Abstract
One of the most common and distressing symptoms after craniotomy is postoperative nausea and vomiting (PONV). PONV could generate delayed postanesthesia care and hospitalization discharge, lower patient satisfaction, and an increase in overall hospitalization costs. The incidence of reported PONV after craniotomy is 22% to 70% without prophylaxis, and a multimodal regimen of medication has been recommended. We conducted a comprehensive literature review of the clinical evidence related to PONV prevention and management after craniotomy. All clinical trials in adult populations relevant to PONV after craniotomy available in English language and indexed in PubMed, Google Scholar and Cochrane Library databases from January 1997 up to September 2018 were retrieved using a combination of free-text words related to PONV in craniotomy. After screening manuscripts identified in the initial search, 23 clinical trials investigating systemic pharmacological intervention versus placebo or active control in patients undergoing craniotomy under general anesthesia met the criteria for inclusion in this comprehensive narrative review. The pathophysiology and mechanisms of PONV after craniotomy could be multifactorial in etiology. Therefore, based on current evidence, PONV management after craniotomy should focus on perioperative patient assessment, surgical, and anesthesia-related risk factors and the selection of systemic pharmacological considerations to reduce its incidence and complications. A multimodal regimen of medication targeting different chemoreceptors in the vomiting center is recommended. Ondansetron and dexamethasone, or their combination, are the most frequently used and effective agents. Further randomized clinical trials comparing different regimens that significantly reduce the incidence of PONV in craniotomy would provide relevant evidence-based data for PONV management in this patient population.
Key Words: postoperative nausea and vomiting (PONV), craniotomy, anesthesia, prophylaxis, supratentorial, infratentorial
Around 41,000 craniotomies for the treatment of intracranial neoplasm were performed in 2009 in the United States.1 The advances in surgery, oncology, anesthesiology, and critical care in neurology are remarkable; nonetheless the morbidity and mortality (40% and 9%, respectively) after craniotomy for tumor surgery remains high.1 One of the most common and distressing symptoms after craniotomy is postoperative nausea and vomiting (PONV).2–4 Both of these conditions generate delayed postanesthesia care and hospitalization discharge associated with lower patient satisfaction and an increase in overall cost of the procedure.2–4 According to the latest Society of Ambulatory Anesthesia guidelines, 50% of the general population is affected by postoperative nausea (PON), 30% by postoperative vomiting (POV) and up to 80% of high-risk patients by PONV.5 In contrast, the incidence of reported PONV after craniotomy is 22% to 70% when prophylaxis is not administered.6–13 In addition, the literature describes PONV after craniotomy varying in incidence between 6% and 60% with prophylaxis.8–30
Several patient, surgical and anesthesia-related risk factors influence the occurrence of PONV after craniotomy. The pathophysiology and mechanisms of PONV manifests could, therefore, be multifactorial in etiology (Table 1).19,20 PONV can result in intravascular volume depletion, electrolyte imbalance (hyponatremia, hypokalemia, hypochloremia, etc.), airway complications (aspirations), venous hypertension, wound dehiscence or hematoma, neurological deterioration and acid-base disturbances.19,20,29,30 The sympathetic preejection phase of the vomiting reflex is associated with systemic hypertension.4 In addition, the ejection phase of vomiting and retching can increase intra-abdominal (>100 mm Hg) and thoracic pressures, leading to increased intracranial pressure, intracranial hemorrhage, and/or cerebral herniation.4,30,31 An abnormal swallow reflex and neurological deterioration in this surgical population could intensify the risk for postvomiting aspiration.4 PONV in the craniotomy population could be a specific sign of intracranial hypertension associated with dislocation of normal brain anatomy, particularly in the posterior fossa. Therefore, the presence of PONV, delayed awakening and neurological focalization (anisocoria and mydriasis) are indications to perform an emergent computed tomography scan.1
TABLE 1.
Postoperative Nausea and Vomiting (PONV) and Studied Risk Factors Considerations
| Risk Factors |
|---|
| Patient-related risk factors |
| Female sex |
| History of motion of sickness or PONV |
| Nonsmoker status |
| Younger age |
| Intracranial hypertension (for PONV after 72 h) |
| Spontaneous postoperative intracranial hypotension |
| Anesthesia-related risk factors |
| Duration of surgery>60 min |
| Higher postoperative analgesic requirements |
| Nontransphenoidal procedure |
| Use of volatile anesthetic agents |
| Neostigmine use (>2.5 mg) |
| Surgery-related risk factors |
| Expected use of opioid medication |
| Nonuse of scalp blocks |
| Infratentorial surgery |
| Microvascular decompression surgery |
| Retrosigmoid vestibular schwannoma |
Several neurosurgical and neuroanesthesia clinical trials have been conducted to assess the safety and efficacy of different regimens to prevent and treat PONV.8,19,27–30 Various modalities have been investigated, and the ideal regimen requires further research. The use of multimodal approaches to the prevention and management of PONV has been proposed as a possible solution to this distressing event.
We conducted a comprehensive literature review of the clinical evidence related to PONV in patients undergoing craniotomy and discuss possible avenues to reduce its incidences in this patient cohort.
METHODS
The first article related to PONV after craniotomy was published in 1997,14 so we searched for all clinical trials relevant to PONV after craniotomy in adults available in the English language and indexed in PubMed, Google Scholar and Cochrane Library databases from January 1997 to September 2018. The search was performed using a combination of medical subject headings and free-text words (“postoperative nausea and vomiting (PONV),” “PONV” or “craniotomy,” “anesthesia” or “craniotomy,” “prophylaxis or PONV,” “infratentorial craniotomy” and “supratentorial craniotomy”). Two independent authors screened all the articles identified by the initial search and assessed them for eligibility, that is, clinical trials published in English and conducted in an adult population that underwent craniotomy where an antiemetic medication was used to prevent or manage PONV. Articles were excluded if they were abstract publications, case reports or series of case reports or investigated a pediatric population. Our final review of all databases was conducted on September 28, 2018.
