Abstract
Patients with diabetes display heightened blood pressure response to exercise but the underlying mechanism are remains to be elucidated. There is no direct evidence that insulin resistance (hyperinsulinemia or hyperglycemia) impact neural cardiovascular control during exercise. We propose a novel paradigm in which hyperinsulinemia or hyperglycemia significantly influence neural regulatory pathways controlling the circulation during exercise in diabetes.
Keywords: Insulin resistance, hyperglycemia, diabetes, exercise blood pressure, sympathetic nerve activity, exercise pressor reflex, central command
Summary:
This perspective for progress aims to examine the role of insulin resistance in abnormal autonomic cardiovascular control in diabetes.
INTRODUCTION
In patients with diabetes mellitus or non-diabetic with insulin resistance, both dynamic and static exercise elicits an excessive increase in blood pressure (BP) [1–10]. To date, the mechanism(s) underlying the exaggerated BP responsiveness to physical activity in this disease remains to be elucidated. Since potentiated BP responses to physical exertion are associated with increased risk for adverse cardiovascular events during or immediately after exercise in individuals that are insulin resistant with or without diabetes mellitus [11–15], elucidating the mechanisms responsible is clinically as well as physiologically important. The aberrant circulatory response also limits the safety of exercise prescription as a non-pharmacological treatment for diabetes which is problematic as habitual physical activity is known to be a viable therapy with demonstrated potential for lowering BP and improving overall cardiovascular health [16–18]. Insulin is a peptide anabolic hormone secreted from pancreatic β cells that plays an important role in the control of blood glucose levels. Evidence suggests that insulin significantly contributes to activation of the sympathetic nervous system [19–21]. In addition, high glucose condition may cause sympathoexcitation that are independent of endogenous insulin in patients with diabetes [22]. However, while insulin resistance is a pathophysiological characteristic of diabetes, the impact of insulin and/or insulin resistance on sympathetically-mediated neural cardiovascular control, especially during exercise, is poorly understood. Thus, the global objective of this review article is to examine historical and recent evidence aimed at determining the mechanisms underlying the heightened BP response to exercise in diabetes. Although insulin modifies neural activity in the central nervous system [23–26] or directly influences nociceptive ion channel function [27], very few studies have directly addressed the impact of insulin on neural cardiovascular control during exercise in diabetes. The key questions to be addressed are whether insulin and/or insulin resistance (i.e., hyperinsulinemia or hyperglycemia) contribute to abnormal BP regulation during exercise after the pathogenesis of this disease.
Exaggerated Exercise BP in Diabetes.
The Table summarizes evidence of abnormal exercise BP in individuals with insulin resistance and/or diabetes. In individuals with insulin resistance or type 2 diabetes mellitus (T2DM), both dynamic [1–3,6,9] and static [4,5,7,8,10] exercise elicits an excessive increase in BP. Likewise, in patients with type 1 diabetes mellitus (T1DM), the systolic and diastolic BP responses to exercise is abnormally heightened [9]. It should be noted that as patients with T1DM don’t have high insulin levels, evidence from T1DM supports the impact of hyperglycemia rather than insulin on the mechanism(s) underling exaggerated exercise BP. Adding to this evidence, we recently investigated the relationship between cardiovascular responses to exercise and insulin resistance-related factors in non-diabetic healthy men and women above 60 years old [10]. We found that hemoglobin A1c (HbA1c), an indicator for glycemic control, is an independent predictor of the diastolic BP response to dynamic handgrip exercise with muscle ischemia when using multivariate models that account for relevant variables and resting systolic BP [10]. In addition, the presence of insulin resistance as evidenced by abnormal homeostatic model assessment of insulin resistance (HOMA-IR) was also determined to be associated with an augmented diastolic BP response to rhythmic dynamic handgrip with muscle ischemia [10]. Despite this increasing evidence, the mechanism(s) underlying the exaggerated BP responsiveness to physical activity in diabetes remain largely elusive.
TABLE 1.
Evidence of abnormal exercise BP in individuals with insulin resistance or diabetes
| Reference | Subject | Age (y) | Glucose | Insulin | Exercise | Cardiovascular response |
|---|---|---|---|---|---|---|
| Brett et al. (2000) | T2DM (M, n=10) |
45±15 | 11±5 mmol/L | 23±7 μU/mL | BIKE 50, 75, 100W |
↑ DBP |
| Petrofsky et al. (2006) | T2DM (M, n=5; F, n=5) |
38±18 | > 126 mg/dL | - | SHG 40% MVC |
↑ SBP/DBP |
| Petrofsky et al. (2005) | T2DM (n=8) |
38±10 | > 126 mg/dL | - | SHG 40% MVC |
↑ SBP/DBP |
| Matteucci et al. (2006) | T1DM (M, n=16; F, n=19) |
36±11 | 12 ±5 mmol/L | - | BIKE 90% HR max |
↑ SBP |
| Scott et al. (2008) | T2DM (M/F, n=73) |
54±10 | 9±3 mmol/L | 18±22 mU/L | RUN Sub-maximal |
↑ Brachial/Central BP |
| Papavasileiou et al. (2009) | Non-diabetes (M, n=27; F, n=40) |
49±5 | 99±5 mg/dL | 13±2 mU/L | RUN Sub-maximal |
↑SBP/DBP associated with glucose, insulin, HOMA-IR |
| Huot et al. (2011) | Non-diabetes (M, n=163; F, n=137) |
35±13 | M, 5±1mmol/L F, 5±1 mmol/L |
M, 61±43 pmol/L W, 64±48 pmol/L |
BIKE PWC150 |
↑ SBP correlated with Insulin AUC |
| Holwerda et al. (2016) | T2DM (M, n=9; F, n=7) |
50±2 | 198±22 mg/dL | 11±2 μIU/mL | SHG 30, 40 % MVC |
↑ MBP, MSNA during exercise and PEMI MSNA correlated with glucose, HbA1c, HOMA-IR |
| Vranish et al. (2020) | T2DM (n=17) |
50±2 | 206±22 mg/dL | 11±2 μIU/mL | SHG 30, 40 % MVC |
↑ MBP, MSNA onset of exercise MSNA correlated with glucose, HbA1c, HOMA-IR |
| Hotta et al. (2020) | Non-diabetes (M, n=23; F, n=22) |
70±6 | 96±13 mg/dL | 6±4 μIU/mL | SHG/ischemic DHG 30% MVC |
↑ DBP during ischemic DHG correlated with HbA1c and associated with HOMA-IR |
T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes mellitus; M, male; F, female; BIKE, cycling exercise; PWC, physical work capacity; RUN, treadmill running; SHG, static handgrip exercise; DHG, dynamic handgrip exercise; MVC, maximal voluntary contraction; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; AUC, area under curve; MSNA, muscle sympathetic nerve activity; PEMI, postexercise muscle ischemia; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; HR, heart rate.
Clinical Implications.
The 2017 American College of Cardiology/American Heart Association high BP guidelines recommended that BP be consistently controlled in patients with high cardiovascular risks, including diabetes mellitus, as guided by home BP and 24-hour BP monitoring during regular activity [28]. Exercise has been shown to improve cardiovascular health [29]. Moreover, exercise training is known to slow the progression of diabetes in pre-diabetic individuals [30], resulting in reductions in mortality rates associated with T2DM. However, there are risks associated with physical activity in T2DM that must be taken into account before its prescription [11–13]. For example, transmission of BP surges to the cerebral circulation is dampened less effectively in patients with T2DM, in particular during high-intensity handgrip exercise, suggesting T2DM patients at greater risk for cerebral events during such activities [31]. Moreover, individuals exhibiting an exaggerated BP response to exercise, like T2DM patients, are more likely to develop future hypertension and are at a greater risk for cardiovascular death [15]. This suggests that early detection of the abnormal circulatory responses to physical activity in pre-diabetic or diabetic patients could also lead to the early treatment and prevention of hypertension in these individuals. Therefore, dissection of the mechanisms underlying the abnormal cardiovascular responses to exercise in diabetes may prove beneficial to the development of the novel therapeutic strategies targeted at reducing the risks associated with physical activity.
Brief Overview of Neural Control of the Circulation during Exercise.
