Abstract
Summary
This study estimates causality of physical activity (PA) on bone mineral density (BMD) by conducting multivariable Mendelian randomization (MR). The findings suggest that habitual vigorous PA increases lumbar spine BMD, and higher overall acceleration average would improve forearm BMD. The results could promote PA intervention targeting individuals with optimized type.
Introduction
Evidence from epidemiologic studies showed type, frequency, and duration of PA influenced BMD. However, these observational studies may be confounded by many factors, resulting in spurious associations. We aimed to conduct multivariable MR to estimate the causal effect of self-reported and device-measured PA on osteoporosis.
Methods
Three self-reported and two device-measured PA-related traits were selected as exposures. Outcomes were BMD at different skeletal sites: femoral neck BMD (FN BMD), lumbar spine BMD (LS BMD), and forearm BMD (FA BMD). Exposure datasets were obtained from UK Biobank with total 377,234 subjects. Outcome datasets were obtained from GEFOS consortium with 53,236 subjects. Standard MR analysis and multivariable MR were conducted to assess the total and direct causal effect of PA on BMD.
Results
For self-reported PA, inverse-normalized moderate-to-vigorous had a direct causal effect on FN BMD independently (β = − 1.116 (95% confidence interval, 95%CI: − 2.210, − 0.023), P = 0.045); vigorous PA showed a direct effect (β = 3.592 (95%CI: 0.310, 6.874), P = 0.032) on LS BMD independently. While overall acceleration average and fraction of accelerations both had a direct causal effect on FA BMD independently.
Conclusions
Habitual vigorous PA could increase LS BMD. Individuals with higher overall acceleration average would have a higher FA BMD.
Keywords: Bone mineral density, Causal effect, Multivariable Mendelian randomization, Physical activity
Introduction
Osteoporosis is the most common metabolic bone disease, especially in the elderly population. The main characteristics of osteoporosis are decreased bone mineral density (BMD) and an increased risk to hip and other fractures, which are associated with increased mortality, concomitant morbidity, and reduced length and quality of life [1, 2]. It is estimated that in 2008 the osteoporosis and osteoporotic fractures caused $22 billion in medical costs in the USA [3]. The prevalence of osteoporosis has increased in the last four decades, which leads to increased health costs and disease burden. Meanwhile, elderly individuals are always more probably accompanied by other chronic diseases, such as hypertension, diabetes, and heart disease, which can cause worse prognosis and low quality of life. Substantial effort has been devoted to assess related risk factors and applicable interventions of osteoporosis. Previous epidemiologic studies suggested that various lifestyle-related factors were associated with bone health. Age, gender, obesity status (both general and abdominal obesity), calcium and milk consumption, caffeine intake, carbonated beverage consumption, cigarette smoking, and physical activity (PA) all have a role in BMD and prognosis of osteoporosis [4, 5]. There are also some other traits associated with BMD, such as sleep quality, menopausal status of women, and leptin [6, 7].
Physical activity is an important factor for health, which protects against a wide range of diseases. Physical activity is associated with cardiometabolic diseases (e.g., obesity, hypertension, diabetes, cardiovascular diseases), bone health, and physical function [8, 9]. Evidence from previous studies showed that PA is associated with bone health: type, frequency, and duration of PA influenced BMD varying in different age and gender groups. In adolescents, vigorous physical activity is the strongest intensity type associated with BMD both in frequency and duration of activity bouts, especially femoral neck BMD [9].
However, these observational studies could be confounded by many factors including age, obesity status, lifestyle (e.g., dairy and calcium consumption), and hormonal characteristics, resulting in spurious associations. Recently, the Mendelian randomization (MR) method was developed to estimate the causality of a risk factor (exposure) on an outcome in epidemiology studies. It has been a powerful tool because unobserved confounding will not affect the results by using genetic variants as instrumental variables (IVs) for the exposure [10]. The details of the main principles and theories of MR have been described previously [10]. In brief, there are three important assumptions for standard MR studies: (1) genetic variables (also called IVs) are associated with exposure traits; (2) genetic variables are not associated with any confounder of the exposure outcome; and (3) IVs are conditionally independent of outcome given the exposure and confounders. Based on these assumptions, genetic variants without horizontal pleiotropy are generally required for MR experiments. However, in reality, many genetic variables are associated with multiple risk factors and affect the outcome via another trait or biological pathway to the one under investigation [11]. As a result, multivariable Mendelian randomization was developed to assess the direct effect of multiple risk factors on an outcome within a single study without the pleiotropic bias in these factors [12]. Multivariable MR could be used to conduct the estimates of causality when the assumptions of standard two-sample MR analysis are violated. This method can also be used to estimate the causal effect of two or more correlated exposures. Furthermore, multivariable MR analysis may help detect whether both exposures have a direct causal effect on the outcome, or whether one exposure mediates the effect of the other on the outcome [13, 14]. Thus, standard MR analysis can assess the total effect of an exposure on an outcome, whereas multivariable MR analysis could evaluate the direct effect of each exposure on the outcome [12].