RESULTS
A total of 2450 articles discussing PONV after craniotomy in adults were identified in our initial search. After duplications were removed, 1029 papers were excluded after the title screen and another 141 after abstract screen. Then, all manuscripts were organized according to relevance, selecting only 300 for full-text review. A total of 277 of these manuscripts were excluded for the following reasons: pediatric population, case reports, series of case reports, non-English language and noncraniotomy related. Following these exclusions, 22 papers were identified as reliable full-text sources pertinent to our clinical review (Fig. 1 32).
FIGURE 1.
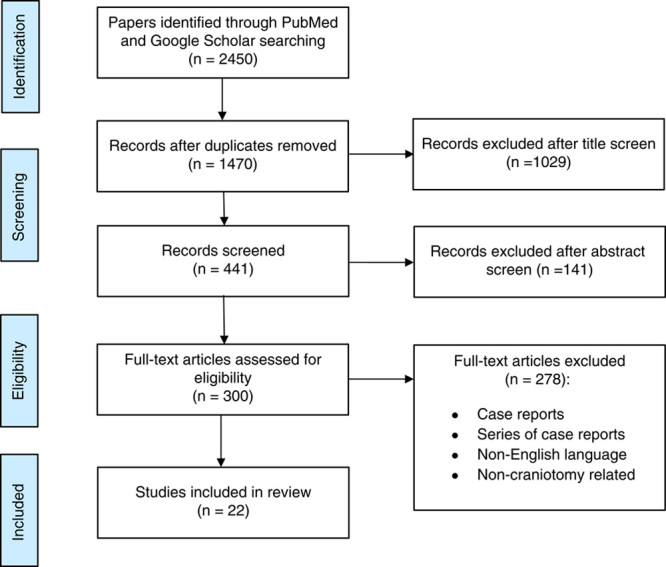
Flowchart diagram of manuscript selection. n indicates number of manuscripts.
Anatomy and Physiology of PONV Mechanisms
The neuroanatomic areas that mediate emesis are the vomiting center and chemoreceptor trigger zone. A significant number of receptors and neurotransmitters are part of this complex emetogenic pathway: dopamine type 2 (D-2), serotonin type 3 (5-HT3), histamine type 1 (H1), muscarinic cholinergic type 1 and neurokinin type 1 (NK1).33,34 In addition, low levels of mean arterial pressure during surgery may cause intermittent hypoperfusion of the brainstem and vestibular system, leading to the production of cytokines, histamine and 5-HT which may stimulate H1 and 5-HT receptors in the chemoreceptor trigger zone.35,36 Low mean arterial pressure may also decrease intestinal perfusion, promoting the release of 5-HT into the systemic circulation which further stimulates 5-HT receptors in the area postrema/chemoreceptor trigger zone, favoring the occurrence of nausea and vomiting.35,37
Risk Factors for PONV
Different PONV risk factors are related to the individual patient, surgical procedure, and anesthesia technique.
Patient-related Factors
The Apfel Score38 identified 4 main risk factors for PONV in patients undergoing general anesthesia; female sex, history of motion sickness or PONV, nonsmoker status and expected use of postoperative opioids. The incidence of PONV increases by 10%, 20%, 40%, 60%, and 80% with 0, 1, 2, 3, or 4 risk factors, respectively.38 Recent publications accord with these rates.5,39
Patient surveys studying PONV risk factors after craniotomy concluded that female sex is the most relevant risk factor in this surgical population.4 Additional risk factors for PONV after craniotomy, such as duration of surgery (>60 min) or younger age have been studied in predictive models with limited validation; individual patient-associated risk factors may play a more important role in the incidence of PONV.4 Lonjaret et al29 suggested that late nausea and vomiting, mainly 72 hours after craniotomy, may be related to intracranial hypertension rather than classic PONV and should be further investigated.
Surgery-related Factors
Different neurosurgical procedures and locations have been described to have a greater or lesser influence on the incidence of PONV.40 However, recent literature suggests that the site of surgery alone has a minimal and unclear predictive value for the development of PONV.4,40 In 1997, Fabling et al28 identified the infratentorial surgical site as a potential risk factor for PONV, although a significant impact has been associated only with nausea. Other PONV risk factors in infratentorial procedures relate to the likely longer duration of surgery and higher postoperative analgesic requirements.4,41 Microvascular decompression and retrosigmoid vestibular schwannoma procedures have been linked to a higher incidence of PONV, with this association being attributed to the proximity of the surgical site to the vomiting center, in the posterior fossa, vestibular and vagus nerves.42–44
In 2006, Flynn and Nemergut45 analyzed 877 patients who underwent microscope-assisted transsphenoidal surgery and reported a 7.5% incidence of PONV, suggesting a protective effect when compared with standard craniotomy approaches. In contrast, Chowdhury et al46 reported an overall 6.7% incidence of PONV when reviewing pituitary surgery complications in 2014; the PONV rates were lower in Cushing disease (4%—protective effect of excessive corticoids production) and apoplexy (0%—high-doses of corticoids administration for optic nerve protection).
