Abstract
A host of novel renal biomarkers have been developed over the past few decades which have enhanced monitoring of renal disease and drug-induced kidney injury in both preclinical studies and in humans. Since chronic kidney disease (CKD) and acute kidney injury (AKI) share similar underlying mechanisms and the tubulointerstitial compartment has a functional role in the progression of CKD, urinary biomarkers of AKI may provide predictive information in chronic renal disease. Numerous studies have explored whether the recent AKI biomarkers could improve upon the standard clinical biomarkers, estimated glomerular filtration rate (eGFR) and urinary albumin to creatinine ratio (UACR), for predicting outcomes in CKD patients. This review is an introduction to alternative assays that can be utilized in chronic (> 3 months duration) nonclinical safety studies to provide information on renal dysfunction, and to demonstrate specific situations where these assays could be utilized in nonclinical drug development. Novel biomarkers such as symmetrical dimethyl arginine (SDMA), dickkopf 3 (DKK3), and cystatin C (CysC) predict chronic renal injury in animals, act as surrogates for GFR, and may predict changes in GFR in patients over time, ultimately providing a bridge from preclinical to clinical renal monitoring. (193 words)
Keywords: chronic renal injury, nonclinical safety, biomarkers, DKK3, SDMA, cystatin C, uromodulin
Introduction
This review will discuss current and novel biomarkers used to monitor chronic renal dysfunction including some biomarkers of acute kidney injury that have also been evaluated in the chronic setting. Through a weight of evidence approach, gleaning information from the published literature regarding biomarkers utilized to monitor chronic renal injury and disease in humans and animals, the intent of this review is to highlight the potential application of some chronic renal injury biomarkers in nonclinical toxicity studies of 4 months or longer duration where they may provide clinical translation, aid pharmaceutical development, and help resolve regulatory hurdles. One of the primary causes for the attrition of promising therapeutic agents from the drug development pipeline is the observation of treatment-related microscopic injury to the kidney in animal toxicology studies.1 Therefore, identifying, evaluating, qualifying, and utilizing relevant biomarkers of renal injury has greatly benefited the drug development process in recent years.2 The inclusion of novel urinary biomarkers and other investigative techniques in nonclinical toxicity studies has successfully identified nephrotoxic signals in animals and, due to their clinical translatability, helped predict and monitor renal toxicity in humans.3 This has helped inform clinical risk assessment and provide enhanced clinical safety monitoring through the establishment of defined entrance and stopping criteria in clinical trials based on information provided by changes in these analytes in laboratory animals in nonclinical studies.4 For many decades, the measurement of blood urea nitrogen (UN or urea nitrogen) and creatinine in serum or plasma have been the basis for assessment of renal function in both animals and humans. The advantage of these two biomarkers is that they progressively increase in both acute and chronic renal toxicity, and the magnitude of response correlates with decreases in renal function as assessed by glomerular filtration rate (GFR).5,6 Unfortunately, both UN and creatinine lack sensitivity in detecting renal disorders as they only begin to increase once approximately 50–60% of nephrons are damaged and/or a similar percentage of renal function is compromised, depending on the species.7–9 This is unacceptable for a clinical biomarker since the goal of any monitoring paradigm in a clinical trial would be to detect the earliest nephrotoxic signal prior to significant kidney injury in order to initiate stopping criteria for kidney injury before damage occurs. There are also a host of extra-renal factors, including dehydration, which can interfere with interpretation of UN and/or creatinine by overestimation of renal injury. In addition, several drugs can compete or interfere with the renal creatinine transporter system resulting in spurious changes.5,10–13 For animals such as dogs that are generally on meat diets, creatinine is vulnerable to diet-related variability (e.g. higher creatinine with meat vs casein-based diets) and other factors including muscle mass and body weight (i.e. higher serum creatinine levels with larger muscle mass), housing, circadian and seasonal variation, hydration status, physical activity, drug treatment, and age.14
In humans, acute kidney injury (AKI) is defined as damage or loss of kidney function for a duration of 7–90 days after an AKI initiating event which would parallel renal injury occurring during the acute/subacute nonclinical toxicity study time frame.15 In contrast, chronic renal failure or chronic kidney disease (CKD) is defined by the persistence of kidney disease for greater than 90 days, which would translate to renal injury that persists in nonclinical toxicity studies of greater than 4 months duration.15 CKD staging in humans is based on biomarkers of glomerular function (estimated GFR, eGFR) and tubuloglomerular injury associated with saturation of the megalin/cubulin resorptive capacity (e.g. urinary albumin to creatinine ratio, UACR).16 Since CKD and AKI share similar underlying mechanisms of functional and structural injury, exist on the same pathophysiologic continuum, and because the tubulointerstitial compartment has a functional role in the progression of CKD17–19, urinary biomarkers of AKI may also provide predictive information for chronic renal injury/disease. Recent efforts have focused on identifying urine or serum biomarkers of renal tubular injury that are either produced by the kidney or accumulate secondary to tubular cell dysfunction. Most of the kidney’s energy expenditure supports the maintenance of non-glomerular functions (i.e. tubular functions), so biomarkers that correlate with tubular injury may act as indicators of kidney pathology or health. In addition, studies have shown that the prognosis of even glomerular origin kidney disease is dependent on the extent of tubulointerstitial fibrosis.19 Renal tubule injury biomarkers also provide information about kidney tubular injury in early CKD where the “renal filtration reserve” is able to compensate for filtration deficits and serum creatinine levels remain unchanged.20 However, once renal filtration reserve is lost, additional renal insults result in further decreases in glomerular filtration and subsequent increases in serum creatinine, limiting the utility of tubular injury biomarkers in patients with diminished renal reserve.21
Numerous studies have explored whether the recent AKI biomarkers (especially kidney injury molecule 1 [KIM-1] and neutrophil gelatinase-associated lipocalin [NGAL]) could improve upon eGFR and UACR for predicting outcomes in CKD patients.(reviewed in21–32) Even though many studies have linked KIM-1 and NGAL to deterioration of renal function, there is conflicting evidence that use of KIM-1 or NGAL improves upon risk assessment beyond the standard renal biomarkers.21,22,25 One possibility is that tubular injury biomarkers may have limited utility in patients with diminished renal reserve because of nephron loss.29 CKD is not a discrete disease entity but rather the common result of diverse disease processes that can occur simultaneously, and it is unlikely that one biomarker will capture all processes.25 Instead, biomarker panels or disease-specific biomarkers may provide better prognostic utility for CKD.30,31 In the future, the use of urinary biomarkers in CKD may involve a more personalized medicine approach, e.g. by targeting patients undergoing therapy for renal fibrosis prevention, or by identifying AKI patients with preserved kidney function who are at risk of progression to CKD.22,30
Over the past 10–20 years, many novel renal biomarkers with increased sensitivity and specificity for renal disease have been developed and used to monitor drug-induced kidney injury (DIKI) in both preclinical studies and human trials.33 Although multiple renal biomarkers are available, currently there is no single defined battery of tests which will identify the pathophysiologic basis for every potential mechanism of renal injury, and most of the established analytes in animals are best suited for acute DIKI because these biomarkers were developed and evaluated for detection and monitoring of renal dysfunction in the setting of AKI. Urinary assays such as KIM-1, albumin, total protein, clusterin (CLU), cystatin-C (CysC), β2 microglobulin (B2M), and renal papillary antigen (RPA-1) have their place in renal investigative studies and have achieved qualification by the Food and Drug Administration (FDA) for defined contexts of use in rat preclinical studies.34–39 In addition, urinary NGAL and osteopontin (OPN) have also received a Letter of Support by the FDA for further evaluation in rat preclinical studies.40 Many of these biomarkers provide nephron segment injury localization and have even shown utility as sensitive AKI immunohistochemical reagents.33,41 In 2018, the FDA qualified the use of a single composite measure of six urine biomarkers (KIM-1, NGAL, CLU, OPN, N-acetyl-beta-D-glucosaminidase [NAG], and CysC) in conjunction with traditional measures of kidney function (creatinine, UN, serum CysC, urine albumin, and urine total protein) to aid in the detection of kidney tubular injury in phase 1 trials in healthy volunteers when there is a prior concern that a drug may cause tubular injury in humans.42–44 However, KIM-1, albumin, and NGAL have demonstrated the most translational applicability and appear to be the most commonly used in acute or subacute nonclinical studies.2,45–52
The purpose of this manuscript is: 1) to provide a comprehensive literature review of current human and animal renal biomarkers of AKI and CKD and how they are being utilized in chronic renal injury/disease (Table 1), 2) to provide a review of alternative assays (Table 2 and Figure 1) that can be utilized in nonclinical safety studies to evaluate chronic renal dysfunction (> 4 months duration), and 3) to identify specific situations where these assays could best be utilized. However, only with increased use of these chronic renal injury biomarkers by multiple institutions and in many nonclinical studies will enough information be generated to establish industry-wide confidence in their use and interpretation. A list of abbreviations used in this review can be found in Supplemental Table 1.
Table 1:
Human Clinical Research Biomarkers Evaluated in Chronic Kidney Injury and Disease in Animals
| Marker | Matrix | Source | Usage | Veterinary Diagnostics | Veterinary Research | Preclinical Research |
|---|---|---|---|---|---|---|
| KIM-1 | Urine | ↑PCT Released | Tubular Injury/Regeneration | X | X | X |
| KIM-1 | Blood | ↑PCT Released | Tubular Injury/Regeneration | - | X | -a |
| NGAL | Urine | ↑Tubular Released | Tubular Injury/Regeneration | X | X | X |
| NGAL | Blood | ↑Tubular Released | Tubular Injury/Regeneration | X | X | - |
| CysC | Urine | ↓PCT Resorption | Tubular Injury | X | X | X |
| CysC | Blood | Glomerular Filtration | eGFR | X | X | -a |
| SDMA | Blood | Glomerular Filtration | eGFR | X | X | X |
| DKK3 | Urine | ↑Tubular Released | Tubular Injury/Fibrosis | - | X | X |
| DKK3 | Blood | ↑Tubular Released | Tubular Injury/Fibrosis | - | X | X |
| B2M | Urine | ↓PCT Resorption | Tubular Injury | - | X | -a |
| B2M | Blood | Glomerular Filtration | eGFR | - | X | -a |
| UMOD | Urine | ↓TAL/DT Released | Tubular Injury/Fibrosis | - | X | -a |
| UMOD | Blood | ↓TAL/DT Released | Tubular Injury/Fibrosis | - | - | - |
| BTP | Blood | Glomerular Filtration | eGFR | - | - | - |
Acute kidney injury reports published, but no chronic injury usage reported. KIM-1: kidney injury molecule 1, NGAL: neutrophil gelatinase-associated lipocalin, CysC: cystatin C, SMDA: symmetric dimethylarginine, DKK3: dickkopf homolog 3, B2M: beta-2 microglobulin, BTP: beta trace protein, UMOD: uromodulin, PCT: proximal convoluted tubule, TAL: thick ascending limb, DT: distal tubule, eGFR: estimated glomerular filtration rate
Table 2:
Summary of Biomarkers of Acute and Chronic Renal Injury Evaluated in Chronic Kidney Injury and Disease
| Biomarker | Regulatory Statusa | Comments | R | D | NP | MP | H |
|---|---|---|---|---|---|---|---|
| Acute Renal Injury Biomarkers | |||||||
| uKIM-1 | Q (H) | Functions in tubular repair as epithelial scavenger receptor for phosphatidyl serine-mediated phagocytosis of apoptotic cells.67 Expression is upregulated with PT injury.87 Chronic PT upregulation was associated with interstitial inflammation and fibrosis in mice suggesting a link between acute and chronic injury.76 Current dog assays are problematic.117 | X | X | X | ||
| uNGAL | LOS (R) / Q (H) | Transport protein originally found in neutrophil specific granules. Located primarily in distal nephron. Induced with injury, inflammation, and neoplastic transformation in many epithelia.127 Functions as Fe-binding siderophore involved in host defense and is involved in nephron development.130 AKI marker with a large dynamic range in many species.38,45,141,146 Good at predicting the progression of AKI to CKD.150 Systemic inflammation or sepsis can cause increased serum levels which can lead to increased urine levels in the absence of renal injury.146 | X | X | X | X | X |
| Acute Renal Injury: LMW Biomarkers of PT Injury with Normal Glomerular Function | |||||||
| uB2M | Q | Found in every nucleated cell and forms the light chain component of class I histocompatibility antigens. Normally filtered through the glomerulus and almost entirely reabsorbed by the PT.357 Urine levels increase with glomerular and/or PT damage due to a variety of causes.362 Has poor stability at room temperature and in acid urine.369 Less useful than other LMW biomarkers such as CysC or retinol binding protein, particularly in dog.161,370 | X | Xb | X | X | |
| uCysC | Q (H) | Produced at a constant rate by all nucleated cells and functions as a lysosomal protease and cysteine proteinase.228 Normally filtered through the glomerulus and almost entirely reabsorbed by the PTs.229 Urine levels increase due to impairment or saturation of PT reabsorption.228 Demonstrated utility in clinic and in animals.31,33,227 | X | X | X | X | |
| Acute Renal Injury and Chronic Renal Dysfunction: Intermediate to HMW Biomarkers of Glomerular / Tubular Damage | |||||||
| uAlbumin | Q (H standard) | High specificity for renal injury. Parallels changes in total urinary protein levels in most species, except in male rats.55,58 Both intact and fragmented albumin is normally reabsorbed in PTs, but immunoassays can underestimate low level injury due to PT resorption/excretion of variably sized non-immunoreactive albumin fragments.51,64 | X | X | X | X | X |
| uProtein | Q (H standard) | Common method for monitoring the progression of glomerular disease in humans and animals.55,56 Can have lower specificity than albumin due to post-renal inflammation or increased production of abnormal LMW proteins.52,53 | X | X | X | X | X |
| Chronic Renal Dysfunction: Candidate Biomarkers | |||||||
| sCysC | NQ (H standard) | Recent eGFR calculations in humans have used cystatin in blood and urine rather than creatinine to more precisely calculate GFR.15 Outperforms creatinine in rats with subacute renal injury and in dogs with decreased mGFR.105,261 | X | X | X | X | |
| sSDMA | NQ | By-product of cellular arginine metabolism and continually released into the circulation.274 Depends on glomerular filtration for excretion and serum levels correlate with renal function.278 Increases progressively with increased renal impairment and progressive nephron loss in animals and humans with CKD.285,286 | X | X | X | ||
| u/pDKK3 | NQ | Member of a family of glycoproteins in the Wnt signaling pathway.309 Demonstrates promising utility as a urinary biomarker of ongoing tubular injury and progressing tubulointerstitial fibrosis.312,314 Another surrogate renal biomarker for GFR.315 | X | X | |||
| Chronic Renal Dysfunction: Other Experimental Biomarkers | |||||||
| u/sUMOD | NQ | Also known as Tamm-Horsfall protein, is the most abundant protein in urine.319 Produced exclusively in the thick ascending limb and the early distal tubule.320 Several studies have reported positive correlations between serum/plasma UMOD levels and kidney function.331 | X | X | X | X | |
| sB2M | NQ | Accumulates in serum when GFR is impaired and increases with stage of renal disease, but use in humans and dogs as a non-creatinine renal filtration marker has been overshadowed by CysC.374,378 | X | X | X | ||
| sBTP | NQ | Also known as lipocalin-type prostaglandin D synthase. Is not physiologically inert. Has ligand-binding, enzymatic properties, and is associated with multiple physiologic functions which can affect its levels.393 In humans, BTP levels have good correlation with mGFR and increase with decreased GFR in CKD especially in early stages.409,414 Limited use in animals. | X | X | |||
Regulatory status refers to rat unless otherwise specified,
Western blot, R: rat, D: dog, NP: nonhuman primate, MP: minipig, H: human, Q: qualified, NQ: not qualified, LOS: letter of support, LMW: low molecular weight, HMW: high molecular weight, u: urine, s: serum, p: plasma, KIM-1: kidney injury molecule-1, PT: proximal tubule, NGAL: neutrophil gelatinase-associated lipocalin, B2M: beta 2 microglobulin, BTP: beta trace protein, CysC: cystatin C, SDMA: symmetrical dimethyl arginine, DKK3: dickkopf homolog 3, UMOD: uromodulin, eGFR: estimated glomerular filtration rate, mGFR: measured glomerular filtration rate, CKD: chronic kidney disease
Figure 1:
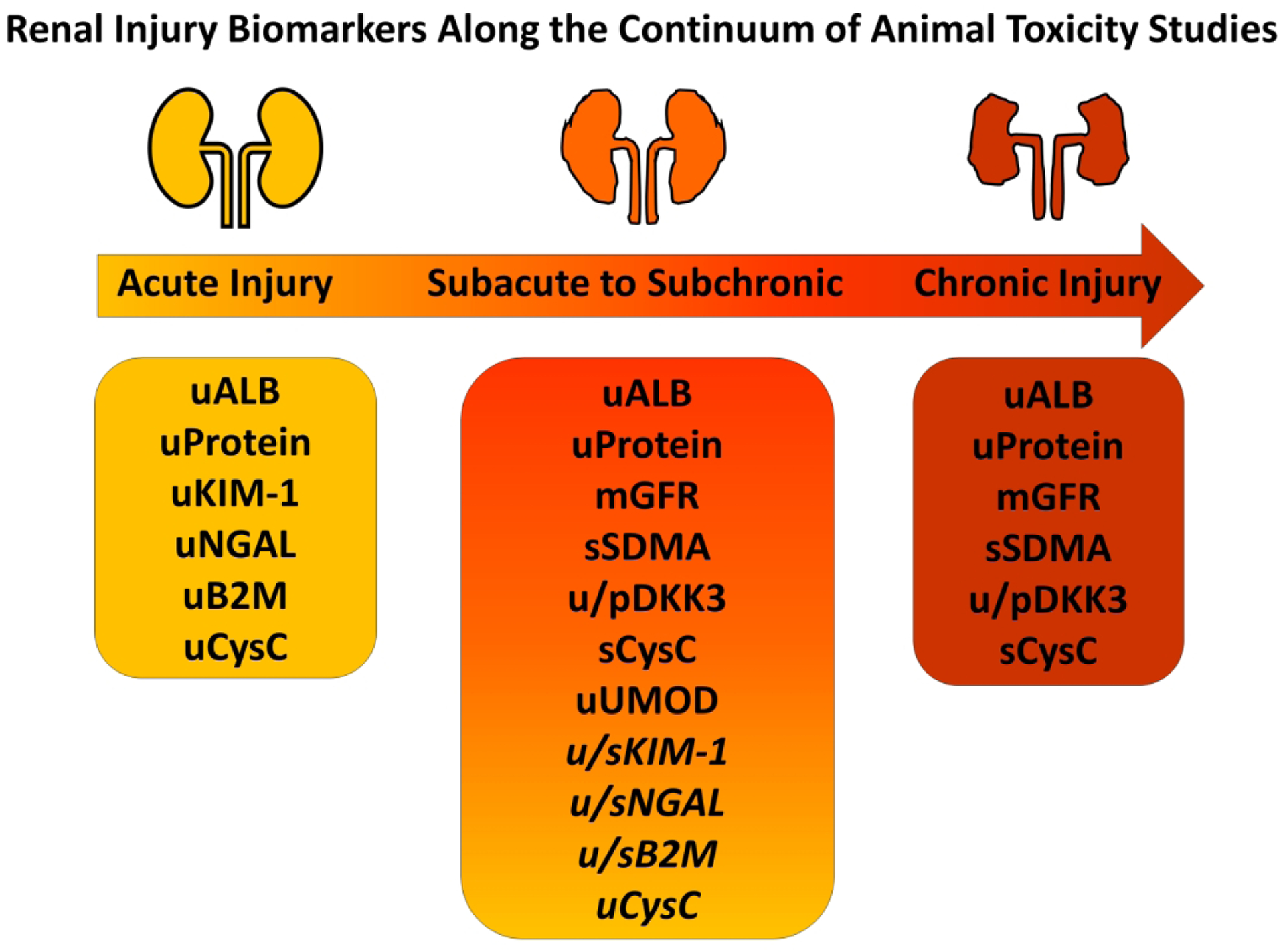
Renal Injury Biomarkers Along the Continuum of Animal Toxicity Studies. Acute renal injury biomarkers (KIM-1, NGAL, B2M, urine CysC) have shown some utility in monitoring the progression of acute to chronic injury, but often their successful usage in subacute to subchronic renal injury has been dependent upon type of injury and/or species (italicized). In addition to the standard renal functional biomarkers (ALB, Protein, mGFR), other biomarkers have shown promise in predicting and monitoring subacute to subchronic (SMDA, DKK3, serum CysC, UMOD) or chronic renal injury (SDMA, DKK3, serum CysC) in animal toxicity studies. u: urine, s: serum, p: plasma, kidney injury molecule 1: KIM-1, neutrophil gelatinase-associated lipocalin: NGAL, beta 2 macroglobulin: B2M, cystatin C: CysC, symmetrical dimethyl arginine: SDMA, dickkopf homolog 3: DKK3, uromodulin: UMOD, albumin: ALB, measured glomerular filtration rate: mGFR.
The following sections first discuss the current clinical standard biomarker of renal function (eGFR) and highlight three established biomarkers of AKI (urine albumin, KIM-1, NGAL) that may also be utilized in the chronic setting. Subsequent sections introduce three promising novel biomarkers of chronic renal dysfunction (CysC, symmetrical dimethyl arginine [SDMA], dickkopf 3 [DKK3]), as well as three other candidate biomarkers (uromodulin [UMOD], B2M, β-Trace Protein [BTP]) that hold promise for detecting chronic renal injury.