The present study aimed to address the causal effect of PA on osteoporosis using standard two-sample Mendelian randomization and multivariable Mendelian randomization analysis. Guidelines of osteoporosis prevention can be improved through this study, by promoting PA intervention and targeting individuals with their own optimized PA, which is a cost-effective approach.
Materials and methods
Data resource
PA GWAS summary statistics
PA generally can be measured by energy expenditure which is scaled in unit of metabolic equivalent (MET). International Physical Activity Questionnaire (IPAQ) based on self-reported and device-measured with wrist-worn triaxial accelerometer are two common methods for the assessment of energy expenditure [15, 16]. In the present study, summary statistics of genome-wide association study (GWAS) from two common types of PA measurements were conducted to estimate the causal effect of PA on BMD.
Summary statistics for self-reported PA were obtained from UK Biobank [17], which measured self-reported levels of PA by a questionnaire [18] in 377,234 European ancestry participants. In UK Biobank, vigorous PA, moderate-to-vigorous PA, and strenuous sports or other exercise were selected as self-reported PA trait assessment [17]. Vigorous PA and strenuous sports or other exercise were calculated as binary outcome to derive effect size for the single nucleotide polymorphisms (SNPs) based on GWAS analysis because of the skewed and zero-inflated distributions. While moderate-to-vigorous PA was measured by summing of total corresponding metabolic equivalents of moderate PA and vigorous PA [18, 19], and was inverse-normalized prior to inclusion of GWAS analyze as exhibiting skewed distribution [17]. Eventually, inverse-normalized moderate-to-vigorous PA, vigorous PA, and strenuous sports or other exercise were included as exposures for self-reported habitual PA traits in the present study.
For device-measured PA traits, summary statistics were obtained from a GWAS in 91,084 UK Biobank participants [17]. Two device-measured PA-related traits (fraction of accelerations > 425 milli-gravities (mg) and overall acceleration average) were selected as exposures to estimate the causal effect on BMD. Fraction of accelerations > 425 mg was also inverse-normalized prior to derive effect size for the SNPs based on GWAS analysis. For both self-reported and device-measured PA traits, each SNP was tested for the association with PA-related traits, with adjusting for sex, age, season of PA measured, research center, first ten genomic principal components, and genotyping chip as covariates. The GWAS summary statistics can be downloaded from https://www.ebi.ac.uk/gwas/publications/29899525.
BMD GWAS summary statistics
As osteoporosis generally can be diagnosed via BMD based on three common skeletal sites in clinical practice, femoral neck BMD (FN BMD), lumbar spine BMD (LS BMD), and forearm BMD (FA BMD) GWAS summary statistics were selected from a large sample meta-analysis conducted by the Genetic Factors for Osteoporosis (GEFOS) consortium among 53,236 European ancestry individuals [20]. The BMD-related GWAS summary statistics can be obtained from the GEFOS website (http://www.gefos.org/?q=content/data-release-2015). The SNPs that are associated with BMD with adjustment for age, age2, sex, and weight was extracted for calculation of MR.
Contributing studies, where the summary statistics was obtained, received ethical approval from their respective institutional review boards. Informed consent was obtained for all participants in original studies.
SNP selection
Exposure-associated SNPs (also called IVs) were extracted from self-reported and device-measured PA-related GWAS studies summary statistics mentioned in the “Data resource” section, respectively. Instrumental variables were selected with a significance (P < 5 × 10−8) according to previous GWASs. For multivariable MR analysis, instruments were extracted for each exposure (PA-related trait) and then were combined as a set of SNPs including all unique instruments. Finally, we extracted each of these unique SNPs from each PA-related trait (exposure).
SNP validation
The selected SNPs associated with PA should not be in linkage disequilibrium (LD) with each other according to assumptions of MR methods. In order to ensure all the selected SNPs obey independence assumption, only these with smaller PA-associated P value were retained among all pairs of SNPs with LD r2 > 0.01 (pairwise-LD was performed among the 1000G European reference panel). Following the purification of selected SNPs, exposure data sets were obtained, which were used to assess the causal effect of PA on the risk of osteoporosis.
Then, these selected SNPs were used to obtain outcome data sets by combining with the BMD-associated GWAS summary statistics mentioned in “Data resource”. If a particular requested SNP (associated with PA, set as target SNP) is not presented in the BMD-related GWAS summary statistics, then a SNP (as a proxy) that is in LD with the target SNP will be searched for instead with LD r2 > 0.8. A harmonization of the effects was conducted to ensure that the effect size estimates of same SNP for exposure and outcome correspond to the same allele. This was performed via corresponding exposure and outcome alleles were on the same strand where possible; if not, we would flip the effect of alleles and infer the palindromic SNPs strand through allele frequency. Then, exposure data and outcome were obtained.
MR estimation
Standard and multivariable MR were conducted to assess the total and direct effect of each PA trait on BMD at different skeletal sites (FA BMD, LS BMD, and FN BMD). Effect size estimates and standard errors for each instrumental SNP available from PA- and BMD-related traits were used to calculate Mendelian randomization estimation.