A retrospective study published by Sato et al43 considered that spontaneous intracranial hypotension might be responsible for an increase in the incidence of nausea and vomiting and that cerebrospinal fluid (CSF) reduction after craniotomy might be associated with PONV. Supporting this hypothesis, cerebrovascular surgery, procedures using a lumbar catheter and transsphenoidal surgery with complicated CSF leaks (all procedures associated with higher volume of CSF removal) have been linked with a higher incidence of PONV.43,45
Anesthesia-related Factors
Current evidence supports the influence of anesthetic techniques on PONV incidence after craniotomies.5 However, the variability of anesthesia regimens, inconsistent postoperative follow-ups among several prospective clinical trials and the limitation of retrospective studies for the reliability of PONV assessments limit the possibility to determine the influence of anesthesia regimens on PONV with certainty.4
The use of volatile anesthetic agents during craniotomies has been associated with a higher incidence of PONV when compared with total intravenous anesthesia (TIVA).44,47 A meta-analysis conducted by Chui et al47 showed that balanced anesthesia with volatile agents (isoflurane, sevoflurane) was associated with an increased incidence of PONV versus propofol-based TIVA in patients undergoing craniotomies. Also, a 25% reduction of PONV incidence has been reported when propofol and air/oxygen are used during TIVA.5 The use of neostigmine (>2.5 mg) during anesthesia management may be a contributing factor for PONV, although there is inconsistent evidence between studies.5,48,49
Dexmedetomidine, a selective α-2-agonist, has been used as a complementary analgesic medication during and after craniotomies and has been associated with a reduction in the incidence of PONV.50,51 In an randomized controlled trial (RCT) including 80 patients undergoing craniotomy with sevoflurane-fentanyl anesthesia, Peng et al51 found that the addition of dexmedetomidine infusion was associated with fewer events requiring PONV rescue medication within the first 90 minutes after surgery compared with placebo (P=0.005). A further RCT conducted by Gupta et al,50 including 50 patients that underwent supratentorial craniotomy under general anesthesia with the administration of intraoperative infusion of dexmedetomidine or fentanyl, reported an 8% and 0% incidence of PONV in the fentanyl and dexmedetomidine groups, respectively.50
Several studies have shown a considerable reduction in PONV incidence when surgical procedures are performed with awake craniotomy techniques rather than with general anesthesia.6,52–54 In a 2002 study of 107 patients undergoing craniotomy for tumor surgery under general anesthesia (n=57) or with an awake technique (n=50), Manninen and Tan52 reported a lower incidence of nausea (4% vs. 23%; P=0.012) and vomiting (0% vs. 11%; P=0.052) in patients having awake craniotomy compared with those having general anesthesia, respectively. Moreover, a retrospective study by Sinha et al6 reported a low (16%) incidence of PONV in 42 patients undergoing awake craniotomy. In a retrospective study of 27 patients who underwent perirolandic glioma resection, Eseonu et al53 reported an incidence of PONV of 11.1% in those who had awake craniotomy compared with 61.3% in those having general anesthesia.53 Furthermore, the adjunct of scalp blocks to the anesthesia technique in supratentorial and infratentorial craniotomies reduces pain, leading to lower opioid consumption and lower PONV incidence for up to 72 hours.55–57
PONV Prophylaxis in Craniotomy Clinical Trials
Nausea and vomiting are the results of several complex pathways involving the gut and the brain.57 For this reason, a multimodal regimen of medication targeting different receptors has been recommended by the latest Society of Ambulatory Anesthesia guidelines for PONV management.5 According to these guidelines, the effectiveness of ondansetron (5-HT3 antagonist) or the combination of aprepitant (NK1 receptor antagonist) and dexamethasone (glucocorticoid) to prevent POV following craniotomy has been confirmed.
In this narrative review, we included studies conducted in adult populations undergoing craniotomy with general anesthesia, with at least 1 intervention to prevent PONV. Intraoperative PONV prophylaxis (before dura closure) was the main intervention in 21 studies, whereas postoperative prophylaxis was reported in 2 RCTs (Table 2). The first attempt to publish evidence on PONV management following craniotomy was made by Sinha et al.14 The use of dexamethasone in combination with P6 acupressure or D1-D2, 5-HT3, NK1, H1 and/or muscarinic receptors antagonists have been tested as part of multimodal strategies to reduce the incidence of PONV.6–30 On the basis of the prophylaxis comparison regimen utilized to prevent PONV after craniotomy, we divided the articles included in this review into pharmacological7–23,25,26,58 and nonpharmacological interventions.9,24,59 Twenty trials of systemic pharmacological intervention versus placebo or active control (1744 subjects) and 3 nonpharmacological studies (294 subjects) were included. Most studies assessed the incidence of PONV following craniotomy and reported at least 24 to 48 hours follow-up. The most frequently used medication in these trials (in 13 studies) was ondansetron.
TABLE 2.
Characteristics of PONV Prophylaxis Following Craniotomy Studies
| References | Study Design | Patient Population | Anesthesia Type | N | Intervention | Time of Administration | Dose Active/Control | N/Groups | Odds Ratio | Efficacy Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Sinha et al14 | RCT | Infratentorial surgery | Inhaled | 40 | Pharmacological | Intraoperative | Ondansetron 4 mg vs. placebo | 20/20 | Not available | 10%/50% of PONV at 24 h |
