Abstract
Immune compromised children are threatened by a higher risk of infections; some of these are preventable by vaccination. Primary care physicians play a fundamental role in optimising vaccination status. In this narrative review, we present the evidence on vaccine safety and immunogenicity in immune compromised children and discuss in which conditions live-attenuated vaccines can possibly be used. Vaccination schedules differ in some of these conditions, including the use of vaccines with higher antigenic contents (e.g. high-dose hepatitis B vaccine), additional vaccine doses (e.g. 2-dose schedule meningococcal vaccine), more frequent booster doses (e.g. life-long pneumococcal vaccine booster), supplementary vaccines (e.g. meningococcal B vaccine) and use of vaccines beyond the age of usual recommendation (e.g. Haemophilus influenza type b vaccine after 5 years of age). Serological monitoring is a useful tool for customizing vaccination schedule in immune compromised children, confirming adequate vaccine response and documenting seroprotection (especially against measles and varicella). Finally, verification of vaccination status of all household members can prevent them being vector of transmission of an infection to the immune compromised children. Conclusion: Intensified information strategies are needed to improve trust, rectify perceived risks and improve vaccine acceptability; primary physicians can play a critical role in the latter.
|
What is Known: • Physician’s awareness is key to success, since it repeatedly correlates with higher vaccination rates | |
|
What is New: • The vaccination status of immunocompromised children is rarely up-to-date • Knowing the latest vaccine recommendations is challenging, as they differ for each medical condition and change periodically • This review summarises the vaccine recommendations for children with compromised immune systems and highlights how paediatricians play a key role in coordinating their application |
Keywords: Immunosuppression, Immunization, Vaccine-preventable diseases, Paediatrician
Introduction
Protecting immune compromised children against infections is challenging and is a problem of growing importance. Indeed, paediatrician are dealing with more and more patients with deficient immune system, as (i) immunosuppressive therapies are increasingly used in various medical conditions and (ii) the life expectancy of patients with these conditions has substantially raised. The quality of life of these children has also improved over the years: they are able to attend school, travel and be active in their community. This inevitably puts them in contact with others and a variety of infectious pathogens. Moreover, the frequent hospital admission and outpatient visits associated with chronic diseases inevitably increase their risk of nosocomial exposure to pathogens.
Vaccination has repeatedly been recognised as one of the most important and most cost-efficient invention in healthcare [1]. Vaccine-preventable diseases occur more frequently and have a worst outcome in immunocompromised individuals. In a retrospective cohort study of nearly 7000 paediatric solid organ recipients, 15.6% were hospitalised for a vaccine-preventable diseases in the first 5 years following transplantation, an 87-fold higher rate compared with the general population [2]. Worst outcomes are well illustrated by the severity of measles infection, which carries a 40% to 70% fatality rate among immunocompromised patients, despite adequate treatment, up to 35-fold higher compared with immunocompetent hosts [3]. Measles and other vaccine-preventable diseases have recently re-emerged in many regions, mostly due to declining vaccine uptake [4, 5]. As herd protection cannot be relied on, prevention of vaccine-preventable diseases in vulnerable population is key.
The aim of this review is to present an overview of the knowledge in the field, provide tables and references that could help primary care physicians when managing immune compromised children. The following questions are addressed: Which fundamental role do primary care physicians play? Who are immune compromised children? Why are their vaccinations status not up-to-date? Are vaccines immunogenic and safe in immune compromised children? Is the vaccination schedule the same than for healthy children? Which additional vaccines are recommended? Why/when should vaccine-preventable diseases serology be monitored? In which situation can live-attenuated vaccine be administrated? Recommendation for passive immunisation are beyond the scope of this review, but details can be find elsewhere [6, 7].
Which fundamental role does the primary care physician play
The primary care physician plays a critical role in optimising their patients’ protection against vaccine-preventable diseases. The first step is to identify within all their patients which are the ones who could benefit from an enhanced protection. When a child has a new diagnosis, and the immune system is likely to be affected, a quick review of the patient’s vaccination history should be automatic. The ascertainment of the patient’s protection status should rely on checking their records or serologies, as trusting oral recall only can lead to undervaccination or overimmunization [44]. Primary care physicians have a key role to play in discussing with families on the importance of vaccination and reassurance on their safety. In collaboration with a specialist team, a customised vaccination schedule could be planed and anticipated, aiming to immunise, for example, early in the disease process, anticipating periods of higher immunosuppression. This schedule should catch up missing vaccination and add the supplementary vaccines when needed. If recommended, vaccine seroresponses should be checked following vaccination and during follow-up visits.
Another fundamental role of the primary care physician is to ensure that all household members have their vaccinations updated. This “cocooning” strategy is a form of indirect protection for non-immune children, or for those who are unable to be vaccinated. Cocooning is, however, usually not sufficient to fully protect these children, especially those with normal lifestyles.
Who are immune compromised children
Immunodeficiency can be primary or acquired, secondary to a disease, infection, medication, chronic organ failure or other state (e.g. malnutrition, young age) [8]. Medications can affect the immune system either as an undesirable side effect (e.g. chemotherapy, drug-induced neutropenia) or intentionally in conditions in which the immune response has to be restrained, e.g. management of autoimmune disorders and immune-mediated diseases, allergic disorders or solid organ transplant. The most common conditions encountered in daily practice are listed in Table 1.
Table 1.