Of prime focus among the many limitations to MR is horizontal pleiotropy of IVs, which is the unprovable assumption that the effect on outcome is mediated by the exposure rather than through independent pathways. Several methods were conducted to detect whether the results of MR analysis were driven by the influence of horizontal pleiotropic effect. The intercept of MR-Egger regression may indicate the potential pleiotropic effects among the IVs [21].
Multivariable MR analysis was conducted to control the pleiotropic effect of IVs associated with PA closely related traits. Two times multivariable MR were performed: first analysis estimated the causal effects of self-reported PA on BMD, and second calculation was performed to assess how device-measured PA affect BMD at different skeletal sites.
Sensitivity analysis
Heterogeneity tests were conducted using Cochran’s Q test to determine if the heterogeneity exist among instrumental variables [22].
We also performed results from a range of sensitivity analyses using robust methods (such as MR-Egger regression, simple mode, and weighted mode), to make the main results more robust [23].
Statistical analysis
All the MR analyses were performed in the R environment. Sensitivity analysis was conducted to make sure results were more robust.
Results
Standard MR analysis
Selected SNPs associated with PA
Self-reported PA-associated SNPs with a GWAS significance (P < 5 × 10−8) and LD clumped are shown in Supplementary Table S1. There were 19 SNPs associated with inverse-normalized moderate-to-vigorous PA, 7 SNPs associated with vigorous physical activity, and 14 SNPs associated with strenuous sports or other exercise. Corresponding information of IVs in the outcome dataset was shown in Supplementary Table S2.
Device-measured PA-associated SNPs with a GWAS significance (P < 5 × 10−8) and LD clumped are shown in Supplementary Table S1. There were 2 SNPs associated with fraction of accelerations > 425 mg; 9 SNPs associated with overall acceleration average. Corresponding information of IVs in the outcome dataset is shown in Supplementary Table S2.
Causal effect of PA on BMD
The directional horizontal pleiotropy effect of IVs can be assessed by the MR-Egger regression method through the intercept term. In MR-Egger regression, if the intercept term has a statistical difference with zero, it means that directional horizontal pleiotropy is driving the results of an MR analysis. Results of horizontal pleiotropy tests according to MR-Egger regression are shown in Supplementary Table S3, which suggested that all self-reported PA-related IVs had no directional horizontal pleiotropy on BMD (with P values more than 0.05).
Then, we calculated standard MR via several methods to assess the causal effect of single PA-related trait on BMD. Results of standard MR analysis for the total effect of each self-reported PA on BMD at different skeletal sites are shown in Table 1 and Supplementary Table S4. The results indicated that inverse-normalized moderate-to-vigorous PA had a casual effect on FN BMD (β = − 0.491 (95% confidence interval (95%CI) − 0.880, − 0.101), P = 0.013), and vigorous PA had a total causal effect on LS BMD (β = 1.014 (95%CI: 0.040, 1.987), P = 0.041). No causal effect of self-reported PA-related traits on FA BMD was found (all P values of these standard MR analyses were more than 0.05).
Table 1.
Total causal effect of self-reported and device-measured PA on BMD in standard MR
| Exposure | Outcome | No. of SNPs | β (95%CI) | SE | P |
|---|---|---|---|---|---|
| Self-reported PA | |||||
| Inverse-normalized moderate-to-vigorous | FA BMD | 19 | −0.579 (−1.364, 0.207) | 0.401 | 0.149 |
| Inverse-normalized moderate-to-vigorous | FN BMD | 18 | −0.491 (−0.880, −0.101) | 0.199 | 0.013* |
| Inverse-normalized moderate-to-vigorous | LS BMD | 18 | −0.053 (−0.508, 0.402) | 0.232 | 0.820 |
| Strenuous sports or other exercise | FA BMD | 13 | 0.268 (−1.014, 1.550) | 0.654 | 0.682 |
| Strenuous sports or other exercise | FN BMD | 13 | 0.321 (−0.297, 0.940) | 0.315 | 0.308 |
| Strenuous sports or other exercise | LS BMD | 13 | −0.305 (−1.234, 0.624) | 0.474 | 0.520 |
| Vigorous | FA BMD | 7 | −0.120 (−1.529, 1.288) | 0.719 | 0.867 |
| Vigorous | FN BMD | 7 | 0.073 (−0.959, 1.105) | 0.526 | 0.890 |
| Vigorous | LS BMD | 7 | 1.014 (0.040, 1.987) | 0.497 | 0.041* |
| Device-measured PA | |||||
| Overall acceleration average | FA BMD | 9 | 0.005 (−0.047, 0.057) | 0.026 | 0.853 |
| Overall acceleration average | FN BMD | 9 | 0.004 (−0.039, 0.048) | 0.022 | 0.845 |
| Overall acceleration average | LS BMD | 9 | −0.013 (−0.065, 0.038) | 0.026 | 0.611 |
| Fraction of accelerations > 425 mg | FA BMD | 2 | −0.791 (−1.686, 0.104) | 0.457 | 0.083 |
| Fraction of accelerations > 425 mg | FN BMD | 2 | −0.197 (−0.628, 0.234) | 0.220 | 0.371 |
| Fraction of accelerations > 425 mg | LS BMD | 2 | −0.123 (−1.540, 1.294) | 0.723 | 0.865 |
FA BMD forearm bone mineral density, FN BMD femoral neck bone mineral density, LS BMD lumbar spine bone mineral density, CI confidence interval
Significance of level at P < 0.05
Results of horizontal test based on MR-Egger regression for device-measured PA trait–related IVs and BMD are shown in Supplementary Table S3, which suggested that all device-measured PA-related IVs had no directional horizontal pleiotropy on BMD (with P value more than 0.05). Then, we calculated standard MR via several methods to assess the causal effect of single PA-related trait on BMD. Results of standard MR analysis for the total effect of each device-measured PA on BMD at different skeletal sites are shown in Table 1 and Supplementary Table S4. No evidence was found that the device-measured PA had a significant effect on BMD.
