheng et al., 2020; Choi et al., 2019; Choi et al., 2016; Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Habib et al., 2019; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jiang et al., 2020; Jin et al., 2020; Khalid et al., 2016; Kim et al., 2017; Kim et al., 2016; Lee et al., 2017; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Malik et al., 2016; Oh et al., 2015; Pan et al., 2020; Qiu et al., 2020; Rhee et al., 2016; Rui et al., 2020; Shalhoub et al., 2018; Shalhoub et al., 2015; Sherbini et al., 2017; Sun et al., 2020; To et al., 2020; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhao et al., 2003; Zhou et al., 2020b). COVID-19, MERS, and SARS studies mentioned 20 (Fan et al., 2020; Liao et al., 2020; Liu et al., 2020a; Lo et al., 2020; Qiu et al., 2020; Rui et al., 2020; Wang et al., 2020a), 42 (Alfaraj et al., 2019; Choi et al., 2016; Khalid et al., 2014), and no asymptomatic patients, respectively. Among MERS studies, one excluded the symptomatic cases (n = 38) from further analysis (Alfaraj et al., 2019).
Furthermore, study characteristics including country, study design, age of participants, comorbidities, symptoms on admission, and type, dosage, and administration route of both IFN and non-IFN treatments have been summarized (Table 1, Table 2, Table 3 ).
Table 1.
Characteristics of included SARS-CoV-2 studies (n = 29).
| Source | Country | Study design | Viral aetiology | Diagnosis | Sample | Reported co-morbidities | Symptoms on admission | Non-intervention treatments | Agea | Intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Rui et al. (2020) | China | Case-series (LOE II) | SARS-CoV-2 | Pharyngeal swab RT-PCR | 28 | DM, HTN, SLE, Hyperthyroidism, Hepatitis B | Fever, Cough, Chest tightness, Runny nose, Sore throat, Myalgia, Headache, Fatigue, Cold | LPV, RTV, IVIG, Methylprednisolone, Antibiotic, Flora | M(-44.5), R(11–68) | IFN-α inhalation 5000000U (injected with 2 ml of sterile water, BD) (28) |
| Jian-ya (2020) | China | Case-series (LOE II) | SARS-CoV-2 | RT-PCR | 51 | CHB, Schizophrenia, HTN, DM | NI | LPV, RTV, Oseltamivir, ARB, IVIG, IM Thymopentin, Glucocorticoid treatment, TCMD, Antibiotics, Bacillus licheniformis capsules, Human Albumin infusion | M(-45), R(16–68), I(34–54) | Inhalation of recombinant human IFN a-1b (51) |
| Liu et al. (2020b) | China | Case-series (LOE II) | SARS-CoV-2 | Swab and BALF RT-PCR | 12 | CHD, COPD, CKD, HTN, DM | Fever, Cough, Diarrhea, Chill, Myalgia | RBV, Oseltamivir, Immunoglobulin, Corticosteroids | R(10–72), Patient 1: 65, Patient 2: 66, Patient 3: 62, Patient 4: 63, Patient 5: 63, Patient 6: 36, Patient 7: 10, Patient 8: 35, Patient 9: 51, Patient 10: 65, Patient 11: 72, Patient 12: 56 | IFN (12) |
| Liao et al. (2020) | China | Retrospective case-series (LOE II) | SARS-CoV-2 | Throat Swab or Lower Respiratory tract RT-PCR | 46 | Obesity, DM, COPD, Hyperthyroidism, Kidney Stones, Arthrolithiasis. | Fever, Cough, Shortness of breath, Chest tightness, Myalgia, Dizziness, Fatigue, Nausea, Diarrhea, Pharyngalgia, Anorexia, Erythra | Budesonide, Antifungal, NAC, Antiviral | R(10–35) | IFN-α inhalation (46) |
| Liu et al. (2020) | China | Retrospective case-series (LOE II) | SARS-CoV-2 | Nasal and Throat Swab RT-PCR | 10 | HTN, CVA, Chronic liver disease | Fever, Cough, Chest pain, Phlegm, Sore throat, Headache, Nausea, Anxiety | LPV, ARB, IVIG, Methylprednisolone, Antibiotic, HSA | M(-42), R(30–62), I(34–50), Patient1: 45, Patient2: 30, Patient3: 62, Patient4: 53, Patient5: 51, Patient6: 47, Patient7: 40, Patient8: 33, Patient9: 34, Patient10: 35 | RH–IFN– a2b 50 μg BD (9) |
| Xiao-Wei et al. (2020) | China | Retrospective case series (LOE II) | SARS-CoV-2 | Sputum and Throat swab RT-PCR | 62 | HTN, DM, COPD, CVA, CKD, liver disease | Fever, Cough, Myalgia, Headache, Diarrhea, Expectoration, Haemoptysis | LPV, RT, ARB, IVIG, Corticosteroid, Quinolones, second generation of β-lactam (oral and IV), Flora therapy | M(-41), I(32–52) | IFN-α-2b inhalation 5000000U BD (33) |
| Huang et al. (2020b) | China | Retrospective case-series (LOE II) | SARS-CoV-2 | RT-PCR | 36 | HTN, Cerebrovascular, Diabetes, CHD, COPD, Chronic renal diseases, Cancer, Hyperlipidemia, ARDS, Electrolyte disturbance, AKI | Fever, Cough, Dyspnea, Sputum production, Myalgia, Fatigue, Diarrhea, Disturbance of consciousness, Haemoptysis | RBV, Oseltamivir, IVIG, Corticosteroid, Antibiotic, Ganciclovir, umifenovir hydrochloride | M(69.22), S(9.64), R(50–90) | IFN-α inhalation (6) |
| Chen/Zhang et al. (2020a) | China | Retrospective cohort (LOE II) | SARS-CoV-2 | RT-PCR OA(57) or clinical diagnostic Criteria OA(44) | 134 | Cerebrovascular and cardiovascular, Endocrine, tumor, Nervous system disease, Respiratory system disease | Fever, Cough, Shortness of breath, Sore throat, Myalgia, Headache, Diarrhea, Haemoptysis, Chill, Malaise | LPV, RTV, RBV, Oseltamivir, IVIG, Corticosteroid, Antibiotic, Ganciclovir, Thymosin, Antifungal treatment | M(60.78), S(12.98), R(24–83) | Yes |
| Cheng et al. (2020) | China | Prospective cohort study (LOE II) | SARS-CoV-2 | RT-PCR | 701 | CKD, COPD, HTN, DM, tumor, AKI | Fever, Respiratory symptoms, Proteinuria, Haematuria | LPV/RTV, RBV, Oseltamivir, Glucocorticoids, Antibiotic, RAAS inhibitors, ARB, Ganciclovir, Antidiabetics, Diuretics | M(-63), I(50–71) | Yes (OA(129), D(169)) |
| Zhou et al. (2020b) | China | Cohort (LOE II) | SARS-CoV-2 | Throat swab RT-PCR | 77 | HTN, diabetes, COPD, chronic bronchitis, heart disease, cancer | Fever, Cough, Chest tightness, Runny nose, Sore throat, Myalgia, Headache, Fatigue, Nausea, Diarrhea | ARB | M(IFN group: 41.3, IFN+ARB group: 40.4, ARB: 64.5), R(IFN group: (27–68), IFN+ARB: (25–80), ARB group (37–73)) | 5 mIU IFN-α-2b (1 ml) was added to 2 ml of sterile water and was nebulized. (53) |
| Qiu et al. (2020) | China | Retrospective cohort (LOE II) | SARS-CoV-2 | Upper nasopharyngeal swabs RT-PCR | 36 | None | Fever, Cough, Shortness of breath, Runny nose, Sore throat, Headache, Vomiting, Diarrhea | LPV/RTV | M(8.3), S(3.5) | IFN-α by aerosolization (b.i.d.) (36) |
| Wan et al. (2020) | China | Case-series (LOE II) | SARS-CoV-2 | Throat Swab RT-PCR | 135 | Diabetes, CVD, HTN, Malignancy, Pulmonary Disease, Chronic liver disease, Malignancy | NI | LPV/RTV, Corticosteroid, TCM, Antibiotic | M(47), I(36–55) | IFN or Kaletra (135) |
| Du et al. (2020) | China | Retrospective case-series (LOE II) | SARS-CoV-2 | RT-PCR | 85 | HTN, DM, CHD, Cerebrovascular diseases, CLD, Malignancy, CKD, COPD | Fever, Cough, Shortness of breath, Chest tightness, Sore throat, Myalgia, Headache, Fatigue, Vomiting, Diarrhea, Anorexia, Abdominal pain | LPV/RTV, RBV, Oseltamivir, ARB, Glucocorticoids, Meropenem, Imipenem/cilastatin, Moxifloxacin, Levofloxacin, Linezolid, Vancomycin, Teicoplanin, Tigecycline, Piperacillin/Tazobactam, Ceftriaxone sodium, Cefoperazone/sulbactam, Ceftazidime tazobactam, Caspofungin, Voriconazole, Fluconazole, Kidney replacement therapy, COVID-19 recovered patient plasma treatment 1 |
M(-65.8), S(14.2), R(14–86) | Recombinant human IFN-2b (32) |
| Fernández‐Ruiz et al. (2020) | Spain | Retrospective case series (LOE II) | SARS-CoV-2 | Nasopharyngeal swab or Sputum RT-PCR | 18 | PKD, HTN, prostaticadenocarcinoma, nephropathy, DM, peripheral artery disease, ESRD, coronary artery disease, obesity, Chronic interstitial nephritis, sleep apnea, Hepatitis, cirrhosis, HCC, asthma, bronchiectasis, splenectomy, Acute liver failure, cardiomyopathy, inflammatory bowel disease, primary sclerosing cholangitis, lung cancer, Congenital heart disease, cardiac allograft vasculopathy |
Fever, Cough, Shortness of breath, Runny nose, Sore throat, Myalgia, Diarrhea, Hyporexia, Epigastric pain, Malaise | LPV, RTV, IVIG, Methylprednisolone, HCQ | M(-71), S(12.8), Patient 1: 78, Patient 2: 73, Patient 3: 80, Patient 4: 71, Patient 5: 72, Patient 6: 76, Patient 7: 39, Patient 8: 65, Patient 9: 63, Patient 10:72, Patient 11: 79, Patient 12: 73, Patient 13: 76, Patient 14: 46, Patient 15: 64, Patient 16: 67, Patient 17: 63, Patient 18: 38 | IFN-β (3) |
| Cai et al. (2020a) | China | Non-randomized Clinical Trial (LOE II) | SARS-CoV-2 | RT-PCR | 80 | NI | NI | LPV/RTV, antipyretics, analgesics, antiemetic drugs | M(Total: −47, FPV+IFN: −43, LPV/RTV+IFN: −49), I(Total: (35.75–61), FPV+IFN: (35.5–59), LPV/RTV+IFN: (36–61)) | IFN-a by aerosol inhalation (5 million U b.i.d.) (80) |
| Wang et al. (2020a) | China | Case reports (LOE III) | SARS-CoV-2 | Throat swab RT-PCR | 2 | NI | Asymptomatic Couple | LPV/RTV, ARB, TCM | Patient 1: 54, Patient 2: 55 | Both Patients: atomization inhalation of recombinant human IFN-α-2b injection (6.0 × 106 IU with 2 ml of sterilized water for injection b.i.d.) |
| Hung et al. (2020) | China | Phase II, Randomized Clinical Trial (LOE I) | SARS-CoV-2 | Nasopharyngeal swab, posterior oropharyngeal, Saliva, Throat RT-PCR | 127 | DM, HTN, Coronary artery disease, cerebrovascular disease, Hyperlipidemia, Thyroid disease, sleep apnoea, Crohn, Epilepsy, TB, hepatitis, Malignancy, smoker | Fever, Cough, Shortness of breath, Chest tightness, Runny nose, Sore throat, Myalgia, Headache, Nausea, Diarrhea, Phlegm, Malaise, Anosmia, Anorexia | LPV/RTV, RBV, Hydrocortisone, Antibiotics | M(LPV/RTV + RBV + IFN-beta (−51), LPV/RTV (−52)), I(LPV/RTV + RBV + IFN-beta (31–61), LPV/RTV(33.5–62.5)) | LPV/RTV + RBV + IFN-β group: Three doses of 8 mIU of IFN-β-1b on alternate days, S.C. (1 ml) (86) |
| Huang et al. (2020a) | China | Retrospective case-series (LOE II) | SARS-CoV-2 | RT-PCR swab | 54, due to incomplete data, 40 were included in further analysis | HTN, Cardiovascular disease, CLD, Chronic bronchitis | Fever, Cough, Shortness of breath, Chest pain, Sore throat, Myalgia, Headache, Dizziness, Fatigue, Nausea, Diarrhea, Phlegm, Anorexia, ARDS | LPV/RTV, Corticosteroid, TCM, Fluoroquinolone or β-lactams, Lactobacillus Bifidus triple live bacteria tablets, Novaferon | M(Total(-41), Common(-41), Severe(-37) (of all cases n = 54), I(Total (31–51), Common (31–51), Severe (27.5–55.5) (of all cases n = 54)) | IFN-α-2b (5 mIU diluted with 2 ml sterile water) (common (18/37), data out of 40 cases comprising 37 common and 3 severe), Novaferon (Common(13/37)), data out of 40 cases comprising 37 common and 3 severe) |
| Wang et al. (2020a) | China | Randomized Clinical trial (LOE I) | SARS-CoV-2 | Nasopharyngeal or oropharyngeal swab RT-PCR | 236 | HTN, Diabetes, Coronary heart disease | Fever | LPV/RTV, Corticosteroid, Antibiotic, Vasopressors, Renal replacement therapy | M(Rem+ IFN(-66), Placebo + IFN(-64) (in this study all data is for Remdesivir+ IFN vs. Placebo(with IFN)), I(Rem+ IFN(57–73), Placebo + IFN(53–70)) | IFN-α-2b (76) |
| Lo et al. (2020) | China | Retrospective cohort (LOE II) | SARS-CoV-2 | Nasopharyngeal swab RT-PCR | 10 | HTN, Dyslipidemia, Past Hep B infection | Fever, Cough, Shortness of breath, Runny nose, Sore throat, Myalgia, Dizziness, Nausea, Diarrhea, Abdominal pain | LPV/RTV, Methylprednisolone, Cephalosporins, Quinolones, Macrolides | M(54), I(27–64) | IFN-β-1b (250mcg) (3) |
| Wang et al. (2020a) | China | Retrospective cohort (LOE II) | SARS-CoV-2 | Throat swab RT-PCR | 80 | HTN, Diabetes, CVD, Cerebrovascular disease, COPD, Renal disease, Liver disease | NI | LPV/RTV, ARB, Corticosteroid, Antibiotic | M(Total:-39, SARS2-Conf:-40, Clinically diagnosed:-39), I(Total:(32–48.5), SARS2-Conf 33–39, Clinically diagnosed: 32–48) | IFN-α (78) |
| Yu et al. (2020) | China | Retrospective case-series (LOE II) | SARS-CoV-2 | Throat swab from the upper respiratory tract, Sputum, and Nasopharyngeal swab RT-PCR | 7 | Hypothyroidism, Polycystic ovary syndrome | Fever, Cough, Shortness of breath, Diarrhea, Manifestations of Obstetrics, Abdominal pain (labour, premonitory labour), increased fetal movement |
Oseltamivir, ARB, Methylprednisolone, Jinyebaidu granules and Lianhuaqingwen capsules, Cephalosporins, Quinolones, or Macrolides, IV Ganciclovir | Patient 1:34, Patient 2: 30, Patient 3: 31, Patient 4: 33, Patient 5: 29, Patient 6: 34, Patient 7: 34 | IFN (40 μg daily, atomization inhalation) (7) |
| Jin et al. (2020) | China | Retrospective cohort (LOE II) | SARS-CoV-2 | Throat swabs and sputum RT-PCR | 651 | Diabetes, Chronic liver disease, Cancer, CKD, CVD, Pregnancy, COPD, Immunosuppression, | Fever, Cough, Shortness of breath, Phlegm, Runny nose, Sore throat, Myalgia, Headache, Fatigue, Nausea, Vomiting, Diarrhea, haemoptysis | LPV/RTV, ARB hydrochloride, Corticosteroid, Antibiotic | M(GI symptoms: 46.14, No GI symptoms: 45.09) I(GI symptoms: 14.19, No GI: 14.45) | IFN-α sprays, ARB hydrochloride capsules (two tab t.i.d. daily), LPV and RTV two tab (500 mg) b.i.d., via the oral route (546) |
| Fan et al. (2020) | China | Retrospective cohort (LOE II) | SARS-CoV-2 | Swab and Sputum RT-PCR | 55 | Diabetes, Coronary artery disease, HTN | Fever, Cough, Shortness of breath, Sore throat, Myalgia, Fatigue, Nausea, Vomiting, Diarrhea | LPV/RTV, RBV, Oseltamivir, Arb, Corticosteroid, Antibiotic, Thymalfasin (Refer to Fig. 1 in original publication for more precise information) | M(46.8) | IFN-α-1b (19) |
| Sun et al. (2020) | China | Cohort (LOE II) | SARS-CoV-2 | Nasopharyngeal swab RT-PCR | 8 | Fever, Cough, Myalgia, Headache, Fatigue, Nausea, Vomiting, Constipation, Polypnea | Oseltamivir, IVIG, Corticosteroid, TCM, Antibiotic, Voriconazole | R(2mon-15yr), Patient 1:8 y, Patient 2: 10 mon Patient3:1 y, 1 mon, Patient4: 2 mon, Patient 5: 2 y, 1 mon, Patient 6: 15 y, Patient7: 13 y, 11 mon, Patient 8: 13 y, 5 mon | Yes (8) | |
| To et al. (2020) | China | Retrospective cohort (LOE II) | SARS-CoV-2 | Nasopharyngeal or Throat swabs RT-PCR | 23 | HTN, Chronic heart disease, Chronic lung disease, Chronic kidney disease, Diabetes, Gout, Hyperlipidemia | Fever, Cough, Shortness of breath, Chest pain, Runny nose, Nose obstruction, Sore throat, Myalgia, Nausea, Diarrhea, Chills, Malaise | LPV, RTV | M (Severe(-66), Mild(-56)) I(Severe (39–75), Mild(37–75)) | LPV/RTV with or without RBV or IFN-β-1b was given in (18) |
| Yuan et al. (2020) | China | Retrospective cohort (LOE II) | SARS-CoV-2 | Nasal and Pharyngeal swab, sputum, and BALF RT-PCR | 94 | HBP, CHD, Diabetes | Fever, Cough, Sore throat, Fatigue, Diarrhea | LPV/RTV, RBV, ARB, Corticosteroid, Favipiravir, IVIG | M(Total(-40), Mild(-19) Moderate(-40), Severe(-63)) I(Total(1–78), Mild(7–39), Moderate(1–78), Severe(32–69)) | IFN in combination with either LPV/RTV or RBV (59) |
| Pan et al. (2020) | China | Cross-Sectional (LOE III) | SARS-CoV-2 | Throat swab from the upper respiratory tract RT-PCR | 204 | Respiratory system disease, Digestive 2, Critical 3)], Digestive system disease, CVD, Nervous system disease, Endocrine system disease, Malignant tumor | Fever, Myalgia, Fatigue, Vomiting, Diarrhea, Abdominal pain, loss of appetite | LPV/RTV, IVIG, Corticosteroid, Antibiotic, Antifungal | M(Total(52.91), no-Digestive symptoms(53.61), Digestive symptoms (52.21[Mild(24), Moderate(47.91), Severe(60.00), Critical(60.87)]), S(Total(15.98), no-Digestive symptoms(16.10), Digestive symptoms(15.92[Moderate(14.85), Severe(9.63), Critical(16.44)]) | Nebulized IFN-α (96) |
| Jiang et al. (2020) | China | Clinical Trialb (LOE II) | SARS-CoV-2 | RT-PCR | 60 | HTN, DM, COPD, CLD | Fever, Cough, Chest tightness, Sore throat, Headache, Fatigue, Vomiting, Diarrhea | LPV/RTV, Oseltamivir, ARB, IVIG, Corticosteroid, Antibiotics | M(Total(-41), non-Severe(40), Severe(-58)), R(Total(12–74), non-Severe(12–69), Severe(37–74)) | IFN-β (60) |
Abbreviations: ADE: adverse drug reaction, AF: atrial fibrillation, AKI: acute kidney injury, ARB: arbidol, ARDS: acute respiratory distress syndrome, b.i.d: 2 times a day, BALF: bronchoalveolar lavage fluid, BD: 2 times a day, CHB: chronic hepatitis B, CHD: coronary heart disease, CHF: congestive heart failure, CKD: chronic kidney disease, CLD: chronic liver disease, COPD: chronic obstructive pulmonary disease, CrCl: creatinine clearance, CRF: chronic renal failure, CVA: Cerebrovascular accident, CVD: Cardiovascular Disease, D: during, DM: diabetes mellitus, ESRD: end stage renal disease, F: female, G6PD: lucose-6-phosphate dehydrogenase, GI: gastrointestinal, GP: group, HBP: high blood pressure, HBV: hepatitis B virus, HCC: Hepatocellular carcinoma, HCQ: hydroxyl chloroquine, HSA: human serum albumin, HTN: hypertension, I: interquartile, IFN: interferon, IHD: Ischemic Heart Disease, IM: intramuscular, IU: international unit, IVIG: intravenous immunoglobulin, LOE: level of evidence, LPV: lopinavir, M(-number): median, M(number): mean, M: male, MERS: middle east respiratory syndrome, mg: milligram, mIU: milli-international unit q12h: every 12 h, mL: milliliter, NAC: N-acetyl cysteine, No.: number, OA: on admission, OD: on discharge, OF: other format, P: patient, PKD: Polycystic kidney disease, PO: per oral, q8h: every 8 h, QID: 4times a day, R: range, RAAS: renin-angiotensin-aldosterone system, RBV: ribavirin, REM: remdesivir, rhIFN: recombinant human interferon RT-PCR: real-time polymerase chain reaction, RTV: ritonavir, S: standard deviation, SARS-CoV: severe acute respiratory syndrome, SARS: severe acute respiratory syndrome, SC: subcutaneous, sec: second, SLE: systematic lupus erythematous, Tab: tablet, TB: tuberculosis, TCM: traditional Chinese medicine, TCMD: traditional Chinese medicine decoction, TDS: 3times a day, μg: microgram.
