Abstract
This review explores the hypothesis that the repetitive contraction-relaxation that occurs during chronic exercise activates skeletal myocyte Nrf2 to upregulate antioxidant enzymes. These proteins are secreted into the circulation within extracellular vesicles and taken up by remote cells thus providing remote organs with cytoprotection against subsequent oxidative stress.
Keywords: Nrf2, antioxidants, exosomes, extracellular vesicles, cardiac protection
Summary for Table of Contents:
We hypothesize that exercise training protects remote tissues by exosomal transport of antioxidant proteins dependent on skeletal muscle Nrf2.
Introduction
Physical activity is fundamental for human health(1). Conversely, inactivity or sedentary behavior predisposes to cardiovascular disease(2). Furthermore, regular exercise not only reduces the risk of diverse chronic diseases, such as heart failure, hypertension, diabetes, cancers, and others, but also improves their prognosis. Indeed, exercise training (ExT) has been recommended as one of the best non-pharmacological strategies for the attenuation, prevention, and even reversal of several pathological conditions(3). Although it is generally accepted that exercise-benefits rely on neurohumoral, metabolic and other mechanisms, the precise mediators of these beneficial effects remain to be identified. Biochemical processes involved in skeletal muscle work lead to the production of metabolic energy in the form of ATP synthesis. As a consequence oxidative stress is increased during contraction. These fundamental mechanisms provide the genesis for the global effects of ExT.
The metabolic hallmark of contracting muscle is the marked increase in oxygen consumption and mitochondrial respiration, resulting in an increase in reactive oxygen species (ROS) consequent to the production of ATP. Excessive ROS (mostly superoxide and hydrogen peroxide generated from cytosolic NADPH oxidase(4)) not only activate antioxidant defenses in skeletal muscle, but may also be responsible for the systemic adaptation to exercise. Considering the unstable nature and short half-life of ROS, it is unlikely that muscle ROS per se can act as remote signaling molecules to provide adaptive protection to other tissues, although exercise certainly evokes increases in plasma lipid peroxides(5). A more reasonable rationale for remote protection following ExT is the transfer of antioxidant proteins, microRNAs and transcription factors from skeletal muscle to condition peripheral organs.
In this review, we develop the novel hypothesis that ExT-induced systemic antioxidant defense relies, in part, on an intact nuclear factor erythroid-derived 2-like 2/ Kelch-like ECH-associated protein 1 (Nrf2/Keap1) system in skeletal muscle. A second hypothesis is that either Nrf2 or antioxidant proteins that are regulated by Nrf2 in muscle communicate with remote tissues through a delivery system dependent on the muscle release of extracellular vesicles (EVs). To support these hypotheses, we provide experimental evidence and evidence from the literature to demonstrate that contracting muscle activates Nrf2 to upregulate a group of cytoprotective proteins, which are released into the blood within extracellular vesicles (EVs) and transferred to remote tissues. This organ-organ protein communication may underlie the beneficial influence of ExT on autonomic function and cardioprotection in chronic and acute cardiovascular disease.
Nrf2 determines the antioxidant defense of skeletal muscle
Contracting muscle produces high levels of ROS (6, 7). Correspondingly, this tissue is equipped with potent protective mechanisms to neutralize excessive ROS via enzymatic and non-enzymatic compounds and pathways, many of which are dynamically regulated by Nrf2, a master transcriptional regulator responsible for cellular redox hemostasis(8, 9). Under basal conditions, Nrf2 is sequestered in the cytoplasm by its tether, Keap1 and rapidly degraded via the Cullin 3 ubiquitin-proteasomal system(10). When ROS are increased, Nrf2 is liberated from Keap1 and translocates to the nucleus where Nrf2 directly binds to antioxidant response elements (AREs) or indirectly binds to ARE-like sequences within the Nrf2 promoter(11), leading to coordinated upregulation of a broad array of genes encoding proteins involved in anti-oxidation, anti-inflammation, detoxification, and metabolism(8, 9).
Emerging evidence from studies in intact animals and cultured myocytes suggests that Nrf2 is the key factor responsible for the exercise-induced antioxidant enzyme expression in skeletal myocytes. In hind limb muscle of C57BL/6J mice, 6 hours of treadmill running led to the release of Nrf2 from Keap1, translocation into the nucleus and upregulated mRNA expression of superoxide dismutase-1 (SOD1), superoxide dismutase-2 (SOD2), catalase (CAT), and hemoxygenase-1 (HO-1), γ-glutamyl cysteine ligase-catalytic (GCLc), γ-glutamyl cysteine ligase-modulatory (GCLm)(12). In cultured skeletal muscle (C2C12) cells, electrical stimulation evoked Nrf2 activation and upregulation of NAD(P)H:quinone oxidoreductase 1 (NQO1), HO-1 and GCLm mRNAs that were significantly impaired when Nrf2 was knocked down by siRNA(13).
Data from Nrf2 deficient mice reinforce the fact that Nrf2 plays a critical role in skeletal muscle redox homeostasis and maintenance of skeletal muscle function. In muscle from adult Nrf2 knockout (KO) mice, antioxidant enzymes (NQO1, G6PD, CAT, GCS, and GSR) are significantly lower whereas ROS are elevated as compared with age-matched wild type (WT) mice(14). Following chronic ExT, Nrf2 KO mice display an impairment of exercise performance and attenuation of exercise-induced antioxidant enzyme expression and activity(15). Moreover, in these Nrf2 KO mice, electrical stimulation-evoked contraction of in situ gastrocnemius muscle was impaired, characterized by a greater rate of fatigue and a decrease in force as compared with WT mice(16). The critical significance of Nrf2 in skeletal muscle was recently validated and confirmed in a study from our laboratory(17). Employing an inducible-skeletal muscle specific-Nrf2 or Keap1 KO mouse model (Figure 1, panel A), we demonstrated that deletion of muscle Nrf2 dramatically impaired treadmill exercise performance and in situ oxidative/glycolytic muscle contractility. In contrast, overexpressing Nrf2, (i.e. deleting Keap1), markedly enhanced these functions. Importantly, mass spectrometric proteomic analysis of skeletal muscle where Nrf2 was overexpressed, resulted in upregulation of more than 100 proteins (Panel B, Figure 1). Deep bioinformatic analysis suggested that these upregulated proteins were involved in cellular detoxification, NADP metabolism, glutathione metabolism, electron transport chain function (Panel C, Figure 1) and activated antioxidant defenses, xenobiotic detoxification, glutathione synthesis, and the apelin adipocyte signaling pathway (Panel D, Figure 1)(17). We postulated that these Nrf2-targeted proteins may not only protect skeletal muscle per se, but also provide non-muscle tissues and organs with additional defense mechanisms, if they could be delivered to remote targets by organ-organ communication. Indeed, skeletal muscle has been identified as an active endocrine organ producing and releasing myokines, cytokines and other signaling molecules(18). These substances can been transported by circulating exosomes and other extracellular vesicles (EVs) from skeletal muscle to distant tissues and organs to exert protection, particularly during exercise(19, 20).
Figure 1.
