Abstract
Japanese rock ptarmigans, Lagopus muta japonica, are classified as an endangered species in Japan and are found only in the Japanese Alps. The number of birds has decreased in the last half century and cage protection projects have been undertaken as in situ conservation strategies (one of the projects for the recovery plan of Japanese rock ptarmigan) in the mountains. During the period with cage protections, some chicks died and two Eimeria spp., E. uekii and E. raichoi, were identified in the chicks. Here, we examined the soil within the cages and in the surrounding environment to assess potential sources of infection between July to August 2020. We found high numbers of oocysts in the cages, especially at the back sides where the ptarmigan family frequently congregated, but soils in other areas outside the cages were less contaminated or not contaminated at all. The time required for more than 50% of the oocysts to sporulate at 15, 20 and 25 °C for E. uekii was 20, 11, and 5 h, respectively, and 72, 48 and 18 h, respectively, for E. raichoi. Our results cast some doubt that coprophagia by chicks is the source of infection because chicks consumed fresh cecal feces (approximately within 1 h) as far as we know, and instead, the protected chicks might be directly or indirectly infected by oocysts in soils or the environment.
Keywords: Japanese Alps, Cage protection, Eimeria uekii, Eimeria raichoi, Japanese rock ptarmigans
Graphical abstract
Highlights
-
•
Cage protection is effective for protecting chicks of Japanese rock ptarmigans.
-
•
Soils at the back sides in the cages were highly contaminated with Eimeria spp.
-
•
E. uekii can rapidly be sporulated at 15 °C in timber regions.
-
•
Protected chicks might be infected by oocysts in soils or the environment.
1. Introduction
Rock ptarmigans (Lagopus muta) are an example of cold-adapted birds as they reside in Arctic ecosystems throughout the year. They tend to inhabit rocky habitats mostly without trees or bushes in the high Arctic (up to 83°N) or the high alpine tundra as far south as southern Europe and Japan (Fuglei et al., 2020). The Japanese rock ptarmigan, Lagopus muta japonica, is one of the 23 to 30 subspecies in the order Galliformes, family Tetraonidae (Johnsgard, 1983; del Hoyo et al., 1994), and it inhabits only alpine areas of the main island of Japan, which is the southernmost habitat area and is isolated from the habitats of other subspecies. The number of the Japanese rock ptarmigans declined from approximately 3000 in the 1980s to about 1700 in the 2000s (Nakamura, 2007) and, thus, they were classified as an endangered species in Japan, and a recovery plan for Japanese rock ptarmigans has been implemented (Japanese Ministry of the Environment, 2012).
Cage protection project has been implemented since 2015 as one of the in situ conservation strategies for the Japanese rock ptarmigan (Kobayashi, 2020). Namely, the hens and chicks are kept in cages placed within their habitats and are allowed to graze freely outside the cages several times during the daytime. To date, owing to a combination of cage protection and removal of predators, the number of territories with brooding rock ptarmigans in the northern part of the southern Japanese Alps, consisting of Mt. Kitadake, Mt. Ainodake and Mt. Noutoridake, increased from 8 in 2014 to 35 in 2019 (Kobayashi, 2020). Cage protection is thought to be effective for protecting chicks from predation and from severe climate conditions in alpine areas. Survivorship of wild chicks over a month was less than 50% without intervention (Kobayashi and Nakamura, 2013), and it improved to up to 80% with cage protection (Kobayashi, 2020). However, approximately 20% of the chicks died during the brooding period even with cage protection. Although the exact reasons for the deaths remain unknown, we speculate that infection with Eimeria spp. could affect the growth and health conditions of the chicks, as described below.
Previously, we reported the high prevalence of two species of Eimeria, E. uekii and E. raichoi, which are protozoan parasites belonging to phylum Apicomplexa, and have high prevalence in Japanese rock ptarmigans (41.7%, 57.5%, 34.3% and 38.5% in 2006, 2007, 2016 and 2017, respectively) (Matsubayashi et al., 2018a, 2018b). Further, developing zoites of Eimeria spp. were frequently detected in the intestines (specifically from the duodenum to colon) of chicks that died during the cage protection (Matsubayashi et al., 2018a, 2020). There was a possibility that hens excreted feces containing oocysts of Eimeria spp. and that the robust oocysts could survive in the environment for a period of several months. Although detailed pathogenicity and infectious dynamics of parasites in the Alps have not been clarified, cage protection might increase the risk of being exposed to eimerian oocysts for chicks within the confined areas of the cages.
