Abstract
Background:
Urea cycle disorders (UCDs) are among the most common inborn errors of liver metabolism. As therapies for hyperammonemia associated with urea cycle dysfunction have improved, chronic complications, such as liver disease, have become increasingly apparent in individuals with UCDs. Liver disease in UCDs may be associated with hepatic inflammation, hepatic fibrosis, portal hypertension, liver cancer and even liver failure. However, except for monitoring serum aminotransferases, there are no clear guidelines for screening and/or monitoring individuals with UCDs for liver disease. Thus, we systematically evaluated the potential utility of several non-invasive biomarkers for liver fibrosis in UCDs.
Methods:
We evaluated grey-scale ultrasonography, liver stiffness obtained from shear wave elastography (SWE), and various serum biomarkers for hepatic fibrosis and necroinflammation, in a cohort of 28 children and adults with various UCDs.
Results:
Overall, we demonstrate a high burden of liver disease in our participants with 46% of participants having abnormal grey-scale ultrasound pattern of the liver parenchyma, and 52% of individuals having increased liver stiffness. The analysis of serum biomarkers revealed that 32% of participants had elevated FibroTest™ score, a marker for hepatic fibrosis, and 25% of participants had increased ActiTest™ score, a marker for necroinflammation. Interestingly, liver stiffness did not correlate with ultrasound appearance or FibroTest™.
Conclusion:
Overall, our results demonstrate the high overall burden of liver disease in UCDs and highlights the need for further studies exploring new tools for identifying and monitoring individuals with UCDs who are at risk for this complication.
Trial registration:
This study has been registered in ClinicalTrials.gov (NCT03721367).
Keywords: hepatic fibrosis, hepatic steatosis, FibroTest™, liver stiffness, shear wave elastography, liver dysfunction, ActiTest™
1.1. INTRODUCTION
Urea cycle disorders (UCDs) are among the most common inborn errors of liver metabolism and have an estimated incidence of 1 in 35,000 live births [1]. These disorders, which are caused by decreased activity of one of the six enzymes that comprise the urea cycle or one of several transporters that transport urea cycle metabolites, are characterized by increased risk for life-threatening hyperammonemia. Increasingly, studies have demonstrated that children and adults with UCDs are also at risk for developing various forms of chronic liver disease [2–6]. This chronic liver disease has been reported even in young children and in some individuals with UCDs who have had few, or even no, documented episodes of recurrent hyperammonemia [7, 8]. The manifestations of chronic liver disease in UCDs are variable and may include elevated serum alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST), hepatomegaly, abnormal grey-scale ultrasound pattern of the liver parenchyma, hepatic steatosis, hepatic fibrosis, cirrhosis with portal hypertension, and impaired liver function, which in some cases, has necessitated liver transplantation [2–4, 6, 7, 9–14]. Moreover, hepatocellular adenomas and hepatocellular carcinomas have been reported in several individuals with various UCDs [15–19]. In addition, severe acute liver injury with features of acute liver failure is a potential presentation of ornithine transcarbamylase (OTC) deficiency, the most common UCD, and has also been described in individuals with other UCDs [20–24]. Although in many cases, the severe liver injury improves with therapy for hyperammonemia, recurrent liver dysfunction has also been reported [21]. The long-term impact of persistent or recurrent liver injury on liver health remains unclear.
Serum aminotransferase levels, serum albumin, and blood coagulation parameters are commonly used measures for assessing liver health in individuals with UCDs. However, advanced fibrosis has been reported even in hepatic biopsies obtained from individuals with only mild elevations of serum aminotransferases in argininosuccinate lyase (ASL) deficiency, one of the UCDs with highest prevalence of liver disease [2, 7, 25]. Thus, these “routine” markers typically used to monitor for liver disease may not always indicate which individuals with UCDs are at increased risk for progressive liver fibrosis or hepatic cirrhosis. The gold standard for diagnosing and staging hepatic fibrosis has traditionally been liver biopsy, an invasive procedure with inherent risks, particularly in the setting of a UCD or compromised coagulation. In addition, the heterogeneous nature of many liver diseases may confound the accuracy of liver biopsy [26–28]. Thus, new and more specific non-invasive tools are necessary to monitor for liver disease in individuals with UCDs.
With the implementation of newborn screening and the availability of pharmacologic and other treatment modalities for the prevention and treatment of hyperammonemia, the overall survival in UCDs has improved. This improvement in survival has highlighted how chronic complications, such as liver disease, contribute to the morbidity of these disorders. From a clinical trial readiness aspect, it is important to develop and validate non-invasive biomarkers that can help assess the severity of liver disease in UCDs and to help predict which individuals are likely to have more advanced liver involvement. Such validated biomarkers would be critical for assessing the utility of established and investigational therapies in preventing liver disease progression in UCDs. Moreover, an accurate assessment of the severity of chronic liver disease may impact decisions and timing of liver transplantation in individuals with UCDs. Thus, in this study, we explored the potential utility of several non-invasive biomarkers for liver fibrosis in a cohort of 28 children and adults with various UCDs. Our study evaluated grey-scale ultrasonography, liver stiffness obtained from shear wave elastography (SWE), hepatic histology when clinically available and various serum biomarkers for hepatic fibrosis and necroinflammation in this cohort.
2. METHODS
2.1. Study procedures.
This study was conducted as a pilot study of the NIH Rare Diseases Clinical Research Network’s (RDCRN) Urea Cycle Disorders Consortium (UCDC) and was monitored by an independent Data/Observational Safety Monitoring Board. The study procedures were performed in accordance with a manual of operations, and data were entered into an electronic data capture system that was developed and maintained by the Data Management and Coordinating Center of the NIH RDCRN. The study was performed at Baylor College of Medicine and Texas Children’s Hospital, Houston, TX. The participants received medical care for their UCD from a variety of providers with biochemical genetics expertise at Texas Children’s Hospital or at medical centers across the U.S. The inclusion criteria for enrollment were: age > 5 years and less than 60 years, weight ≥ to 11 kg, and a diagnosis of UCD. For enrollment, a diagnosis of OTC deficiency was confirmed by molecular testing or enzymatic testing while the diagnosis of citrullinemia type I (ASS1 deficiency), ASL deficiency, or arginase (ARG1) deficiency was confirmed based on biochemical, molecular, or enzymatic testing. Participants were excluded if they had a history of: hyperammonemia (blood ammonia > 100 μmol/L) documented in the medical record or reported by the participant in the 30 days preceding enrollment visit, history of liver transplantation, current pregnancy, and/or confirmed diagnosis of chronic viral hepatitis, autoimmune liver disease, or alcohol-related liver disease. Individuals who had hyperammonemia within 30 days preceding enrollment could be enrolled at a later date. Clinical and previous laboratory data for each participant were collected by retrospective review of the medical record. Body mass index (BMI) was calculated using standard equations, and each participant was assigned to a BMI category (underweight, healthy weight, overweight, or obese) with different thresholds for adults and children. For those over 20 years of age, the thresholds for underweight, overweight and obesity were 18.5 kg/m2, 25 kg/m2, and 30 kg/m2, respectively. For those under 20 years of age, the thresholds were based on the CDC growth chart percentiles (5th, 85th, 95th). Thirteen participants had a study visit for repeat biomarker studies at least 12 months after the date of the initial study visit.
2.2. Serum Biomarkers.
All participants were fasted for approximately four hours prior to blood collection to assess routine markers of hepatic function and new biomarkers of liver disease. FibroTest™ and ActiTest™ were performed on serum by a commercial laboratory (Quest Diagnostics, San Juan Capistrano, CA). FibroTest™ scores were calculated using the individual’s age, sex, and serum levels of α2-macroglobulin, haptoglobin, apolipoprotein A1, and γ-glutamyl transferase (GGT) [29–31]. For FibroTest™, a numerical score between 0 and 1 was reported along with the predicted Metavir score for fibrosis (Supplemental Table 3) [32]. A FibroTest™ score less than 0.21 corresponds to a predicted Metavir score of F0 (no fibrosis) while a score greater than 0.74 corresponds to a predicted Metavir score of F4 (cirrhosis). The ActiTest™ uses ALT in addition to the components of FibroTest™ and has been demonstrated to correlate with necroinflammatory activity on liver biopsy in various disease states [30]. For ActiTest™, a numerical score between 0 and 1 was reported along with the predicted Metavir score for necroinflammatory activity (Supplemental Table 4) [32]. An ActiTest™ score less than 0.17 corresponds to a predicted Metavir score of A0 (no necroinflammatory activity) while a score greater than 0.62 corresponds to a predicted Metavir score of A3 (severe necroinflammatory activity). The APRI (AST-to-platelet ratio index) was calculated using the following equation: APRI = (AST in units per liter/AST upper limit of normal in units per liter)/platelet count (109 per liter) [33]. For the upper limit of normal for AST, a value of 40 units per liter was used [34, 35]. An APRI score greater than 0.7 has been shown to have a sensitivity of 77% and specificity of 72% for predicting significant hepatic fibrosis in individuals with chronic hepatitis C [36]. Fibrosis-4 (FIB-4) was calculated using the following equation: FIB-4 = (Age in years x AST level in units per liter) / (platelet count (109 per liter) x √ ALT in units per liter) [37]. A FIB-4 score of less than 1.45 has been shown to have a negative predictive value of 90% for advanced fibrosis in individuals with hepatitis C [37].
