Abstract
Background:
Asthma affects millions of people worldwide. Lima, Peru is one of the most polluted cities in the Americas but has insufficient ground PM2.5 (particulate matter that are 2.5 microns or less in diameter) measurements to conduct epidemiologic studies regarding air pollution. PM2.5 estimates from a satellite-driven model have recently been made, enabling a study between asthma and PM2.5.
Objective:
We conducted a daily time-series analysis to determine the association between asthma emergency department (ED) visits and estimated ambient PM2.5 levels in Lima, Peru from 2010 to 2016.
Methods:
We used Poisson generalized linear models to regress aggregated counts of asthma on district-level population weighted PM2.5. Indicator variables for hospitals, districts, and day of week were included to account for spatial and temporal autocorrelation while assessing same day, previous day, day before previous and average across all 3-day exposures. We also included temperature and humidity to account for meteorology and used dichotomous percent poverty and gender variables to assess effect modification.
Results:
There were 103,974 cases of asthma ED visits during the study period across 39 districts in Lima. We found a 3.7% (95% CI: 1.7%-5.8%) increase in ED visits for every interquartile range (IQR, 6.02 μg/m3) increase in PM2.5 same day exposure with no age stratification. For the 0 to 18 years age group, we found a 4.5% (95% CI: 2.2%-6.8%) increase in ED visits for every IQR increase in PM2.5 same day exposure. For the 19 to 64 years age group, we found a 6.0% (95% CI: 1.0%-11.0%) increase in ED visits for every IQR in average 3-day exposure. For the 65 years and up age group, we found a 16.0% (95% CI: 7.0%-24.0%) decrease in ED visits for every IQR increase in PM2.5 average 3-day exposure, although the number of visits in this age group was low (4,488). We found no effect modification by SES or gender.
Discussion:
Results from this study provide additional literature on use of satellite-driven exposure estimates in time-series analyses and evidence for the association between PM2.5 and asthma in a low- and middle-income (LMIC) country.
Keywords: PM2.5, asthma, time-series, ED visits, remote sensing
Introduction
According to the 2018 Global Asthma Report (GBA), approximately 339 million people are living with asthma worldwide [1]. Asthma results in not only premature deaths but also reduced quality of life in people of all ages [2]. Asthma is a chronic disease that affects the airways, especially those of children and the elderly due to early-life development of lung function and subsequent decline in function as age increases. Since the comparison of prevalence and trends of asthma between countries requires large-scale surveys that have not been implemented since the early 2000s, much of the statistics on the burden of asthma are provided by the GBA report. The GBA report also notes that asthma prevalence has been increasing in the past few decades by as much as 50% per decade [3, 4]. Emergency department (ED) visits due to asthma exacerbation incur both direct and indirect economic costs including diagnostic tests, medication, and work and school days lost [5]. Although estimates are not available for many of the developing countries, the economic burden of asthma in the United States is between $150 to $3,000 in direct costs per patient, totaling more than $56 billion annually [6]. Indirect costs, including lost pay from sickness and lost work output from missed school and work days, total $3 billion between 2008–2013 in the U.S. [6]. Air pollution, including PM2.5 (particulate matter with an aerodynamic diameter of 2.5 μm or less), has been shown to be associated with many cardiovascular and respiratory diseases. Furthermore, epidemiologic studies have indicated that exposure to PM2.5 may exacerbate asthmatic symptoms [7–9]. Moreover, past studies on the association between air pollution and asthma have relied on ground monitor measurements, and more recently, on modeled estimates that are more spatially and temporally resolved [10–12].
To date, studies on the association between PM2.5 and asthma were largely conducted in developed countries with sufficient numbers of daily ground monitoring measurements of PM2.5 [12–15]. However, results from these studies may not provide sufficient guidance for low- and middle-income countries (LMIC) where ground monitors are scarce and the composition and concentrations of PM2.5 may differ. Lima, Peru is the third most populous and one of the most polluted cities in the Americas. Lima’s air pollution is largely driven by an aging vehicular fleet in the urban center [16, 17]. Furthermore, particulate matter in Lima may be comprised mainly of black or elemental carbon, nitrogen oxide (NOx), and carbon dioxide from biomass burning, and diesel and gasoline combustion [18]. Conversely, composition of PM2.5 in developed countries may contain more sulfur due to power generation and industrial sources. PM2.5 in developed countries may also have reduced NOx and carbon dioxide due to newer vehicular engines that burn fossil fuels more cleanly [18]. As such, PM2.5 concentrations in developed countries tend to be lower than in LMICs. A study by Silva et al. reported an annual average PM2.5 of 26 μg/m3 in Lima from 2010 to 2015, which exceeds the World Health Organization’s (WHO) annual guidelines of 10 μg/m3 [19]. Sparse daily monitoring data in Lima between March 2014 through December 2016 indicate that at least one in ten PM2.5 monitors exceeds WHO daily guidelines of 25 μg/m3 on 93% of those days. Furthermore, Lima consistently ranks among the top five most polluted urban centers in South America [16]. The spatial distribution of PM2.5 in Lima is impacted by local wind conditions. Air pollution generated in more urban districts near the coast is pushed and trapped against the Andes Mountains in the east by winds blowing from the west. These high PM2.5 levels in conjunction with the meteorological and topographical characteristics of Lima pose a major public health threat and warrant further investigations on air pollution and adverse health outcomes in Lima, Peru.
As one of the most rapidly developing urban centers in South America, one-third of the population of Peru resides in Lima. Although Peru has a decentralized healthcare system and 60% of Peruvians have free medical coverage maintained by the Ministry of Health (MINSA), access to healthcare may be hindered by the large gap in health status between the poor and the rich [20]. Recent studies suggests that the asthma prevalence among children and adolescents in Lima hovers around 13% while other studies indicate asthma prevalence as high as 19.6% for the entire Lima population [21]. Yet, limited monitor measurements have made epidemiologic studies of air pollution in Lima difficult, and there exist few studies pertaining to asthma and air pollution in Lima. There is one prior cross-sectional study estimating the impact of traffic flow on the prevalence of asthma among schoolchildren, and one cohort study over an 8-month period in one neighborhood of Lima [22, 23]. Both studies found a significant association between asthma prevalence and increased exposure to PM2.5; however, these studies are only representative of relatively short time-frames.