The sympathetic nervous system plays a crucial role in the regulation of the cardiovascular system during exercise. Adjustments in the autonomic nervous system regulating the cardiovascular response to physical activity are primarily mediated by integrating input from central command (CC), the exercise pressor reflex (EPR), the arterial and cardiopulmonary baroreflexes and arterial chemoreflex [32–35]. Of these, CC and the EPR are engaged during exercise only. CC is a neural drive that originates in the cerebral cortex and regulates both locomotor and cardiovascular systems [34,36–38]. The EPR generates somatosensory signals from working skeletal muscle that modulate autonomic activity [32,39,40]. Skeletal muscle Group III afferents are predominantly mechanically-sensitive A-δ fibers that primarily mediate the muscle mechanoreflex component of the EPR [40]. Skeletal muscle Group IV afferents are primarily chemically-sensitive C fibers associated with the muscle metaboreflex component of the EPR [40]. Both group III and IV afferent fibers synapse in the dorsal horn of the spinal cord and subsequently project to the brainstem [41–44]. Evidence suggests that the first site critical for the processing of EPR sensory information in the brainstem is the nucleus tractus solitarius (NTS) within the medulla oblongata [42,45–47]. NTS GABAergic neurons communicate with neurons in the caudal ventrolateral medulla (CVLM) with subsequent interconnections with the rostral ventrolateral medulla (RVLM) [48–51]. The RVLM generates basal sympathetic cardiac and vasomotor activity and is a critical synaptic relay for input from both CC and the EPR [52]. The net result of these interactions is an increase in sympathetic outflow and decrease in parasympathetic activity during exercise.
Abnormal Exercise BP in Diabetic Animal Models.
As stated previously, although increasing evidence suggests that the pressor response to exercise is exaggerated in diabetes, the underlying mechanisms causing this abnormality remain largely unknown. Specifically, the contributions of CC vs. the EPR in mediating the augmented increase in BP in response to exercise in this disease have not been determined. Given this background, we have utilized a rat model of T2DM generated by administering a high fat diet (HFD) in combination with a low dose (25-35 mg/kg) of streptozotocin (STZ). Using this model, we recently examined the impact of T2DM on CC and EPR function [53]. Consistent with previous studies, the combination of a HFD and a low dose of STZ significantly increased fasting blood glucose as well as plasma insulin concentration [53]. Importantly, the body weights of T2DM rats were comparable with control animals eliminating the potential influence of obesity. In these studies, compared to healthy controls, the generation of T2DM markedly augmented the cardiovascular and sympathetic responses to the separate stimulation of both the mesencephalic locomotor region (a putative component of the CC pathway) as well as the EPR [53]. Consistent with this, importantly, the effects of T2DM occurred in the absence of the development of hypertension, a diabetic co-morbidity known to exaggerate CC [54,55] and EPR [56–62] activity. Our research group has extensively studied the altered cardiovascular responses to exercise induced by chronic high blood pressure using non-diabetic spontaneously hypertensive rats [56–63]. Given that spontaneously hypertensive rats are known to display peripheral insulin resistance as well as central insulin signaling defects [64], these previous findings in hypertensive animals have served as a major impetus for the investigation of the role insulin resistance plays in the abnormal regulation of the cardiovascular system during exercise in diabetes. It is likewise noted that potentiated EPR function has been observed in other models of diabetes mellitus such as University of California Davis (UCD) T2DM rats [65] or STZ-induced T1DM [66,67]. With regard to the latter, we have recently demonstrated that the sympathetic response to activation of the EPR, as well as its mechanically (i.e., skeletal muscle mechanoreflex) and chemically (i.e., skeletal muscle metaboreflex) sensitive components, was abnormally potentiated in STZ-induced T1DM [68].
MECHANISMS UNDERLYING ABNORMAL METABOREFLEX FUNCTION IN DIABETES
Sensitized Afferent Discharge to Chemical Stimulation as well as Potentiated Skeletal Muscle Metaboreflex Activity in T1DM.
In patients with T1DM, the systolic BP response to exercise is abnormally exaggerated [9]. Moreover, it has been demonstrated in STZ induced T1DM rats that the circulatory and sympathetic responses to intra-arterial administration of the transient receptor potential cation channel subfamily V member 1 (TRPV1) agonist, capsaicin, is significantly enhanced as compared to control animals [68]. In addition, the response of group IV afferents to capsaicin exposure has been shown to be significantly greater in T1DM than control [68]. TRPV1 is a metaboreceptor widely expressed in muscle afferents[69] and recent human studies support that TRPV1 contributes to EPR function in healthy subjects [70,71]. Importantly, a recent study[72] using a novel TRPV1 null mouse model to directly study the EPR demonstrated that the metaboreflex is, in part, mediated by activation of TRPV1 in skeletal muscle afferents consistent with a large number of previous studies in healthy[73] and hypertensive rats[60], with some exceptions [74]. Therefore, sensitization of muscle afferents by TRPV1 action may contribute to the abnormal circulatory responsiveness manifest in T1DM (see Figure 1). STZ-induced T1DM animals display hyperglycemia but not hyperinsulinemia since STZ treatment mitigates and/or eliminates pancreatic production of insulin. This suggests that hyperglycemia, rather than insulin, contributes to the generation of abnormal cardiovascular responses in T1DM. Hyperglycemia leads to the formation and accumulation of advanced glycation end products (AGE) [75] and the inflammatory cytokine high-mobility group box-protein 1 (HMGB1) [76]. Both AGE and HMGB1 bind to the receptors for advanced glycation end products (RAGE) [75,77]. RAGE is known to stimulate phosphorylation of protein kinase C (PKC) [77], leading to induction of peripheral neuronal damage [78]. Interestingly, evidence suggests that TRPV1 is activated by PKC in sensory neurons [79,80]. In a previous study from our lab, plasma HMGB1 was found to be significantly higher in rats with T1DM than control, while plasma AGE did not differ [68]. These findings are consistent with reports that HMGB1 levels are increased in the plasma [81] and skin [82] of patients and animals with T1DM. It is known that HMGB1 acts more rapidly to activate RAGE [77] whereas AGE must accumulate over time [75]. In a previous investigation, we additionally observed that phosphorylated PKCα protein expression is upregulated in the dorsal root ganglia (DRG) of T1DM rats [68]. Thus, in hyperglycemic T1DM, it is plausible to suggest that HMGB1 activation of RAGE rather than AGE contributes to the phosphorylation of PKC in DRG neurons thereby contributing to augmentations in metaboreflex function.
Figure 1. Peripheral mechanisms underlying exaggerated skeletal muscle metaboreflex function in diabetes.

As depicted, it is proposed that potentiated skeletal muscle metaboreflex activity in diabetes is induced by the sensitization of TRPV1 in skeletal muscle afferents. Hyperglycemia and/or hyperinsulinemia increase insulin, AGE, and HMGB1. These substrates activate the PKC-TRPV1 pathway in sensory neurons. TRPV1 sensitization leads to an increase in Na+/Ca2+ influx, which induces action potential firing in the afferent fiber. The action potentials in muscle afferents are transduced via the DRG to the spinal cord and relayed to cardiovascular control areas in the brainstem which, in diabetes, evoke an abnormal BP response to exercise (i.e., TRPV1-induced metaboreflex overactivity). AGE, advanced glycation end products; DRG, dorsal root ganglion; IR, insulin receptor; HMGB1, high mobility group box 1; PKC, protein kinase C; RAGE, receptor for advanced glycation end products; TRPV1, transient receptor potential vanilloid 1.
Sensitized Afferent Discharge to Chemical Stimulation as well as Potentiated Skeletal Muscle Metaboreflex Activity in T2DM.