2.3. Ultrasound with SWE.
SWE (GE Logiq E9, GE Healthcare, Boston, MA) was performed after a four-hour fast as previously described within two weeks of the blood sample collection [2]. The liver shear wave speed was reported with a predicted degree of fibrosis using the Metavir scoring system for fibrosis [32]. The IQR/median was used to assess the quality of each SWE. In accordance with the Society of Radiologists in Ultrasound recommendation in 2015, an IQR/median < 0.30 for 9–11 valid measurements of liver stiffness was used as a standard to assess quality and reliability of the SWE exam [38]. For the data analysis, the manufacturer’s cutoff values for fibrosis were used as follows: F>0: 1.35 m/s, F>1: 1.66 m/s, F>2: 1.77 m/s, F>3: 1.99 m/s, and F>4: 2.2 m/s (Supplemental Table 1) [38]. These cutoff values have been established using measurements from adults with hepatitis C [38]. Additionally, comparisons were made using a published pediatric control data set obtained from healthy children [39]. Although these published data were collected outside of our institution, they were generated using the same SWE equipment (GE Logiq E9) used in our study [39]. The mean and 97.5 percentiles for shear wave stiffness were available for healthy children ages 4–7 years, 8–11 years, 12–14 years, and 15–17 years [39]. Grey-scale ultrasound was performed using standard techniques. Participants with coarse heterogenous echotexture or homogenously increased echogenicity were categorized as having abnormal liver parenchyma.
2.4. Histologic Analysis.
Histologic analysis of the liver was performed for two participants who had undergone liver transplantation for their disease. This was performed by one pediatric pathologist with specialization in hepatopathology. Representative sections of their liver explants were examined by routine H&E as well as more specialized histochemical stains including Masson trichrome, Periodic acid-Schiff (PAS) with diastase and PAS without diastase. The Masson trichrome stain was used to evaluate liver fibrosis stage using the Metavir scoring system as described above. The PAS without/with diastase was used to assess the presence or absence of glycogen in these sections.
2.5. Statistical Analysis.
Demographic characteristics, medical history, and liver biomarkers were summarized using means and standard deviations for continuous outcomes and counts and frequencies for categorical outcomes. For each outcome, we stratified subject characteristics summaries by normal or abnormal readings. For the strength of association between outcome measures and subject characteristics, univariate analyses were used (independent t-test for comparison of means and chi-square test for comparison of proportions). P < 0.05 (nominal) was considered as statistically significant. All statistical analyses were conducted using R or Graphpad Prism v.8.4.2 software (San Diego, CA).
2.6. Study Approval.
All study procedures were approved by the Institutional Review Boards (IRB) of Baylor College of Medicine and/or Children’s National Hospital, Washington, D.C., which serves as the central IRB of record for the UCDC. Written informed consent was obtained from participants or their parent(s).
3. RESULTS
3.1. Study population.
A total of 28 children and adults with various UCDs were enrolled in the study (Table 1). The age range for participants was 5 years to 52 years. At the enrollment visit, 57% of participants had a prior history of at least one episode of hyperammonemia > 100 μmol/L recorded in the medical record, and 54% of participants were overweight or obese based on BMI. Of the 28 participants, 13 (46%) participants had a repeat evaluation one year after the enrollment evaluation. No participants had liver biopsy during the course of the study, but two participants underwent liver transplantation within one year of enrollment in the study. For these two participants, liver tissue was available for review and comparison with serum biomarkers and SWE results. Data from the enrollment visit for the participants with ASL deficiency have been previously published [2].
Table 1. Study Participants.
Baseline characteristics of all participants enrolled in the study.
| Total | OTC deficiency (Male) | OTC deficiency (Female) | ASS1 deficiency | ASL deficiency | ARG1 deficiency | ||
|---|---|---|---|---|---|---|---|
| N | 28 | 3 | 7 | 4 | 8 | 6 | |
| Median age in yrs (range) | 15 (5 – 52) | 41 (26 – 51) | 16 (5 – 52) | 8 (5 – 15) | 10 (5 – 31) | 16 (6 – 35) | |
| BMI Category N (%) | Underweight | 1 (4%) | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 0 (0%) |
| Healthy | 12 (43%) | 0 (0%) | 2 (29%) | 1 (25%) | 6 (75%) | 3 (50%) | |
| Overweight | 5 (18%) | 2 (67%) | 0 (0%) | 0 (0%) | 2 (25%) | 1 (17%) | |
| Obese | 10 (36%) | 1 (33%) | 5 (71%) | 2 (50%) | 0 (0%) | 2 (33%) | |
| Sex | |||||||
| Male | 12 (43%) | 3 (100%) | 0 (0%) | 0 (0%) | 5 (63%) | 4 (67%) | |
| Female | 16 (57%) | 0 (0%) | 7 (100%) | 4 (100%) | 3 (38%) | 2 (33%) | |
| Diagnosis N (%) | |||||||
| Neonatal or NBS | 9 (32%) | 0 (0%) | 0 (0%) | 4 (100%) | 5 (63%) | 0 (0%) | |
| Family History | 4 (14%) | 1 (33%) | 2 (29%) | 0 (0%) | 1 (13%) | 0 (0%) | |
| Late-Onset | 15 (54%) | 2 (67%) | 5 (71%) | 0 (0%) | 2 (25%) | 6 (100%) | |
| Documented history of HA N (%) | 16 (57%) | 2 (67%) | 4 (57%) | 4 (100%) | 4 (50%) | 2 (33%) | |
Numbers may not equal 100% because of rounding.
Abbreviations: ARG1, arginase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthase; BMI, body-mass index; HA, hyperammonemia; N, number; NBS, newborn screening; OTC, ornithine transcarbamylase; yrs, years;
3.2. Grey-Scale Ultrasonography at Enrollment.
Thirteen participants (46%) had grey-scale ultrasound pattern showing abnormal hepatic parenchyma (Table 2). Participants with abnormal hepatic parenchyma were, on average, older than those with normal-appearing hepatic parenchyma. Moreover, eight of the nine participants greater than 18 years of age in our study had abnormal hepatic parenchyma by ultrasonography. However, there were no significant differences in BMI categories or in the proportion of individuals with history of at least one prior episode of hyperammonemia > 100 μmol/L between individuals with and without an appearance of abnormal hepatic parenchyma by ultrasound. The mean values of the routine biomarkers (ALT, AST, GGT) were also not different between the normal and abnormal groups. Interestingly, whereas FibroTest™ and FIB-4 were significantly higher in individuals with abnormal ultrasound findings, APRI and ActiTest™ were not. Seven of the ten participants with OTC deficiency, including all three male participants with OTC deficiency, had abnormal appearing hepatic parenchyma, while all four individuals with ASS1 deficiency had normal appearing hepatic parenchyma by ultrasound. Incidental findings identified on ultrasound included renal calculi and hydronephrosis in one participant with ASL deficiency, hepatic mass determined to be a likely hemangioma by MRI in one individual with ARG1 deficiency, findings consistent with focal fat deposition in one individual with ARG1 deficiency, and a unilocular cystic lesion that appeared to be a simple hepatic cyst by MRI in one individual with ARG1 deficiency. No tumors were identified in any participant.
Table 2. Liver Grey Scale Ultrasound Findings.
Summary statistics are mean +/− SD for continuous variables and counts with percentages for categorical variables. Results are stratified by the appearance of the hepatic parenchyma on ultrasound (normal and abnormal). To evaluate (univariate) association of each characteristic with echotexture, we used t-test to compare means of continuous variables and chi-square tests to compare proportions of categorical variables.
| Normal Hepatic Parenchyma | Abnormal Hepatic Parenchyma | p value | |
|---|---|---|---|
| N (%) | 15 (54%) | 13 (46%) | |
| Baseline Information | |||
| Age (years) | 11.0 +/− 6.9 | 26.5 +/− 16.4 | <0.01 |
| Males (%) | 3 (20%) | 9 (69%) | 0.02 |
| History of HA | 11 (73%) | 5 (39%) | 0.14 |
| BMI Category | 0.25 | ||
| Healthy | 8 (53%) | 4 (31%) | |
| Overweight or Obese | 6 (40%) | 9 (69%) | |
| Underweight | 1 (7%) | 0 (0%) | |
| UCD Diagnosis | 0.04 | ||
| OTC Deficiency | 3 (20%) | 7 (54%) | |
| ASS1 Deficiency | 4 (27%) | 0 (0%) | |
| ASL Deficiency | 6 (40%) | 2 (15%) | |
| ARG1 Deficiency | 2 (13%) | 4 (31%) | |
| Liver Biochemistries | |||
| ALT (units/L) | 39 +/− 30 | 43 +/− 20 | 0.74 |
| AST (units/L) | 37 +/− 14 | 44 +/− 22 | 0.29 |
| GGT (units/L) | 21 +/− 18 | 22 +/− 12 | 0.80 |
| Biomarkers | |||
| APRI | 0.33 +/− 0.16 | 0.44 +/− 0.23 | 0.15 |
| FIB-4 | 0.24 +/− 0.17 | 0.71 +/− 0.57 | <0.01 |
| FibroTest™ | 0.12 +/− 0.07 | 0.25 +/− 0.16 | <0.01 |
| ActiTest™ | 0.11 +/− 0.13 | 0.14 +/− 0.11 | 0.60 |
| Shear wave elastography | |||
| Liver Stiffness (m/s)1 | 1.34 +/− 0.19 | 1.46 +/− 0.24 | 0.14 |
Abbreviations: ALT, alanine aminotransferase; APRI, AST to platelet ratio; ARG1, arginase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthase; AST, aspartate aminotransferase; BMI, body-mass index; GGT, gamma glutamyltransferase; HA, hyperammonemia; N, number; OTC, ornithine transcarbamylase;
For liver stiffness, the values for one participant with IQR/median > 0.3 were not included in the analysis.