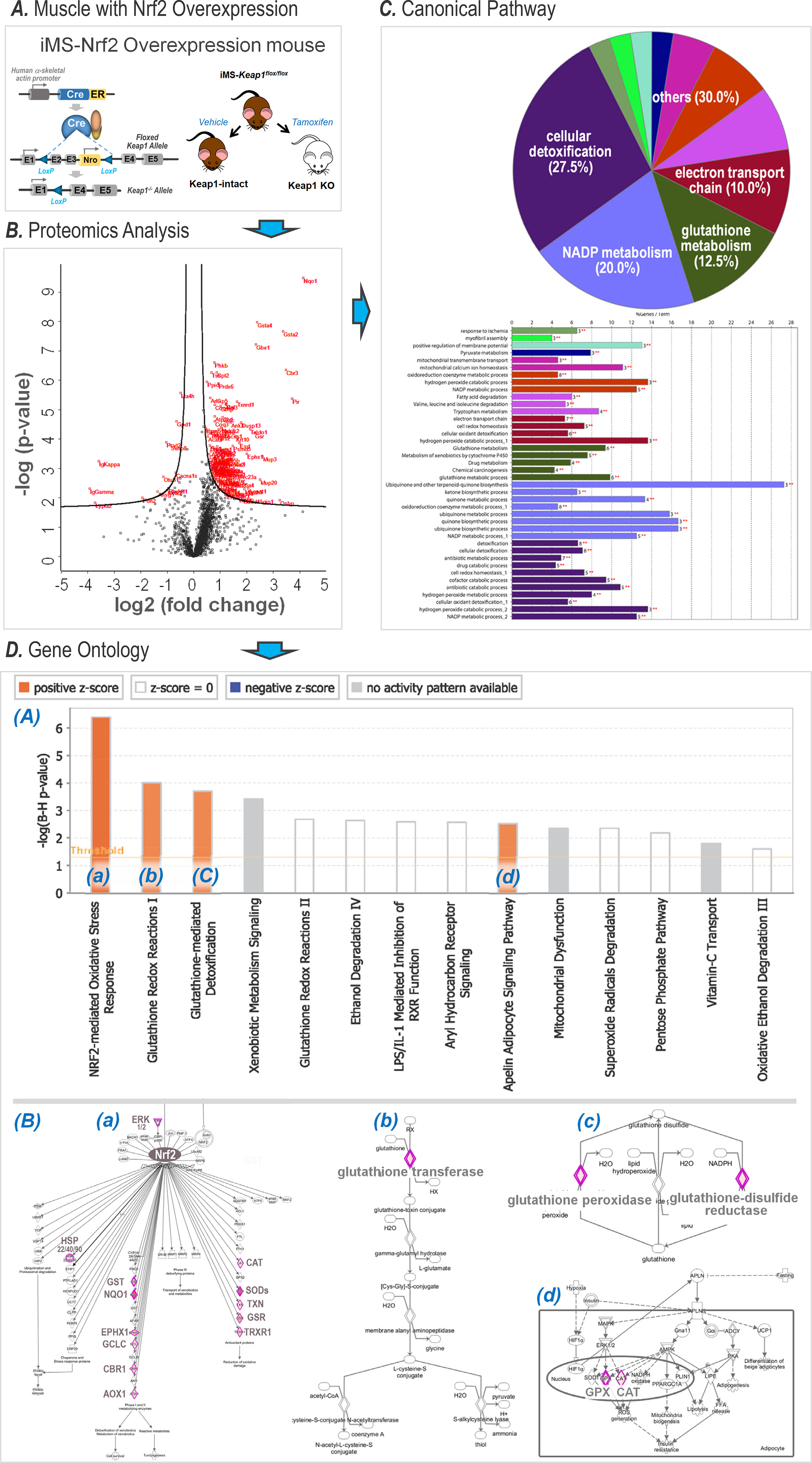
Proteomic and bioinformatic analyses of skeletal muscle in animals with Nrf2 overexpression (Modified from (17). Copyright © 2020 John Wiley & Sons. Used with permission.). A. Transgenic mouse construct with selective muscle Nrf2 overexpression by deletion of exons 2 and 3 of the Keap1 gene. B. Proteomic analysis revealing over 100 proteins that are upregulated; C. Canonical pathway analysis suggesting 4 major metabolic processes potentially impacted; D. Gene ontology analysis suggesting 4 intracellular signaling pathways that are activated (orange bars in subpanel (A)) via upregulating over 20 pivotal proteinss (red rhombi in subpanel (B)) in each of the corresponding pathways labeled as (a), (b), (c), and (d).
Exercise training increases circulating extracellular vesicles
Extracellular Vesicles are small heterogeneous particles composed of a lipid bi-layer containing multiple biomolecules derived from the cytosol and/or plasma membrane of the parent cells(21). They were initially described as “garbage bags” by which the cells eliminate their unneeded products(22). We now know that EVs are involved in a vast array of functional intercellular communication and biomolecule transfer networks(23, 24). Based on their size and biogenesis, EVs have been classified into three main categories. Apoptotic bodies are large vesicles (50 – 5000 nm), released from cells during the programmed cell death process. Apoptotic bodies harbor primarily apoptotic nuclear materials surrounded by a permeable membrane. Exosomes are the smallest EVs (40 – 150 nm) and diffuse into the extracellular medium when intracellular multivesicular bodies fuse with the cellular plasma membrane via the active process of exocytosis. Classical microvesicles are distinguished from the other two EVs by size (150 – 1000 nm) and mechanisms regulating vesicle formation, such as cytoskeleton remodeling and externalization of phosphatidylserine(25). Although the relative concentration of each vesicle subtype is unknown, analysis of EV size distribution in body fluids showed a greater abundance of small vesicles (< 300 nm in diameter) than of larger vesicles(26).
Evidence from animal and human experiments have suggested that exercise can significantly increase circulating EV levels. Fruhbeis, et al.(27) demonstrated that, after a single bout of exhaustive cycling exercise, the total amount of EVs in venous blood of healthy subjects increased, on average, 2.7 times immediately and returned to baseline levels after 90 min. This exercise-triggered rapid release of EVs into the circulation was further confirmed by several additional human experiments(28–32). A milestone study on exercise-released EVs was performed by Whitham, et al.(33), who demonstrated that ExT not only elevated circulating EV concentration, but also enriched their functional protein cargo. They further suggested that these ExT-released EVs exerted remote biological effects, particularly on glycolytic processes in hepatocytes.
Although it is assumed that EVs released following ExT are derived from skeletal muscle, identification of the precise origin of circulating EVs is still unclear. One strategy to help unravel this issue is to assay tissue-specific proteins carried by EVs, for example, using CD105, CD62E, CD14, CD61, FABP, and SCGA as markers of EVs released by the total endothelium, activated endothelium, monocytes/macrophages, platelets, adipose tissue, and striated skeletal muscle, respectively(34). However, these protein markers cannot be used to quantify the amount of EVs released. To overcome this challenge, we recently created a muscle-specific membrane-GFP+ transgenic mouse line, the MS-mG, by crossing the MLC-cre mice with the mT/mG mice. MLC-cre mice express Cre recombinase driven by the skeletal muscle specific myosin light polypeptide 1 promoter(35). The mT/mG line is a double-fluorescent Cre reporter model that expresses membrane-targeted tandem dimer Tomato (mT) prior to Cre-mediated excision and membrane-targeted green fluorescent protein (mG) after excision(36). Thus, the MS-mG model expresses a dual fluorescent protein reporter whose skeletal myocytes exclusively express green fluorescent protein (GFP) while all other tissues express the red fluorescent protein, tdTomato. Accordingly, their EVs are identifiable based on their origins, as illustrated in Panel A of Figure 2. Panel B shows confocal images taken from one MS-mG mouse, demonstrating that GFP is exclusively expressed in skeletal muscle and tdTomato is expressed in all non-muscle tissues. Subpanel (a) is a cross section of the tail, indicating that only tail muscles (six masses) exhibit green fluorescence, whereas all other tissues, including skin, subcutaneous tissues, blood vessels, adipose, nerves, and caudal vertebra exhibit red fluorescence. Subpanels (b) and (c) show a cross section of the gastrocnemius and a longitudinal image of muscle fibers demonstrating GFP in myocytes whereas non-muscle tissues surrounded by myocytes, such as blood vessels, nerve trunks and terminals, capillaries, and endplates express tdTomato. Subpanel (d) shows EVs isolated from plasma grouped into green and red particles, suggesting that their sources are either skeletal muscle or non-muscle tissues. While dtTomato/GFP cre mice have been described in the literature(37), to our knowledge, this is the only animal model currently available to distinguish skeletal muscle-derived EVs from those derived from other tissues. Indeed, the EV populations isolated from plasma of MS-mG mice can be recognized and characterized by nanoscale flow cytometry based on their fluorescent signals. Accordingly, the influence of exercise on skeletal muscle- and non-muscle tissue-derived EVs can be determined in number and size. More importantly, employing this reporter mouse, we can separate and enrich these two types of EVs by fluorescence-activated cell sorting (FACS) for subsequent proteomic analysis, enabling us to present a protein profile of circulating EVs derived from skeletal muscle and non-muscle tissues, respectively. Accordingly, we can provide insight into the skeletal muscle mechanisms responsible for exercise-induced systemic benefits.
Figure 2.