Age of participants is reported as reported in each study. Estimated mean values may be found in (supplementary material).
Randomization process not stated.
Table 2.
Characteristics of included MERS-CoV studies (n = 26a).
| Source | Country | Study design | Viral aetiology | Diagnosis | Sample | Reported co-morbidities | Symptoms on admission | Ageb | Intervention | Non-intervention treatments |
|---|---|---|---|---|---|---|---|---|---|---|
| Habib et al. (2019) | Saudi Arabia | Retrospective cohort study (LOE II) | MERS | PCR from respiratory tract samples | 63 | Diabetes, HTN, hepatitis C, chronic renal failure, and chronic heart disease | Fever, Diarrhea, Abdominal pain, Organ failure | M(59.7) S(18.2) | IFN- α (61) | RBV |
| Arabi et al. (2019)† | Saudi Arabia | Retrospective cohort study (LOE II) | MERS | Swab RT-PCR | 349 | DM, Malignancy, CPD, Moderate to severe liver disease, CKD, Chronic Cardiac, Chronic neurological disease, Rheumatological disease | NI | M(IFN and/or RBV (−57.5), No IFN and/or RBV (−58)) I(IFN and/or RBV (47–70), No IFN and/or RBV (41–70)) | Combination of RBV and rIFN (117), rIFN alone (9), (rINF type: α 2a 73, α 2b 22, β-1a 31) | RBV, Oseltamivir, Corticosteroid, NO, Renal replacement therapy, Vasopressors, Neuromuscular blockade |
| Choi et al. (2019) | South Korea | Case report (LOE III) | MERS | Patient 1: RT-PCR of nasopharyngeal aspirate, Patients 2 and 3: RT-PCR | 3 | None | Fever, Cough, Shortness of breath, Phlegm, Sore throat, Myalgia, Headache, Diarrhea | Patient1 38, Patient 2 33, Patient 3 45 | Patient 1: interferon α2a (180 μg/week), Patients 2 and 3: interferon-α2a | LPV, RTV, RBV |
| Alfaraj et al. (2019) | Saudi Arabia | Retrospective cohort study (LOE II) | MERS | RT-PCR of respiratory samples | 314 | NI | Fever, Cough, Shortness of breath, Sore throat | M(48.0), S(17.3) | Yes | RBV, Corticosteroid |
| Shalhoub et al. (2018)† | Mainly Saudi Arabia | Retrospective cohort study (LOE II) | MERS | RT-PCR from a respiratory tract sample (nasopharyngeal swab, sputum, deep tracheal aspirate or BAL | 32 | Diabetes, Chronic cardiac disease, CRD, CPD, Malignancies including leukemia, lymphoma or solid tumors | Fever, Cough, Shortness of breath, Chest tightness, Runny nose, Sore throat, Myalgia, Headache, Fatigue, Nausea, Vomiting, Diarrhea, Altered consciousness, Wheezing, Abdominal pain | M(-39), I(32–48) | Yes (13) | RBV, Oseltamivir, IVIG, Vasopressors, Renal replacement therapy |
| Garout et al. (2018) | Saudi Arabia | Retrospective Cohort (LOE II) | MERS | Swab RT-PCR | 52 | HTN, DM, CRF | NI | R(15–35) for (9), (35–55) for (24), (55–75) for (16), (75–85) for (3) | IFN-α (35) | RBV |
| Al-Tawfiq et al. (2014) | Saudi Arabia | Case report (LOE III) | MERS | Nasopharyngeal dacron-flocked swabs or sputum samples RT-PCR | 3 | Rheumatoid arthritis, DM, Dyslipidemia, Chronic HBV carrier | Fever, Cough, Dizziness, Fatigue, Nausea, Vomiting, Diarrhea, | Patient 1: 56, Patient 2: 52, Patient 3: 53 | Patients 2 and 3: IFN-α 2b | Patients 2 and 3: RBV, Patient 2: Oseltamivir |
| Sherbini et al. (2017) | Saudi Arabia | Retrospective cohort (LOE II) | MERS | Swab RT-PCR | 29 | DM, CKD | Fever, Cough, Shortness of breath, Vomiting, Diarrhea | M(45), S(12) | Yes (19) | RBV, Corticosteroid, Levofloxacin, Meropenem, Linezolid, Piperacillin, Azithromycin |
| Lee et al. (2017) | South Korea | Retrospective case report (LOE III) | MERS | Swab RT-PCR | 1 | HTN, Dyslipidemia | Fever, Myalgia, Chills, Dyspnea, Malaise | Patient 1: 68 | Pegylated IFN-α-2b 180 mcg Daily | RBV, Oseltamivir, IV ceftriaxone, azithromycin Vancomycin, and meropenem, Tigecycline, IV colistin, Amikacin, and Fluconazole |
| Kim et al. (2017) | South Korea | Retrospective case report (selected from a cohort) (LOE II) | MERS | RT-PCR for specimen from the lower respiratory tract (collected sputum and endotracheal aspirates) |
23, 4 were included in further analysis | DM, HTN, CHD, CKD, Bronchiectasis, Malignancy, Psychiatric disorder, Ankylosing spondylitis | Fever, Cough, Shortness of breath, Myalgia, Headache, Nausea, Confusion | M(-46), I(27–46) Patient 1: 55, Patient 2: 43, Patient 3: 46, Patient 4: 38 | Pegylated IFN α -2a S.C (180 μg/week for 2 weeks)(23) | LPV, RTV, RBV, Ceftazidime, Teicoplanin, Meropenem, and Moxifloxacin, Patient2: Antiemetic, Antitussive, and Non-steroidal anti-inflammatory drugs |
| Arabi et al. (2017)† | Saudi Arabia | Retrospective cohort (LOE II) | MERS | RT-PCR | 349 | Diabetes, CKD, chronic liver disease | NI | RBV/rIFN M (−57.5) I(47.0–70.0), No RBV/rIFN M (−58.0) I(41.0–70.0) |
rIFN α-2a (73) rIFN α-2b (22) rIFN-β-1a (31) |
RBV |
| Rhee et al. (2016) | South Korea | Retrospective case-series (LOE II) | MERS | Oropharyngeal swab sputum, and tracheal aspiration RT-PCR | 5 | NI | Fever, Cough, Myalgia, Headache, Diarrhea, Abdominal pain, Loose stool | Patient 1: 46, Patient 2: 47, Patient 3: 65, Patient 4: 27, Patient 5: 35 | Pegylated IFN α -2a SC 180 mg/week for 2 weeks (2) | Patients 1, 2, 3, 4, and 5: LPV, Patients 1, 2, 3, 4, and 5: RTV, Patient 2: RBV, Patient 5: Corticosteroid, Patients 1, 2, 3, 4, and 5: Antibiotic, patient3: Ionotropic, Patient 5: Convalescent plasma |
| Malik et al. (2016) | UAE | Case report (LOE III) | MERS | Nasopharyngeal aspirate RT-PCR | 1 | 32 week pregnant | Fever, Back pain | Patient 1: 32 | Peg IFN-α (180 μg/week) (1) | RBV, Oseltamivir, Vancomycin, Meropenem |
| Kim et al. (2016) | South Korea | Case report (LOE III) | MERS | nasopharyngeal/oropharyngeal, sputum RT-PCR | 1 | HTN, DM, Distal Pancreatectomy due to benign pancreatic neoplasm, Chronic dry cough, and Diagnosed with mycobacterium intracellulare | Fever, Cough, Weakness, | Patient 1: 64 | IFN-α2a SC180 microg/0.5 ml (1) | LPV, RTV, RBV |
| Khalid et al. (2016) | Saudi Arabia | Retrospective cohort (LOE II) | MERS | Swab RT-PCR | 32 (14 final inclusion in further analysis) | HTN, DM, Respiratory diseases, Obesity, CHF, CKD, Dialysis, IHD, Stroke, Immunosuppression | Fever, Cough, Shortness of breath, Chest pain, Sore throat, Myalgia, Headache, Nausea, Vomiting, Diarrhea, Hemoptysis, Abdominal pain | M(-54), R(23–79) | Peg IFN-α-2b (11) | RBV, Oseltamivir, Methylprednisolone, Antibiotic, NO, Neuromuscular Blockade, Renal replacement therapy, Vasopressor |
| Choi et al. (2016) | South Korea | Retrospective cohort (LOE II) | MERS | RT-PCR | 186 | HTN, DM, Malignancy, COPD, CHD, Cerebrovascular disease, CLD, CKD, Hematologic malignancy | Fever, Cough, Shortness of breath, Runny nose, Sore throat, Myalgia, Headache, Nausea, Vomiting, Diarrhea, Sputum, Abdominal pain, Decreased consciousness | M(-55), R(16–86) | Yes (183) | LPV/RTV,RBV, IVIG, Antibiotic, Convalescent serum |
| Cha et al. (2016) | South Korea | Case report (LOE III) | MERS | Urine, stool, and sputum RT-PCR | 1 | HTN | Fever, Cough, Shortness of breath, Myalgia, Weakness, Nausea, Vomiting | 68 | Pegylated IFN- α 180 μg (1) | RBV, Vancomycin, Tigecycline, Colistimethate |
| Al-Hameed et al. (2016) | Saudi Arabia | Prospective cohort (LOE II) | MERS | Swab RT-PCR | 8 | DM, HTN, CHF or IHD, Cirrhosis, G6PD deficiency | Fever, Cough, Shortness of breath, Chest pain, Myalgia, Headache, Diarrhea, Sputum production, Altered mental state | M(-56.5), R(26–94) | IFN- α-2b (8) | RBV, Oseltamivir, Corticosteroid, Antibiotic, Norepinephrine, Renal replacement therapy |
| Al Ghamdi et al. (2016) | Saudi Arabia | Retrospective cohort (LOE II) | MERS | PCR from clinical nasal swabs or nasopharyngeal aspirates | 51 | DM, HTN, End stage renal disease, Coronary artery disease, Immunosuppression, Pregnant | Fever, Cough, Runny nose, Sore throat, Vomiting, Diarrhea | M(-54), I(36.5–58) | IFN-β (23, 10 in combination with RBV, 11 IFN- β alone), IFN-α (8, 5 in combination with RBV, 2 IFN-α alone) | RBV, Oseltamivir, Antibiotic, Mycophenolate mofetil |
| Shalhoub et al. (2015) | Saudi Arabia | Sequential retrospective cohort study (LOE II) | MERS | RT–PCR from a respiratory tract sample | 32, 24 included in further analysis (received IFN) | DM, HTN, Chronic renal impairment, Renal failure on hemodialysis, Low ejection fraction | Fever, Cough, Shortness of breath, Chest pain, Phlegm, Vomiting, Diarrhea, Abdominal pain, Confusion | M(IFNa (−65), IFNb (−67), I(IFNa (33–84), IFNb (25–84)) | IFN-α-2a (180 mg S.C. once a week) combined with RBV (loading dose of 2 g orally followed by 600 mg orally q12 h): 13, IFN-b1a (44 mg S.C. three times a week) combined with RBV, dosed as above: 11 |
RBV |
| Oh et al. (2015) | South Korea | Case Report (LOE III) | MERS | RT–PCR on a sputum specimen | 1 | NI | Fever, Cough | Patient 1: 35 | Pegylated IFN α-2a via S.C. injection at a dose of 180 μg per week for 2 weeks (1) |
RBV, Antibiotic, IV Methylprednisolone 1 |
| Khalid et al. (2015) | Saudi Arabia | Case Report (LOE III) | MERS | Patient 1: RT-PCR (UPE, ORF 1b) Patient 2: RT-PCR Patient Routine clinical laboratory tests for influenza, parainfluenza, respiratory syncytial virus, adenovirus, rhino- virus, enterovirus, Epstein–Barr virus, cytomegalovirus, human metapneumovirus, urinary Legionella antigen and serology for Mycoplasma pneumoniae and Chlamydia pneumoniae (no serological results for MERS-CoV) | 2 | NI | Shortness of breath | Patient 1:52, Patient 2: 42 | Pegylated IFN α-2b (2) | RBV, Corticosteroid (Patient 1: IV methylprednisolone, Antibiotic (Patient 1: Broad-spectrum antibiotics like ceftriaxone and azithromycin), Patient 2: Cefuroxime and Azithromycin) |
| Al-Qaseer (2015) | Kuwait | Case Report (LOE III) | MERS | BAL endotracheal RT-PCR | 3 | DM, HTN, peptic ulcer, DM, HTN, IHD | Fever, Cough, Shortness of breath, Diarrhea, Night sweats, loss of appetite | Patient 1: 47, Patient 2: 52, Patient 3: 60 | Patient 1: IFN-α-2a μg S.C., Patient 2: IFN-α-2b 1.5 μg/kg S.C. | RBV, Patient 1: Oseltamivir, Patient 2: IVIG, Patients 1 and 2: Corticosteroid, Patients 1, 2, and 3: Antibiotic |
| Omrani et al. (2014) | Saudi Arabia | Retrospective cohort (LOE II) | MERS | RT-PCR testing of respiratory tract samples | 44 | CHF, Dementia, COPD, Asthma, Rheumatological disease, CLD, DM, Hemiplegia, CKD, Malignant disorder | NI | M(IFN/RBV: 67·4, No IFN/RBV: 64·0) S(IFN/RBV: 18·5, No IFN/RBV: 18·1) |
Pegylated IFN-α -2a S.C. (180 μg/week for 2 weeks) (20 from treatment group) | RBV, Oseltamivir, Hydrocortisone, Antibiotics, Vasopressor Therapy, IVIG |
| Khalid et al. (2014) | Saudi Arabia | Case-series (LOE II) | MERS | Swab RT-PCR | 6 | IHD, heart failure, Right bundle branch block, Cardiomyopathy heart failure | NI | Patient 1: 74, Patient 2: 84, Patient 3: 76, Patient 4: 54, Patient 5: 48, Patient 6: 17 | IFN-α-2b 180 μg S.C. once per weeks for 2 weeks in CrCl 20–50 ml/min (6) | RBV, Methylprednisolone pulse, Antibiotic |
| Al-Tawfiq et al. (2014) | Saudi Arabia | Case reports (LOE III) | MERS | Swab RT-PCR | 5 | HTN, DM, CKD, Dialysis, Asthma, Obstructive sleep apnea, Coronary artery disease, AF, ESRD | Fever, Cough, Shortness of breath, Respiratory failure | M(-62), R(24–81) Patient 1: 62, Patient 2: 58, Patient 3: 63, Patient 4: 81, Patient 5: 24) | IFN-α-2b 130 μg S.C. (1), 100 μg S.C.(3), 144 μg S.C.(1) | RBV, Oseltamivir, IV Methylprednisolone, Imipenem, Levofloxacin |
Abbreviations: AF: atrial fibrillation, BAL: bronchoalveolar lavage, CHD: coronary heart disease, CKD: chronic kidney disease, CoV: coronavirus, CPD: cephalopelvic disproportion, CRD: chronic respiratory disease, CRF: chronic renal failure, DM: diabetes melitus, ESRD: end stage renal disease, G6PD: Glucose-6-phosphate dehydrogenase, g: grams, HBV: hepatitis B virus, HTN: hypertension, I: interquartile, IFN: interferon, IHD: ischemic heart disease, IVIG: intravenous immunoglobulin, LOE: level of evidence, M: mean, MERS: middle east respiratory syndrome, mL: milliliter, NI: not identified/indicated, NO: nitric oxide, ORF: open reading frame, q12h: every 12 h, RBV: ribavirin, rIFN: recombinant interferon, RT-PCR: reverse transcription polymerase chain reaction, S: standard deviation, SARS: severe acute respiratory syndrome, SC: subcutaneous, μg: microgram, upE: envelope gene.