One of two ultrasound patterns were evident in the individuals who had an abnormal appearance of the hepatic parenchyma by grey-scale ultrasound. Eight participants (29%) had a diffuse homogeneous increase in echogenicity including one participant with ASL deficiency, four participants with OTC deficiency, and three participants with ARG1 deficiency. In contrast, five participants (18%) had a coarse, heterogeneous echotexture including one participant with ASL deficiency, three individuals with OTC deficiency, and one individual with ARG1 deficiency. Given the small samples sizes, further statistical analyses of the characteristics of these two groups could not be performed.
3.3. SWE at Enrollment.
All but one participant had valid SWE performed. The participant with IQR/median > 0.3 was removed from the SWE analysis but was included in grey-scale ultrasound and serum biomarker analysis. Of note, this individual had a BMI of 35 kg/m2 and abnormal appearance of the liver parenchyma. Using the manufacturer’s cutoff for normal liver stiffness (Supplemental Table 1) [38], 52% (14/27) of all participants had elevated liver stiffness (Table 3). Ten individuals had values corresponding to a prediction of minimal fibrosis (>1.35 m/s, >F0), three had values that were predicted to correspond to mild fibrosis (>F1), while one individual was predicted to have significant fibrosis (>F3). The >F1 values were observed in one individual with ASL deficiency (age 11 years) and one female with OTC deficiency (16 years). A female adult with OTC deficiency had liver stiffness of 2.01 m/s (>F3). This individual also had an elevated BMI (35 kg/m2) and abnormal grey-scale ultrasound pattern of the liver parenchyma. There were otherwise no significant differences between demographics and clinical characteristics between individuals with and without increased liver stiffness as assessed by SWE. Interestingly, only two of the eight adult participants with valid SWE had normal liver stiffness.
Table 3. Liver stiffness as assessed by SWE.
Summary statistics are mean +/− SD for continuous variables and counts with percentages for categorical variables. Results are stratified by liver stiffness (normal and abnormal). To evaluate (univariate) association of each characteristic with liver stiffness, we used t-test to compare means of continuous variables and chi-square tests to compare proportions of categorical variables. The data from one participant was excluded because the IQR/median was greater than 0.3, thus making the assessment unreliable. Percentages have been rounded to the nearest whole number.
| Normal liver Stiffness (<1.35 m/s) | Abnormal liver Stiffness (≥ 1.35 m/s) | p value | |
|---|---|---|---|
| N (%) | 13 (48%) | 14 (52%) | |
| Baseline Information | |||
| Age (years) | 14.9 +/− 14.8 | 20.8 +/− 14.3 | 0.30 |
| Males (%) | 4 (31%) | 7 (50%) | 0.53 |
| History of HA | 8 (62%) | 7 (50%) | 0.83 |
| BMI Category | 0.53 | ||
| Healthy | 6 (46%) | 6 (43%) | |
| Overweight or Obese | 6 (46%) | 8 (57%) | |
| Underweight | 1 (8%) | 0 (0%) | |
| UCD Diagnosis | 0.37 | ||
| OTC Deficiency | 3 (23%) | 6 (43%) | |
| ASS1 Deficiency | 3 (23%) | 1 (7%) | |
| ASL Deficiency | 3 (23%) | 5 (36%) | |
| ARG1 Deficiency | 4 (31%) | 2 (14%) | |
| Liver Biochemistries | |||
| ALT (units/L) | 43 +/− 29 | 39 +/− 23 | 0.69 |
| AST (units/L) | 44 +/− 14 | 37 +/− 22 | 0.37 |
| GGT (units/L) | 20 +/− 11 | 22 +/− 19 | 0.76 |
| Calculated Biomarkers | |||
| APRI | 0.42 +/− 0.19 | 0.34 +/− 0.22 | 0.35 |
| FIB-4 | 0.37 +/− 0.33 | 0.53 +/− 0.58 | 0.40 |
| FibroTest™ | 0.18 +/− 0.12 | 0.18 +/− 0.16 | 0.98 |
| ActiTest™ | 0.14 +/− 0.13 | 0.11 +/− 0.12 | 0.52 |
| Ultrasonography | |||
| Abnormal Hepatic Parencyhma | 5 (38 %) | 7 (50%) | 0.83 |
Abbreviations: ALT, alanine aminotransferase; APRI, AST to platelet ratio; ARG1, arginase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthase; AST, aspartate aminotransferase; BMI, body-mass index; GGT, gamma glutamyltransferase; HA, hyperammonemia; N, number; OTC, ornithine transcarbamylase;
Given that the manufacturer’s cutoffs for liver stiffness were generated using adult hepatitis C populations, we also compared the results from the pediatric participants in this study to previously published data of liver stiffness generated in healthy children (n=243) [39]. Eleven (57.9%) of 19 pediatric participants (age ≤ 17 years) in our study had a liver stiffness measurement greater than the 97.5th percentile for their age based on these published data (Figure 1) compared to eight (42%) using the manufacturer’s adult cutoff. The published 97.5th %tiles for healthy children were similar to the adult cutoff (1.35 m/s or above) for all age ranges except for the 4–7 year age range.
Figure 1. Liver stiffness in participants ages 5–17 years.
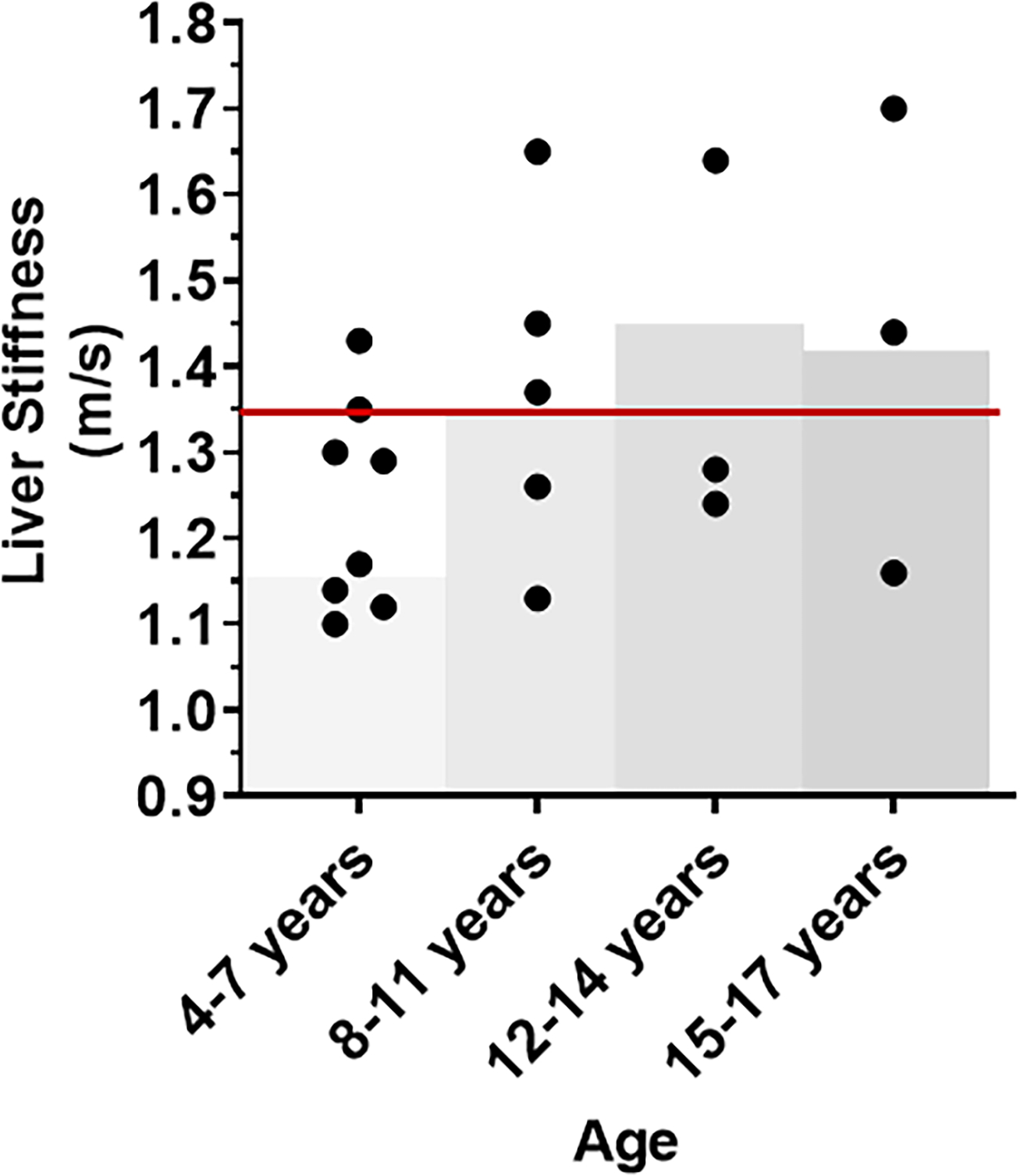
The median liver shear wave velocity in m/s, a marker of liver stiffness, for each participant is plotted based on age. The top of each grey box represents the 97.5th %tile of the liver stiffness from a published cohort of healthy children of the same age using the GE Logiq E9 equipment used in this study[39]. The red line indicates the manufacturer’s cutoff for normal stiffness in adults.