Summary of vaccine recommendations in children with chronic illness and/or immunosuppression
| Medical condition | How is the immune system affected | Non-live vaccines recommendation | Live-attenuated vaccines recommendation a | Additional vaccine(s) recommendation | Serological monitoring | Guidelines, references |
|---|---|---|---|---|---|---|
| Primary immunodeficiency disorders | Genetic abnormality affecting various pathway of the immune response | Routine b | Permitted in certain situations only |
IIV PCV (± PPSV23) MCV4 MenB if complement deficiency |
“Regularly”, but no guidance on how often |
ACIP [29] |
| Oncological diseases | Most cancers and their treatment affect the immune system |
Routine during chemotherapy c Re-start vaccination as of 3m to 6m after completion of chemotherapy (including Hib, regardless of age) |
Permitted as of 3m to 6m after completion of chemotherapy |
IIV (even during chemotherapy) PCV (± PPSV23) MCV |
No indication Could be useful to monitor seroprotection against measles and varicella |
CCLG [48] IDSA [22] ACIP [29] AIEOP [55] |
| Hematopoietic stem-cell transplantation | Impaired and immature immune cells, loss of Ig | Revaccination starting 3m to 6m after HSCT (including Hib, regardless of age) | Revaccination permitted in certain condition as of 1.5y to 2y after HSCT |
IIV PCV, 3d (± PPSV23) MCV, 2d |
No indication Could be useful to monitor seroprotection against measles and varicella |
CCLG [48] EBMT [49] IDSA [22] ACIP [29] |
| Solid organ transplantation | Immunosuppressive treatment | Accelerated schedule before SOT. Continue after SOT (as of 2m to 6m post-SOT) | Accelerated schedule if > 4w before SOT. Permitted in certain situation after SOT, as of 1y post-SOT [47] |
IIV PCV (± PPSV23) |
Frequent monitoring to guide vaccination; it can also inform on protection against measles and varicella |
AST, IPTA [47] IDSA [22] ACIP [29] |
|
Asplenia/hyposplenia Sickle cell disease |
Higher risk of fulminant infection with encapsulated bacteria and parasite (highest risk in the first 2y of asplenia but persist life-long) | Routine, catch-up Hib vaccination regardless of age, HBV vaccination highly recommended if frequent transfusion. Anticipate 2w between vaccination and elective splenectomy | Permitted, as of a few days after splenectomy |
IIV PCV (± PPSV23) MCV4 2d 2m apart, then every 5y MenB |
Frequent monitoring of serotype-specific pneumococcal IgG to guide booster doses |
IDSA [22] ACIP [29] |
| Human immunodeficiency virus infection | Lower CD4+ T-cell | Delay vaccination until viral load < 50 copies/mL and CD4 > 15% for 6m. Use high-dose HBV vaccine (40 μg) in adolescents. Give Hib vaccine regardless of age if not immune. DT booster at least 1×/10y. | Permitted only if CD4 > 200 cells/μl (or > 15–24% in infants and children) for > 6m |
IIV PCV (± PPSV23) MCV4 2d 2m apart |
Anti-HBs Ig periodically (if ongoing exposure) Anti-tetanus, anti-diphtheria 1×/5y Anti-measles, anti-rubella 1×/3–5y |
PENTA [56] CHIVA IDSA [22] ACIP [29] |
| Immunosuppressive treatment for rheumatologic, renal, neurologic, gastrointestinal conditions | Underlying disease with dysregulated immune system, immunosuppressive treatment to control disease activity | Accelerate schedule before immunosuppression, but continue during and after | Permitted if low immunosuppression |
IIV PCV (± PPSV23) |
No indication but monitoring could guide booster doses and inform on protection, in particular against measles and varicella |
IDSA [22] Review [16] |
| Complement inhibitors (eculizumab) | Medication inhibiting the deployment of the terminal complement system, high risk of meningococcal disease | Routine | Permitted |
IIV PCV MCV4 MenB |
No indication | Review [57] |
| Inflammatory bowel disease | Underlying defect in immune system, immunosuppressive treatment | Accelerate schedule before immunosuppression, but continue during and after | Permitted if low immunosuppression |
IIV PCV (± PPSV23) |
No indication, but monitoring could guide booster doses and inform on protection, in particular against measles and varicella |
IDSA [22] |
| Nephrotic syndrome | Urinary loss of IgG, oedema, immunosuppressive treatment | Accelerate schedule before immunosuppression, but continue during and after | Permitted if low immunosuppression, VZV vaccine highly recommended |
IIV PCV (± PPSV23) |
Monitoring of serotype-specific pneumococcal antibody useful to guide booster. Monitor seroprotection against measles and varicella could be useful as well. |
ACIP [29] Review [18] |
| Prematurity |
Immune cell immaturity Low IgG level (not had time to transfer from the mother) |
Accelerated schedule, based on chronological age | Accelerated schedule, based on chronological age |
IIV PCV MCV RSV (cf country) |
No indication |
Review [58] AAP (RSV) [7] |
| Diabetes mellitus | Impaired phagocytic and neutrophil function, worsen with inadequate glycaemic control | Routine, HBV vaccination highly recommended | Permitted |
IIV PCV (± PPSV23) |
Documentation of protection against HBV. No other indication, antibody response to vaccinations seems to be normal overall |
ACIP [29] Review [59] CDA (adults) [60] |
| Renal failure, chronic kidney disease (including dialysis) | Mild defects in T cell function, immune response impaired by various factor; Ig loss in dialysate | Accelerate schedule before dialysis, but continue during and after, HBV vaccination highly recommended | Permitted |
IIV PCV (± PPSV23) |
No indication, but monitoring could guide booster doses and inform on protection (vaccine responses likely to be impaired) |
ACIP [29] Review [18] |
| Chronic liver disease | Impaired phagocyte function and defects in opsonising antibody, Ig loss in ascites, hyposplenism (with severe liver disease), higher risk of severe superimposed viral hepatitis | Routine, HAV and HBV vaccination highly recommended | Permitted |
IIV PCV (± PPSV23) |
No indication, but monitoring could guide booster doses and inform on protection | ACIP [29] |
| Chronic heart disease or malformation | Infections may precipitate cardiac decompensation | Routine | Permitted |
IIV PCV (± PPSV23) RSV (cf country and underlying disease) |
No indication |
ACIP [29] AAP (RSV) [7] |
|
Chronic lung disease Cystic fibrosis Bronchopulmonary dysplasia Asthma |
Increased risk of severe respiratory infections. Severe lung diseases lead to poor mucociliary clearance, bronchiectasis, defects in pulmonary macrophage function | Routine | Permitted |
IIV PCV (± PPSV23) RSV (cf country and underlying disease severity) |
No indication |
ACIP [29] AAP (RSV) [7] |