24 distinct studies are listed. The three non-distinct studies are marked as †. Among the studies with common participants (2, 5, 11), Arabi et al. is considered the most complete, and was considered for analyses.
Age of participants is reported as reported in each study. Estimated mean values may be found in (supplementary material).
Table 3.
Characteristics of included SARS-CoV studies (n = 2).
| Source | Country | Study design | Viral aetiology | Diagnosis | Sample | Reported co-morbidities | Symptoms on admission | Agea | Intervention | Non-intervention treatments |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhao et al. (2003) | China | Randomized Clinical Trial |
SARS-CoV-1 | SARS clinical inclusion criteria | 190 | NI | Fever, Cough, Shortness of breath, Chest pain, Myalgia, Headache, Dizziness, Fatigue, Diarrhea, palpitation, Chills/Rigor | M(28.6) (group A:(33.6), group B:(32.4), group C:(32.5), group D: (30.5)), S(10.3) (group A: (13.9), group B:(12.4), group C:(12.1), group D: (12.3)), R(16–84) |
30 cases in group B: recombinant IFN-a, I.M. 3,000,000 U/day, Some cases in group C: IFN-a IM. 3,000,000 U/day), 45 cases in group D: IFN-a I.M. 3000000U/day | RBV, Methylprednisolone, Antibiotic |
| Loutfy et al. (2003) | Canada | Cohort | SARS-CoV-1 | Clinical inclusion criteria and IgG sample testing | 22 | HTN | NI | M (IFN Alfacon-1 group (−48), Corticosteroids Alone group (−42), R(IFN Alfacon-1 (27–56), Corticosteroids Alone (16–86)) |
IFN- alfacon-1 (9) | IVIG, Corticosteroids, High-dose methylprednisolone, Antibiotics, OF (Maximum steroid dose, mg (IFN Alfacon-1 group 500 (50–500) Corticosteroids Alone group 70 (40–500))) |
Abbreviations: CoV: coronavirus, IFN: interferon, igG: immunoglobulin G, IM: intramuscular, IVIG: intravenous immune globulin, M: mean, O: other formats, R: range, RBV: ribavirin, SARS: severe acute respiratory syndrome, U: unit.
Age of participants is reported as reported in each study. Estimated mean values may be found in (supplementary material).
3.3. Assessment of risk of bias
29 COVID-19 studies (Cai et al., 2020; Chen et al., 2020; Cheng et al., 2020; Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Pan et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhou et al., 2020b) were included, four of which were clinical trials (Cai et al., 2020; Hung et al., 2020; Jiang et al., 2020; Wang et al., 2020c). Among trials, two were randomized (Hung et al., 2020; Wang et al., 2020c). An RCT was of a high risk of bias; due to that, the assessors were aware of the intervention, and no efforts to resolve the possibility of bias were discussed (Hung et al., 2020). The other was of low risk of bias (Wang et al., 2020c). Also, there were two non-randomized trials (Cai et al., 2020; Jiang et al., 2020), which had a moderate risk of bias (Cai et al., 2020), and one was not assessed due to no statement on the randomization process (in the protocol or the publication) (Jiang et al., 2020). A poor quality cross-sectional study was also included (Pan et al., 2020). 11 cohorts of COVID-19 cases were critically appraised (Cheng et al., 2020; Fan et al., 2020; Huang et al., 2020a; Jin et al., 2020; Lo et al., 2020; Qiu et al., 2020; Sun et al., 2020; To et al., 2020; Wang et al., 2020b; Yuan et al., 2020; Zhou et al., 2020b). Of those, three had good quality (Cheng et al., 2020; Jin et al., 2020; Zhou et al., 2020b), two had a fair quality (Fan et al., 2020; Sun et al., 2020), and others had a poor quality (Cai et al., 2020; Huang et al., 2020a; Lo et al., 2020; To et al., 2020; Wang et al., 2020b; Yuan et al., 2020). 12 case-series (Chen et al., 2020; Du et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020a; Jian-ya, 2020; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Rui et al., 2020; Wan et al., 2020; Xiao-Wei et al., 2020; Yu et al., 2020) were also assessed. Results showed seven to be of good quality, while four had a fair quality (Du et al., 2020; Liao et al., 2020; Liu et al., 2020b; Xiao-Wei et al., 2020), and one was of poor quality (Jian-ya, 2020). Importantly, a case-series was pre-printed (Chen et al., 2020), but was later published with a comparator group. The published version showed poor quality due to a lack of comparability according the NOS tool (Zhang et al., 2020a). The only included case report was of a high risk of bias (Wang et al., 2020a) (Table 4, Table 5, Table 6, Table 7, Table 8 ).
Table 4.
Critical appraisal for cross-sectional studies using the NIH tool.
| Source | subject | Clarifying question | Clarifying population | 50%eligible persons | Selection from similar population | size justification, power description, or variance and effect estimates provided | the exposure(s) of interest measured prior to the outcome(s) being measured | timeframe sufficient | study examine different levels of the exposure as related to the outcome | the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants | the exposure(s) assessed more than once over time | outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants | the outcome assessors blinded to the exposure status of participants | loss to follow-up after baseline 20% or less | key potential confounding variables measured | Final quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pan et al. (1) | SARS-CoV-2 | yes | yes | yes | yes | no | NR | NR | yes | CD | NR | yes | no | no | no | Poor quality |
Abbreviations: CD: Cannot be determined, NR: Not Reported, severe acute respiratory syndrome coronavirus.
1. Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. The American journal of gastroenterology. 2020;115.
Table 5.
a and b. Critical appraisal of clinical trials using ROBINS-I and RoB2 tools (n = 1, 3).
| Source | Aetiology | Randomization | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Cai et al.(1) | SARS-CoV-2 | Non-randomized | low | moderate | NI | low | low | moderate | NI | Moderate risk of bias |
| Source | Aetiology | Randomization | Risk of bias arising from the randomization process | Risk of bias due to deviations from the intended interventions (effect of assignment to intervention) | Risk of bias due to deviations from the intended interventions (effect of adhering to intervention) | Missing outcome data | Risk of bias in measurement of the outcome | Risk of bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Hung et al.(2) | SARS-CoV-2 | Randomized | low | some concerns | NA | low | high | low | High risk of bias |
| Wang et al.(3) | SARS-CoV-2 | Randomized | low | low | NA | low | low | low | Low risk of bias |
| Zhao(4) | SARS-CoV-1 | Randomized | some concerns | high | NA | low | high | some concerns | High risk of bias |
Abbreviations: CD: Cannot be determined, NA: Not applicable, NI: not indicated/identified, NR: Not Reported, SARS-CoV: severe acute respiratory syndrome coronavirus.
References.
1. Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020.
2. Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. The Lancet. 2020.
3. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020.
4. Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. Journal of medical microbiology. 2003;52(8):715-20.
Table 6.
Critical appraisal for case-series using NIH tool.
| Source | Subject | Clarifying question | Clarifying population | cases consecutive | Comparability of subjects | Clarifying interventions | Clarifying outcome | Length of follow up | Statistical method | Result | Final quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wan et al. (1) | SARS-CoV-2 | yes | yes | no | no | yes | yes | yes | yes | yes | Good |
| Du et al. (2) | SARS-CoV-2 | yes | yes | yes | no | no | yes | yes | yes | yes | Fair |
| Ruiz et al. (3) | SARS-CoV-2 | yes | yes | no | no | yes | yes | yes | yes | yes | Good |
| Huang et al. (4) | SARS-CoV-2 | yes | yes | no | no | yes | yes | yes | yes | yes | Good |
| Yu et al. (5) | SARS-CoV-2 | yes | yes | no | no | yes | yes | yes | yes | yes | Good |
| Rui et al. (6) | SARS-CoV-2 | yes | yes | yes | no | yes | CD | No/CD | no | CD | Poor |
| Jian-ya (7) | SARS-CoV-2 | yes | yes | yes | CD | yes | yes | yes | yes | yes | Good |
| Liu et al. (8) | SARS-CoV-2 | yes | yes | yes | yes/CD | No/NA | yes | no | yes | yes | Fair |
| Liao et al.(9) | SARS-CoV-2 | yes | yes | No/NR | yes/CD | No/NA | yes | yes | yes | yes | Fair |
| Liu et al. (10) | SARS-CoV-2 | yes | yes | yes | yes/CD | yes | yes/no | no | yes | yes | Good |
| Xu et al. (11) | SARS-CoV-2 | yes | yes | yes | yes | yes | yes | no | yes | yes | Good |
| Chen/Zhang et al.(12, 13)a | SARS-CoV-2 | yes | yes | yes | CD | yes/no | yes | yes | yes | yes | Fair |
| Khalid et al. (14) | MERS-CoV | yes | yes | yes | yes | yes | yes | yes | no | yes | Good |
| Rhee et al. (15) | MERS-CoV | no | yes | yes | yes/CD | yes | yes | yes | yes | yes | Good |
Abbreviations: CD: Cannot be determined, MERS-CoV: Middle-Eastern respiratory syndrome coronavirus, NA: Not applicable, NR: Not Reported, SARS-CoV: severe acute respiratory syndrome coronavirus.
References.
1. Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. Journal of medical virology. 2020.
2. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan: A Retrospective Observational Study. American Journal of Respiratory and Critical Care Medicine. 2020.
3. Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. American Journal of Transplantation. 2020.
4. Huang Q, Deng X, Li Y, Sun X, Chen Q, Xie M, et al. Clinical characteristics and drug therapies in patients with the common-type coronavirus disease 2019 in Hunan, China. International journal of clinical pharmacy. 2020:1.
5. Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. The Lancet Infectious Diseases. 2020.
6. Rui Z, Yunguang L, Yanrong L, Ning L, Qiulian L, Youling L, et al. Clinical characteristics of 28 patients with novel coronavirus pneumonia. Chinese Journal of Infectious Diseases. 2020(12):006-.
7. Jian-ya G. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv. 2020.
8. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Science China Life Sciences. 2020;63(3):364-74.
9. Liao J, Fan S, Chen J, Wu J, Xu S, Guo Y, et al. Epidemiological and clinical characteristics of COVID-19 in adolescents and young adults. medRxiv. 2020.
10. Liu F, Xu A, Zhang Y, Xuan W, Yan T, Pan K, et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. International Journal of Infectious Diseases. 2020.
11. Xu YD, Jiang M, Chen RC, Fang JQ. [Evaluation of the efficacy and safety of corticosteroid in the treatment of severe SARS in Guangdong province with multi-factor regression analysis]. Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue. 2008;20(2):84-7.
12. Chen J, Fan H, Zhang L, Huang B, Zhu M, Zhou Y, et al. Retrospective Analysis of Clinical Features in 101 Death Cases with COVID-19. medRxiv. 2020.
13. Zhang L, Huang B, Xia H, Fan H, Zhu M, Zhu L, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiology and Infection. 2020;148:e199.
14. Khalid M, Khan B, Al Rabiah F, Alismaili R, Saleemi S, Rehan-Khaliq AM, et al. Middle Eastern Respiratory Syndrome Corona Virus (MERS CoV): case reports from a tertiary care hospital in Saudi Arabia. Annals of Saudi medicine. 2014;34(5):396–400.
15. Rhee J-Y, Hong G, Ryu KM. Clinical implications of five cases of Middle East respiratory syndrome coronavirus infection in South Korea Outbreak. Japanese journal of infectious diseases. 2016:JJID. 2015.445.
This paper was published as Zhang et al. (13) with 134 cases, and comparative study design. Please refer to results section of the manuscript for quality assessment of the extended version of this study.
Table 7.
Critical appraisal for included Cohorts via the NOS tool.
| Source | Aetiology | Selection 1-representativeness | Selection 2-non exposed | Selection 3- Ascertainment of exposure | Selection 4- outcome of interest | Comparability | Outcome 1- assessment | Outcome 2- length of follow up | Outcome 3- adequate length of following | Final quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. (1) | SARS-CoV-2 | 1(b) | 1(a) | 1(a) | Yes(a) | 2(a-b) | 1(a) | 1(a) | 0(d) | Good quality |
| Qui et al. (2) | SARS-CoV-2 | 1(a) | 1(a) | 0(e) | 1(a) | 1(b) | 1(b) | 1(a) | 0(d) | Poor quality |
| Lo et al. (3) | SARS-CoV-2 | 1(b) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 0(d) | Poor quality |
| Wang et al. (4) | SARS-CoV-2 | 1(b) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 0(d) | Poor quality |
| Jin et al. (5) | SARS-CoV-2 | 1(a) | 1(a) | 1(a) | 0(b) | 0(c) | 1(b) | 1(a) | 1(b) | Good quality |
| Fan et al. (6) | SARS-CoV-2 | 1(a) | 1(a) | 1(a) | 0(b) | 0(c) | 1(a) | 1(a) | 1(a) | Fair quality |
| Sun et al. (7) | SARS-CoV-2 | 1(a) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 0(d) | Fair quality |
| To et al. (8) | SARS-CoV-2 | 1(a) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 0(d) | Poor quality |
| Yuan et al. (9) | SARS-CoV-2 | 1(a) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 0(d) | Poor quality |
| Cheng et al. (10) | SARS-CoV-2 | 1(a) | 1(a) | 1(a) | 1(a) | 2(a-b) | 1(a) | 1(a) | 1(a) | Good quality |
| Huang et al. (11) | SARS-CoV-2 | 0(c) | 0(c) | 1(a) | 1(a) | 0(c) | 1(a) | 1(a) | 0(d) | Poor quality |
| Habib et al. (12) | MERS-CoV | 0(c) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 1(a) | Poor quality |
| Arabi et al. (13) | MERS-CoV | 1(a) | 1(a) | 1(a) | 1(a) | 2(a-b) | 1(b) | 1(a) | 1(a) | Good quality |
| Alfaraj et al. (14) | MERS-CoV | 0(d) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 1(a) | Poor quality |
| Garout et al. (15) | MERS-CoV | 0(d) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 1(a) | Poor quality |
| Sherbini et al. (16) | MERS-CoV | 0(d) | 1(a) | 1(a) | 1(a) | 0(c) | 1(a) | 1(a) | 1(a) | Poor quality |
| Khalid et al. (17) | MERS-CoV | 0(d) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 1(a) | Poor quality |
| Choi et al. (18) | MERS-CoV | 0(d) | 1(a) | 1(a) | 1(a) | 0(c) | 1(a) | 1(a) | 1(a) | Poor quality |
| Al-Hameed et al.(19) | MERS-CoV | 1(b) | 0(c) | 1(a) | 1(a0 | 0(c) | 0(c) | 1(a) | 1(a) | Poor quality |
| Al-Ghamdi et al.(20) | MERS-CoV | 0(d) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 1(a) | Poor quality |
| Shalhoub (2015) et al. (21) | MERS-CoV | 1(a) | 1(a) | 1(a) | 1(a) | 2(a-b) | 1(b) | 1(a) | 1(a) | Good quality |
| Omrani et al. (22) | MERS-CoV | 1(a) | 1(a) | 1(a) | 1(a) | 0(c) | 1(b) | 1(a) | 1(a) | Poor quality |
| Loutfy et al. (23) | SARS-CoV-1 | 1(a) | 1(a) | 1(a) | 1(a) | 2(a-b) | 1(b) | 1(a) | 1(a) | Good quality |
Abbreviations: CD: Cannot be determined, MERS-CoV: Middle-Eastern respiratory syndrome coronavirus, NA: Not applicable, NR: Not Reported, SARS-CoV: severe acute respiratory syndrome coronavirus.
References.
1. Zhou Q, Chen V, Shannon CP, Wei X-S, Xiang X, Wang X, et al. Interferon-α2b Treatment for COVID-19. Frontiers in Immunology. 2020;11:1061.
2. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. The Lancet Infectious Diseases. 2020.
3. Lo IL, Lio CF, Cheong HH, Lei CI, Cheong TH, Zhong X, et al. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. International journal of biological sciences. 2020;16(10):1698.
4. Wang X, Liu W, Zhao J, Lu Y, Wang X, Yu C, et al. Clinical characteristics of 80 hospitalized frontline medical workers infected with COVID-19 in Wuhan, China. Journal of Hospital Infection. 2020.
5. Jin X, Lian J-S, Hu J-H, Gao J, Zheng L, Zhang Y-M, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002-9.
6. Fan L, Liu C, Li N, Liu H, Gu Y, Liu Y, et al. Medical treatment of 55 patients with COVID-19 from seven cities in northeast China who fully recovered: a single-center, retrospective, observational study. medRxiv. 2020.
7. Sun D, Li H, Lu X-X, Xiao H, Ren J, Zhang F-R, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World Journal of Pediatrics. 2020:1-9.
8. To KK-W, Tsang OT-Y, Leung W-S, Tam AR, Wu T-C, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. The Lancet Infectious Diseases. 2020.
9. Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflammation Research. 2020:1-8.
10. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney impairment is associated with in-hospital death of COVID-19 patients. medRxiv. 2020.
11. Huang Y, Zhou H, Yang R, Xu Y, Feng X, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020.
12. Habib AMG, Ali MAE, Zouaoui BR, Taha MAH, Mohammed BS, Saquib N. Clinical outcomes among hospital patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. BMC infectious diseases. 2019;19(1):1–6.
13. Arabi YM, Shalhoub S, Mandourah Y, Al-Hameed F, Al-Omari A, Al Qasim E, et al. Ribavirin and Interferon Therapy for Critically Ill Patients With Middle East Respiratory Syndrome: A Multicenter Observational Study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2019.
14. Alfaraj SH, Al-Tawfiq JA, Assiri AY, Alzahrani NA, Alanazi AA, Memish ZA. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: A cohort study. Travel Medicine and Infectious Disease. 2019;29:48-50.
15. Garout MA, Jokhdar HA, Aljahdali IA, Zein AR, Goweda RA, Hassan-Hussein A. Mortality rate of ICU patients with the Middle East Respiratory Syndrome-Coronavirus infection at King Fahad Hospital, Jeddah, Saudi Arabia. Central European journal of public health. 2018;26(2):87–91.
16. Sherbini N, Iskandrani A, Kharaba A, Khalid G, Abduljawad M, Al-Jahdali H. Middle East respiratory syndrome coronavirus in Al-Madinah City, Saudi Arabia: Demographic, clinical and survival data. Journal of Epidemiology and Global Health. 2017;7(1):29–36.
17. Khalid I, Alraddadi BM, Dairi Y, Khalid TJ, Kadri M, Alshukairi AN, et al. Acute Management and Long-Term Survival Among Subjects With Severe Middle East Respiratory Syndrome Coronavirus Pneumonia and ARDS. Respir Care. 2016;61(3):340-8.
18. Choi WS, Kang C-I, Kim Y, Choi J-P, Joh JS, Shin H-S, et al. Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infection & chemotherapy. 2016;48(2):118-26.
19. Al-Hameed F, Wahla AS, Siddiqui S, Ghabashi A, Al-Shomrani M, Al-Thaqafi A, et al. Characteristics and outcomes of Middle East respiratory syndrome coronavirus patients admitted to an intensive care unit in Jeddah, Saudi Arabia. Journal of intensive care medicine. 2016;31(5):344-8.
20. Al Ghamdi M, Alghamdi KM, Ghandoora Y, Alzahrani A, Salah F, Alsulami A, et al. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC infectious diseases. 2016;16(1):174.
21. Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N, et al. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother. 2015;70(7):2129-32.
22. Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. The Lancet Infectious Diseases. 2014;14(11):1090-5.
23. Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. Jama. 2003;290(24):3222-8.
Table 8.
Critical appraisal for case-reports via the tool recently suggested by Murad et al. (1).
| Source | Aetiology | Selection | Ascertainment | Causality | Reporting | Final quality |
|---|---|---|---|---|---|---|
| Wang et al. (2) | SARS-CoV-2 | unclear | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Choi et al. (3) | MERS-CoV | unclear | Yes/no | No/no/no/yes | yes | High Risk of Bias |
| Al-Tawfiq et al. (4) | MERS-CoV | unclear | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Kim et al. (5) | MERS-CoV | Yes | Yes/yes | Yes/no/no/yes | yes | Low risk of bias |
| Malik et al. (6) | MERS-CoV | unclear | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Kim et al. (7) | MERS-CoV | unclear | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Cha et al. (8) | MERS-CoV | unclear | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Oh et al. (9) | MERS-CoV | Yes | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Khalid et al. (10) | MERS-CoV | Yes | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Lee et al. (11) | MERS-CoV | Yes | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Al-Tawfiq et al. (12) | MERS-CoV | unclear | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
| Tawalah et al. (13) | MERS-CoV | unclear | Yes/yes | No/no/no/yes | yes | High Risk of Bias |
Abbreviations: CD: Cannot be determined, MERS-CoV: Middle-Eastern respiratory syndrome coronavirus, NA: Not applicable, NR: Not Reported, SARS-CoV: severe acute respiratory syndrome coronavirus.
References.
1. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ evidence-based medicine. 2018;23(2):60-3.
2. Wang L, Xu X, Ruan J, Lin S, Jiang J, Ye H. Quadruple therapy for asymptomatic COVID-19 infection patients. Expert Review of Anti-infective Therapy. 2020:1-8.
3. Choi JY, Oh JO, Ahn JY, Choi H, Kim JH, Seong H, et al. Absence of neutralizing activity in serum 1 year after successful treatment with antivirals and recovery from mers in south korea. Clinical and Experimental Vaccine Research. 2019;8(1):86-8.
4. Al-Tawfiq JA, Hinedi K. The calm before the storm: clinical observations of Middle East respiratory syndrome (MERS) patients. Journal of Chemotherapy. 2018;30(3):179-82.
5. Kim J-E, Heo J-H, Kim H-O, Song S-H, Park S-S, Park T-H, et al. Neurological Complications during Treatment of Middle East Respiratory Syndrome. Journal of Clinical Neurology. 2017;13(3):227-33.
6. Malik A, El Masry KM, Ravi M, Sayed F. Middle east respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerging infectious diseases. 2016;22(3):515.
7. Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-alpha for Middle East respiratory syndrome. Antiviral therapy. 2016;21(5):455-9.
8. Choi WS, Kang C-I, Kim Y, Choi J-P, Joh JS, Shin H-S, et al. Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infection & chemotherapy. 2016;48(2):118-26.
9. Oh M-d, Choe PG, Oh HS, Park WB, Lee S-M, Park J, et al. Middle East respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015. Journal of Korean Medical Science. 2015;30(11):1701-5.
10. Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt TS, Al Mutairy E. Case report Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antiviral therapy. 2015;20:87-91.
11. Lee JY, Kim YJ, Chung EH, Kim DW, Jeong I, Kim Y, et al. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC infectious diseases. 2017;17(1):498.
12. Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. International Journal of Infectious Diseases. 2014;20:42-6.
13. Al-Qaseer M. The most effective therapeutic regimen for patients with severe Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection. Journal of Infectious Diseases and Therapy. 2015.
From 26 studies of MERS (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2017, 2019; Cha et al., 2016; Choi et al., 2016, 2019; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2014, 2015, 2016; Kim et al., 2016, 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Rhee et al., 2016; Shalhoub et al., 2015, 2018; Sherbini et al., 2017), two were good quality case-series (Khalid et al., 2014; Rhee et al., 2016). There were 11 cohorts (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Alfaraj et al., 2019; Arabi et al., 2019; Choi et al., 2016; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2016; Omrani et al., 2014; Shalhoub et al., 2015; Sherbini et al., 2017), two of which were good quality (Arabi et al., 2019; Shalhoub et al., 2015), while the other nine were of poor quality, mostly due to low comparability scores and not adjusting for confounding factors (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Alfaraj et al., 2019; Choi et al., 2016; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2016; Omrani et al., 2014; Sherbini et al., 2017). From 11 case reports in MERS (Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Cha et al., 2016; Choi et al., 2019; Khalid et al., 2015; Kim et al., 2016, 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015), ten were of a high risk of bias, mainly because of lacking any description of suitable selection processes (Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Cha et al., 2016; Choi et al., 2019; Khalid et al., 2015; Kim et al., 2016; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015). Furthermore, only one study had a low risk of bias (Kim et al., 2017).
Two studies were included in SARS (Loutfy et al., 2003; Zhao et al., 2003). One was a randomized clinical trial (Zhao et al., 2003) with a high risk of bias, and the other was a cohort study of good quality (Loutfy et al., 2003).
Finally, we provided the results for the quality of evidence by the LOE tool (Table 1, Table 2, Table 3). For COVID-19 studies, only one study declared a conflict of interest (Wang et al., 2020c), and others did not have any competing interests to declare. For MERS studies, two studies declared the existence of a conflict of interest (Arabi et al., 2020; Omrani et al., 2014). Finally, SARS studies declared no conflict of interest. A few studies did not mention whether competing interests were present (Table S2).
3.4. Mortality
3.4.1. COVID-19
Out of 29 clinical COVID-19 studies, three did not specify mortality (Cai et al., 2020a; Fan et al., 2020; Zhou et al., 2020b). A total of 414 cases expired in 26 studies (Chen et al., 2020; Cheng et al., 2020; Du et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Pan et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020). Interestingly, 14 studies reported no mortality (Huang et al., 2020a; Hung et al., 2020; Jiang et al., 2020; Liao et al., 2020; Liu et al., 2020a, 2020b; Lo et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; Wang et al., 2020a; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020), and all cases in three studies died (Chen et al., 2020; Du et al., 2020; Huang et al., 2020b). This was, in part, due to that some studies strictly sampled cases with a fatal outcome or survivors. Interestingly a study of 101 non-survivors was published with 134 cases, comprising a new comparator group of 33 survivors (Zhang et al., 2020a).
A recent open-label RCT showed no mortality in both LPV/RTV + RBV + IFN-β (n = 86) and LPV/RTV groups (n = 41) (P = 1.00) (Hung et al., 2020). A double-blind, placebo-controlled, multicenter RCT included 158 and 78 cases as intention-to-treat population in REM + IFN and Placebo + IFN groups, respectively. Results showed a 28-day Mortality of 22 (14%) in REM group (for REM + IFN: 29 (18%)) and 10 (13%) in the placebo group (for placebo + IFN: 15 (19%)) (risk difference = 1·1%, 95% CI: (−8·1,10·3)) (Wang et al., 2020c).
3.4.2. MERS
A total of 494 patients expired in all 24 studies (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Arabi et al., 2019; Cha et al., 2016; Choi et al., 2016, 2019; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2014, 2015, 2016; Kim et al., 2016, 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015; Omrani et al., 2014; Rhee et al., 2016; Shalhoub et al., 2015; Sherbini et al., 2017). A multi-center study, after adjusting for diabetes with chronic complications, liver disease, renal disease, any malignancy, SOFA score on day 1, source of infection, and year, indicated an increased day 90 mortality in the group receiving rIFN vs. the no rIFN group (logistic regression adjusted OR = 2.53, 95% CI: (1.32,4.85) (P = 0.005)) (Arabi et al., 2019). The combination therapy of IFN/RBV was not associated with death in a recent cohort (Alfaraj et al., 2019). Another study reported a CFR of 31.5% in patients who received IFN treatments, and a CFR of 40% in patients who did not receive IFN (P = 0.698) (Sherbini et al., 2017).
3.4.3. SARS
In two studies, 12 (5.67%) patients died (Loutfy et al., 2003; Zhao et al., 2003). A randomized trial of 190 patients treated SARS cases with the following regimens: Group A (n = 40): RBV and Cefoperazone/Sulbactam, and oxygen therapy; Group B: fluoroquinolone, rIFN-α and restricted steroid use (n = 30); Group C (n = 60): quinolone, azithromycin, rIFN-α for some patients, and steroids when symptoms worsened; and Group D (n = 60): levofloxacin, azithromycin, 45 patients were given rIFN-α, high-dose methylprednisolone was given when infiltrates affected more than one pulmonary segment or when consolidation was expanded, and broad-spectrum antibiotics if a bacterial infection was confirmed after culture. In four groups, 2 (5%), 2 (6.67%), 7 (11.67%), and 0 (0%) patients died, respectively (Zhao et al., 2003). In the other SARS study, 1 (7.7%) patient in the corticosteroid group (n = 13) died, while all patients in the corticosteroid + IFN-Alfacon-1 group (n = 9) survived (Loutfy et al., 2003).
3.5. Discharge
3.5.1. COVID-19
953 hospital discharges were reported in 24 studies (Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Pan et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020b; Wang et al., 2020c; Xiao-Wei et al., 2020; Yu et al., 2020; Yuan et al., 2020; Zhou et al., 2020b). A RCT using a six-category ordinal scale previously defined by the authors showed that at the first level of the scale, day 28 discharge (alive) was 92 (61%) in the REM + IFN group compared to 45 (58%) in the placebo + IFN group (OR = 1·15, 95% CI: (0·67,1·96)) (Wang et al., 2020c).
3.5.2. MERS
A total of 33 cases were discharged in 18 studies (Al Ghamdi et al., 2016; Al-Hameed et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Cha et al., 2016; Choi et al., 2016, 2019; Garout et al., 2018; Khalid et al., 2014, 2015, 2016; Kim et al., 2016, 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015; Rhee et al., 2016). However, six studies did not report a clear discharge outcome (Alfaraj et al., 2019; Arabi et al., 2019; Habib et al., 2019; Omrani et al., 2014; Shalhoub et al., 2015; Sherbini et al., 2017). A recent observation, in which all cases were either discharged or deceased by the end of the study period, showed a discharge rate of 20% in the RBV + IFN-α (n = 35) group vs. 35.2% in the no RBV + IFN-α group (n = 17) (Garout et al., 2018).
3.6. Chest imaging and X-ray presentations
3.6.1. COVID-19
Consolidation of pneumonia was indicated in six studies (Cai et al., 2020a; Fernández-Ruiz et al., 2020; Huang et al., 2020a; Jian-ya, 2020; Liao et al., 2020; Liu et al., 2020a), and local or diffuse infiltrates were reported in two studies (Fan et al., 2020; Hung et al., 2020). Some publications reported ground glassy shadows (Chen et al., 2020; Du et al., 2020; Huang et al., 2020a; Jian-ya, 2020; Jin et al., 2020; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020a). Blurred edges were also reported by one study (Rui et al., 2020). Speckles and patchy shadows were observed in nine studies (Chen et al., 2020; Huang et al., 2020a; Liao et al., 2020; Liu et al., 2020a; Lo et al., 2020; Rui et al., 2020; Sun et al., 2020; Wan et al., 2020; Wang et al., 2020a). Thickening or disorder of textures was observed in three distinct reports (Huang et al., 2020a; Jian-ya, 2020; Rui et al., 2020). Other reported categories included unilateral or bilateral CXR involvement, pleural effusion, pneumothorax, white lung appearance, lung streak shadow, single lobe lesions, multiple solid nodules, visible band shadows, and bronchial shadow with air (Cai et al., 2020a; Cheng et al., 2020; Du et al., 2020; Fan et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020a; Huang et al., 2020b; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Liao et al., 2020; Liu et al., 2020a; Liu et al., 2020b; Lo et al., 2020; Pan et al., 2020; ; Rui et al., 2020; Sun et al., 2020 Qiu et al., 2020, To et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020b; Xiao-Wei et al., 2020; Yu et al., 2020; Zhou et al., 2020b).
A recent non-randomized open-label trial investigated the efficacy of combination therapy of IFN with FPV, and included a total of 80 patients, who received IFN-α1b in two arms of the study (FPV + IFN group (n = 35), LPV/RTV + IFN (n = 45). The results showed that CT scan scores (median, range) were 12 (4.0–14.0) for FPV + IFN group, and 10 (4.5–13.5) for the LPV/RTV + IFN group (P = 0.78). Chest CT changes showed improvement in 32 cases (91.43%) vs. 28 (62.22%) cases, deterioration in 1 case (3.23%) vs. 9 (20.00%) cases, and was constant in 2 cases (6.45%) vs. 8 (17.78%) cases in FPV + IFN group and LPV/RTV + IFN group after 2 weeks, respectively (P = 0.004) (Cai et al., 2020a).
3.6.2. MERS
Of 24 distinct reports, lung consolidation was present in 12 studies (Al Ghamdi et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Arabi et al., 2019; Choi et al., 2016, 2019; Kim et al., 2017; Lee et al., 2017; Malik et al., 2016; Oh et al., 2015; Rhee et al., 2016; Sherbini et al., 2017), while 11 studies showed infiltrates (Al Ghamdi et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Arabi et al., 2019; Cha et al., 2016; Khalid et al., 2014, 2015; Kim et al., 2016; Lee et al., 2017; Oh et al., 2015; Rhee et al., 2016). Eight studies reported ground glass shadows (Cha et al., 2016; Choi et al., 2016; Khalid et al., 2015; Kim et al., 2017; Lee et al., 2017; Oh et al., 2015; Rhee et al., 2016; Sherbini et al., 2017). Patchy shadows were observed in two studies (Al-Tawfiq and Hinedi, 2018; Oh et al., 2015). Other reported categorizations included atelectasis, hilar vascular shadow, bronchovascular marking, acute pulmonary embolism, multiple solid nodules, single or multiple lobe lesion, and pleural effusion (Al-Qaseer, 2015; Cha et al., 2016; Choi et al., 2016; Khalid et al., 2014, 2015, 2016; Kim et al., 2016, 2017; Malik et al., 2016; Rhee et al., 2016; Sherbini et al., 2017).
3.6.3. SARS
In a randomized trial with four treatment groups (described in the SARS mortality section), the number of cases with unabsorbed pulmonary infiltrates was 12, 11, 13, and 4 for groups A, B, C, and D, respectively. Moreover, the difference between groups was significant (P = 0.003). This study also reported infiltrates localized in one pulmonary segment, signs in one pulmonary field, the involvement of both lungs, diffuse damage, as well as reported cases with only interstitial changes (Zhao et al., 2003). In a recent preliminary study, patients were treated with IFN-Alfacon-1 + corticosteroid or corticosteroid alone. In this study, all cases in both groups showed abnormal chest imaging (P > 0.99). Eighteen patients did not show a full resolution of CXR abnormalities. Interestingly, the IFN-Alfacon-1 treatment group showed a reduced duration to 50% resolution of lung imaging abnormalities. The median for this duration was 4 in the IFN-Alfacon-1 + corticosteroid group vs. 9 in the corticosteroid only group (P = 0.001) (Loutfy et al., 2003).