Estimated Glomerular Filtration Rate (eGFR)
GFR is the gold standard used to diagnose, manage, and predict risk of ESRD in humans and animals. It is also used clinically to adjust dosing of renally excreted medications in patients with renal disease.16 GFR often serves as an outcome measure in clinical trials and is used to determine prevalence of CKD in epidemiologic studies.53–55 Unfortunately, GFR is not readily measured directly and is generally assessed using the clearance of bolus-injected surrogates including creatinine, inulin, iohexol, or radiolabeled molecules at specific timepoints.14,56 However, this is rarely done in the clinic due to cost, availability, difficulty of measurement, and inconvenience.57,58 Likewise, it is logistically difficult and expensive to include in animal studies. To the authors’ knowledge, measured GFR (mGFR) has not been included routinely in toxicologic and safety pharmacology studies59,60, and certainly would not be a practical endpoint to add to a routine good laboratory practice (GLP) study. Additionally, for regulatory toxicology studies, there could be a hypothetical concern for potential toxicity or drug-drug interactions with these surrogate molecules.61 Technological advances in imaging equipment (optoacoustic tomography and transcutaneous fluorescent-sinistrin decay) have enabled use of portable units for more direct measurement of GFR in investigational experiments in animals and in institutional diagnostic applications, but these techniques are equally difficult to incorporate into nonclinical toxicology studies where large numbers of animals and strict time-sensitive protocols are in place.3,62–64 This instrumentation requires advanced training and practice to effectively standardize or equilibrate between animals, and the time it takes to measure even one animal requires prospective planning for staggered dosing if multiple dose groups are to be measured on study. Even in human nephrology practice, it is extremely uncommon to measure GFR directly, and in clinical trials, GFR is routinely estimated using endogenous biomarkers in a variety of specific formulas that estimate urinary clearance (eGFR).65–68 All endogenous filtration biomarkers have determinants other than GFR (non-GFR determinants), which include production, tubular secretion or reabsorption, and extra-renal clearance which could affect the accuracy of GFR estimates.69,70 Since these processes are usually not measured, surrogate variables to adjust for characteristics such as age and sex have been incorporated into eGFR equations.71 Additionally, non-GFR determinants in one population might differ from the source population used to derive the equation, so estimating equations should be used in the populations in which they were developed.72 For example, since urea and creatinine are eliminated through the dialysis treatment due to their small molecular weight, other LMW proteins (CysC, B2M, BTP) that have higher molecular mass are more useful to estimate residual kidney function in ESRD patients.72
Some of the more commonly used eGFR equations include: Cockcroft-Gault (CG), Modification of Diet in Renal Disease (MDRD) Study, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.73–75 The CG is no longer recommended for clinical use since it does not use standardized creatinine values, does not account for body surface area (obese and elderly individuals), lacks inclusion of racial diversity, and overestimates kidney function.75–77 In contrast, the MDRD Study and CDK-EPI equations use creatinine measurement procedures that are traceable to an isotope dilution mass spectrometry reference and contain variables that adjust the eGFR to account for creatinine differences due to age, sex, and ethnicity.78 The CKD-EPI equation is currently recommended by Kidney Disease Outcomes Quality Initiative (KDOQI) for estimation of GFR79 and is more accurate and less biased than the MDRD Study equation in populations with eGFR near and above 60 mL/min/1.73 m2 or older than 70 years of age.76,77,80 In addition, the CKD-EPI equation more accurately predicts adverse kidney, cardiovascular, and mortality outcomes for patients with CKD.81 However, the CKD-EPI equation does not work well for all patient populations including renal transplant patients, whereas some studies have shown better performance in Asians vs the MDRD.81,82 All estimating equations have errors relative to mGFR, and errors will be greater for people who have non-GFR determinants that differ from the populations used to derive the equations (e. g. vegetarians vs. bodybuilders).78 Attempts to adapt human eGFR equations for use in veterinary species have been unsuccessful, and currently there are no widely accepted eGFR formulas for preclinical species.83–86
One of the measured parameters that are included in the estimation of eGFR in both animals and humans has historically been creatinine, therefore creatinine clearance is a major determinant of eGFR. However, many other non-GFR determinants can influence creatinine levels, including age, sex, race, liver function, and intestinal flora (since creatinine is metabolized by intestinal bacteria).11,12,70,87–93 While the majority of creatinine is filtered unchanged through the glomerulus into the urinary filtrate, over 40% may be secreted via renal transporters depending on the species, and this transport may be affected by drugs.12,94–96 Tubular secretion can be increased to up to 50% of total renal elimination as GFR declines and can be supplemented with increased extra-renal elimination via the gastrointestinal tract.11,89,97,98 Creatinine production differs between patients with stable CKD and those with progressive disease due to comorbidities, further limiting its utility.30 In addition, patients with glomerular disease have progressive fractional hypersecretion of creatinine by renal tubules as the disease worsens leading to overestimation of GFR, so the use of creatinine as a filtration biomarker is inaccurate in these individuals.91 Another gap in clinical monitoring during early GFR decline referred to as the “creatinine blind region” occurs when there is increased creatinine secretion and decreased creatinine generation.99
Even though all current eGFR equations in humans include demographic adjustments of creatinine concentrations, GFR estimation remains imprecise for both under and overestimation of mGFR.100 In order to improve GFR estimation, several alternate LMW biomarkers (CysC, BTP, and B2M) have been proposed and studied. Since these LMW proteins are mainly cleared from the blood through glomerular filtration, are almost completely reabsorbed by proximal tubular cells, and are degraded to smaller peptides and amino acids that are reabsorbed into peritubular circulation with minimal urinary excretion101, they were proposed as potential serum biomarkers of impaired GFR, and their increased urinary excretion was considered to be an indicator of tubular damage.97,102,103 Several GFR estimation equations have incorporated these molecules alone or in combination.104 The 2012 KDIGO CKD guidelines recommend the use of the CKD-EPI CysC or creatinine/CysC equation in certain circumstances where eGFR accuracy is critical or when CKD diagnosis using creatinine alone is uncertain.16 Recently, the CKD-EPI collaboration has developed equations using the additional serum biomarkers BTP and B2M. Although BTP and B2M slightly underestimate mGFR in the higher eGFR range, combining these biomarkers has similar accuracy as that of the combined CKD-EPI creatinine and CysC equation.58,105 In a recent study that evaluated the performance of six CKD-EPI GFR estimation equations (eGFR-EPI) against the gold standard urinary inulin clearance in patients with CKD, the combination creatinine/CysC equation provided the best overall estimate of mGFR with the lowest bias, highest precision and accuracy, and greatest classification.104 The BTP and B2M equations displayed the worst performance with significantly less precision at all levels of GFR, and averaging the creatinine/CysC and BTP/B2M equations did not improve or worsen the performance compared to the creatine/CysC equation alone.104
Estimated GFR is absolutely critical to chronic renal functional assessment in human patients, but it is clear that to date mGFR is impractical to institute in nonclinical safety assessment studies, and eGFR calculations and interpretation in laboratory animals are still in their infancy.83,84,106 Thus, surrogate indicators of chronic renal function are essential for providing a basis for a reliable nephrotoxic signal in animal trials that can be translated into clinical patients.
Established Biomarkers of Acute Kidney Disease That May Also Be Utilized in the Chronic Setting
Urinary Protein and Urinary Albumin
Urine protein excretion is frequently assessed in nonclinical toxicity studies, and even in many clinical trials using rapid dipstick techniques that are qualitative, but only semi-quantitative. However, in the chronic setting, a more quantitative approach is often necessary to properly assess renal function. Both urine total protein and urine albumin are typically represented as concentrations and, like other urine analytes, are subsequently normalized to creatinine as a ratio. In humans, spot urine samples are usually taken from a first morning void, but in laboratory animals, urine is collected in a metabolism cage and the total volume collected is recorded for a defined time period of up to 16 hours depending on species or experimental design.107 Thus, in laboratory animals, urine creatinine or urine volume can be used as normalization parameters. Urinary protein to creatinine ratio (UPCR) and UACR are two of the few biomarkers that are used to monitor the progression of chronic renal failure in humans, generally in association with calculation of eGFR. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for the evaluation and management of CKD includes proteinuria in the staging of CKD.16 Proteinuria can be differentiated on the basis of amount of protein (nephrotic [>3.5 g protein excreted over 24 hours] vs non-nephrotic), type of protein (albuminuria or low molecular weight [LMW] proteins), and underlying pathology (glomerular vs non-glomerular).108,109 Proteinuria of renal origin can be classified into one or more of the following categories: tubular, overflow, or glomerular.109,110 Tubular proteinuria results from tubulointerstitial disease affecting the proximal tubules and interstitium resulting in decreased proximal tubule reabsorption of proteins, especially LMW proteins (< 25,000 Daltons) which under normal conditions are completely reabsorbed in the proximal tubules.109,110 Overflow proteinuria is mostly associated with increased production or circulation of large quantities of proteins in disease states (e.g., light chains in multiple myeloma, myoglobin in rhabdomyolysis) that exceed the reabsorption capacity of the proximal tubule, leading to excessive urinary protein which can be toxic to the tubules and cause AKI.109,110 Glomerular proteinuria is associated with damage to the glomerulus, and more significant glomerular disease results in more severe proteinuria. Post-renal proteinuria can occur with inflammation, hemorrhage, and/or mucosal secretions from the genitourinary tract (e.g., infections, nephrolithiasis, tumors) and resolves with resolution of the underlying condition.109 Urinary protein levels can fluctuate considerably in both acute and chronic renal failure, and may vary considerably even in patients with relatively normal renal function.111 Changes in urinary protein levels remain the most reliable method for monitoring the progression of glomerular disease in human patients and animals.79,112 In general, urinary albumin levels tend to parallel changes in total protein in most species.79,113 The rat is an exception, as rat urine is protein-rich due to the presence of large quantities of proteins known as “major urinary proteins” including alpha2-urinary-globulin.114 This is especially true in male rats.114 Consequently, urinary albumin excretion can be more specific as a biomarker of renal disease than urinary protein.114–118 Based on a rat renal micropuncture study, albumin absorption is distributed almost equally across the three segments of the proximal tubule.119 Albumin in the filtrate occurs as both intact (immunoreactive) albumin and variably sized albumin fragments. However, an elegant study using125 I-albumin intravenously administered to rats demonstrated that only 1–2% of the albumin recovered in urine was the intact form and that the vast majority of recovered albumin (98–99%) was fragmented and exocytosed both apically and basolaterally from proximal tubule cells.120 Thus, contrary to past dogma, more albumin than previously thought crosses the glomerular barrier in health, and not all urinary albumin is completely resorbed into the blood stream.120 This observation also has clinical implications in that immunoturbidimetric assays can underestimate severity of albuminuria since only intact albumin is detected by these assays.108,121 In addition, there are situations, particularly in chronic renal disorders, where urine albumin does not parallel total protein in the filtrate. Plasma albumin levels can decrease with chronic loss and, with a higher percentage of renal uptake, changes in urinary albumin may be overshadowed by large protein spillage. Therefore, in these situations (e.g. certain tubulointerstitial and vascular nephropathies including polycystic kidney disease), changes in urinary albumin levels may not detect progressive renal damage or dysfunction with advanced chronic renal disease.21,22 Thus, monitoring urinary protein and albumin levels is of clear benefit in the analysis of acute, subchronic, or chronic nonclinical toxicity studies where renal toxicity is predicted or suspected. However, there remains a substantial need for biomarkers which can provide more sensitive and specific data on the progressive renal dysfunction that may accompany drug or chemical administration with more chronic duration.
Kidney Injury Molecule 1 (KIM-1)
KIM-1 is a type 1 transmembrane glycoprotein on the cell surface of epithelial and immune cells that serves as a scavenger receptor for oxidized low-density lipoproteins and phosphatidylserine-mediated phagocytosis of apoptotic cells.122–126 It is virtually absent in healthy kidneys, but its expression is upregulated in proximal tubule cells following acute injury. This is especially true after ischemia-reperfusion injury and drug-related renal toxicity.127–133 The upregulation of KIM-1 following an ischemic insult is thought to be associated with the renewal of functional and morphological integrity of kidneys.127 KIM-1 upregulation has an anti-inflammatory effect by mediating epithelial cell phagocytosis of apoptotic cells leading to downregulation of innate immunity and inflammation through effects on NF-κB signaling.134 In addition to its role in acute tubular damage, glomerular KIM-1 expression was increased in parallel with proteinuria and podocytopenia (decreased podocytes in the glomerular tuft) in a diabetic animal model supporting its use as a potential biomarker for glomerular injury in proteinuric kidney disease.135 KIM-1 has also been shown preclinically to be upregulated in the later phases of AKI suggesting a role in renal repair.136 However, chronic proximal tubule overexpression of KIM-1 in a mouse model caused interstitial inflammation and fibrosis linking acute and recurrent injury in the progression of chronic renal disease.132 Thus, KIM-1 can be used as a biomarker of tubular damage, but it can also cause tubular damage by promoting interstitial fibrosis, so chronic elevations in KIM-1 may warrant additional monitoring for ongoing renal damage.
Following acute injury, the membrane bound domain of KIM-1 is cleaved via a metalloproteinase-dependent process releasing it into the urine and extracellular space.137 Urinary KIM-1 has been shown to be a good predictor of acute renal injury prior to detectable changes in eGFR.127,138 Numerous recent studies in humans have evaluated the biomarker potential of urinary KIM-1 in CKD.22 KIM-1 was useful in the detection of early CKD of uncertain etiology and was better than conventional biomarkers.28 Urinary KIM-1 was also higher in patients with proteinuria and normal renal function, suggesting that urinary KIM-1 should be evaluated in conjunction with urine protein excretion in patients with pre-existing nephropathy.139,140 Antihypertensive therapy in a rat model and in patients decreased urinary KIM-1 in parallel with lowering urinary protein excretion suggesting an improvement of proteinuria-induced tubular injury, however there was no change in creatinine clearance despite decreased KIM-1 excretion.141,142 KIM-1 may also be a potential biomarker of CKD caused by tubulointerstitial damage since its expression correlates with the degree of interstitial fibrosis and inflammation in affected patient kidneys and in a rodent model.143–147 In addition, several studies in various patient disease cohorts including diabetics demonstrated the ability of urinary KIM-1 levels to predict the development and progression of CKD.21,147–154 A meta-analysis of 10 studies found that urinary KIM-1 had borderline significance as an independent predictor of stage 3 CKD.155 KIM-1 in combination with urinary NGAL improved the ability to predict renal outcomes in other human chronic renal disease cohorts (glomerulonephritis, vasculitis, lupus nephritis).27,156 In a longer term study, plasma KIM-1 predicted future eGFR decline and CKD risk in healthy middle-aged patients.157 Serum/plasma KIM-1 was also elevated in patients with CKD of various etiologies.158 In longitudinal studies in normoalbuminuric diabetic patients, serum/plasma KIM-1 was found to be strongly associated with renal decline (eGFR) and/or onset of CKD stage 3 or higher.158–160
Numerous rodent DIKI studies demonstrated the utility of urinary KIM-1 for detection of AKI47,52,161–168, but performance of urinary KIM-1 in nonrodent species has been less consistent. Early increases in urine KIM-1 were seen in some AKI studies in monkeys,45,48,165,169 but not in others.170 In the dog, some authors describe diagnostic utility for KIM-1171,172, while others, including the current authors, report poor accuracy of KIM-1 in dog DIKI studies.3,4,167,173,174
Urinary KIM-1 is rarely used in veterinary clinical practice due to questionable single analyte assay performance in dogs. Also, canine-specific KIM-1 enzyme-linked immunosorbent assay (ELISA)167,171,172,174 and/or multiplex assays with KIM-1175 have underperformed other biomarkers including NGAL in dogs. Nevertheless, in dogs, urine KIM-1 has been shown to be increased in AKI and in conditions such as babesiosis171, but not in CKD.172 In cats, KIM-1 was detected by a qualitative urine immunoassay in those at risk of AKI, but not in cats with CKD.176
In rat investigational studies of DIKI, elevated KIM-1 levels have persisted during regeneration phases following dosing with gentamicin and carbapenem A reflecting ongoing repair.161 However, in other investigational studies in rodents, peak values of KIM-1 in DIKI have occurred at 3–6 weeks, with values steadily declining afterwards despite microscopic evidence of ongoing renal degeneration or fibrosis. KIM-1 urine and serum levels rose initially in a mouse ischemia-reperfusion injury model, but steadily declined from day 7 post injury to day 28 despite ongoing tubulointerstitial fibrosis.177 Additionally, in a rat model of Adriamycin nephrotoxicity, urinary KIM-1 was elevated at 12 weeks and reversed following 6 weeks antiproteinuric treatment (renin-angiotensin system blockade), but was not accompanied by reversal of interstitial fibrosis.142 KIM-1 has also been less consistent when utilized in chronic nonclinical drug studies and has often been undetectable in rodents when assessed in toxicity studies of 6 months or longer duration (unpublished data). In some rodent subchronic studies, KIM-1 levels increased following toxicant exposure concordant with renal histopathology and declined to normal or near normal levels during the recovery phases.163,178 In other rodent subchronic studies, KIM-1 values remained elevated after 90 days of Ochratoxin A dosing where it outperformed other biomarkers52, and at 12 weeks following Adriamycin administration179, suggesting it could be a biomarker of subchronic nephrotoxicity. Interestingly, in a subclinical adenine or aristocholic acid-induced CKD model where AKI was induced following a recovery period by low-dose cisplatin180, KIM-1 responses to AKI were lower and delayed in CKD rats compared to non-CKD rats, and were inversely linked to the extent of prior cortical damage.180 These data suggest that in the presence of known CKD in rats, urinary KIM-1 levels may vary indicating the need for thresholds to be lowered, combined with delaying and/or including serial sampling. The totality of these findings indicate that urine KIM-1 alone may not be ideal for detecting chronic renal injury in longer term animal toxicity studies, but may add value as part of a panel of other biomarkers.
Neutrophil Gelatinase-Associated Lipocalin (NGAL)
NGAL, also known as lipocalin-2, is a glycoprotein bound to matrix metalloproteinase-9 in human neutrophils and is involved in transport of hydrophilic substances through membranes to maintain cellular homeostasis.181 NGAL is involved in the early stages of nephron development182 and is widely expressed in adult tissues (lung, gastrointestinal tract, liver, and kidney), but its main sites of expression are white blood cells and kidney loop of Henle and collecting ducts.183,184 NGAL inhibits bacterial growth via binding bacterial siderophores and sequestering iron.185,186 Tubule cells process the NGAL-Fe-siderophore complex leading to increased cytoplasmic iron concentrations, activation of iron-dependent processes, and decreased oxidative stress.187,188 NGAL is markedly induced in epithelial cells in response to injury, inflammation, and neoplastic transformation.183,188,189 NGAL was identified as one of the earliest and most upregulated genes in rodent tubular and ischemic renal injury models, especially in the distal nephron.190,191 However, in a mouse model of cisplatin AKI, NGAL was induced predominantly in proximal tubules.192 NGAL was also shown to be an early biomarker of tubular injury in dogs with experimentally induced AKI165,174,193–195 or naturally occurring AKI.196–201 In an experimental model of septic AKI in pigs, both serum and urine NGAL increased in association with tubular histopathology in septic animals compared to controls.202 Even though most research supports a protective role for NGAL in AKI, studies in mice revealed that NGAL upregulation is involved in the progression of chronic renal injury in humans and certain strains of mice by modulation of epidermal growth factor-mediated tubular mitogenesis and cyst formation.189 In a mouse model of ischemia-reperfusion injury, NGAL increased continuously during the AKI-CKD transition (from day 3 to day 28 post injury); whereas KIM-1 declined gradually in the chronic phase suggesting that NGAL may be able to dynamically monitor AKI-CKD progression.177 Similarly, in a rat model of adenine-induced tubulointerstitial injury, urinary NGAL levels were increased during the AKI phase and remained elevated for 4–8 weeks in the CKD phase.203
NGAL exists in multiple forms, with 25 kD monomers considered to be of renal tubular origin and dimers of the 25 kD monomer of neutrophilic origin.204 It should also be noted that certain NGAL forms of systemic origin can cross the glomerular filtration barrier, contributing to urinary NGAL excretion. In a study comparing the utility of serum NGAL in AKI and chronic kidney injury models in rats and mice, serum NGAL had variable success in detection of kidney injury across all of the models, which is not surprising given that serum NGAL can lack specificity for renal injury due to production and release by neutrophils and non-renal epithelia (liver, bladder, lung, stomach, small intestine, pancreas, prostate gland, mammary gland, adrenal gland, thymus) in response to injury.205,206 In a mouse ischemia-reperfusion kidney injury model, serum and urine NGAL increased continuously during progression of AKI to CKD compared with serum and urine KIM-1 which peaked in the acute phase and declined during the chronic phase.177 Similarly in humans, serum and urine NGAL were good prognostic biomarkers for predicting the progression of AKI to CKD in sepsis-associated AKI patients.207 Because of potential non-renal sources of serum NGAL, the association of serum NGAL changes with renal injury is ideally informed by effects on biomarkers of systemic inflammation as well as changes in other renal injury biomarkers.
In humans, urinary NGAL has been shown to be a good predictor of renal injury prior to detectable changes in eGFR28,147,208, and has also been shown to be inversely correlated with eGFR while being directly correlated with interstitial fibrosis and tubular atrophy.147,209 Investigators have also shown in CKD patients that increased urine NGAL reflects residual renal function and is directly correlated with serum creatinine and proteinuria, and inversely with GFR.210 As GFR decreases and urine protein and serum creatinine increase in early CKD, urine NGAL increased proportionately in the majority of patients until late in disease progression when advanced stages of CKD and severe renal tubular degeneration and atrophy resulted in diminished renal reserve.210 Unlike KIM-1 and NAG, urine NGAL was shown to be an independent predictor of end stage renal disease (ESRD) risk and of mortality for patients with CKD.155 Similarly, in elderly CKD patients, increased serum NGAL reflected renal impairment and was an independent risk biomarker for progression of ESRD.211 However, other studies have shown that urine NGAL did not provide additional information beyond the standard renal biomarkers in predicting outcomes in patients with ESRD, but did improve prediction of CKD progression in elderly patients with low-grade proteinuria212 and in diabetic patients.213 Similarly plasma/serum NGAL improved upon serum creatinine and eGFR in predicting the severity of tubulointerstitial infiltrates and fibrosis in patients with early CKD and predicted renal dysfunction in patients with early diabetes and children with CKD.214–216
In the clinical veterinary setting, urinary and to a lesser extent serum NGAL have shown promise as biomarkers of CKD progression. In a study of naturally occurring CKD in dogs, higher urine and serum NGAL were associated with death, and serum NGAL was better than serum creatinine at predicting clinical outcomes.199 In another study in dogs with naturally occurring CKD, serum NGAL was a good indicator of CKD severity and correlated with serum creatinine, but the serum NGAL values in dogs with advanced CKD had a wide range of variation suggesting that dogs with more stable CKD may not have on-going renal tubular injury leading to serum NGAL increases, and that urine NGAL may be a more sensitive disease biomarker in these dogs.217 Likewise, in dogs presenting with azotemia, plasma NGAL levels were able to differentiate between those with AKI (higher NGAL) versus CKD.200 Increased urinary NGAL was shown to occur early in the development of CKD, correlate with glomerular and tubulointerstitial lesions, and/or be associated with shorter survival in dogs.198–200,218–223 In dogs with x-linked hereditary nephropathy, urinary NGAL differentiated affected from unaffected dogs early in the disease process, with increases during early to mid-stages of disease, but it was not helpful for monitoring mid- to late-stage renal failure.218 Similarly, cats with higher urinary NGAL concentrations have more rapid CKD progression.224
Urinary NGAL to creatinine ratios have been quite successful in detecting DIKI in animals including mice, rats, dogs and nonhuman primates.3,4,40,45,47,51,52,163–165,195,211,225,226 However, the ability of NGAL to detect minimal kidney lesions in monkeys was inferior to KIM-1 and CLU.45 In addition, NGAL was the most nonspecific biomarker evaluated in a series of rat studies comparing renal and non-renal toxins, and increased urine and plasma NGAL were observed in a variety of different end-organ toxicities in the absence of renal toxicity.40,47 In bacterial nephritis, NGAL was elevated up to 6 weeks post infection in rodents.227 Similarly, dogs with lower urinary tract infections or diseases and pyuria can have increased urinary NGAL.198,228,229 In cats, three forms of urinary NGAL were detected and two were found to be associated with renal diseases of either the upper (monomeric form) or the lower (dimeric form) urinary tract.230 Increased NGAL concentrations in the urine of animals with lower urinary tract diseases having inflammation could be due to the presence of neutrophils, and as with sepsis or systemic inflammation, determining the molecular forms of urine NGAL may allow for more specific and precise detection of urinary tract disease.221
In chronic animal safety assessment studies of 3 months or longer, urinary NGAL values tend to wane over time similar to KIM-1. Therefore, urinary NGAL values appear to be ineffective in monitoring the progression of chronic renal injury or fibrosis as NGAL levels have been inconsistently elevated despite sustained injury (unpublished data). Both KIM-1 and NGAL were not increased at any time point throughout a 36-week study tracking the progression of nephropathy in hypertensive and obese rats.231 One explanation for this negative result is the relatively more severe glomerular damage than tubular injury in these rats231, but transiently increased NGAL expression may have been missed. The poor performance of NGAL in longer term nonclinical safety studies has led to the search for other renal biomarkers that may augment current biomarkers used in chronic animal studies. In addition to KIM-1 and NGAL, uromodulin (UMOD, discussed later) has also been shown to predict AKI before renal functional decline.127,147,210
Promising Novel Biomarkers of Chronic Renal Dysfunction That Can Be Added to Nonclinical Studies
Cystatin C (CysC)
As the authors noted, currently utilized biomarkers generally have limitations in the chronic setting, but there are some potential alternatives available to fill this important niche. CysC is one of the promising new analytes that may make the transition from AKI to CKD biomarker and, in the context of nonclinical safety, work as both an acute and chronic renal biomarker.45,47,51,167,211,232 As noted above, recent eGFR calculations in humans have utilized cystatin in blood and urine rather than creatinine to more precisely calculate GFR, but this has not generally been an accepted practice in nonclinical safety assessment due to limited availability of CysC testing and the overall difficulty in validating eGFR calculations in animals.