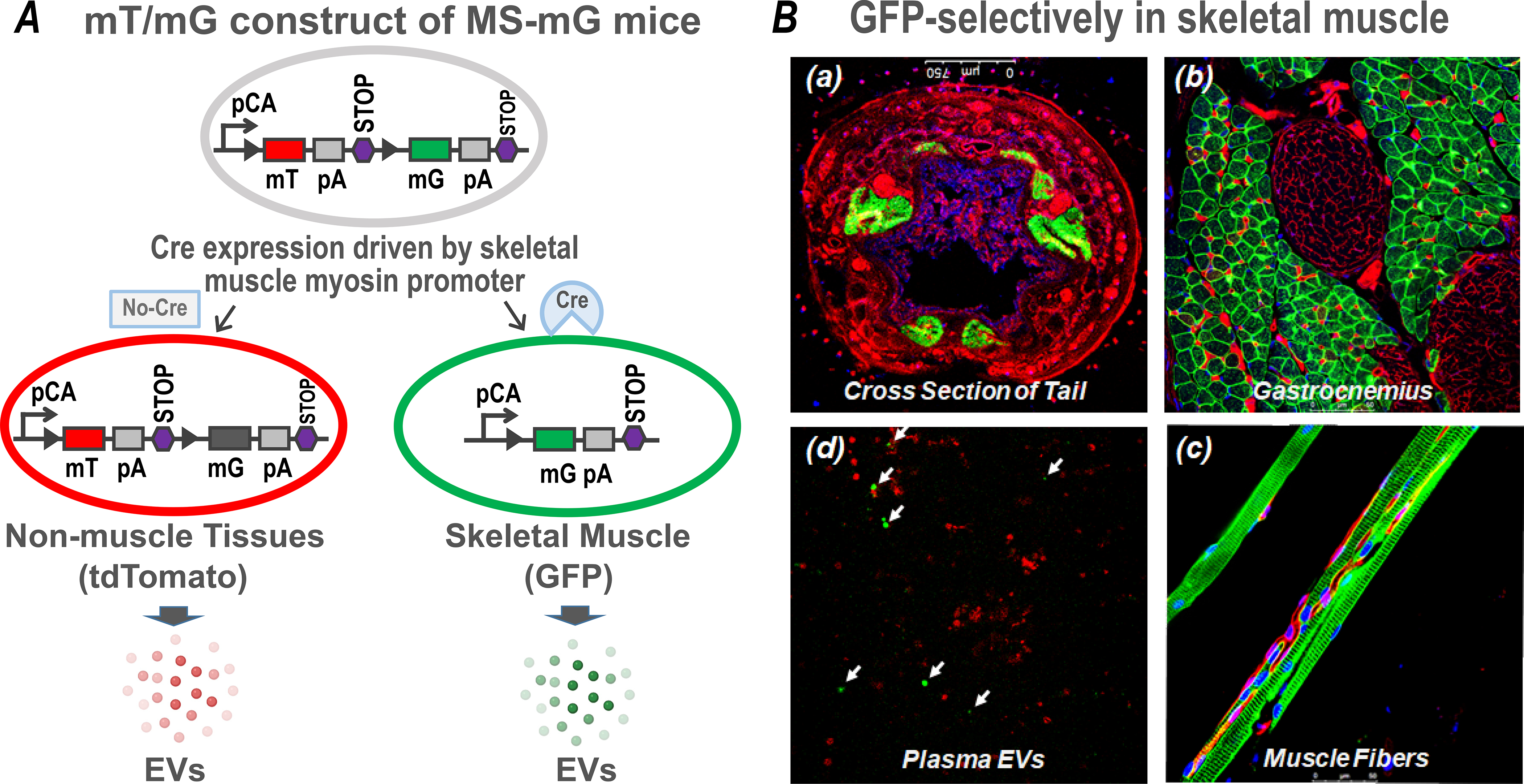
Schematic diagram of gene construct for the double-fluorescent protein expression in MS-mG mice (A) and confocal images from one MS-mG mouse (B) showing that GFP is exclusively expressed in tail muscles (a), gastrocnemius (b), muscle fibers (c), and muscle-derived EVs in plasma (d; arrows indicate the EVs generated by skeletal muscle), while tdTomato is expressed in non-muscle tissues.
Figure 3, Panel A shows the effects of a single bout of exhaustive exercise on plasma EVs of MS-mG mice, demonstrating that acute exercise significantly increases circulating skeletal muscle-EVs by 4-fold (GFP+-particles) and non-muscle-EVs by 2-fold (tdTomato+-particles).
Figure 3.
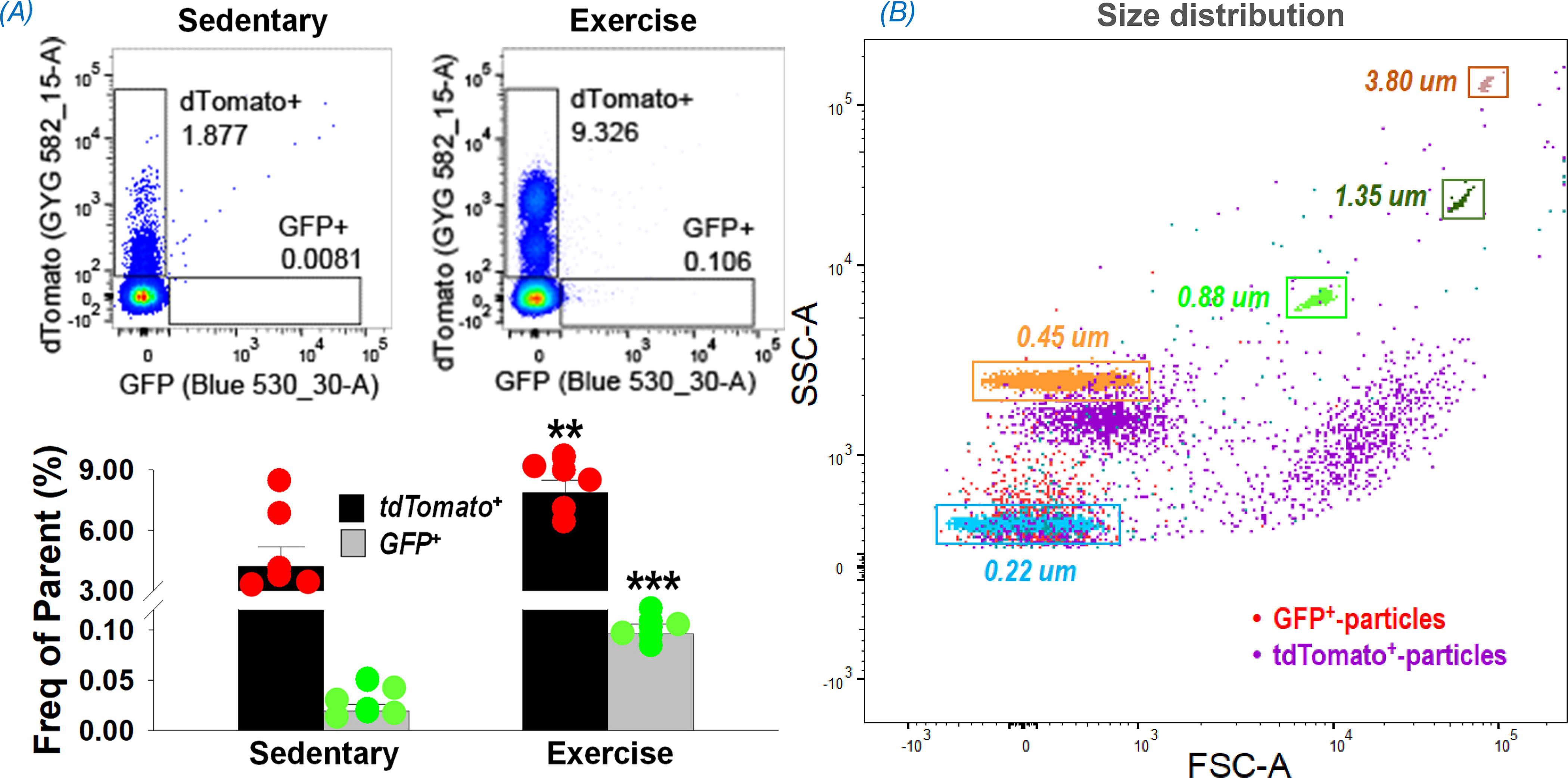
Flow cytometry data showing an increase in skeletal muscle- (GFP+) and non-muscle tissue- (tdTomato+) derived EVs in the plasma following a single bout of exhaustive exercise (A). **p<0.01 and ***p<0.001 vs sedentary mice; n = 6/group. (B) shows size distribution of plasma EVs by overlaying particles on the control beads (dots surrounded by rectangles). GFP+-particles (red) are present in one group, whereas tdTomato+-particles (purple) are present as three distinct size groups. SSC-A: side scatter area; FSC-A: forward scatter area.
Panel B of Figure 3 shows an overlay of the EVs detected in MS-mG mouse plasma on the size of control beads ranging from 0.22 to 3.80 um. Although covering the whole range of control beads, skeletal muscle-derived EVs (GFP+ particles shown as red dots) concentrated in one cluster around 0.22 um, whereas non-muscle tissue-derived EVs (tdTomato+ particles shown as purple dots) were separated into three main groups close to 0.22, 0.45, and 0.88 um areas, implying a single origin of GFP+ particles and multi-organ origins for non-muscle tissue-derived EVs, the tdTomato+ particles.