| Haemophilia | Historical increased risk of transfusion-related transmission of viral infection | Routine,d HAV and HBV vaccination highly recommended | Permitted d | No indication, adequate response to HBV vaccine could be documented | WFH [61] | |
|
Malnutrition Anorexia nervosa |
Immune response impaired due to malnutrition | Routine | Permitted |
IIV? Insufficient data to date |
No indication | Review [62] |
| Obesity | Immune response slightly impaired due to overweight (and insulin resistance), higher risk of respiratory infection | Routine | Permitted | IIV | No indication, few studies reported lower vaccine responses | Reviews [63–65] |
| Coeliac disease | Functional hyposplenism (reversible), impaired immune response | Routine, HBV vaccination highly recommended | Permitted |
IIV PCV (± PPSV23) ± MCV if hyposplenism confirmed |
HBV serology (data suggest poor response to HBV vaccine administered prior to gluten-free diet) | Review [66, 67] |
| Chronic neurological disease and neurodevelopmental disorder | Decreased protection of airways increases risk of infection, higher risk of complication for some VPD (e.g. influenza, pneumococcus, varicella, pertussis) | Routine | Permitted, VZV vaccination highly recommended (higher risk of neurological complications) |
IIV PCV |
No indication | Recent article [68] |
| Inborn errors of metabolism | Neurological defect, concomitant immunodeficiency, metabolic decompensation | Routine | Permitted |
IIV? PCV? Insufficient data to date |
Unpredictable vaccine responses, depending on underlying immune defect | Review [21] |
|
CNS anatomic barrier defect (e.g. CSF leak, inner ear dysplasia, or cochlear implant) |
Deficient anatomical barrier leads to higher risk of CNS infection | Routine | Permitted | PCV (± PPSV23) | No indication |
IDSA [22] ACIP [29] |
| Severe dermatologic conditions (severe eczema, psoriasis) | Chickenpox particularly prone to bacterial superinfection; severe dermatologic possibly require immunosuppressive treatment | Routine | Permitted if low immunosuppression, VZV vaccination highly recommended | No indication | Review [69] | |
| Parents, close contact of immune compromised individuals | ‘Cocooning’ strategy, to decrease the risk to transmit VPD to the immunocompromised children | Routine | Highly recommended if not immune, OPV and smallpox vaccine are the only LAV contra-indicated in close contact | IIV or LAIV | Documentation of immunity against measles and varicella if disease/vaccination history uncertain (or immunise regardless) |
IDSA [22] Review [53] |
The table summarises the vaccine recommendations available for various health conditions. Recommendations can differ between guidelines and between countries. In some countries, the cost of some vaccines may not be reimbursed. Recommendation for serological monitoring is rarely discussed in guidelines and the ones presented in this table summarise experts’ advices
aThe live-attenuated influenza vaccine should never be given to immune compromised children as they can receive the inactivated influenza vaccine
bEffectiveness doubtful, depend on underlying disease and whether IVIG are given regularly
cPostpone if lymphocyte count < 1.0 × 109/L. Non-live vaccine permitted during chemotherapy but will not be considered as “valid dose”
dReduce the risk of bleeding by subcutaneous injection, use smallest gauge needle and applying pressure and/or ice for 3–5 min after injection
AAP American Academy of Paediatrics, ACIP Advisory Committee on Immunization Practices, AIEOP Italian Association Paediatric Haematology Oncology, CDA Canadian Diabetes Association, CHIVA Children’s HIV Association, CSF cerebrospinal fluid, d dose, DTaP diphtheria-tetanus-pertussis vaccine, EBMT European Society for Blood and Marrow Transplantation, HAV hepatitis A virus, HBV hepatitis B virus, Hib Haemophilus influenzae type b, HSCT hematopoietic stem cell transplantation, IDSA Infectious Disease Society of America, Ig immunoglobulin, IIV inactivated influenza vaccine, IPTA International Paediatric Transplant Association, IPV inactivated poliovirus vaccine, IVIG intravenous immunoglobulins, LAIV live-attenuated influenza vaccine, LAV live-attenuated vaccine, m month, MCV meningococcal conjugated vaccine, MenB meningococcus type B vaccine, MMR measles-mumps-rubella vaccine, NLV non-live vaccine, OPV oral polio vaccine, PCV pneumococcal conjugate vaccine, PPSV23 23-valent pneumococcal polysaccharide vaccine, PENTA Paediatric European Network for Treatment of AIDS, RSV respiratory syncytial virus, SOT solid organ transplantation, VPD vaccine-preventable disease, VZV varicella vaccine, w week, WFH World Federation of Hemophilia, y year
Why are immune compromised children’s vaccination status not up-to-date
Although vaccinations seem particularly indicated in this high-risk population, immune compromised children are often less adequately vaccinated than healthy children [9–11]. As vaccines and booster doses are given regularly throughout childhood, most children may not have completed their schedule before the onset of immunosuppression. But the main reasons underlying non-vaccination are summarised in Fig. 1 [11–13]. Moreover, as vaccination guidelines change frequently, and differ for each different medical condition, it is challenging to stay up-to-date with the most recent, specific recommendations [14]. As an example, it was recently reported in patients with inflammatory bowel disease (IBD) that vaccination was the least frequently followed quality of care recommendation [15]. In Italy, vaccination rates in children with HIV, cystic fibrosis, liver transplantation or diabetes were low against pneumococcus (< 25%) and highly variable for influenza (21% to 90%) [11]. Information and better communication appear to be key components for increasing vaccination uptake; primary care physician usually excels in both, being trusted by and close to the patient’s family (Fig. 2).
Fig. 1.

Common barriers to vaccination
Fig. 2.
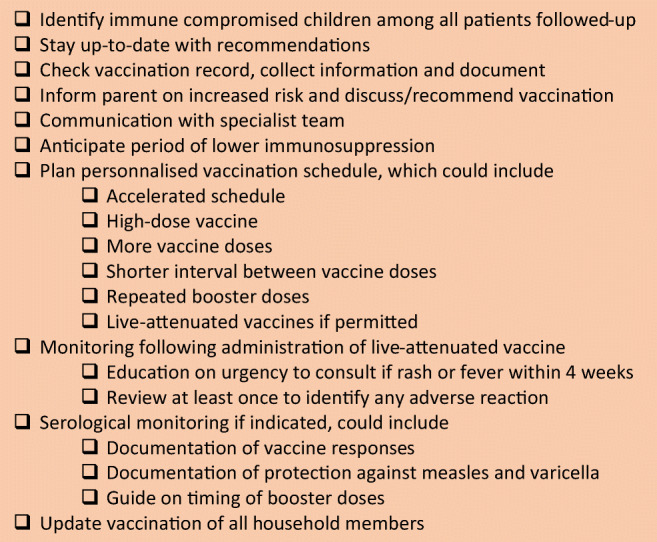
Checklist for primary care physician in optimising patients’ protection
Are vaccines immunogenic in immune compromised children
Concern on vaccine effectiveness is often an obstacle to vaccination in immune compromised children; immune response to vaccination can be suboptimal [16]. Vaccine responses may be reduced in both magnitude and durability, explaining the need for repeated monitoring of antibody levels during follow-up. However, even in highly immunocompromised hosts, vaccination may induce at least some immune response that could be beneficial in case of further encounter with the pathogen. Vaccines should therefore be administered despite possible non-responsiveness; in some medical conditions, monitoring of antibody concentration is recommended (Table 1).