3.7. Disease severity
3.7.1. COVID-19
Seven studies that did not report the number of severe and non-severe cases (Chen et al., 2020; Fernández-Ruiz et al., 2020; Huang et al., 2020b; Liu et al., 2020b; Wang et al., 2020b; Xiao-Wei et al., 2020; Yu et al., 2020) were excluded. 22 distinct reports, including 766 severe and 2007 non-severe cases, were studied (Cai et al., 2020a; Cheng et al., 2020; Du et al., 2020; Du et al., 2020, Fan et al., 2020; Huang et al., 2020a; Hung et al., 2020; Jian-ya, 2020; Jiang et al., 2020; Jin et al., 2020; Liao et al., 2020; Liu et al., 2020a; Lo et al., 2020; Pan et al., 2020; Qiu et al., 2020; Rui et al., 2020; Sun et al., 2020; To et al., 2020; Wan et al., 2020; Wang et al., 2020a; Wang et al., 2020c; Yuan et al., 2020; Zhou et al., 2020b).
A retrospective cohort reported mean IFN treatment durations (days) in various levels of COVID-19 severity were 10.88, 95% CI: (8.00,13.75) in the mild group (n = 8), 14.24, 95% CI: (13.45,15.03) in the moderate group (n = 75), and 15.55, 95% CI: (13.84,17.25) in the severe group (n = 11), which were significantly different (one-way ANOVA, P = 0.01) (Yuan et al., 2020). The number of non-severe (n = 52) and severe (n = 8) patients receiving various combination IFN regimens were reported in a trial. Among cases treated with IFN-β + LPV/RTV, 39 (80%) were non-severe and 3 (38%) were severe (P = 0.045). Also, among cases treated with IFN-β + LPV/RTV + ARB, 10 (19%) were non-severe and 5 (63%) were severe (P = 0.019) (Jiang et al., 2020).
3.7.2. MERS
454 severe cases were reported in 10 studies (Al-Hameed et al., 2016; Al-Qaseer, 2015; Arabi et al., 2019; Cha et al., 2016; Choi et al., 2016, 2019; Kim et al., 2017; Lee et al., 2017; Omrani et al., 2014; Rhee et al., 2016). The rest of studies had an unclear number of severe cases (Al Ghamdi et al., 2016; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2014, 2015, 2016; Kim et al., 2016; Malik et al., 2016; Oh et al., 2015; Shalhoub et al., 2015; Sherbini et al., 2017). In total, 9 non-severe cases were also reported in 10 studies (Al-Hameed et al., 2016; Arabi et al., 2019; Cha et al., 2016; Choi et al., 2016, 2019; Khalid et al., 2015; Kim et al., 2017; Lee et al., 2017; Omrani et al., 2014; Rhee et al., 2016), and the rest of studies had an unclear number of non-severe cases (Al Ghamdi et al., 2016; Al-Qaseer, 2015; Al-Tawfiq and Hinedi, 2018; Al-Tawfiq et al., 2014; Alfaraj et al., 2019; Garout et al., 2018; Habib et al., 2019; Khalid et al., 2014, 2016; Kim et al., 2016; Malik et al., 2016; Oh et al., 2015; Shalhoub et al., 2015, 2018; Sherbini et al., 2017).
Univariable analysis of the influence of severity of disease on medications administered showed a significant negative risk association of – 4.62, 95% CI: (−8.40,−0.84) (P = 0.018) for IFN-α, and a negative but non-significant risk association of – 1.24, 95% CI: (−6.71,4.24) (P = 0.652) for IFN-β. Moreover, a multivariable analysis, which included a biomarker of disease severity, showed a strong association between disease severity and decreased survival, and no association between treatment with IFN-β and mortality (OR = 0.68, 95% CI: (0.04,10.28)) (P = 0.778) (Al Ghamdi et al., 2016). However, another study that did not discuss the severity of included patients showed a reduction in mortality was significantly associated with IFN-α (OR = 0.16, 95% CI: (0.02,1.38)) (P = 0.09). The lower mortality did not reach statistical significance for IFN-β (OR = 0.28, 95% CI (0.03,2.33)) (P = 0.24) (Shalhoub et al., 2015).
3.8. Inflammatory cytokines
3.8.1. COVID-19
A cohort study showed that during the time interval of day 0–20 (upon onset of symptoms), on average, cases receiving the ARB only regimen had higher CRP levels than cases treated with IFN alone or both IFN and ARB, by 25.7 mg/l. Also, over the time interval between day 12 and day 42 (upon onset of symptoms), on average, cases receiving the ARB only regimen showed higher IL-6 levels than the cases who received IFN alone or both IFN and ARB, by 33.5 pg/ml. These effects were not influenced by co-morbidities for IL-6 (P = 0.456), or CRP (P = 0.420) levels (Zhou et al., 2020b). In a recent phase II trial, IL-6 levels (log10 pg/ml, median, (Q1,Q3)) were 1·4, (1·0–1·4) in LPV/RTV + RBV + IFN-β group (n = 86), and 1·4, (1·0–1·6) in LPV/RTV group (n = 41). These results did not show any significant differences between the trial arms (P = 0.43). Also, in this trial, TNF-α levels were measured for both trial arms (P = 1.00) (Hung et al., 2020). In another clinical study, CRP (mg/dl, median, (Q1,Q3) level was 18.6 (5.0–20.0) for all patients (n = 80). CRP levels were 15.0, (3.0–19.2) and 21.4, (5.0–23.2) in the FPV + IFN-α (n = 35) and LPV/RTV + IFN-α arm (n = 45) of the study, respectively (P = 0.33). IL-6 (ng/l, median, (Q1,Q3)) was 13.4, (4.4–16.2) in all patients. IL-6 levels were 14.0, (3.5–11.0) and 12.9, (5.3–16.8) in FPV + IFN-α and LPV/RTV + IFN-α arm, respectively (P = 0.77) (Cai et al., 2020).
3.8.2. MERS
There were higher CRP levels (mg/l, median, (Q1,Q3)) in cases treated with IFN-α (n = 13) (86.5, (25,226)) compared to cases treated with IFN-β (80, (19.3346)); However, this difference did not reach statistical significance (P = 0.61) (Shalhoub et al., 2015).
3.9. Hospitalization duration
3.9.1. COVID-19
In a recent cohort, duration from the symptom onset to hospital admission (days, median, (Q1,Q3)) was 8.0, (5.5, 15.5), 6.5, (3.0, 10.0), and 10.0, (4.5, 19.5) for IFN, IFN + ARB, and ARB groups, respectively. This difference, however, was not statistically significant (P = 0.087) (Zhou et al., 2020b). A placebo-controlled RCT of IFN therapy in combination with REM showed a similar duration of hospitalization (days, median, (Q1,Q3)) in the two arms of the trial 25·0, (16·0,38·0) in intention-to-treat populations of REM group (for REM + IFN: 29 (18%)) vs. 24·0 (18·0,36·0) in placebo group (for placebo + IFN: 15 (19%)) (risk difference = 0·0, 95% CI: (−4·0,4·0)) (Wang et al., 2020c). A lately surfaced cohort indicated that the duration of hospitalization was significantly correlated with PCR negative conversion durations in the IFN-α + LPV/RTV + RBV group (P = 0.0215), as well as the IFN-α + LPV/RTV group (P = 0.012) (Yuan et al., 2020). A recent study divided the cohort of study into patients who experienced symptoms for more or less than ten days; Furthermore, 15 (45.5%) cases with symptoms lasting longer than ten days and 19 (65.5%) cases with symptoms lasting shorter than or equal to 10 days received IFN alone or in combination with ARB, RBV, or LPV/RTV (Xiao-Wei et al., 2020). In a recent phase II RCT, the duration of hospital stay (days, median (Q1,Q3)) was significantly lower in the LPV/RTV + RBV + IFN-β 9·0, (7·0–13·0), compared with 14·5, (9·3–16·0) in the LPV/RTV (control) group (P = 0·016) (Hung et al., 2020).
3.9.2. MERS
The length of hospital stay (days, median, (Q1,Q3)) in a recent multi-center study was reported 17 (10, 28) in RBV/IFN group compared to 20 (10, 36) in the no RBV/IFN group (P = 0.48) (Arabi et al., 2019).
3.9.3. SARS
In a randomized trial of 4 treatment groups (regimens were described in SARS mortality section), time to discharge (days, (S.D.)) was 24·8, (5·5) in group A, 24·8, (6·4) in group B, 22·4, (5·9) in group C, and 20·7, (4·6) in group D. Also, the difference between groups was not reported significant (Zhao et al., 2003).
3.10. Unfavorable drug events in CoV infections
3.10.1. COVID-19
The total number of ADEs were significantly lower in the FPV + IFN-α (4) compared to the LPV/RTV arm of the trial (25) (P = 0.001); Also, nausea in patients in the FPV + IFN-α group was lower significantly in the FPV + IFN-α (0) compared to the LPV/RTV arm of the trial (6) (P = 0.03) (Cai et al., 2020). In another study, self-limited nausea and diarrhea were similar between the two groups. Furthermore, in this study, ADEs were reported by 41 (48%) of cases in the LPV/RTV + RBV + IFN group and 20 (49%) of cases in the LPV/RTV group (Hung et al., 2020). A placebo-controlled multi-center trial for the efficacy of REM + IFN compared with placebo + IFN found that ADEs were reported in 102 (66%) of REM receivers compared to 50 (64%) of placebo receivers. Importantly, REM was stopped early because of ADEs in 18 (12%) cases compared to 4 (5%) patients who discontinued placebo early (Wang et al., 2020c). Finally, ADEs have been provided (Table S2).
3.10.2. MERS
MERS patients showed several ADEs during treatment with IFNs and other drugs. ADEs also included pancreatitis, which was reported in one patient (Al-Tawfiq et al., 2014), kidney injuries (e.g., AKI) in 5 cases (Khalid et al., 2016), and renal failure in 3 cases (Khalid et al., 2014). Hepatic jaundice occurred in one patient during therapy (Choi et al., 2019). Changes in laboratory data were mentioned in some studies. Alterations in the laboratory profile of patients included increased amylase and lipase in seven patients who were treated with IFN (Al-Tawfiq et al., 2014; Rhee et al., 2016), increased bilirubin (Kim et al., 2016), decrease in Hb in 45 cases (Al-Tawfiq et al., 2014; Omrani et al., 2014), Scr and AST-ALT elevation in one patient with IFN therapy (Al-Tawfiq et al., 2014), thrombocytopenia in 2 patients (Al-Tawfiq et al., 2014; Cha et al., 2016), anemia in 46 cases (Al-Qaseer, 2015; Omrani et al., 2014), and hemodynamic instability in 3 patients during treatment with IFN and other drugs were reported as ADEs (Khalid et al., 2014). Fever was mentioned in one IFN receiver (Cha et al., 2016). Also, multi-organ damage as a severe side effect was reported in 5 cases treated with IFN (Omrani et al., 2014).
3.10.3. SARS
In SARS patients, fever, neutropenia with an absolute neutrophil count (ANC) of less than 1000/μl on the last day of treatment, a minor transient decrease in ANC, and elevation of serum transaminase levels were reported during IFN therapy in both IFN-Alfacon-1 and corticosteroid alone groups (Loutfy et al., 2003).
3.11. Quantitative analysis
Three observational COVID-19 investigations were eligible for meta-analysis of IFN treatment and severity. The studies showed moderate certainty (Table 9 ). Fixed-effects (Mantel-Haenszel) (I2 <35%) approach was employed. Results showed the relation between receiving IFN and severity to be inconclusive. Effect size in all three studies crossed the line of no effect, indicating inconclusiveness of the current data (log OR = −0.44, 95% CI: (−1.13,0.25), I2 = 31.42%) (Fig. 3 ).
Table 9.
Summary of findings for COVID-10 severity and IFN administration.
| IFN compared to No IFN for COVID-19 | ||||||
|---|---|---|---|---|---|---|
| Patient or population: COVID-19 Setting: Observational studies Intervention: IFN Comparison: No IFN | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with No IFN | Risk with IFN | |||||
| Severity | 318 per 1000 | 145 per 1000 (33–453) | OR 0.363 (0.074–1.778) | 168 (3 observational studies) | ⨁⨁⨁◯ MODERATE |
(Lo et al., 2020), (Fan et al., 2020), (Pan et al., 2020) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; COVID-19: coronavirus 2019; GRADE: Grading of Recommendations Assessment, Development and Evaluation; IFN: interferon OR: Odds ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Fig. 3.
Quantitative analysis for COVID-19 severity and IFN administration vs. placebo.
Six MERS-CoV cohorts were included in the quantitative synthesis. Evaluation of the risk of bias across studies via GRADE showed the evidence was low certainty, indicating that further research could change our estimation (Table 10 ). Random-effects (DerSimonian-Laird) (I2 >35%) approach was selected. Mortality was not clearly affected by the administration of RBV/IFN treatment vs. no RBV/IFN (log OR = −0.05, 95% CI: (−0.71,0.62), I2 = 44.71%) (Fig. 4 ). Publication bias was not analyzed due to the insufficient number of studies (n < 10). Finally, summary of findings table for GRADE assessments for narrative synthesis outcomes were conducted according to a new study (Murad et al., 2017), and results were provided in Table 11, Table 12, Table 13 for COVID-19, MERS, and SARS, respectively.
Table 10.
Summary of Findings table for MERS-CoV mortality and IFN/RBV administration.
| Bias across studies for mortality in MERS studies | ||||||
|---|---|---|---|---|---|---|
| Patient or population: MERS-CoV patients Setting: Observational studies Intervention: RBV/IFN Comparison: No RBV/IFN | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | References | |
| Risk with No RBV/IFN | Risk with RBV/IFN | |||||
| Mortality | 580 per 1000 | 552 per 1000 (211–852) | OR 0.891 (0.194–4.168) | 708 (6 observational studies) | ⨁⨁◯◯ LOW a |
(Habib et al., 2019), (Arabi et al., 2019), (Omrani et al., 2014), (Garout et al., 2018), (Khalid et al., 2016), (Choi et al., 2016) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CoV: coronavirus; IFN: interferon; MERS: middle east respiratory syndrome; OR: Odds ratio; RBV: ribavirin. | ||||||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Explanations.
References.
Many did not control for age, sex, and disease severity.
Fig. 4.
Quantitative analysis for MERS-CoV mortality and IFN/RBV administration vs. no IFN/RBV.
Table 11.
GRADE assessment for narrative synthesis outcomes in COVID-19 studies.
| Summary of findings: | |||
|---|---|---|---|
| Combination or non-combination IFN treatment compared to No IFN/A different treatment protocol including IFN for COVID-19 | |||
| Patient or population: COVID-19 Setting: RCTs, non-randomized trials, and observational studies Intervention: Combination or non-combination IFN treatment Comparison: No IFN/A different treatment protocol including IFN | |||
| Outcomes | Impact | Studies | Certainty of the evidence (GRADE) |
| Mortality | Both studies did not report a remarkable difference in total mortality. Considerable variations were present among study designs. | (2 RCTs) | ⨁⨁⨁◯ MODERATEa |
| Comparative assessments are lacking. | (2 non-randomized trials) | – | |
| Comparative assessments are lacking. | (25 observational studies) | ⨁⨁◯◯ LOW |
|
| Discharge | Wang et al. showed higher discharge rate in IFN + REM group compared to IFN + Placebo. | (2 RCTs) | ⨁⨁⨁⨁ HIGH |
| Comparative assessments are lacking. | (2 non-randomized trials) | – | |
| Comparative assessments are lacking. | (25 observational studies) | – | |
| Chest X-ray | Comparative assessments are lacking. | (2 RCTs) | – |
| Cai et al. showed FPV + IFN-α was significantly linked to improvement in CXR compared with LPV/RTV + IFN-α treatment (p = 0.004). | (2 non-randomized trials) | ⨁⨁⨁⨁ HIGH |
|
| Comparative assessments are lacking. | (25 observational studies) | – | |
| Severity | Meta-analysis conducted. | (2 RCTs) | -b |
| Jiang et al. showed cases treated with IFN-β + LPV/RTV, 39 (80%) were non-severe and 3 (38%) were severe (p = 0.045). Also, among cases treated with IFN-β + LPV/RTV + ARB, 10 (19%) were non-severe and 5 (63%) were severe (p = 0.019) | (2 non-randomized trials) | ⨁⨁⨁◯ MODERATEc |
|
| A cohort by Yuan et al. showed IFN treatment durations varied significantly among severe, moderate, and mild cases (p = 0.01). | (25 observational studies) | ⨁◯◯◯ VERY LOWd |
|
| Inflammatory profile | Hung et al. did not find any significant difference in inflammatory profile. | (2 RCTs) | ⨁⨁⨁◯ MODERATEe |
| In a study with FPV + IFN-α and LPV/RTV + IFN-α arms, Cai et al. did not show a significant different in IL-6 and CRP. | (2 non-randomized trials) | ⨁⨁⨁◯ MODERATEe |
|
| Zhou et al. showed a higher efficacy for IFN and its combination therapies vs. therapy without IFN in reducing inflammatory elements. | (25 observational studies) | ⨁◯◯◯ VERY LOWe |
|
| Hospital durations | Comparative assessments are lacking. | (2 non-randomized trials) | – |
| Hung et al. showed LPV/RTV + RBV + IFN-β group had a reduced hospitalization time compared to LPV/RTV. Wang et al. included two arms both of which used IFN, and detected no difference in hospitalization duration. | (2 RCTs) | ⨁⨁⨁⨁ HIGH |
|
| No significant effects were detected in 3 studies. Xu et al. 15 (45.5%) cases with symptoms lasting longer than ten days and 19 (65.5%) cases with symptoms lasting shorter than or equal to 10 days received IFN alone or in combination with ARB, RBV, or LPV/RTV. In the study by Zhou et al. IFN, IFN + ARB, and ARB arms were not significantly different in regards to symptom onset to hospital admission (p = 0.87). Yuan et al. showed that the duration of hospitalization was significantly correlated with PCR negative conversion durations in the IFN-α + LPV/RTV + RBV group (p = 0.0215), as well as the IFN-α + LPV/RTV group (p = 0.012) | (25 observational studies) | ⨁◯◯◯ VERY LOWf,g |
|
| ADEs | Significant and clear association was not established. | (2 RCTs) | ⨁⨁⨁◯ MODERATEh |
| Cai et al. showed FPV + IFN-α was significantly linked to less total ADEs compared with LPV/RTV + IFN-α treatment (p = 0.001). | (2 non-randomized trials) | ⨁⨁⨁⨁ HIGH |
|
| Comparative assessments are lacking. | (25 observational studies) | – | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADEs: adverse drug events, ARB: arbidol, CI: Confidence interval, FPV: favipiravir, IL: interleukin, LPV: lopinavir, RCT: randomized controlled trial, REM: remdesivir, RTV: ritonavir. | |||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
Explanations.