Recently, Vu et al. developed a machine learning satellite-driven model that estimated daily PM2.5 at 1 km2 spatial resolution, enabling the possibility of conducting time-series analyses which requires daily estimates of PM2.5 [24]. The time-series analysis has several advantages including the ability to assess if short-term temporal variation in the exposure of interest is associated with changes in the outcome of interest [25]. The newly developed exposure model provides daily PM2.5 estimates from 2010 through 2016, enabling studies with a longer study period, a finer spatial resolution, a larger population size, and stronger statistical power. Several studies have begun to utilize the satellite-derived PM2.5 estimates to study health effects in Lima [7, 26, 27]. A time-series conducted by Davila et al. found a significant positive association between PM2.5 and acute lower respiratory infections, pneumonia, and acute bronchiolitis/asthma in outpatient clinic visits, across different age groups in children up to age five between 2011–2015 [26]. Davila et al.’s study considered asthma together with acute bronchiolitis, was conducted using weekly visits as the outcome, and only examined children under age five. Studies by Tapia et al. found significant associations between PM2.5 and cardiorespiratory outcomes; however, none focused on asthma morbidity [7, 27]. Here, we conduct daily time-series analyses to determine the association between counts of asthma ED visits from nine Lima hospitals and estimated ambient PM2.5 levels in Lima, Peru from 2010 to 2016 across all ages.
Materials and Methods
Study Domain
Lima is nestled 154 meters above sea level between the Pacific Ocean in the west and the Andes Mountains in the east. Home to about 10 million inhabitants (30% of Peru’s entire population), Lima’s air pollution stems from an aging vehicular fleet, biomass burning, and distinct topography. The study domain includes the 43 districts within the province of Lima as well as the Seaport of Callao, and is divided into five different zones (North, South, East, West, and Central) (Figure 1). Since Vu et al.’s model was calibrated to ground stations that are all located below 375 meters in altitude, extrapolation of PM2.5 levels to locations above this height may contain large uncertainties. Thus, four districts (Carabayllo, Chaclacayo, Cienguilla, and Lurigancho) with an average altitude higher than 570 meters were excluded from this study; however, these districts only represented 4% of the total population of the study domain.
Figure 1.
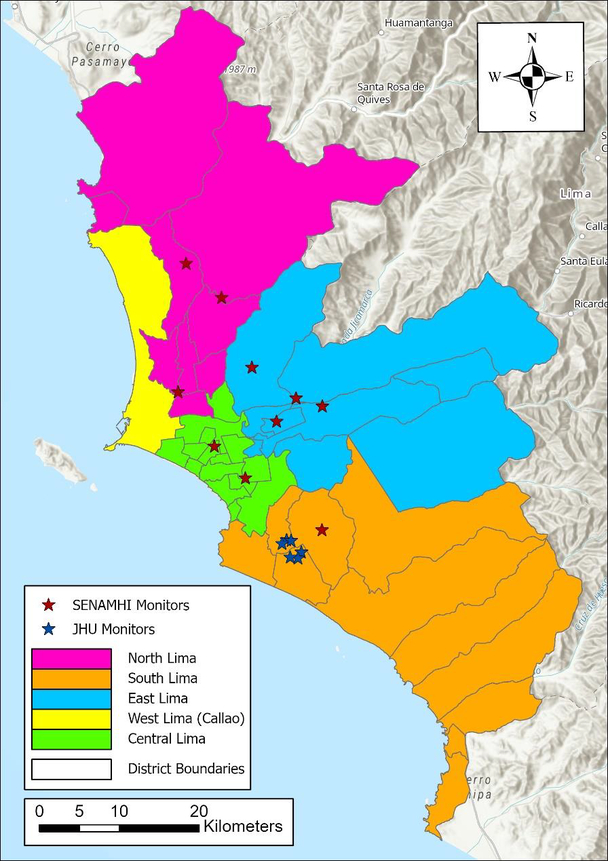
Study domain with the province of Lima and the Seaport of Callao divided into five different zones. The locations of the ten SENMAHI ground monitors and six Johns Hopkins University (JHU) monitor sites used to develop the exposure model that provided daily estimates for the present study are also included.
Satellite-derived PM2.5 Estimates
Ground PM2.5 measurements exist from ten monitors in the Servicio Nacional de Meteorología e Hidrología del Perú (SENAMHI) network, maintained by Peru’s Ministry of Environment from April 2014 through December 2016. Ground measurements also exist from six Johns Hopkins University monitoring sites from November 2011 through March 2013. However, the total number of daily measurements only account for 19% of the days from March 2010 through December 2016 (our study period) where any monitor recorded a measurement on a given day. This results in an ineffective coverage of both spatial and temporal variability of PM2.5 and therefore would not be sufficient for a time-series analysis. Consequently, daily ambient PM2.5 concentrations from March 2010 to December 2016 were estimated by a random forest (RF) model developed by Vu et al. The RF model calibrated satellite aerosol optical depth (AOD), meteorological parameters from chemical transport models, and land use variables with available ground measurements from the two monitoring networks (SENAMHI and Johns Hopkins) [24].