Are vaccines safe in immune compromised children
Whereas immunogenicity is an important aspect, vaccine safety is often the main concern of parents and healthcare practitioners. Live-attenuated vaccines (LAV), in particular, are usually avoided in the immunocompromised hosts as they could theoretically induce vaccine-strain infections; these are discussed in detail in a section below. In contrast, non-live vaccines are incapable of causing infection, since they consist in inactivated toxins (protein), in pathogen that have been killed (inactivated) or in only specific segments of the pathogen (subunit, polysaccharides) that may be conjugated to a protein (conjugate vaccine) to enhance the immunological response (Table 2). These vaccines can be given to immunocompromised patient without any safety concerns, as demonstrated in many studies in patients with chronic diseases, e.g. in HIV-infected individuals [17], patients with immune-mediated diseases [16], chronic kidney diseases [18] and solid organ transplantation recipients [19]. Therefore, information is critical to clearly explain the expected benefit to the patient.
Table 2.
Summary of recommendation for vaccine administration and serological monitoring
| Pathogen | Vaccine type | Vaccine recommendation | Rational for serological monitoring | Test used to measure seroprotection | Level required | Mechanism prevented |
|---|---|---|---|---|---|---|
| Diphtheria | Protein | Booster doses may be required more frequently; accelerated schedule in preterm or before onset of immunosuppression; effectiveness doubtful during cancer treatment and in children with primary immunodeficiency | Monitor vaccine response and guide for booster indication | Toxin neutralisation | 0.01–0.1 IU/mL | Toxin production |
| Tetanus | Protein | Toxin neutralisation | 0.1–0.1 IU/mL | Toxin production | ||
| Pertussis | Protein | No indication | ELISA | Not defined | Mucosal replication | |
| Polio | Inactivated | Not routinely indicated | Serum neutralisation | 1/4–1/8 dilution | Viremia | |
| Haemophilus influenzae b | Conjugate | Catch-up regardless of age in some high-risk situation (hypo-/asplenia, HIV, after chemotherapy, after HSCT) | Could be used to document protection in high-risk situation | ELISA |
1 ng/mL (polysaccharide) 0.15 ng/mL (conjugated) |
Bacteraemia |
| Hepatitis A | Inactivated | Mainly recommended in travellers or if high risk of hepatitis | Not routinely indicated | ELISA | 20 IU/L | Viremia |
| Hepatitis B | Subunit | Particularly recommended in cases of increased risk of needle-/transfusion-related transmission; supplementary vaccine doses and/or use of vaccine with higher antigenic dose may be required | Monitor vaccine response as poorly immunogenic in immunocompromised individuals | ELISA |
10 IU/L (protective) >100–1000 IU/L (optimal) |
Viremia |
| Human papillomavirus | Subunit | Strongly recommended in all immunocompromised conditions, with a 3-dose schedule regardless of age | No indication | ELISA | Not defined | Mucosal replication |
| Influenza | Inactivated | Recommended in all chronic diseases and immunocompromised conditions; clinical studies are ongoing to evaluate the need of high-dose vaccine in certain conditions | No indication | HAI |
1/40 dilution (1/320 dilution in children) |
Mucosal replication |
| Pneumococcus |
Conjugate Polysaccharide |
Recommended in all chronic diseases and immunocompromised conditions; indication for booster is less clear, mainly indicated in hypo-/asplenic patients | Could be used to guide for booster indication |
Serotype-specific ELISA Serotype-specific OPA |
0.35 μg/mL 1/8 dilution (differ among serotypes) |
Bacteraemia |
| Meningococcus |
Conjugate Polysaccharide |
Mainly recommended when complement is affected, in oncological, HSCT, HIV-infected and hypo-/asplenic individuals, with a 2-doses schedule | No indication |
ELISA Bactericidal test |
2 μg/mL 1/4 dilution (human serum) |
Bacteraemia |
| Measles | Live-attenuated | Accelerated schedule finishing at least 4 weeks before onset of immunosuppression. Permitted in some situations during immunosuppression (low immunosuppression, specific criteria for HIV and SOT) | Could be used to document protection in high risk situation |
Microneutralisation assay ELISA |
120 mIU/mL 150–200 mIU/mL |
Viremia |
| Mumps | Live-attenuated | No indication | Serum neutralisation | Not defined | Viremia | |
| Rubella | Live-attenuated | Could be used to document protection prior to pregnancy | Immunoprecipitation | 10–15 mIU/mL | Viremia | |
| Varicella | Live-attenuated | Accelerated schedule finishing at least 4 weeks before onset of immunosuppression. Permitted in some situations during immunosuppression (low immunosuppression, specific criteria for HIV and SOT). Highly recommended in some medical condition (e.g. nephrotic syndrome if low immunosuppression, neurological disorders, skin disorders) | Could be used to document protection in high risk situation |
Serum neutralization Glycoprotein ELISA |
1/64 dilution 5 IU/mL |
Viremia |
Proper communication is particularly important for patients with immune-mediated diseases, inborn error of metabolism or solid organ transplantation, for which questions about the inflammation induced by vaccination could play a role in modulating the auto-immune response or inducing a metabolic crisis. Moreover, since the medical conditions of these children will be lifelong, vaccinations cannot be postponed indefinitely. Therefore, in these children, while it is important as for all children to monitor possible side effects of vaccination, the effect of the vaccine on the underlying condition should also be reported, such as signs of graft rejection or flare in disease activity. However, there is increasing data suggesting that concerns regarding the risk of disease exacerbation are unfounded, with numerous studies showing that immunization did not induce significant worsening of underlying disease [16, 19–21].
Is the vaccination schedule the same than for healthy children
The vaccination schedules are usually the same; they slightly differ from those of healthy children in that they may include supplementary vaccinations (for example usually not given beyond a certain age), accelerated schedule, extra doses for primary vaccination, extra boosters, as well as specific conditions for administration of LAV. These are detailed in Tables 1 and 2, and in the following sections.
Recommendations are somewhat different between immunocompromising conditions: they are determined by the individual risk of infection and the data available. Among the various guidelines available, the Infectious Diseases Society of America provides a good overview of the current evidence available and covers most medical conditions (Table 1) [22]. The different national immunisation schedules can also be found online [23].
Which supplementary non-live vaccines are indicated in which situation
Most immune compromised children benefit from protection against pneumococcus, influenza, meningococcus and human papilloma virus (HPV). These vaccines are included in many national guidelines for healthy children as well, so may not necessarily be considered as “supplementary vaccines”.