As indicated in the Table, Holwerda et al. [7] demonstrated that BP and muscle sympathetic nerve activity responses were heighted during post exercise muscle ischemia, an experimental paradigm designed to isolate the muscle metaboreflex, in T2DM patients. The study further revealed that the increases in muscle sympathetic nerve activity during skeletal muscle metaboreflex activation were significantly correlated with fasting glucose, HbA1c, and HOMA-IR [7]. This suggests that a heightened metabolic component of the EPR that appears to be related to the severity of T2DM. However, the exact mechanisms underlying the potentiation in metaboreflex function in T2DM remain to be fully elucidated. To address this gap, recent preliminary data from our laboratory demonstrated that, in vivo, the BP and sympathetic responses to intra-arterial administration of the TRPV1 agonist capsaicin were abnormally potentiated in T2DM rats [83,84]. Further, in vitro, TRPV1-induced action potential discharge was markedly increased in skeletal muscle group IV fibers isolated from T2DM animals [84,85]. In addition, the expression of phosphorylated TRPV1 and PKCα in DRG were enhanced in diabetic animals [84,85]. These findings provide the first evidence suggesting that the function/activation of TRPV1 is increased in the skeletal muscle afferents of T2DM animals. Interestingly, neither AGE, HMGB1, nor RAGE were different between controls and T2DM rats [84,85]. Unlike reported in T1DM, TRPV1 sensitization in mildly-hyperglycemic early-stage T2DM might not occur as a result of RAGE/PKC signaling but rather a completely different pathway. Most importantly, the discharge response to capsaicin administration was significantly associated with fasting blood glucose [84,85]. Bestall et al. [82] recently demonstrated that in DRG neural cells (50B11) exposed to high-glucose conditions for 24 hours, phosphorylation of TRPV1 (S800 site) was increased compared with basal glucose conditions. Moreover, high glucose induced phosphorylation of PKC in DRG neurons in vitro [86]. We also recently confirmed that the discharge to capsaicin was potentiated by acute exposure of group IV afferents to a high-glucose environment [84]. Thus, in mildly-hyperglycemic, early state T2DM, glucose/PKC signaling may contribute to neuronal sensitization via TRPV1. Taken together, it is suggested that the alterations observed in T2DM rats are associated with hyperglycemia and likely play a role in mediating the enhanced BP response characteristics of this disease.
Peripheral hyperinsulinemia is also a pathophysiological characteristic of T2DM. In pilot studies, using whole cell patch-clamp techniques, we investigated the impact of insulin on chemically activated currents in DRG neurons in normal animals. The total charge transfer induced by capsaicin-activated current was significantly higher after insulin exposure than that of control [87]. These changes were blocked by pretreatment with the insulin receptor inhibitor GSK1838705 [87]. Likewise, in a muscle-nerve preparation, insulin administration also significantly increased the response magnitude of 1µM capsaicin [87]. Given these initial findings, it is possible that insulin also could potentiate TRPV1 activity significantly contributing to an exaggerated expression of the muscle metaboreflex in T2DM.
MECHANISMS UNDERLYING ABNORMAL MECHANOREFLEX FUNCTION IN DIABETES
Mechano-gated Channels and the Skeletal Muscle Mechanoreflex.
The mechanism of mechanotransduction in skeletal muscle sensory afferents remains to be determined although several possibilities have been suggested. TRPV2 was recently demonstrated to be required for mechanical nociception as well as the stretch-evoked response in primary sensory neurons [88]. It is also worth noting that insulin has been shown to facilitate capsaicin-evoked TRPV1 responses as well as translocation of TRPV1 and TRPV2 to the cell surface in DRG neurons [27] and pancreatic cells [89]. Thermally and chemically sensitive TRPV1 is likewise proposed to be mechanosensitive because it responds to cell deformation induced by hypertonicity [90]. Therefore, it is possible that the insulin/TRPV pathways could be associated with the sensitization of mechanosensitive-muscle afferents. Recent evidence suggests that mechano-gated PIEZO2 channels contribute to the expression of the muscle mechanoreflex in normal rats [91], as well as in rat models of peripheral artery disease [92] and T1DM [66]. The PIEZO2 channel has been shown to be directly gated by mechanical stimuli [93]. However, to our knowledge, there is no study investigating the possible interactions between PIEZO2 ion channels and the insulin signaling pathway. Cleary, further investigation is required.
Mechanisms Underlying the Sensitization of the Skeletal Muscle Mechanoreflex in T1DM.
As shown in the Table, the BP response to exercise is exaggerated in T1DM [9]. As mentioned previously, it is likely that an overactive EPR contributes to the potentiated pressor response [67]. Consistent with the pioneering work of Grotle et al. [67], we recently demonstrated in rats with STZ-induced T1DM that the BP and renal sympathetic nerve activity (RSNA) responses to passive muscle stretch (a maneuver designed to preferentially stimulate the mechanoreflex) were significantly augmented compared to control animals [68]. In addition, using an isolated muscle-nerve preparation, it was determined that the response of mechanosensitive-group IV afferents to mechanical stimulation were likewise significantly enhanced in T1DM [68]. As mentioned, rats with T1DM exhibit hyperglycemia due to the lack of insulin production/secretion in the pancreas. Thus, it is probable that hyperglycemia, rather than insulin, plays a crucial role in the generation of abnormal cardiovascular responses to exercise in T1DM. High glucose [86] and elevated glucose-induced increases in HMGB1 [76,78,94] are known to activate PKC in sensory neurons. Importantly, PKC activates PIEZO channels, one of the known mechanoreceptors [95]. Moreover, PIEZO2 channels contribute to the expression of the mechanical component of the EPR in normal rats [91] and T1DM [66]. As mentioned, we have previously observed an upregulation of PKCα protein in the DRG of animals with induced T1DM [68] and T2DM [96]. Additionally, we have demonstrated that plasma HMGB1 is increased in T1DM [96]. Although speculative, it is possible that the glucose/PKC/PIEZO2 pathway could sensitize mechanosensitive-sensory afferents in T1DM. Additional studies in this area are warranted in the future.
Mechanisms Underlying the Sensitization of the Skeletal Muscle Mechanoreflex in T2DM.
In pilot studies with T2DM rats, mechanoreflex activation by passive stretch evoked significantly greater increases in BP as well as RSNA [96]. The response to mechanical stimulation was likewise significantly greater in group IV afferents from T2DM as compared to those from control animals [96]. Collectively, these findings suggest that T2DM exacerbated the cardiovascular and sympathetic responses to stimulation of the mechanoreflex. Peripheral hyperinsulinemia associated with insulin resistance is a pathophysiological characteristic of T2DM. Insulin receptors are known to be expressed on DRG neurons [97–100] and peripheral nerves [98,100]. In the presence of insulin, the sensitivity of TRPV1 is increased and its activation threshold reduced making it more responsive to stimuli [27]. Furthermore, TRPV2 is expressed in mechanosensitive primary afferent neurons [101,102]. Taken together, it is hypothesized that insulin potentiates the response of thin fiber afferents to mechanical stimuli. To test this hypothesis, we recently performed whole-cell patch clamp studies obtaining recordings from cultured small DRG neurons observing mechanically-activated currents induced by mechanical stimuli applied to the cell surface [103]. Utilizing this experimental paradigm, it was determined that insulin injection significantly augmented the amplitude of mechanically-activated currents and decreased the mechanical threshold [103]. More importantly, pretreatment with the insulin receptor antagonist, GSK1838705, significantly suppressed the insulin-induced potentiation of the mechanical responses [103]. We further examined the impact of insulin on thin fiber muscle afferent activity in response to mechanical stimuli using rat muscle-nerve preparation. Likewise, insulin increased the sensitivity of mechanosensitive-group IV muscle afferents as evidenced by a significant reduction in the response threshold to mechanical stimuli [103]. Although speculative in nature, the observed insulin-induced mechanical sensitization of somatosensory thin fiber afferents may contribute to an augmentation in muscle mechanoreflex activity in hyperinsulinemic T2DM [8].
CENTRAL MECHANISMS OF ABNORMAL EXERISE BP IN DIABETES
Pioneering work by Holwerda et al. [7] showed that the muscle sympathetic nerve response to a cold pressor test were potentiated in patients with T2DM. This suggests that a heightened central sympathetic reactivity may be involved in exaggerated cardiovascular responses to exercise in this disease.
Role of Brain Insulin in the Regulation of the Sympathetic Nervous System during Exercise.