Utilizing estimates derived from an advanced exposure model allows two advantages. First, there is extensive temporal coverage of daily estimates from March 2010 through December 2016, enabling a larger sample size and stronger statistical power to detect any association. Second, the modeled PM2.5 estimates are also spatially resolved, with one estimate for every 1 km2 of the study domain. Such specifications likely reduce many of the uncertainties and biases associated with traditional methods which assign single levels of PM2.5 to all of Lima based on a limited number of monitors. Vu et al.’s RF model achieved a model training R2 and cross-validation (CV) R2 and root mean square prediction error (RMSE) of 0.70 (5.95 μg/m3 model training RMSE and 5.97 μg/m3 CV RMSE). These results indicate good fit and stable prediction capabilities. Since only the district of residence was available as the address for each participant in the health data, the PM2.5 exposure estimates were aggregated to the district level. Each daily 1 km2 PM2.5 estimate was multiplied by the population density of the corresponding 1 km2 LandScan™ population grid. These grids were then summed within each district and divided by the total population of that district to derive a daily population-weighted district-average PM2.5 estimate. In total, the exposure model provided PM2.5 estimates for 2,236 (91%) of the 2,465 days during the study period.
Health Data
Electronic patient records for ED visits were obtained from nine large public hospitals belonging to the Ministry of Health of Peru (MINSA) in Lima during the period of March 2010 through December 2016. Information for each ED visit included the patient’s primary International Classification of Disease 10th Revision (ICD10) diagnosis code, their district of residence, age, and gender. Asthma cases included ICD10 codes J45-J46. Validity of the electronic patient visit records were evaluated by comparing a random sample of 100 electronic medical records with hardcopies of medical history at each hospital. Since personal addresses within districts were not available in the electronic records, the number of visits were aggregated to the district level for each day.
Time-series Analysis
This study utilized a time-series approach through Poisson generalized linear models (GLMs) to estimate the associations between daily district-level PM2.5 and counts of ED visits for asthma. An advantage of a time-series approach is that only time-varying variables can be assessed as confounders. We assessed the effects of same day (lag 0), previous day (lag 1), the day before previous (lag 2), and an average across all 3 prior days (3-day avg.) PM2.5 exposure in separate models. Additionally, a categorical variable for district was added to control for spatial variability and allows the regression to be based only on temporal effects. The district indicator variables also control for spatial autocorrelation in the baseline asthma ED visits across all the districts as well as unmeasured factors that may vary between districts [18]. We also included indicator variables for each day of the week to account for daily fluctuations in PM2.5, and added parametric cubic splines with monthly knots to control for long-term trends in ER visit rates. We controlled for meteorology using same day mean temperature (in linear, quadratic, and cubic forms), and same day mean relative humidity. We also included indicator variables for each hospital to indicate if that hospital contributed any cases for each day. Count data is usually over or under dispersed, meaning the mean and variance of the Poisson counts do not equal each other. Since the variance of the asthma ED visits is larger than the mean, we added a dispersion parameter (pscale in SAS) to account for overdispersion in all of our models.
We assessed effect modification by socioeconomic status (SES), gender, and age in our models. To assess interaction by SES, we obtained estimates of the percent of households above the poverty level for each district from the National Institute of Statistics and Informatics of Peru (INEI). A dichotomous variable was created to indicate whether districts were above or below the median poverty percentage (12.9%). To assess interaction by age, we stratified the asthma ED visit counts into three age strata: 1) 0 to 18 years, 2) 19 to 64 years, and 3) 65 years and above. We ran a separate model for each age-specific group, and effect modification would be indicated by differences in the observed association of PM2.5 between the different age strata. Since 62% of the participants age 18 years and under were also age 6 years and under, we ran a separate model for the participants age 6 years and under as a sensitivity analysis. To assess gender as an effect modifier, we aggregated the number of asthma ED visits by district by gender and included gender as a dichotomous variable. We also aggregated the asthma ED visit counts by gender and district for all three age groups as a sensitivity analysis. Finally, we conducted sensitivity analyses using Zero-Inflated Poisson (ZIP) models to ensure robust results in the 65 years and above age group when many districts carried zero counts due to low ED visits (206 days out of 1,845 days contained zero counts in all districts). We conducted the ZIP model sensitivity analyses on all age groups to ensure results did not deviate from our original models. Data processing was conducted in R© (version 3.6.2) and model analyses were done using SAS v9.4 (SAS Institute Inc., Cary, NC, USA). Best model fit was determined via the lowest Akaike Information Criterion (AIC) value within each age strata.
Results
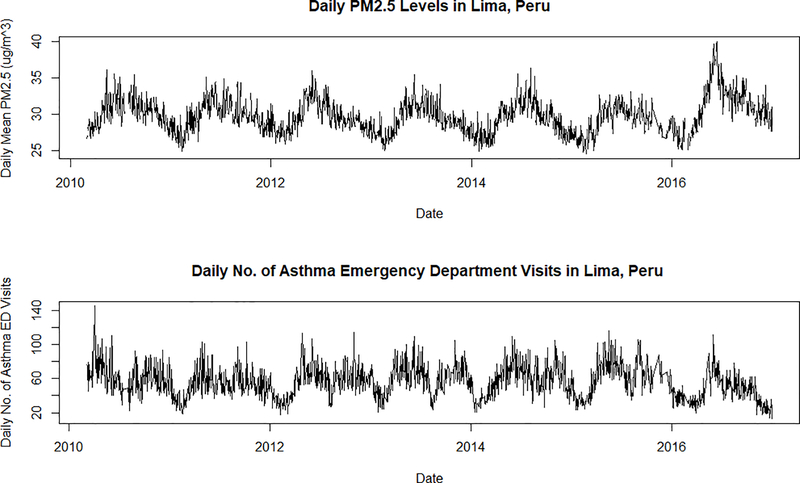
In total, there were 103,974 cases of asthma ED visits in Lima from March 2010 through December 2016. Table S1 in the supplemental shows the number of asthma ED visits for each district in Lima along with the mean and standard deviation of the estimated population-weighted PM2.5 during the entire study period. Table S1 also includes the median and interquartile range of the estimated population-weighted PM2.5. Figure 2 shows the daily Lima-wide average PM2.5 between 2010 and 2016 as well as the daily number of asthma ED visits during the same period. In general, the annual trends of PM2.5 are usually high during the winter months of June-August and low during the summer months of December-March of each year. Asthma ED visits, on the other hand, follow a biannual pattern and peak during the fall and spring months.