CysC is a LMW protein produced at a constant rate by all nucleated cells and functions as a lysosomal protease and cysteine proteinase.233 It is freely filtered at the glomerulus with almost complete reabsorption and catabolism in the proximal tubules and it is not secreted by the tubules, so it is minimally present or absent in urine under normal circumstances.234,235 However, similar to other LMW proteins, impairment or saturation of proximal tubule reabsorption can lead to marked increases in levels of urinary CysC in both animals and humans and thus is biomarker of acute tubular injury when glomerular function is not affected.236,237 Since the sieving coefficient (measure of equilibration of a substance through a semipermeable membrane) of CysC is close to unity, the renal clearance of CysC approximates GFR, and serum CysC has been considered to be an effective alternative to creatinine as a biomarker of GFR.238,239 In addition, CysC–based GFR estimates do not require correction for muscle bulk, diet, age, gender, and race.102,240–242 However, some researchers found that older age, male gender, greater weight and height, cigarette smoking, thyroid disease, malignancy, corticosteroid therapy, pregnancy, inflammation and/or higher C-reactive protein (CRP) levels are associated with increased serum CysC values.58,243–247 In addition, the non-renal clearance of CysC in both healthy humans and rats was found to be substantial and approximately 15% of the total clearance.238,248 The relative proportion of CysC eliminated extra-renally increases with decreasing GFR and was shown to overestimate renal clearance in conditions such as dialysis patients with advanced renal failure.248,249 Despite these limitations, serum CysC has been shown to be useful for detecting slight decreases in renal function in early renal failure in various patient groups78,250–252, and to predict mortality in CKD independent of GFR.253,254 Serum CysC also correlated better with mGFR than creatinine concentration in stage 3 CKD patients and was a good predictor of kidney dysfunction in patients with portal hypertension.70,255 In addition, serum CysC was better able to predict adverse outcomes compared to serum creatinine.256,257 Equations utilizing CysC combined with creatinine improve the accuracy of GFR estimation compared to equations based on single biomarkers.58,71,81,104,254,258 Serum CysC has also been shown to be independently associated with cardiovascular disease, ESRD, and mortality.259,260 Even though no causal relationship has been established to date, these associations may provide a better understanding of the common pathways of morbidity and mortality in cardiovascular and renal disease.261 As the 2012 KDIGO CKD guideline recommends assessment of CysC based on evidence that eGFR with CysC instead of creatinine may better classify CKD in human patients16,244,254,258,259,262, there is promise for using CysC in chronic animal safety studies.
In the rat, serum CysC was shown to be more sensitive, specific, and reliable than serum creatinine and was able to detect different types of acute drug-induced lesions in various nephron segments (proximal and distal tubules) independent of the mechanisms of nephrotoxicity and even at levels of minimal injury.161 In addition, in rat acute and subacute nephrotoxicity studies, urine CysC levels were increased, often earlier than other urine biomarkers.47,52,166,167,178,263,264 In dogs with renal disease, serum CysC outperformed serum creatinine when compared to mGFR and it was a more sensitive indicator of decreased GFR.265,266 Serum CysC has also been proposed as an early biomarker for kidney dysfunction in critically ill dogs, and in dogs with leishmaniasis, chronic nephritis, and diabetes mellitus.267–269 In addition, serum CysC had better sensitivity and specificity for detection of early stage I CKD in dogs than serum creatinine or symmetrical dimethyl arginine (SMDA).270 Serum CysC was also increased in an AKI study in monkeys given gentamicin.170 In dogs220,271 and cats272,273, urinary CysC was found to be higher in animals with renal disease than in healthy animals or animals with non-renal disease, but it was only increased in the urine of dogs with leishmaniasis during the azotemic stages of CKD.274 In dogs with gentamicin-induced AKI, urinary CysC was found to be the most sensitive indicator of both structural and functional kidney injury relative to traditional and novel urinary biomarkers including urinary albumin, Kim-1, and NGAL.174
Although these results are encouraging, considerable biological variability of serum CysC concentrations in dogs has been reported, suggesting that serum CysC may not be superior to serum creatinine for GFR determination, and further studies will be needed to confirm the value of serum CysC compared to serum creatinine.275,276 As CysC analysis becomes more routine in the assessment of chronic renal disease in human practice, and eGFR estimation is improved in animals, there is the potential that nonclinical use of CysC in both urine and serum could also become routine.
Symmetrical Dimethyl Arginine (SDMA)
SDMA, one of the most promising candidates for a chronic renal biomarker, was discovered over 40 years ago.277 The essential amino acid arginine is metabolized within mitochondria into two structural isomers: SDMA and asymmetrical dimethyl arginine (ADMA).278 SDMA and ADMA are released into the cytoplasm and subsequently transported out of the cell and into the circulation.279 SDMA is excreted primarily through the kidney (>90%) and undergoes minimal metabolism280,281, but ADMA is highly protein-bound, extensively metabolized enzymatically, and only 20% is excreted in the urine making it unsuitable as a renal biomarker.281,282 In contrast, SDMA depends almost entirely on glomerular filtration for excretion, thus its serum levels correlate directly with renal function (GFR).283,284 In addition, SDMA is not affected by non-renal factors that influence creatinine and/or CysC such as lean body mass, food intake, inflammation, diabetes, or estrogen therapy.285,286 Most importantly, SDMA levels have been demonstrated to rise progressively with increased renal impairment and progressive nephron loss in animals and humans with chronic renal disease.283,287–295 Chronic SDMA infusion does not affect renal function or kidney histology, so SDMA is not a direct cause of kidney injury.296 In the absence of renal impairment, SDMA is not affected by acute inflammatory responses, liver and cardiovascular disease, or by diabetes mellitus.297,298
Multiple studies in human CKD patients have demonstrated significant and progressive increases in SDMA relative to control populations with normal renal function.284,285,291,299 In humans with impaired renal function, there was a strong correlation between SDMA and mGFR, and SDMA outperformed creatinine but was equivalent to CysC and eGFR equations.283,285 Interestingly, in a study in healthy dogs of various ages fed prescription diets designed to maintain lean body mass, SDMA concentrations decreased significantly from baseline at 3 months and decreased further at 6 months, suggesting a dietary effect on renal function, and that SDMA could have clinical advantages over serum creatinine in monitoring responses to nutritional interventions.300 Based on total biological variability (analytical, intra- and interindividual) in healthy dogs, use of SDMA as a single measurement is not recommended to screen for early renal functional loss, rather serial monitoring and use of the critical difference (CD) is recommended for monitoring kidney function instead of comparison with population-based reference values.301 In renal ischemia models in both rats and mice, and in nephrectomized dog and rat models, serum SDMA levels increased progressively as renal mass was reduced.296,302–305 This increase in SDMA associated with nephron loss is not significantly diminished over time in contrast to NGAL, KIM-1, or OPN for which responses wane over time.
SDMA has been routinely used in companion veterinary practice for over 10 years as a reliable biomarker of progressive kidney disease in dogs and cats with a variety of chronic conditions including Lyme nephropathy, familial amyloidosis, renal dysplasia, and polycystic kidney disease.287,306,307 One of the advantages of this assay is that it is performed in plasma or serum rather than urine, eliminating the need for urine collection or use of a metabolism cage. Venipuncture can be easily scheduled in GLP studies at specific timepoints weeks or months apart. In animals, SDMA increases compare favorably with declines in GFR. An investigative study in dogs comparing SDMA to mGFR demonstrated 90% sensitivity and specificity, similar to creatinine and much better than CysC.308 In a separate study in dogs, similar sensitivity (90%) and specificity (83%) were noted compared with GFR as assessed by iohexol, but this depended upon the threshold used.309 It has been noted that the optimal SDMA cutoff for detecting greater than a 40% decrease in GFR was serum SDMA >18 μg/dL.309 SDMA has begun to be incorporated in target animal safety studies in dogs for detection of drug-induced nephrotoxicity by drugs intended for veterinary use in this species (personal communication). SDMA has been evaluated in human studies and in rare clinical trials, but the liquid chromatography-mass spectrometry analytical method required for the assay in humans has limited its availability and utility.283 In animals, the liquid chromatography-mass spectrometry method is still the gold standard, but a more recent proprietary ELISA assay appears to outperform the older DLD® ELISA.310 While there is extensive experience in dogs and cats, this assay has only recently become commercially available in rodents, and is scaled to be able to be run on large numbers of animals with a required serum volume of 50–100 ul.311,312
Dickkopf Homolog 3 (DKK3)
Another surrogate biomarker for GFR that has received increased attention in the human literature is DKK3. DKK3 belongs to a family of glycoproteins that modulate the Wnt signaling pathway and has promising utility as a urinary biomarker of ongoing tubular injury and progressing tubulointerstitial fibrosis in animals and humans. DKK3 has been shown to be a stress-induced, profibrotic molecule of renal tubular origin with dual roles in promoting repair/regeneration acutely or facilitating progression to CKD after AKI, depending on the magnitude and duration of activation.313,314 Although regenerative processes may occur in the early stages of renal injury via the Wnt pathway, continuous Wnt activation is detrimental and induces epithelial-mesenchymal transition and tubulointerstitial fibrosis.315,316 DKK3 is expressed in the developing kidney, is suppressed in adult life, and re-expressed in tubular epithelium under pathological conditions, eliciting a profibrotic T cell response.313,317
AKI is a known and common complication in patients after cardiac surgery. In preoperative cardiac patients at risk for AKI and subsequent loss of renal function, DKK3 has been shown to be an effective biomarker of renal tubular stress.318 In a study of 733 patients, preoperative urinary DKK3 was found to significantly improve prediction of risk of AKI and kidney function loss after cardiac surgery (p<0.0001).318 In one study to investigate the potential use of urinary DKK3 as a biomarker of short-term eGFR loss (over 12 months), eGFR and urinary DKK3 levels were prospectively assessed in 481 patients with CKD of various etiologies and compared with a general population sample and also with samples from patients with confirmed IgA nephropathy.319 The investigators were able to successfully identify patients at high risk for eGFR decline regardless of the cause of kidney injury. Thus, DKK3 was identified as a potential tool to monitor CKD progression and assess effects of interventions in patients with short-term risk of eGFR loss beyond what established biomarkers were able to do. In IgA nephropathy patients, urinary DKK3 levels (normalized to creatinine) were significantly higher in patients with CKD than in the control population, closely followed declines in eGFR over 12 months, were related to extent of renal fibrosis, and significantly improved prediction of eGFR decline and disease progression when compared to urinary albumin levels alone.319 In addition to urinary levels, elevated plasma levels of DKK3 were found to be associated with renal graft versus host disease progression, sclerosis, and higher mortality.320
DKK3 also appears to be important in progression of renal disease in animals. Using DKK3-deficient mice, tubular damage and renal interstitial fibrosis were significantly reduced compared with wildtype mice after unilateral ureteral ligation, as well as in a mouse adenine nephropathy model.313 This study also used antibody-mediated blockade of DKK3 to elicit similar findings. Urinary levels of DKK3 in rodents follow disease progression, tubule atrophy, and interstitial fibrosis.317 In an Adriamycin nephropathy model in mice, DKK3 expression was upregulated in the tubule epithelium and interstitium.321 Taken together, these findings suggest that DKK3 may be a useful diagnostic biomarker for both acute tubular injury and chronic renal disease involving tubulointerstitial fibrosis. DKK3 is just beginning to be assessed in rodent and nonrodent toxicology studies (unpublished data), so it has yet to be determined whether this potential biomarker will be successful for prediction and monitoring of chronic renal disease progression in nonclinical studies, but since the biology of the Wnt pathway is similar in humans and animals, the future for this analyte is promising. An ELISA assay is available in humans that apparently will cross react with most species of laboratory animals (personal communication), although other immunoassays and mass spectrometry have also been used for analysis of urinary and plasma DKK3.319,320,322
In summary, DKK3 is a stress-induced, profibrotic, tubular epithelium secreted glycoprotein that may be a useful marker for ongoing tubular injury and progressing tubulointerstitial fibrosis as it has dual roles in the promotion of acute repair/regeneration and facilitating progression to CKD.
Other Experimental Biomarkers That May Have Potential in Chronic Renal Dysfunction
Uromodulin (UMOD)
UMOD, also known as Tamm-Horsfall protein, is a glycosylphosphatidylinositol (GPI) anchored glycoprotein. It is the most abundant protein excreted in urine and is the predominant protein in hyaline casts.323,324 UMOD is produced exclusively in the thick ascending limb and early distal tubule, and is released via proteolytic cleavage of the GPI anchored ectodomain into the tubule where it coats the tubular lumen.325 In addition to this canonical apical targeting, UMOD also sorts, to lesser degree, to the basolateral domain of the tubular epithelial cells and is released into the interstitium where it enters the circulation.326 UMOD has been implicated in the pathophysiology of multiple human kidney diseases including polycystic kidney disease and nephrolithiasis, and may have a protective role in urinary tract infections.327–331 It has a tendency to form gels in aggregates, and this is increased by the presence of urinary constituents including albumin, sodium and calcium ions, as well as acidic pH.332 In patients with chronic renal failure, urinary UMOD has been shown to be decreased suggesting that serum UMOD levels may somehow reflect the amount of intact nephrons being linked to the “functional renal mass”.333,334 Long-term studies in elderly adults, demonstrated that low urine UMOD may identify persons at risk of progressive kidney disease and mortality.335,336 In a study comparing urine and plasma UMOD levels in patients with and without CKD, decreases in plasma UMOD paralleled decreases in eGFR and outperformed urinary UMOD in the identification of early stages of CKD, discrimination between non-CKD versus advanced CKD, and correlation with eGFR.8 Several studies have reported direct correlations between serum/plasma UMOD levels and kidney function.337–340 Because serum UMOD often correlates significantly with albuminuria, which is itself a strong predictor of CKD progression, UMOD may also play an important role in the prediction of CKD progression.341 One prospective study showed that low serum UMOD concentrations in CKD patients was associated with an increased risk of ESRD, independent of the traditional risk factors for progression of CKD.342 On the other hand, higher serum UMOD levels have been associated with better mortality outcomes for cardiovascular disease.341,343 Thus, the evidence suggests that high levels of urine and serum UMOD are associated with better kidney, cardiovascular disease, and mortality outcomes.329 Recent investigations in humans and transgenic mice demonstrated that UMOD inhibits the generation of reactive oxygen species in the kidney and systemically by suppressing transient receptor potential cation channel, subfamily M, member 2 (TRPM2) activity, which may explain why lower UMOD levels are linked to poor outcomes and increased mortality.344
Both animal and human studies suggest that UMOD may be involved in inflammation and progression of chronic urinary disease by entering the renal interstitium, either through basolateral secretion or urinary back-leakage in damaged tubules, and stimulating cells of the immune system.345 Rare mutations in UMOD can result in autosomal dominant tubulointerstitial kidney disease, leading to CKD.346 Another study showed that tubulointerstitial nephritis could be produced in rabbits by intravenous injection of urine or UMOD, and was the result of a predominately cellular immune response directed against UMOD.347 UMOD also has renoprotective effects, as shown in a mouse model of ischemia-reperfusion injury where UMOD knockout mice had more severe renal injury and increased toll-like receptor 4 (TLR4) expression that was localized on the basolateral rather than apical membrane of the proximal tubule S3 segment where it is normally expressed in wild-type mice.348 These findings suggest that UMOD protects the outer medulla during renal injury by reducing inflammation through decreased interaction between interstitial proinflammatory TLR4 ligands and the TLR4 receptor.348 However, in a rat model of pyelonephritis-induced AKI, urinary UMOD levels decreased, but paradoxically increased after 3 weeks, and the authors concluded urine UMOD was not a suitable biomarker in this model.263 In a few AKI studies in primates, urine UMOD was shown to decrease early following injury, but its use in chronic studies or CKD has not been reported for this species.170,349
In veterinary clinical practice, UMOD performance has been inconsistent in CKD. A few reports in dogs with chronic renal failure or urolithiasis have shown reduced renal excretion of UMOD.350–353 In dogs with stage 4 CKD, UMOD was undetectable or lower than controls in a long-term prospective study.354 In dogs with babesiosis, UMOD did not distinguish between diseased and normal dogs, and did not correlate with urine protein increases, in contrast with other biomarkers including KIM-1 and SDMA.171,355 In a study comparing dogs with various degrees of CKD, only the most azotemic and proteinuric animals showed significant decreases in urine UMOD compared with controls.356 However, in a recent study, decreased UMOD was observed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in stage 1 non-proteinuric CKD dogs, suggesting that UMOD may be an early biomarker of renal dysfunction in dogs.357 In a study of familial glomerular nephritis in Doberman dogs, the levels of UMOD changed throughout the course of disease such that UMOD levels increased above normal during the early course of nephropathy, but declined as disease progressed.358 Similar observations have been made in patients with early diabetes without GFR impairment, animal models of diabetes, and patients prior to the onset of CKD.8,341,359–361 However, as CKD progresses and GFR declines, continued fibrosis causes additional nephrons to drop out leading to decreased UMOD production.8,337,361
Various animal knockout and transgenic studies have been done to further characterize UMOD and its role in CKD.362 Recently, the use of urinary UMOD as a diagnostic biomarker of chronic renal disease in humans has been questioned since an analysis of UMOD in 77 CKD patients demonstrated that as many as 22% had normal urinary levels.333,362 Although more studies are required to fully understand the biological function of UMOD, the fact that UMOD levels may either increase or decrease following injury makes use of UMOD as a biomarker of CKD challenging. At present, UMOD alone may not be a candidate chronic renal biomarker for nonclinical toxicity studies, but it might add value in a panel of chronic analytes.
In summary, decreases in plasma UMOD have been shown to parallel decreases in eGFR and outperform urinary UMOD in the identification of early stages of CKD, discrimination between non-CKD versus advanced CKD, and correlation with eGFR. Because UMOD is produced exclusively in the thick ascending limb and early distal tubule, it may be decreased in chronic renal disease and aid in the identification of early stages of CKD.
Beta 2 Microglobulin (B2M)
Early in the search for renal biomarkers of AKI and tubule injury, urinary B2M was proposed as an effective biomarker in humans. B2M is a LMW protein that is found in every nucleated cell and forms the light chain component of class I histocompatibility antigens. It is normally filtered through the glomerulus and then almost entirely reabsorbed by the proximal tubules where it is subsequently catabolized into its constituent amino acids.363 B2M renal handling studies in rats364, dogs365 and humans366 suggested that extraction of B2M may also occur from the peritubular capillary circulation into proximal tubules; however, another study in rats was unable to show that the extraction rate of B2M exceeded the rate of filtration.367 B2M urine levels increase following proximal tubular damage due to causes ranging from cardiac surgery, renal transplantation or drug-induced renal injury.368 In a prospective study of 252 children, urine B2M, NGAL and KIM-1 all demonstrated good accuracy in predicting AKI in the emergency room situation.369
In rats, urine B2M is sensitive to functional alterations of the proximal tubules, and albumin to B2M ratios may be used to determine the origin of proteinuria since glomerular injury is expected to have a higher albumin to B2M ratio than tubular injury.370 Because urinary albumin levels increase 10-fold more than B2M in rats between the ages of 2 and 20 months, B2M may be of benefit in evaluation of renal injury in rats with chronic progressive nephropathy (CPN) since tubular function is thought to be preserved more than glomerular permeability in CPN.370 Over the past decade, urine B2M has been studied extensively and qualified for preclinical use in tubular and glomerular DIKI in rat.47,162,166 In a few cases, urine B2M was better able to detect glomerular injury than tubular damage in rats162, paralleling findings in human renal diseases with predominantly glomerular lesions (IgA nephropathy and systemic lupus erythematosus).371,372 Thus, caution is warranted when interpreting high urine B2M levels as increased filtered protein load may also be due to glomerular injury and competitive tubular uptake.349,373 B2M assays are not widely available for nonrodent laboratory species, and even in the rat, values can be highly variable,47,162,166 with normal values in males approximately 3 times those in females.114 B2M is degraded in acidic urine or after short periods at room temperature.374,375 A few studies have demonstrated increased concentrations of urine B2M via western blot in dogs with X-linked hereditary nephropathy and progressive renal disease, but B2M (as well as urine NGAL) was not helpful for monitoring mid- to late-stage renal failure, which was best monitored using retinol binding protein (RBP), another LMW protein.218,376 For these reasons, B2M has not been very successful or widely used in nonrodent laboratory animals or veterinary nephrology. Other LMW biomarkers such as alpha 1 microglobulin or RBP can be substituted to assess proteinuria of tubular origin in AKI, however many have not been evaluated in CKD. Despite these caveats, there is some evidence, mostly from the human literature, that urine B2M might provide some support for monitoring renal injury as an additive parameter in the clinic, e.g. with lithium-treatment, cadmium toxicity, and human immunodeficiency virus patients treated with tenofovir.377–379
B2M accumulates in the serum when GFR is impaired380; however, serum B2M concentrations can be increased in conditions other than CKD such as neoplastic and inflammatory conditions.381 Systolic blood pressure, age, weight, gender, total cholesterol, smoking, and urine protein excretion have been shown to be associated with increased serum B2M independent of GFR.70,382 In humans serum B2M has shown good correlation with eGFR, especially in genetic kidney diseases, but poor correlation with urine protein or albumin.383 In human nephrology, its use as a non-creatinine renal filtration marker has been surpassed by CysC. Similarly, serum B2M was outperformed by serum CysC in the detection of renal damage in dogs with CKD and other kidney diseases.384 This is because there are many non-renal diseases that can affect serum values and there are conflicting data about the relationship between B2M and disease progression.385,386 For example, catabolism in the kidney results in a very small percentage of B2M left in the filtrate under normal conditions, and altered cell binding from interaction with proteins that B2M chaperones in serum may cause fluctuations of serum B2M during periods of inflammation.385,386 Since it has been known for over 50 years that B2M can be catabolized in rat proximal tubules, there are additional reasons that it is not a good substitute for creatinine or CysC in GFR equations.364 These poorly understood factors may therefore affect both serum and subsequently urine levels387, and the degree of correlation between B2M and GFR varies greatly with the nephrotoxin studied.385,388
In human CKD, B2M increased with the stage of disease389, and could reliably predict GFR in many situations.390,391 However, in a recent meta-analysis by the CKD Biomarkers Consortium, it was determined that B2M and BTP (discussed below) did not provide “substantial additional prognostic information over eGFR-creatinine or albuminuria, but might be appropriate in certain circumstances where eGFR was inaccurate or albuminuria was unavailable”.392 Given the current data, we believe other biomarkers are better suited for use in monitoring chronic kidney injury in nonclinical safety studies although B2M may have added value in a panel of biomarkers in certain situations.