It was previously thought that insulin in the brain did not play a significant regulatory role especially in cardiovascular control during exercise. However, recent evidence suggests that insulin not only regulates glucose and lipid metabolism but also modulates neural activity in the brain. To date, whether brain insulin modulates EPR activity remains undetermined although likely. Insulin enters the central nervous system via a saturable transport system [104,105] and its receptors are widely expressed in the brain including the medulla oblongata, the site of both the NTS and RVLM [106]. The binding of insulin to its receptor activates phosphoinositide 3-kinase (PI3K) phosphorylating PIP2 (phosphatidylinositol 4,5-bisphosphate) to form PIP3 (phosphatidylinositol (3,4,5)-trisphosphate)[107,108]. PIP3 directly activates KATP channels [109,110] resulting in hyperpolarization and decreased neuronal firing rate. PIP3 also indirectly activates Akt signaling [107,108] resulting in activation of nitric oxide synthase (NOS). The resultant nitric oxide (NO) produced also activates KATP channels in neurons [111]. A more recent study demonstrated that activation of KATP channels in the RVLM decreases basal BP and RSNA [23,112]. The NOS inhibitor, N-Nitroarginine methyl ester, partially attenuates these sympathoinhibitory effects[23]. As more direct evidence, insulin administration into the RVLM significantly attenuates BP and sympathetic responses to electrical stimulation of the RVLM in cats[113]. Further, insulin-stimulated production of NO[26] increases its bioavailability in the brain. An increase in NO bioavailability in the NTS and RVLM decreases blood pressure and sympathetic nervous system activity [114]. It is plausible that the insulin signaling pathway in the brain contributes to the modulation of the sympathetic and pressor responses to exercise via its actions on the NTS and RVLM in the brainstem.
Dysfunctional Brain Insulin Signaling in T2DM.
Numerous studies demonstrate that brain insulin signaling is significantly altered in T2DM. For example, insulin binding to brain capillaries is reduced in genetically obese hyperinsulinemic Zucker rats as compared to lean Zucker rats [115]. Further, in dogs, increased adiposity induced by high-fat feeding is associated with reduced insulin delivery to the central nervous system[116]. These reports suggest that the chronic peripheral hyperinsulinemia associated with insulin resistance in T2DM results in hypoinsulinemia in the central nervous system [115,116]. In contrast, insulin receptor expression in the brain is unlikely to be affected by T2DM [117,118]. However, its downstream signaling cascade is impaired. For example, down-regulation of key enzymes in the insulin signaling pathway, such as PI3K, Akt, and NOS have been observed in human [118] and rat studies [64,117]. Further, insulin has been shown to activate KATP channels in hypothalamic neurons of lean but not obese rats [25]. In addition, normotensive T2DM patients exhibit sympathetic hyperactivity at rest which is likely contributory to the development of essential hypertension [119]. Together, these alterations in brain insulin signaling in T2DM may contribute to the exaggerated cardiovascular response to exercise.
FUTURE PERSPECTIVES
An increasing number of studies in diabetic patients and various animal models of diabetes suggest that EPR and/or CC dysfunction contributes significantly to the potentiated hemodynamic responsiveness during physical activity characteristic of the disease. To date, evidence elucidating both central and peripheral mechanisms underlying the pathogenesis of abnormal exercise BP in diabetes is just beginning to emerge. Aerobic exercise is more commonly prescribed in clinical practice rather than forms of resistance exercise. On the other hand, diabetes mellitus is an independent risk factor for low muscular strength. As such, a position statement by the American Diabetes Association recommends resistance training (e.g. static/ischemic) as well for T2DM patients [120]. Combining endurance exercise with resistance exercise may provide greater improvements [121], and high-intensity interval training may be superior to continuous aerobic training in adults with diabetes [122]. In addition, this is clinically important because isometric contractions are a component of many daily activities and are capable of inducing marked increases in BP even when performed with a small muscle mass [123]. Therefore, dissection of the mechanisms underlying the abnormal cardiovascular response to both static and dynamic exercise in T2DM may prove beneficial to the development of novel therapeutic strategies targeted at reducing the risks associated with physical activity. This could lead to the prescription of exercise of greater frequency, intensity and duration allowing the benefits of exercise training to be fully realized in this disease.
Specifically, studies have demonstrated that EPR overactivity in diabetes is mediated, in part, by insulin, insulin resistance or hyperglycemia. Continuous research will likely be beneficial to the development of novel therapies targeted at reducing the risks associated with physical activity in this disease. For example, it has been recently demonstrated that desensitization of TPRV1 in cardiac afferent neurons by resiniferatoxin exhibits protective effects against autonomic dysfunction in heart failure animals [124,125]. Further, ablation of cardiac afferent nerves by resiniferatoxin at the level of the upper thoracic DRG can prevent the development of hypertension in spontaneously hypertensive rats [126]. We previously demonstrated that blockade of TRPV1, which contributes significantly to EPR activation [73], significantly reduced pressor and sympathetic responses to supra-stimulation of the metaboreflex (via ischemic hindlimb muscle contraction) in spontaneously hypertensive rats [60]. Therefore, further investigation as to whether antagonism of hyperinsulinemia/hyperglycemia-induced sensitization of TRPV and mechano-gated channels ameliorates abnormal EPR function in diabetes may prove valuable in facilitating favorable clinical outcomes when physical activity is part of the prescribed treatment regimen (see Figure 1 and Figure 2). Hyperglycemia activate superoxide production [127,128]. Increasing evidence suggests that oxidative stress in the exercising muscle modulates the EPR function in disease states associated with excessive oxidative stress such as hypertension [129], heart failure [130], peripheral arterial disease [131]. Further investigation is needed to examine if hyperglycemia-induced oxidative stress potentiate EPR function in diabetes.
Figure 2. Peripheral mechanisms underlying exaggerated skeletal muscle mechanoreflex function in diabetes.
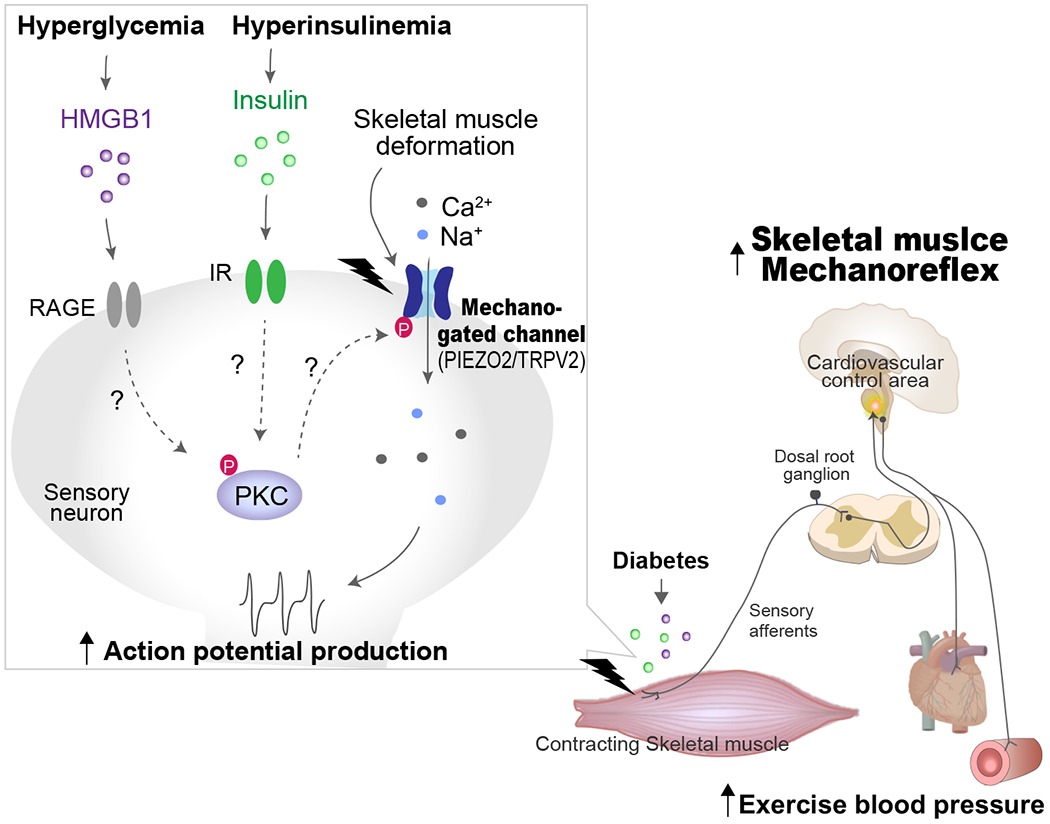
As depicted, it is proposed that augmented skeletal muscle mechanoreflex activity in diabetes is induced by the sensitization of mechano-gated channels in skeletal muscle afferents. Hyperglycemia and/or hyperinsulinemia increase insulin and HMGB1. These substrates may activate PKC phosphorylated-mechanoreceptors in sensory neurons. The sensory signals generated by activation of mechanoreceptors (PIEZO and TRPV2) in skeletal muscle are transduced via the DRG to the spinal cord and relayed to cardiovascular control areas in the brainstem which, in diabetes, evoke an abnormal BP response to exercise (i.e., TRPV1-induced mechanoreflex overactivity). AGE, advanced glycation end products; DRG, dorsal root ganglion; IR, insulin receptor; HMGB1, high mobility group box 1; PKC, protein kinase C; RAGE, receptor for advanced glycation end products; TRPV2, transient receptor potential vanilloid 2.