Different study designs with considerably different combination or non-combination IFN treatments may introduce inconsistency.
No explanation of randomization process both in trial document and the article.
IFN durations are indirectly related to our question. Our question is whether IFN use vs. a different treatment therapy is linked to COVID-19 severity. Duration of therapy is related but may not provide a direct answer.
All important inflammatory elements, such as major inflammatory cytokines are required for proper assessment of inflammatory state.
Different hospital durations have been comparatively described in different arms of 2 studies, which can not be assessed with consistency.
Hospital durations and IFN use is the main question. However, Xu et al. synthesized IFN use by duration of symptoms. Although related, this might be seriously indirect in answering the research question.
Assessment depending on specific ADEs, serious ADEs, or any ADEs could result in different interpretations of the same study.
Table 12.
GRADE assessment for narrative synthesis outcomes in MERS studies.
| Summary of findings: | |||
|---|---|---|---|
| Combination or non-combination IFN treatment compared to No IFN/A different treatment protocol including IFN for MERS | |||
| Patient or population: MERS Setting: Observational studies Intervention: Combination or non-combination IFN treatment Comparison: No IFN/A different treatment protocol including IFN | |||
| Outcomes | Impact | Studies | Certainty of the evidence (GRADE) |
| Mortality | Meta-analysis conducted. Also, 2 additional studies for narrative synthesis detected the use of IFN therapy was possibly of no use (Alfaraj et al and Sherbini et al.). | (26 observational studies) | ⨁◯◯◯ VERY LOWa |
| Discharge | Garout et al. showed a discharge rate of 20% in the RBV + IFN-α (n = 35) group vs. 35.2% in the no RBV + IFN-α group (n = 17). | (26 observational studies) | ⨁◯◯◯ VERY LOWa |
| Chest x-ray | Comparative assessments are lacking. | (26 observational studies) | – |
| Inflammatory profile | A study compared IFN-α with IFN-β, and found the difference in CRP levels was not significant (p = 0.61) (Shalhoub et al.). | (26 observational studies) | ⨁◯◯◯ VERY LOWb |
| Severity | Al Ghamdi et al. showed a negative relation with severity for IFN-α but not IFN-β. Precisely, Univariable analysis of the influence of severity of disease on medications administered showed a significant negative risk association of – 4.62, 95% CI: (−8.40,−0.84) (p = 0.018) for IFN-α, and a negative but non-significant risk association of – 1.24, 95% CI: (−6.71,4.24) (p = 0.652) for IFN-β. Moreover, a multivariable analysis, which included a biomarker of disease severity, showed a strong association between disease severity and decreased survival, and no association between treatment with IFN-β and mortality (OR = 0.68, 95% CI: (0.04,10.28)) (p = 0.778) | (26 observational studies) | ⨁⨁◯◯ LOW |
| Hospital durations | The length of hospital stay in RBV/IFN vs no RBV/IFN was not significantly different (p = 0.48) (Arabi et al.). | (26 observational studies) | ⨁⨁◯◯ LOW |
| ADEs | In 7 studies, ADEs were recorded while using regimen containing IFNs, including multi-organ damage, adverse change in blood profile, thrombocytopenia, kidney disease, fever, and pancreatitis (Al-Tawfiq et al., Rhee et al., Kim et al., Cha et al., Al-Qaseer et al., Omrani et al., Khalid et al.). | (26 observational studies) | ⨁◯◯◯ VERY LOWa,c |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADEs: adverse drug events, CI: Confidence interval, CRP: C-reactive protein, IFN: interferon, MERS: Middle-Eastern respiratory syndrome, RBV: ribavirin. | |||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
Explanations.
Different IFN-based regimen were used, and were compared to varied treatment options. Results should be taken as speculative, rather than as for net efficacy of IFN.
All important inflammatory elements, such as major inflammatory cytokines are required for proper assessment of inflammatory state.
Combination therapies of IFN as well as lack of a comparator group makes it difficult to determine whether such adverse events are a direct result of IFNs administration.
Table 13.
GRADE assessment for narrative synthesis outcomes in SARS studies.
| Summary of findings: | |||
|---|---|---|---|
| Combination or non-combination IFN treatment compared to No IFN/A different treatment protocol including IFN for SARS | |||
| Patient or population: SARS Setting: RCTs and observational studies Intervention: Combination or non-combination IFN treatment Comparison: No IFN/A different treatment protocol including IFN | |||
| Outcomes | Impact | No. of participants (studies) | Certainty of the evidence (GRADE) |
| Mortality | One randomized trial study was included, that gives a good idea of effect of specific regimen used in the study, However, assessment of efficacy of IFN therapies, and in particular the role of IFN is not feasible in such settings. Precisely, in a trial In four groups, 2 (5%), 2 (6.67%), 7 (11.67%), and 0 (0%) patients died, respectively (Zhao et al.). (See explanation for regimens in each group.)a | (1 RCT) | ⨁⨁⨁⨁ HIGHb |
| In the other SARS study, 1 (7.7%) patient in the corticosteroid group (n = 13) died, while all patients in the corticosteroid + IFN-Alfacon-1 group (n = 9) survived (Loutfy et al.). | (1 observational study) | ⨁⨁◯◯ LOW |
|
| Discharge | Comparative assessments are lacking. | (1 RCT) | – |
| Comparative assessments are lacking. | (1 observational study) | – | |
| Chest x-ray | Regimen (A, B, C, and D) are could be linked to unabsorbed infiltrates (p = 0.003) (Zhao et al.). However, this may be of little help for a broad range of IFN regimen due to that regimen given in the study are combinations of numerous agents. | (1 RCT) | ⨁⨁⨁⨁ HIGH |
| Addition of IFN-Alfacon-1 to corticosteroid regimen may enhance its therapeutic efficacy. In a cohort, IFN-Alfacon-1 treatment group showed a reduced duration to 50% resolution of lung imaging abnormalities (p = 0.001) (Loutfy et al.). | (1 observational study) | ⨁⨁◯◯ LOW |
|
| Inflammatory profile | Comparative assessments are lacking. | (1 RCT) | – |
| Comparative assessments are lacking. | (1 observational study) | – | |
| Severity | Comparative assessments are lacking. | (1 RCT) | – |
| Comparative assessments are lacking. | (1 observational study) | – | |
| Hospital durations | No significant difference was detected in time to discharge (Zhao et al.). | (1 RCT) | ⨁⨁⨁⨁ HIGH |
| Comparative assessments are lacking. | (1 observational study) | – | |
| ADEs | Comparative assessments are lacking. | (1 RCT) | – |
| A cohort revealed several adverse effects while treatment. However, it may not be feasible to attribute these, merely to IFN administration. Fever, neutropenia with an absolute neutrophil count (ANC) of less than 1000/μL on the last day of treatment, a minor transient decrease in ANC, and elevation of serum transaminase levels were reported during IFN therapy in both IFN-Alfacon-1 and corticosteroid alone groups (Loutfy et al.). | (1 observational study) | ⨁⨁◯◯ LOW |
|
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ANC: absolute neutrophil count, CI: Confidence interval, IFN: interferon, RCT: randomized controlled trial, SARS: severe acute respiratory syndrome. | |||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
Explanations.
The randomized trial of 190 patients by Zhao et al. treated SARS cases with the following regimens: Group A (n = 40): RBV and Cefoperazone/Sulbactam, and oxygen therapy; Group B: fluoroquinolone, rIFN-α and restricted steroid use (n = 30); Group C (n = 60): quinolone, azithromycin, rIFN-α for some patients, and steroids when symptoms worsened; and Group D (n = 60): levofloxacin, azithromycin, 45 patients were given rIFN-α, high-dose methylprednisolone was given when infiltrates affected more than one pulmonary segment or when consolidation was expanded, and broad-spectrum antibiotics if a bacterial infection was confirmed after culture.
Different IFN-based regimen were used, and were compared to varied treatment options. Results should be taken as speculative, rather than as for net efficacy of IFN.
4. Discussion
SARS-CoV, MERS-CoV, and SARS-CoV2 are human CoVs (hCoVs) that have been the cause of three outbreaks during the last two decades (Jabbari et al., 2020). All of them have shown the potential to manifest as multisystem difficult to treat infections (Goudarzi et al., 2020; Heidarpour et al., 2020; Jahanshahlu and Rezaei, 2020a; Jenab et al., 2020; Nejadghaderi et al., 2020; Rahmani et al., 2020; Sadeghmousavi and Rezaei, 2020; Yazdanpanah et al., 2020b). In particular, multisystem involvement in COVID-19 has been associated with anti-viral immunity paralysis on the one hand, and on the other hand release of pro-inflammatory cytokines, e.g., interleukin-6, and hyperinflammatory shock (Bahrami et al., 2020; Fathi and Rezaei, 2020; Mojtabavi et al., 2020; Nasab et al., 2020; Rokni et al., 2020; Saghazadeh and Rezaei, 2020a; Sarzaeim and Rezaei, 2020; Yazdanpanah et al., 2020a). Such an unpleasant event is orchestrated by genetic and environmental factors that make individuals susceptible to develop hyper inflammatory responses (Darbeheshti and Rezaei, 2020; Yousefzadegan and Rezaei, 2020). Supporting this, inborn errors of immunity have not been shown to increase the risk of developing severe COVID-19 and dying from that. However, there are sporadic reports of death in patients with combined immunodeficiency (Ahanchian et al., 2020a, 2020b; Babaha and Rezaei, 2020). For this, anti-inflammatory and immunomodulatory treatments along with monoclonal antibodies appear as potential candidates (Basiri et al., 2020b; Fathi and Rezaei, 2020; Jahanshahlu and Rezaei, 2020b; Mansourabadi et al., 2020; Pashaei and Rezaei, 2020; Pourahmad et al., 2020; Saghazadeh and Rezaei, 2020b; Seyedpour et al., 2020; Shojaeefar et al., 2020).
The ongoing COVID-19 pandemic has led the scientific community to consider repurposing previously approved treatments such as convalescent plasma, antivirals like IFN, and LPV/RTV, and the clinical reapplication of the experience learned from previous global epidemics caused by hCoVs (Guy et al., 2020). The present research has systematically investigated the efficacy of combinational or mere IFN therapy. We have reviewed the clinical literature regarding the clinical efficacy of IFN for three deadly human CoVs by analyzing the mortality, discharge, CXR presentations, onset-to-treatment duration, ADEs, and other clinically essential outcomes.
Understandably, mortality is of high clinical interest. Mortality in COVID-19 and SARS cases was not significantly affected by IFN therapy, as studies reported no mortality in all study subgroups of similar mortality. Moreover, a poor-quality cohort of SARS patients showed lower mortality and faster CXR improvement in patients receiving IFN-Alfacon-1 compared to IFN-Alfacon-1 + corticosteroids group. However, the results of this uncontrolled study should be taken with its small sample size and lack of randomization in mind. The higher discharge was indicated in a high-quality trial for combinational IFN therapy with REM vs. IFN alone in COVID-19. However, lower discharge rates for taking RBV/IFN were indicated in a poor-quality cohort. In addition to a lack of high-quality evidence backing up the use of RBV/IFN for COVID-19 patients, the calculated effect size for six MERS-CoV studies shows that IFN/RBV treatment did not prove beneficial compared with no RBV/IFN in terms of mortality. Cytokine storm in COVID-19 cases has been known for inducing a destructive immune response and is possibly responsible for unfavored clinical outcomes in COVID-19 (Nile et al., 2020). For COVID-19, inflammatory cytokines (e.g., IL-6, TNF-α, and CRP) were lower in patients who received IFN with or without ARB. The quality of this study was good. Moreover, a randomized trial of a high risk of bias indicated no difference between inflammatory cytokine levels. Furthermore, our results should be interpreted in light of competing interests of included literature.
Also, a comparison between the anti-inflammatory potential of two IFN types was not significant in MERS patients. A wide range of chest radiography presentations was found in both COVID-19 and MERS patients. Interestingly, a moderately biased trial by Cai et al. showed a significant improvement in CXRs in FPV + IFN-α group vs. LPV/RTV +IFN-α group (P = 0.004), while also showing significantly fewer ADEs (P < 0.001). The median for onset-to-treat times was mostly under two weeks for both MERS and COVID-19. Interestingly, combination ARB + IFN treatment was clinically effective in a cohort via reducing inflammatory cytokines despite the relatively long onset-to-treatment interval (days, median (Q1,Q3)) 17.0 (10.0, 22.0). ADEs were mostly reported for IFN in combination with other antivirals. Therefore, despite that some studies showed certain combinations are less likely to result in ADEs, they were inconclusive for the use of IFN.
Studies strictly including patients according to a strictly fatal or non-fatal outcome do not help compare drug effects. In general, we indicate that although IFN has been commonly given in combination with antiviral therapies (e.g., with RBV in MERS cases), most studies have not reported a definitive benefit for the inclusion of IFN in administered regimens. Therefore, we suggest that more placebo-controlled RCTs with larger populations are required to clarify further the efficacy of IFN for a reduction in improving clinical status, and more importantly, mortality in COVID-19 patients. Also, we recommend that in order to reduce bias and increase usability in practice, comparative observational studies should control for confounding factors, especially severity.
The major restriction in our synthesis was the high risk of bias in many observational studies. Also, most articles were observational studies, many of which were case reports or case series, and did not include a control group. Also, many cohort studies did not control any confounders, resulting in a high risk of bias. Most studies used a combination of pharmacological and non-pharmacological treatments, and utilized highly varied administration protocols along with IFN therapy or did not report outcomes classified by receiving IFN, complicating the distinguish of IFN-related harms or benefits from other interventions. MERS studies mostly reported RBV/IFN treatment groups, which does not assess the net effect of IFN therapy. Despite calling authors, six of SARS studies could not be retrieved due to the unavailability of the full-text. A limitation in SARS studies was using solely clinical criteria for inclusion rather than confirmed laboratory results. This may lead to the dampening of any actual treatment effects of antiviral therapeutics. We highlighted our assessments in light of lessons that can be learned from past CoVs, as they share principal similarities with the novel SARS-CoV2 (Gilbert, 2020; Peeri et al., 2020). However, though IFN treatments may hold significant potential for the management of hospitalized COVID-19 patients, it is challenging to approximate their worldwide acceptance in regards to evidence of this treatment, irrespective of the efficacy of such therapeutics in past outbreaks.
5. Conclusion
In conclusion, the present systematic review reveals that the efficacy of IFN alone has not been investigated sufficiently for three deadly human CoVs. Still, we found that combination therapy of IFN with antivirals such as FPV, ARB, REM, or corticosteroids can have potential benefits (e.g., faster CXR improvement, lower level of inflammatory cytokines). These potentials need to be tested in larger RCTs. Also, the data regarding mortality, a crucially determining clinical outcome, seem insufficient for assessing treatment efficacy. Further investigation considering potential benefits and harms (e.g., ADEs) discussed in the present research can shed light on the path, leading to more successful conclusive trials in the strict time researchers possess during rapidly evolving outbreaks.
It is notable that many of the available therapeutic options are not specific to the COVID-19 condition and the death tolls are rising (Mohamed and Rezaei, 2020). This lack of specificity has brought about numerous efforts towards understanding the origin of the virus (Lundstrom et al., 2020; Sharifkashani et al., 2020) and discovering more targeted approaches for treatment of the disease (Ahanchian et al., 2020b; Fathi and Rezaei, 2020; Lotfi et al., 2020; Mansourabadi et al., 2020; Rabiee et al., 2020; Rezaei, 2020b; Saghazadeh and Rezaei, 2020b; Seyedpour et al., 2020; Sharifkashani et al., 2020), and the management of comorbid diseases (Moazzami et al., 2020; Sahu et al., 2020). Definitely, such efforts need great scientific collaborations to occur and get presented (Mohamed et al., 2020b; Momtazmanesh et al., 2020; Moradian et al., 2020; Rzymski et al., 2020). During this pandemic in which people are at risk of infection and re-infection (Jabbari and Rezaei, 2020; Rezaei, 2020a) and social distancing is the most important method of prevention, utilization of hybrid methods for holding scientific events might help the data to be shared and the information to be exchanged (Hanaei et al., 2020; Samieefar et al., 2020).
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the final version of the manuscript.
Availability of data and materials
Not applicable.
Funding
There is no funding for the present study.