Beta Trace Protein (BTP)
BTP is also known as lipocalin-type prostaglandin D synthase. Glycoforms of varying molecular weights exist due to differences in post-translational glycosylation that occur between tissues.99,393–395 The smaller “brain” origin glycoform predominates in the cerebrospinal fluid (CSF); whereas, the larger “plasma” type glycoform predominates in serum and urine.394 BTP was identified first in human CSF396 and in rat brain.397,398,395,399 BTP represents almost 3% of the total CSF protein making it a useful diagnostic biomarker of CSF leakage.396,400 It is also found in smaller concentrations in serum, breast milk, aqueous and vitreous humor, seminal fluid, and amniotic fluid.401 In monkey, BTP was localized via in situ hybridization and immunohistochemistry to the proximal tubules, loop of Henle, and glomerulus.402 Similarly in a diabetic rat model, BTP was detected in the proximal tubules, but was not present in glomeruli.403 Unlike serum creatinine, BTP is not physiologically inert and has both ligand-binding and enzymatic properties. BTP binds with high affinity to a number of lipophilic substances including thyroid hormones, retinoids, β amyloid and bilirubin.399,404,405 It catalyzes the conversion of prostaglandin H2 to prostaglandin D2 (PGD2).399 PGD2 has several important physiologic functions including sleep regulation, nociception, inflammation, vasodilation, bronchoconstriction, platelet aggregation, bone remodeling and adipogenesis.99 Thus, there are many clinical conditions that could influence BTP levels.406–408 In addition, many of these non-GFR determinants of BTP are not included as variables in the eGFR equations.70,99,406 However, unlike serum creatinine which can be decreased with hepatic dysfunction due to reduced hepatic production of the precursor creatine, BTP concentrations are not affected by liver dysfunction.409
BTP is almost completely excreted by the kidneys. It is freely filtered by the glomeruli, reabsorbed and metabolized in the proximal tubule, with minimal tubular secretion.387,393,399,410,411 Unlike CysC and B2M, BTP is not completely reabsorbed by tubular cells and is detectable in urine of healthy individuals, which argues against tubular dysfunction as the sole cause of increased urinary levels.103,393,394 In humans, 90–100% of radiolabeled BTP was recovered in urine indicating almost total renal elimination.412 In a similar study in dogs, only 10.3% of intravenous radiolabeled BTP was recovered in the urine, suggesting that BTP was primarily degraded in the tissues.413 However, both studies demonstrated rapid elimination of BTP from the serum, with a half-life of <1 hour.412,413 The reason for the discordance between these two studies is not known. BTP is highly lipophilic with a distribution volume in the dog comparable to the volume of canine plasma indicating primarily distribution within the blood,413 but different BTP isoforms used in the two studies could have had different filtration properties.99 Others speculated that BTP could be metabolized by tubular cells or other cell types, so additional studies are needed to understand renal BTP handling.414
Serum BTP levels in humans were shown to have a good correlation with mGFR based on inulin clearance and nuclear medicine methods.415 BTP serum levels were found to be elevated in patients with decreased kidney function suggesting that this protein could be used as a diagnostic biomarker in kidney disease.394,416 Multiple studies showed that increased BTP in serum and urine correlated well with decreased GFR in patients with CKD, and that the increase was observed in the early stages of the disease.417–422 Studies of diverse patient groups including kidney transplant recipients, children, and patients with CKD found that BTP was better for detecting reduced GFR than serum creatinine423–425; whereas others did not.417,419,426,427 BTP has been reported to be associated with higher mortality in patients with CKD and ESRD.392,428–430 Studies suggest age387,417,427,431,432, muscle mass433, gender93, ethnicity434, serum albumin levels70, urine protein excretion70 and body weight406 may have an effect on BTP serum concentrations. BTP was not considered to be an appropriate biomarker of GFR in pregnancy435–437, but serum BTP for estimating GFR was reliable for assessment of kidney function in neonates.438 BTP is less affected by glucocorticoids than CysC as a biomarker of GFR in patients, especially after renal transplantation.395 However, high dose (but not low dose) steroid therapy has been shown to lower serum BTP.426,439 In studies of elderly patients comparing the novel eGFR equations to mGFR, the BTP equation was less precise and accurate than the creatinine/CysC equation.432,440 In other studies, the BTP equation alone or added to the creatinine/CysC equation did not improve upon mGFR.380,441,442 White et al.99 found that the BTP equations provided no clear advantage over the traditional creatinine-based equations in adults due to imprecision of the equations and substantial non-GFR-dependent influences on serum BTP concentrations. As predicted, in a recent study comparing multiple CKD-EPI-GFR equations against the gold standard of inulin clearance, the BTP and B2M equations displayed the worst performance with significantly less precision at all levels of GFR and did not improve GFR estimation when added to the creatinine/CysC equation.104 Taken together, these data suggest that increased serum BTP may occur due to non-renal pathophysiologic processes or biological functions of BTP beyond its association with GFR, therefore the utility of BTP for evaluation of chronic renal disease in patients is questionable.99
Limited information is available regarding serum, urine, or tissue BTP levels in normal or diseased animals.397,402,403,413,443 To the authors’ knowledge, neither urine nor serum BTP have been assessed in nonclinical studies. However, given the abundant human literature demonstrating its many issues, BTP is not likely as useful as CysC for GFR estimation. Based on current information, its use in animals for GFR estimation or as a biomarker of chronic DIKI cannot be recommended until further data regarding its utility in chronic renal toxicity in nonclinical species becomes available.
Other Alternative Methods for Assessing Chronic Renal Function
A variety of other biomarker modalities have been considered for noninvasive monitoring of CKD and chronic renal diseases including microRNA assessment444–446, metabolomics357,447, and peptidomics448–452, as well as biomarkers originally intended for detection of glomerular diseases (e.g. slit diaphragm proteins453–455). Unfortunately, while these approaches may have some utility in some chronic renal diseases in humans, they do not lend themselves to the high throughput and rapid analysis required for nonclinical GLP studies, nor can they be extrapolated to GFR estimation. Also, they provide no translational advantages in the nonclinical safety domain to help monitor potential DIKI in clinical trials. Such approaches, despite their promise in clinical CKD, are not discussed in this review.
Applications for Biomarkers of CKD
When it comes to renal biomarker implementation in nonclinical safety, the most frequent question from toxicologists and clinical pathologists is “In what situation should I utilize these biomarkers where they are most appropriate, and can they provide clinically translatable information without encumbering study logistics?” It is inappropriate to include these or any other analytes in a toxicity study simply because they are available. It is likely that these parameters will be part of a panel of renal biomarkers that include acute biomarkers such as urinary KIM-1, NGAL, and albumin to cover both acute and chronic renal events. However, there are specific situations where the judicious use of the SDMA, CysC, DKK3 or other assays discussed above may be valuable to clinicians overseeing patients in clinical trials. The first situation is probably the most obvious and common, but potentially has the most potential for overuse. This occurs when a potentially progressive tubulointerstitial kidney lesion is noted in 2 to 4-week acute or subacute toxicity studies in animals and there is concern that the lesion could progress to chronic renal failure. If the lesion initiated in the acute period, these chronic biomarkers can be evaluated in studies of 6 months or longer where NGAL or KIM-1 might no longer be increased since these biomarkers tend to wane over time. In other cases, a renal lesion is found that is very subtle when it is noted in 2 to 4-week studies and there is no meaningful increase in biomarkers such as urinary KIM-1, NGAL, or albumin. It is possible that progression could result in a more severe lesion by 3 or 6 months of dosing, and that a panel of renal biomarkers which would include both acute and chronic biomarkers could provide some confidence for clinicians that the lesion could be monitored in the clinic, particularly if the safety margin was small and the lesions occurred at or near clinical parity. It should be stressed that the clinical safety margin, nature of the lesion, and potential clinical translatability are all important factors in the decision to add chronic biomarkers in subsequent studies. If a nephrotoxic signal occurs only at animal doses that represent 20-fold or higher multiples of clinical exposure or was limited to non-tolerated doses resulting in morbidity in animals, there is little to be gained by adding any renal biomarkers in longer term animal studies. If the renal toxicity is represented by recurrent tubule degeneration and/or necrosis typical of AKI, then acute renal biomarkers are likely to be as effective in detecting renal injury as the novel biomarkers discussed above, and may be useful in the clinic to monitor for potential nephrotoxicity as additional nephrons undergo degenerative changes. In contrast, a very subtle lesion or changes that are distinct from AKI (e.g. fibrosis or atrophy as noted below) may be better candidates for inclusion of chronic renal functional biomarkers than acute injury biomarkers which are less likely to demonstrate modest structural changes.
As another example, a problematic gap in long-term safety evaluation occurs when the lesion is not typical of AKI and necrosis or degeneration are not the primary features. In this circumstance, most acute renal biomarkers would not be expected to change significantly. Examples include animal studies where drugs that have induced minimal interstitial fibrosis and tubular atrophy after several weeks of dosing may cause regulatory or clinical concern for progressive renal fibrosis in humans, or inflammatory conditions that raise concern for potential chronic interstitial nephritis in patients. The authors are familiar with two different proprietary drug candidates that induced mild fibrosis in definitive GLP studies in rats or dogs, but while the lesions were still present after 3 months, neither progressed in severity in longer-term chronic studies based on histopathology. However, regulatory concern over the lesions resulted in clinical holds and a project cancellation due to a lack of a reliable translatable biomarker in the clinic to augment eGFR alone. There are also examples of drugs which have induced transient vascular ischemia in the kidney causing very small infarcts at irregular and unpredictable intervals. In these cases, acute renal biomarkers could miss the lesion if sampling is not performed near the time of injury, and the long-term decline in renal function would be difficult to monitor or prevent due to minimal severity, renal compensatory changes, or sampling at a time that is distant from the peak injury or functional defect. With the trend among pharmaceutical companies for developing highly immunomodulatory molecules/biologics as anticancer therapeutics, we have noted a large number of compounds (proprietary information) which induce enhanced inflammatory infiltration into the renal interstitium replacing parenchyma as a function of suprapharmacology. There are similar examples in the literature, including lymphohistocytic inflammation reported in the kidneys of monkeys treated with an anti-cytotoxic T-lymphocyte-associated protein 4 antibody,456 and there are several clinical reports of acute interstitial nephritis associated with check point inhibitor administration.457–461 In many of these examples, urinary biomarkers have not been effective in identifying lesions except in the most severe cases, and regulatory concern has extended to the possibility that these may mimic the syndrome of drug-induced interstitial nephritis, which is life threatening in humans (albeit very rare) and generally not predicted in animals.462 Antisense oligonucleotide therapies, particularly of the 2’-O-methyl or 2’-O-methoxyethyl class, have often been associated with perivascular inflammatory infiltrates, and the kidney is one of the more common locations for this lesion.463,464 There have been several highly publicized regulatory issues in the kidney with these compounds, and the practical use of chronic renal biomarkers would help alleviate some of this regulatory concern.465
Applications for Biomarkers of Glomerular Injury
Another relatively common situation where the use of chronic renal biomarkers in nonclinical studies would be extremely beneficial to safety scientists overseeing clinical trials involves nephrotoxic compounds that affect the glomerulus rather than the tubules. While by far the most common glomerular toxicity encountered by nonclinical toxicologic pathologists is related to immune complex disease associated with the administration of humanized antibodies and peptides to nonhuman primates, this is not a situation that lends itself to the use of chronic renal biomarkers.465–467 The rapidly progressive glomerulonephritis (RPGN) produced by anti-drug antibody-related immune complexes are too fulminant to require chronic dosing to observe a signal, and these preclinical immune reactions are not predictive in humans.465 Also, most cases of immune-mediated glomerulonephritis due to drug or chemical treatment may have a rapid onset and fulminant course typical of human RPGN, thus human trials would not benefit from the addition of chronic renal biomarkers in longer term animal studies. In contrast, there are other types of glomerular disease in toxicologic studies that have a more protracted progression and where the clinical translatability is less clear, including glomerulosclerotic lesions and/or those that are related to intracellular accumulation of xenobiotic substances.465,468 In these situations, serial UPCR measurement along with surrogate biomarkers of GFR such as SDMA, CysC, or DKK3 could track the decline in renal function that accompanies glomerular injury enabling assessment of the development of the lesion in individual animals over time, instead of comparing microscopic lesions across dose groups at the end of dosing. While these analytes may be utilized directly as human biomarkers in clinical trials, the information derived from animal data can also be directly compared to alterations in eGFR in humans, which assesses the same factors. Thus, direct comparison of biomarker data or clinical translation would not be necessary.
Applications in Discovery Pathology
The final area of unexplored utility for these chronic renal biomarkers is in discovery pathology. Clinical pharmacology studies of renal impairment in human patients are often required in phase II or III trials when the intended target population for a drug includes patients with below normal renal function. In general, supportive studies in animal models of renal disease are not necessary to begin these trials. However, in early phases of candidate selection of potential drugs that may be intended for these renally impaired populations, it is quite common to compare candidate compounds or to run investigational studies with lead candidates in rodent renal models looking for efficacy in restoring or maintaining renal function, as well as eliminating candidates with unacceptable nephrotoxic profiles. In these situations, SMDA, DKK3, CysC, or even GFR measurement are an excellent means of determining potential effects on renal function over weeks or months. For some drug indications such as lysosomal storage diseases or polycystic kidney disease, serial use of these biomarkers in the same animal is probably the most critical area where information might be obtained that could have a direct effect on the prospects of drug success. Currently, histopathology and urine protein are the main parameters utilized in these long-term animal model studies to compare between drug candidates, but the variability of morphologic changes between individual animals often makes histopathologic assessment difficult and inadequate.
Summary and Conclusion
Estimation of GFR is a critical component in assessing chronic renal function in human patients and along with UPCR and UACR is the cornerstone of monitoring potential kidney adverse events during clinical trials. Unfortunately, GFR measurement is often logistically impractical to implement in nonclinical safety assessment studies, and eGFR calculations in animals are not yet available.83,84,106 Thus, surrogate analytes of chronic renal function are needed to provide a basis for reliable translation of potential nephrotoxic signals in chronic animal safety studies to clinical trials and for monitoring potential adverse renal events.
Biomarkers that have promising potential applicability in chronic nonclinical studies which may directly parallel changes in GFR currently include SDMA, CysC, and DKK3 alone or in combination in a panel of analytes along with proven AKI biomarkers such as KIM-1 or NGAL. These individual biomarkers and/or biomarker panels may provide substantial advantages over using KIM-1 or NGAL alone in studies > 3 months duration, increasing the sensitivity and specificity of current approaches. Other biomarkers such as UMOD or BTP may be included in a panel as additional parameters to support a weight-of-evidence approach in certain situations. This additional information on renal function may highlight potential clinical risk and provide a direct bridge to predict potential adverse declines in GFR in patients. Therefore, although there is a translational aspect to their use and they may be added to clinical trials to monitor changes observed in nonclinical studies, the fact that they can act as surrogate GFR biomarkers indicates that significant changes in SDMA, DKK3, CysC, KIM-1, and/or NGAL (with changes in urine protein) in chronic animal studies may reflect or predict possible changes in GFR in patients over time. Therefore, routine eGFR and UPCR measurements in clinical trial patients may be sufficient to provide confidence that chronic renal signals in animals are not occurring in clinical patients, even if the same biomarkers are not used in both animal and human studies. Additional toxicity studies with these exploratory biomarkers will demonstrate which of the alternative chronic renal biomarkers hold the most promise or, like acute renal biomarkers, if a panel of biomarkers will best monitor progression of chronic renal injury. The use of several of these chronic renal biomarkers can be implemented almost immediately in drug discovery or animal model studies and should provide a great benefit over current renal biomarkers for assessment of safety and efficacy of drugs intended to target CKD and inherited chronic renal diseases.
Supplementary Material
Acknowledgments
The authors thank Rick Adler (GSK), Karen Lynch (GSK), John Kreeger (Inotiv), Greg Travlos (NTP), and Michelle Cora (NTP) for their helpful review and suggestions concerning the manuscript.
Funding
This work was support in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (SE). The authors (LO, DE, KF) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential, real or perceived conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Redfern WS, Ewart E, Hammond TG, et al. Impact and frequency of different toxicities throughout the pharmaceutical life cycle. Toxicologist. 2010;114:1081. [Google Scholar]
- 2.Troth SP, Vlasakova K, Amur S, Amin RP, Glaab WE. Translational Safety Biomarkers of Kidney Injury. Semin Nephrol. 2019;39(2):202–214. [DOI] [PubMed] [Google Scholar]
- 3.Ennulat D, Ringenberg M, Frazier KS. Toxicologic Pathology Forum Opinion Paper*: Recommendations for a Tiered Approach to Nonclinical Mechanistic Nephrotoxicity Evaluation. Toxicol Pathol. 2018;46(6):636–646. [DOI] [PubMed] [Google Scholar]
- 4.Frazier KS, Ryan AM, Peterson RA, Obert LA. Kidney Pathology and Investigative Nephrotoxicology Strategies Across Species. Semin Nephrol. 2019;39(2):190–201. [DOI] [PubMed] [Google Scholar]
- 5.Hosten AO. BUN and Creatinine. In: rd, Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 6.Provenzano M, Rotundo S, Chiodini P, et al. Contribution of Predictive and Prognostic Biomarkers to Clinical Research on Chronic Kidney Disease. Int J Mol Sci. 2020;21(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum N, Dichoso CC, Carlton CE. Blood urea nitrogen and serum creatinine. Physiology and interpretations. Urology. 1975;5(5):583–588. [DOI] [PubMed] [Google Scholar]
- 8.Steubl D, Block M, Herbst V, et al. Plasma Uromodulin Correlates With Kidney Function and Identifies Early Stages in Chronic Kidney Disease Patients. Medicine (Baltimore). 2016;95(10):e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finco DR, Duncan JR. Evaluation of blood urea nitrogen and serum creatinine concentrations as indicators of renal dysfunction: a study of 111 cases and a review of related literature. J Am Vet Med Assoc. 1976;168(7):593–601. [PubMed] [Google Scholar]
- 10.Lee DH, Malat GE, Bias TE, Harhay MN, Ranganna K, Doyle AM. Serum creatinine elevation after switch to dolutegravir in a human immunodeficiency virus-positive kidney transplant recipient. Transpl Infect Dis. 2016;18(4):625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bargnoux A-S, Kuster N, Cavalier E, et al. Serum creatinine: advantages and pitfalls. Journal of Laboratory and Precision Medicine. 2018;3:71–71. [Google Scholar]
- 12.Nigam PK, Chandra A. Positive and negative false estimates of serum creatinine. Interventional-Cardiology. 2017;9(4):163–167. [Google Scholar]
- 13.Mehta AR. Why does the plasma urea concentration increase in acute dehydration? Adv Physiol Educ. 2008;32(4):336. [DOI] [PubMed] [Google Scholar]
- 14.Braun JP, Lefebvre HP, Watson AD. Creatinine in the dog: a review. Vet Clin Pathol. 2003;32(4):162–179. [DOI] [PubMed] [Google Scholar]
- 15.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. [DOI] [PubMed] [Google Scholar]
- 16.KDIGO. 2012 Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International. 2013;Supplement(3):136–150. [Google Scholar]
- 17.Nath KA, Salahudeen AK, Clark EC, Hostetter MK, Hostetter TH. Role of cellular metabolites in progressive renal injury. Kidney Int Suppl. 1992;38:S109–113. [PubMed] [Google Scholar]
- 18.Sawhney S, Mitchell M, Marks A, Fluck N, Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015;5(1):e006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takaori K, Nakamura J, Yamamoto S, et al. Severity and Frequency of Proximal Tubule Injury Determines Renal Prognosis. J Am Soc Nephrol. 2016;27(8):2393–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosch JP. Renal reserve: a functional view of glomerular filtration rate. Semin Nephrol. 1995;15(5):381–385. [PubMed] [Google Scholar]
- 21.Hsu CY, Xie D, Waikar SS, et al. Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int. 2017;91(1):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo-Rodriguez E, Fernandez-Prado R, Martin-Cleary C, et al. Kidney Injury Marker 1 and Neutrophil Gelatinase-Associated Lipocalin in Chronic Kidney Disease. Nephron. 2017;136(4):263–267. [DOI] [PubMed] [Google Scholar]
- 23.Coca SG. Kidney Injury Biomarkers with Clinical Utility: Has Godot Finally Arrived? Am J Nephrol. 2019;50(5):357–360. [DOI] [PubMed] [Google Scholar]
- 24.Schwab S, Marwitz T, Woitas RP. The role of prognostic assessment with biomarkers in chronic kidney disease: a narrative review. Journal of Laboratory and Precision Medicine,. 2018;3(12):7. [Google Scholar]
- 25.Ntrinias T, Papasotiriou M, Balta L, et al. Biomarkers in Progressive Chronic Kidney Disease. Still a Long Way to Go. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2019;40(3):27–39. [DOI] [PubMed] [Google Scholar]
- 26.Alderson HV, Ritchie JP, Pagano S, et al. The Associations of Blood Kidney Injury Molecule-1 and Neutrophil Gelatinase-Associated Lipocalin with Progression from CKD to ESRD. Clin J Am Soc Nephrol. 2016;11(12):2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seibert FS, Sitz M, Passfall J, et al. Prognostic Value of Urinary Calprotectin, NGAL and KIM-1 in Chronic Kidney Disease. Kidney Blood Press Res. 2018;43(4):1255–1262. [DOI] [PubMed] [Google Scholar]
- 28.De Silva PM, Mohammed Abdul KS, Eakanayake EM, et al. Urinary Biomarkers KIM-1 and NGAL for Detection of Chronic Kidney Disease of Uncertain Etiology (CKDu) among Agricultural Communities in Sri Lanka. PLoS Negl Trop Dis. 2016;10(9):e0004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang WR, Parikh CR. Biomarkers of Acute and Chronic Kidney Disease. Annu Rev Physiol. 2019;81:309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caplin B, Nitsch D. Urinary biomarkers of tubular injury in chronic kidney disease. Kidney Int. 2017;91(1):21–23. [DOI] [PubMed] [Google Scholar]
- 31.Rysz J, Gluba-Brzozka A, Franczyk B, Jablonowski Z, Cialkowska-Rysz A. Novel Biomarkers in the Diagnosis of Chronic Kidney Disease and the Prediction of Its Outcome. Int J Mol Sci. 2017;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernando B, Alli-Shaik A, Hemage RKD, et al. Pilot Study of Renal Urinary Biomarkers for Diagnosis of CKD of Uncertain Etiology. Kidney Int Rep. 2019;4(10):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardenas-Gonzalez M, Pavkovic M, Vaidya VS. 14.07 - Biomarkers of Acute Kidney Injury. In: McQueen CA, ed. Comprehensive Toxicology (Third Edition). Oxford: Elsevier; 2018:147–163. [Google Scholar]
- 34.Sistare FD, Dieterle F, Troth S, et al. Towards consensus practices to qualify safety biomarkers for use in early drug development. Nat Biotechnol. 2010;28(5):446–454. [DOI] [PubMed] [Google Scholar]
- 35.Dieterle F, Sistare F, Goodsaid F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28(5):455–462. [DOI] [PubMed] [Google Scholar]
- 36.FDA. Urinary Clusterin and Renal Papillary Antigen (RPA-1) Biomarker Qualification Letter. 2010:https://www.fda.gov/media/82521/download.