Figure 2.
Daily Lima-wide population-weighted average PM2.5 during the study period of March 2010 through December 2016. The bottom panel shows the total number of daily asthma ED visits in all districts during the same time period.
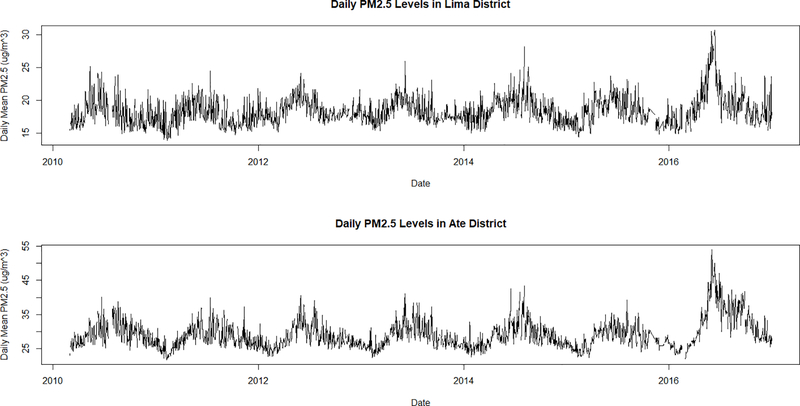
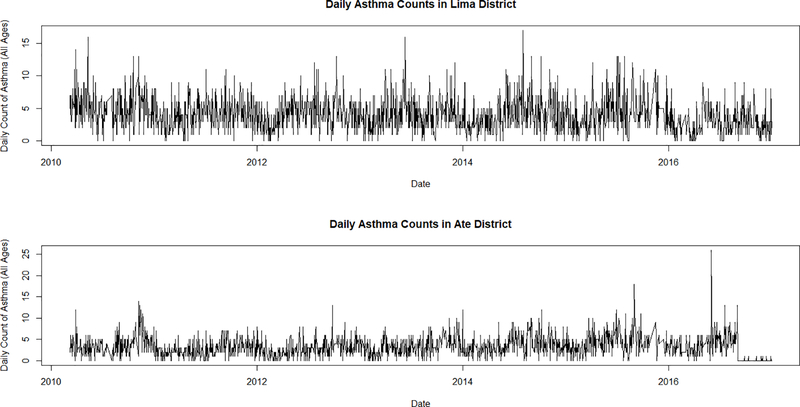
Daily statistics stratified by districts show varying results. Figure 3 shows the time-series of mean daily PM2.5 levels in Lima District and Ate District for the entire study period. Both districts show distinct seasonal patterns of high values in the summer months and lower values in the winter months. However, Lima District is closer to the coast and therefore has a smaller range compared to Ate District located further inland towards the Andes Mountains. Figure 4 shows the time-series of daily counts of asthma for all ages in Lima District and Ate District. Unlike PM2.5, there does not seem to be a marked trend in seasonal or annual patterns of asthma ED visits in Lima during the study period, although in Lima District, asthma does appear somewhat higher in the summer.
Figure 3.
Daily population-weighted district-averaged PM2.5 levels in Lima District (top) and Ate District (bottom) from March 2010 through December 2016. Lima District is closer to the coast and has a lower and smaller range compared to Ate District located further inland. Both districts show similar seasonal and annual trends with higher peaks during the winter and lower peaks in the summer.
Figure 4.
Total number of daily asthma ED visits in Lima District (top) and Ate District (bottom) from March 2010 through December 2016. There does not seem to be a significant difference in the number and trends of asthma ED visits between coastal and mountainous districts.
Validation of patient electronic medical records against paper copies indicate that the date of the patient’s emergency visit had the highest matching rate at 94%. Records with mismatched dates typically disagreed by one to two days, with the hardcopy record date occurring earlier than the date in the digital record. In contrast, ICD10 diagnosis code had the lowest matching rate at 86%. The discrepancy in ICD10 code matching rate was largely due to ambiguous diagnoses when patients presented a wide variety of symptoms during the visit and were subsequently hospitalized. These non-definitive diagnoses were recorded in the hard copy’s emergency room records, but hospital discharge records matched the electronic patient records that we used. Thus, for most cases with discrepancy in diagnosis, we felt the electronic records are likely to be correct. Mismatch between electronic and hardcopy patient records for district, age, and sex, was a result of missing data in the hardcopy of the patient’s records.
Table 1 shows the number of asthma ED visits stratified by sex, age groups, and region for the entire study period. The vast majority of the visits were from young people ages 18 years and below (73.0%) compared to 22.7% for adults ages 19 to 64 years and 4.3% for people ages 65 and older. For all cases of asthma ED visits across all age groups, same day (lag 0) PM2.5 levels produced the best fitting model between daily air pollution and ED visits compared to models assessing lag 1, lag 2, and 3-day average effects. When stratified by age groups; however, we found that lag 0 produced the best fitting model in the 0 to 18 years age group. For the 19 to 64 years and the 65 years and older age groups, the 3-day average produced the best fitting model. All model results are shown in Table 2 in the form of rate ratios (RR) and their 95% confidence intervals for an interquartile range (IQR) increase of 6.02 μg/m3 of PM2.5 exposure. The overdispersion parameter (pscale) ranged from 1.05 to 1.13 indicating that asthma ED counts were not overly dispersed.
Table 1.
Number of asthma ED visits by sex, age groups, and región for the entire study period.
| Total | 103,974 |
| Sex | n (%) |
| Males | 53,944 (51.9) |
| Females | 50,030 (48.1) |
| Age Group | n (%) |
| 0–18 | 75,917 (73.0) |
| 19–64 | 23,569 (22.7) |
| 65+ | 4,488 (4.3) |
| Region | n (%) |
| Central | 28,207 (27.1) |
| East | 19,958 (19.2) |
| North | 22,863 (22.0) |
| South | 20,457 (19.7) |
| West | 12,489 (12.0) |
Table 2.