Evidence suggests that central insulin resistance is a potentially important factor in the pathophysiology of obesity, systemic insulin resistance (a common co-morbidity of T2DM) and cognitive impairments (e.g. Alzheimer’s disease). Intranasal delivery of insulin is a non-invasive technique that bypasses the blood-brain barrier and delivers insulin from the nasal cavity to the CNS via intraneuronal and extraneuronal pathways. Intranasal administration of insulin to the brain greatly impacts cognitive function and peripheral metabolism [132]. For example, intranasal insulin treatment for 21 days improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia [133]. In animal studies, long-term treatment with intranasal insulin ameliorates cognitive impairment in a streptozotocin-induced Alzheimer’s rat model[134]. To date, there is no report suggesting that nasal insulin treatment improves insulin resistance-induced autonomic nervous system dysfunction in T2DM. However, it is logical to suggest that central insulin treatment may attenuate the overactive exercise BP in T2DM by improving brain insulin signaling (see Figure 3). Pilot studies in our laboratory have demonstrated that the pressor and sympathetic responses to activation of the EPR were significantly attenuated two hours after intracerebroventricular injection of insulin in T2DM but not control animals [63]. This finding suggests that brain insulin contributes to the modulation of the BP response to EPR activation in T2DM. Moreover, central delivery of insulin ameliorates CC and/or EPR overactivity in T2DM. It is of note, pilot data was obtained using an acute delivery of insulin centrally and the impact of a more chronic administration needs to be investigated. In addition, further study is required to determine which brain regions (e.g. NTS and/or RVLM) are involved in this response. As recent studies suggest that glutamatergic N-methyl-D-aspartate [135] or melanocortin receptor 3/4 [136] in the hypothalamic paraventricular nucleus contribute to insulin-induced sympathetic responses, these receptor could be specific target for central insulin treatment in this disease. It has been reported that lumbar but not renal sympathetic nerve is particularly sensitive to insulin in rodents [21]. It is physiologically and clinically relevant to clarify impacts of central insulin on differential sympathetic outflow to internal organs or exercising skeletal muscle especially during exercise.
Figure 3. Central mechanisms of exaggerated cardiovascular responses to exercise in diabetes.
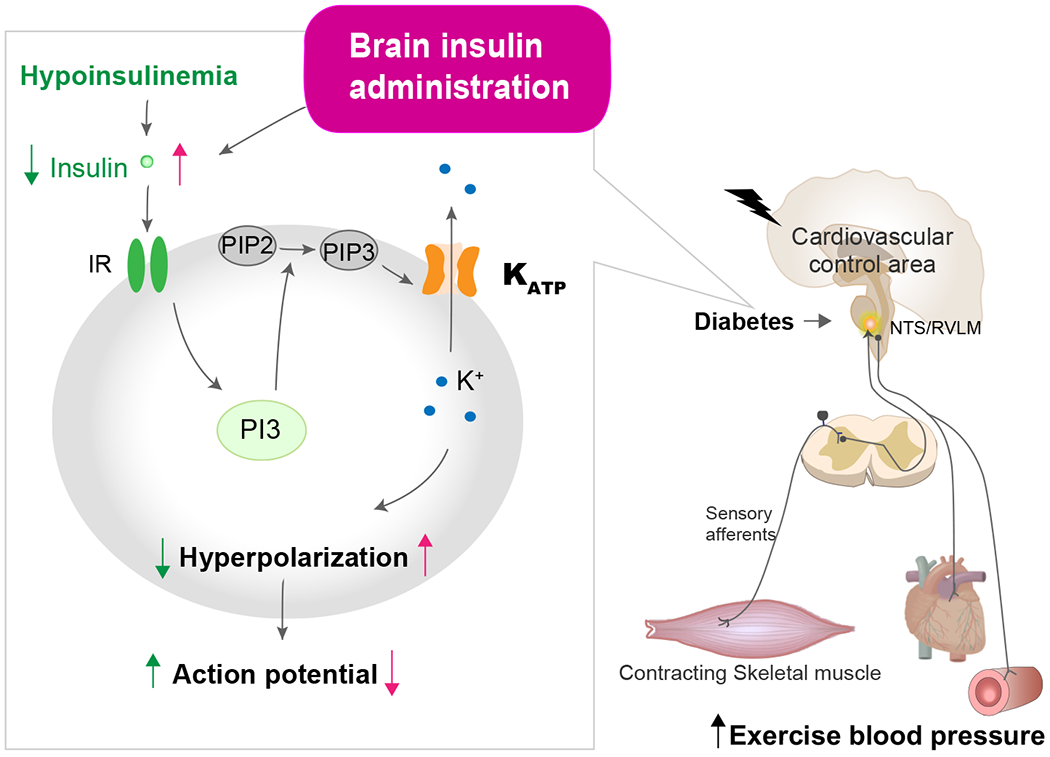
As depicted, it is proposed that the brain insulin signaling pathway contributes to the modulation of the BP responses to exercise in diabetes. The binding of insulin to IR activates phosphorylating PIP2 to form PIP3. PIP3 directly activates KATP channels resulting in hyperpolarization and decreased neuronal firing rate. Central hypoinsulinemia induced by diabetes, therefore, may lead to the pathogenesis of abnormally enhanced cardiovascular responses to physical activity via the central nervous system. Administration of insulin to the whole brain or the NTS/RVLM may be effective in ameliorating this exaggerated exercise BP response in diabetes. IR, insulin receptor; KATP, ATP-sensitive potassium; NTS, nucleus tractus solitarius; PI3K, phosphoinositide 3-kinase; PIP, phosphatidylinositol 4,5-bisphosphate; RVLM, rostral ventrolateral medulla.
Key Points.
In patients with diabetes, exercise elicits an excessive increase in blood pressure (BP). Since such an exaggerated BP response to physical exertion increases the risk for the development of an unfavorable cardiovascular event, elucidating the mechanisms responsible is clinically important.
Insulin resistance (hyperinsulinemia or hyperglycemia) is one of the pathophysiological characteristics of diabetes. As such, peripheral and central insulin resistance may contribute importantly to the development of abnormal autonomic cardiovascular control in diabetes.
Sensitization of skeletal muscle sensory neurons by insulin or glucose as well as impairment of insulin transport to the central nervous system (decreasing the activity of the insulin signaling pathway in the brain) may potentiate the BP response to exercise.
Identifying potential treatments that improve abnormally high exercise BP responses in diabetes may allow the safe prescription of physical activity as a beneficial therapeutic intervention in this disease.
ACKNOWLEDGEMENTS
Disclosure of funding received for this work from: M.M. is supported by the National Institutes of Health Grants R01-HL-151632. S.A.S and W.V. are supported by R01-HL-133179 and W.V. by P30DK079328.