3.4. FibroTest™ at Enrollment.
Overall, nine participants (32%) had FibroTest™ scores corresponding to Metavir predicted score > F0. Eight individuals had scores corresponding to F1 or F1-F2 fibrosis and one individual with ARG1 deficiency had a score suggestive of F3 fibrosis (Table 4). This individual with ARG1 deficiency had a heterogeneous, coarse echotexture of the liver on ultrasound with monophasic and dampened hepatic venous waveforms noted in all hepatic veins (Supplemental Table 2). All other individuals with FibroTest™ scores corresponding to >F0 had OTC deficiency or ASL deficiency. No individuals with ASS1 deficiency had elevated FibroTest™ scores predicting any degree of fibrosis.
Table 4. FibroTest™ results.
Summary statistics are mean +/− SD for continuous variables and counts with percentages for categorical variables. Results are stratified by FibroTest™ score. To evaluate (univariate) association of each characteristic with FibroTest™ score, we used t-test to compare means of continuous variables and chi-square tests to compare proportions of categorical variables. Percentages have been rounded to the nearest whole number.
| Score Indicating F0 | Score Indicating >F0 | p value | |
|---|---|---|---|
| N (%) | 19 (68%) | 9 (32%) | |
| Baseline Information | |||
| Age (years) | 13.6 +/− 10.3 | 28.0 +/− 17.4 | 0.01 |
| Males (%) | 5 (26%) | 7 (78%) | 0.03 |
| History of HA | 12 (63%) | 4 (44%) | 0.60 |
| BMI Category | 0.78 | ||
| Healthy | 8 (42%) | 4 (44%) | |
| Overweight or Obese | 10 (53%) | 5 (56%) | |
| Underweight | 1 (5%) | 0 (0%) | |
| UCD Diagnosis | 0.41 | ||
| OTC deficiency | 7 (37%) | 3 (33%) | |
| ASS1 deficiency | 4 (21%) | 0 (0%) | |
| ASL deficiency | 5 (26%) | 3 (33%) | |
| ARG1 Deficiency | 3 (16%) | 3 (33%) | |
| Liver Biochemistries | |||
| ALT (units/L) | 36 +/− 26 | 51 +/− 23 | 0.14 |
| AST (units/L) | 41 +/− 20 | 40 +/− 15 | 0.92 |
| GGT (units/L) | 20 +/− 16 | 25 +/− 12 | 0.43 |
| Calculated Biomarkers | |||
| APRI | 0.37 +/− 0.21 | 0.41 +/− 0.19 | 0.59 |
| FIB-4 | 0.36 +/− 0.44 | 0.66 +/− 0.48 | 0.12 |
| ActiTest™ | 0.09 +/− 0.11 | 0.19 +/− 0.12 | 0.04 |
| Ultrasonography | |||
| Abnormal Hepatic Parenchyma | 5 (26%) | 8 (89%) | <0.01 |
| Liver Stiffness (m/s)1 | 1.41 (0.25) | 1.36 (0.14) | 0.63 |
Abbreviations: ALT, alanine aminotransferase; APRI, AST to platelet ratio; ARG1, arginase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthase; AST, aspartate aminotransferase; BMI, body-mass index; GGT, gamma glutamyltransferase; HA, hyperammonemia; N, number; OTC, ornithine transcarbamylase;
For liver stiffness, the values for one participant with IQR/median > 0.3 were not included in the analysis.
Individuals with FibroTest™ scores corresponding to >F0 were significantly older and more likely to be male (Table 4). However, no significant differences in BMI or liver biochemistries were noted between individuals with normal vs elevated FibroTest™. Interestingly, 8/9 participants with elevated FibroTest™ scores also had an abnormal grey-scale ultrasound pattern, but the median liver stiffness as assessed by SWE was similar in the participants with normal vs abnormal FibroTest™.
3.5. ActiTest™ at Enrollment.
Of the 28 participants, seven participants (25%) had an ActiTest™ score suggesting a necroinflammatory activity grade > A0. There were no significant differences in age, gender, or BMI category in participants with scores suggesting a necroinflammatory grade of A0 vs. scores suggesting a grade > A0 (Table 5). APRI but not FIB-4 was higher in the >A0 group. Participants with ActiTest™ scores > A0 also had higher FibroTest™ scores. The distribution of diagnoses was different in the >A0 group. Of note, 4 of 6 participants with ARG1 deficiency had an ActiTest™ score >A0 whereas all but one participant with OTC deficiency (male or female) and all participants with ASS1 deficiency had ActiTest™ scores suggestive of A0. Despite these trends, there were no significant differences in liver stiffness or ultrasound findings between the >A0 or A0 groups.
Table 5. ActiTest™.
Summary statistics are mean +/− SD for continuous variables and counts with percentages for categorical variables. Results are stratified by ActiTest™ score. To evaluate (univariate) association of each characteristic with ActiTest™ score, we used t-test to compare means of continuous variables and chi-square tests to compare proportions of categorical variables. Percentages have been rounded to the nearest whole number.
| Score Indicating A0 | Score Indicating >A0 | p value | |
|---|---|---|---|
| N (%) | 21 (75%) | 7 (25%) | |
| Baseline Information | |||
| Age (years) | 19.6 +/− 15.4 | 14 +/− 10.5 | 0.38 |
| Males (%) | 9 (43%) | 3 (43%) | 1.00 |
| History of HA | 11 (52%) | 5 (71%) | 0.66 |
| BMI Category | 0.84 | ||
| Healthy | 9 (43%) | 3 (43%) | |
| Overweight or Obese | 11 (52%) | 4 (57%) | |
| Underweight | 1 (5%) | 0 (0%) | |
| UCD Diagnosis | 0.04 | ||
| OTC Deficiency | 9 (43%) | 1 (14%) | |
| ASS1 Deficiency | 4 (19%) | 0 (0%) | |
| ASL Deficiency | 6 (29%) | 2 (29%) | |
| ARG1 Deficiency | 2 (10%) | 4 (57%) | |
| Liver Biochemistries | |||
| ALT (units/L) | 29 +/− 10 | 76 +/− 26 | <0.001 |
| AST (units/L) | 37 +/− 19 | 52 +/− 8 | 0.06 |
| GGT (units/L) | 19 +/− 10 | 30 +/− 24 | 0.07 |
| Calculated Biomarkers | |||
| APRI | 0.33 +/− 0.19 | 0.53 +/− 0.14 | 0.02 |
| FIB-4 | 0.48 +/− 0.49 | 0.40 +/− 0.43 | 0.70 |
| FibroTest™ | 0.15 +/− 0.11 | 0.26 +/− 0.19 | 0.06 |
| Ultrasonography | |||
| Abnormal Hepatic Parenchyma | 10 (48%) | 3 (43%) | 1.00 |
| Liver Stiffness (m/s)1 | 1.43 (0.24) | 1.29 (0.14) | 0.16 |
Abbreviations: ALT, alanine aminotransferase; APRI, AST to platelet ratio; ARG1, arginase; ASL, argininosuccinate lyase; ASS1, argininosuccinate synthase; AST, aspartate aminotransferase; BMI, body-mass index; GGT, gamma glutamyltransferase; HA, hyperammonemia; N, number; OTC, ornithine transcarbamylase;
For liver stiffness, the values for one participant with IQR/median > 0.3 were not included in the analysis.