| Fabling et al7 | RCT | Supratentorial surgery | Inhaled | 60 | Pharmacological | Intraoperative | Ondansetron 4 mg vs. droperidol 0.625 mg vs. placebo | 20/20/20 | Not available | 40%/40%/70% of PONV at 48 h |
| Kathirvel et al15 | RCT | Craniotomy | Inhaled | 152 | Pharmacological | Intraoperative | Ondansetron 4 mg vs. placebo | 78/74 | Not available | 11%/39% of POV at 24 h |
| Wang et al9 | RCT | Supratentorial surgery | Unknown | 70 | Pharmacological | Intraoperative | Granisetron 3 mg vs. placebo | 35/35 | Not available | 25.7%/57.1% of PONV at 72 h |
| Fabling et al8 | RCT | Infratentorial surgery | Inhaled | 46 | Pharmacological | Intraoperative | Ondansetron 8 mg vs. placebo | 23/23 | OR: 3.24, P=0.366 | 40%/40% of PON at 48 h |
| Madenoglu et al10 | RCT | Supratentorial surgery | Inhaled | 60 | Pharmacological | Intraoperative | Tropisetron 2 g vs. placebo | 30/30 | Not available | 30%/46.7% of PON and 26.7%/60% of POV at 24 h |
| Hartsell et al11 | RCT | Acoustic Neuroma | Inhaled | 60 | Pharmacological | Postoperative | Ondansetron 8 mg (oral) bid vs. placebo | 28/32 | Not available | 57.1%/81.3% of POV at 24 h |
| Jellish et al16 | RCT | Skull base surgery | Inhaled | 120 | Pharmacological | Postoperative | PCA placebo vs. PCA morphine 5 mg/mL vs. PCA morphine+30 mg ondansetron | 40/40/40 | Not available | 28.6%/35.7%/33.3% of PON at 24 h |
| Wig et al12 | RCT | Supratentorial surgery | Inhaled | 70 | Pharmacological | Intraoperative | Ondansetron 4 mg vs. placebo | 35/35 | Not available | 23%/46% of POV at 24 h |
| Jain et al17 | RCT | Supratentorial surgery | Inhaled | 90 | Pharmacological | Intraoperative | Ondansetron 4 mg vs. granisetron 1 mg vs. placebo | 27/30/30 | Not available | 7.4%/6.6%/60% of POV at 24 h and 33.3%/16.7%/53% of PON at 24 h |
| Habib et al18 | RCT | Craniotomy | Balanced | 104 | Pharmacological | Intraoperative | Aprepitant 40 mg (oral) vs. ondansetron 4 mg | 51/53 | Not available | 16% vs. 38% of POV at 48 h and 14% vs. 36% of POV at 24 h |
| Tsutsumi et al21 | RCT | Craniotomy | TIVA | 64 | Pharmacological | Intraoperative | Fosaprepitant 150 mg vs. ondansetron 4 mg | 32/32 | Vomiting: OR=0.067, P<0.001 Complete response: OR=2.790, P=0.045 | 6%/50% of POV at 24-48 h and 63%/38% complete response at 24 h |
| Gupta et al13 | RCT | Craniotomy | Inhaled | 75 | Pharmacological | Intraoperative | Granisetron 1 mg vs. ondansetron 4 mg vs. placebo | 25/25/25 | Not available | 4%/12%/56% of PONV at 24 h and 8%/12%/8% of PONV at 48 h |
| Ryu et al20 | RCT | Craniotomy | TIVA | 160 | Pharmacological | Intraoperative | Ondansetron 4 mg vs. ondansetron 8 mg vs. ramosetron 0.3 mg | 55/54/51 | Not available | 59%/41%/14% of PONV at 48 h |
| Bergese et al22 | Prospective single-arm study | Craniotomy | Balanced | 36 | Pharmacological | Intraoperative | Scopolamine patch 1.5 mg+ondansetron 4 mg+dexamethasone 10 mg | 36 | Not available | 31% of PONV at 24 h |
| Ha et al23 | RCT | Microvascular decompression | Balanced | 62 | Pharmacological | Intraoperative | Ondansetron 8 mg vs. ramosetron 0.3 mg | 31/31 | Not available | 51.6% of PON at 48 h |
| Bergese et al26 | RCT | Craniotomy | Inhaled | 95 | Pharmacological | Intraoperative | Aprepitant (oral) 40 mg vs. ondansetron 4 mg+promethazine 25 mg—dexamethasone 10 mg | 48/47 | Not available | 31%/36.2% of PONV at 24 h |
| Bergese et al25 | Prospective single-arm study | Craniotomy | Balanced | 40 | Pharmacological | Intraoperative | Palonosetron 0.075 mg+dexamethasone 10 mg+promethazine 25 mg | 40 | Not available | 30% of PONV at 24 h |
| Atsuta et al58 | RCT | Craniotomy | TIVA | 186 | Pharmacological | Intraoperative | Fosaprepitant 150 vs. 1.25 mg droperidol | 94/92 | RR: 0.336 for POV RR: 0.822 for PONV at 72 h | 12.8%/38% for POV and 44.7%/54.3% of PONV at 72 h |
| Wang et al9 | RCT | Supratentorial surgery | Inhaled | 80 | Nonpharmacological | Intraoperative | P6 acupressure vs. sham | 40/40 | Not available | 18%/37% of PON at 24 h |
| Xu et al59 | RCT | Infratentorial surgery | Balanced | 119 | Nonpharmacological | Intraoperative | P6 acupressure vs. sham | 60/59 | Not available | 22%/41% of POV at 24 h |
| Nilsson et al24 | RCT | Craniotomy | Balanced | 95 | Nonpharmacological | Intraoperative | P6 acupressure vs. sham | 43/52 | Not available | 72%/64% of PONV at 48 h |
OR indicates odds ratio; PCA, patient-controlled analgesia; PON, postoperative nausea; PONV, postoperative nausea and vomiting; POV, postoperative vomiting; RCT, randomized controlled trial; RR, risk ratio; TIVA, total intravenous anesthesia.
5-HT3 receptor antagonists, alone or in combination, have proven effectiveness for PONV prophylaxis/treatment in several trials of craniotomy procedures.7–15,17–21,25,26 The lack of sedative effects makes 5-HT3 antagonists the “gold-standard” drugs for PONV prophylaxis in craniotomies when postoperative clinical neurological assessments are required.8,20
Two trials assessed postcraniotomy administration of ondansetron versus placebo for PONV prophylaxis.11,16 Hartsell et al11 investigated the postoperative administration of ondansetron (4 mg) twice a day for up to 72 hours in acoustic neuroma surgical patients receiving inhaled general anesthesia and found that ondansetron was associated with a lower incidence of POV at 24 hours compared with placebo (57.1% vs. 81.3%, respectively). Conversely, Jellish et al16 compared the postoperative administration of morphine alone, morphine plus ondansetron (30 mg) and placebo using the patient-controlled analgesia technique. The addition of ondansetron did not reduce the incidence of PONV significantly, and these authors concluded that the use of this technique is not justifiable.