Comprehensive model results in Rate Ratios (RR) for an interquartile range (IQR) increase of 6.02 μg/m3 of PM2.5 for all cases of asthma ED visits and stratified by age groups along with the model AIC values.
| All Cases | RR (95% CI) | AIC |
| Lag 0 | 1.04 (1.02, 1.06) | 167,411.5 |
| Lag 1 | 1.03 (1.01, 1.05) | 167,419.5 |
| Lag 2 | 1.02 (1.00, 1.04) | 167,422.3 |
| 3-day avg. | 1.04 (1.02, 1.06) | 167,412.9 |
| Aged 0–18 Years | RR | AIC |
| Lag 0 | 1.04 (1.02, 1.07) | 143,285.5 |
| Lag 1 | 1.03 (1.01, 1.06) | 143,294.3 |
| Lag 2 | 1.03 (1.01, 1.05) | 143,294.6 |
| 3-day avg. | 1.05 (1.02, 1.07) | 143,287.1 |
| Aged 19–64 Years | RR | AIC |
| Lag 0 | 1.05 (1.01, 1.10) | 83,374.8 |
| Lag 1 | 1.05 (1.00, 1.09) | 83,376.0 |
| Lag 2 | 1.02 (0.98, 1.07) | 83,379.6 |
| 3-day avg. | 1.06 (1.01, 1.11) | 83,374.1 |
| Aged 65+ Years | RR | AIC |
| Lag 0 | 0.86 (0.79, 0.95) | 27,863.6 |
| Lag 1 | 0.87 (0.80, 0.95) | 27,864.6 |
| Lag 2 | 0.87 (0.80, 0.95) | 27,864.4 |
| 3-day avg. | 0.84 (0.76, 0.93) | 27,861.7 |
We found that an IQR increase in PM2.5 exposure was associated with a 3.7% increase in asthma ED visits. For the 0 to 18 years age group, we found a corresponding 4.5% increase in asthma ED visits and for the 19 to 64 years age group, a 6.0% increase for every IQR increase in PM2.5. In the 65 years and above age group, we found a 16% decrease in asthma ED visits for every IQR increase in PM2.5 exposure. However, these results may be imprecise since the total number of asthma ED visits in this age group for the entire study period is relatively low (4,488 across 7 years). A sensitivity analysis for the 65 years and above age group using the ZIP model yielded similar results with a 18% decrease in asthma ED visits for every IQR increase in PM2.5 exposure. We found no effect modification by SES for all ages combined or specific age groups with p-values ranging from 0.07 (18 years and under age group) to 0.29 (19 to 64 years age group). Similarly, we found no effect modification by gender for all ages combined or specific age groups with p-values ranging from 0.22 (all ages) to 0.32 (18 years and under age group).
Discussion
Although many South American countries have set up official air quality monitoring stations, only 104 cities record measurements for PM10 and 57 cities record measurements for PM2.5 [28]. PM2.5 is smaller and can be transported deeper into lung tissue leading to deadlier adverse health consequences. Many of the epidemiologic studies pertaining to air pollution and asthma have centered on ozone and children under 18 since children’s immune systems and lungs are not fully developed at the start of exposure [29, 30]. Additionally, studies of particulate matter in South America largely focus on PM10 since measurements are more readily available, or are based on estimated particulate matter using chemical models such as CATT-BRAMS [31–33]. Before the development of the PM2.5 exposure model, epidemiologic studies in Lima were hindered by sparse ground and mobile-based measurements and often utilized cross-sectional study designs that make causal inference difficult [22, 34]. One of the main strengths of the present study is the use of daily PM2.5 estimates at 1 km2 spatial resolution, which enables an investigation of the association between asthma and ambient air pollution in not only children but also adults using a time-series approach.
This study found moderately strong associations between ambient PM2.5 exposure and asthma ED visits. The mean population-weighted PM2.5 in Lima was 21.0 μg/m3, while mean population-weighted PM2.5 for individual districts ranged from 16.6 μg/m3 in Magdalena del Mar (Central Lima) to 32.3 μg/m3 in San Juan de Lurigancho (East Lima). This distribution is largely due to strong prevailing winds from the Pacific Ocean. Coastal winds drive the air pollution generated in the urban central region of Lima to the east and northeast regions against the Andes Mountains. Lima’s topography and meteorological conditions lead to a thermal inversion layer that traps air pollution and reduces its dispersion resulting in lower PM2.5 concentrations near the coast and rising concentrations towards the east. The heterogeneity in the spatial distribution of PM2.5 among the districts in Lima indicates that district-specific PM2.5 estimates should be used instead of Lima-wide averages. We conducted a sensitivity analysis using Lima-wide PM2.5 estimates compared to district-specific estimates. Results from this sensitivity analysis indicate that the district-specific associations were stronger, suggesting lower exposure measurement error than the traditional assignment of central or nearest monitor measurement or city-wide averages. We also assessed different lags including same-day, previous day, day before previous, and an average of the three days since studies have shown that the timing of exacerbation of asthma by air pollution varies across ages and geographic locations [35–37].
We found a 3.7% (95% CI: 1.7%, 5.8%) increase in asthma ED visits per IQR increase in PM2.5 exposure in all ages. These results are consistent with previous studies that also utilized a time-series approach with a similar IQR range [38]. A meta-analysis conducted by Zheng et al. looking at 37 studies found a 2.3% (95% CI: 1.5%, 3.1%) increase in asthma cases for a 10 μg/m3 increase in PM2.5 exposure [39]. All the studies in that meta-analysis were conducted in developed countries (20 in N. America, 7 in Europe, 7 in Asia, and 3 in Australia). These authors reported similar results for all age groups [39].