3.6. Hepatic Histology.
Two participants had liver transplantation between 3 and 7 months after their enrollment and completion of serum biomarkers and ultrasound studies; their explant liver samples were available for analysis (Figures 2 and 3).
Figure 2. Liver explant tissue from individual with OTC deficiency who had liver transplantation after biomarker evaluations.
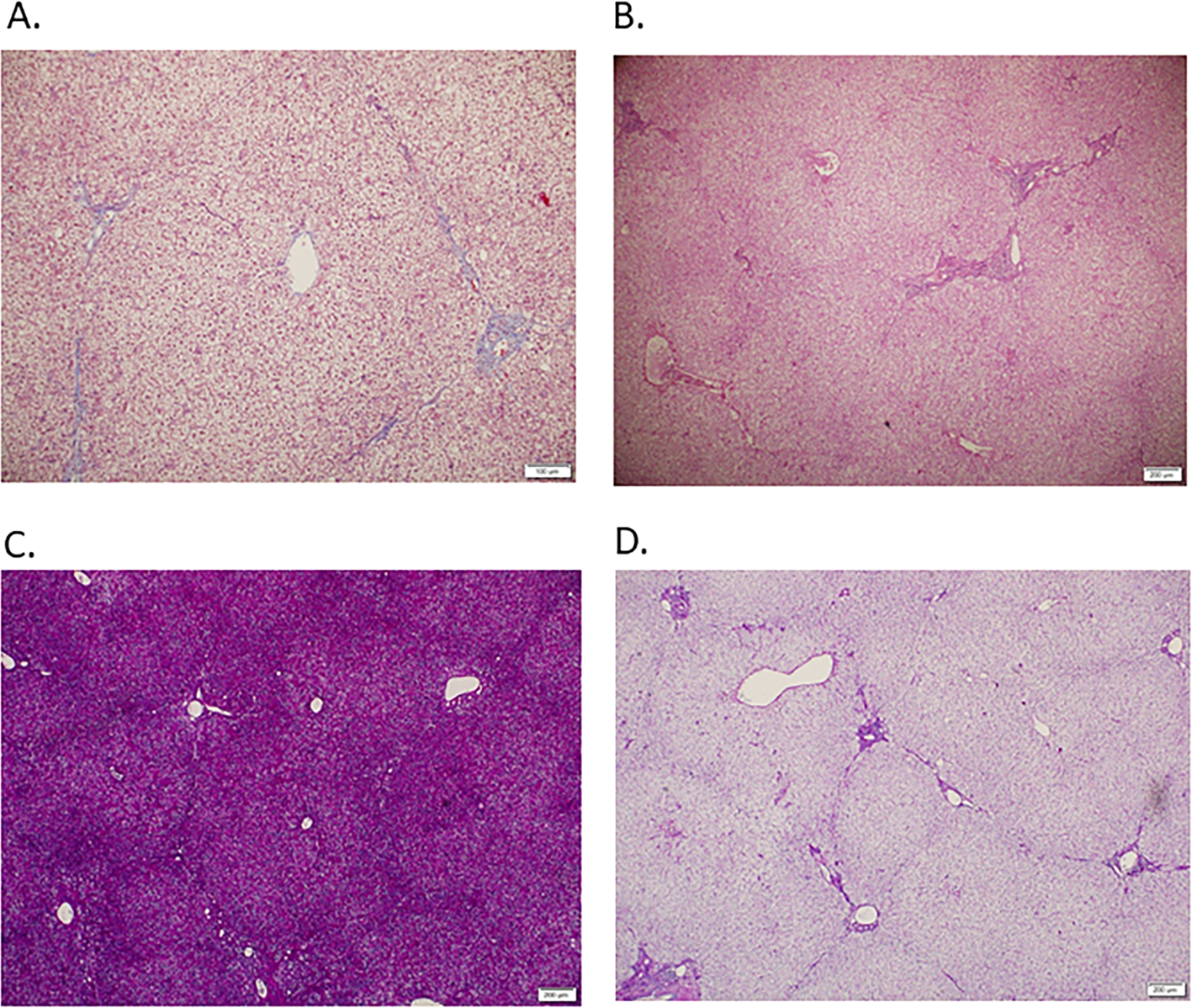
A. Trichrome staining (100x) shows stage 1 fibrosis. B. Hematoxylin and eosin staining (40x) shows mild expansion of portal tracts by fibroconnective tissue. C. PAS stain without diastase (40x) demonstrates strong staining suggestive of hepatic glycogen accumulation. D. PAS with diastase (40x) shows markedly reduced intra-hepatic PAS staining which is consistent with hepatic glycogen.
Figure 3. Liver explant tissue from individual with ARG1 deficiency who had liver transplantation after biomarker evaluations.
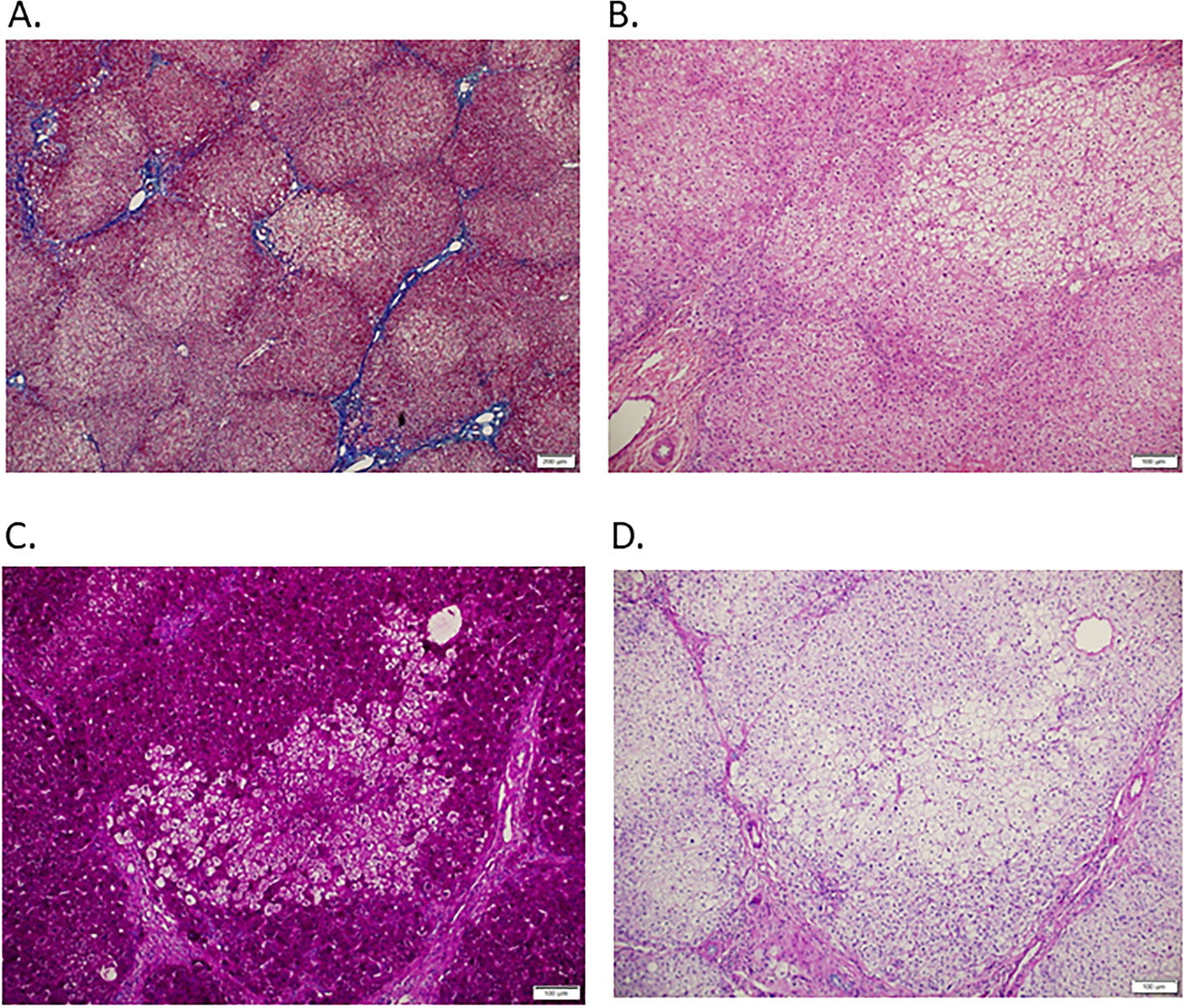
A. Trichrome staining (40x) shows stage 3 fibrosis. B. Hematoxylin and eosin staining (100x) shows variable hepatocyte tinctorial change with many hepatocytes showing pale to moderate eosinophilic cytoplasmic staining with clusters of hepatocytes with clear cytoplasm. C. PAS stain without diastase (100x) suggests excess glycogen with patchy areas with little to no glycogen accumulation corresponding to the areas of clear cytoplasm on H&E. D. PAS stain with diastase (100x) indicates that the PAS stained positive material noted in the absence of diastase is consistent with hepatic glycogen.