Multivariable MR analysis
Following the conventional MR analysis, we conducted multivariable MR analysis to estimate the causal association between PA-related traits and BMD. This process of analysis contained two parts: the effect of self-reported PA traits and the effect of device-measured PA traits.
Effect of self-reported PA traits on BMD
Direct effect of self-reported PA on FN BMD is shown in Table 2 and Fig. 1. Three PA-related traits based on self-report were included in this multivariable MR analysis (containing measurement of PA type, frequency, and intensity) as exposures. All three exposures were used to assess the causal effect on FA BMD, FN BMD, and LS BMD, respectively. Results suggested that inverse-normalized moderate-to-vigorous PA had a direct causal effect on FN BMD independently (effect coefficient was − 1.116 (95%CI: − 2.210, −0.023), P = 0.045), which indicated that inverse-normalized moderate-to-vigorous PA had an independent negative effect on FN BMD. For vigorous LS BMD, vigorous PA showed a direct effect (β = 3.592 (95%CI: 0.310, 6.874), P = 0.032), which suggested that vigorous PA could increase LS BMD independently. While results suggested no evidence was found that three self-reported PA had direct causal effect on FA BMD. There was also no evidence that either strenuous sports or other exercise nor vigorous PA had a causal effect on FN BMD or LS BMD.
Table 2.
Direct causal effect of self-reported and device-measured PA on BMD in multivariable MR
| Exposure | Outcome | No. of SNPs | β (95%CI) | SE | P |
|---|---|---|---|---|---|
| Self-reported PA | |||||
| Inverse-normalized moderate-to-vigorous | FA BMD | 17 | −0.240 (−2.142, 1.662) | 0.970 | 0.805 |
| Strenuous sports or other exercise | FA BMD | 11 | 1.532 (−1.190, 4.253) | 1.388 | 0.270 |
| Vigorous | FA BMD | 5 | −1.163 (−5.913, 3.586) | 2.423 | 0.631 |
| Inverse-normalized moderate-to-vigorous | FN BMD | 16 | −1.116 (−2.210, −0.023) | 0.558 | 0.045* |
| Strenuous sports or other exercise | FN BMD | 11 | −0.089 (−1.648, 1.469) | 0.795 | 0.911 |
| Vigorous | FN BMD | 5 | 1.705 (−1.000, 4.410) | 1.380 | 0.217 |
| Inverse-normalized moderate-to-vigorous | LS BMD | 16 | −1.295 (−2.616, 0.027) | 0.674 | 0.055 |
| Strenuous sports or other exercise | LS BMD | 11 | −1.556 (−3.465, 0.353) | 0.974 | 0.110 |
| Vigorous | LS BMD | 5 | 3.592 (0.310, 6.874) | 1.675 | 0.032* |
| Device-measured PA | |||||
| Overall acceleration average | FA BMD | 8 | 0.147 (0.011, 0.283) | 0.069 | 0.034* |
| Fraction of accelerations > 425 mg | FA BMD | 2 | −1.817 (−3.353, −0.281) | 0.784 | 0.020* |
| Overall acceleration average | FN BMD | 8 | 0.055 (−0.081, 0.191) | 0.069 | 0.430 |
| Fraction of accelerations > 425 mg | FN BMD | 2 | −0.570 (−2.113, 0.973) | 0.787 | 0.469 |
| Overall acceleration average | LS BMD | 8 | 0.028 (−0.160, 0.217) | 0.096 | 0.768 |
| Fraction of accelerations > 425 mg | LS BMD | 2 | −0.546 (−2.698, 1.571) | 1.089 | 0.605 |
FA BMD forearm bone mineral density, FN BMD femoral neck bone mineral density, LS BMD lumbar spine bone mineral density
Significance level at P < 0.05
Fig. 1.