Footnotes
Disclosure of conflicts of interest: No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCE
- 1.Brett SE, Ritter JM, Chowienczyk PJ. Diastolic blood pressure changes during exercise positively correlate with serum cholesterol and insulin resistance. Circulation. 2000. February 15;101(6):611–5. [DOI] [PubMed] [Google Scholar]
- 2.Huot M, Arsenault BJ, Gaudreault V, et al. Insulin resistance, low cardiorespiratory fitness, and increased exercise blood pressure: contribution of abdominal obesity. Hypertension. 2011. December;58(6):1036–42. [DOI] [PubMed] [Google Scholar]
- 3.Papavasileiou MV, Thomopoulos C, Antoniou I, et al. Impaired glucose metabolism and the exaggerated blood pressure response to exercise treadmill testing in normotensive patients. J Clin Hypertens (Greenwich). 2009. November;11(11):627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrofsky JS, Lee S. The impact of rosiglitazone on cardiovascular responses and endurance during isometric exercise in patients with Type 2 diabetes. Med Sci Monit. 2006. January;12(1):CR21–26. [PubMed] [Google Scholar]
- 5.Petrofsky JS, Stewart B, Patterson C, et al. Cardiovascular responses and endurance during isometric exercise in patients with Type 2 diabetes compared to control subjects. Med Sci Monit. 2005. October;11(10):CR470–7. [PubMed] [Google Scholar]
- 6.Scott JA, Coombes JS, Prins JB, et al. Patients with type 2 diabetes have exaggerated brachial and central exercise blood pressure: relation to left ventricular relative wall thickness. Am J Hypertens. 2008. June;21(6):715–21. [DOI] [PubMed] [Google Scholar]
- 7.Holwerda SW, Restaino RM, Manrique C, et al. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2016. January 15;310(2):H300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vranish JR, Holwerda SW, Kaur J, et al. Augmented pressor and sympathoexcitatory responses to the onset of isometric handgrip in patients with type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2020. February 1;318(2):R311–R319. [DOI] [PubMed] [Google Scholar]
- 9.Matteucci E, Rosada J, Pinelli M, et al. Systolic blood pressure response to exercise in type 1 diabetes families compared with healthy control individuals. J Hypertens. 2006. September;24(9):1745–51. [DOI] [PubMed] [Google Scholar]
- 10.Hotta N, Hori A, Okamura Y, et al. Insulin resistance is associated with an exaggerated blood pressure response to ischemic rhythmic handgrip exercise in non-diabetic older adults. J Appl Physiol (1985). 2020. June 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoberg E, Schuler G, Kunze B, et al. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990. March 1;65(9):583–9. [DOI] [PubMed] [Google Scholar]
- 12.Mittleman MA, Maclure M, Tofler GH, et al. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993. December 2;329(23):1677–83. [DOI] [PubMed] [Google Scholar]
- 13.Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin. 1996. May;14(2):263–70. [DOI] [PubMed] [Google Scholar]
- 14.Lewis GD, Gona P, Larson MG, et al. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). Am J Cardiol. 2008. June 1;101(11):1614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss SA, Blumenthal RS, Sharrett AR, et al. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010. May 18;121(19):2109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brook RD, Appel LJ, Rubenfire M, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension. 2013. June;61(6):1360–83. [DOI] [PubMed] [Google Scholar]
- 17.Dengel DR, Hagberg JM, Pratley RE, et al. Improvements in blood pressure, glucose metabolism, and lipoprotein lipids after aerobic exercise plus weight loss in obese, hypertensive middle-aged men. Metabolism. 1998. September;47(9):1075–82. [DOI] [PubMed] [Google Scholar]
- 18.Vina J, Sanchis-Gomar F, Martinez-Bello V, et al. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012. September;167(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan DA, Balon TW, Ginsberg BH, et al. Nonuniform regional sympathetic nerve responses to hyperinsulinemia in rats. Am J Physiol. 1993. February;264(2 Pt 2):R423–7. [DOI] [PubMed] [Google Scholar]
- 20.Muntzel MS, Morgan DA, Mark AL, et al. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol. 1994. November;267(5 Pt 2):R1350–5. [DOI] [PubMed] [Google Scholar]
- 21.Ward KR, Bardgett JF, Wolfgang L, et al. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011. March;57(3):435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marfella R, Nappo F, De Angelis L, et al. Hemodynamic effects of acute hyperglycemia in type 2 diabetic patients. Diabetes Care. 2000. May;23(5):658–63. [DOI] [PubMed] [Google Scholar]
- 23.Guo Q, Jin S, Wang XL, et al. Hydrogen sulfide in the rostral ventrolateral medulla inhibits sympathetic vasomotor tone through ATP-sensitive K+ channels. J Pharmacol Exp Ther. 2011. August;338(2):458–65. [DOI] [PubMed] [Google Scholar]
- 24.Hirooka Y, Kishi T, Sakai K, et al. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am J Physiol Regul Integr Comp Physiol. 2011. April;300(4):R818–26. [DOI] [PubMed] [Google Scholar]
- 25.Spanswick D, Smith MA, Mirshamsi S, et al. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000. August;3(8):757–8. [DOI] [PubMed] [Google Scholar]
- 26.Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996. August 15;98(4):894–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Buren JJ, Bhat S, Rotello R, et al. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005. April 27;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018. June;71(6):1269–1324. [DOI] [PubMed] [Google Scholar]
- 29.Marwick TH, Hordern MD, Miller T, et al. Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009. June 30;119(25):3244–62. [DOI] [PubMed] [Google Scholar]
- 30.Jadhav RA, Hazari A, Monterio A, et al. Effect of Physical Activity Intervention in Prediabetes: A Systematic Review With Meta-analysis. J Phys Act Health. 2017. September;14(9):745–755. [DOI] [PubMed] [Google Scholar]
- 31.Vianna LC, Deo SH, Jensen AK, et al. Impaired dynamic cerebral autoregulation at rest and during isometric exercise in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2015. April 1;308(7):H681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman M, Forester HV. Relexes controlling circulatory, vetilatory and airway responses to exercsie. In: Rowell L, Shepard JT, editor. Handbook of Physiology: Section 12, Exercise: Regulation and Integration of Multiple Systems. Vol. Bethesda, MD, USA: American Physiological Society; 1996. p. 381–447. [Google Scholar]
- 33.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–42. [DOI] [PubMed] [Google Scholar]
- 34.Waldrop TG, Eldridge FL, Iwamoto GA, et al. Central neural control of respiration and circulation during exercise. In: Rowell L, Shepard JT, editor. Handbook of Physiology: Section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD, USA: American Physiological Society; 1996. p. 333–380. [Google Scholar]
- 35.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol. 2015. April;5(2):475–512. [DOI] [PubMed] [Google Scholar]
- 36.Eldridge FL, Millhorn DE, Kiley JP, et al. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol. 1985. March;59(3):313–37. [DOI] [PubMed] [Google Scholar]
- 37.Kerman IA, Enquist LW, Watson SJ, et al. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003. June 1;23(11):4657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neuroscience. 2003;118(3):853–66. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman MP, Longhurst JC, Rybicki KJ, et al. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983. July;55(1 Pt 1):105–12. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman MP, Waldrop TG, Rybicki KJ, et al. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res. 1984. November;18(11):663–8. [DOI] [PubMed] [Google Scholar]
- 41.Iwamoto GA, Waldrop TG, Kaufman MP, et al. Pressor reflex evoked by muscular contraction: contributions by neuraxis levels. Journal of Applied Physiology. 1985;59(2):459–467. [DOI] [PubMed] [Google Scholar]
- 42.Kalia M, Mei SS, Kao FF. Central projections from ergoreceptors (C fibers) in muscle involved in cardiopulmonary responses to static exercise. Circulation Research. 1981;48:I48–I62. [PubMed] [Google Scholar]
- 43.Light AR, Perl ER. Re-examination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. Journal of Comparative Neurology. 1979;186:117–132. [DOI] [PubMed] [Google Scholar]
- 44.Potts JT, Lee SM, Anguelov PI. Tracing of projection neurons from the cervical dorsal horn to the medulla with the anterograde tracer biotinylated dextran amine. Autonomic Neuroscience. 2002;98:64–69. [DOI] [PubMed] [Google Scholar]
- 45.Person RJ. Somatic and vagal afferent convergence on solitary tract neurons in cat: electrophysiological characteristics. Neuroscience. 1989;30:283–295. [DOI] [PubMed] [Google Scholar]
- 46.Toney GM, Mifflin SW. Time-dependent inhibition of hindlimb somatic afferent input to nucleus tractus solitarius. Journal of Neurophysiology. 1994;72(1):63–71. [DOI] [PubMed] [Google Scholar]