A 9-year-old female with OTC deficiency had a liver transplantation 2.5 months after enrollment. The reason for transplantation was metabolic instability with hyperammonemia and history of metabolic stroke. The ultrasound with SWE had shown normal–appearing hepatic parenchyma with median liver stiffness of 1.45 m/s suggestive of F>0 fibrosis. In contrast, the FibroTest™ score predicted no hepatic fibrosis. AST (48 U/L) and ALT (59 U/L) were slightly above the normal range, and ActiTest™ predicted A0-A1 score for necroinflammatory activity. Histopathologic evaluation of the liver explant confirmed stage 1 fibrosis (Figures 2A and 2B). In addition, PAS staining with and without diastase indicated excess glycogen accumulation (Figures 2C and 2D). Microvesicular steatosis was also noted. Thus, increased liver stiffness, elevated ActiTest™, and mild elevations of AST and ALT were associated with the finding of F1 fibrosis in this participant.
A 6-year-old with ARG1 deficiency had liver transplantation 7 months after enrollment. The reason for liver transplantation was metabolic instability and repeated hyperammonemia. Ultrasound with SWE had shown normal-appearing hepatic parenchyma with median liver stiffness of 1.1 m/s, and the FibroTest™ score predicted no hepatic fibrosis. A CT scan performed as a part of clinical care approximately one year prior to enrollment visit had also not demonstrated evidence for hepatic fibrosis or portal hypertension (Figure 3). However, a repeat ultrasound performed four months after the enrollment visit showed a possible mild, diffuse, non-specific, increase in echogenicity. Interestingly, the liver stiffness of 1.1 m/s is above the mean for age (0.98 m/s) and just below the 97.5th%tile for age (1.15 m/s) using the pediatric published data.[39] AST (53 U/L) and ALT (72 U/L) were slightly above the normal range, and ActiTest™ indicated A0-A1 score for necroinflammatory activity. A previous liver biopsy performed approximately 5 years prior to enrollment and prior to the diagnosis of ARG1 deficiency demonstrated portal to portal fibrosis (F2). Trichrome and hematoxylin & eosin staining of liver sections from the explant tissue confirmed stage 3 fibrosis (Figure 3A and 3B). Moreover, PAS staining with and without diastase demonstrated excessive glycogen but with patchy foci of clustered hepatocytes with little to no glycogen (Figure 3C and 3D). No steatosis was noted. Thus, liver stiffness above mean for age, abnormal appearance to the hepatic parenchyma, AST and ALT elevations, and mild elevation in ActiTest™ were associated with the finding of F3 fibrosis in this participant.
3.7. Longitudinal Analysis.
Of the 26 participants who did not have a liver transplantation within one year of the initial evaluation, 13 participants had a repeat evaluation approximately one year after the initial studies (Figure 4). Overall, hepatic echotexture readings by grey-scale ultrasound were stable in 70% (9/13) of participants. The median change for FibroTest™ score was 0 (range: −0.04 to 0.06), a finding which suggests that the FibroTest™ score was relatively stable for most participants during the one-year interval. Likewise, ActiTest™ scores demonstrated a median change of 0.01 (range: −0.04 to 0.15). FIB-4 increased in nearly every participant from year 1 to year 2 (median change of 0.11, range: −1.24 to 0.31). The median change in liver stiffness as assessed by SWE was −0.01, and the range was large (−0.29 to 0.29).
Figure 4. Repeat biomarker evaluation.
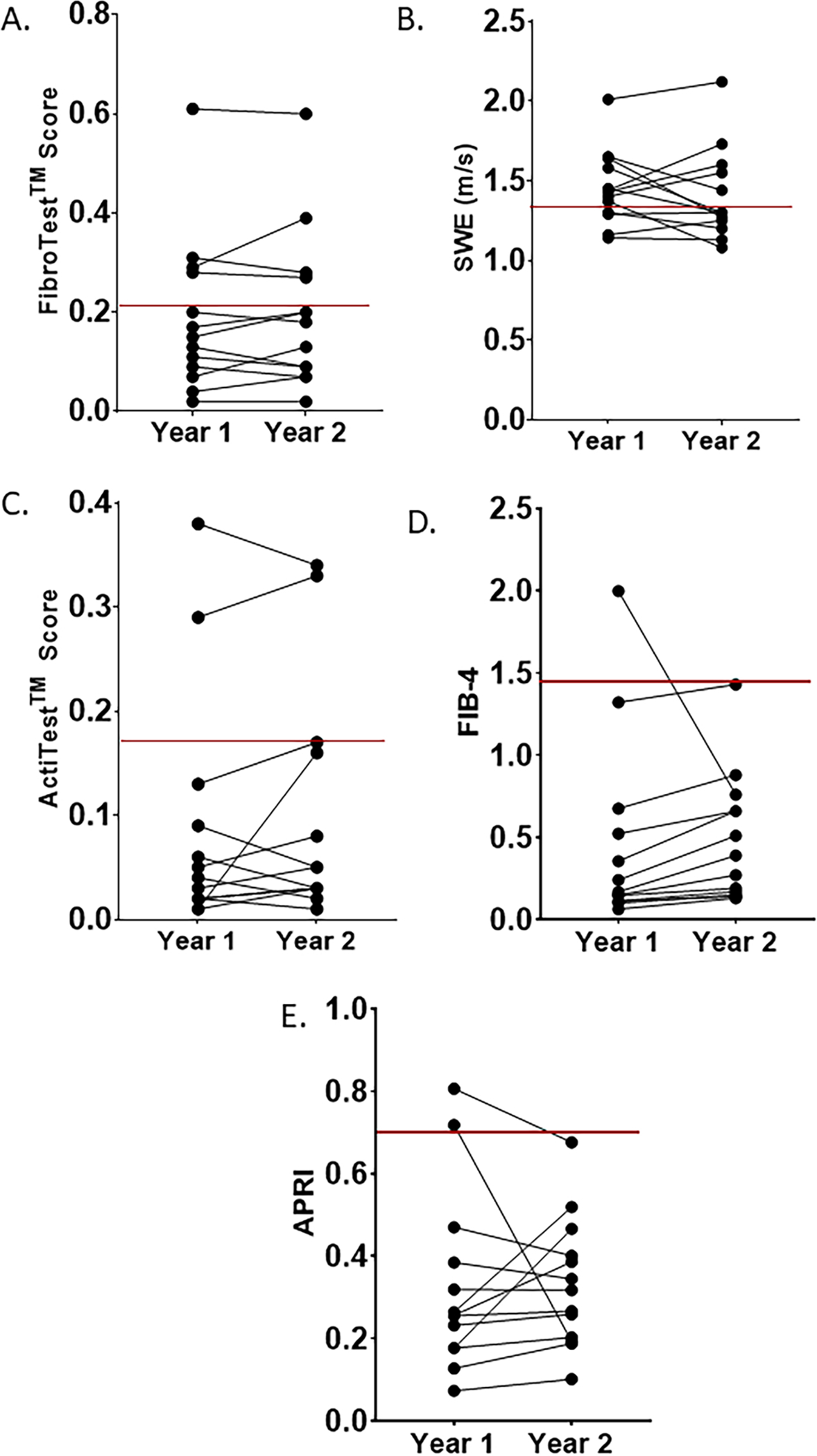
Repeat biomarker evaluation was performed on 13 participants approximately one year after initial evaluation. The year 1 and year 2 values for each biomarker for each individual are shown. The red line indicates the cutoff for normal vs. abnormal values provided by manufacturer or laboratory for liver stiffness obtained by SWE, ActiTest™ and FibroTest™. For FIB-4 and APRI, the red line indicates cutoffs determined in individuals with hepatitis C [36, 37].
4. DISCUSSION
To our knowledge, this is the largest systematic analysis of various biomarkers of liver disease and fibrosis in individuals with UCDs. Overall, our findings provide evidence indicating a high burden of liver abnormalities in individuals with UCDs, including individuals with no known history of liver disease, no known history of hyperammonemia, and normal or near-normal levels of serum AST and ALT. In nearly half of participants, we observed an abnormal grey-scale ultrasound pattern of the hepatic parenchyma. Whereas the exact implication of this finding in UCDs is yet to be understood, heterogeneity of liver on ultrasound and especially a heterogeneous, coarse echotexture and an irregular, nodular margin has been shown to be a marker for advanced liver disease in another Mendelian disorder, cystic fibrosis [40]. In children with cystic fibrosis, heterogenous liver on ultrasound was associated with a 9-fold increase in risk for developing a nodular ultrasound pattern, which is a surrogate marker for advanced cystic fibrosis liver disease [40]. Additionally, in our cohort, regardless of which liver stiffness threshold was used (adult or pediatric), a majority of participants demonstrated increased liver stiffness. Furthermore, ActiTest™-predicted necroinflammation and FibroTest™-predicted fibrosis in 25% and 32%, respectively. This burden of liver disease may be particularly high in adults with UCDs as 8 of 9 adult participants had abnormal appearance of the liver by ultrasound and 6 of 8 adult participants who had valid SWE had increased liver stiffness. This study, together with the increasing number of reports describing the prevalence and impact of liver disease in individuals with UCDs [2–4] demonstrate a need for effective monitoring of progressive liver disease among individuals with UCDs [41].