Four RCTs comparing ondansetron to placebo for PONV management were identified. Sinha et al14 initially proposed ondansetron (4 mg) as an ideal prophylactic medication for preventing PONV postcraniotomy in a study comparing ondansetron (4 mg) with placebo in patients undergoing infratentorial craniotomy under inhaled anesthesia; ondansetron reduced the incidence of PONV by 40% at 24 hours when compared with placebo (PONV incidence 10% vs. 50%, respectively (P<0.05). Another RCT by Kathirvel et al15 compared the use of ondansetron (4 mg) and placebo in patients undergoing supratentorial craniotomy under inhaled anesthesia and found that the incidence of POV at 24 hours was 11% in the ondansetron group compared with 39% in the placebo group (P=0.01). A similar study comparing ondansetron (4 mg) with placebo in patients scheduled to undergo supratentorial surgery under inhaled anesthesia found an incidence of POV at 24 hours of 23% and 46% (P<0.05), respectively.12 Last, Fabling et al8 conducted an RCT comparing the efficacy of ondansetron versus placebo for PONV prevention in 46 patients undergoing infratentorial craniotomy under inhaled general anesthesia. In this study a single dose of intravenous ondansetron (4 mg) at incision closure was moderately effective in decreasing early PONV when compared with placebo (17% vs. 22% 8 h after surgery), without effect on delayed PONV incidence after 48 hours.
Four RCTs tested the use of 5-HT3 receptor antagonists versus placebo in craniotomy patients. Wang et al9 reported that granisetron (3 mg) reduced the incidence of PONV at 72 hours by 31% compared with placebo in patients who underwent infratentorial surgery under general anesthesia; the incidence of PONV was 25.7% and 57.1% (P<0.01) in the granisetron and placebo groups, respectively. Furthermore, in a 3-group RCT comparing the administration of ondansetron (4 mg), granisetron (1 mg), and placebo in patients undergoing supratentorial surgery, Jain et al17 concluded that ondansetron and granisetron are similarly effective at preventing vomiting without reducing the incidence of nausea; in this study the incidence of POV was 7.4%, 6.6%, and 60% in the ondansetron, granisetron, and placebo groups, respectively (P<0.001). Conversely, a study by Gupta et al13 in patients undergoing craniotomy with inhaled anesthesia found that both granisetron (1 mg) and ondansetron (4 mg) administered at dural closure provided superior PONV prophylaxis than placebo; PONV rates were 4%, 12%, and 56% in the granisetron, ondansetron, and placebo groups, respectively (P<0.05). Last, in an RCT including patients undergoing supratentorial tumor resections under inhaled general anesthesia and receiving tropisetron (2 mg) or placebo at dural closure, Madenoglu et al10 found a similar incidence of PON in the 2 groups (30.0% in the tropisetron group versus 46.7% in the placebo group).
In addition, 2 RCTs investigated different regimens of 5-HT3 receptor antagonists. Ha et al23 compared the preventive antiemetic effects of ramosetron (0.3 mg) versus ondansetron (8 mg)—two 5-HT3 receptor antagonists—administered at dural closure after microvascular decompression with retromastoid craniotomy under balanced anesthesia. At 48 hours postcraniotomy, the overall PONV occurrence was similar in the 2 groups; the incidence of nausea was 87.1% and 93.6% and incidence of vomiting 51.6% and 61.3% in the ramosetron and ondansetron groups, respectively. However, Ryu et al20 found that ramosetron was more effective (83%) than ondansetron (37% and 59% for ondansetron 4 and 8 mg, respectively) in providing a complete PONV response at 0 to 48 hours after surgery; ramosetron decreased the incidence of PONV (14% vs. 59% to 41%) and the need for rescue medication when compared with ondansetron at 48 hours in patients undergoing craniotomy with TIVA. In an observational study including 229 patients undergoing craniotomy with general anesthesia (inhaled anesthesia, balanced anesthesia or TIVA) and receiving granisetron (1 mg) and/or dexamethasone (4 to 8 mg), Latz et al19 reported a PONV incidence of 47% at 24 hours postcraniotomy.
The prophylaxis efficacy of NK1 receptor antagonist drugs was assessed in 3 RCTs, which demonstrated the superiority of NK1 to 5-HT3 receptor antagonist drugs for POV prevention following craniotomies, when administered alone or as part of multimodal therapy. Habib et al18 reported that the combination of oral aprepitant (40 mg) and intravenous dexamethasone (10 mg) was more effective in preventing POV than the combination of intravenous ondansetron (4 mg) and dexamethasone (10 mg) during the first 48-hour postcraniotomy (16% vs. 38%, respectively). Similarly, Tsutsumi et al21 found that fosaprepitant (150 mg) administration in patients undergoing craniotomies with TIVA reduced the incidence of POV by 44% in the first 24 to 48 hours after surgery when compared with ondansetron (4 mg). In addition, the incidence of complete response 24-hour postcraniotomy was higher in the fosaprepitant group (63%) compared with the ondansetron group (38%). Atsuta et al58 demonstrated that the incidence of vomiting 0 to 72 hours after craniotomy was also significantly lower in patients receiving fosaprepitant (150 mg) immediately after induction of anesthesia than in those receiving droperidol (1.25 mg) at the end of surgery (12.8% and 38%, respectively).
Nonpharmacological interventions for PONV prevention, such as transcutaneous electrical acupoint stimulation at the P6 meridian points, can be effective adjuncts to standard PONV prophylaxis medication in patients undergoing craniotomy under general anesthesia.9,24,59,60 In a 2014 meta-analysis, including 3 RCTs incorporating 3 to 6 acupuncture points on the same side of the craniotomy, Asmussen at al61 reported a PONV incidence of 6.9% in the acupuncture groups versus 14.8% in control groups (P=0.017).