Skeletal muscle secretes extracellular vesicles by two mechanisms
EVs can be released from all tissues by different mechanisms depending on cell type, physiological circumstances, and pathological challenges. For instance, in endothelial cells, laminar shear stress (SS) has been suggested as a physiological regulator of EV release, where the sustained low SS stimulates EV release through activation of Rho kinases and ERK1/2 pathways, while atheroprotective high SS limits EV release in a nitric oxide (NO)-dependent regulation of ATP-binding cassette subfamily A, member 1- (ABCA1) expression and of cytoskeletal reorganization(38). Redox status (an important metabolic mechanism) is another key factor influencing EV biogenesis and release. Exposure of human alveolar and bronchial epithelial cells to 100 uM H2O2 causes a significant increase in EV release, which was completely abolished by preincubation with the antioxidant N-acetyl-cysteine (NAC)(39). H2O2 treatment also led to time- and dose-dependent EV release from cultured human retinal pigment epithelial cells that was strongly correlated to apoptosis(40). Benedikter, et al. demonstrated that oxidative stress was the major mechanism underlying cigarette smoke extract-augmented EV release of human bronchial epithelial cells(41).
Given an association of EV release with increases in the levels of intracellular calcium(42) and a rapid release of Ca2+ from the sarcoplasmic reticulum following motor neuron stimulation of skeletal muscle fibers(43), it has been proposed that a transient Ca2+ increase during skeletal muscle contraction is likely involved in EV release(44). While not extensively studied in skeletal muscle, data from cancer cells indicates a sarcoplasmic reticulum mechanism that is calcium and calpain dependent(45). Skeletal muscle denervation significantly altered the miRNA component of released EVs with an increase in miR-206 and a decrease in miRs 1, 133a, and 133b(46) however, it is not clear if EV concentration or amount was changed by this intervention. By comparing cultured mouse soleus with extensor digitorum longus, Nie, et al. found that oxidative muscle produces significantly more EVs than glycolytic muscle(47), strongly suggesting that intrinsic metabolic characterization can influence myocyte EV secretion. A recent study by our laboratory assayed circulating EVs of mice with muscle-specific Nrf2 deletion or overexpression. These data provide clues to understanding the mechanisms underlying EV release by skeletal muscle during exercise. Panel A of Figure 4 shows representative electronmicrographic images of isolated plasma EVs. Panel B shows representative NanoSight® results indicating concentration and size distribution of EVs isolated from plasma of WT sedentary mice or following 3 weeks of ExT. Panel C shows group data, where it can be seen that although there were no differences in EV size among groups, plasma EV concentration was dramatically altered by manipulating skeletal muscle Nrf2 and the effects of exercise training. As predicted, we found a significantly higher EV concentration in the plasma of sedentary mice with muscle Nrf2 deficiency that may be attributed to oxidative stress-stimulated EV release from skeletal muscle. In contrast, we unexpectedly found that circulating EVs were also increased in sedentary mice with muscle Nrf2 overexpression (i.e. Keap1 KO). Obviously, this cannot be explained by an increased oxidative stress. Given that this model is characterized by increased expression of antioxidant enzymes, glutathione synthetase, and glutathione in skeletal muscle(17), redox-associated metabolic stressors are not likely the cause of this phenomenon. Indeed, glutathione has been used to prevent ROS-induced EV secretion by scavenging thiol-reactive compounds in cigarette smoke extract-treated airway epithelial cells(41) and preventing ROS from reacting with cellular thiols in alcohol-mediated hepatic injury(48). Alternatively, we believe that the enhanced mechanical activity of skeletal muscle contractility may explain the increased EVs in this Nrf2 overexpression model. Indeed, we found that ExT further increased the EV concentration of Keap1 KO mice as compared with sedentary animals. In contrast, ExT failed to change EVs of Nrf2 KO mice due to their poor exercise performance during training(17). In summary, our data suggest that both metabolic and mechanic factors reluate the EV secretion from skeletal muscle.
Figure 4.
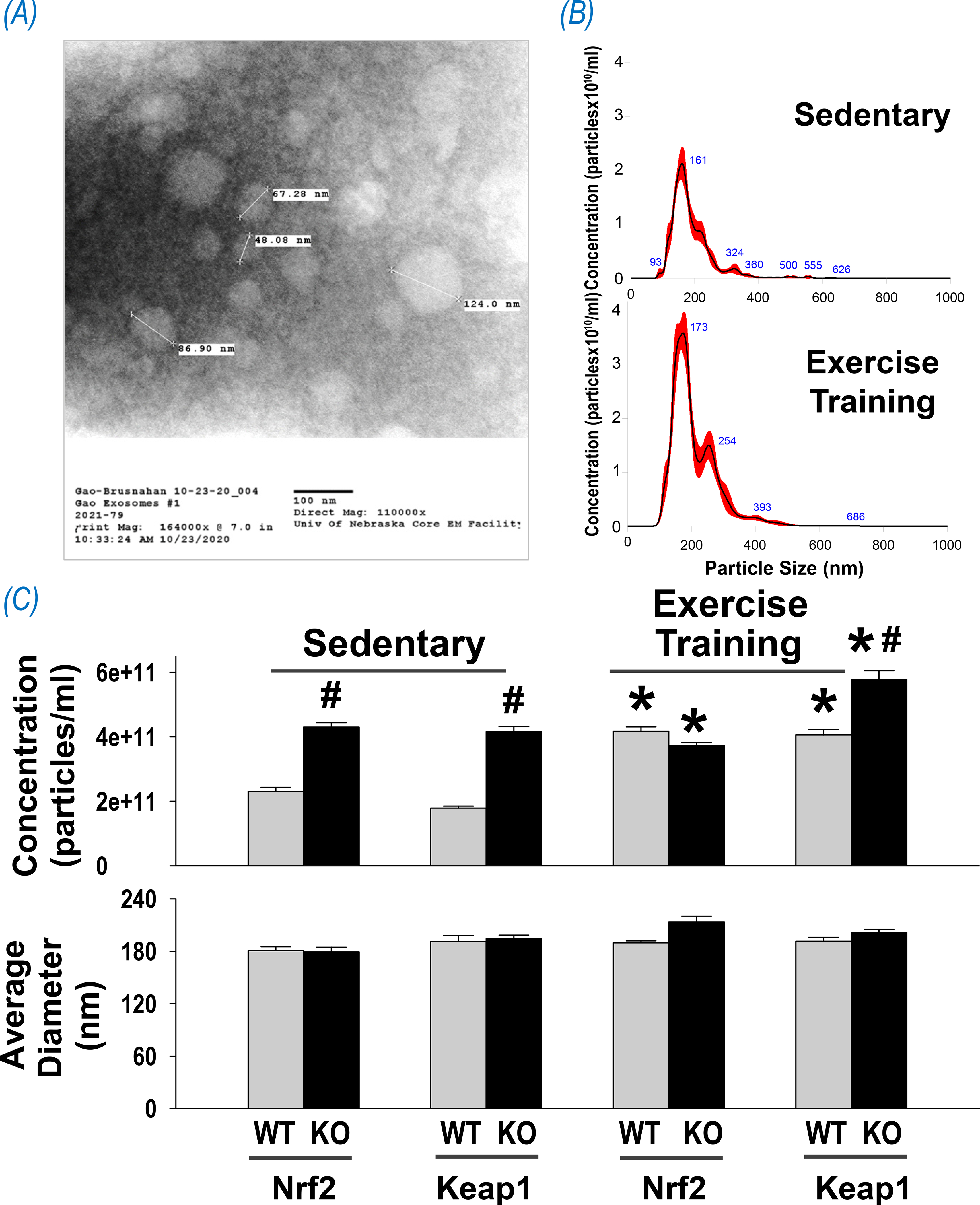
Effects of exercise on circulating EVs of mice with skeletal muscle Nrf2 deficiency or overexpression. (A) Representative electron micrograph of isolated exosomes; (B) Representative results of nanoparticle tracking analysis showing concentration and size distribution of EVs purified from the plasma of sedentary and exercised mice. (C) Mean data of EV concentration and average diameter. *P < 0.05 compared with corresponding mice in sedentary cohort; #P < 0.05 compared with WT mice in the same category. n = 4/group.