Invasive pneumococcal diseases carry a high mortality rate (11–30%) [24] and are more frequent in immunocompromised individuals, or those with chronic diseases, such as IBD [25], nephrotic syndrome [26] or a-/hyposplenic conditions [27, 28]. The pneumococcal conjugate vaccine (PCV) is usually recommended in healthy children before the age of 5, but also in all medical conditions with immunosuppression, regardless of age. Although some guidelines also recommend to subsequently administer the 23-valent polysaccharide vaccine (PPSV23) to those at high risk [29], many experts disagree, since PPSV23 do not induce memory cells and become less effective after repeated administrations (hyporesponsiveness) [30].
Influenzais probably the most common vaccine-preventable diseases leading to hospitalisation, accounting for 3.4% of all critical care admissions in the USA during the flu season [31]. In a retrospective cohort study in paediatric solid organ transplantation recipient, 40% of the hospitalisation for vaccine-preventable diseases were due to influenza infection [2]. Given the high burden of influenza disease, the vaccine is recommended in virtually all immune compromised children, as of 6 months of age. Moreover, preventing influenza also helps preventing secondary pneumococcal infection. Immune compromised children should always receive the inactivated vaccine and not the live-attenuated influenza vaccine (in Europe, the latter is only available in the UK).
Meningococcal vaccines are recommended to asplenic patients, HIV-infected individuals, those with complement deficiencies or receiving a treatment affecting the complement (such as eculizumab) [32]. .Most guidelines recommend a 2-dose schedule of the 4-valent conjugate vaccine (MCV4), and, when available, vaccination against serogroup B as well (Table 1).
As the risk of malignancy related to HPV is highly increased (up to 100-fold) in immunocompromised individuals [33], a 3-dose schedule is strongly recommended for all. The 2-doseschedule—used routinely in immunocompetent 11–15-year-old individuals—may not be sufficiently immunogenic, reason why the 3-dose schedule should be preferred [34].
Do immune compromised children need more or higher doses
As vaccination may be less immunogenic in immune compromised children and immunity may wane faster, it is sometimes useful to administer vaccines with higher antigenic contents, additional vaccine doses or more frequent booster doses to ensure adequate response (via serological monitoring, as discussed below) and subsequent protection against vaccine-preventable diseases.
High-dose vaccine
For vaccination against HBV per example, use of high-dose vaccine is recommended by some experts in HIV-infected adolescents (and adult), haemodialysis adult, and studies involving adults suggest it could be beneficial for oncological patients, or those with immune-mediated diseases [22]. Another example is the high-dose influenza vaccine being currently evaluated in immunocompromised individuals, including oncological patients, solid organ transplantation recipients and haemodialysis patients [35–37]. Data in paediatric patients, however, is scarce.
More vaccine doses
Regarding schedule, 3-dose(rather than 2-doses) schedule are recommended for HPV in all immunocompromised condition, and a 2-dose (rather than single dose) schedule is recommended for MCV4 [22].
More boosters
Regular MCV and PCV booster are recommended in some immunocompromised condition, whereas they are not recommended in healthy children. Diphtheria-tetanus booster doses are recommended more often as well, as guided by serological monitoring.
The rationale behind serological monitoring
One of the most useful tools for customization of vaccination schedule in immune compromised children is to regularly monitor their serologies [38]. In children receiving chemotherapy for example, there is strong evidence to suggest that antibody concentrations wane more rapidly during treatment [39]. Cut-off values for seroprotection (i.e. correlates of protection) are available for most vaccine-preventable diseases (Table 2), but may vary slightly between laboratories. These measures allow to (i) confirm adequate vaccine response, (ii) guide when to administer a booster dose and (iii) document current protection against vaccine-preventable diseases. The latter is of particular importance for varicella and measles viruses, for which the reported mortality rates in infected immunocompromised hosts are up to 25% and 70%, respectively [3, 40, 41]. For both viruses, absence of seroprotection would require prompt management following contact (intravenous immunoglobulins and/or antiviral therapy), whereas documentation of highly seroprotective titres could suggest a “wait and see” attitude [42]. Physician can therefore inform individually on the risk of severe disease following contact with varicella or measles and provide guidance on what to do if this situation occurs. Regular monitoring of serologies against vaccine-preventable diseases has been adopted by many as an important part of the regular follow-up of immunocompromised patient. Although this test does not measure the other actors of the immune response, it is the only indirect measure of protection available. There is, however, no clear recommendation on when, in whom and how often should serology be assessed (Tables 1 and 2). Annual monitoring of serologies may be indicated in highly immunocompromised patients, or when the immunosuppressing regimen has recently been increased, whereas less frequent monitoring (i.e. once every 5 years) should probably be enough in well-controlled HIV-infected individuals, for example.
In which situation can live-attenuated vaccines be administered
When vaccinating immunocompromised individuals, the most important safety issue concerns LAV. They consist in live pathogens that have been ‘weakened’ so that they can still replicate but with difficulty and without having the capacity to cause the disease in an immunocompetent host. Given the fear of a theoretical uncontrolled replication that could lead to severe vaccine-induced disease, LAV are mostly contraindicated in immune compromised children. In patients with severe primary immunodeficiency disease (e.g. severe combined immunodeficiency), LAV carry a significant risk of vaccine-strain infections, which have been reported following the oral rotavirus or poliovirus vaccines, measles-mumps-rubella(MMR) vaccine and bacille Calmette-Guérin vaccine [43, 44]. However, there is growing evidence documenting the safety of immunising immunocompromised hosts with different types of LAV in carefully selected settings.
MMR and varicella vaccines are usually well tolerated in case of milder immunosuppression, such as in children with DiGeorge syndrome (if lymphocyte count is > 500 cells/μL) [43], HIV-infected individuals (if CD4 count is > 200 cells/μL) [45, 46], liver or kidney transplant recipients (strict conditions [47]), after hematopoietic stem cell transplantation [48, 49], or in individuals with immune-mediated diseases on low/no immune suppression [16, 22], including children with nephrotic syndrome [50]. MMR and varicella vaccine have indeed the potential to protect patients against threatening pathogen that are endemic or linked with epidemics in many places around the world. However, extra caution should be taken and close safety monitoring is highly recommended following the administration of LAV in any situation when the immune system is affected [22, 47]. In the setting of solid organ transplantation, a consensus of worldwide experts has recommended the following surveillance: (i) education on urgency to seek medical attention in case of new onset of rash or fever within 4 weeks following vaccination and (ii) at least one contact with the patient’s caregiver in the month following vaccination to identify any adverse event that might have occurred [47].
Concluding discussion
As many questions remain, clinical trials are still needed to refine the study of the immune response induced by each vaccine in all immunocompromising conditions to determine whether, when and for whom there is a need for a specific immunisation schedule. Moreover, additional guidance regarding the serological monitoring of vaccine response and persistence of protection is required.