Given the limited sample size and the variable ages of participants, it is not possible to discern whether these results represent pathology that is unique to a specific UCD diagnosis. We and others have previously demonstrated that a higher proportion of individuals with ASL deficiency and ARG1 deficiency have elevations of serum AST and ALT as well as increased risk for hepatomegaly and abnormal ultrasound pattern of the hepatic parenchyma [2, 3, 6]. Interestingly, four of six individuals with ARG1 deficiency, and two out of eight individuals with ASL deficiency had elevated ActiTest™, a finding which is consistent with our previous work suggesting a high prevalence of liver inflammation in these disorders [2]. However, increased liver stiffness was also noted in at least one individual from each UCD studied. Likewise, abnormal appearance of the hepatic parenchyma and abnormal markers of fibrosis and necroinflammatory activity were observed in individuals with all disorders except ASS1 deficiency.
Participants with abnormal FibroTest™ (which incorporates age) or abnormal hepatic ultrasound appearance of the hepatic parenchyma were significantly older than individuals with normal results. However, age was not associated with abnormal ActiTest™ or increased liver stiffness. Thus, studies in larger cohorts with balanced ages, where possible, are needed to confirm whether age is a risk factor for these complications in individuals with UCDs. Matched ages may be especially important given that cutoffs for several biomarkers used in our study are not available for children. For instance, FibroTest™ scores have been demonstrated to correlate with degree of hepatic fibrosis on liver biopsy in various adult liver diseases but specific cutoffs in children have not been established [29–31]. In addition, previous studies have suggested that UCD severity may be associated with liver disease in UCDs [2, 3]. However, in this study, a history of hyperammonemia was not associated with any of the biomarkers that we evaluated.
An important limitation in the field of rare diseases is the paucity of validated biomarkers that can be used to assess disease severity and to predict which subset of individuals are more likely to develop complications. From a clinical trial readiness aspect, it is important to understand whether biomarkers that have been developed for common disorders are valid when used in specific rare diseases. Furthermore, assessing the correlation between the various biomarkers in rare disease populations is pivotal for selecting appropriate endpoints for clinical investigation as well as clinical care. Thus, in this study, we assessed the association between the presence or absence of abnormalities based on the use of different serum and radiological biomarkers in individuals with UCDs. For instance, there was a trend suggesting elevated FibroTest™ scores in participants with abnormal (>A0) necroinflammatory activity as assessed by ActiTest™. This is not surprising as ActiTest™ scores encompass ALT plus all components of FibroTest™. Likewise, individuals classified categorically into the group with higher than normal (>A0) ActiTest™ scores had higher APRI scores compared to those with normal ActiTest™ scores (A0). However, there were no similar associations between FIB-4 and other serum biomarkers. All but one participant with abnormal FibroTest™ score (>F0) had abnormal appearance of the hepatic parenchyma. Although FibroTest™ was not predictive of fibrosis in the two study participants with liver histology, abnormalities in multiple other biomarkers were noted in these participants including mild elevations in ALT and AST, elevated ActiTest™, and liver stiffness above the mean for age.
The lack of association of liver stiffness as assessed by SWE with the abnormal appearance of the hepatic parenchyma or Metavir fibrosis score predicted by FibroTest™ observed in our study is likely due to the following several factors. First, increased hepatic glycogen and steatosis have been reported in individuals with UCDs [2, 14, 42]. However, SWE does not assess liver fat or glycogen. Whereas the impact of glycogen and fat on liver stiffness in this population is unknown, it is possible that these could be confounding covariates. Likewise, two distinct patterns of abnormal hepatic parenchyma were observed by ultrasound. Although a diffuse, homogenous increase in echogenicity may be more consistent with steatosis and a coarse, heterogeneous echotexture may be more consistent with fibrosis, the impact of hepatic glycogen on the appearance of the liver in the setting of a UCD is unclear. Thus, given the high percentage of individuals (54%) with overweight or obesity in our cohort and the high percentage of individuals (29%) with hepatic ultrasound findings compatible with steatosis, future studies of liver stiffness in UCDs should utilize transient elastography or magnetic resonance elastography as these tools have the capability of quantifying liver fat in addition to liver stiffness. Such tools may facilitate the assessment of the contribution of steatosis to liver stiffness in UCDs, which is important as steatosis is an independent risk factor for hepatic fibrosis [43]. Second, although the collection and processing of samples and the ultrasound measurements were done in a standardized manner as outlined in the manual of operations to assure consistency and minimize variation, SWE is known to be quite variable and operator-dependent [38]. Moreover, there is a marked overlap of fibrosis stages when using the standard published cutoff values for liver stiffness [38]. Lastly, adult-specific manufacturer cutoffs may not accurately reflect increased stiffness in all children. For instance, the use of the 97.5 percentile from published data identified three additional participants from the youngest cohort with higher than expected liver stiffness who were not identified as having elevated liver stiffness using the manufacturer’s cutoffs [39]. Conversely, the participant with stage 3 fibrosis in their explant had liver stiffness that was above the mean for age but not above the 97.5 percentile. It is possible that increased hepatic glycogen may be a confounding factor in the interpretation of liver stiffness in this participant and in other individuals with UCDs.
Of the three individuals (11%) who were predicted to have more than minimal risk for clinically significant fibrosis (>F1) by liver stiffness, two had abnormal appearance of the hepatic parenchyma on ultrasound, but none had abnormal FibroTest™. These results suggest that there may be a better association between increased stiffness and abnormal ultrasound findings in individuals with liver stiffness values that predict more than minimal risk for significant fibrosis. This finding is consistent with our hypothesis that liver stiffness in UCDs may not always reflect fibrosis.
Despite the finding of a high burden of liver disease in UCDs in our study and others [2–6], the mechanisms underlying liver disease in UCDs remain unclear. Hyperammonemia has been suggested to be a risk factor for hepatic fibrosis in more common forms of liver disease as ammonia may activate hepatic stellate cells, which play a key role in the development and progression of hepatic fibrosis [44, 45]. The finding of worsening transaminase levels after exposure to high dose arginine in ASL deficiency supports the hypothesis that exposure to toxic metabolites such as argininosuccinic acid and arginine may also contribute to liver disease in specific disorders, such as ASL deficiency and ARG1 deficiency [46]. In addition, citric acid cycle dysfunction, mitochondrial dysfunction, oxidative stress, and deficiency of metabolites have also been suggested as possible contributing factors to liver disease in this population [4, 6, 47]. Clearly, future mechanistic studies are needed to elucidate the underlying mechanisms for liver disease in the various UCDs as understanding the mechanisms is necessary for the future development of therapeutic strategies targeting this complication.
In conclusion, this evaluation of serum and radiological biomarkers of liver disease in UCDs demonstrates a high burden of liver abnormalities in individuals with UCDs. A majority of participants had mildly increased liver stiffness. However, given the prevalence of elevated hepatic glycogen and possibly steatosis in this population, it is unclear whether liver stiffness always accurately reflects hepatic fibrosis in UCDs. Thus, more studies are needed to further investigate the use of more sophisticated radiological biomarkers and serum biomarkers to assess for risk of fibrosis in this population. The correlation of various biomarkers with explant histopathology should be a focus of future work to validate the utility of various serum and radiologic biomarkers for hepatic disease in UCDs. From a clinical perspective, we recommend that physicians providing care for individuals with UCDs be aware of the burden of liver disease in this population. We encourage physicians to discuss measures to promote liver health including vaccinations against hepatotropic viruses, and avoidance of hepatotoxic medications, alcohol, and excessive weight gain with their patients who have UCDs.
Supplementary Material
Supplemental Figure 1. CT scan of liver from participant with arginase deficiency who had liver transplantation. Abdominal CT scan performed as a part of clinical care approximately one year prior to enrollment visit did not demonstrate evidence for hepatic fibrosis or pericapsular collateral vessels suggestive of portal hypertension
ACKNOWLEDGEMENTS
We thank the participants and families for their participation. LCB holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. This work was also supported by NIH K08DK106453 (to LCB) and by the Urea Cycle Disorders Consortium (UCDC) (U54HD061221). The UCDC (U54HD061221) is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the Office of Rare Diseases Research (ORDR), the National Center for Advancing Translational Science (NCATS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The UCDC is also supported by the O’Malley Foundation, the Kettering Fund, and the National Urea Cycle Disorders Foundation. The project was supported in part by the Clinical Translational Core (CTC) of the Baylor College of Medicine (BCM) Intellectual and Developmental Disabilities Research Center (IDDRC). The BCM IDDRC is supported by P50 HD103555 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The contents of this manuscript are solely the responsibility of the authors and they do not necessarily represent the official views of the NICHD or the National Institutes of Health.