- 47.Toney GM, Mifflin SW. Time-dependent inhibition of hindlimb somatic afferent transmission with nucleus tractus solitarius: an in vivo intracellular recording study. Neuroscience. 1995;68(2):445–453. [DOI] [PubMed] [Google Scholar]
- 48.Potts JT, Paton JF, Mitchell JH, et al. Contraction-sensitive skeletal muscle afferents inhibit arterial baroreceptor signalling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience. 2003;119(1):201–14. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira AL, Ramos PS, Samora M, et al. GABAergic contribution to the muscle mechanoreflex-mediated heart rate responses at the onset of exercise in humans. Am J Physiol Heart Circ Physiol. 2018. April 1;314(4):H716–H723. [DOI] [PubMed] [Google Scholar]
- 50.Teixeira AL, Fernandes IA, Vianna LC. GABAA receptors modulate sympathetic vasomotor outflow and the pressor response to skeletal muscle metaboreflex activation in humans. J Physiol. 2019. August;597(16):4139–4150. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira AL, Fernandes IA, Vianna LC. Cardiovascular Control During Exercise: The Connectivity of Skeletal Muscle Afferents to the Brain. Exerc Sport Sci Rev. 2020. April;48(2):83–91. [DOI] [PubMed] [Google Scholar]
- 52.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994. April;74(2):323–64. [DOI] [PubMed] [Google Scholar]
- 53.Kim HK, Hotta N, Ishizawa R, et al. Exaggerated pressor and sympathetic responses to stimulation of the mesencephalic locomotor region and exercise pressor reflex in type 2 diabetic rats. Am J Physiol-Reg I. 2019. August;317(2):R270–R279. [DOI] [PubMed] [Google Scholar]
- 54.Liang N, Iwamoto GA, Downey RM, et al. The Pressor Response to Concurrent Stimulation of the Mesencephalic Locomotor Region and Peripheral Sensory Afferents Is Attenuated in Normotensive but Not Hypertensive Rats. Front Physiol. 2019;10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang N, Mitchell JH, Smith SA, et al. Exaggerated sympathetic and cardiovascular responses to stimulation of the mesencephalic locomotor region in spontaneously hypertensive rats. American journal of physiology Heart and circulatory physiology. 2016. January 01;310(1):H123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuno M, Downey RM, Mitchell JH, et al. Aldosterone and Salt Loading Independently Exacerbate the Exercise Pressor Reflex in Rats. Hypertension. 2015. September;66(3):627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizuno M, Lozano G, Siddique K, et al. Enalapril attenuates the exaggerated sympathetic response to physical stress in prenatally programmed hypertensive rats. Hypertension. 2014. February;63(2):324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mizuno M, Mitchell JH, Crawford S, et al. High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am J Physiol Regul Integr Comp Physiol. 2016. July 01;311(1):R39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mizuno M, Murphy MN, Mitchell JH, et al. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011. March;300(3):H968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizuno M, Murphy MN, Mitchell JH, et al. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol. 2011. December 15;589(Pt 24):6191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizuno M, Siddique K, Baum M, et al. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension. 2013. January;61(1):180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy MN, Mizuno M, Downey RM, et al. Neuronal nitric oxide synthase expression is lower in areas of the nucleus tractus solitarius excited by skeletal muscle reflexes in hypertensive rats. Am J Physiol Heart Circ Physiol. 2013. June 01;304(11):H1547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mizuno M, Mitchell J, Smith SA. Exaggerated sympathetic and pressor responses to exercise pressor reflex activation are attenuated by intracerebroventricular insulin administration in type 2 diabetic rats. Faseb J. 2017. April;31. [Google Scholar]
- 64.Hsiao M, Lu PJ, Huang HN, et al. Defective phosphatidylinositol 3-kinase signaling in central control of cardiovascular effects in the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens Res. 2008. June;31(6):1209–18. [DOI] [PubMed] [Google Scholar]
- 65.Grotle AK, Crawford CK, Huo Y, et al. Exaggerated cardiovascular responses to muscle contraction and tendon stretch in UCD type-2 diabetes mellitus rats. Am J Physiol Heart Circ Physiol. 2019. August 1;317(2):H479–H486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grotle AK, Garcia EA, Harrison ML, et al. Exaggerated mechanoreflex in early-stage type 1 diabetic rats: role of Piezo channels. Am J Physiol Regul Integr Comp Physiol. 2019. May 1;316(5):R417–R426. [DOI] [PubMed] [Google Scholar]
- 67.Grotle AK, Garcia EA, Huo Y, et al. Temporal changes in the exercise pressor reflex in type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2017. October 1;313(4):H708–H714. [DOI] [PubMed] [Google Scholar]
- 68.Ishizawa R, Kim HK, Hotta N, et al. Skeletal Muscle Reflex-Induced Sympathetic Dysregulation and Sensitization of Muscle Afferents in Type 1 Diabetic Rats. Hypertension. 2020. April;75(4):1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin DS, Kim EH, Song KY, et al. Neurochemical Characterization of the TRPV1-Positive Nociceptive Primary Afferents Innervating Skeletal Muscles in the Rats. J Korean Neurosurg Soc. 2008;43(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Notay K, Klingel SL, Lee JB, et al. TRPV1 and BDKRB2 receptor polymorphisms can influence the exercise pressor reflex. J Physiol. 2018. November;596(21):5135–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vianna LC, Fernandes IA, Barbosa TC, et al. Capsaicin-based analgesic balm attenuates the skeletal muscle metaboreflex in healthy humans. J Appl Physiol (1985). 2018. August 1;125(2):362–368. [DOI] [PubMed] [Google Scholar]
- 72.Li Q, Garry MG. A murine model of the exercise pressor reflex. The Journal of physiology. 2020. May 13. [DOI] [PubMed] [Google Scholar]
- 73.Smith SA, Leal AK, Williams MA, et al. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol. 2010. April 1;588(Pt 7):1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ducrocq GP, Estrada JA, Kim JS, et al. Blocking the transient receptor potential vanilloid-1 does not reduce the exercise pressor reflex in healthy rats. American journal of physiology Regulatory, integrative and comparative physiology. 2019. October 1;317(4):R576–r587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh VP, Bali A, Singh N, et al. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014. February;18(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maeda T, Ozaki M, Kobayashi Y, et al. HMGB1 as a potential therapeutic target for neuropathic pain. J Pharmacol Sci. 2013;123(4):301–5. [DOI] [PubMed] [Google Scholar]
- 77.Saleh A, Smith DR, Tessler L, et al. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Exp Neurol. 2013. November;249:149–59. [DOI] [PubMed] [Google Scholar]
- 78.Zochodne DW. Mechanisms of diabetic neuron damage: Molecular pathways. Handb Clin Neurol. 2014;126:379–99. [DOI] [PubMed] [Google Scholar]
- 79.Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005. January 7;280(1):618–27. [DOI] [PubMed] [Google Scholar]
- 80.Wang S, Joseph J, Ro JY, et al. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain. 2015. May;156(5):931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Devaraj S, Dasu MR, Park SH, et al. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia. 2009. August;52(8):1665–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bestall SM, Hulse RP, Blackley Z, et al. Sensory neuronal sensitisation occurs through HMGB-1-RAGE and TRPV1 in high-glucose conditions. J Cell Sci. 2018. July 26;131(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ishizawa R, Kim HK, Hotta N, et al. An Exaggerated Muscle Metaboreflex In Diabetic Rats Is Mediated By Potentiated Skeletal Muscle Afferent Responsiveness. Med Sci Sport Exer. 2019. June;51(6):494–494. [Google Scholar]
- 84.Ishizawa R, Kim HK, Hotta N, et al. TRPV1 (Transient Receptor Potential Vanilloid 1) Sensitization of Skeletal Muscle Afferents in Type 2 Diabetic Rats With Hyperglycemia. Hypertension. 2021. March 1:HYPERTENSIONAHA12015672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishizawa R, Hotta N, Iwamoto GA, et al. Sensory Neuron Sensitization By Pkc-induced Trpv1 Phosphorylation In Type 2 Diabetic Rats. Med Sci Sport Exer. 2020;52(7):227–227. [Google Scholar]
- 86.Chattopadhyay M, Mata M, Fink DJ. Continuous delta-opioid receptor activation reduces neuronal voltage-gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. J Neurosci. 2008. June 25;28(26):6652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hotta N, Katanosaka K, Mizumura K, et al. Responses to Mechanical and Chemical Stimuli are Augmented by Insulin Administration in Neurons Innervating Skeletal Muscle. Faseb J. 2019. April;33. [Google Scholar]