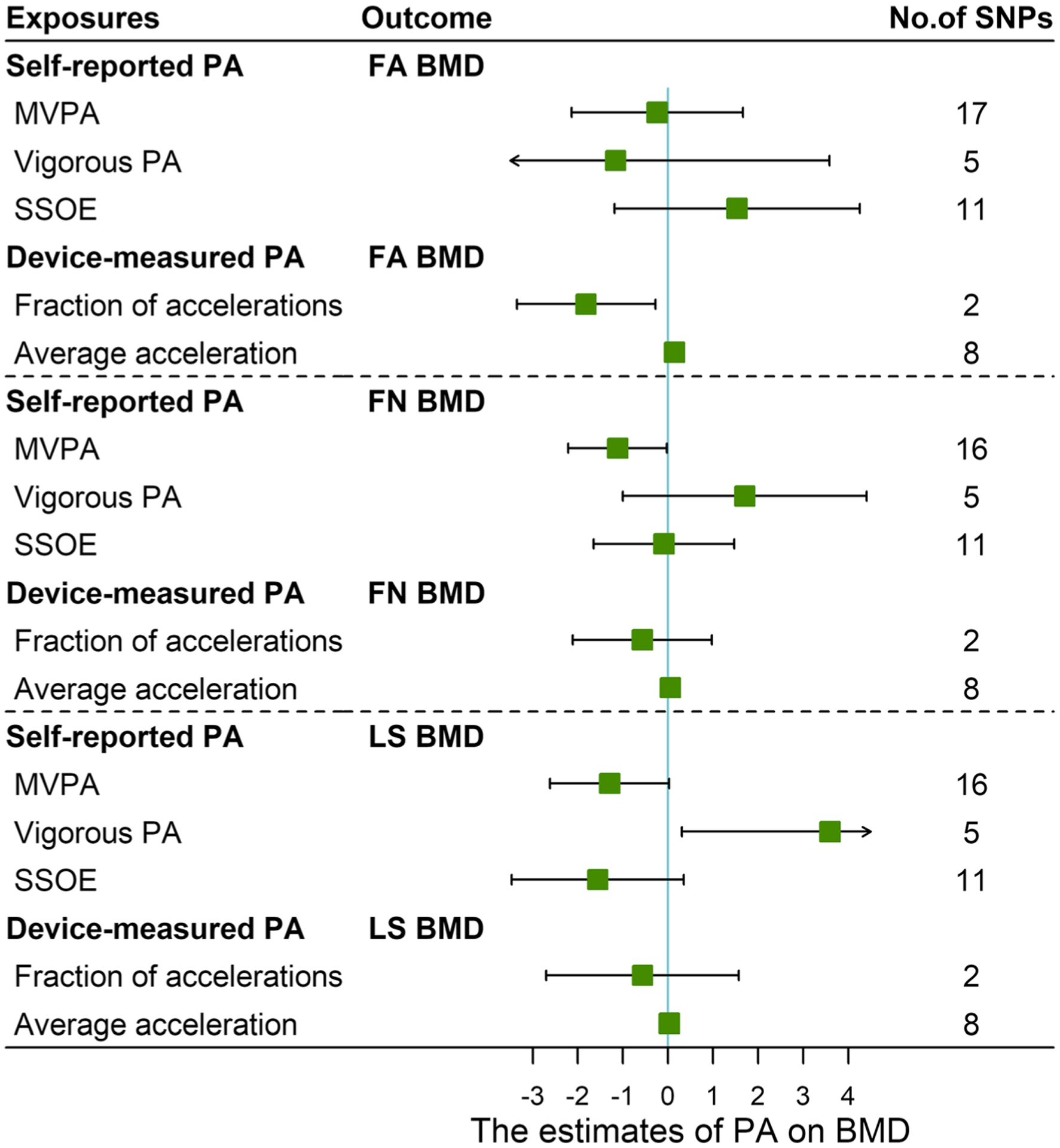
Forest plot for effects of physical activity of bone mineral density estimated by multivariable Mendelian randomization. FA BMD forearm bone mineral density, FN BMD femoral neck bone mineral density, LS BMD lumbar spine bone mineral density, MVPA inverse-normalized moderate-to-vigorous physical activity, PA physical activity, SSOE strenuous sports or other exercise. Clip confidence intervals to arrows when they exceed specified limits
Effect of device-measured PA traits on BMD
Results for direct effect of device-measured PA traits on different skeletal sites BMD are shown in Table 2 and Fig. 1. Two PA-related traits (containing fraction of accelerations > 425 mg and overall acceleration average) were included as exposures in multivariable MR analysis. Both two PA-related traits were used to estimate the causal effect of device-measured PA on FA BMD, FN BMD, and LS BMD, respectively. Results suggested that both overall acceleration average and fraction of accelerations had a direct causal effect on FA BMD independently. Effect coefficients of these two traits were 0.147 (95%CI: 0.011, 0.283) (P = 0.034) and −1.817 (95%CI: − 3.353, − 0.281) (P = 0.020), which indicated that overall acceleration average can increase FA BMD; however, fraction of accelerations > 425 mg may cause the decrease FA BMD. While results suggested no evidence was found that two device-measured PA traits had a direct causal effect on LS BM or FN BMD.