As previously noted, the mechanism of nausea and vomiting is complex, involving receptors and pathways located in the gut and brain.57 For this reason, a multimodal regimen of medication targeting different receptors has been recommended for the prevention of PONV in consensus guidelines for the management of PONV.5 Three clinical trials conducted by our group demonstrated an acceptable incidence of PONV following craniotomies when a novel triple therapy regimen was implemented.22,25,26 This multimodal regimen, which includes transdermal scopolamine (1.5 mg) administration before surgery and the combination of ondansetron (4 mg) and dexamethasone (10 mg) at anesthesia induction, has proven to be effective in PONV prevention (with an incidence of 33%) during the first 24 hours postcraniotomy.22 When discussing the potential benefits of transdermal scopolamine in reducing PONV incidence, we should consider the risk:benefit ratio of its use in craniotomy patients due to the potential for side effects (mydriasis and sedation) which could limit postoperative neurological evaluation and differential diagnosis of intracranial hypertension.1,22,62 Furthermore, in a single-arm study using a triple therapy of palonosetron (0.075 mg), dexamethasone (10 mg), and promethazine (25 mg) we reported a 30% incidence of PON and 7.5% incidence of PONV after craniotomy, without evidence of QT interval prolongation, a common adverse effect associated with palonosetron use.25 Last, an RCT conducted by our group found that the combination of intravenous promethazine (25 mg) and dexamethasone (10 mg) with oral aprepitant (40 mg) had similar efficacy in the prevention of PONV to intravenous promethazine (25 mg), dexamethasone (10 mg) and ondansetron (4 mg) (PONV rates of 31% and 36.2%, respectively).26 The use of promethazine in the craniotomy population should also be considered cautiously when immediate postoperative neurological evaluation is required because of its potential sedating effect.1,25 The results of the aforementioned trials using triple therapy are consistent in reporting significantly lower rates of PONV following craniotomy when compared with previously published data.7–15,17–21,25,26
This comprehensive narrative literature review has highlighted several limitations with published clinical studies that impact efforts to create specific guidelines or strategies to manage PONV. The diversity of study methodology, systemic pharmacological interventions, multipoint electroacupunctures, and follow-up times restricted our review from reaching a definitive conclusion. We identified several factors that could influence a higher incidence of PONV after craniotomy, but there are no comparable estimates among all the reviewed clinical trials due to the variability of patient population and regimens used.
CONCLUSIONS
The pathophysiology and mechanisms of postcraniotomy PONV are multifactorial in etiology and related to factors associated with anatomic-physiological mechanisms, patient populations, surgery type, and anesthesia technique. The literature reports that infratentorial craniotomies require a longer duration of surgery and higher exposure to anesthetic drugs and analgesics, and consequently are associated with higher rates of PONV. In addition, anesthesia technique can play an important role in reducing the incidence of PONV after craniotomy; there is robust evidence of lower rates of PONV with TIVA or awake craniotomy compared with inhalational anesthesia. On the basis of current evidence, prevention and management of PONV after craniotomy should focus on perioperative patient assessment, surgical, and anesthesia-related risk factors and the selection of systemic pharmacological agents to reduce its incidence and potential complications. In addition, a multimodal regimen of medication targeting different chemoreceptors in the vomiting center has been recommended. Ondansetron and dexamethasone, or their combination, are the most frequently used and effective. Further randomized clinical trials comparing different regimens that significantly reduce the incidence of PONV in craniotomy are required to provide relevant evidence-based data for PONV management in patients undergoing craniotomy.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Jordan N. Keels, Parna Thakkar, MD, Andrew Costa, and Brian E. Estevez for their writing and editing collaboration that greatly improved our manuscript.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1. Popugaev KA, Lubnin AY. Wartenberg KE, Shukri K, Abdelhak T. Postoperative care in neurooncology. Neurointensive Care. Cham, Switzerland: Springer; 2015:95–123. [Google Scholar]
- 2. Johnson WC, Seifi A. Trends of the neurosurgical economy in the United States. J Clin Neurosci. 2018;53:20–26. [DOI] [PubMed] [Google Scholar]
- 3. Cowan JA, Jr, Chandler WF. Changing trends in the use and costs of procedures performed by neurosurgeons in the United States. Clin Neurosurg. 2007;54:209–211. [PubMed] [Google Scholar]
- 4. Eberhart LH, Morin AM, Kranke P, et al. Prevention and control of postoperative nausea and vomiting in post-craniotomy patients. Best Pract Res Clin Anaesthesiol. 2007;21:575–593. [DOI] [PubMed] [Google Scholar]
- 5. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. [DOI] [PubMed] [Google Scholar]
- 6. Sinha PK, Koshy T, Gayatri P, et al. Anesthesia for awake craniotomy: a retrospective study. Neurol India. 2007;55:376–381. [DOI] [PubMed] [Google Scholar]
- 7. Fabling JM, Gan TJ, El-Moalem HE, et al. A randomized, double-blinded comparison of ondansetron, droperidol, and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. Anesth Analg. 2000;91:358–361. [DOI] [PubMed] [Google Scholar]
- 8. Fabling JM, Gan TJ, El-Moalem HE, et al. A randomized, double-blind comparison of ondansetron versus placebo for prevention of nausea and vomiting after infratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14:102–107. [DOI] [PubMed] [Google Scholar]