- 88.Katanosaka K, Takatsu S, Mizumura K, et al. TRPV2 is required for mechanical nociception and the stretch-evoked response of primary sensory neurons. Sci Rep. 2018. November 14;8(1):16782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hisanaga E, Nagasawa M, Ueki K, et al. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes. 2009. January;58(1):174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishihara E, Hiyama TY, Noda M. Osmosensitivity of transient receptor potential vanilloid 1 is synergistically enhanced by distinct activating stimuli such as temperature and protons. PLoS One. 2011;6(7):e22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Copp SW, Kim JS, Ruiz-Velasco V, et al. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J Physiol. 2016. February 1;594(3):641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Copp SW, Kim JS, Ruiz-Velasco V, et al. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in rats with ligated femoral arteries. Am J Physiol Heart Circ Physiol. 2016. May 1;310(9):H1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parpaite T, Coste B. Piezo channels. Curr Biol. 2017. April 3;27(7):R250–R252. [DOI] [PubMed] [Google Scholar]
- 94.Meents JE, Neeb L, Reuter U. TRPV1 in migraine pathophysiology. Trends Mol Med. 2010;16(4):153–159. [DOI] [PubMed] [Google Scholar]
- 95.Dubin AE, Schmidt M, Mathur J, et al. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell reports. 2012. September 27;2(3):511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ishizawa R, Kim HK, Hotta N, et al. An Exaggerated Muscle Mechanoreflex in Type 2 Diabetic Rats Is Mediated by Potentiated Skeletal Muscle Afferent Discharge to Mechanical Stimulation. Faseb J. 2019. April;33. [Google Scholar]
- 97.Baiou D, Santha P, Avelino A, et al. Neurochemical characterization of insulin receptor-expressing primary sensory neurons in wild-type and vanilloid type 1 transient receptor potential receptor knockout mice. J Comp Neurol. 2007. July 10;503(2):334–47. [DOI] [PubMed] [Google Scholar]
- 98.Grote CW, Groover AL, Ryals JM, et al. Peripheral nervous system insulin resistance in ob/ob mice. Acta Neuropathol Commun. 2013. May 10;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lazar BA, Jancso G, Palvolgyi L, et al. Insulin Confers Differing Effects on Neurite Outgrowth in Separate Populations of Cultured Dorsal Root Ganglion Neurons: The Role of the Insulin Receptor. Front Neurosci. 2018;12:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sugimoto K, Murakawa Y, Sima AA. Expression and localization of insulin receptor in rat dorsal root ganglion and spinal cord. J Peripher Nerv Syst. 2002. March;7(1):44–53. [DOI] [PubMed] [Google Scholar]
- 101.Lawson JJ, McIlwrath SL, Woodbury CJ, et al. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008. April;9(4):298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woodbury CJ, Zwick M, Wang S, et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004. July 14;24(28):6410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hotta N, Katanosaka K, Mizumura K, et al. Insulin potentiates the response to mechanical stimuli in small dorsal root ganglion neurons and thin fibre muscle afferents in vitro. J Physiol. 2019. October;597(20):5049–5062. [DOI] [PubMed] [Google Scholar]
- 104.Schwartz MW, Bergman RN, Kahn SE, et al. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest. 1991. October;88(4):1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rhea EM, Rask-Madsen C, Banks WA. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J Physiol. 2018. October;596(19):4753–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978. April 27;272(5656):827–9. [DOI] [PubMed] [Google Scholar]
- 107.Muniyappa R, Montagnani M, Koh KK, et al. Cardiovascular actions of insulin. Endocr Rev. 2007. August;28(5):463–91. [DOI] [PubMed] [Google Scholar]
- 108.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005. March;16(2):59–65. [DOI] [PubMed] [Google Scholar]
- 109.MacGregor GG, Dong K, Vanoye CG, et al. Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proc Natl Acad Sci U S A. 2002. March 5;99(5):2726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Plum L, Ma X, Hampel B, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006. July;116(7):1886–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kawano T, Zoga V, Kimura M, et al. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation. Mol Pain. 2009;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duan XC, Guo R, Liu SY, et al. Gene transfer of cystathionine beta-synthase into RVLM increases hydrogen sulfide-mediated suppression of sympathetic outflow via KATP channel in normotensive rats. Am J Physiol Heart Circ Physiol. 2015. March 15;308(6):H603–11. [DOI] [PubMed] [Google Scholar]
- 113.Kuo SW, Hsieh JH, Wu WC, et al. Effects of insulin on the cardiovascular integrating mechanisms of brain stem in cats. Am J Physiol. 1993. October;265(4 Pt 1):E609–16. [DOI] [PubMed] [Google Scholar]
- 114.Kishi T, Hirooka Y, Ito K, et al. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension. 2002. February;39(2):264–8. [DOI] [PubMed] [Google Scholar]
- 115.Schwartz MW, Figlewicz DF, Kahn SE, et al. Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides. 1990. May-Jun;11(3):467–72. [DOI] [PubMed] [Google Scholar]
- 116.Kaiyala KJ, Prigeon RL, Kahn SE, et al. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000. September;49(9):1525–33. [DOI] [PubMed] [Google Scholar]
- 117.Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007. July;56(7):1817–24. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y, Liu F, Grundke-Iqbal I, et al. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011. September;225(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huggett RJ, Scott EM, Gilbey SG, et al. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003. December 23;108(25):3097–101. [DOI] [PubMed] [Google Scholar]
- 120.Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016. November;39(11):2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007. September 18;147(6):357–69. [DOI] [PubMed] [Google Scholar]
- 122.Jelleyman C, Yates T, O’Donovan G, et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. 2015. November;16(11):942–61. [DOI] [PubMed] [Google Scholar]
- 123.Mitchell JH, Wildenthal K. Static (isometric) exercise and the heart: physiological and clinical considerations. Annu Rev Med. 1974;25:369–81. [DOI] [PubMed] [Google Scholar]
- 124.Wang HJ, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent reflex control of cardiac function in normal and chronic heart failure states. J Physiol. 2017. April 15;595(8):2519–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang HJ, Wang W, Cornish KG, et al. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension. 2014. October;64(4):745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shanks J, de Morais SDB, Gao L, et al. TRPV1 (Transient Receptor Potential Vanilloid 1) Cardiac Spinal Afferents Contribute to Hypertension in Spontaneous Hypertensive Rat. Hypertension. 2019. October;74(4):910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Russell JW, Golovoy D, Vincent AM, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. Faseb J. 2002. November;16(13):1738–48. [DOI] [PubMed] [Google Scholar]
- 128.Sedeek M, Callera G, Montezano A, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010. December;299(6):F1348–58. [DOI] [PubMed] [Google Scholar]
- 129.Koba S, Watanabe R, Kano N, et al. Oxidative stress exaggerates skeletal muscle contraction-evoked reflex sympathoexcitation in rats with hypertension induced by angiotensin II. Am J Physiol Heart Circ Physiol. 2013. January 1;304(1):H142–53. [DOI] [PubMed] [Google Scholar]
- 130.Koba S, Gao Z, Sinoway LI. Oxidative stress and the muscle reflex in heart failure. J Physiol. 2009. November 1;587(Pt 21):5227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harms JE, Kuczmarski JM, Kim JS, et al. The role played by oxidative stress in evoking the exercise pressor reflex in health and simulated peripheral artery disease. J Physiol. 2017. July 1;595(13):4365–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ott V, Benedict C, Schultes B, et al. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab. 2012. March;14(3):214–21. [DOI] [PubMed] [Google Scholar]
- 133.Claxton A, Baker LD, Hanson A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44(3):897–906. [DOI] [PubMed] [Google Scholar]
- 134.Guo Z, Chen Y, Mao YF, et al. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci Rep. 2017. April 06;7:45971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stocker SD, Gordon KW. Glutamate receptors in the hypothalamic paraventricular nucleus contribute to insulin-induced sympathoexcitation. J Neurophysiol. 2015. March 1;113(5):1302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi Z, Zhao D, Cassaglia PA, et al. Sites and sources of sympathoexcitation in obese male rats: role of brain insulin. Am J Physiol Regul Integr Comp Physiol. 2020. March 1;318(3):R634–R648. [DOI] [PMC free article] [PubMed] [Google Scholar]