ExT-induced systemic antioxidant defense relies on skeletal muscle Nrf2
Regular physical activity confers potent antioxidant effects universally(49). Accordingly, ExT has been recommended as an important intervention to enhance systemic and organ antioxidant capacity in aging(50) and in pathological conditions, such as cardiovascular diseases(51), type 2 diabetes mellitus(52), obesity(53), and Alzheimer’s disease(54). More than 10 years ago, we found a significant increase in oxidative stress in the rabbit rostral ventrolateral medulla (RVLM), a primary central nucleus located in the lower brainstem responsible for the sympathetic regulation, in a model of pacing-induced chronic heart failure (CHF)(55). We further demonstrated that this disturbed central redox balance could be rectified by a four-week treadmill ExT program by enhancing antioxidant defenses that reduced CHF progression(56). These observations were recently reinforced by several studies from our laboratory utilizing transgenic mouse models with coronary artery ligation-induced CHF. We found that the redox status in the RVLM determined the central regulation on sympathetic outflow(57). Impaired antioxidant capacity in the RVLM contributed to sympatho-excitation in CHF(58), and more importantly, ExT ameliorated autonomic activity in the CHF state by upregulating antioxidant enzymes in the RVLM(59). Although the underlying mechanisms are not completely clear, we postulated that this ExT-enhanced central antioxidant effect could be attributed to at least two potential mechanisms. One is induced activation of endogenous antioxidant defenses intracellularly in RVLM neurons by increased neuronal and metabolic activity(60), a well-known phenomenon observed during exercise(61). Another mechanism is the transfer of contracting muscle-derived enzymatic and non-enzymatic antioxidants into the brain from the peripheral circulation; an organ-organ communication pathway with the assistance of circulating EVs. Indeed, it has been proposed that there is a muscle-brain endocrine loop by which the myokine cathepsin B, when increased peripherally by exercise, can pass through the blood-brain barrier (BBB) and enhance brain-derived neurotrophic factor (BDNF) production and hence neurogenesis, memory and learning(62). In addition, it has been demonstrated that circulating EVs derived from different species (human vs mouse) and cell types (cancerous vs non-cancerous cell lines) can cross the BBB and be taken up by the brain(63). Exercise-released EVs contains a vast array of signaling proteins and other functional molecules that targets the brain to slow or prevent the progression of neurodegenerative diseases such as Alzheimer’s disease(33, 64). Accordingly, we propose that contracting muscle-generated antioxidant proteins also enter the brain by similar mechanisms and enhance central antioxidant capacity, resulting in an improvement of autonomic regulation of cardiovascular diseases such as CHF. Consistent with this notion, we found a dramatic enrichment of antioxidant cargo, including NQO1 and SOD2, in circulating EVs following ExT, which relies on intact Nrf2 in skeletal muscle. As can be seen in Figure 5, NQO1 and SOD2 in EVs isolated from plasma of ExT-WT mice was significantly higher than that in sedentary controls, whereas this effect was completely abolished when skeletal muscle Nrf2 was deleted. In this study, we also found a significantly lower NQO1 and SOD2 in the EVs of sedentary Nrf2-deficient mice as compared with sedentary WT mice, suggesting that skeletal muscle Nrf2 is responsible for not only the exercise-induced but also baseline enzymatic antioxidants in circulating EVs.
Figure 5.
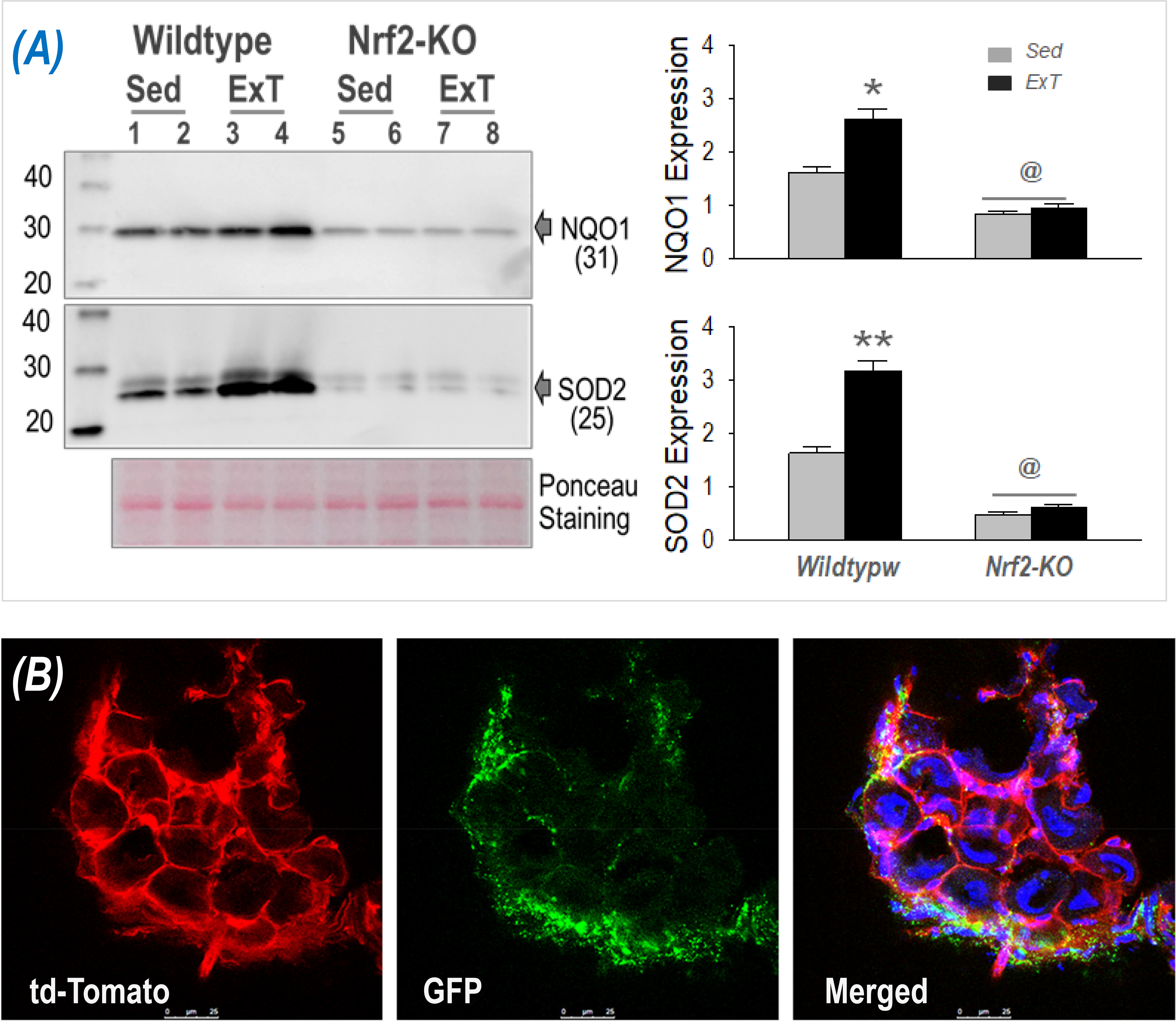
(A) Western blot data showing NQO1 and SOD2 proteins from circulating EVs of WT and skeletal muscle Nrf2-deficient mice that were either sedentary or following ExT. *p<0.05 and **p<0.01 vs sedentary; @p<0.05 vs WT ExT mice; n = 7/group. (B) Confocal image showing appearance of skeletal muscle-derived EVs in T3 dorsal root ganglia following ExT.
Exercise training protects the cardiovascular system by influencing autonomic regulation, as well as having direct effects on the myocardium. Oxidative stress is a hallmark of myocardial ischemia/reperfusion (I/R) injury(65, 66). Reperfusion of ischemic myocardium generates a massive increase in ROS from the mitochondrial respiratory chain and cytosol/cytomembrane NOX family of enzymes(65). These excessive ROS elicit cardiomyocyte death by opening the mitochondrial permeability transition pore, acting as neutrophil chemo-attractants, mediating dysfunction of the sarcoplasmic reticulum and contributing to intracellular Ca2+ overload, damaging the cell membrane by lipid peroxidation, inducing enzyme denaturation, and causing direct oxidative damage to DNA(67, 68). Therefore, supplementation with exogenous antioxidants have been demonstrated to salvage the ischemic myocardium from impending infarction and reduce infarct size(65, 66). Similarly, contracting muscle-generated antioxidant enzymes, once transported to heart, are able to provide the myocardium with additional antioxidant capacity and protect against I/R injury. Panel A of Figure 6 provides experimental evidence showing transfer of skeletal muscle-derived EVs to the heart in skeletal muscle-specific GFP reporter mice, where increased GFP signal in the myocardium following ExT can be observed. Panel B of Figure 6 shows Evans blue/TTC double staining data of mouse hearts indicating that ExT significantly decreased infarct area in WT mice subjected to cardiac ischemia/reperfusion injury as compared with the sedentary controls. On the other hand, in mice with skeletal muscle Nrf2 deficiency, this ExT-evoked cardioprotective effect was impaired (i.e. infarct size was larger), suggesting that skeletal muscle Nrf2 plays a critical role in ExT-protection of the myocardium against I/R injury. The literature suggests that EVs can transfer diverse protective signals from remote conditioned organs to ischemic myocardium(69–72). These include miRNAs(73), cytokines(74), and growth factors(75).
Figure 6.