Discussion
PA plays an important role in health and incidence of various diseases [8]. In the present study, GWASs summary statistics were used to estimate the causal effect of self-reported PA and device-measured PA on BMD at different sites (FA BMD, FN BMD, and LS BMD) through standard MR and multivariable MR analysis. Sensitivity analyses were also conducted to assess the reliability and stability of the results. As shown in self-reported PA traits results, inverse-normalized moderate-to-vigorous PA was detected to be associated with the reduction of FN BMD, which was in disagreement with previous studies [9, 24–26]. This may be caused by the following reasons: first, in the original GWAS study, inverse-normalized moderate-to-vigorous PA was calculated following inverse normalization transportation [17], which would make information lost compared with original moderate-to-vigorous PA data; second, potential unannounced pleiotropic effect of IVs may still exist even pleiotropy test had been conducted; third, this result probably reflected the real direct effect of inverse-normalized moderate-to-vigorous PA on FN BMD. While vigorous PA showed a significant direct effect on LS BMD, which suggested individuals with vigorous PA would have a higher level of LS BMD compared to the population without vigorous PA. For epidemiologic studies, PA was generally considered to affect health via increasing energy expenditure level to regular carbohydrate and lipid metabolism, which could impact health status and a large number of metabolic conditions. Effect of PA on bone health may be complex and related with several different pathways: (1) PA is the key stimulus for bone growth and remodeling through the bone cell response to biomechanical load which resolves into a direct stimulation of the segment(s) [27, 28]; (2) PA increases bone resistance and improves bone quality by inducing changes in bone geometry and architecture [29]; (3) mechanical effort induced by muscle pressure and tension is an physiological stimulus for bone formation, which would act as an indirect load to mediate the endocrine stimulation [30]; (4) PA could regular vitamin D and calcium absorption via affecting lipid metabolism and body composition [31, 32]; (5) habitual PA stimulates the endocrine glands which increases serum calcium and has positive effects on BMD [4]; (6) PA could regular microenvironment between bone and skeletal muscle through impacting muscle strength [33, 34]; (7) PA is able to modify bone functions, passes through the immune system activation: and (8) people with habitual PA may have more opportunities to expose to outside sunshine.
Meanwhile, for device-measured PA based on 7 days of wrist-wear accelerometer, the result suggested that a higher overall acceleration average could increase FA BMD independently, which indicated that it had a direct causal effect on FA BMD. The effect of fraction of accelerations > 425 mg on FA BMD was reverse. This meant fraction of accelerations > 425 mg was associated with reduced FA BMD. Meanwhile, multivariable MR analysis showed that no evidence was found about the direct causal effect of two device-measured PA on the other two BMD traits (FN BMD and LS BMD). This situation may be caused by the wrist-wearing device which only records the movement of the upper limb rather than the whole body.
Previous observational epidemiology studies demonstrated that PA may improve BMD in different age and sex groups [4, 6, 9, 35, 36], especially in postmenopausal women [24, 35, 37]; however, these observational studies could be confounded by many factors. There were a few randomized controlled trials (RCTs) and meta-analysis of the PA on BMD which were mostly conducted in specific populations, such as elder people [38] and postmenopausal women [25]. These studies concluded that physical activity may improve LS and FN BMD in older adults and postmenopausal women, which may because bone responds positively to impact of PA and high-intensity progressive resistance training, and the optimization of muscle strength, balance, and mobility minimizes the risk of falls (and thereby fracture) that is particularly relevant for individuals with limited functional capacity and/or a very high risk of osteoporotic fracture such as osteoporosis patients and individuals with osteopenia. However, the exact type of activity, its intensity, its duration, and its frequency are still unclear. Further studies are needed to determine the precise training protocol for specific population (elder adults and postmenopausal women) and general population. Besides, concepts and knowledge on the association and effect of physical activity/exercise on osteoporosis from epidemiological studies had been conducted and updated in recent years among different genders, different age ranges, different races, and different countries.
The results of this study support the hypothesis that habitual PA with vigorous and moderate-to-vigorous intensity may have a causal role in the pathogenesis of osteoporosis. This study may provide authentic and dependable evidence of causal association between PA and osteoporosis since standard and multivariable Mendelian randomization analysis used in this study can make the influence of complex economic and social confounding factors minimized/eliminated. Compared with conventional clinical randomized control trials that cost a lot of time and resources, MR analysis can be an ideal alternative with the same confounder control. In this study, the large sample-sized and publicly available GWAS summary statistics for self-reported PA, device-measured PA, and BMD were used to maximize statistical power. Furthermore, multivariable MR was conducted following standard MR analysis, which could reveal the total and direct causal effect of exposures on outcome.
These findings could help to elucidate the genetic relationship between PA and osteoporosis, which will better appeal for promoting PA and potentially targeting the optimized PA for different individuals. It means the results may give a new view of “Precision Exercise Prescription” for the populations with increased fracture risk. This study may also implement a great epidemic and clinical effect. Releasing the potential causal effect between PA and BMD could promote osteoporosis preventive guidelines, give precise exercise prescriptions for the target population, and eventually reduce fracture risk of those individuals who do not have enough intense PA. Increasing energy metabolism is the main pathway of PA affecting human health, which can bring a variety of beneficial effects on cardiovascular diseases, Alzheimer’s, osteoporosis, and metabolism disorders such as obesity and diabetes. Physical activity has been an important component in different recommendations and guidelines for various metabolic diseases according to the evidence obtained from observed studies.