Authors’ contributions
K.S. conceptualized the study, performed data curation and analysis, and prepared the initial draft. S.Y. conceptualized the study, performed data curation, and prepared the initial draft. M.B. conceptualized the study and performed data curation. E.H. conceptualized the study and performed data curation. M.G. conceptualized the study and performed data curation. A.S. conceptualized the study, designed the project, and prepared the final draft. N.R. conceptualized the study, supervised the project, and critically appraised the manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejphar.2021.174248.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ahanchian H., Moazzen N., Faroughi M.S.D., Khalighi N., Khoshkhui M., Aelami M.H., Haghi N.S.M., Rezaei N. 2020. COVID-19 in a Child with Primary Specific Antibody Deficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanchian H., Moazzen N., Joghatayi S.H., Saeidinia A., Khoshkhui M., Aelami M.H., Rezaei N., Haghi N.S.M. Death due to COVID-19 in an infant with combined immunodeficiencies. Endocr. Metab. Immune Disord. - Drug Targets. 2020;9:755–758. doi: 10.2174/1871530320666201021142313. [DOI] [PubMed] [Google Scholar]
- Al Ghamdi M., Alghamdi K.M., Ghandoora Y., Alzahrani A., Salah F., Alsulami A., Bawayan M.F., Vaidya D., Perl T.M., Sood G. Treatment outcomes for patients with middle eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect. Dis. 2016;16(1):174. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hameed F., Wahla A.S., Siddiqui S., Ghabashi A., Al-Shomrani M., Al-Thaqafi A., Tashkandi Y. Characteristics and outcomes of Middle East respiratory syndrome coronavirus patients admitted to an intensive care unit in Jeddah, Saudi Arabia. J. Intensive Care Med. 2016;31:344–348. doi: 10.1177/0885066615579858. [DOI] [PubMed] [Google Scholar]
- Al-Osail A.M., Al-Wazzah M.J. The history and epidemiology of Middle East respiratory syndrome corona virus. Multidiscip. Respir. Med. 2017;12 doi: 10.1186/s40248-017-0101-8. 20-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qaseer M. The most effective therapeutic regimen for patients with severe Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection. J. Infect. Dis. Ther. 2015;3(4) doi: 10.4172/2332-0877.1000223. [DOI] [Google Scholar]
- Al-Tawfiq J.A., Hinedi K. The calm before the storm: clinical observations of Middle East respiratory syndrome (MERS) patients. J. Chemother. 2018;30(3):179–182. doi: 10.1080/1120009X.2018.1429236. [DOI] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Momattin H., Dib J., Memish Z.A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int. J. Infect. Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaraj S.H., Al-Tawfiq J.A., Assiri A.Y., Alzahrani N.A., Alanazi A.A., Memish Z.A. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: a cohort study. Trav. Med. Infect. Dis. 2019;29:48–50. doi: 10.1016/j.tmaid.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Shalhoub S., AlOmari A., Mandourah Y., Al-Hameed F., Sindi A., Alraddadi B., Al Motairi A., Al Khatib K., Mommin A.A., et al. C63 Viral Respiratory Infections. Conference: american thoracic society international conference, ATS 2017; 2017. Effect of Ribavirin and Interferon on the Outcome of Critically Ill Patients with Mers. American Journal of Respiratory and Critical Care Medicine. United states 195. [Google Scholar]
- Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., Jose J., Alraddadi B., Almotairi A., Al Khatib K., Abdulmomen A., Qushmaq I., Sindi A.A., Mady A., Solaiman O., Al-Raddadi R., Maghrabi K., Ragab A., Al Mekhlafi G.A., Balkhy H.H., Al Harthy A., Kharaba A., Gramish J.A., Al-Aithan A.M., Al-Dawood A., Merson L., Hayden F.G., Fowler R. 70(9) Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2019. Ribavirin and Interferon Therapy for Critically Ill Patients with Middle East Respiratory Syndrome: A Multicenter Observational Study; pp. 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., Jose J., Alraddadi B., Almotairi A., Al Khatib K. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin. Infect. Dis. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaha F., Rezaei N. Primary immunodeficiency diseases in COVID-19 pandemic: a predisposing or protective factor? Am. J. Med. Sci. 2020;360(6):740–741. doi: 10.1016/j.amjms.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami A., Vafapour M., Moazzami B., Rezaei N. Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J. Paediatr. Child Health. 2020;57(6):922–925. doi: 10.1111/jpc.15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Basiri A., Heidari A., Nadi M.F., Fallahy M.T.P., Nezamabadi S.S., Sedighi M., Saghazadeh A., Rezaei N. Reviews in Medical Virology n/a; 2020. Microfluidic Devices for Detection of RNA Viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basiri A., Pazhouhnia Z., Beheshtizadeh N., Hoseinpour M., Saghazadeh A., Rezaei N. Regenerative medicine in COVID-19 treatment: real opportunities and range of promises. Stem Cell Rev. Rep. 2020:1–13. doi: 10.1007/s12015-020-09994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost P., Giladi A., Liu Y., Bendjelal Y., Xu G., David E., Blecher-Gonen R., Cohen M., Medaglia C., Li H. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020;181:1475–1488. doi: 10.1016/j.cell.2020.05.006. e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y. Experimental Treatment with Favipiravir for COVID-19: an Open-Label Control Study. Engineering. 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. 32346491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing, StatPearls Publishing LLC; Treasure Island (FL): 2020. Features, Evaluation and Treatment Coronavirus (COVID-19), StatPearls. [PubMed] [Google Scholar]
- Cha R.-h., Yang S.H., Moon K.C., Joh J.-S., Lee J.Y., Shin H.-S., Kim D.K., Kim Y.S. A case report of a Middle East respiratory syndrome survivor with kidney biopsy results. J. Kor. Med. Sci. 2016;31 (4):635–640. doi: 10.3346/jkms.2016.31.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Fan H., Zhang L., Huang B., Zhu M., Zhou Y., Yu W., Zhu L., Cheng S., Tao X. 2020. Retrospective Analysis of Clinical Features in 101 Death Cases with COVID-19. medRxiv. [Google Scholar]
- Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney impairment is associated with in-hospital death of COVID-19 patients. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W.S., Kang C.-I., Kim Y., Choi J.-P., Joh J.S., Shin H.-S., Kim G., Peck K.R., Chung D.R., Kim H.O. Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infect. Chemother. 2016;48(2):118–126. doi: 10.3947/ic.2016.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.Y., Oh J.O., Ahn J.Y., Choi H., Kim J.H., Seong H., Jeong S.J., Ku N.S., Yeom J.S., Choi J.P. Absence of neutralizing activity in serum 1 year after successful treatment with antivirals and recovery from mers in South Korea. Clin. Exper. Vacc. Res. 2019;8:86–88. doi: 10.7774/cevr.2019.8.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- College Station T.S.L., StataCorp . vol. 15. StataCorp LLC; College Station, TX: 2017. (Stata Statistical Software: Release). [Google Scholar]
- Darbeheshti F., Rezaei N. Genetic predisposition models to COVID-19 infection. Med. Hypotheses. 2020;142:109818. doi: 10.1016/j.mehy.2020.109818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoudi-Monfared E., Rahmani H., Khalili H., Hajiabdolbaghi M., Salehi M., Abbasian L., Kazemzadeh H., Yekaninejad M.S. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Liu C., Li N., Liu H., Gu Y., Liu Y., Chen Y. Medical treatment of 55 patients with COVID-19 from seven cities in northeast China who fully recovered: a single-center, retrospective, observational study. medRxiv. 2020 doi: 10.1101/2020.03.28.20045955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Liu H., Li N., Liu C., Gu Y., Liu Y., Chen Y. Medical treatment of 55 patients with COVID-19 from seven cities in northeast China who fully recovered: a single-center, retrospective, observational study. Medicine (Baltim.) 2021;100(2) doi: 10.1097/MD.0000000000023923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi N., Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol. Int. 2020;44:1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Ruiz M., Andrés A., Loinaz C., Delgado J.F., López‐Medrano F., San Juan R., González E., Polanco N., Folgueira M.D., Lalueza A. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am. J. Transplant. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z.C., Zhu J.H., Sun Y., Ding X.L., Ma J.S., Cui Y.X., Du X.K., Gao T., He Q.Y. [Clinical investigation of outbreak of nosocomial severe acute respiratory syndrome] Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue. 2003;15:332–335. [PubMed] [Google Scholar]
- Garout M.A., Jokhdar H.A., Aljahdali I.A., Zein A.R., Goweda R.A., Hassan-Hussein A. Mortality rate of ICU patients with the Middle East respiratory syndrome-coronavirus infection at king Fahad hospital, Jeddah, Saudi Arabia. Cent. Eur. J. Publ. Health. 2018;26(2):87–91. doi: 10.21101/cejph.a4764. [DOI] [PubMed] [Google Scholar]
- Gilbert G.L. Commentary: SARS, MERS and COVID-19—new threats; old lessons. Int. J. Epidemiol. 2020;49:726–728. doi: 10.1093/ije/dyaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi S., Dehghani Firouzabadi F., Dehghani Firouzabadi M., Rezaei N. Cutaneous lesions and COVID-19: cystic painful lesion in a case with positive SARS-CoV-2. Dermatol. Ther. 2020 doi: 10.1111/dth.14266. [DOI] [PubMed] [Google Scholar]
- GradePro M.U. McMaster University; 2020. GRADEpro GDT: GRADEpro Guideline Development Tool [Software] 2020 (developed by Evidence Prime, Inc.). Available from gradepro.org. [Google Scholar]
- Guy R.K., DiPaola R.S., Romanelli F., Dutch R.E. Rapid repurposing of drugs for COVID-19. Science. 2020;368:829–830. doi: 10.1126/science.abb9332. [DOI] [PubMed] [Google Scholar]
- Habib A.M.G., Ali M.A.E., Zouaoui B.R., Taha M.A.H., Mohammed B.S., Saquib N. Clinical outcomes among hospital patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection. BMC Infect. Dis. 2019;19(1):1–6. doi: 10.1186/s12879-019-4555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Ye Q. Kidney involvement in COVID‐19 and its treatments. J. Med. Virol. 2021;93:1387–1395. doi: 10.1002/jmv.26653. [DOI] [PubMed] [Google Scholar]
- Hanaei S., Rezaei N. Archives of medical research; 2020. COVID-19: Developing from an Outbreak to A Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaei S., Takian A., Majdzadeh R., Maboloc C.R., Grossmann I., Gomes O., Milosevic M., Gupta M., Shamshirsaz A.A., Harbi A., Burhan A.M., Uddin L.Q., Kulasinghe A., Lam C.M., Ramakrishna S., Alavi A., Nouwen J.L., Dorigo T., Schreiber M., Abraham A., Shelkovaya N., Krysztofiak W., Ebrahimi Warkiani M., Sellke F., Ogino S., Barba F.J., Brand S., Vasconcelos C., Salunke D.B., Rezaei N. Emerging standards and the hybrid model for organizing scientific events during and after the COVID-19 pandemic. Disaster Med. Public Health Prep. 2020:1–17. doi: 10.1017/dmp.2020.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidarpour M., Vakhshoori M., Abbasi S., Shafie D., Rezaei N. Adrenal insufficiency in coronavirus disease 2019: a case report. J. Med. Case Rep. 2020;14:134. doi: 10.1186/s13256-020-02461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. National Academies Press (US), Institute of Medicine (US) Forum on Microbial Threats; Washington (DC): 2004. THE SARS EPIDEMIC AND ITS AFTERMATH IN CHINA: A POLITICAL PERSPECTIVE. [Google Scholar]
- Huang Q., Deng X., Li Y., Sun X., Chen Q., Xie M., Liu S., Qu H., Liu S., Wang L. Clinical characteristics and drug therapies in patients with the common-type coronavirus disease 2019 in Hunan, China. Int. J. Clin. Pharm. 2020:1. doi: 10.1007/s11096-020-01031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhou H., Yang R., Xu Y., Feng X., Gong P. medRxiv; 2020. Clinical Characteristics of 36 Non-survivors with COVID-19 in Wuhan, China. [Google Scholar]
- Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari P., Rezaei N. With risk of reinfection, is COVID-19 here to stay? Disaster Med. Public Health Prep. 2020;1 doi: 10.1017/dmp.2020.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari P., Jabbari F., Ebrahimi S., Rezaei N. 2020. COVID-19: A Chimera of Two Pandemics. Disaster Medicine and Public Health Preparedness; pp. 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahlu L., Rezaei N. Archives of medical research; 2020. Central Nervous System Involvement in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahlu L., Rezaei N. Monoclonal antibody as a potential anti-COVID-19. Biomed. Pharmacother. 2020;129:110337. doi: 10.1016/j.biopha.2020.110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenab Y., Rezaei N., Hedayat B., Naderian M., Shirani S., Hosseini K. 2020. Occurrence of Acute Coronary Syndrome, Pulmonary Thromboembolism, and Cerebrovascular Event in COVID-19. Clinical Case Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian-ya G. medRxiv; China: 2020. Clinical Characteristics of 51 Patients Discharged from Hospital with COVID-19 in Chongqing. [Google Scholar]
- Jiang Y., He S., Zhang C., Wang X., Chen X., Jin Y., He Z., Cai M., Lin Z., Ying L. Clinical characteristics of 60 discharged cases of 2019 novel coronavirus-infected pneumonia in Taizhou, China. Ann. Transl. Med. 2020;8(8) doi: 10.21037/atm.2020.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Lian J.-S., Hu J.-H., Gao J., Zheng L., Zhang Y.-M., Hao S.-R., Jia H.-Y., Cai H., Zhang X.-L. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M., Khan B., Al Rabiah F., Alismaili R., Saleemi S., Rehan-Khaliq A.M., Weheba I., Al Abdely H., Halim M., Nadri Q.J., Al Dalaan A.M., Zeitouni M., Butt T., Al Mutairy E. Middle eastern respiratory syndrome corona virus (MERS CoV): case reports from a tertiary care hospital in Saudi Arabia. Ann. Saudi Med. 2014;34(5):396–400. doi: 10.5144/0256-4947.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid M., Al Rabiah F., Khan B., Al Mobeireek A., Butt T.S., Al Mutairy E. Case report Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir. Ther. 2015;20:87–91. doi: 10.3851/IMP2792. [DOI] [PubMed] [Google Scholar]
- Khalid I., Alraddadi B.M., Dairi Y., Khalid T.J., Kadri M., Alshukairi A.N., Qushmaq I.A. Acute management and long-term survival among Subjects with severe Middle East respiratory syndrome coronavirus pneumonia and ARDS. Respir. Care. 2016;61:340–348. doi: 10.4187/respcare.04325. [DOI] [PubMed] [Google Scholar]
- Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol. Health. 2015;37 doi: 10.4178/epih/e2015033. e2015033-e2015033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U.J., Won E.J., Kee S.J., Jung S.I., Jang H.C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-alpha for Middle East respiratory syndrome. Antivir. Ther. 2016;21(5):455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- Kim J.-E., Heo J.-H., Kim H.-O., Song S.-H., Park S.-S., Park T.-H., Ahn J.-Y., Kim M.-K., Choi J.-P. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitar-Jerala N. The role of interferons in inflammation and inflammasome activation. Front. Immunol. 2017;8:873. doi: 10.3389/fimmu.2017.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Kim Y.J., Chung E.H., Kim D.W., Jeong I., Kim Y., Yun M.R., Kim S.S., Kim G., Joh J.S. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect. Dis. 2017;17(1):498. doi: 10.1186/s12879-017-2576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xiao S.Y. Hepatic involvement in COVID‐19 patients: pathology, pathogenesis, and clinical implications. J. Med. Virol. 2020;92:1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- Liao J., Fan S., Chen J., Wu J., Xu S., Guo Y., Li C., Zhang X., Wu C., Mou H. medRxiv; 2020. Epidemiological and Clinical Characteristics of COVID-19 in Adolescents and Young Adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K., Yu W., Zhang J. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. 32173576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo I.L., Lio C.F., Cheong H.H., Lei C.I., Cheong T.H., Zhong X., Tian Y., Sin N.N. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16(10):1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi M., Rezaei N. SARS-CoV-2: a comprehensive review from pathogenicity of the virus to clinical consequences. J. Med. Virol. 2020;92(10):1864–1874. doi: 10.1002/jmv.26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi M., Hamblin M.R., Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clinica chimica acta; international journal of clinical chemistry. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., Pham D.H., Deif H., LaMere E.A., Chang M., Kain K.C., Farcas G.A., Ferguson P., Latchford M., Levy G., Dennis J.W., Lai E.K., Fish E.N. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. Jama. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Lundstrom K., Seyran M., Pizzol D., Adadi P., Mohamed Abd El-Aziz T., Hassan S.S., Soares A., Kandimalla R., Tambuwala M.M., Aljabali A.A.A., Kumar Azad G., Pal Choudhury P., Uversky V.N., Sherchan S.P., Uhal B.D., Rezaei N., Brufsky A.M. 2020. Viewpoint: Origin of SARS-CoV-2. Viruses 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A., El Masry K.M., Ravi M., Sayed F. Middle east respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg. Infect. Dis. 2016;22(3):515. doi: 10.3201/eid2203.151049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansourabadi A.H., Sadeghalvad M., Mohammadi-Motlagh H.R., Rezaei N. The immune system as a target for therapy of SARS-CoV-2: a systematic review of the current immunotherapies for COVID-19. Life Sci. 2020;258:118185. doi: 10.1016/j.lfs.2020.118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020;179:104811. doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., Lopez-Granados E., Gianelli C., Robles-Marhuenda A., Jeandel P.-Y. Coronavirus Disease 2019 in patients with inborn errors of immunity: an international study. J. Allergy Clin. Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazzami B., Razavi-Khorasani N., Dooghaie Moghadam A., Farokhi E., Rezaei N. COVID-19 and telemedicine: immediate action required for maintaining healthcare providers well-being. J. Clin. Virol. 2020;126:104345. doi: 10.1016/j.jcv.2020.104345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed K., Rezaei N. COVID-19 pandemic is not the time of trial and error. Am. J. Emerg. Med. 2020;S0735-6757(20)30814-7 doi: 10.1016/j.ajem.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed K., Rezaei N., Rodríguez-Román E., Rahmani F., Zhang H., Ivanovska M., S A.M., Joya M., Makuku R., Md Shahidul I., Nesrine R., Laila R., Goda R., Sunny O.A., Mujtaba S., Zoghi S., Irtsyan S., Ling I., Orsolya C., Attig-Bahar F., Hazar Sayar E., Soloukey C., Giulia G. International efforts to save healthcare personnel during COVID-19. Acta Biomed. : Atenei Parmensis. 2020;91 doi: 10.23750/abm.v91i3.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed K., Rodríguez-Román E., Rahmani F., Zhang H., Ivanovska M., Makka S.A., Joya M., Makuku R., Islam M.S., Radwan N., Rahmah L., Goda R., Abarikwu S.O., Shaw M., Zoghi S., Irtsyan S., Ling I., Cseprekal O., Faten A.B., Hazar Sayar E., Soloukey C., Grancini G., Rezaei N. Borderless collaboration is needed for COVID-19-A disease that knows no borders. Infect. Contr. Hosp. Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabavi H., Saghazadeh A., Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur. Cytokine Netw. 2020;31:44–49. doi: 10.1684/ecn.2020.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtazmanesh S., Ochs H.D., Uddin L.Q., Perc M., Routes J.M., Vieira D.N., Al-Herz W., Baris S., Prando C., Rosivall L., Abdul Latiff A.H., Ulrichs T., Roudenok V., Aldave Becerra J.C., Salunke D.B., Goudouris E., Condino-Neto A., Stashchak A., Kryvenko O., Stashchak M., Bondarenko A., Rezaei N. All together to fight COVID-19. Am. J. Trop. Med. Hyg. 2020;102:1181–1183. doi: 10.4269/ajtmh.20-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradian N., Ochs H.D., Sedikies C., Hamblin M.R., Camargo C.A., Jr., Martinez J.A., Biamonte J.D., Abdollahi M., Torres P.J., Nieto J.J., Ogino S., Seymour J.F., Abraham A., Cauda V., Gupta S., Ramakrishna S., Sellke F.W., Sorooshian A., Wallace Hayes A., Martinez-Urbistondo M., Gupta M., Azadbakht L., Esmaillzadeh A., Kelishadi R., Esteghamati A., Emam-Djomeh Z., Majdzadeh R., Palit P., Badali H., Rao I., Saboury A.A., Jagan Mohan Rao L., Ahmadieh H., Montazeri A., Fadini G.P., Pauly D., Thomas S., Moosavi-Movahed A.A., Aghamohammadi A., Behmanesh M., Rahimi-Movaghar V., Ghavami S., Mehran R., Uddin L.Q., Von Herrath M., Mobasher B., Rezaei N. The urgent need for integrated science to fight COVID-19 pandemic and beyond. J. Transl. Med. 2020;18:205. doi: 10.1186/s12967-020-02364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra M.E., Van Thanh L., Kamel M.G., Ghazy A.A., Altibi A.M., Dat L.M., Thy T.N.X., Vuong N.L., Mostafa M.R., Ahmed S.I. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and meta‐analysis. Rev. Med. Virol. 2018;28 doi: 10.1002/rmv.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad M.H., Mustafa R.A., Schünemann H.J., Sultan S., Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid. Base Med. 2017;22:85–87. doi: 10.1136/ebmed-2017-110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasab M.G., Saghazadeh A., Rezaei N. Archives of medical research; 2020. SARS-CoV-2-A Tough Opponent for the Immune System. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, L., and Blood Institute, Quality Assessment Tool for Case Series Studies.