- 9. Wang YJ, Cheng ZG, Guo QL. Clinical observation of granisetron in preventing postoperative nausea and vomiting following supratentorial craniotomy. Hunan Yi Ke Da Xue Xue Bao. 2002;27:545–546. [PubMed] [Google Scholar]
- 10. Madenoglu H, Yildiz K, Dogru K, et al. Randomized, double-blinded comparison of tropisetron and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. J Neurosurg Anesthesiol. 2003;15:82–86. [DOI] [PubMed] [Google Scholar]
- 11. Hartsell T, Long D, Kirsch JR. The efficacy of postoperative ondansetron (Zofran) orally disintegrating tablets for preventing nausea and vomiting after acoustic neuroma surgery. Anesth Analg. 2005;101:1492–1496. [DOI] [PubMed] [Google Scholar]
- 12. Wig J, Chandrashekharappa KN, Yaddanapudi LN, et al. Effect of prophylactic ondansetron on postoperative nausea and vomiting in patients on preoperative steroids undergoing craniotomy for supratentorial tumors. J Neurosurg Anesthesiol. 2007;19:239–242. [DOI] [PubMed] [Google Scholar]
- 13. Gupta P, Sabharwal N, Kale S, et al. Granisetron versus ondansetron for post-operative nausea and vomiting prophylaxis in elective craniotomies for brain tumors: a randomized controlled double-blind study. Anesth Essays Res. 2014;8:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinha PK, Tripathi M, Ambesh SP. Efficacy of ondansetron in prophylaxis of postoperative nausea and vomiting in patients following infratentorial surgery: a placebo-controlled prospective double-blind study. J Neurosurg Anesthesiol. 1999;11:6–10. [DOI] [PubMed] [Google Scholar]
- 15. Kathirvel S, Dash HH, Bhatia A, et al. Effect of prophylactic ondansetron on postoperative nausea and vomiting after elective craniotomy. J Neurosurg Anesthesiol. 2001;13:207–212. [DOI] [PubMed] [Google Scholar]
- 16. Jellish WS, Leonetti JP, Sawicki K, et al. Morphine/ondansetron PCA for postoperative pain, nausea, and vomiting after skull base surgery. Otolaryngol Head Neck Surg. 2006;135:175–181. [DOI] [PubMed] [Google Scholar]
- 17. Jain V, Mitra JK, Rath GP, et al. A randomized, double-blinded comparison of ondansetron, granisetron, and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. J Neurosurg Anesthesiol. 2009;21:226–230. [DOI] [PubMed] [Google Scholar]
- 18. Habib AS, Keifer JC, Borel CO, et al. A comparison of the combination of aprepitant and dexamethasone versus the combination of ondansetron and dexamethasone for the prevention of postoperative nausea and vomiting in patients undergoing craniotomy. Anesth Analg. 2011;112:813–818. [DOI] [PubMed] [Google Scholar]
- 19. Latz B, Mordhorst C, Kerz T, et al. Postoperative nausea and vomiting in patients after craniotomy: incidence and risk factors. J Neurosurg. 2011;114:491–496. [DOI] [PubMed] [Google Scholar]
- 20. Ryu JH, Lee JE, Lim YJ, et al. A prospective, randomized, double-blind, and multicenter trial of prophylactic effects of ramosetronon postoperative nausea and vomiting (PONV) after craniotomy: comparison with ondansetron. BMC Anesthesiol. 2014;14:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsutsumi YM, Kakuta N, Soga T, et al. The effects of intravenous fosaprepitant and ondansetron for the prevention of postoperative nausea and vomiting in neurosurgery patients: a prospective, randomized, double-blinded study. Biomed Res Int. 2014;2014:307025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergese SD, Antor MA, Uribe AA, et al. Triple therapy with scopolamine, ondansetron, and dexamethasone for prevention of postoperative nausea and vomiting in moderate to high-risk patients undergoing craniotomy under general anesthesia: a pilot study. Front Med. 2015;2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ha SH, Kim H, Ju HM, et al. Comparison of the antiemetic effect of ramosetron with ondansetron in patients undergoing microvascular decompression with retromastoid craniotomy: a preliminary report. Korean J Anesthesiol. 2015;68:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nilsson I, Karlsson A, Lindgren L, et al. The efficacy of P6 acupressure with sea-band in reducing postoperative nausea and vomiting in patients undergoing craniotomy: a randomized, double-blinded, placebo-controlled study. J Neurosurg Anesthesiol. 2015;27:42–50. [DOI] [PubMed] [Google Scholar]
- 25. Bergese SD, Puente EG, Antor MA, et al. The effect of a combination treatment using palonosetron, promethazine, and dexamethasone on the prophylaxis of postoperative nausea and vomiting and qtc interval duration in patients undergoing craniotomy under general anesthesia: a pilot study. Front Med. 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bergese SD, Puente EG, Antor MA, et al. A prospective, randomized, double-blinded, double-dummy pilot study to assess the preemptive effect of triple therapy with aprepitant, dexamethasone, and promethazine versus ondansetron, dexamethasone and promethazine on reducing the incidence of postoperative nausea and vomiting experienced by patients undergoing craniotomy under general anesthesia. Front Med. 2016;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742–753. [DOI] [PubMed] [Google Scholar]
- 28. Fabling JM, Gan TJ, Guy J, et al. Postoperative nausea and vomiting. A retrospective analysis in patients undergoing elective craniotomy. J Neurosurg Anesthesiol. 1997;9:308–312. [PubMed] [Google Scholar]
- 29. Lonjaret L, Guyonnet M, Berard E, et al. Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. 2017;36:213–218. [DOI] [PubMed] [Google Scholar]
- 30. Jangra K, Kumari K, Panda NB, et al. Postoperative nausea and vomiting in neurosurgical patients: current concepts and management. Neurol India. 2018;66:1117–1123. [DOI] [PubMed] [Google Scholar]
- 31. Andrews PL. Physiology of nausea and vomiting. Br J Anaesth. 1992;69(suppl 1):2S–19S. [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iqbal IM, Spencer R. Postoperative nausea and vomiting. Anaesth Intens Care Med. 2012;13:613–616. [Google Scholar]