Strengths and limitations
This study estimated the causal effect of PA on BMD based on MR analysis, which is also the first genetic research for the association between PA and BMD. The estimates of causal effect in MR studies will not be affected by confounders, which pushes traditional studies to the limit, as alleles distribute randomly when forming gametes at meiosis [39]. Sensitivity analyses including heterogeneity tests and pleiotropy assessment were conducted to improve the reliability and stability of the results. Different MR methods (such as MR-Egger and Inverse variance weighted) were used to avoid bias caused by different estimate methods. In order to disentangle the pleiotropic effect among various PA-related traits, multivariable MR was also analyzed [40]. Although it is the first time to assess the genetic association between PA and BMD, some limitations need to be addressed. First, the summary-level data of PA and BMD from several large GWASs was adopted rather than the individual-level data. Previous studies had demonstrated that using summary statistics data to perform the MR analysis had similar efficiency as using individual-level data [39]. It is more convenient to obtain summary statistics for PA and BMD comparing with individual-level data. Second, this study did not assess the effect depending on different gender and age separately due to data availability though physiological structure and hormone levels are quite different between them, which may need further research to detect the effect and influence of PA on BMD among different age and gender. Third, there were previous RCT and meta-analyses on this topic. However, these previous studies mainly focused on specific population (the elderly and postmenopausal women) and were conducted several years before. Concepts and knowledge on the association and effect of PA/exercise on osteoporosis from epidemiological studies had been conducted and updated in recent years among different genders, different age ranges, different races, and different countries. MR analyses conducted in this study could help to elucidate the genetic relationship between PA and osteoporosis, which will better appeal for promoting PA and potentially targeting the optimized PA for general individuals.
Conclusions
In conclusion, habitual vigorous PA could increase LS BMD. Individuals with higher overall acceleration average would have a higher FA BMD.
Supplementary Material
Acknowledgments
The authors would like to thank the participants, the coordinators, and administrators for their support during the study. The authors would like to thank Jing Zhang for her suggestions and revision of the manuscripts.
Funding Hong-Wen Deng was partially supported by grants from the National Institutes of Health [AR069055, U19 AG055373, R01 MH104680, R01AR059781, and P20GM109036], and the Edward G. Schlieder Endowment fund to Tulane University. Chang-Qing Sun was partially supported by grants from the Key Research & Development and Promotion Projects of Henan Province [192102310191], and the Major Project of Basic Research of Philosophy and Social Science of Henan Education Department [2015-JCZD-009]. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00198-020-05786-2.
Data availability The datasets used and/or analyzed during the current study are publicly available. For BMD, data could be downloaded from http://www.gefos.org/?q=content/data-release-2015 and PA data could be downloaded from https://www.ebi.ac.uk/gwas/publications/29899525.
Ethics approval and consent to participate Contributing studies, where the summary statistics was obtained, received ethical approval from their respective institutional review boards. Informed consent was obtained for all participants in original studies.
Conflicts of interest None.
Publisher's Disclaimer: Disclaimers The views expressed in the submitted article are authors own and not an official position of the institution or funder.
References
- 1.Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393:364–376 [DOI] [PubMed] [Google Scholar]
- 2.Papadimitriou N, Tsilidis KK, Orfanos P, Benetou V, Ntzani EE, Soerjomataram I, Künn-Nelen A, Pettersson-Kymmer U, Eriksson S, Brenner H, Schöttker B, Saum KU, Holleczek B, Grodstein FD, Feskanich D, Orsini N, Wolk A, Bellavia A, Wilsgaard T, Jørgensen L, Boffetta P, Trichopoulos D, Trichopoulou A (2017) Burden of hip fracture using disability-adjusted life-years: a pooled analysis of prospective cohorts in the CHANCES consortium. Lancet Public Health 2:e239–e246 [DOI] [PubMed] [Google Scholar]
- 3.Blume SW, Curtis JR (2011) Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int 22:1835–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alghadir AH, Gabr SA, Al-Eisa E (2015) Physical activity and lifestyle effects on bone mineral density among young adults: sociodemographic and biochemical analysis. J Phys Ther Sci 27: 2261–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amiri AM, Hosseini SR, Rahmaninia F, Nooreddini H, Bijani A (2015) Relationship between bone mineral density and physical activity level in the elderly. Ann Appl Sport Sci 3:23–32 [Google Scholar]
- 6.Lin J, Chen L, Ni S, Ru Y, Ye S, Fu X, Gan D, Li J, Zhang L, Han S, Zhu S (2019) Association between sleep quality and bone mineral density in Chinese women vary by age and menopausal status. Sleep Med 53:75–80 [DOI] [PubMed] [Google Scholar]
- 7.Meng XH, Tan LJ, Xiao HM, Tang BS, Deng HW (2019) Examining the causal role of leptin in bone mineral density: a Mendelian randomization study. Bone 125:25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW (2012) Correlates of physical activity: why are some people physically active and others not? Lancet 380:258–271 [DOI] [PubMed] [Google Scholar]