Funding: Funding was provided by NIH, Burroughs Wellcome Fund, the O’Malley Foundation, the Kettering Fund, and the National Urea Cycle Disorders Foundation
Footnotes
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Summar ML, et al. The incidence of urea cycle disorders. Mol Genet Metab. 2013;110(1–2):179–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burrage LC, et al. Chronic liver disease and impaired hepatic glycogen metabolism in argininosuccinate lyase deficiency. JCI Insight. 2020;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ranucci G, et al. Chronic liver involvement in urea cycle disorders. J Inherit Metab Dis. 2019;42(6):1118–27. [DOI] [PubMed] [Google Scholar]
- [4].Bigot A, Tchan MC, Thoreau B, Blasco H, and Maillot F. Liver involvement in urea cycle disorders: a review of the literature. J Inherit Metab Dis. 2017;40(6):757–69. [DOI] [PubMed] [Google Scholar]
- [5].Batshaw ML, Tuchman M, Summar M, Seminara J, and Members of the Urea Cycle Disorders C. A longitudinal study of urea cycle disorders. Mol Genet Metab. 2014;113(1–2):127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kolker S, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J Inherit Metab Dis. 2015;38(6):1059–74. [DOI] [PubMed] [Google Scholar]
- [7].Mori T, et al. Progressive liver fibrosis in late-onset argininosuccinate lyase deficiency. Pediatr Dev Pathol. 2002;5(6):597–601. [DOI] [PubMed] [Google Scholar]
- [8].Trevisson E, et al. Argininosuccinate lyase deficiency: mutational spectrum in Italian patients and identification of a novel ASL pseudogene. Hum Mutat. 2007;28(7):694–702. [DOI] [PubMed] [Google Scholar]
- [9].Zimmermann A, Bachmann C, and Baumgartner R. Severe liver fibrosis in argininosuccinic aciduria. Arch Pathol Lab Med. 1986;110(2):136–40. [PubMed] [Google Scholar]
- [10].Santos Silva E, et al. Liver transplantation in a case of argininaemia. J Inherit Metab Dis. 2001;24(8):885–7. [DOI] [PubMed] [Google Scholar]
- [11].Schlune A, Vom Dahl S, Haussinger D, Ensenauer R, and Mayatepek E. Hyperargininemia due to arginase I deficiency: the original patients and their natural history, and a review of the literature. Amino Acids. 2015;47(9):1751–62. [DOI] [PubMed] [Google Scholar]
- [12].Marble M, et al. Living related liver transplant in a patient with argininosuccinic aciduria and cirrhosis: metabolic follow-up. J Pediatr Gastroenterol Nutr. 2008;46(4):453–6. [DOI] [PubMed] [Google Scholar]
- [13].Szymanska E, et al. Polish Experience with Liver Transplantation and Post-Transplant Outcomes in Children with Urea Cycle Disorders. Ann Transplant. 2017;22(555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Badizadegan K, and Perez-Atayde AR. Focal glycogenosis of the liver in disorders of ureagenesis: its occurrence and diagnostic significance. Hepatology. 1997;26(2):365–73. [DOI] [PubMed] [Google Scholar]
- [15].Koo M, Lipshutz GS, Cederbaum SD, and Lassman C. Biopsy-proven Hepatocellular Carcinoma in a 53-year-old Woman With Arginase Deficiency. Pediatric and Developmental Pathology. 2017. [DOI] [PubMed] [Google Scholar]
- [16].Wilson JM, Shchelochkov OA, Gallagher RC, and Batshaw ML. Hepatocellular carcinoma in a research subject with ornithine transcarbamylase deficiency. Mol Genet Metab. 2012;105(2):263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tsang JP, et al. Arginase deficiency with new phenotype and a novel mutation: contemporary summary. Pediatr Neurol. 2012;47(4):263–9. [DOI] [PubMed] [Google Scholar]
- [18].Baruteau J, et al. Expanding the phenotype in argininosuccinic aciduria: need for new therapies. J Inherit Metab Dis. 2017;40(3):357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheng L, Liu Y, Wang W, Merritt JL, and Yeh M. Hepatocellular Adenoma in a Patient with Ornithine Transcarbamylase Deficiency. Case Reports Hepatol. 2019;2019(2313791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Laemmle A, et al. Frequency and Pathophysiology of Acute Liver Failure in Ornithine Transcarbamylase Deficiency (OTCD). PLoS One. 2016;11(4):e0153358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gallagher RC, Lam C, Wong D, Cederbaum S, and Sokol RJ. Significant hepatic involvement in patients with ornithine transcarbamylase deficiency. J Pediatr. 2014;164(4):720–5 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yucel H, et al. Recurrent hepatic failure and status epilepticus: an uncommon presentation of hyperargininemia. Metab Brain Dis. 2018;33(5):1775–8. [DOI] [PubMed] [Google Scholar]
- [23].Faghfoury H, Baruteau J, de Baulny HO, Haberle J, and Schulze A. Transient fulminant liver failure as an initial presentation in citrullinemia type I. Mol Genet Metab. 2011;102(4):413–7. [DOI] [PubMed] [Google Scholar]
- [24].de Groot MJ, et al. Metabolic investigations prevent liver transplantation in two young children with citrullinemia type I. J Inherit Metab Dis. 2010;33 Suppl 3(S413–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Newnham T, et al. Liver transplantation for argininosuccinic aciduria: clinical, biochemical, and metabolic outcome. Liver Transpl. 2008;14(1):41–5. [DOI] [PubMed] [Google Scholar]
- [26].Colloredo G, Guido M, Sonzogni A, and Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39(2):239–44. [DOI] [PubMed] [Google Scholar]
- [27].Janiec DJ, Jacobson ER, Freeth A, Spaulding L, and Blaszyk H. Histologic variation of grade and stage of non-alcoholic fatty liver disease in liver biopsies. Obes Surg. 2005;15(4):497–501. [DOI] [PubMed] [Google Scholar]
- [28].Regev A, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614–8. [DOI] [PubMed] [Google Scholar]
- [29].Myers RP, et al. Biochemical markers of liver fibrosis: a comparison with historical features in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97(9):2419–25. [DOI] [PubMed] [Google Scholar]
- [30].Poynard T, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Imbert-Bismut F, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357(9262):1069–75. [DOI] [PubMed] [Google Scholar]
- [32].Bedossa P, and Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–93. [DOI] [PubMed] [Google Scholar]
- [33].Wai CT, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. [DOI] [PubMed] [Google Scholar]
- [34].Estey MP, et al. CLSI-based transference of the CALIPER database of pediatric reference intervals from Abbott to Beckman, Ortho, Roche and Siemens Clinical Chemistry Assays: direct validation using reference samples from the CALIPER cohort. Clin Biochem. 2013;46(13–14):1197–219. [DOI] [PubMed] [Google Scholar]
- [35].Colantonio DA, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58(5):854–68. [DOI] [PubMed] [Google Scholar]
- [36].Lin ZH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726–36. [DOI] [PubMed] [Google Scholar]
- [37].Sterling RK, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–25. [DOI] [PubMed] [Google Scholar]
- [38].Barr RG, et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2015;276(3):845–61. [DOI] [PubMed] [Google Scholar]
- [39].Mjelle AB, et al. Normal Liver Stiffness Values in Children: A Comparison of Three Different Elastography Methods. J Pediatr Gastroenterol Nutr. 2019;68(5):706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Siegel MJ, et al. Heterogeneous Liver on Research Ultrasound Identifies Children with Cystic Fibrosis at High Risk of Advanced Liver Disease: Interim Results of a Prospective Observational Case-Controlled Study. J Pediatr. 2020;219(62–9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Haberle J, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis. 2019;42(6):1192–230. [DOI] [PubMed] [Google Scholar]
- [42].Yaplito-Lee J, Chow CW, and Boneh A. Histopathological findings in livers of patients with urea cycle disorders. Mol Genet Metab. 2013;108(3):161–5. [DOI] [PubMed] [Google Scholar]
- [43].Kabbany MN, et al. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol. 2017;112(4):581–7. [DOI] [PubMed] [Google Scholar]
- [44].De Chiara F, et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J Hepatol. 2018;69(4):905–15. [DOI] [PubMed] [Google Scholar]
- [45].Jalan R, et al. Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension. J Hepatol. 2016;64(4):823–33. [DOI] [PubMed] [Google Scholar]
- [46].Nagamani SC, et al. A randomized controlled trial to evaluate the effects of high-dose versus low-dose of arginine therapy on hepatic function tests in argininosuccinic aciduria. Mol Genet Metab. 2012;107(3):315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Erez A, et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med. 2011;17(12):1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. CT scan of liver from participant with arginase deficiency who had liver transplantation. Abdominal CT scan performed as a part of clinical care approximately one year prior to enrollment visit did not demonstrate evidence for hepatic fibrosis or pericapsular collateral vessels suggestive of portal hypertension