- Nejadghaderi S.A., Heidari A., Shakerian N., Saghazadeh A., Rezaei N. Cardiovascular system is at higher risk of affecting by COVID-19. Acta bio-medica. Atenei Parmensis. 2020;91 doi: 10.23750/abm.v91i3.9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. Cytokine & growth factor reviews; 2020. COVID-19: Pathogenesis, Cytokine Storm and Therapeutic Potential of Interferons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.-d., Choe P.G., Oh H.S., Park W.B., Lee S.-M., Park J., Lee S.K., Song J.-S., Kim N.J. Middle East respiratory syndrome coronavirus superspreading event involving 81 persons, Korea 2015. J. Kor. Med. Sci. 2015;30(11):1701–1705. doi: 10.3346/jkms.2015.30.11.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani A., Shalhoub S. Middle East respiratory syndrome coronavirus (MERS-CoV): what lessons can we learn? J. Hosp. Infect. 2015;91:188–196. doi: 10.1016/j.jhin.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., Almakhlafi G.A., Albarrak M.M., Memish Z.A., Albarrak A.M. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14(11):1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onabajo O.O., Banday A.R., Stanifer M.L., Yan W., Obajemu A., Santer D.M., Florez-Vargas O., Piontkivska H., Vargas J.M., Ring T.J., Kee C., Doldan P., Tyrrell D.L., Mendoza J.L., Boulant S., Prokunina-Olsson L. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat. Genet. 2020;52:1283–1293. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. World Health Organization; 2011. Clinical Care for Severe Actue Respiratory Infection. [Google Scholar]
- Organization W.H. World Health Organization; 2019. Clinical Management of Severe Acute Respiratory Infection when Middle East Respiratory Syndrome Coronavirus ( MERS-CoV) Infection Is Suspected: Interim Guidance. [Google Scholar]
- Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., Li P., Hu B., Wang J., Hu C. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020;115 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashaei M., Rezaei N. Immunotherapy for SARS-CoV-2: potential opportunities. Expet Opin. Biol. Ther. 2020:1–5. doi: 10.1080/14712598.2020.1807933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S., Baghbanzadeh M., Aghamohammadi N., Zhang W., Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int. J. Epidemiol. 2020;49:717–726. doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penson D., Krishnaswami S., Jules A. 2018. Newcastle–Ottawa Quality Assessment Form for Cohort Studies; p. 2012. [Google Scholar]
- Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. the official publication of the European Society of Clinical Microbiology and Infectious Diseases; 2020. COVID-19, SARS and MERS: Are They Closely Related? Clinical Microbiology and Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourahmad R., Moazzami B., Rezaei N. Efficacy of plasmapheresis and immunoglobulin replacement therapy (IVIG) on patients with COVID-19. SN Comp. Clin. Med. 2020:1–5. doi: 10.1007/s42399-020-00438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutanen S.M. In: Principles and Practice of Pediatric Infectious Diseases. fourth ed. Long S.S., editor. Content Repository Only!; London: 2012. pp. 1547–1712. [Google Scholar]
- Qing C., Li-lan Z., De-xian Y.U., Zhi-ai Y.U., Yi L.I.U., Li-ping Z., Zhi-feng L.I., Zhao-jun D., Bin-hui W., Xue-jun W.E.I., Gui-fang H.U., Yu-qing L.I.U., Xin-wei C.H.U., Yan-hong H.A.N., Min W.U., Xiao-ling J., Jian-dong L.I., Ying-chun D.A.I., Jun N.I.E., Jun L., Li Z.H.U., Su-xia S.U.N., Yong-yu R.U.I., Ding-kang Z., Shou-yi Y.U., Yun-de H.O.U. A field trial for evaluating the safety of recombinant human interferon alpha-2b for nasal spray. Chin. J. Exp. Clin. Virol. 2005:211–215. [PubMed] [Google Scholar]
- Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiee N., Rabiee M., Bagherzadeh M., Rezaei N. COVID-19 and picotechnology: potential opportunities. Med. Hypotheses. 2020;144:109917. doi: 10.1016/j.mehy.2020.109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani A., Saleki K., Javanmehr N., Khodaparast J., Saadat P., Nouri H.R. Mesenchymal stem cell-derived extracellular vesicle-based therapies protect against coupled degeneration of the central nervous and vascular systems in stroke. Ageing Res. Rev. 2020;62:101106. doi: 10.1016/j.arr.2020.101106. [DOI] [PubMed] [Google Scholar]
- Rahmani A., Baee M., Saleki K., Moradi S., Nouri H.R. Applying high throughput and comprehensive immunoinformatics approaches to design a trivalent subunit vaccine for induction of immune response against emerging human coronaviruses SARS-CoV, MERS-CoV and SARS-CoV-2. J. Biomol. Struct. Dyn. 2021:1–17. doi: 10.1080/07391102.2021.1876774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D., Zumla A., Locatelli F., Ippolito G., Kroemer G. Coronavirus infections: epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4:66–74. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasoulinejad S.A., Karkhah A., Paniri A., Saleki K., Pirzadeh M., Nouri H.R. Contribution of inflammasome complex in inflammatory-related eye disorders and its implications for anti-inflammasome therapy. Immunopharmacol. Immunotoxicol. 2020;42:400–407. doi: 10.1080/08923973.2020.1808986. [DOI] [PubMed] [Google Scholar]
- Rezaei N. COVID-19 affects healthy pediatricians more than pediatric patients. Infect. Contr. Hosp. Epidemiol. 2020;1 doi: 10.1017/ice.2020.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei N. COVID-19 and medical biotechnology. Avicenna J. Med. Biotechnol. (AJMB) 2020;12:139. [PMC free article] [PubMed] [Google Scholar]
- Rhee J.-Y., Hong G., Ryu K.M. Clinical implications of five cases of Middle East respiratory syndrome coronavirus infection in South Korea Outbreak. Jp. J. Infect. Dis. JJID. 2016;69(5):361–366. doi: 10.7883/yoken.JJID.2015.445. 2015.2445. [DOI] [PubMed] [Google Scholar]
- Rokni M., Hamblin M.R., Rezaei N. Cytokines and COVID-19: friends or foes? Hum. Vaccines Immunother. 2020:1–3. doi: 10.1080/21645515.2020.1799669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostamtabar M., Esmaeilzadeh S., Tourani M., Rahmani A., Baee M., Shirafkan F., Saleki K., Mirzababayi S.S., Ebrahimpour S., Nouri H.R. Pathophysiological roles of chronic low‐grade inflammation mediators in polycystic ovary syndrome. J. Cell. Physiol. 2021;236:824–838. doi: 10.1002/jcp.29912. [DOI] [PubMed] [Google Scholar]
- Rui Z., Yunguang L., Yanrong L., Ning L., Qiulian L., Youling L., et al. Clinical characteristics of 28 patients with novel coronavirus pneumonia. Chin. J. Infect. Dis. 2020;(12) E006-E006. [Google Scholar]
- Rzymski P., Nowicki M., Mullin G.E., Abraham A., Rodríguez-Román E., Petzold M.B., Bendau A., Sahu K.K., Ather A., Naviaux A.-F. Quantity does not equal quality: scientific principles cannot be sacrificed. Int. Immunopharm. 2020;86:106711. doi: 10.1016/j.intimp.2020.106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghmousavi S., Rezaei N. COVID-19 and multiple sclerosis: predisposition and precautions in treatment. SN Compr. Clin. Med. 2020:1–6. doi: 10.1007/s42399-020-00504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus - a perspective. Expet Rev. Clin. Immunol. 2020;16:465–470. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh A., Rezaei N. Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharm. 2020;84:106560. doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu K.K., Siddiqui A.D., Rezaei N., Cerny J. Challenges for management of immune thrombocytopenia during COVID-19 pandemic. J. Med. Virol. 2020;92(11):2277–2282. doi: 10.1002/jmv.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleki K., Banazadeh M., Saghazadeh A., Rezaei N. The involvement of the central nervous system in patients with COVID-19. Rev. Neurosci. 2020;31:453. doi: 10.1515/revneuro-2020-0026. [DOI] [PubMed] [Google Scholar]
- Samieefar N., Yari Boroujeni R., Jamee M., Lotfi M., Golabchi M.R., Afshar A., Miri H., Khazeei Tabari M.A., Darzi P., Abdullatif Khafaie M., Amirheidari B., Tamadon A., Rambod Rad N., Samimi N., Farjam M., Shiravi F., Farshidi N., Hedayati Ch M., Doostkamel D., Alikhani R., Razmkhah M., Abdollahifard S., Nasiri Kalmarzi R., Kelishadi R., Khazaei H., Aghamohammadi A., Jafari Mousavi F.S., Shamsizadeh M., Khojasteh A., Rezaei N. Country quarantine during COVID-19: critical or not? Disaster Med. Public Health Prep. 2020:1–6. doi: 10.1017/dmp.2020.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzaeim M., Rezaei N. Kawasaki disease and multisystem inflammatory syndrome in children with COVID-19. SN Compr. Clin. Med. 2020:1–6. doi: 10.1007/s42399-020-00558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedpour S., Khodaei B., Loghman A.H., Seyedpour N., Kisomi M.F., Balibegloo M., Nezamabadi S.S., Gholami B., Saghazadeh A., Rezaei N. Targeted therapy strategies against SARS-CoV-2 cell entry mechanisms: a systematic review of in vitro and in vivo studies. J. Cell. Physiol. 2020;236(4):2364–2392. doi: 10.1002/jcp.30032. [DOI] [PubMed] [Google Scholar]
- Shalhoub S., Farahat F., Al-Jiffri A., Simhairi R., Shamma O., Siddiqi N., Mushtaq A. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J. Antimicrob. Chemother. 2015;70(7):2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalhoub S., Al-Hameed F., Mandourah Y., Balkhy H.H., Al-Omari A., Al Mekhlafi G.A., Kharaba A., Alraddadi B., Almotairi A., Al Khatib K. Critically ill healthcare workers with the middle east respiratory syndrome (MERS): a multicenter study. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0206831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifkashani S., Bafrani M.A., Khaboushan A.S., Pirzadeh M., Kheirandish A., Yavarpour Bali H., Hessami A., Saghazadeh A., Rezaei N. Angiotensin-converting enzyme 2 (ACE2) receptor and SARS-CoV-2: potential therapeutic targeting. Eur. J. Pharmacol. 2020;884:173455. doi: 10.1016/j.ejphar.2020.173455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbini N., Iskandrani A., Kharaba A., Khalid G., Abduljawad M., Al-Jahdali H. Middle East respiratory syndrome coronavirus in Al-Madinah City, Saudi Arabia: Demographic, clinical and survival data. J. Epidemiol. Glob. Health. 2017;7(1):29–36. doi: 10.1016/j.jegh.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaeefar E., Malih N., Rezaei N. The possible double-edged sword effects of vitamin D on COVID-19: a hypothesis. Cell Biol. Int. 2020;45(1):54–57. doi: 10.1002/cbin.11469. [DOI] [PubMed] [Google Scholar]
- Sterne J.A.C., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., Carpenter J.R., Chan A.-W., Churchill R., Deeks J.J., Hróbjartsson A., Kirkham J., Jüni P., Loke Y.K., Pigott T.D., Ramsay C.R., Regidor D., Rothstein H.R., Sandhu L., Santaguida P.L., Schünemann H.J., Shea B., Shrier I., Tugwell P., Turner L., Valentine J.C., Waddington H., Waters E., Wells G.A., Whiting P.F., Higgins J.P.T. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Li H., Lu X.-X., Xiao H., Ren J., Zhang F.-R., Liu Z.-S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J. Pediatr. 2020:1–9. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., Yip C.C.-Y., Cai J.-P., Chan J.M.-C., Chik T.S.-H. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totura A.L., Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expet Opin. Drug Discov. 2019;14:397–412. doi: 10.1080/17460441.2019.1581171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lang C., Huang D., Sun Q., Xiong Y. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J. Med. Virol. 2020 doi: 10.1002/jmv.25783. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xu X., Ruan J., Lin S., Jiang J., Ye H. Quadruple therapy for asymptomatic COVID-19 infection patients. Expert Rev. Anti-infect. Ther. 2020:1–8. doi: 10.1080/14787210.2020.1758066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu W., Zhao J., Lu Y., Wang X., Yu C., Hu S., Shen N., Liu W., Sun Z. Clinical characteristics of 80 hospitalized frontline medical workers infected with COVID-19 in Wuhan, China. J. Hosp. Infect. 2020;105(3):399–403. doi: 10.1016/j.jhin.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir C.J., Butcher I., Assi V., Lewis S.C., Murray G.D., Langhorne P., Brady M.C. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med. Res. Methodol. 2018;18:25. doi: 10.1186/s12874-018-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Who . 2020. Coronavirus Disease (COVID-19) Travel Advice. [Google Scholar]
- Wu W., Wang J., Liu P., Chen W., Yin S., Jiang S., Yan L., Zhan J., Chen X., Huang Z. Clinical features of 96 patients with severe acute respiratory syndrome from a hospital outbreak. Zhonghua nei ke za zhi. 2003;42:453–457. [PubMed] [Google Scholar]
- Wu W., Wang J., Liu P., Chen W., Yin S., Jiang S., Yan L., Zhan J., Chen X., Li J., Huang Z., Huang H. A hospital outbreak of severe acute respiratory syndrome in Guangzhou, China. Chin. Med. J. 2003;116:811–818. [PubMed] [Google Scholar]
- Xiao-Wei X., Xiao-Xin W., Xian-Gao J., Kai-Jin X., Ling-Jun Y., Chun-Lian M., Shi-Bo L., Hua-Ying W., Zhang S., Gao H.-N. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ Br. Med. J. (Clin. Res. Ed.) 2020;368 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.D., Jiang M., Chen R.C., Fang J.Q. [Evaluation of the efficacy and safety of corticosteroid in the treatment of severe SARS in Guangdong province with multi-factor regression analysis] Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue. 2008;20:84–87. [PubMed] [Google Scholar]
- Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256:117900. doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah N., Saghazadeh A., Rezaei N. Anosmia: a missing link in the neuroimmunology of coronavirus disease 2019 (COVID-19) Rev. Neurosci. 2020;31(7):691–701. doi: 10.1515/revneuro-2020-0039. [DOI] [PubMed] [Google Scholar]
- Yousefzadegan S., Rezaei N. Case report: death due to COVID-19 in three brothers. Am. J. Trop. Med. Hyg. 2020;102:1203–1204. doi: 10.4269/ajtmh.20-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., Liu Y., Xiao J., Liu H., Deng D. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Zou R., Zeng L., Kou S., Lan J., Li X., Liang Y., Ding X., Tan G., Tang S. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm. Res. 2020:1–8. doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Huang B., Xia H., Fan H., Zhu M., Zhu L., Zhang H., Tao X., Cheng S., Chen J. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiol. Infect. 2020;148:e199. doi: 10.1017/S0950268820002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K., Hodeib S., Korol C. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Zhang F., Xu M., Huang K., Zhong W., Cai W., Yin Z., Huang S., Deng Z., Wei M. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J. Med. Microbiol. 2003;52(8):715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Chen V., Shannon C.P., Wei X.-S., Xiang X., Wang X., Wang Z.-H., Tebbutt S.J., Kollmann T.R., Fish E.N. Corrigendum: interferon-α2b treatment for COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Chen V., Shannon C.P., Wei X.-S., Xiang X., Wang X., Wang Z.-H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-α2b treatment for COVID-19. Front. Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., MacArthur M.R., He X., Wei X., Zarin P., Hanna B.S., Wang Z.-H., Xiang X., Fish E.N. Interferon-α2b treatment for COVID-19 is associated with improvements in lung abnormalities. Viruses. 2021;13:44. doi: 10.3390/v13010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.-E., Barbry P., Leslie A., Kiem H.-P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., Banovich N., Barbry P., Brazma A., Desai T., Duong T.E., Eickelberg O., Falk C., Farzan M., Glass I., Haniffa M., Horvath P., Hung D., Kaminski N., Krasnow M., Kropski J.A., Kuhnemund M., Lafyatis R., Lee H., Leroy S., Linnarson S., Lundeberg J., Meyer K., Misharin A., Nawijn M., Nikolic M.Z., Ordovas-Montanes J., Pe’er D., Powell J., Quake S., Rajagopal J., Tata P.R., Rawlins E.L., Regev A., Reyfman P.A., Rojas M., Rosen O., Saeb-Parsy K., Samakovlis C., Schiller H., Schultze J.L., Seibold M.A., Shalek A.K., Shepherd D., Spence J., Spira A., Sun X., Teichmann S., Theis F., Tsankov A., van den Berge M., von Papen M., Whitsett J., Xavier R., Xu Y., Zaragosi L.-E., Zhang K. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human Airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.