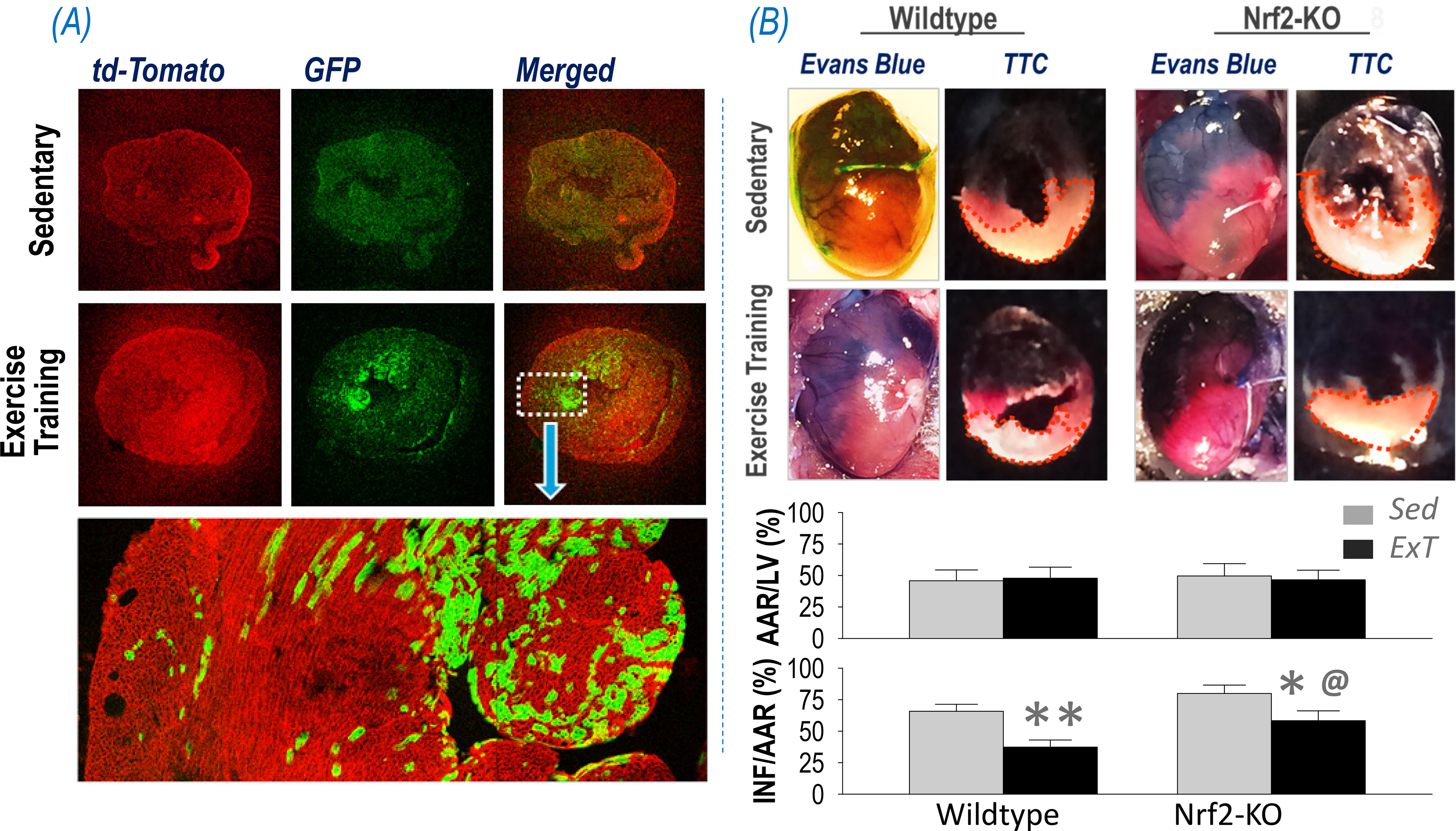
(A) Confocal images showing transfer of skeletal muscle-derived EVs (GFP+) to the myocardium following ExT. (B) Representative images of whole heart/heart sections and quantified data showing the area at risk (AAR) and infarct area (INF) of the left ventricle (LV) subjected to ischemia/reperfusion of mice with muscle Nrf2 intact or deleted following ExT or being sedentary. *p<0.05 and **p<0.01 vs sedentary mice; @p<0.05 vs wildtype ExT mice; n = 7/group.
While there is evidence that mechanical forces can mediate the release of EVs from muscle (20, 76, 77) the specific role of Nrf2 in mediating these events is completely unknown thus opening up a wide field of investigation into specific mechanisms. It will be important to determine the role of oxidative stress and antioxidant proteins in mediating EV release from skeletal muscle. Our data suggest a novel cardioprotective signal from EVs derived from contacting muscle containing a group of enzymatic antioxidants under the governance of Nrf2.
Summary
Global benefits of ExT have been recognized for centuries, particularly on the cardiovascular system(78). This effect, we believe, is an orchestrated result involving multiple systems and diverse mechanisms. In this brief review, we propose a novel mechanism to explain the protective effects of ExT on neural and cardiac function based on the published literature and new data obtained in our laboratory that is dependent on skeletal muscle Nrf2-activated antioxidant genes and EV-mediated organ-organ communication. Certainly, this review is not exhaustive and there are multiple mechanisms to explain the beneficial effects of ExT. A simplified schematic of this pathway is shown in Figure 7. These concepts integrate exercise training, skeletal muscle Nrf2, and EV-mediated inter-organ communication to provide insight into the mechanisms underpinning the benefits of ExT and a new strategy for developing pharmacological therapy to those patients who may benefit from, but are intolerant of ExT.
Figure 7.
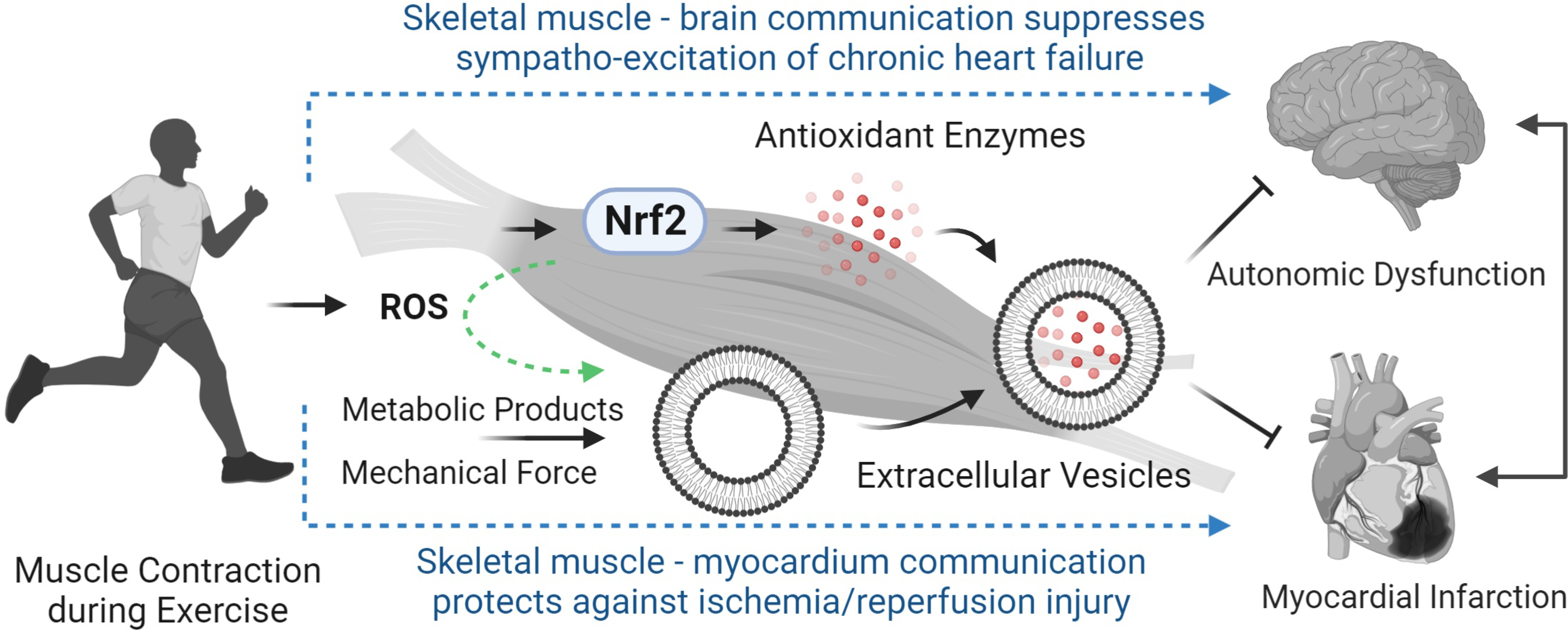
Proposed mechanisms underlying ExT-evoked protection of the cardiovascular system via intercellular communication from contracting skeletal muscle with the central nervous system and the myocardium in the chronic heart failure state and during acute cardiac ischemia.
Key points.
Skeletal muscle nuclear factor erythroid-derived 2-like 2 (Nrf2) plays a crucial role in maintaining redox homeostasis by upregulating more than 100 proteins, that not only protect skeletal myocytes against oxidative stress locally but may also provide distant cells with an additional cytoprotective defense via tissue to tissue communication.