- 37.Sauer J-M, Walker EG, Porter AC. The Predictive Safety Testing Consortium: safety biomarkers, collaboration, and qualification. Journal of Medicines Development Sciences,. 2015;1(1):34–45. [Google Scholar]
- 38.FDA. List of qualified biomarkers. https://www.fda.gov/drugs/cder-biomarker-qualification-program/biomarker-qualification-submissions.
- 39.Harpur E, Ennulat D, Hoffman D, et al. Biological qualification of biomarkers of chemical-induced renal toxicity in two strains of male rat. Toxicol Sci. 2011;122(2):235–252. [DOI] [PubMed] [Google Scholar]
- 40.Phillips JA, Holder DJ, Ennulat D, et al. Rat Urinary Osteopontin and Neutrophil Gelatinase-Associated Lipocalin Improve Certainty of Detecting Drug-Induced Kidney Injury. Toxicol Sci. 2016;151(2):214–223. [DOI] [PubMed] [Google Scholar]
- 41.Wadey RM, Pinches MG, Jones HB, Riccardi D, Price SA. Tissue Expression and Correlation of a Panel of Urinary Biomarkers Following Cisplatin-induced Kidney Injury. Toxicologic Pathology. 2013;42(3):591–602. [DOI] [PubMed] [Google Scholar]
- 42.FDA. Qualification of biomarkers: clusterin (CLU), cystatin-C (CysC), kidney injury molecule-1 (KIM-1), N-acetyl- beta-D-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin (NGAL), and osteopontin (OPN). 2016:https://www.fda.gov/media/115671/download.
- 43.FDA. Letter of support for drug-induced (DIKI) renal tubular injury biomarker(s). 2016:https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM535972.pdf.
- 44.FDA. Center for Drug Evaluation and Research Qualification Determination Letter. 2018:https://www.fda.gov/media/119799/download.
- 45.Gu YZ, Vlasakova K, Troth SP, et al. Performance Assessment of New Urinary Translational Safety Biomarkers of Drug-induced Renal Tubular Injury in Tenofovir-treated Cynomolgus Monkeys and Beagle Dogs. Toxicol Pathol. 2018;46(5):553–563. [DOI] [PubMed] [Google Scholar]
- 46.Gui S, Gathiaka S, Li J, Qu J, Acevedo O, Hevel JM. A remodeled protein arginine methyltransferase 1 (PRMT1) generates symmetric dimethylarginine. J Biol Chem. 2014;289(13):9320–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlasakova K, Erdos Z, Troth SP, et al. Evaluation of the relative performance of 12 urinary biomarkers for renal safety across 22 rat sensitivity and specificity studies. Toxicol Sci. 2014;138(1):3–20. [DOI] [PubMed] [Google Scholar]
- 48.Vlasakova K, Troth SP, Sistare FD, Glaab WE. Evaluation of 10 Urinary Biomarkers for Renal Safety With 5 Nephrotoxicants in Nonhuman Primates. Toxicol Pathol. 2020;48(5):633–648. [DOI] [PubMed] [Google Scholar]
- 49.Pais GM, Avedissian SN, O’Donnell JN, et al. Comparative Performance of Urinary Biomarkers for Vancomycin-Induced Kidney Injury According to Timeline of Injury. Antimicrob Agents Chemother. 2019;63(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo QH, Chen ML, Chen ZL, et al. Evaluation of KIM-1 and NGAL as Early Indicators for Assessment of Gentamycin-Induced Nephrotoxicity In Vivo and In Vitro. Kidney Blood Press Res. 2016;41(6):911–918. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann D, Adler M, Vaidya VS, et al. Performance of novel kidney biomarkers in preclinical toxicity studies. Toxicol Sci. 2010;116(1):8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann D, Fuchs TC, Henzler T, et al. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology. 2010;277(1–3):49–58. [DOI] [PubMed] [Google Scholar]
- 53.White CA, Siegal D, Akbari A, Knoll GA. Use of kidney function end points in kidney transplant trials: a systematic review. Am J Kidney Dis. 2010;56(6):1140–1157. [DOI] [PubMed] [Google Scholar]
- 54.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821–835. [DOI] [PubMed] [Google Scholar]
- 55.Grams ME, Juraschek SP, Selvin E, et al. Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C-based estimates. Am J Kidney Dis. 2013;62(2):253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bueters R, Bael A, Gasthuys E, Chen C, Schreuder MF, Frazier KS. Ontogeny and Cross-species Comparison of Pathways Involved in Drug Absorption, Distribution, Metabolism, and Excretion in Neonates (Review): Kidney. Drug Metab Dispos. 2020;48(5):353–367. [DOI] [PubMed] [Google Scholar]
- 57.Levey AS, Inker LA. Assessment of Glomerular Filtration Rate in Health and Disease: A State of the Art Review. Clin Pharmacol Ther. 2017;102(3):405–419. [DOI] [PubMed] [Google Scholar]
- 58.Inker LA, Levey AS, Coresh J. Estimated Glomerular Filtration Rate From a Panel of Filtration Markers-Hope for Increased Accuracy Beyond Measured Glomerular Filtration Rate? Adv Chronic Kidney Dis. 2018;25(1):67–75. [DOI] [PubMed] [Google Scholar]
- 59.Benjamin A, da Costa AN, Delaunois A, Rosseels M-L, Valentin J-P. Renal Safety Pharmacology in Drug Discovery and Development. In: Pugsley MK, Curtis MJ, eds. Principles of Safety Pharmacology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015:323–352. [DOI] [PubMed] [Google Scholar]
- 60.Benjamin A, Nogueira da Costa A, Delaunois A, Rosseels ML, Valentin JP. Renal Safety Pharmacology in Drug Discovery and Development. Handb Exp Pharmacol. 2015;229:323–352. [DOI] [PubMed] [Google Scholar]
- 61.Redfern WS, Ewart LC, Lainée P, Pinches M, Robinson S, Valentin J-P. Functional assessments in repeat-dose toxicity studies: the art of the possible. Toxicology Research. 2013;2(4):209. [Google Scholar]
- 62.Scarfe L, Rak-Raszewska A, Geraci S, et al. Measures of kidney function by minimally invasive techniques correlate with histological glomerular damage in SCID mice with adriamycin-induced nephropathy. Sci Rep. 2015;5:13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedemann J, Heinrich R, Shulhevich Y, et al. Improved kinetic model for the transcutaneous measurement of glomerular filtration rate in experimental animals. Kidney Int. 2016;90(6):1377–1385. [DOI] [PubMed] [Google Scholar]
- 64.Wang E, Meier DJ, Sandoval RM, et al. A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals. Kidney Int. 2012;81(1):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porrini E, Ruggenenti P, Luis-Lima S, et al. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol. 2019;15(3):177–190. [DOI] [PubMed] [Google Scholar]
- 66.Porrini E, Ruggenenti P, Luis-Lima S, et al. Author Correction: Estimated GFR: time for a critical appraisal. Nat Rev Nephrol. 2019;15(2):121. [DOI] [PubMed] [Google Scholar]
- 67.Porrini E, Ruggenenti P, Luis-Lima S, et al. Reply to ‘Strengths and limitations of estimated and measured GFR’. Nat Rev Nephrol. 2019;15(12):785–786. [DOI] [PubMed] [Google Scholar]
- 68.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16(1):51–64. [DOI] [PubMed] [Google Scholar]
- 69.Foster MC, Levey AS, Inker LA, et al. Non-GFR Determinants of Low-Molecular-Weight Serum Protein Filtration Markers in the Elderly: AGES-Kidney and MESA-Kidney. Am J Kidney Dis. 2017;70(3):406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X, Foster MC, Tighiouart H, et al. Non-GFR Determinants of Low-Molecular-Weight Serum Protein Filtration Markers in CKD. Am J Kidney Dis. 2016;68(6):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steubl D, Inker LA. How best to estimate glomerular filtration rate? Novel filtration markers and their application. Curr Opin Nephrol Hypertens. 2018;27(6):398–405. [DOI] [PubMed] [Google Scholar]
- 73.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 74.Florkowski CM, Chew-Harris JS. Methods of Estimating GFR - Different Equations Including CKD-EPI. Clin Biochem Rev. 2011;32(2):75–79. [PMC free article] [PubMed] [Google Scholar]
- 75.Stevens LA, Padala S, Levey AS. Advances in glomerular filtration rate-estimating equations. Curr Opin Nephrol Hypertens. 2010;19(3):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coresh J, Stevens LA. Kidney function estimating equations: where do we stand? Curr Opin Nephrol Hypertens. 2006;15(3):276–284. [DOI] [PubMed] [Google Scholar]
- 78.Miller WG, Bachmann LM, Delanghe JR, Inker LA, Jones GRD, Vassalotti JA. Optimal Use of Biomarkers for Chronic Kidney Disease. Clin Chem. 2019;65(8):949–955. [DOI] [PubMed] [Google Scholar]
- 79.NKF. National Kidney Foundation, Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. GUIDELINE 5. ASSESSMENT OF PROTEINURIA 2002; http://kidneyfoundation.cachefly.net/professionals/KDOQI/guidelines_ckd/index.htm.
- 80.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delanaye P, Pottel H, Botev R, Inker LA, Levey AS. Con: Should we abandon the use of the MDRD equation in favour of the CKD-EPI equation? Nephrol Dial Transplant. 2013;28(6):1396–1403; discussion 1403. [DOI] [PubMed] [Google Scholar]
- 82.Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finch NC, Syme HM, Elliott J. Development of an estimated glomerular filtration rate formula in cats. J Vet Intern Med. 2018;32(6):1970–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McKenna M, Pelligand L, Elliott J, Walker D, Jepson R. Clinical utility of estimation of glomerular filtration rate in dogs. J Vet Intern Med. 2020;34(1):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Von Hendy-Willson VE, Pressler BM. An overview of glomerular filtration rate testing in dogs and cats. Vet J. 2011;188(2):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sadick M, Attenberger U, Kraenzlin B, et al. Two non-invasive GFR-estimation methods in rat models of polycystic kidney disease: 3.0 Tesla dynamic contrast-enhanced MRI and optical imaging. Nephrol Dial Transplant. 2011;26(10):3101–3108. [DOI] [PubMed] [Google Scholar]
- 87.Thomas D, Zachariah S, Elamin AEE, Hasim ALO. Limitations of serum creatinine as a marker of renal function. Scholars Academic Journal of Pharmacy. 2017;6(5):168–170. [Google Scholar]
- 88.Delanaye P, Cavalier E, Pottel H. Serum Creatinine: Not So Simple! Nephron. 2017;136(4):302–308. [DOI] [PubMed] [Google Scholar]
- 89.Wang K, Kestenbaum B. Proximal Tubular Secretory Clearance: A Neglected Partner of Kidney Function. Clin J Am Soc Nephrol. 2018;13(8):1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kher K, Mistry K. Assessment of glomerular and tubular function. Curr Pediatr Rev. 2014;10(2):142–150. [DOI] [PubMed] [Google Scholar]
- 91.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28(5):830–838. [DOI] [PubMed] [Google Scholar]
- 92.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–1953. [PubMed] [Google Scholar]
- 93.Juraschek SP, Coresh J, Inker LA, et al. Comparison of serum concentrations of beta-trace protein, beta2-microglobulin, cystatin C, and creatinine in the US population. Clin J Am Soc Nephrol. 2013;8(4):584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chu X, Bleasby K, Chan GH, Nunes I, Evers R. Transporters affecting biochemical test results: Creatinine-drug interactions. Clin Pharmacol Ther. 2016;100(5):437–440. [DOI] [PubMed] [Google Scholar]
- 95.Sjostrom PA, Odlind BG, Wolgast M. Extensive tubular secretion and reabsorption of creatinine in humans. Scand J Urol Nephrol. 1988;22(2):129–131. [DOI] [PubMed] [Google Scholar]
- 96.Chiou WL. Nonrenal Elimination, Extensive Renal Tubular Reabsorption and Secretion of Creatinine in Humans and Animals. Boston, MA: Springer; 1984. [Google Scholar]
- 97.Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem. 2012;58(4):680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hankins DA, Babb AL, Uvelli DA, Scribner BH. Creatinine degradation I: the kinetics of creatinine removal in patients with chronic kidney disease. Int J Artif Organs. 1981;4(1):35–39. [PubMed] [Google Scholar]
- 99.White CA, Ghazan-Shahi S, Adams MA. beta-Trace protein: a marker of GFR and other biological pathways. Am J Kidney Dis. 2015;65(1):131–146. [DOI] [PubMed] [Google Scholar]
- 100.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156(11):785–795, W-270, W-271, W-272, W-273, W-274, W-275, W-276, W-277, W-278. [DOI] [PubMed] [Google Scholar]
- 101.Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979;16(3):251–270. [DOI] [PubMed] [Google Scholar]
- 102.George JA, Gounden V. Novel glomerular filtration markers. Adv Clin Chem. 2019;88:91–119. [DOI] [PubMed] [Google Scholar]
- 103.Dajak M, Ignjatovic S, Stojimirovic B, Gajic S, Majkic-Singh N. Urinary beta-trace protein as a tubular marker of renal dysfunction in patients with chronic kidney disease. Clin Chim Acta. 2010;411(15–16):1154–1155. [DOI] [PubMed] [Google Scholar]
- 104.White CA, Allen CM, Akbari A, et al. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin Chim Acta. 2019;488:189–195. [DOI] [PubMed] [Google Scholar]
- 105.Foster MC, Inker LA, Hsu CY, et al. Filtration markers as predictors of ESRD and mortality in Southwestern American Indians with type 2 diabetes. Am J Kidney Dis. 2015;66(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Linnetz EH, Graves TK. Glomerular filtration rate in general small animal practice. Compend Contin Educ Vet. 2010;32(10):E1–5; quiz E6. [PubMed] [Google Scholar]
- 107.Frazier KS. Renal System. In: Steinbach TJ, Patrick DJ, Cozenza ME, eds. Toxicologic Pathology for Non-Pathologists. 1st ed. New York, NY: Humana Press; 2019:201–250. [Google Scholar]
- 108.Russo LM, Bakris GL, Comper WD. Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis. 2002;39(5):899–919. [DOI] [PubMed] [Google Scholar]
- 109.D’Aguilar SK, Skandhan A. Proteinuria: A Guide to Diagnosis and Assessment. Internal Medicine Open Journal. 2020;4(1):3–9. [Google Scholar]
- 110.Carroll MF, Temte JL. Proteinuria in adults: a diagnostic approach. Am Fam Physician. 2000;62(6):1333–1340. [PubMed] [Google Scholar]
- 111.Carter JL, Parker CT, Stevens PE, et al. Biological Variation of Plasma and Urinary Markers of Acute Kidney Injury in Patients with Chronic Kidney Disease. Clin Chem. 2016;62(6):876–883. [DOI] [PubMed] [Google Scholar]
- 112.Vaden SL. Glomerular disease. Top Companion Anim Med. 2011;26(3):128–134. [DOI] [PubMed] [Google Scholar]
- 113.Sumida K, Nadkarni GN, Grams ME, et al. Conversion of Urine Protein-Creatinine Ratio or Urine Dipstick Protein to Urine Albumin-Creatinine Ratio for Use in Chronic Kidney Disease Screening and Prognosis: An Individual Participant-Based Meta-analysis. Ann Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hard GC. Species comparison of the content and composition of urinary proteins. Food Chem Toxicol. 1995;33(9):731–746. [DOI] [PubMed] [Google Scholar]
- 115.Malard V, Gaillard JC, Berenguer F, Sage N, Quemeneur E. Urine proteomic profiling of uranium nephrotoxicity. Biochim Biophys Acta. 2009;1794(6):882–891. [DOI] [PubMed] [Google Scholar]
- 116.Vettorazzi A, Wait R, Nagy J, Monreal JI, Mantle P. Changes in male rat urinary protein profile during puberty: a pilot study. BMC Res Notes. 2013;6:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cavaggioni A, Mucignat-Caretta C. Major urinary proteins, alpha(2U)-globulins and aphrodisin. Biochim Biophys Acta. 2000;1482(1–2):218–228. [DOI] [PubMed] [Google Scholar]
- 118.Imafidon EC, Akomolafe RO, Oladele AA. Sexually dimorphic proteinuria in Wistar rats: Relevance to clinical models. Pathophysiology. 2016;23(1):51–59. [DOI] [PubMed] [Google Scholar]
- 119.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol. 1992;263(4 Pt 2):F601–606. [DOI] [PubMed] [Google Scholar]
- 120.Gudehithlu KP, Pegoraro AA, Dunea G, Arruda JA, Singh AK. Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kidney Int. 2004;65(6):2113–2122. [DOI] [PubMed] [Google Scholar]
- 121.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20(3):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuroda M, Fujikura D, Nanbo A, et al. Interaction between TIM-1 and NPC1 Is Important for Cellular Entry of Ebola Virus. J Virol. 2015;89(12):6481–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118(5):1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ichimura T, Brooks CR, Bonventre JV. Kim-1/Tim-1 and immune cells: shifting sands. Kidney Int. 2012;81(9):809–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rennert PD. Novel roles for TIM-1 in immunity and infection. Immunol Lett. 2011;141(1):28–35. [DOI] [PubMed] [Google Scholar]
- 126.Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care. 2010;16(6):556–561. [DOI] [PubMed] [Google Scholar]
- 127.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. [DOI] [PubMed] [Google Scholar]
- 128.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552–563. [DOI] [PubMed] [Google Scholar]
- 129.Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc. 2014;125:293–299; discussion 299. [PMC free article] [PubMed] [Google Scholar]
- 130.Prozialeck WC, Vaidya VS, Liu J, et al. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72(8):985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lim AI, Chan LY, Lai KN, et al. Distinct role of matrix metalloproteinase-3 in kidney injury molecule-1 shedding by kidney proximal tubular epithelial cells. Int J Biochem Cell Biol. 2012;44(6):1040–1050. [DOI] [PubMed] [Google Scholar]
- 132.Humphreys BD, Xu F, Sabbisetti V, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123(9):4023–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sayanthooran S, Magana-Arachchi DN, Gunerathne L, Abeysekera T. Potential diagnostic biomarkers for chronic kidney disease of unknown etiology (CKDu) in Sri Lanka: a pilot study. BMC Nephrol. 2017;18(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125(4):1620–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao X, Zhang Y, Li L, et al. Glomerular expression of kidney injury molecule-1 and podocytopenia in diabetic glomerulopathy. Am J Nephrol. 2011;34(3):268–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ko GJ, Grigoryev DN, Linfert D, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol. 2010;298(6):F1472–1483. [DOI] [PubMed] [Google Scholar]
- 137.Lim AI, Tang SC, Lai KN, Leung JC. Kidney injury molecule-1: more than just an injury marker of tubular epithelial cells? J Cell Physiol. 2013;228(5):917–924. [DOI] [PubMed] [Google Scholar]
- 138.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. [DOI] [PubMed] [Google Scholar]
- 139.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212(2):209–217. [DOI] [PubMed] [Google Scholar]
- 140.Huang Y, Tian Y, Likhodii S, Randell E. Baseline urinary KIM-1 concentration in detecting acute kidney injury should be interpreted with patient pre-existing nephropathy. Pract Lab Med. 2019;15:e00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Waanders F, Vaidya VS, van Goor H, et al. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. Am J Kidney Dis. 2009;53(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kramer AB, van Timmeren MM, Schuurs TA, et al. Reduction of proteinuria in adriamycin-induced nephropathy is associated with reduction of renal kidney injury molecule (Kim-1) over time. Am J Physiol Renal Physiol. 2009;296(5):F1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28(5):478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schroppel B, Kruger B, Walsh L, et al. Tubular expression of KIM-1 does not predict delayed function after transplantation. J Am Soc Nephrol. 2010;21(3):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu PC, Wei L, Shang WY, et al. Urinary kidney injury molecule-1 is related to pathologic involvement in IgA nephropathy with normotension, normal renal function and mild proteinuria. BMC Nephrol. 2014;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Timmeren MM, Bakker SJ, Vaidya VS, et al. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am J Physiol Renal Physiol. 2006;291(2):F456–464. [DOI] [PubMed] [Google Scholar]
- 147.Peralta CA, Katz R, Bonventre JV, et al. Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2012;60(6):904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jungbauer CG, Uecer E, Stadler S, et al. N-acteyl-ss-D-glucosaminidase and kidney injury molecule-1: New predictors for long-term progression of chronic kidney disease in patients with heart failure. Nephrology (Carlton). 2016;21(6):490–498. [DOI] [PubMed] [Google Scholar]
- 149.de Carvalho JA, Tatsch E, Hausen BS, et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin Biochem. 2016;49(3):232–236. [DOI] [PubMed] [Google Scholar]
- 150.Satirapoj B, Aramsaowapak K, Tangwonglert T, Supasyndh O. Novel Tubular Biomarkers Predict Renal Progression in Type 2 Diabetes Mellitus: A Prospective Cohort Study. J Diabetes Res. 2016;2016:3102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nielsen SE, Reinhard H, Zdunek D, et al. Tubular markers are associated with decline in kidney function in proteinuric type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97(1):71–76. [DOI] [PubMed] [Google Scholar]
- 152.Coca SG, Nadkarni GN, Huang Y, et al. Plasma Biomarkers and Kidney Function Decline in Early and Established Diabetic Kidney Disease. J Am Soc Nephrol. 2017;28(9):2786–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Conway BR, Manoharan D, Manoharan D, et al. Measuring urinary tubular biomarkers in type 2 diabetes does not add prognostic value beyond established risk factors. Kidney Int. 2012;82(7):812–818. [DOI] [PubMed] [Google Scholar]