As new vaccines become available and the epidemiology of vaccine-preventable diseases evolves, it is increasingly important for all those caring for children to be up to date with the recent changes to guidelines, in order to improve the usual low uptake of additional immunisations in high-risk groups [51]; physician‘s awareness is key, since it repeatedly correlates with higher vaccination rates [11].
Abbreviations
- IBD
Inflammatory bowel disease
- HBV
Hepatitis B virus
- HIV
Human immunodeficiency virus
- HPV
Human papilloma virus
- LAV
Live-attenuated vaccine
- MCV
Meningococcal conjugate vaccine
- MMR
Measles-mumps-rubella
- PCV
Pneumococcal conjugate vaccine
- RSV
Respiratory syncytial virus
Author contribution
LFP drafted the initial manuscript. KMPB critically revised the manuscript and both authors approved the final version as submitted.
Funding
Open Access funding provided by Université de Genève. LFP is supported by the Swiss National Science Foundation (Early Postdoc.Mobility grant number P2GEP3_178155).
There is no funding source for this review
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laure F. Pittet, Email: laure.pittet@hcuge.ch
Klara M. Posfay-Barbe, Email: Klara.PosfayBarbe@hcuge.ch
References
- 1.van Wijhe M, McDonald SA, de Melker HE, Postma MJ, Wallinga J. Effect of vaccination programmes on mortality burden among children and young adults in the Netherlands during the 20th century: a historical analysis. Lancet Infect Dis. 2016;16:592–598. doi: 10.1016/S1473-3099(16)00027-X. [DOI] [PubMed] [Google Scholar]
- 2.Feldman AG, Beaty BL, Curtis D, Juarez-Colunga E, Kempe A. Incidence of hospitalization for vaccine-preventable infections in children following solid organ transplant and associated morbidity, mortality, and costs. JAMA Pediatr. 2019;173:260–268. doi: 10.1001/jamapediatrics.2018.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe measles in immunocompromised patients. JAMA. 1992;267:1237–1241. [PubMed] [Google Scholar]
- 4.Pittet LF, Abbas M, Siegrist CA, Pittet D. Missed vaccinations and critical care admission: all you may wish to know or rediscover-a narrative review. Intensive Care Med. 2020;46:202–214. doi: 10.1007/s00134-019-05862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VanderEnde K, Gacic-Dobo M, Diallo MS, Conklin LM, Wallace AS. Global routine vaccination coverage - 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1261–1264. doi: 10.15585/mmwr.mm6745a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luna MS, Manzoni P, Paes B, Baraldi E, Cossey V, Kugelman A, Chawla R, Dotta A, Rodriguez Fernandez R, Resch B, Carbonell-Estrany X. Expert consensus on palivizumab use for respiratory syncytial virus in developed countries. Paediatr Respir Rev. 2020;33:35–44. doi: 10.1016/j.prrv.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 8.Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2010;125:S195–S203. doi: 10.1016/j.jaci.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman AG, Curtis DJ, Moore SL, Kempe A. Under-immunization of pediatric transplant recipients: a call to action for the pediatric community. Pediatr Res. 2020;87:277–281. doi: 10.1038/s41390-019-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinelli M, Giugliano FP, Strisciuglio C, Urbonas V, Serban DE, Banaszkiewicz A, Assa A, Hojsak I, Lerchova T, Navas-Lopez VM, Romano C, Sladek M, Veres G, Aloi M, Kucinskiene R, Miele E (2019) Vaccinations and immunization status in pediatric inflammatory bowel disease: a multicenter study from the Pediatric IBD Porto Group of the ESPGHAN. Inflamm Bowel Dis [DOI] [PubMed]
- 11.Giannattasio A, Squeglia V, Lo Vecchio A, Russo MT, Barbarino A, Carlomagno R, Guarino A. Pneumococcal and influenza vaccination rates and their determinants in children with chronic medical conditions. Ital J Pediatr. 2010;36:28. doi: 10.1186/1824-7288-36-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty M, Schmidt-Ott R, Santos JI, Stanberry LR, Hofstetter AM, Rosenthal SL, Cunningham AL. Vaccination of special populations: Protecting the vulnerable. Vaccine. 2016;34:6681–6690. doi: 10.1016/j.vaccine.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Dipasquale V, Romano C. Vaccination strategies in pediatric inflammatory bowel disease. Vaccine. 2017;35:6070–6075. doi: 10.1016/j.vaccine.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Eibl MM, Wolf HM. Vaccination in patients with primary immune deficiency, secondary immune deficiency and autoimmunity with immune regulatory abnormalities. Immunotherapy. 2015;7:1273–1292. doi: 10.2217/IMT.15.74. [DOI] [PubMed] [Google Scholar]
- 15.Feuerstein JD, Castillo NE, Siddique SS, Lewandowski JJ, Geissler K, Martinez-Vazquez M, Thukral C, Leffler DA, Cheifetz AS. Poor documentation of inflammatory bowel disease quality measures in academic, community, and private practice. Clin Gastroenterol Hepatol. 2016;14:421–428.e422. doi: 10.1016/j.cgh.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Papp KA, Haraoui B, Kumar D, Marshall JK, Bissonnette R, Bitton A, Bressler B, Gooderham M, Ho V, Jamal S, Pope JE, Steinhart AH, Vinh DC, Wade J. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23:50–74. doi: 10.1177/1203475418811335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obaro SK, Pugatch D, Luzuriaga K. Immunogenicity and efficacy of childhood vaccines in HIV-1-infected children. Lancet Infect Dis. 2004;4:510–518. doi: 10.1016/S1473-3099(04)01106-5. [DOI] [PubMed] [Google Scholar]
- 18.Esposito S, Mastrolia MV, Prada E, Pietrasanta C, Principi N. Vaccine administration in children with chronic kidney disease. Vaccine. 2014;32:6601–6606. doi: 10.1016/j.vaccine.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Mulley WR, Dendle C, Ling JEH, Knight SR. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta-analysis. J Heart Lung Transplant. 2018;37:844–852. doi: 10.1016/j.healun.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Klein NP, Aukes L, Lee J, Fireman B, Shapira SK, Slade B, Baxter R, Summar M. Evaluation of immunization rates and safety among children with inborn errors of metabolism. Pediatrics. 2011;127:e1139–e1146. doi: 10.1542/peds.2010-3706. [DOI] [PubMed] [Google Scholar]
- 21.Menni F, Chiarelli G, Sabatini C, Principi N, Esposito S. Vaccination in children with inborn errors of metabolism. Vaccine. 2012;30:7161–7164. doi: 10.1016/j.vaccine.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, Kang I. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:309–318. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 23.European Centre for Disease Prevention and Control. Vaccine schedules in all countries of the European Union 2020 [Available from: https://vaccine-schedule.ecdc.europa.eu/. Accessed 18 April 2020].