We also found a 4.5% (95% CI: 2.2%, 6.8%) increase in asthma ED visits in people ages 18 years and under for every IQR change in same day (lag 0) PM2.5 exposure. Our findings are consistent with studies Journal conducted in cities in developed countries. A prior multi-city study conducted in Dallas, St. Louis, and Atlanta in the United States reported a 2.0% (95% CI: 1.0%, 4.0%) increase in asthma ED visits per 8.0 μg/m3 increase in PM2.5 exposure in 5 to 18 year-olds [40]. Additionally, Gleason et al. found a 3.0% (95% CI: 2.0%, 4.0%) increase in asthma ED visits in children ages 3 to 17 years living in New Jersey, U.S. per 10 μg/m3 increase in same day PM2.5 exposure [41]. It is expected that same day exposure would result in the best model fit for the 0 to 18 years age group as well as the overall model since 73% of the total sample size consists of children. Studies have shown that the effects of PM2.5 on asthma exacerbation may last up to six days, and that the most significant effects happen during same-day or day-before exposures [42]. The greater effect of PM2.5 on asthma exacerbation in children may partly be explained by the biological mechanisms including inflammation in the alveolar region of the lung caused by deposition of finer particles [43].
We found a 6.0% (95% CI: 1.0%, 11.0%) increase in ED visits per IQR change in PM2.5 for adults ages 19 to 64 years based on the previous 3-day average. Conversely, PM2.5 appeared to be protective for people ages 65 years and above using exposure based on the previous 3-day average. Since the 65 years and above age group had a very low sample size (4,488 cases across 1,845 study period days and 39 districts), and a large number of days contained no cases, sensitivity analyses including ZIP models were conducted to ensure robust model performance. Results from the ZIP analyses were similar although less pronounced. The protective effect in the oldest age group contradicts previous studies including one by Park et al. that reported a cumulative risk greater than one for each lag strata from zero to 15 days per 10 μg/m3 of PM2.5 exposure in people above 65 years of age. It is possible that the 65 years and above population are less likely to present to the ER in general than other ages which may hinder the assessment of the effects of PM2.5 on this age group. Another possibility is that people 65 years and above may have a less responsive immune system and more commonly have allergic asthma. Finally, another possibility is that misclassification of asthma is more common in the 65 years and above age group. Nonetheless, results for the 65 years and above population in this study should be interpreted with caution and that further studies are needed to investigate how to parse out asthma from other comorbidities often associated with the older adult population [44].
The associations found in this study pertain to PM2.5 levels at or well below the Peruvian permitted 24h and annual standards, 50 μg/m3 and 25 μg/m3, respectively. The Peruvian standards are also higher than the 24h and annual standards set by the WHO, 25 μg/m3 and 10 μg/m3, respectively [45]. These two sets of standards highlight the differences in standard levels permissible in a LMIC compared to developed countries. Results from this study are consistent with others previously published that utilized the new PM2.5 exposure estimates. Davila Cordova et al. found a 10% increase in weekly asthma outpatient visits in children under 5 years of age from 2001 to 2015 for every 7.1 μg/m3 increase in PM2.5 exposure [26]. Tapia et al. found a 4% increase in all respiratory ED visits for every 6.1 μg/m3 increase in PM2.5 exposure between 2010 through 2016 [7].
A strength of this study is the use of comprehensive health data from MINSA. We estimate that our population from nine large public hospitals represents about half the population of Lima. We think it is unlikely that the relationship between air pollution and ER visits for this part of the population differs substantially from the rest of Lima, although we have no data to confirm this. Another strength of this study is the ability to obtain large datasets for both the exposure and the outcome of interest which cover a long study period, something made possible by the ecologic design of our study. While we lack some individual level information, such that our study is potentially susceptible to confounding and ecologic bias, the time series design compares the same population to itself over time, lessening these concerns.
There are several limitations in our study. We did not have data to further stratified asthma by phenotypes (e.g. allergic, non-allergic, severe, etc.) which may differ in their association with PM2.5. In addition, we did not have data on ozone and NOx (nitric oxide, NO, and nitrogen dioxide, NO2). Ozone and NOx have been shown to be significantly associated with asthma, independent of the effects of PM2.5. NOx is one of the major chemicals emitted from vehicle exhaust and is a precursor of ozone and the absence of both these compounds are a major limitation to this study. Body mass index (BMI) has also been shown to be associated with both outdoor air pollution exposure and asthma exacerbation. However, information on BMI for each ED visit case was not available and the availability of this information may modify the association between air pollution and asthma ED visits in Lima. Although SES is an effect modifier in the relationship between PM2.5 and asthma in the literature, this study found no such relationship. One possibility might be due to having only limited ecological data on the percentage of the population living in poverty in each district. Furthermore, the effects of gender as an effect modifier on the association between air pollution and asthma has been unclear and results from our study suggests that gender is not an effect modifier in the Lima population. Lastly, another limitation of this study is that health records provided only district-level information on residence, not the exact address of residence, and we were unable to fully utilize the benefits of the 1 km2 PM2.5 exposure model. The aggregation of the PM2.5 estimates to the district level may further pull the associations toward the null due to misclassification of exposure.
Past studies have indicated that asthma exacerbation may also be moderated by both indoor and outdoor PM2.5 concentrations [46]. However, since Lima is a city with moderate climate, and windows are often open, we might expect indoor and outdoor air pollution to be similar [47]. Indoor air can have much higher PM2.5 levels in Peru, but this occurs in the countryside where biomass fuel is used for cooking, which is very uncommon in Lima [48]. Although we do not have indoor air pollution measurements in Lima, differences in outdoor and indoor PM2.5 concentrations may be assessed through a tracer element such as sulfur, which has few indoor generating sources [49].