Exercise training (ExT) dramatically increases circulating extracellular vesicles (EVs), that are derived from both skeletal muscle and non-muscle tissues. Skeletal muscle-released EVs mediate delivery of cytoprotective proteins from contracting muscle to remote tissues and may be responsible for ExT-induced beneficial effects.
Nrf2 indirectly influences EV release of skeletal muscle at the rest and during ExT by altering intracellular redox status of myocytes (metabolic mechanism for EV release) and muscle contractility (mechanical mechanism for EV release).
ExT-evoked systemic antioxidant defense is associated with contracting skeletal muscle-released EVs enriched with antioxidant enzymes under the control of skeletal muscle Nrf2 (both cargo and number of circulating EVs). Antioxidant protein transfer from contracting muscle to the central nervous system and myocardium represents a novel mechanism underpinning ExT-induced suppression of sympathetic nerve hyperactivity in the chronic heart failure state and protection of the heart during ischemia/reperfusion injury.
Acknowledgements
The results presented here were supported, in part, by National Institutes of Health (NIH) grant P01 HL62222 (IHZ) and UNMC Frances E. Lageschulte and Evelyn B. Weese New Frontiers in Medical Research Fund (LG). We thank Ms Tara L. Rudebush and Ms Li Yu for their expert technical assistance. We appreciate the professional service of Mass Spectrometry & Proteomics Core, Bioinformatics and Systems Biology Core, Flow Cytometry research Core, Nanomaterials Characterization Facility, and Electron Microscopy Facility of the University of Nebraska Medical Center.
References
- 1.Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol 2017;32(5):541–56. Epub 2017/07/15. doi: 10.1097/HCO.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 2.Katzmarzyk PT, Ross R, Blair SN, Despres JP. Should we target increased physical activity or less sedentary behavior in the battle against cardiovascular disease risk development? Atherosclerosis. 2020;311:107–15. Epub 2020/08/11. doi: 10.1016/j.atherosclerosis.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–8. Epub 2018/11/13. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriquez-Olguin C, Knudsen JR, Raun SH, Li Z, Dalbram E, Treebak JT, et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat Commun 2019;10(1):4623. Epub 2019/10/13. doi: 10.1038/s41467-019-12523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kliszczewicz B, Quindry CJ, Blessing LD, Oliver DG, Esco RM, Taylor JK. Acute Exercise and Oxidative Stress: CrossFit() vs. Treadmill Bout. J Hum Kinet 2015;47:81–90. Epub 2015/11/12. doi: 10.1515/hukin-2015-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers SK, Hamilton K. Antioxidants and exercise. Clin Sports Med 1999;18(3):525–36. [DOI] [PubMed] [Google Scholar]
- 7.Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules. 2015;5(2):356–77. doi: 10.3390/biom5020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev 2018;98(3):1169–203. Epub 2018/05/03. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruns DR, Drake JC, Biela LM, Peelor FF 3rd, Miller BF, Hamilton KL. Nrf2 Signaling and the Slowed Aging Phenotype: Evidence from Long-Lived Models. Oxid Med Cell Longev 2015;2015:732596. doi: 10.1155/2015/732596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol 2006;26(1):221–9. Epub 2005/12/16. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol 2002;22(9):2883–92. Epub 2002/04/10. doi: 10.1128/mcb.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T, He S, Liu S, Kong Z, Wang J, Zhang Y. Effects of different exercise durations on Keap1-Nrf2-ARE pathway activation in mouse skeletal muscle. Free Radic Res 2015;49(10):1269–74. doi: 10.3109/10715762.2015.1066784. [DOI] [PubMed] [Google Scholar]
- 13.Horie M, Warabi E, Komine S, Oh S, Shoda J. Cytoprotective Role of Nrf2 in Electrical Pulse Stimulated C2C12 Myotube. PLoS One. 2015;10(12):e0144835. doi: 10.1371/journal.pone.0144835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller CJ, Gounder SS, Kannan S, Goutam K, Muthusamy VR, Firpo MA, et al. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim Biophys Acta 2012;1822(6):1038–50. doi: 10.1016/j.bbadis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol 2016;594(18):5195–207. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crilly MJ, Tryon LD, Erlich AT, Hood DA. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol (1985). 2016;121(3):730–40. doi: 10.1152/japplphysiol.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao L, Kumar V, Vellichirammal NN, Park SY, Rudebush TL, Yu L, et al. Functional, proteomic and bioinformatic analyses of Nrf2- and Keap1- null skeletal muscle. J Physiol 2020;598(23):5427–51. Epub 2020/09/08. doi: 10.1113/JP280176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann C, Weigert C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb Perspect Med 2017;7(11). Epub 2017/04/09. doi: 10.1101/cshperspect.a029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safdar A, Tarnopolsky MA. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb Perspect Med 2018;8(3). Epub 2017/05/12. doi: 10.1101/cshperspect.a029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vechetti IJ Jr., Valentino T, Mobley CB, McCarthy JJ. The role of extracellular vesicles in skeletal muscle and systematic adaption to exercise. J Physiol 2020. Epub 2020/01/17. doi: 10.1113/JP278929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 2013;113(1):1–11. Epub 2013/03/05. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987;262(19):9412–20. [PubMed] [Google Scholar]
- 23.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018;19(4):213–28. Epub 2018/01/18. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 24.Kalra H, Drummen GP, Mathivanan S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int J Mol Sci 2016;17(2):170. Epub 2016/02/11. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol 2011;31(1):15–26. Epub 2010/12/17. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 26.Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3. Epub 2014/10/04. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fruhbeis C, Helmig S, Tug S, Simon P, Kramer-Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:28239. Epub 2015/07/05. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Just J, Yan Y, Farup J, Sieljacks P, Sloth M, Veno M, et al. Blood flow-restricted resistance exercise alters the surface profile, miRNA cargo and functional impact of circulating extracellular vesicles. Sci Rep 2020;10(1):5835. Epub 2020/04/05. doi: 10.1038/s41598-020-62456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garner RT, Solfest JS, Nie Y, Kuang S, Stout J, Gavin TP. Multivesicular body and exosome pathway responses to acute exercise. Exp Physiol 2020;105(3):511–21. Epub 2020/01/10. doi: 10.1113/EP088017. [DOI] [PubMed] [Google Scholar]
- 30.Brahmer A, Neuberger E, Esch-Heisser L, Haller N, Jorgensen MM, Baek R, et al. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J Extracell Vesicles. 2019;8(1):1615820. Epub 2019/06/14. doi: 10.1080/20013078.2019.1615820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helmig S, Fruhbeis C, Kramer-Albers EM, Simon P, Tug S. Release of bulk cell free DNA during physical exercise occurs independent of extracellular vesicles. Eur J Appl Physiol 2015;115(11):2271–80. Epub 2015/07/02. doi: 10.1007/s00421-015-3207-8. [DOI] [PubMed] [Google Scholar]
- 32.Guescini M, Canonico B, Lucertini F, Maggio S, Annibalini G, Barbieri E, et al. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PLoS One. 2015;10(5):e0125094. Epub 2015/05/09. doi: 10.1371/journal.pone.0125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitham M, Febbraio MA. Redefining Tissue Crosstalk via Shotgun Proteomic Analyses of Plasma Extracellular Vesicles. Proteomics. 2019;19(1–2):e1800154. Epub 2018/10/24. doi: 10.1002/pmic.201800154. [DOI] [PubMed] [Google Scholar]
- 34.Rigamonti AE, Bollati V, Pergoli L, Iodice S, De Col A, Tamini S, et al. Effects of an acute bout of exercise on circulating extracellular vesicles: tissue-, sex-, and BMI-related differences. Int J Obes (Lond). 2020;44(5):1108–18. Epub 2019/10/04. doi: 10.1038/s41366-019-0460-7. [DOI] [PubMed] [Google Scholar]
- 35.Bothe GW, Haspel JA, Smith CL, Wiener HH, Burden SJ. Selective expression of Cre recombinase in skeletal muscle fibers. Genesis. 2000;26(2):165–6. Epub 2000/03/21. [PubMed] [Google Scholar]
- 36.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. Epub 2007/09/18. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 37.Hong J, Lisco AM, Rudebush TL, Yu L, Gao L, Kitzerow O, et al. Identification of Cardiac Expression Pattern of Transient Receptor Potential Vanilloid Type 1 (TRPV1) Receptor using a Transgenic Reporter Mouse Model. Neurosci Lett 2020;737:135320. Epub 2020/08/26. doi: 10.1016/j.neulet.2020.135320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vion AC, Ramkhelawon B, Loyer X, Chironi G, Devue C, Loirand G, et al. Shear stress regulates endothelial microparticle release. Circ Res 2013;112(10):1323–33. Epub 2013/03/29. doi: 10.1161/CIRCRESAHA.112.300818. [DOI] [PubMed] [Google Scholar]