- 24.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 25.Kantso B, Simonsen J, Hoffmann S, Valentiner-Branth P, Petersen AM, Jess T. Inflammatory bowel disease patients are at increased risk of invasive pneumococcal disease: a nationwide Danish cohort study 1977-2013. Am J Gastroenterol. 2015;110:1582–1587. doi: 10.1038/ajg.2015.284. [DOI] [PubMed] [Google Scholar]
- 26.Lebel A, Kropach N, Ashkenazi-Hoffnung L, Huber-Yaron A, Davidovits M (2020) Infections in children with nephrotic syndrome: twenty years of experience. Clin Pediatr (Phila):9922820908583 [DOI] [PubMed]
- 27.Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86–97. doi: 10.1016/S0140-6736(10)61493-6. [DOI] [PubMed] [Google Scholar]
- 28.Di Sabatino A, Rosado MM, Ciccocioppo R, Cazzola P, Morera R, Corazza GR, Carsetti R. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol. 2005;100:1788–1795. doi: 10.1111/j.1572-0241.2005.41939.x. [DOI] [PubMed] [Google Scholar]
- 29.Robinson CL, Bernstein H, Poehling K, Romero JR, Szilagyi P. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:130–132. doi: 10.15585/mmwr.mm6905a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borrow R, Heath PT, Siegrist CA. Use of pneumococcal polysaccharide vaccine in children: what is the evidence? Curr Opin Infect Dis. 2012;25:292–303. doi: 10.1097/QCO.0b013e3283531b0f. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz JR, Neuzil KM, Shay DK, Rue TC, Neradilek MB, Zhou H, Seymour CW, Hooper LG, Cheng PY, Goss CH, Cooke CR. The burden of influenza-associated critical illness hospitalizations. Crit Care Med. 2014;42:2325–2332. doi: 10.1097/CCM.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, de Wals P, Dinleyici EC, Echaniz-Aviles G, Hakawi A, Kamiya H, Karachaliou A, Lucidarme J, Meiring S, Mironov K, Sáfadi MAP, Shao Z, Smith V, Steffen R, Stenmark B, Taha MK, Trotter C, Vázquez JA, Zhu B. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: Epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18:15–30. doi: 10.1080/14760584.2019.1557520. [DOI] [PubMed] [Google Scholar]
- 33.Reusser NM, Downing C, Guidry J, Tyring SK. HPV carcinomas in immunocompromised patients. J Clin Med. 2015;4:260–281. doi: 10.3390/jcm4020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garland SM, Brotherton JML, Moscicki AB, Kaufmann AM, Stanley M, Bhatla N, Sankaranarayanan R, de Sanjose S, Palefsky JM. HPV vaccination of immunocompromised hosts. Papillomavirus Res. 2017;4:35–38. doi: 10.1016/j.pvr.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai JJ, Lin C, Ho CL, Chen PH, Lee CH (2019)Alternative-dose versus standard-dose trivalent influenza vaccines for immunocompromised patients: A meta-analysis of randomised control trials. J Clin Med:8 [DOI] [PMC free article] [PubMed]
- 36.Miskulin D, Weiner DE, Manley HJ. High-dose versus standard-dose influenza vaccine in hemodialysis patients. Am J Kidney Dis. 2020;75:456. doi: 10.1053/j.ajkd.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Mombelli M, Kampouri E, Manuel O. Influenza in solid organ transplant recipients: epidemiology, management, and outcomes. Expert Rev Anti Infect Ther. 2020;18:103–112. doi: 10.1080/14787210.2020.1713098. [DOI] [PubMed] [Google Scholar]
- 38.Moore DL. Immunization of the immunocompromised child: Key principles. Paediatr Child Health. 2018;23:203–205. doi: 10.1093/pch/pxx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Top KA, Vaudry W, Morris SK, Pham-Huy A, Pernica JM, Tapiero B, Gantt S, Price VE, Rassekh SR, Sung L, McConnell A, Rubin E, Chawla R, Halperin SA (2020) Waning vaccine immunity and vaccination responses in children treated for acute lymphoblastic leukemia: A Canadian Immunization Research Network Study. Clin Infect Dis [DOI] [PMC free article] [PubMed]
- 40.Lynfield R, Herrin JT, Rubin RH. Varicella in pediatric renal transplant recipients. Pediatrics. 1992;90:216–220. [PubMed] [Google Scholar]
- 41.Feldhoff CM, Balfour HH, Jr, Simmons RL, Najarian JS, Mauer SM. Varicella in children with renal transplants. J Pediatr. 1981;98:25–31. doi: 10.1016/s0022-3476(81)80527-6. [DOI] [PubMed] [Google Scholar]
- 42.Lachiewicz AM, Srinivas ML. Varicella-zoster virus post-exposure management and prophylaxis: A review. Prev Med Rep. 2019;16:101016. doi: 10.1016/j.pmedr.2019.101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobh A, Bonilla FA. Vaccination in primary immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4:1066–1075. doi: 10.1016/j.jaip.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Fekrvand S, Yazdani R, Olbrich P, Gennery A, Rosenzweig SD, Condino-Neto A, Azizi G, Rafiemanesh H, Hassanpour G, Rezaei N, Abolhassani H, Aghamohammadi A (2020) Primary immunodeficiency diseases and Bacillus Calmette-Guerin (BCG)-vaccine-derived complications: a systematic review. J Allergy Clin Immunol Pract [DOI] [PubMed]
- 45.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Recommended immunization schedule for adults and adolescents with HIV infection. [updated 13 Sept 2017, available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed 17 May 2020].