Conclusion
This study is the first to utilize a time-series approach to investigate the association between satellite-derived PM2.5 estimates and asthma ED visits in Lima, Peru. We found that short-term exposure to ambient PM2.5 is associated with moderate increases in asthma ED visits in children under 19 years of age and among adults ages 19 to 64 years. Results from this study provide new support for such associations in the literature pertaining to LMIC, and also provides evidence for the Peruvian government to investigate the need to lower the current PM2.5 standards in Lima. While we do not know whether lowering Peruvian standards for PM2.5 would result in different findings for the exposure-response of asthma and PM2.5, our findings indicate PM2.5 increases asthma risk. Precautionary principle would argue for lowering PM2.5 levels to lower asthma risk as well as risks for other health endpoints linked to PM2.5 in Lima including ER visits for all cardiorespiratory diseases, overall mortality, and reproductive outcomes.
Future studies in LMICs should attempt to obtain more detailed address information for participants and improved data on potential effect modifiers like SES and BMI. They should also seek to incorporate exposure to ozone and NOx. In Lima specifically, information on the use of emergency rooms for the older adults with asthma and possible mid-diagnoses may help further investigate the seemingly protective effect of PM2.5 in this study. Although the generalizability from our results to other LMICs are uncertain, the methods from this study hopefully provide guidance on how one can conduct epidemiologic studies in developing countries with high air pollution exposure but limited ground monitoring measurements.
Supplementary Material
Highlights.
Asthma ED visits increased 4% per IQR increase in PM2.5 exposure for people in Lima
Children experienced a 5% increase in ED visits per IQR increase in PM2.5 exposure
Adults experienced a 6% increase in ED visits per IQR increase in PM2.5 exposure
Acknowledgement
Research reported in this publication was supported by the NIH Fogarty International Center, National Institutes of Environmental Health Sciences, National Cancer Institute, Centers for Disease Control and the NIH under Award Number U01 TW0101 07 and 5T32ES12870. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work of Vu and Liu were partially supported by NASA Applied Science Program (Grant # 80NSSC19K0191 and #NNX16AQ28G). This research was also supported by the HERCULES Center Pilot Project Program.
Footnotes
Competing Interests
The authors declared that they have no competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Network GA, The Global Asthma Report 2018. 2018: Auckland, New Zealand. [Google Scholar]
- 2.Anenberg Susan C, et al. , Estimates of the Global Burden of Ambient PM2.5, Ozone, and NO2 on Asthma Incidence and Emergency Room Visits. Environmental Health Perspectives, 2018. 126(10): p. 107004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoli M, et al. , The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy, 2004. 59(5): p. 469–78. [DOI] [PubMed] [Google Scholar]
- 4.Braman SS, The global burden of asthma. Chest, 2006. 130 (1 Suppl): p. 4s–12s. [DOI] [PubMed] [Google Scholar]
- 5.Zhang FY, et al. , Estimation of the Effects of Air Pollution on Hospitalization Expenditures for Asthma. International Journal of Health Services, 2020. 50(1): p. 100–109. [DOI] [PubMed] [Google Scholar]
- 6.Nurmagambetov T, Kuwahara R, and Garbe P, The cost of asthma in the United States. America Thoracic Society, 2017. [DOI] [PubMed]
- 7.Tapia V, et al. , Time-series analysis of ambient PM2.5 and cardiorespiratory emergency room visits in Lima, Peru during 2010–2016. Journal of Exposure Science & Environmental Epidemiology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vardoulakis S and Osborne N, Air pollution and asthma. Archives of Disease in Childhood, 2018. 103(9): p. 813–+. [DOI] [PubMed] [Google Scholar]
- 9.Bouazza N, et al. , Fine particulate pollution and asthma exacerbations. Archives of Disease in Childhood, 2018. 103(9): p. 828–831. [DOI] [PubMed] [Google Scholar]
- 10.Alhanti BA, et al. , Ambient air pollution and emergency department visits for asthma: a multi-city assessment of effect modification by age. Journal of Exposure Science and Environmental Epidemiology, 2016. 26 (2): p. 180–188. [DOI] [PubMed] [Google Scholar]
- 11.Bose S, et al. , Association of traffic air pollution and rhinitis quality of life in Peruvian children with asthma. Plos One, 2018. 13(3): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang HH, et al. , Time-series analysis of satellite-derived fine particulate matter pollution and asthma morbidity in Jackson, MS. Environmental Monitoring and Assessment, 2019. 191: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo BQ, et al. , Associations between short-term exposure to fine particulate matter and acute exacerbation of asthma in Yancheng, China. Chemosphere, 2019. 237: p. 6. [DOI] [PubMed] [Google Scholar]
- 14.Strosnider HM, et al. , Age-Specific Associations of Ozone and Fine Particulate Matter with Respiratory Emergency Department Visits in the United States. American Journal of Respiratory and Critical Care Medicine, 2019. 199(7): p. 882–890. [DOI] [PubMed] [Google Scholar]
- 15.Abrams JY, et al. , Associations between Ambient Fine Particulate Oxidative Potential and Cardiorespiratory Emergency Department Visits. Environmental Health Perspectives, 2017. 125(10): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO (World health Organization). WHO Global Urban Ambient Air Pollution Database. 2016 August 25, 2017]; Available from: http://www.who.int/phe/health_topics/outdoorair/databases/cities/en/.
- 17.Fischer K, Improving Sustainable Development in Lima Through Public Transportation. Perspectives on Business and Economics, 2017. 35(Leveraging Peru's Economic Potential): p. 6. [Google Scholar]
- 18.Ventura LMB, et al. , Chemical composition of fine particles (PM2.5): water-soluble organic fraction and trace metals. Air Quality, Atmosphere & Health, 2017. 10(7): p. 845–852. [Google Scholar]
- 19.Silva J, et al. , Particulate matter levels in a South American megacity: the metropolitan area of Lima-Callao, Peru. Environmental Monitoring and Assessment, 2017. 189(12): p. 635. [DOI] [PubMed] [Google Scholar]
- 20.Global Health Workforce Alliance, Peru W 2020. 06/15/2020]; Available from: https://www.who.int/workforcealliance/countries/per/en/#:∼:text=Peru%20has%20a%20decentralized%20health,together%20provide%20services%20to%20the.