- 154.Vaidya VS, Niewczas MA, Ficociello LH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-beta-D-glucosaminidase. Kidney Int. 2011;79(4):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou LT, Lv LL, Pan MM, et al. Are Urinary Tubular Injury Markers Useful in Chronic Kidney Disease? A Systematic Review and Meta Analysis. PLoS One. 2016;11(12):e0167334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ding Y, Nie LM, Pang Y, et al. Composite urinary biomarkers to predict pathological tubulointerstitial lesions in lupus nephritis. Lupus. 2018;27(11):1778–1789. [DOI] [PubMed] [Google Scholar]
- 157.Schulz CA, Engstrom G, Nilsson J, et al. Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol Dial Transplant. 2020;35(2):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sabbisetti VS, Waikar SS, Antoine DJ, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25(10):2177–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nowak N, Skupien J, Niewczas MA, et al. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int. 2016;89(2):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Colombo M, McGurnaghan SJ, Blackbourn LAK, et al. Comparison of serum and urinary biomarker panels with albumin/creatinine ratio in the prediction of renal function decline in type 1 diabetes. Diabetologia. 2020;63(4):788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ozer JS, Dieterle F, Troth S, et al. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol. 2010;28(5):486–494. [DOI] [PubMed] [Google Scholar]
- 162.Dieterle F, Perentes E, Cordier A, et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28(5):463–469. [DOI] [PubMed] [Google Scholar]
- 163.Rouse RL, Zhang J, Stewart SR, Rosenzweig BA, Espandiari P, Sadrieh NK. Comparative profile of commercially available urinary biomarkers in preclinical drug-induced kidney injury and recovery in rats. Kidney Int. 2011;79(11):1186–1197. [DOI] [PubMed] [Google Scholar]
- 164.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol. 2012;40(7):1031–1048. [DOI] [PubMed] [Google Scholar]
- 165.Burt D, Crowell SJ, Ackley DC, Magee TV, Aubrecht J. Application of emerging biomarkers of acute kidney injury in development of kidney-sparing polypeptide-based antibiotics. Drug Chem Toxicol. 2014;37(2):204–212. [DOI] [PubMed] [Google Scholar]
- 166.Kuwata K, Nakamura I, Ide M, Sato H, Nishikawa S, Tanaka M. Comparison of changes in urinary and blood levels of biomarkers associated with proximal tubular injury in rat models. J Toxicol Pathol. 2015;28(3):151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McDuffie JE, Lee S, Ma JY, Chen Y, Snook S. Acute biomarker panel changes associated with amphotericin B nephrotoxicity in female Sprague-Dawley rats. J Toxicol Sci. 2016;41(4):459–468. [DOI] [PubMed] [Google Scholar]
- 168.O’Donnell JN, Rhodes NJ, Miglis CM, et al. Dose, duration, and animal sex predict vancomycin-associated acute kidney injury in preclinical studies. Int J Antimicrob Agents. 2018;51(2):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen Y, Dale Thurman J, Kinter LB, Bialecki R, Eric McDuffie J. Perspectives on using a multiplex human kidney safety biomarker panel to detect cisplatin-induced tubular toxicity in male and female Cynomolgus monkeys. Toxicol Appl Pharmacol. 2017;336:66–74. [DOI] [PubMed] [Google Scholar]
- 170.Gautier JC, Zhou X, Yang Y, et al. Evaluation of novel biomarkers of nephrotoxicity in Cynomolgus monkeys treated with gentamicin. Toxicol Appl Pharmacol. 2016;303:1–10. [DOI] [PubMed] [Google Scholar]
- 171.Kules J, Bilic P, Beer Ljubic B, et al. Glomerular and tubular kidney damage markers in canine babesiosis caused by Babesia canis. Ticks Tick Borne Dis. 2018;9(6):1508–1517. [DOI] [PubMed] [Google Scholar]
- 172.Lippi I, Perondi F, Meucci V, Bruno B, Gazzano V, Guidi G. Clinical utility of urine kidney injury molecule-1 (KIM-1) and gamma-glutamyl transferase (GGT) in the diagnosis of canine acute kidney injury. Vet Res Commun. 2018;42(2):95–100. [DOI] [PubMed] [Google Scholar]
- 173.Wagoner MP, Yang Y, McDuffie JE, et al. Evaluation of Temporal Changes in Urine-based Metabolomic and Kidney Injury Markers to Detect Compound Induced Acute Kidney Tubular Toxicity in Beagle Dogs. Curr Top Med Chem. 2017;17(24):2767–2780. [DOI] [PubMed] [Google Scholar]
- 174.Sasaki A, Sasaki Y, Iwama R, et al. Comparison of renal biomarkers with glomerular filtration rate in susceptibility to the detection of gentamicin-induced acute kidney injury in dogs. J Comp Pathol. 2014;151(2–3):264–270. [DOI] [PubMed] [Google Scholar]
- 175.Davis J, Raisis AL, Miller DW, Rossi G. Validation of a commercial magnetic bead-based multiplex assay for 5 novel biomarkers of acute kidney injury in canine serum. J Vet Diagn Invest. 2020;32(5):656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bland SK, Cote O, Clark ME, DeLay J, Bienzle D. Characterization of kidney injury molecule-1 in cats. J Vet Intern Med. 2014;28(5):1454–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dong Y, Zhang Q, Wen J, et al. Ischemic Duration and Frequency Determines AKI-to-CKD Progression Monitored by Dynamic Changes of Tubular Biomarkers in IRI Mice. Front Physiol. 2019;10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jeong M, Kim YW, Min JR, et al. Kidney Toxicity Induced by 13 Weeks Exposure to the Fruiting Body of Paecilomyces sinclairii in Rats. Toxicol Res. 2012;28(3):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hussein AM, Malek HA, Saad MA. Renoprotective effects of aliskiren on adenine-induced tubulointerstitial nephropathy: possible underlying mechanisms. Can J Physiol Pharmacol. 2016;94(8):829–837. [DOI] [PubMed] [Google Scholar]
- 180.Succar L, Pianta TJ, Davidson T, Pickering JW, Endre ZH. Subclinical chronic kidney disease modifies the diagnosis of experimental acute kidney injury. Kidney Int. 2017;92(3):680–692. [DOI] [PubMed] [Google Scholar]
- 181.Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318 (Pt 1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang J, Blum A, Novak T, Levinson R, Lai E, Barasch J. An epithelial precursor is regulated by the ureteric bud and by the renal stroma. Dev Biol. 2002;246(2):296–310. [DOI] [PubMed] [Google Scholar]
- 183.Soni SS, Cruz D, Bobek I, et al. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42(1):141–150. [DOI] [PubMed] [Google Scholar]
- 184.Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clin Chim Acta. 2015;438:350–357. [DOI] [PubMed] [Google Scholar]
- 185.Nasioudis D, Witkin SS. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med Microbiol Immunol. 2015;204(4):471–479. [DOI] [PubMed] [Google Scholar]
- 186.Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–1056. [DOI] [PubMed] [Google Scholar]
- 187.Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2):407–413. [DOI] [PubMed] [Google Scholar]
- 188.Paragas N, Qiu A, Hollmen M, Nickolas TL, Devarajan P, Barasch J. NGAL-Siderocalin in kidney disease. Biochim Biophys Acta. 2012;1823(9):1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Viau A, El Karoui K, Laouari D, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120(11):4065–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63(5):1714–1724. [DOI] [PubMed] [Google Scholar]
- 191.Yuen PS, Jo SK, Holly MK, Hu X, Star RA. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics. 2006;25(3):375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24(3):307–315. [DOI] [PubMed] [Google Scholar]
- 193.Kai K, Yamaguchi T, Yoshimatsu Y, Kinoshita J, Teranishi M, Takasaki W. Neutrophil gelatinase-associated lipocalin, a sensitive urinary biomarker of acute kidney injury in dogs receiving gentamicin. J Toxicol Sci. 2013;38(2):269–277. [DOI] [PubMed] [Google Scholar]
- 194.Luo XQ, Yang X, Hu R, et al. [Effects of methyl cantharidimide tablets on urinary protein and enzymes in Beagle dogs]. Zhongguo Zhong Yao Za Zhi. 2014;39(22):4426–4429. [PubMed] [Google Scholar]
- 195.Zhou X, Ma B, Lin Z, et al. Evaluation of the usefulness of novel biomarkers for drug-induced acute kidney injury in beagle dogs. Toxicol Appl Pharmacol. 2014;280(1):30–35. [DOI] [PubMed] [Google Scholar]
- 196.Kuwabara T, Mori K, Mukoyama M, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75(3):285–294. [DOI] [PubMed] [Google Scholar]
- 197.Lee YJ, Hu YY, Lin YS, et al. Urine neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute canine kidney injury. BMC Vet Res. 2012;8:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Segev G, Palm C, LeRoy B, Cowgill LD, Westropp JL. Evaluation of neutrophil gelatinase-associated lipocalin as a marker of kidney injury in dogs. J Vet Intern Med. 2013;27(6):1362–1367. [DOI] [PubMed] [Google Scholar]
- 199.Hsu WL, Lin YS, Hu YY, Wong ML, Lin FY, Lee YJ. Neutrophil gelatinase-associated lipocalin in dogs with naturally occurring renal diseases. J Vet Intern Med. 2014;28(2):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Steinbach S, Weis J, Schweighauser A, Francey T, Neiger R. Plasma and urine neutrophil gelatinase-associated lipocalin (NGAL) in dogs with acute kidney injury or chronic kidney disease. J Vet Intern Med. 2014;28(2):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cortellini S, Pelligand L, Syme H, Chang YM, Adamantos S. Neutrophil Gelatinase-Associated Lipocalin in Dogs With Sepsis Undergoing Emergency Laparotomy: A Prospective Case-Control Study. J Vet Intern Med. 2015;29(6):1595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Izquierdo-Garcia JL, Nin N, Cardinal-Fernandez P, et al. Identification of novel metabolomic biomarkers in an experimental model of septic acute kidney injury. Am J Physiol Renal Physiol. 2019;316(1):F54–F62. [DOI] [PubMed] [Google Scholar]
- 203.Gil A, Brod V, Awad H, Heyman SN, Abassi Z, Frajewicki V. Neutrophil gelatinase-associated lipocalin in a triphasic rat model of adenine-induced kidney injury. Ren Fail. 2016;38(9):1448–1454. [DOI] [PubMed] [Google Scholar]
- 204.Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5(12):2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31(7):433–441. [DOI] [PubMed] [Google Scholar]
- 206.Wang W, Li Z, Chen Y, Wu H, Zhang S, Chen X. Prediction Value of Serum NGAL in the Diagnosis and Prognosis of Experimental Acute and Chronic Kidney Injuries. Biomolecules. 2020;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tuan PNH, Quyen DBQ, Van Khoa H, et al. Serum and Urine Neutrophil Gelatinase-Associated Lipocalin Levels Measured at Admission Predict Progression to Chronic Kidney Disease in Sepsis-Associated Acute Kidney Injury Patients. Dis Markers. 2020;2020:8883404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bolignano D, Coppolino G, Aloisi C, Romeo A, Nicocia G, Buemi M. Effect of a single intravenous immunoglobulin infusion on neutrophil gelatinase-associated lipocalin levels in proteinuric patients with normal renal function. J Investig Med. 2008;56(8):997–1003. [DOI] [PubMed] [Google Scholar]
- 209.Nickolas TL, Forster CS, Sise ME, et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012;82(6):718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bolignano D, Coppolino G, Campo S, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant. 2008;23(1):414–416. [DOI] [PubMed] [Google Scholar]
- 211.Gu YZ, Vlasakova K, Darbes J, et al. Urine kidney safety biomarkers improve understanding of indirect intra-renal injury potential in dogs with a drug-induced prerenal azotemia. Toxicology. 2020;439:152462. [DOI] [PubMed] [Google Scholar]
- 212.Smith ER, Lee D, Cai MM, et al. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD). Nephrol Dial Transplant. 2013;28(6):1569–1579. [DOI] [PubMed] [Google Scholar]
- 213.Duan S, Chen J, Wu L, et al. Assessment of urinary NGAL for differential diagnosis and progression of diabetic kidney disease. J Diabetes Complications. 2020:107665. [DOI] [PubMed] [Google Scholar]
- 214.Moriya H, Mochida Y, Ishioka K, et al. Plasma neutrophil gelatinase-associated lipocalin (NGAL) is an indicator of interstitial damage and a predictor of kidney function worsening of chronic kidney disease in the early stage: a pilot study. Clin Exp Nephrol. 2017;21(6):1053–1059. [DOI] [PubMed] [Google Scholar]
- 215.Bacci MR, Chehter EZ, Azzalis LA, Costa de Aguiar Alves B, Fonseca FLA. Serum NGAL and Cystatin C Comparison With Urinary Albumin-to-Creatinine Ratio and Inflammatory Biomarkers as Early Predictors of Renal Dysfunction in Patients With Type 2 Diabetes. Kidney Int Rep. 2017;2(2):152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22(1):101–108. [DOI] [PubMed] [Google Scholar]
- 217.Ahn HJ, Hyun C. Evaluation of serum neutrophil gelatinase-associated lipocalin (NGAL) activity in dogs with chronic kidney disease. Vet Rec. 2013;173(18):452. [DOI] [PubMed] [Google Scholar]
- 218.Nabity MB, Lees GE, Cianciolo R, Boggess MM, Steiner JM, Suchodolski JS. Urinary biomarkers of renal disease in dogs with X-linked hereditary nephropathy. J Vet Intern Med. 2012;26(2):282–293. [DOI] [PubMed] [Google Scholar]
- 219.Hokamp JA, Cianciolo RE, Boggess M, et al. Correlation of Urine and Serum Biomarkers with Renal Damage and Survival in Dogs with Naturally Occurring Proteinuric Chronic Kidney Disease. J Vet Intern Med. 2016;30(2):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hezzell MJ, Foster JD, Oyama MA, et al. Measurements of echocardiographic indices and biomarkers of kidney injury in dogs with chronic kidney disease. Vet J. 2020;255:105420. [DOI] [PubMed] [Google Scholar]
- 221.Hsu WL, Chiou HC, Tung KC, et al. The different molecular forms of urine neutrophil gelatinase-associated lipocalin present in dogs with urinary diseases. BMC Vet Res. 2014;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim YM, Polzin DJ, Rendahl A, Granick JL. Urinary neutrophil gelatinase-associated lipocalin in dogs with stable or progressive kidney disease. J Vet Intern Med. 2019;33(2):654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cobrin AR, Blois SL, Abrams-Ogg AC, et al. Neutrophil gelatinase-associated lipocalin in dogs with chronic kidney disease, carcinoma, lymphoma and endotoxaemia. J Small Anim Pract. 2016;57(6):291–298. [DOI] [PubMed] [Google Scholar]
- 224.Wang IC, Hsu WL, Wu PH, Yin HY, Tsai HJ, Lee YJ. Neutrophil Gelatinase-Associated Lipocalin in Cats with Naturally Occurring Chronic Kidney Disease. J Vet Intern Med. 2017;31(1):102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fuchs TC, Hewitt P. Preclinical perspective of urinary biomarkers for the detection of nephrotoxicity: what we know and what we need to know. Biomark Med. 2011;5(6):763–779. [DOI] [PubMed] [Google Scholar]
- 226.Marrer E, Dieterle F. Impact of biomarker development on drug safety assessment. Toxicol Appl Pharmacol. 2010;243(2):167–179. [DOI] [PubMed] [Google Scholar]
- 227.Ichino M, Kuroyanagi Y, Kusaka M, et al. Increased urinary neutrophil gelatinase associated lipocalin levels in a rat model of upper urinary tract infection. J Urol. 2009;181(5):2326–2331. [DOI] [PubMed] [Google Scholar]
- 228.Daure E, Belanger MC, Beauchamp G, Lapointe C. Elevation of neutrophil gelatinase-associated lipocalin (NGAL) in non-azotemic dogs with urinary tract infection. Res Vet Sci. 2013;95(3):1181–1185. [DOI] [PubMed] [Google Scholar]
- 229.Proverbio D, Spada E, Baggiani L, et al. Short communication: Relationship between urinary neutrophil gelatinase-associated lipocalin and noninfectious pyuria in dogs. Dis Markers. 2015;2015:387825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu PH, Hsu WL, Tsai PJ, Wu VC, Tsai HJ, Lee YJ. Identification of urine neutrophil gelatinase-associated lipocalin molecular forms and their association with different urinary diseases in cats. BMC Vet Res. 2019;15(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang Q, Davis KJ, Hoffmann D, Vaidya VS, Brown RP, Goering PL. Urinary biomarkers track the progression of nephropathy in hypertensive and obese rats. Biomark Med. 2014;8(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Uchino H, Fujishima J, Fukuoka K, et al. Usefulness of urinary biomarkers for nephrotoxicity in cynomolgus monkeys treated with gentamicin, cisplatin, and puromycin aminonucleoside. J Toxicol Sci. 2017;42(5):629–640. [DOI] [PubMed] [Google Scholar]
- 233.Grubb AO. Cystatin C--properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34. [DOI] [PubMed] [Google Scholar]
- 235.Chew JS, Saleem M, Florkowski CM, George PM. Cystatin C--a paradigm of evidence based laboratory medicine. Clin Biochem Rev. 2008;29(2):47–62. [PMC free article] [PubMed] [Google Scholar]
- 236.Togashi Y, Sakaguchi Y, Miyamoto M, Miyamoto Y. Urinary cystatin C as a biomarker for acute kidney injury and its immunohistochemical localization in kidney in the CDDP-treated rats. Exp Toxicol Pathol. 2012;64(7–8):797–805. [DOI] [PubMed] [Google Scholar]
- 237.Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74(8):1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56(5):409–414. [DOI] [PubMed] [Google Scholar]
- 239.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. [DOI] [PubMed] [Google Scholar]
- 240.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sjostrom P, Tidman M, Jones I. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. 2005;65(2):111–124. [DOI] [PubMed] [Google Scholar]
- 242.Madero M, Sarnak MJ. Association of cystatin C with adverse outcomes. Curr Opin Nephrol Hypertens. 2009;18(3):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65(4):1416–1421. [DOI] [PubMed] [Google Scholar]
- 244.Teo BW, Sabanayagam C, Liao J, et al. Comparison of CKD-EPI Cystatin C and Creatinine Glomerular Filtration Rate Estimation Equations in Asian Indians. Int J Nephrol. 2014;2014:746497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bokenkamp A, van Wijk JA, Lentze MJ, Stoffel-Wagner B. Effect of corticosteroid therapy on serum cystatin C and beta2-microglobulin concentrations. Clin Chem. 2002;48(7):1123–1126. [PubMed] [Google Scholar]
- 246.Kimmel M, Braun N, Alscher MD. Influence of thyroid function on different kidney function tests. Kidney Blood Press Res. 2012;35(1):9–17. [DOI] [PubMed] [Google Scholar]
- 247.Risch M, Purde MT, Baumann M, et al. High first-trimester maternal blood cystatin C levels despite normal serum creatinine predict pre-eclampsia in singleton pregnancies. Scand J Clin Lab Invest. 2017;77(8):634–643. [DOI] [PubMed] [Google Scholar]
- 248.Sjostrom PA, Jones IL, Tidman MA. Cystatin C as a filtration marker--haemodialysis patients expose its strengths and limitations. Scand J Clin Lab Invest. 2009;69(1):65–72. [DOI] [PubMed] [Google Scholar]
- 249.Vilar E, Boltiador C, Viljoen A, Machado A, Farrington K. Removal and rebound kinetics of cystatin C in high-flux hemodialysis and hemodiafiltration. Clin J Am Soc Nephrol. 2014;9(7):1240–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mussap M, Dalla Vestra M, Fioretto P, et al. Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int. 2002;61(4):1453–1461. [DOI] [PubMed] [Google Scholar]
- 251.Herget-Rosenthal S, Trabold S, Pietruck F, Holtmann M, Philipp T, Kribben A. Cystatin C: efficacy as screening test for reduced glomerular filtration rate. Am J Nephrol. 2000;20(2):97–102. [DOI] [PubMed] [Google Scholar]
- 252.Aksun SA, Ozmen D, Ozmen B, et al. Beta2-microglobulin and cystatin C in type 2 diabetes: assessment of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2004;112(4):195–200. [DOI] [PubMed] [Google Scholar]
- 253.Tangri N, Inker LA, Tighiouart H, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. 2012;23(2):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013;62(3):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Torner M, Mangal A, Scharnagl H, et al. Sex specificity of kidney markers to assess prognosis in cirrhotic patients with TIPS. Liver Int. 2020;40(1):186–193. [DOI] [PubMed] [Google Scholar]
- 256.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–246. [DOI] [PubMed] [Google Scholar]
- 257.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22(1):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305(15):1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.van der Laan SW, Fall T, Soumare A, et al. Cystatin C and Cardiovascular Disease: A Mendelian Randomization Study. J Am Coll Cardiol. 2016;68(9):934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Benoit SW, Ciccia EA, Devarajan P. Cystatin C as a biomarker of chronic kidney disease: latest developments. Expert Rev Mol Diagn. 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhang M, Cao X, Cai G, et al. Clinical evaluation of serum cystatin C and creatinine in patients with chronic kidney disease: a meta-analysis. J Int Med Res. 2013;41(4):944–955. [DOI] [PubMed] [Google Scholar]
- 263.Skowron B, Baranowska A, Dobrek L, et al. Urinary neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, uromodulin, and cystatin C concentrations in an experimental rat model of ascending acute kidney injury induced by pyelonephritis. J Physiol Pharmacol. 2018;69(4). [DOI] [PubMed] [Google Scholar]
- 264.Pianta TJ, Succar L, Davidson T, Buckley NA, Endre ZH. Monitoring treatment of acute kidney injury with damage biomarkers. Toxicol Lett. 2017;268:63–70. [DOI] [PubMed] [Google Scholar]
- 265.Ghys L, Paepe D, Smets P, Lefebvre H, Delanghe J, Daminet S. Cystatin C: a new renal marker and its potential use in small animal medicine. J Vet Intern Med. 2014;28(4):1152–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Almy FS, Christopher MM, King DP, Brown SA. Evaluation of cystatin C as an endogenous marker of glomerular filtration rate in dogs. J Vet Intern Med. 2002;16(1):45–51. [DOI] [PubMed] [Google Scholar]