- 46.Scott P, Moss WJ, Gilani Z, Low N. Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity. J Infect Dis. 2011;204(Suppl 1):S164–S178. doi: 10.1093/infdis/jir071. [DOI] [PubMed] [Google Scholar]
- 47.Suresh S, Upton J, Green M, Pham-Huy A, Posfay-Barbe KM, Michaels MG, Top KA, Avitzur Y, Burton C, Chong PP, Danziger-Isakov L, Dipchand AI, Hebert D, Kumar D, Morris SK, Nalli N, Ng VL, Nicholas SK, Robinson JL, Solomon M, Tapiero B, Verma A, Walter JE, Allen UD (2019) Live vaccines after pediatric solid organ transplant: proceedings of a consensus meeting, 2018. Pediatr Transplant:e13571 [DOI] [PubMed]
- 48.Patel S, Heath P, Skinner R. Vaccinations for paediatric patients treated with standard-dose chemotherapy and hematopoietic stem cell transplantation (HSCT) recipients 2017 [updated Sept 2017, available from: https://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/Vaccinations_for_Children_treated_with_Standard-dose_Chemotherapy_and_HSCT_Recipients-Sept_2014-FINAL_CCLG.pdf. Accessed 8 Feb 2020]
- 49.Ljungman P, Cordonnier C, Einsele H, Englund J, Machado CM, Storek J, Small T, Center for International B, Marrow Transplant R, National Marrow Donor P, European B, Marrow Transplant G, American Society of B, Marrow T, Canadian B, Marrow Transplant G, Infectious Disease Society of A, Society for Healthcare Epidemiology of A, Association of Medical M, Infectious Diseases C, Centers for Disease C, Prevention (2009) Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant 44:521-526 [DOI] [PubMed]
- 50.Kamei K, Miyairi I, Ishikura K, Ogura M, Shoji K, Funaki T, Ito R, Arai K, Abe J, Kawai T, Onodera M, Ito S (2018) Prospective study of live attenuated vaccines for patients with nephrotic syndrome receiving immunosuppressive agents. J Pediatr 196:217-222.e211 [DOI] [PubMed]
- 51.Pinto MV, Bihari S, Snape MD. Immunisation of the immunocompromised child. J Infect. 2016;72(Suppl):S13–S22. doi: 10.1016/j.jinf.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Bonilla FA. Update: Vaccines in primary immunodeficiency. J Allergy Clin Immunol. 2018;141:474–481. doi: 10.1016/j.jaci.2017.12.980. [DOI] [PubMed] [Google Scholar]
- 53.Medical Advisory Committee of the Immune Deficiency Foundation, Shearer WT, Fleisher TA, Buckley RH, Ballas Z, Ballow M, Blaese RM, et al. (2014) Recommendations for live viral and bacterial vaccines in immunodeficient patients and their close contacts. J Allergy Clin Immunol 133:961-966 [DOI] [PMC free article] [PubMed]
- 54.Martire B, Azzari C, Badolato R, Canessa C, Cirillo E, Gallo V, Graziani S, Lorenzini T, Milito C, Panza R, Moschese V, with Italian Network for Primary Immunodeficiencies Vaccination in immunocompromised host: Recommendations of Italian Primary Immunodeficiency Network Centers (IPINET) Vaccine. 2018;36:3541–3554. doi: 10.1016/j.vaccine.2018.01.061. [DOI] [PubMed] [Google Scholar]
- 55.Cesaro S, Giacchino M, Fioredda F, Barone A, Battisti L, Bezzio S, Frenos S, De Santis R, Livadiotti S, Marinello S, Zanazzo AG, Caselli D. Guidelines on vaccinations in paediatric haematology and oncology patients. Biomed Res Int. 2014;2014:707691. doi: 10.1155/2014/707691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menson EN, Mellado MJ, Bamford A, Castelli G, Duiculescu D, Marczynska M, Navarro M, Scherpbier HJ, Heath PT. Guidance on vaccination of HIV-infected children in Europe. HIV Med. 2012;13(333-336):e331–e314. doi: 10.1111/j.1468-1293.2011.00982.x. [DOI] [PubMed] [Google Scholar]
- 57.Benamu E, Montoya JG. Infections associated with the use of eculizumab: recommendations for prevention and prophylaxis. Curr Opin Infect Dis. 2016;29:319–329. doi: 10.1097/QCO.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 58.Gagneur A, Pinquier D, Quach C. Immunization of preterm infants. Hum Vaccin Immunother. 2015;11:2556–2563. doi: 10.1080/21645515.2015.1074358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calliari LE, Almeida FJ, Noronha RM. Infections in children with diabetes. J Pediatr (Rio J) 2020;96:39–46. doi: 10.1016/j.jped.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Husein N, Chetty A. Influenza, pneumococcal, hepatitis b and herpes zoster vaccinations. Can J Diabetes. 2018;42(Suppl 1):S142–s144. doi: 10.1016/j.jcjd.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 61.World Federation of Hemophilia. Guidelines for the Management of Hemophilia 2012 [Available from: https://elearning.wfh.org/resource/treatment-guidelines/. Accessed 18 April 2020].
- 62.Gibson D, Mehler PS (2019) Anorexia nervosa and the immune system-a narrative review. J Clin Med 8 [DOI] [PMC free article] [PubMed]
- 63.Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelishadi R, Roufarshbaf M, Soheili S, Payghambarzadeh F, Masjedi M. Association of childhood obesity and the immune system: a systematic review of reviews. Child Obes. 2017;13:332–346. doi: 10.1089/chi.2016.0176. [DOI] [PubMed] [Google Scholar]
- 65.Tagliabue C, Principi N, Giavoli C, Esposito S. Obesity: impact of infections and response to vaccines. Eur J Clin Microbiol Infect Dis. 2016;35:325–331. doi: 10.1007/s10096-015-2558-8. [DOI] [PubMed] [Google Scholar]
- 66.Snyder J, Butzner JD, DeFelice AR, Fasano A, Guandalini S, Liu E, Newton KP (2016)Evidence-informed expert recommendations for the management of celiac disease in children. Pediatrics:138 [DOI] [PubMed]
- 67.Simons M, Scott-Sheldon LAJ, Risech-Neyman Y, Moss SF, Ludvigsson JF, Green PHR. Celiac disease and increased risk of pneumococcal infection: a systematic review and meta-analysis. Am J Med. 2018;131:83–89. doi: 10.1016/j.amjmed.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 68.Dinleyici M, Carman KB, Kilic O, Laciner Gurlevik S, Yarar C, Dinleyici EC. The immunization status of children with chronic neurological disease and serological assessment of vaccine-preventable diseases. Hum Vaccin Immunother. 2018;14:1970–1976. doi: 10.1080/21645515.2018.1460986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kienast AK, Kreth HW, Hoger PH. Varicella vaccination in children with atopic eczema. J Dtsch Dermatol Ges. 2007;5:875–880. doi: 10.1111/j.1610-0387.2007.06488.x. [DOI] [PubMed] [Google Scholar]
- 70.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 72.Plotkin SA, Orenstein WA, Offit PA (2012) Vaccines.6th edition edn. Elsevier, Philadelphia, PA
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.