- 21.Lai CKW, et al. , Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax, 2009. 64(6): p. 476. [DOI] [PubMed] [Google Scholar]
- 22.Rivero KMR, et al. , Effects Of Long-Term Exposure To Pm2.5 On Asthma Control In Children: Longitudinal Study In A Peri-Urban Community In Lima, Peru. American Journal of Respiratory and Critical Care Medicine, 2016. 193: p. 1.26720781 [Google Scholar]
- 23.Carbajal-Arroyo L, et al. , Impact of traffic flow on the asthma prevalence among school children in Lima, Peru. Journal of Asthma, 2007. 44(3): p. 197–202. [DOI] [PubMed] [Google Scholar]
- 24.Vu BN, et al. , Developing an Advanced PM(2.5) Exposure Model in Lima, Peru. Remote sensing, 2019. 11(6): p. 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhaskaran K, et al. , Time series regression studies in environmental epidemiology. International journal of epidemiology, 2013. 42(4): p. 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davila Cordova JE, et al. , Association of PM(2.5) concentration with health center outpatient visits for respiratory diseases of children under 5 years old in Lima, Peru. Environmental health: a global access science source, 2020. 19(1): p. 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tapia V, et al. , PM(2.5) exposure on daily cardio-respiratory mortality in Lima, Peru, from 2010 to 2016. Environ Health, 2020. 19(1): p. 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riojas-Rodríguez H, et al. , Air pollution management and control in Latin America and the Caribbean: implications for climate change. Rev Panam Salud Publica, 2016. 40(3): p. 150–159. [PubMed] [Google Scholar]
- 29.Schwartz J, Air pollution and children's health. Pediatrics, 2004. 113(4): p. 1037–1043. [PubMed] [Google Scholar]
- 30.Peters JM, et al. , A study of twelve southern California communities with differing levels and types of air pollution - II. Effects on pulmonary function. American Journal of Respiratory and Critical Care Medicine, 1999. 159(3): p. 768–775. [DOI] [PubMed] [Google Scholar]
- 31.Cesar ACG, Nascimento LFC, and Carvalho J.A. d. Jr., Association between exposure to particulate matter and hospital admissions for respiratory disease in children. Revista de saude publica, 2013. 47(6): p. 1209–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuan TS, Venâncio TS, and Nascimento LF, Air pollutants and hospitalization due to pneumonia among children. An ecological time series study. Sao Paulo Med J, 2015. 133(5): p. 408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousa SIV, et al. , Short-term effects of air pollution on respiratory morbidity at Rio de Janeiro — Part II: Health assessment. Environment International, 2012. 43: p. 1–5. [DOI] [PubMed] [Google Scholar]
- 34.Underhill JL, et al. , Association of Roadway Proximity with Indoor Air Pollution in a Peri-Urban Community in Lima, Peru. International Journal of Environmental Research and Public Health, 2015. 12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien L-C, Chen Y-A, and Yu H-L, Lagged Influence of Fine Particulate Matter and Geographic Disparities on Clinic Visits for Children's Asthma in Taiwan. International journal of environmental research and public health, 2018. 15(4): p. 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schildcrout JS, et al. , Ambient Air Pollution and Asthma Exacerbations in Children: An Eight-City Analysis. American Journal of Epidemiology, 2006. 164(6): p. 505–517. [DOI] [PubMed] [Google Scholar]
- 37.Rosenquist NA, et al. , Acute associations between PM2.5 and ozone concentrations and asthma exacerbations among patients with and without allergic comorbidities. Journal of Exposure Science & Environmental Epidemiology, 2020. 30(5): p. 795–804. [DOI] [PubMed] [Google Scholar]
- 38.Castner J, Guo L, and Yin Y, Ambient air pollution and emergency department visits for asthma in Erie County, New York 2007–2012. International Archives of Occupational and Environmental Health, 2018. 91(2): p. 205–214. [DOI] [PubMed] [Google Scholar]
- 39.Zheng X.-y, et al. , Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PloS one, 2015. 10(9): p. e0138146–e0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alhanti BA, et al. , Ambient air pollution and emergency department visits for asthma: a multi-city assessment of effect modification by age. Journal of Exposure Science & Environmental Epidemiology, 2016. 26(2): p. 180–188. [DOI] [PubMed] [Google Scholar]
- 41.Gleason JA, Bielory L, and Fagliano JA, Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: A case-crossover study. Environmental Research, 2014. 132: p. 421–429. [DOI] [PubMed] [Google Scholar]
- 42.Slaughter JC, et al. , Effects of ambient air pollution on symptom severity and medication use in children with asthma. Ann Allergy Asthma Immunol, 2003. 91(4): p. 346–53. [DOI] [PubMed] [Google Scholar]
- 43.Anderson PJ, Wilson JD, and Hiller FC, Respiratory tract deposition of ultrafine particles in subjects with obstructive or restrictive lung disease. Chest, 1990. 97(5): p. 1115–20. [DOI] [PubMed] [Google Scholar]
- 44.Gillman A and Douglass JA, Asthma in the elderly. Asia Pacific allergy, 2012. 2(2): p. 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bicentenario DOD, Approve Environmental Quality Standards (EQS) for Air and established Complementary Provisions. 2017.
- 46.Jie Y, et al. , Do indoor environments influence asthma and asthma-related symptoms among adults in homes? A review of the literature. Journal of the Formosan Medical Association, 2011. 110(9): p. 555–563. [DOI] [PubMed] [Google Scholar]
- 47.Organization) WWH. Background information on urban outdoor air pollution. 2021. [cited 2021 February 26, 2021]; Available from: https://www.who.int/phe/health_topics/outdoorair/databases/background_information/en/.
- 48.Kephart JL, et al. , Indoor air pollution concentrations and cardiometabolic health across four diverse settings in Peru: a cross-sectional study. Environmental Health, 2020. 19(1): p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habre R, et al. , The effects of PM2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. Journal of Exposure Science & Environmental Epidemiology, 2014. 24(4): p. 380–387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.