- 39.Novelli F, Neri T, Tavanti L, Armani C, Noce C, Falaschi F, et al. Procoagulant, tissue factor-bearing microparticles in bronchoalveolar lavage of interstitial lung disease patients: an observational study. PLoS One. 2014;9(4):e95013. Epub 2014/04/30. doi: 10.1371/journal.pone.0095013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carver KA, Yang D. N-Acetylcysteine Amide Protects Against Oxidative Stress-Induced Microparticle Release From Human Retinal Pigment Epithelial Cells. Invest Ophthalmol Vis Sci 2016;57(2):360–71. Epub 2016/02/05. doi: 10.1167/iovs.15-17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benedikter BJ, Volgers C, van Eijck PH, Wouters EFM, Savelkoul PHM, Reynaert NL, et al. Cigarette smoke extract induced exosome release is mediated by depletion of exofacial thiols and can be inhibited by thiol-antioxidants. Free Radic Biol Med. 2017;108:334–44. Epub 2017/04/01. doi: 10.1016/j.freeradbiomed.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 42.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem 2003;278(22):20083–90. Epub 2003/03/18. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 43.Melzer W, Rios E, Schneider MF. Time course of calcium release and removal in skeletal muscle fibers. Biophys J 1984;45(3):637–41. Epub 1984/03/01. doi: 10.1016/S0006-3495(84)84203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louzada RA, Bouviere J, Matta LP, Werneck-de-Castro JP, Dupuy C, Carvalho DP, et al. Redox Signaling in Widespread Health Benefits of Exercise. Antioxid Redox Signal. 2020. Epub 2020/03/17. doi: 10.1089/ars.2019.7949. [DOI] [PubMed] [Google Scholar]
- 45.Taylor J, Azimi I, Monteith G, Bebawy M. Ca(2+) mediates extracellular vesicle biogenesis through alternate pathways in malignancy. J Extracell Vesicles. 2020;9(1):1734326. Epub 2020/03/21. doi: 10.1080/20013078.2020.1734326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Gasperi R, Hamidi S, Harlow LM, Ksiezak-Reding H, Bauman WA, Cardozo CP. Denervation-related alterations and biological activity of miRNAs contained in exosomes released by skeletal muscle fibers. Sci Rep 2017;7(1):12888. Epub 2017/10/19. doi: 10.1038/s41598-017-13105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nie Y, Sato Y, Garner RT, Kargl C, Wang C, Kuang S, et al. Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-kappaB signalling. Exp Physiol 2019;104(8):1262–73. Epub 2019/05/23. doi: 10.1113/EP087396. [DOI] [PubMed] [Google Scholar]
- 48.Cho YE, Im EJ, Moon PG, Mezey E, Song BJ, Baek MC. Increased liver-specific proteins in circulating extracellular vesicles as potential biomarkers for drug- and alcohol-induced liver injury. PLoS One. 2017;12(2):e0172463. Epub 2017/02/23. doi: 10.1371/journal.pone.0172463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simoes HG. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med 2017;47(2):277–93. Epub 2016/06/05. doi: 10.1007/s40279-016-0566-1. [DOI] [PubMed] [Google Scholar]
- 50.Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, et al. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018;9(24):17181–98. Epub 2018/04/24. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penna C, Alloatti G, Crisafulli A. Mechanisms Involved in Cardioprotection Induced by Physical Exercise. Antioxid Redox Signal. 2020;32(15):1115–34. Epub 2020/01/02. doi: 10.1089/ars.2019.8009. [DOI] [PubMed] [Google Scholar]
- 52.de Lemos ET, Oliveira J, Pinheiro JP, Reis F. Regular physical exercise as a strategy to improve antioxidant and anti-inflammatory status: benefits in type 2 diabetes mellitus. Oxid Med Cell Longev 2012;2012:741545. Epub 2012/08/29. doi: 10.1155/2012/741545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab 2007;9(6):813–39. Epub 2007/10/11. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 54.Valenzuela PL, Castillo-Garcia A, Morales JS, de la Villa P, Hampel H, Emanuele E, et al. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res Rev 2020;62:101108. Epub 2020/06/21. doi: 10.1016/j.arr.2020.101108. [DOI] [PubMed] [Google Scholar]
- 55.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 2004;95(9):937–44. Epub 2004/10/02. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 56.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115(24):3095–102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 57.Gao L, Zimmerman MC, Biswal S, Zucker IH. Selective Nrf2 Gene Deletion in the Rostral Ventrolateral Medulla Evokes Hypertension and Sympathoexcitation in Mice. Hypertension. 2017;69(6):1198–206. doi: 10.1161/HYPERTENSIONAHA.117.09123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma A, Hong J, Shanks J, Rudebush T, Yu L, Hackfort BT, et al. Upregulating Nrf2 in the RVLM ameliorates sympatho-excitation in mice with chronic heart failure. Free Radic Biol Med 2019;141:84–92. Epub 2019/06/11. doi: 10.1016/j.freeradbiomed.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wafi AM, Yu L, Gao L, Zucker IH. Exercise training upregulates Nrf2 protein in the rostral ventrolateral medulla of mice with heart failure. J Appl Physiol (1985). 2019;127(5):1349–59. Epub 2019/09/27. doi: 10.1152/japplphysiol.00469.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 2018;15:490–503. Epub 2018/02/08. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perrey S. Promoting motor function by exercising the brain. Brain Sci 2013;3(1):101–22. Epub 2013/01/01. doi: 10.3390/brainsci3010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol 2019;15(7):383–92. Epub 2019/03/07. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 63.Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int J Mol Sci 2020;21(12). Epub 2020/06/25. doi: 10.3390/ijms21124407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitham M, Febbraio MA. The ever-expanding myokinome: discovery challenges and therapeutic implications. Nat Rev Drug Discov 2016;15(10):719–29. Epub 2016/09/13. doi: 10.1038/nrd.2016.153. [DOI] [PubMed] [Google Scholar]
- 65.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med 2018;117:76–89. Epub 2018/01/27. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 66.Kurian GA, Rajagopal R, Vedantham S, Rajesh M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid Med Cell Longev 2016;2016:1656450. Epub 2016/06/18. doi: 10.1155/2016/1656450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357(11):1121–35. Epub 2007/09/15. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 68.Zhou T, Chuang CC, Zuo L. Molecular Characterization of Reactive Oxygen Species in Myocardial Ischemia-Reperfusion Injury. Biomed Res Int 2015;2015:864946. Epub 2015/10/29. doi: 10.1155/2015/864946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res 2014;114(2):325–32. Epub 2014/01/18. doi: 10.1161/CIRCRESAHA.113.300636. [DOI] [PubMed] [Google Scholar]
- 70.Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 2016;228(2):R57–71. Epub 2016/01/09. doi: 10.1530/JOE-15-0201. [DOI] [PubMed] [Google Scholar]
- 71.Sluijter JPG, Davidson SM, Boulanger CM, Buzas EI, de Kleijn DPV, Engel FB, et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2018;114(1):19–34. Epub 2017/11/07. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davidson SM, Yellon DM. Exosomes and cardioprotection - A critical analysis. Mol Aspects Med 2018;60:104–14. Epub 2017/11/11. doi: 10.1016/j.mam.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bartekova M, Jelemensky M, Dhalla NS. Emerging role of non-coding RNAs and extracellular vesicles in cardioprotection by remote ischemic conditioning of the heart. Rev Cardiovasc Med 2019;20(2):59–71. Epub 2019/07/28. doi: 10.31083/j.rcm.2019.02.54. [DOI] [PubMed] [Google Scholar]
- 74.Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 2017;14(5):259–72. Epub 2017/02/06. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 75.Davidson SM, Takov K, Yellon DM. Exosomes and Cardiovascular Protection. Cardiovasc Drugs Ther 2017;31(1):77–86. Epub 2016/11/01. doi: 10.1007/s10557-016-6698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bittel DC, Jaiswal JK. Contribution of Extracellular Vesicles in Rebuilding Injured Muscles. Front Physiol 2019;10:828. Epub 2019/08/06. doi: 10.3389/fphys.2019.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vechetti IJ Jr. Emerging role of extracellular vesicles in the regulation of skeletal muscle adaptation. J Appl Physiol (1985). 2019;127(2):645–53. Epub 2019/06/14. doi: 10.1152/japplphysiol.00914.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreira JBN, Wohlwend M, Wisloff U. Exercise and cardiac health: physiological and molecular insights. Nat Metab 2020;2(9):829–39. Epub 2020/08/19. doi: 10.1038/s42255-020-0262-1. [DOI] [PubMed] [Google Scholar]