- 267.Pasa S, Atasoy A, Derincegoz OO, Karul A. Serum Cystatin C Concentration as a Marker Acute Renal Dysfunction in Critically Ill Dogs. Journal of Animal and Veterinary Advances. 2008;7(11):1410–1412. [Google Scholar]
- 268.Wehner A, Hartmann K, Hirschberger J. Utility of serum cystatin C as a clinical measure of renal function in dogs. J Am Anim Hosp Assoc. 2008;44(3):131–138. [DOI] [PubMed] [Google Scholar]
- 269.Antognoni MT, Siepi D, Porciello F, Rueca F, Fruganti G. Serum cystatin-C evaluation in dogs affected by different diseases associated or not with renal insufficiency. Vet Res Commun. 2007;31 Suppl 1:269–271. [DOI] [PubMed] [Google Scholar]
- 270.Kim J, Lee CM, Kim HJ. Biomarkers for chronic kidney disease in dogs: a comparison study. J Vet Med Sci. 2020;82(8):1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Monti P, Benchekroun G, Berlato D, Archer J. Initial evaluation of canine urinary cystatin C as a marker of renal tubular function. J Small Anim Pract. 2012;53(5):254–259. [DOI] [PubMed] [Google Scholar]
- 272.Ghys LF, Meyer E, Paepe D, Delanghe J, Daminet S. Analytical validation of a human particle-enhanced nephelometric assay for cystatin C measurement in feline serum and urine. Vet Clin Pathol. 2014;43(2):226–234. [DOI] [PubMed] [Google Scholar]
- 273.Paepe D, Ghys LF, Smets P, Lefebvre HP, Croubels S, Daminet S. Routine kidney variables, glomerular filtration rate and urinary cystatin C in cats with diabetes mellitus, cats with chronic kidney disease and healthy cats. J Feline Med Surg. 2015;17(10):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Garcia-Martinez JD, Martinez-Subiela S, Tvarijonaviciute A, Caldin M, Ceron JJ. Urinary ferritin and cystatin C concentrations at different stages of kidney disease in leishmaniotic dogs. Res Vet Sci. 2015;99:204–207. [DOI] [PubMed] [Google Scholar]
- 275.Pagitz M, Frommlet F, Schwendenwein I. Evaluation of biological variance of cystatin C in comparison with other endogenous markers of glomerular filtration rate in healthy dogs. J Vet Intern Med. 2007;21(5):936–942. [DOI] [PubMed] [Google Scholar]
- 276.Keevil BG, Kilpatrick ES, Nichols SP, Maylor PW. Biological variation of cystatin C: implications for the assessment of glomerular filtration rate. Clin Chem. 1998;44(7):1535–1539. [PubMed] [Google Scholar]
- 277.Kakimoto Y, Akazawa S. Isolation and identification of N-G,N-G- and N-G,N’-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem. 1970;245(21):5751–5758. [PubMed] [Google Scholar]
- 278.Tang J, Frankel A, Cook RJ, et al. PRMT1 is the predominant type I protein arginine methyltransferase in mammalian cells. J Biol Chem. 2000;275(11):7723–7730. [DOI] [PubMed] [Google Scholar]
- 279.Tojo A, Welch WJ, Bremer V, et al. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int. 1997;52(6):1593–1601. [DOI] [PubMed] [Google Scholar]
- 280.Ogawa T, Kimoto M, Watanabe H, Sasaoka K. Metabolism of NG,NG-and NG,N’G-dimethylarginine in rats. Arch Biochem Biophys. 1987;252(2):526–537. [DOI] [PubMed] [Google Scholar]
- 281.Schwedhelm E, Boger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol. 2011;7(5):275–285. [DOI] [PubMed] [Google Scholar]
- 282.Tran CT, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atheroscler Suppl. 2003;4(4):33–40. [DOI] [PubMed] [Google Scholar]
- 283.Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function--a meta-analysis. Nephrol Dial Transplant. 2006;21(9):2446–2451. [DOI] [PubMed] [Google Scholar]
- 284.Marescau B, Nagels G, Possemiers I, et al. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism. 1997;46(9):1024–1031. [DOI] [PubMed] [Google Scholar]
- 285.El-Khoury JM, Bunch DR, Hu B, Payto D, Reineks EZ, Wang S. Comparison of symmetric dimethylarginine with creatinine, cystatin C and their eGFR equations as markers of kidney function. Clin Biochem. 2016;49(15):1140–1143. [DOI] [PubMed] [Google Scholar]
- 286.Pedersen LG, Tarnow I, Olsen LH, Teerlink T, Pedersen HD. Body size, but neither age nor asymptomatic mitral regurgitation, influences plasma concentrations of dimethylarginines in dogs. Res Vet Sci. 2006;80(3):336–342. [DOI] [PubMed] [Google Scholar]
- 287.Hall JA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med. 2014;28(6):1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hall JA, Yerramilli M, Obare E, Yerramilli M, Yu S, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L-carnitine, and medium-chain triglycerides. Vet J. 2014;202(3):588–596. [DOI] [PubMed] [Google Scholar]
- 289.Jepson RE, Syme HM, Vallance C, Elliott J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J Vet Intern Med. 2008;22(2):317–324. [DOI] [PubMed] [Google Scholar]
- 290.Relford R, Robertson J, Clements C. Symmetric Dimethylarginine: Improving the Diagnosis and Staging of Chronic Kidney Disease in Small Animals. Vet Clin North Am Small Anim Pract. 2016;46(6):941–960. [DOI] [PubMed] [Google Scholar]
- 291.Oliva-Damaso E, Oliva-Damaso N, Rodriguez-Esparragon F, et al. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int J Mol Sci. 2019;20(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Le Sueur ANV, Geraldes SS, Melchert A, et al. Symmetric dimethylarginine concentrations in dogs with International Renal Interest Society stage 4 chronic kidney disease undergoing intermittent hemodialysis. J Vet Intern Med. 2019;33(6):2635–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Guess SC, Yerramilli M, Obare EF, Grauer GF. Longitudinal Evaluation of Serum Symmetric Dimethylarginine (SDMA) and Serum Creatinine in Dogs Developing Chronic Kidney Disease. International Journal Applied Research Veterinary Medicine. 2018;16(2):122–130. [Google Scholar]
- 294.Braff J, Obare E, Yerramilli M, Elliott J, Yerramilli M. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med. 2014;28(6):1699–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hall JA, Yerramilli M, Obare E, Yerramilli M, Almes K, Jewell DE. Serum Concentrations of Symmetric Dimethylarginine and Creatinine in Dogs with Naturally Occurring Chronic Kidney Disease. J Vet Intern Med. 2016;30(3):794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Veldink H, Faulhaber-Walter R, Park JK, et al. Effects of chronic SDMA infusion on glomerular filtration rate, blood pressure, myocardial function and renal histology in C57BL6/J mice. Nephrol Dial Transplant. 2013;28(6):1434–1439. [DOI] [PubMed] [Google Scholar]
- 297.Blackwell S, O’Reilly DS, Reid D, Talwar D. Plasma dimethylarginines during the acute inflammatory response. Eur J Clin Invest. 2011;41(6):635–641. [DOI] [PubMed] [Google Scholar]
- 298.Meinitzer A, Kielstein JT, Pilz S, et al. Symmetrical and asymmetrical dimethylarginine as predictors for mortality in patients referred for coronary angiography: the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2011;57(1):112–121. [DOI] [PubMed] [Google Scholar]
- 299.Kielstein JT, Boger RH, Bode-Boger SM, et al. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol. 2002;13(1):170–176. [DOI] [PubMed] [Google Scholar]
- 300.Hall JA, Yerramilli M, Obare E, Yerramilli M, Melendez LD, Jewell DE. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med. 2015;29(3):808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kopke MA, Burchell RK, Ruaux CG, Burton SE, Lopez-Villalobos N, Gal A. Variability of Symmetric Dimethylarginine in Apparently Healthy Dogs. J Vet Intern Med. 2018;32(2):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Betz B, Moller-Ehrlich K, Kress T, et al. Increased symmetrical dimethylarginine in ischemic acute kidney injury as a causative factor of renal L-arginine deficiency. Transl Res. 2013;162(2):67–76. [DOI] [PubMed] [Google Scholar]
- 303.Tatematsu S, Wakino S, Kanda T, et al. Role of nitric oxide-producing and -degrading pathways in coronary endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2007;18(3):741–749. [DOI] [PubMed] [Google Scholar]
- 304.Al Banchaabouchi M, Marescau B, Possemiers I, D’Hooge R, Levillain O, De Deyn PP. NG, NG-dimethylarginine and NG, NG-dimethylarginine in renal insufficiency. Pflugers Arch. 2000;439(5):524–531. [DOI] [PubMed] [Google Scholar]
- 305.Carello KA, Whitesall SE, Lloyd MC, Billecke SS, D’Alecy LG. Asymmetrical dimethylarginine plasma clearance persists after acute total nephrectomy in rats. Am J Physiol Heart Circ Physiol. 2006;290(1):H209–216. [DOI] [PubMed] [Google Scholar]
- 306.Nabity MB. Traditional Renal Biomarkers and New Approaches to Diagnostics. Toxicol Pathol. 2018;46(8):999–1001. [DOI] [PubMed] [Google Scholar]
- 307.Nabity MB, Lees GE, Boggess MM, et al. Symmetric Dimethylarginine Assay Validation, Stability, and Evaluation as a Marker for the Early Detection of Chronic Kidney Disease in Dogs. J Vet Intern Med. 2015;29(4):1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Pelander L, Haggstrom J, Larsson A, et al. Comparison of the diagnostic value of symmetric dimethylarginine, cystatin C, and creatinine for detection of decreased glomerular filtration rate in dogs. J Vet Intern Med. 2019;33(2):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.McKenna M, Pelligand L, Elliott J, Cotter D, Jepson R. Relationship between serum iohexol clearance, serum SDMA concentration, and serum creatinine concentration in non-azotemic dogs. J Vet Intern Med. 2020;34(1):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ernst R, Ogeer J, McCrann D, et al. Comparative performance of IDEXX SDMA Test and the DLD SDMA ELISA for the measurement of SDMA in canine and feline serum. PLoS One. 2018;13(10):e0205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.IDEXX. SDMA Research Diagnostics An Introduction. 2019; https://www.idexxbioanalytics.com/hubfs/Discovery-Resource%20Materials/Patho/SDMA%20INFO%20SHEET%20v10F-1.pdf
- 312.Cross J, Prusevich P, Yerramilli M, Li J, Riley L, Hamlin D. Validation of a high-throughput immunoassay for the quantitation of symmetric dimethylarginine (SDMA) in rats. The Toxicologist: Supplement to Toxicological Sciences. 2017;156(1):Abstract no. 2027. [Google Scholar]
- 313.Federico G, Meister M, Mathow D, et al. Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight. 2016;1(1):e84916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Schunk SJ, Speer T, Petrakis I, Fliser D. Dickkopf 3-a novel biomarker of the ‘kidney injury continuum’. Nephrol Dial Transplant. 2020. [DOI] [PubMed] [Google Scholar]
- 315.Hong F, Hong J, Wang L, et al. Chronic exposure to nanoparticulate TiO2 causes renal fibrosis involving activation of the Wnt pathway in mouse kidney. J Agric Food Chem. 2015;63(5):1639–1647. [DOI] [PubMed] [Google Scholar]
- 316.Wong DW, Yiu WH, Wu HJ, et al. Downregulation of renal tubular Wnt/beta-catenin signaling by Dickkopf-3 induces tubular cell death in proteinuric nephropathy. Cell Death Dis. 2016;7:e2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Grone EF, Federico G, Nelson PJ, Arnold B, Grone HJ. The hormetic functions of Wnt pathways in tubular injury. Pflugers Arch. 2017;469(7–8):899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Schunk SJ, Zarbock A, Meersch M, et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet. 2019;394(10197):488–496. [DOI] [PubMed] [Google Scholar]
- 319.Zewinger S, Rauen T, Rudnicki M, et al. Dickkopf-3 (DKK3) in Urine Identifies Patients with Short-Term Risk of eGFR Loss. J Am Soc Nephrol. 2018;29(11):2722–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Inamoto Y, Martin PJ, Lee SJ, et al. Dickkopf-related protein 3 is a novel biomarker for chronic GVHD after allogeneic hematopoietic cell transplantation. Blood Adv. 2020;4(11):2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lipphardt M, Dihazi H, Jeon NL, et al. Dickkopf-3 in aberrant endothelial secretome triggers renal fibroblast activation and endothelial-mesenchymal transition. Nephrol Dial Transplant. 2019;34(1):49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yu B, Kiechl S, Qi D, et al. A Cytokine-Like Protein Dickkopf-Related Protein 3 Is Atheroprotective. Circulation. 2017;136(11):1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lynn KL, Shenkin A, Marshall RD. Factors affecting excretion of human urinary Tamm-Horsfall glycoprotein. Clin Sci (Lond). 1982;62(1):21–26. [DOI] [PubMed] [Google Scholar]
- 324.Rutecki GJ, Goldsmith C, Schreiner GE. Characterization of proteins in urinary casts. Fluorescent-antibody identification of Tamm-Horsfall mucoprotein in matrix and serum proteins in granules. N Engl J Med. 1971;284(19):1049–1052. [DOI] [PubMed] [Google Scholar]
- 325.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003;42(4):658–676. [DOI] [PubMed] [Google Scholar]
- 326.El-Achkar TM, McCracken R, Liu Y, et al. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol. 2013;304(8):F1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Carvalho M, Mulinari RA, Nakagawa Y. Role of Tamm-Horsfall protein and uromodulin in calcium oxalate crystallization. Braz J Med Biol Res. 2002;35(10):1165–1172. [DOI] [PubMed] [Google Scholar]
- 328.Eckardt KU, Alper SL, Antignac C, et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int. 2015;88(4):676–683. [DOI] [PubMed] [Google Scholar]
- 329.Micanovic R, LaFavers K, Garimella PS, Wu XR, El-Achkar TM. Uromodulin (Tamm-Horsfall protein): guardian of urinary and systemic homeostasis. Nephrol Dial Transplant. 2020;35(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Raffi HS, Bates JM Jr., Laszik Z, Kumar S. Tamm-horsfall protein protects against urinary tract infection by proteus mirabilis. J Urol. 2009;181(5):2332–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Garimella PS, Bartz TM, Ix JH, et al. Urinary Uromodulin and Risk of Urinary Tract Infections: The Cardiovascular Health Study. Am J Kidney Dis. 2017;69(6):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Kumar S, Muchmore A. Tamm-Horsfall protein--uromodulin (1950–1990). Kidney Int. 1990;37(6):1395–1401. [DOI] [PubMed] [Google Scholar]
- 333.Prajczer S, Heidenreich U, Pfaller W, Kotanko P, Lhotta K, Jennings P. Evidence for a role of uromodulin in chronic kidney disease progression. Nephrol Dial Transplant. 2010;25(6):1896–1903. [DOI] [PubMed] [Google Scholar]
- 334.Halankar A, SHalia K. Uromodulin Levels in Chronic Kidney Disease. International Journal of Biomedical and Advance Research. 2016;7(8):383–387. [Google Scholar]
- 335.Garimella PS, Biggs ML, Katz R, et al. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney Int. 2015;88(5):1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Garimella PS, Katz R, Ix JH, et al. Association of urinary uromodulin with kidney function decline and mortality: the health ABC study. Clin Nephrol. 2017;87(6):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Risch L, Lhotta K, Meier D, Medina-Escobar P, Nydegger UE, Risch M. The serum uromodulin level is associated with kidney function. Clin Chem Lab Med. 2014;52(12):1755–1761. [DOI] [PubMed] [Google Scholar]
- 338.Then C, Then HL, Lechner A, et al. Serum uromodulin and risk for cardiovascular morbidity and mortality in the community-based KORA F4 study. Atherosclerosis. 2020;297:1–7. [DOI] [PubMed] [Google Scholar]
- 339.Tachibana S, Iyoda M, Suzuki T, et al. Serum uromodulin is associated with the severity of clinicopathological findings in ANCA-associated glomerulonephritis. PLoS One. 2019;14(11):e0224690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Fedak D, Kuzniewski M, Fugiel A, et al. Serum uromodulin concentrations correlate with glomerular filtration rate in patients with chronic kidney disease. Pol Arch Med Wewn. 2016;126(12):995–1004. [DOI] [PubMed] [Google Scholar]
- 341.Leiherer A, Muendlein A, Saely CH, et al. The value of uromodulin as a new serum marker to predict decline in renal function. J Hypertens. 2018;36(1):110–118. [DOI] [PubMed] [Google Scholar]
- 342.Lv L, Wang J, Gao B, et al. Serum uromodulin and progression of kidney disease in patients with chronic kidney disease. J Transl Med. 2018;16(1):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Delgado GE, Kleber ME, Scharnagl H, Kramer BK, Marz W, Scherberich JE. Serum Uromodulin and Mortality Risk in Patients Undergoing Coronary Angiography. J Am Soc Nephrol. 2017;28(7):2201–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.LaFavers KA, Macedo E, Garimella PS, et al. Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci Transl Med. 2019;11(512). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lhotta K. Uromodulin and chronic kidney disease. Kidney Blood Press Res. 2010;33(5):393–398. [DOI] [PubMed] [Google Scholar]
- 346.Bleyer AJ, Hart PS, Kmoch S. Autosomal Dominant Tubulointerstitial Kidney Disease, UMOD-Related. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews((R)). Seattle (WA)1993. [Google Scholar]
- 347.Mayrer AR, Kashgarian M, Ruddle NH, et al. Tubulointerstitial nephritis and immunologic responses to Tamm-Horsfall protein in rabbits challenged with homologous urine or Tamm-Horsfall protein. J Immunol. 1982;128(6):2634–2642. [PubMed] [Google Scholar]
- 348.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008;295(2):F534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Chen H, Avital Y, Segev G. Biomarkers of Acute Kidney Injury. Israel Journal of Veterinary Medicine. 2017;72(1):3–12. [Google Scholar]
- 350.Raila J, Aupperle H, Raila G, Schoon HA, Schweigert FJ. Renal pathology and urinary protein excretion in a 14-month-old Bernese mountain dog with chronic renal failure. J Vet Med A Physiol Pathol Clin Med. 2007;54(3):131–135. [DOI] [PubMed] [Google Scholar]
- 351.Raila J, Forterre S, Kohn B, Brunnberg L, Schweigert FJ. Effects of chronic renal disease on the transport of vitamin A in plasma and urine of dogs. Am J Vet Res. 2003;64(7):874–879. [DOI] [PubMed] [Google Scholar]
- 352.Forterre S, Raila J, Schweigert FJ. Protein profiling of urine from dogs with renal disease using ProteinChip analysis. J Vet Diagn Invest. 2004;16(4):271–277. [DOI] [PubMed] [Google Scholar]
- 353.Argade S, Chen T, Shaw T, et al. An evaluation of Tamm-Horsfall protein glycans in kidney stone formers using novel techniques. Urolithiasis. 2015;43(4):303–312. [DOI] [PubMed] [Google Scholar]
- 354.Chacar F, Kogika M, Sanches TR, et al. Urinary Tamm-Horsfall protein, albumin, vitamin D-binding protein, and retinol-binding protein as early biomarkers of chronic kidney disease in dogs. Physiol Rep. 2017;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Winiarczyk D, Adaszek L, Bartnicki M, et al. Utility of urinary markers in the assessment of renal dysfunction in canine babesiosis. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2017;45(2):84–88. [DOI] [PubMed] [Google Scholar]
- 356.Raila J, Schweigert FJ, Kohn B. Relationship between urinary Tamm-Horsfall protein excretion and renal function in dogs with naturally occurring renal disease. Vet Clin Pathol. 2014;43(2):261–265. [DOI] [PubMed] [Google Scholar]
- 357.Ferlizza E, Isani G, Dondi F, et al. Urinary proteome and metabolome in dogs (Canis lupus familiaris): The effect of chronic kidney disease. J Proteomics. 2020;222:103795. [DOI] [PubMed] [Google Scholar]
- 358.Winiarczyk D, Adaszek L, Madany J, Winiarczyk M, Winiarczyk S. Utility of Urinary Markers in the Assessment of Renal Dysfunction in Familial Glomerulonephritis in Dobermann Dogs. J Vet Res. 2020;64(1):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Scherberich JE, Gruber R, Nockher WA, et al. Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol Dial Transplant. 2018;33(2):284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zimmerhackl LB, Pfleiderer S, Kinne R, Manz F, Schuler G, Brandis M. Tamm-Horsfall-Protein excretion as a marker of ascending limb transport indicates early renal tubular damage in diabetes mellitus type I. J Diabet Complications. 1991;5(2–3):112–114. [DOI] [PubMed] [Google Scholar]
- 361.Thornley C, Dawnay A, Cattell WR. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond). 1985;68(5):529–535. [DOI] [PubMed] [Google Scholar]
- 362.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011;80(4):338–347. [DOI] [PubMed] [Google Scholar]
- 363.Bethea M, Forman DT. Beta 2-microglobulin: its significance and clinical usefulness. Ann Clin Lab Sci. 1990;20(3):163–168. [PubMed] [Google Scholar]
- 364.Bernier GM, Conrad ME. Catabolsm of human beta-2-microglobulin by the rat kidney. Am J Physiol. 1969;217(5):1359–1362. [DOI] [PubMed] [Google Scholar]
- 365.Hall PW 3rd, Chung-Park M, Vacca CV, London M, Crowley AQ. The renal handling of beta 2-microglobulin in the dog. Kidney Int. 1982;22(2):156–161. [DOI] [PubMed] [Google Scholar]
- 366.Ravnskov U, Johansson BG, Gothlin J. Renal extraction of 2 -microglobulin. Scand J Clin Lab Invest. 1972;30(1):71–75. [DOI] [PubMed] [Google Scholar]
- 367.Fredriksson A. Renal handling of beta2-microglobulin in experimental renal disease. Scand J Clin Lab Invest. 1975;35(6):591–600. [DOI] [PubMed] [Google Scholar]
- 368.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Du Y, Zappitelli M, Mian A, et al. Urinary biomarkers to detect acute kidney injury in the pediatric emergency center. Pediatr Nephrol. 2011;26(2):267–274. [DOI] [PubMed] [Google Scholar]