- 34. Horn CC, Wallisch WJ, Homanics GE, et al. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur J Pharmacol. 2014;722:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borgeat A, Ekatodramis G, Schenker CA. Postoperative nausea and vomiting in regional anesthesiaa review. Anesthesiology. 2003;98:530–547. [DOI] [PubMed] [Google Scholar]
- 36. Pusch F, Berger A, Wilding E, et al. Preoperative orthostatic dysfunction is associated with an increased incidence of postoperative nausea and vomiting. Anesthesiology. 2002;96:1381–1385. [DOI] [PubMed] [Google Scholar]
- 37. Gan TJ, Mythen MG, Glass PSA. Intraoperative gut hypoperfusion may be a risk factor for postoperative nausea and vomiting. Br J Anaesth. 1997;78:476. [DOI] [PubMed] [Google Scholar]
- 38. Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. [DOI] [PubMed] [Google Scholar]
- 39. Dewinter G, Staelens W, Veef E, et al. Simplified algorithm for the prevention of postoperative nausea and vomiting: a before-and-after study. Br J Anaesth. 2018;120:156–163. [DOI] [PubMed] [Google Scholar]
- 40. Apfel CC, Kranke P, Eberhart LH. Comparison of surgical site and patient’s history with a simplified risk score for the prediction of postoperative nausea and vomiting. Anaesthesia. 2004;59:1078–1082. [DOI] [PubMed] [Google Scholar]
- 41. Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2002;46:921–928. [DOI] [PubMed] [Google Scholar]
- 42. Meng L, Quinlan JJ. Assessing risk factors for postoperative nausea and vomiting: a retrospective study in patients undergoing retromastoid craniectomy with microvascular decompression of cranial nerves. J Neurosurg Anesthesiol. 2006;18:235–239. [DOI] [PubMed] [Google Scholar]
- 43. Sato K, Sai S, Adachi T. Is microvascular decompression surgery a high risk for postoperative nausea and vomiting in patients undergoing craniotomy? J Anesth. 2013;27:725–730. [DOI] [PubMed] [Google Scholar]
- 44. Tan C, Ries CR, Mayson K, et al. Indication for surgery and the risk of postoperative nausea and vomiting after craniotomy: a case-control study. J Neurosurg Anesthesiol. 2012;24:325–330. [DOI] [PubMed] [Google Scholar]
- 45. Flynn BC, Nemergut EC. Postoperative nausea and vomiting and pain after transsphenoidal surgery: a review of 877 patients. Anesth Analg. 2006;103:162–167. [DOI] [PubMed] [Google Scholar]
- 46. Chowdhury T, Prabhakar H, Bithal PK, et al. Immediate postoperative complications in transsphenoidal pituitary surgery: a prospective study. Saudi J Anaesth. 2014;8:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chui J, Mariappan R, Mehta J, et al. Comparison of propofol and volatile agents for maintenance of anesthesia during elective craniotomy procedures: systematic review and meta-analysis. Can J Anaesth. 2014;61:347–356. [DOI] [PubMed] [Google Scholar]
- 48. Cheng CR, Sessler DI, Apfel CC. Does neostigmine administration produce a clinically important increase in postoperative nausea and vomiting? Anesth Analg. 2005;101:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tramer MR, Fuchs-Buder T. Omitting antagonism of neuromuscular block: effect on postoperative nausea and vomiting and risk of residual paralysis. A systematic review. Br J Anaesth. 1999;82:379–386. [DOI] [PubMed] [Google Scholar]
- 50. Gupta A, Dwivedi Y, Saxena S, et al. A randomized control study of dexmedetomidine versus fentanyl as an anesthetic adjuvant in supratentorial craniotomies. Anaesth Pain Intens Care. 2017;21:306–311. [Google Scholar]
- 51. Peng K, Jin XH, Liu SL, et al. Effect of intraoperative dexmedetomidine on post-craniotomy pain. Clin Ther. 2015;37:1114.e1–1121.e1. [DOI] [PubMed] [Google Scholar]
- 52. Manninen PH, Tan TK. Postoperative nausea and vomiting after craniotomy for tumor surgery: a comparison between awake craniotomy and general anesthesia. J Clin Anesth. 2002;14:279–283. [DOI] [PubMed] [Google Scholar]
- 53. Eseonu CI, Rincon-Torroella J, ReFaey K, et al. Awake craniotomy vs craniotomy under general anesthesia for perirolandic gliomas: evaluating perioperative complications and extent of resection. Neurosurgery. 2017;81:481–489. [DOI] [PubMed] [Google Scholar]
- 54. Ouyang M, McDonagh DL, Phillips-Bute B, et al. Does midline shift predict postoperative nausea in brain tumor patients undergoing awake craniotomy? A retrospective analysis. Curr Med Res Opin. 2013;29:1033–1038. [DOI] [PubMed] [Google Scholar]
- 55. Akcil EF, Dilmen OK, Vehid H, et al. Which one is more effective for analgesia in infratentorial craniotomy? The scalp block or local anesthetic infiltration. Clin Neurol Neurosurg. 2017;154:98–103. [DOI] [PubMed] [Google Scholar]
- 56. Hwang JY, Bang JS, Oh CW, et al. Effect of scalp blocks with levobupivacaine on recovery profiles after craniotomy for aneurysm clipping: a randomized, double-blind, and controlled study. World Neurosurg. 2015;83:108–113. [DOI] [PubMed] [Google Scholar]
- 57. Tsaousi GG, Pourzitaki C, Bilotta F. Prophylaxis of postoperative complications after craniotomy. Curr Opin Anaesthesiol. 2017;30:534–539. [DOI] [PubMed] [Google Scholar]
- 58. Atsuta J, Inoue S, Tanaka Y, et al. Fosaprepitant versus droperidol for prevention of PONV in craniotomy: a randomized double-blind study. J Anesth. 2017;31:82–88. [DOI] [PubMed] [Google Scholar]
- 59. Xu M, Zhou SJ, Jiang CC, et al. The effects of P6 electrical acustimulation on postoperative nausea and vomiting in patients after infratentorial craniotomy. J Neurosurg Anesthesiol. 2012;24:312–316. [DOI] [PubMed] [Google Scholar]
- 60. Stoicea N, Gan TJ, Joseph N, et al. Alternative therapies for the prevention of postoperative nausea and vomiting. Front Med. 2015;2:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Asmussen S, Maybauer DM, Chen JD, et al. Effects of acupuncture in anesthesia for craniotomy: a meta-analysis. J Neurosurg Anesthesiol. 2017;29:219–227. [DOI] [PubMed] [Google Scholar]
- 62. Antor MA, Uribe AA, Erminy-Falcon N, et al. The effect of transdermal scopolamine for the prevention of postoperative nausea and vomiting. Front Pharmacol. 2014;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]