- 9.Marin-Puyalto J, Maestu J, Gomez-Cabello A, Latt E, Remmel L, Purge P, Vicente-Rodriguez G, Jurimae J (2019) Frequency and duration of vigorous physical activity bouts are associated with adolescent boys’ bone mineral status: a cross-sectional study. Bone 120:141–147 [DOI] [PubMed] [Google Scholar]
- 10.Davey Smith G, Hemani G (2014) Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23:R89–R98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess S, Thompson SG (2015) Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 181:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanderson E, Davey Smith G, Windmeijer F, Bowden J (2019) An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol 48:713–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess S, Thompson DJ, Rees JMB, Day FR, Perry JR, Ong KK (2017) Dissecting causal pathways using Mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Genetics 207:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Relton CL, Davey Smith G (2012) Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol 41:161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bull FC, Maslin TS, Armstrong T (2009) Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 6:790–804 [DOI] [PubMed] [Google Scholar]
- 16.Rowlands AV, Mirkes EM, Yates T, Clemes S, Davies M, Khunti K, Edwardson CL (2018) Accelerometer-assessed physical activity in epidemiology: are monitors equivalent? Med Sci Sports Exerc 50:257–265 [DOI] [PubMed] [Google Scholar]
- 17.Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, Alexander GE, Chen Z, Going SB (2018) Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes 42:1161–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig CL, Marshall AL, Sjostrom M et al. (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395 [DOI] [PubMed] [Google Scholar]
- 19.Ekelund U, Sepp H, Brage S, Becker W, Jakes R, Hennings M, Wareham NJ (2006) Criterion-related validity of the last 7-day, short form of the International Physical Activity Questionnaire in Swedish adults. Public Health Nutr 9:258–265 [DOI] [PubMed] [Google Scholar]
- 20.Zheng HF, Forgetta V, Hsu YH et al. (2015) Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature 526:112–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S, Dudbridge F, Thompson SG (2016) Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med 35:1880–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW (2019) Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther 23:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segev D, Hellerstein D, Dunsky A (2018) Physical activity-does it really increase bone density in postmenopausal women? A review of articles published between 2001–2016. Curr Aging Sci 11:4–9 [DOI] [PubMed] [Google Scholar]
- 26.Benedetti MG, Furlini G, Zati A, Letizia Mauro G (2018) The effectiveness of physical exercise on bone density in osteoporotic patients. Biomed Res Int 2018:4840531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin CT, Rubin J, Judex S (2013) Exercise and the prevention of osteoporosis. In: Rosen CJ (ed) Primer on the metabolic bone diseases and disorders of mineral metabolism, Wiley, Hoboken, pp 396–402 [Google Scholar]
- 28.Klein-Nulend J, Bacabac RG, Bakker AD (2012) Mechanical loading and how it affects bone cells: the role of the osteocyte cytoskeleton in maintaining our skeleton. Eur Cell Mater 24:278–291 [DOI] [PubMed] [Google Scholar]
- 29.Forwood MR (2001) Mechanical effects on the skeleton: are there clinical implications? Osteoporos Int 12:77–83 [DOI] [PubMed] [Google Scholar]
- 30.Duncan RL, Turner CH (1995) Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57: 344–358 [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Xu D (2017) Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis 16:132–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Si S, Liu J, Wang Z, Jia H, Feng K, Sun L, Song SJ (2016) The associations of serum lipids with vitamin D status. PLoS One 11:e0165157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott D, Johansson J, McMillan LB, Ebeling PR, Nordstrom A, Nordstrom P (2019) Mid-calf skeletal muscle density and its associations with physical activity, bone health and incident 12-month falls in older adults: the healthy ageing initiative. Bone 120:446–451 [DOI] [PubMed] [Google Scholar]
- 34.Zymbal V, Baptista F, Letuchy EM, Janz KF, Levy SM (2019) Mediating effect of muscle on the relationship of physical activity and bone. Med Sci Sports Exerc 51:202–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muir JM, Ye C, Bhandari M, Adachi JD, Thabane L (2013) The effect of regular physical activity on bone mineral density in postmenopausal women aged 75 and over: a retrospective analysis from the Canadian multicentre osteoporosis study. BMC Musculoskelet Disord 14:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendez-Gallegos E, Caire-Juvera G, Astiazaran-Garcia H, Mendez-Estrada RO (2018) Comparison of measurements of bone mineral density in young and middle-aged adult women in relation to dietary, anthropometric and reproductive variables. Nutrients 10: 1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson S, Weeks B, Weis L, Harding A, Horan S, Beck B (2019) High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J Bone Miner Res 34:572. [DOI] [PubMed] [Google Scholar]
- 38.Marques EA, Mota J, Carvalho J (2012) Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Dordr) 34:1493–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess S, Freitag DF, Khan H, Gorman DN, Thompson SG (2014) Using multivariable Mendelian randomization to disentangle the causal effects of lipid fractions. PLoS One 9:e108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