- 370.Viau C, Bernard A, Ouled A, Lauwerys R. Determination of rat beta 2-microglobulin in urine and in serum. II. Application of its urinary measurement to selected nephrotoxicity models. J Appl Toxicol. 1986;6(3):191–195. [DOI] [PubMed] [Google Scholar]
- 371.Shin JR, Kim SM, Yoo JS, et al. Urinary excretion of beta2-microglobulin as a prognostic marker in immunoglobulin A nephropathy. Korean J Intern Med. 2014;29(3):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Choe JY, Park SH, Kim SK. Urine beta2-microglobulin is associated with clinical disease activity and renal involvement in female patients with systemic lupus erythematosus. Lupus. 2014;23(14):1486–1493. [DOI] [PubMed] [Google Scholar]
- 373.Thielemans N, Lauwerys R, Bernard A. Competition between albumin and low-molecular-weight proteins for renal tubular uptake in experimental nephropathies. Nephron. 1994;66(4):453–458. [DOI] [PubMed] [Google Scholar]
- 374.Ennulat D, Adler S. Recent successes in the identification, development, and qualification of translational biomarkers: the next generation of kidney injury biomarkers. Toxicol Pathol. 2015;43(1):62–69. [DOI] [PubMed] [Google Scholar]
- 375.Blumsohn A, Morris BW, Griffiths H, Ramsey CF. Stability of beta 2-microglobulin and retinol binding protein at different values of pH and temperature in normal and pathological urine. Clin Chim Acta. 1991;195(3):133–137. [DOI] [PubMed] [Google Scholar]
- 376.Vinge L, Lees GE, Nielsen R, Kashtan CE, Bahr A, Christensen EI. The effect of progressive glomerular disease on megalin-mediated endocytosis in the kidney. Nephrol Dial Transplant. 2010;25(8):2458–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Gatanaga H, Tachikawa N, Kikuchi Y, et al. Urinary beta2-microglobulin as a possible sensitive marker for renal injury caused by tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2006;22(8):744–748. [DOI] [PubMed] [Google Scholar]
- 378.Kim YD, Yim DH, Eom SY, et al. Temporal changes in urinary levels of cadmium, N-acetyl-beta-d-glucosaminidase and beta2-microglobulin in individuals in a cadmium-contaminated area. Environ Toxicol Pharmacol. 2015;39(1):35–41. [DOI] [PubMed] [Google Scholar]
- 379.Rybakowski JK, Abramowicz M, Chlopocka-Wozniak M, Czekalski S. Novel markers of kidney injury in bipolar patients on long-term lithium treatment. Hum Psychopharmacol. 2013;28(6):615–618. [DOI] [PubMed] [Google Scholar]
- 380.Inker LA, Tighiouart H, Coresh J, et al. GFR Estimation Using beta-Trace Protein and beta2-Microglobulin in CKD. Am J Kidney Dis. 2016;67(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Miyata T, Jadoul M, Kurokawa K, Van Ypersele de Strihou C. Beta-2 microglobulin in renal disease. J Am Soc Nephrol. 1998;9(9):1723–1735. [DOI] [PubMed] [Google Scholar]
- 382.Stanga Z, Nock S, Medina-Escobar P, Nydegger UE, Risch M, Risch L. Factors other than the glomerular filtration rate that determine the serum beta-2-microglobulin level. PLoS One. 2013;8(8):e72073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Karki M, Nanto-Salonen K, Niinikoski H, Tanner LM. Urine Beta2-Microglobulin Is an Early Marker of Renal Involvement in LPI. JIMD Rep. 2016;25:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Navarro L, Verde MT, Pardo M, Villaneuva S, Ferreira C. Comparison Between Cystatin C and Beta-2-Microglobulin as New Serum Biomarkers of Renal Disease in Dogs. Paper presented at: The 40th Congress of the World Small Animal Veterinary Association Proceeding Online2015; Zaragoza, Spain. [Google Scholar]
- 385.Argyropoulos CP, Chen SS, Ng YH, et al. Rediscovering Beta-2 Microglobulin As a Biomarker across the Spectrum of Kidney Diseases. Front Med (Lausanne). 2017;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Portman RJ, Kissane JM, Robson AM. Use of beta 2 microglobulin to diagnose tubulo-interstitial renal lesions in children. Kidney Int. 1986;30(1):91–98. [DOI] [PubMed] [Google Scholar]
- 387.Filler G, Priem F, Lepage N, et al. Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem. 2002;48(5):729–736. [PubMed] [Google Scholar]
- 388.Kaye WA, Griffiths WC, Camara PD, Trebbin WM, Solomon RJ, Diamond I. The significance of beta-2 microglobulinuria associated with gentamicin therapy. Ann Clin Lab Sci. 1981;11(6):530–537. [PubMed] [Google Scholar]
- 389.Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–1303. [DOI] [PubMed] [Google Scholar]
- 390.Astor BC, Shafi T, Hoogeveen RC, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59(5):653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Ikezumi Y, Honda M, Matsuyama T, et al. Establishment of a normal reference value for serum beta2 microglobulin in Japanese children: reevaluation of its clinical usefulness. Clin Exp Nephrol. 2013;17(1):99–105. [DOI] [PubMed] [Google Scholar]
- 392.Inker LA, Coresh J, Sang Y, et al. Filtration Markers as Predictors of ESRD and Mortality: Individual Participant Data Meta-Analysis. Clin J Am Soc Nephrol. 2017;12(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Hoffmann A, Conradt HS, Gross G, Nimtz M, Lottspeich F, Wurster U. Purification and chemical characterization of beta-trace protein from human cerebrospinal fluid: its identification as prostaglandin D synthase. J Neurochem. 1993;61(2):451–456. [DOI] [PubMed] [Google Scholar]
- 394.Hoffmann A, Nimtz M, Conradt HS. Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology. 1997;7(4):499–506. [DOI] [PubMed] [Google Scholar]
- 395.Orenes-Pinero E, Manzano-Fernandez S, Lopez-Cuenca A, Marin F, Valdes M, Januzzi JL. beta-Trace protein: from GFR marker to cardiovascular risk predictor. Clin J Am Soc Nephrol. 2013;8(5):873–881. [DOI] [PubMed] [Google Scholar]
- 396.Clausen J. Proteins in normal cerebrospinal fluid not found in serum. Proc Soc Exp Biol Med. 1961;107:170–172. [DOI] [PubMed] [Google Scholar]
- 397.Urade Y, Fujimoto N, Hayaishi O. Purification and characterization of rat brain prostaglandin D synthetase. J Biol Chem. 1985;260(23):12410–12415. [PubMed] [Google Scholar]
- 398.Watanabe K, Urade Y, Mader M, Murphy C, Hayaishi O. Identification of beta-trace as prostaglandin D synthase. Biochem Biophys Res Commun. 1994;203(2):1110–1116. [DOI] [PubMed] [Google Scholar]
- 399.Urade Y, Hayaishi O. Biochemical, structural, genetic, physiological, and pathophysiological features of lipocalin-type prostaglandin D synthase. Biochim Biophys Acta. 2000;1482(1–2):259–271. [DOI] [PubMed] [Google Scholar]
- 400.Risch L, Lisec I, Jutzi M, Podvinec M, Landolt H, Huber AR. Rapid, accurate and non-invasive detection of cerebrospinal fluid leakage using combined determination of beta-trace protein in secretion and serum. Clin Chim Acta. 2005;351(1–2):169–176. [DOI] [PubMed] [Google Scholar]
- 401.Melegos DN, Diamandis EP, Oda H, Urade Y, Hayaishi O. Immunofluorometric assay of prostaglandin D synthase in human tissue extracts and fluids. Clin Chem. 1996;42(12):1984–1991. [PubMed] [Google Scholar]
- 402.Nagata N, Fujimori K, Okazaki I, et al. De novo synthesis, uptake and proteolytic processing of lipocalin-type prostaglandin D synthase, beta-trace, in the kidneys. FEBS J. 2009;276(23):7146–7158. [DOI] [PubMed] [Google Scholar]
- 403.Ogawa M, Hirawa N, Tsuchida T, et al. Urinary excretions of lipocalin-type prostaglandin D2 synthase predict the development of proteinuria and renal injury in OLETF rats. Nephrol Dial Transplant. 2006;21(4):924–934. [DOI] [PubMed] [Google Scholar]
- 404.Tanaka T, Urade Y, Kimura H, Eguchi N, Nishikawa A, Hayaishi O. Lipocalin-type prostaglandin D synthase (beta-trace) is a newly recognized type of retinoid transporter. J Biol Chem. 1997;272(25):15789–15795. [DOI] [PubMed] [Google Scholar]
- 405.Beuckmann CT, Aoyagi M, Okazaki I, et al. Binding of biliverdin, bilirubin, and thyroid hormones to lipocalin-type prostaglandin D synthase. Biochemistry. 1999;38(25):8006–8013. [DOI] [PubMed] [Google Scholar]
- 406.White CA, Akbari A, Doucette S, et al. Effect of clinical variables and immunosuppression on serum cystatin C and beta-trace protein in kidney transplant recipients. Am J Kidney Dis. 2009;54(5):922–930. [DOI] [PubMed] [Google Scholar]
- 407.Gallant MA, Chamoux E, Bisson M, et al. Increased concentrations of prostaglandin D2 during post-fracture bone remodeling. J Rheumatol. 2010;37(3):644–649. [DOI] [PubMed] [Google Scholar]
- 408.Barcelo A, de la Pena M, Barbe F, Pierola J, Bosch M, Agusti AG. Prostaglandin D synthase (beta trace) levels in sleep apnea patients with and without sleepiness. Sleep Med. 2007;8(5):509–511. [DOI] [PubMed] [Google Scholar]
- 409.Chakraborty D, Akbari A, Knoll GA, et al. Serum BTP concentrations are not affected by hepatic dysfunction. BMC Nephrol. 2018;19(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Watanabe T, Narumiya S, Shimizu T, Hayaishi O. Characterization of the biosynthetic pathway of prostaglandin D2 in human platelet-rich plasma. J Biol Chem. 1982;257(24):14847–14853. [PubMed] [Google Scholar]
- 411.Bianchi C, Donadio C, Tramonti G, et al. High and preferential accumulation in the kidney of anionic and cationic small proteins. Contrib Nephrol. 1990;83:39–46. [DOI] [PubMed] [Google Scholar]
- 412.Olsson JE, Link H, Nosslin B. Metabolic studies on 125I-labelled beta-trace protein, with special reference to synthesis within the central nervous system. J Neurochem. 1973;21(5):1153–1159. [DOI] [PubMed] [Google Scholar]
- 413.Li W, Mase M, Inui T, et al. Pharmacokinetics of recombinant human lipocalin-type prostaglandin D synthase/beta-trace in canine. Neurosci Res. 2008;61(3):289–293. [DOI] [PubMed] [Google Scholar]
- 414.Shafi T, Pluznick JL. Renal Handling of beta-Trace Protein: Interpreting the Evidence. Am J Kidney Dis. 2015;65(6):967. [DOI] [PubMed] [Google Scholar]
- 415.Tramonti G, Ferdeghini M, Donadio C, et al. Renal function and serum concentration of five tumor markers (TATI, SCC, CYFRA 21–1, TPA, and TPS) in patients without evidence of neoplasia. Cancer Detect Prev. 2000;24(1):86–90. [PubMed] [Google Scholar]
- 416.Melegos DN, Grass L, Pierratos A, Diamandis EP. Highly elevated levels of prostaglandin D synthase in the serum of patients with renal failure. Urology. 1999;53(1):32–37. [DOI] [PubMed] [Google Scholar]
- 417.Donadio C, Lucchesi A, Ardini M, Donadio E, Giordani R. Serum levels of beta-trace protein and glomerular filtration rate--preliminary results. J Pharm Biomed Anal. 2003;32(4–5):1099–1104. [DOI] [PubMed] [Google Scholar]
- 418.Serum Donadio C. and urinary markers of early impairment of GFR in chronic kidney disease patients: diagnostic accuracy of urinary beta-trace protein. Am J Physiol Renal Physiol. 2010;299(6):F1407–1423. [DOI] [PubMed] [Google Scholar]
- 419.Spanaus KS, Kollerits B, Ritz E, et al. Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem. 2010;56(5):740–749. [DOI] [PubMed] [Google Scholar]
- 420.Donadio C, Bozzoli L. Urinary beta-trace protein: A unique biomarker to screen early glomerular filtration rate impairment. Medicine (Baltimore). 2016;95(49):e5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Bacci MR, Alves B, Cavallari MR, et al. Urinary beta-trace protein gene expression analysis in type 2 diabetes mellitus patients. Einstein (Sao Paulo). 2017;15(4):441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Kobata M, Shimizu A, Rinno H, et al. Beta-trace protein, a new marker of GFR, may predict the early prognostic stages of patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2004;18(4):237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Filler G, Kusserow C, Lopes L, Kobrzynski M. Beta-trace protein as a marker of GFR--history, indications, and future research. Clin Biochem. 2014;47(13–14):1188–1194. [DOI] [PubMed] [Google Scholar]
- 424.Priem F, Althaus H, Birnbaum M, Sinha P, Conradt HS, Jung K. Beta-trace protein in serum: a new marker of glomerular filtration rate in the creatinine-blind range. Clin Chem. 1999;45(4):567–568. [PubMed] [Google Scholar]
- 425.Giessing M Beta-trace protein as indicator of glomerular filtration rate. Urology. 1999;54(5):940–941. [PubMed] [Google Scholar]
- 426.Poge U, Gerhardt TM, Stoffel-Wagner B, et al. beta-Trace protein is an alternative marker for glomerular filtration rate in renal transplantation patients. Clin Chem. 2005;51(8):1531–1533. [DOI] [PubMed] [Google Scholar]
- 427.Woitas RP, Stoffel-Wagner B, Poege U, Schiedermaier P, Spengler U, Sauerbruch T. Low-molecular weight proteins as markers for glomerular filtration rate. Clin Chem. 2001;47(12):2179–2180. [PubMed] [Google Scholar]
- 428.Foster MC, Coresh J, Hsu CY, et al. Serum beta-Trace Protein and beta2-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults With CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2016;68(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Shafi T, Parekh RS, Jaar BG, et al. Serum beta-trace protein and risk of mortality in incident hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(9):1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Bhavsar NA, Appel LJ, Kusek JW, et al. Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis. 2011;58(6):886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Bokenkamp A, Franke I, Schlieber M, et al. Beta-trace protein--a marker of kidney function in children: “Original research communication-clinical investigation”. Clin Biochem. 2007;40(13–14):969–975. [DOI] [PubMed] [Google Scholar]
- 432.Werner K, Pihlsgard M, Elmstahl S, Legrand H, Nyman U, Christensson A. Combining Cystatin C and Creatinine Yields a Reliable Glomerular Filtration Rate Estimation in Older Adults in Contrast to beta-Trace Protein and beta2-Microglobulin. Nephron. 2017;137(1):29–37. [DOI] [PubMed] [Google Scholar]
- 433.Pham-Huy A, Leonard M, Lepage N, Halton J, Filler G. Measuring glomerular filtration rate with cystatin C and beta-trace protein in children with spina bifida. J Urol. 2003;169(6):2312–2315. [DOI] [PubMed] [Google Scholar]
- 434.Tin A, Astor BC, Boerwinkle E, Hoogeveen RC, Coresh J, Kao WH. Genome-wide significant locus of beta-trace protein, a novel kidney function biomarker, identified in European and African Americans. Nephrol Dial Transplant. 2013;28(6):1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Asgeirsson D, Venturoli D, Rippe B, Rippe C. Increased glomerular permeability to negatively charged Ficoll relative to neutral Ficoll in rats. Am J Physiol Renal Physiol. 2006;291(5):F1083–1089. [DOI] [PubMed] [Google Scholar]
- 436.Deen WM. Cellular contributions to glomerular size-selectivity. Kidney Int. 2006;69(8):1295–1297. [DOI] [PubMed] [Google Scholar]
- 437.Roberts M, Lindheimer MD, Davison JM. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol. 1996;270(2 Pt 2):F338–343. [DOI] [PubMed] [Google Scholar]
- 438.Khosravi N, Asgari M, Khalessi N, Hoseini R, Khosravi N. Serum Beta-Trace Protein for Assessment of Kidney Function in Neonates. Iran J Kidney Dis. 2018;12(1):11–13. [PubMed] [Google Scholar]
- 439.Abbink FC, Laarman CA, Braam KI, et al. Beta-trace protein is not superior to cystatin C for the estimation of GFR in patients receiving corticosteroids. Clin Biochem. 2008;41(4–5):299–305. [DOI] [PubMed] [Google Scholar]
- 440.Ebert N, Koep C, Schwarz K, et al. Beta Trace Protein does not outperform Creatinine and Cystatin C in estimating Glomerular Filtration Rate in Older Adults. Sci Rep. 2017;7(1):12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Pottel H, Schaeffner E, Ebert N. Evaluating the diagnostic value of rescaled beta-trace protein in combination with serum creatinine and serum cystatin C in older adults. Clin Chim Acta. 2018;480:206–213. [DOI] [PubMed] [Google Scholar]
- 442.den Bakker E, Gemke R, Pottel H, et al. Estimation of GFR in children using rescaled beta-trace protein. Clin Chim Acta. 2018;486:259–264. [DOI] [PubMed] [Google Scholar]
- 443.Song WL, Wang M, Ricciotti E, et al. Tetranor PGDM, an abundant urinary metabolite reflects biosynthesis of prostaglandin D2 in mice and humans. J Biol Chem. 2008;283(2):1179–1188. [DOI] [PubMed] [Google Scholar]
- 444.Nassirpour R, Raj D, Townsend R, Argyropoulos C. MicroRNA biomarkers in clinical renal disease: from diabetic nephropathy renal transplantation and beyond. Food Chem Toxicol. 2016;98(Pt A):73–88. [DOI] [PubMed] [Google Scholar]
- 445.Ichii O, Ohta H, Horino T, et al. Urinary exosome-derived microRNAs reflecting the changes of renal function and histopathology in dogs. Sci Rep. 2017;7:40340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Khurana R, Ranches G, Schafferer S, et al. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA. 2017;23(2):142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Davies R. The metabolomic quest for a biomarker in chronic kidney disease. Clin Kidney J. 2018;11(5):694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Pontillo C, Zhang ZY, Schanstra JP, et al. Prediction of Chronic Kidney Disease Stage 3 by CKD273, a Urinary Proteomic Biomarker. Kidney Int Rep. 2017;2(6):1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Pontillo C, Mischak H. Urinary peptide-based classifier CKD273: towards clinical application in chronic kidney disease. Clin Kidney J. 2017;10(2):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Chen L, Smith J, Mikl J, et al. A Multiplatform Approach for the Discovery of Novel Drug-Induced Kidney Injury Biomarkers. Chem Res Toxicol. 2017;30(10):1823–1834. [DOI] [PubMed] [Google Scholar]
- 451.Ferlizza E, Campos A, Neagu A, et al. The effect of chronic kidney disease on the urine proteome in the domestic cat (Felis catus). Vet J. 2015;204(1):73–81. [DOI] [PubMed] [Google Scholar]
- 452.Zurbig P, Siwy J, Mischak H. Emerging urine-based proteomic biomarkers as valuable tools in the management of chronic kidney disease. Expert Rev Mol Diagn. 2019;19(10):853–856. [DOI] [PubMed] [Google Scholar]
- 453.da Silva CA, Araujo LS, Dos Reis Monteiro MLG, et al. Evaluation of the Diagnostic Potential of uPAR as a Biomarker in Renal Biopsies of Patients with FSGS. Dis Markers. 2019;2019:1070495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Kwon SK, Kim SJ, Kim HY. Urine synaptopodin excretion is an important marker of glomerular disease progression. Korean J Intern Med. 2016;31(5):938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Sekulic M, Pichler Sekulic S. A compendium of urinary biomarkers indicative of glomerular podocytopathy. Patholog Res Int. 2013;2013:782395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Price KD, Simutis F, Fletcher A, et al. Nonclinical safety evaluation of two distinct second generation variants of anti-CTLA4 monoclonal antibody, ipilimumab, in monkeys [abstract]. In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; Oct 26–30, 2017 2018; Philadelphia, PA. [Google Scholar]
- 457.Ryuzaki M, Tokuyama H, Uchiyama K, et al. Acute Interstitial Nephritis With Karyomegalic Epithelial Cells After Nivolumab Treatment-Two Case Reports. Clin Med Insights Case Rep. 2019;12:1179547619853647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Shirali AC, Perazella MA, Gettinger S. Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am J Kidney Dis. 2016;68(2):287–291. [DOI] [PubMed] [Google Scholar]
- 459.Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90(3):638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Perazella MA, Nolin TD. Adverse Drug Effects in Patients with CKD: Primum Non Nocere. Clin J Am Soc Nephrol. 2020;15(8):1075–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Perazella MA, Shirali AC. Nephrotoxicity of Cancer Immunotherapies: Past, Present and Future. J Am Soc Nephrol. 2018;29(8):2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Frazier KS, Seely JC, Hard GC, et al. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 2012;40(4 Suppl):14S–86S. [DOI] [PubMed] [Google Scholar]
- 463.Frazier KS, Sobry C, Derr V, et al. Species-specific inflammatory responses as a primary component for the development of glomerular lesions in mice and monkeys following chronic administration of a second-generation antisense oligonucleotide. Toxicol Pathol. 2014;42(5):923–935. [DOI] [PubMed] [Google Scholar]
- 464.Frazier KS. Antisense oligonucleotide therapies: the promise and the challenges from a toxicologic pathologist’s perspective. Toxicol Pathol. 2015;43(1):78–89. [DOI] [PubMed] [Google Scholar]
- 465.Frazier KS, Obert LA. Drug-induced Glomerulonephritis: The Spectre of Biotherapeutic and Antisense Oligonucleotide Immune Activation in the Kidney. Toxicol Pathol. 2018;46(8):904–917. [DOI] [PubMed] [Google Scholar]
- 466.Naylor SW, Czajkowski M, Harvey W, Smith M, Bradley AE, Cary M. Histopathological Findings in Cynomolgus Macaques (Macaca fascicularis) Consistent with Secondary Immunological Reaction to Biotherapeutics with an Emphasis on the CNS and Eye. Toxicol Pathol. 2019;47(2):165–173. [DOI] [PubMed] [Google Scholar]
- 467.Rojko JL, Evans MG, Price SA, et al. Formation, clearance, deposition, pathogenicity, and identification of biopharmaceutical-related immune complexes: review and case studies. Toxicol Pathol. 2014;42(4):725–764. [DOI] [PubMed] [Google Scholar]
- 468.Frazier KS. Species Differences in Renal Development and Associated Developmental Nephrotoxicity. Birth Defects Res. 2017;109(16):1243–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