In the present study, we examined Eimeria spp. in soils within the cages as well as in the surrounding environment and in the feces of chicks, and the intestines of dead chicks in order to assess the potential sources of infection. Hens brood chicks for 4 months after hatching and chicks at 3–18 days of age show coprophagy toward freshly defecated cecal feces of their mothers (Kobayashi et al., 2019). Oocysts that were shed in feces were sporulated under favorable conditions as mature infective forms. Thus, we determined the sporulation time of the oocysts to evaluate whether coprophagy is the source of infection and we discuss parasitic circulation of infection in the timber regions.
2. Materials and methods
2.1. Study site
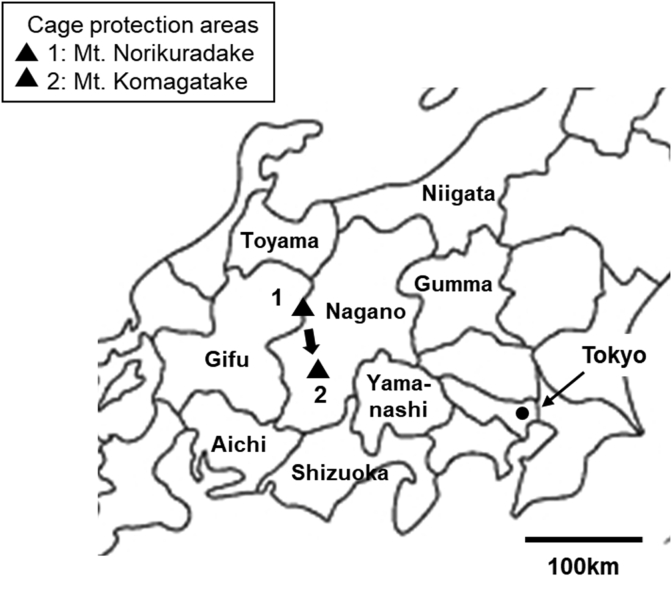
The Northern Japanese Alps are a mountain range at the prefectural borders of Niigata, Toyama, Nagano, and Gifu Prefectures. Mt. Norikuradake consists of the range located between Gifu Prefecture and Nagano Prefecture, Japan (No. 1 area of Fig. 1). In the Central Japanese Alps (Nagano Prefecture), the Japanese rock ptarmigan had been noted as being locally extinct, and one female was found at Mt. Komagatake in 2019 (No. 2 area of Fig. 1). Thus, the Environmental Ministry of Japan started this repopulation project in the Central Japanese Alps in 2020.
Fig. 1.
Location of Mt. Norikuradake (36°06′N, 137°33′E) in the Northern Japanese Alps (1) and Mt. Komagatake (35°79′N, 137°80′E) in the Central Japanese Alps (2). Three broods were transported from Mt. Norikuradake to Mt. Komagatake.
2.2. Cage protection and birds
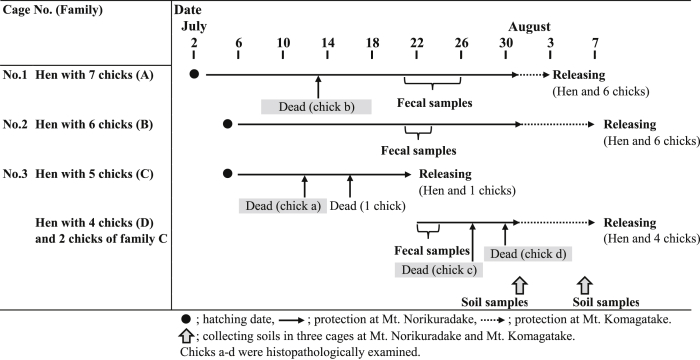
Cage protection was conducted for a total of four broods between 3 and 31 July at Mt. Norikuradake (36°06′N, 137°33′E) in the southern tip of the Northern Japanese Alps (Fig. 2A–B). Three broods were transported by helicopter to Mt. Komagatake (35°79′N, 137°80′E) in Nagano Prefecture in the Central Japanese Alps on 1 August, and we continued cage protection for 3–7 days until releasing the chicks (see Table 1 and Fig. 1).
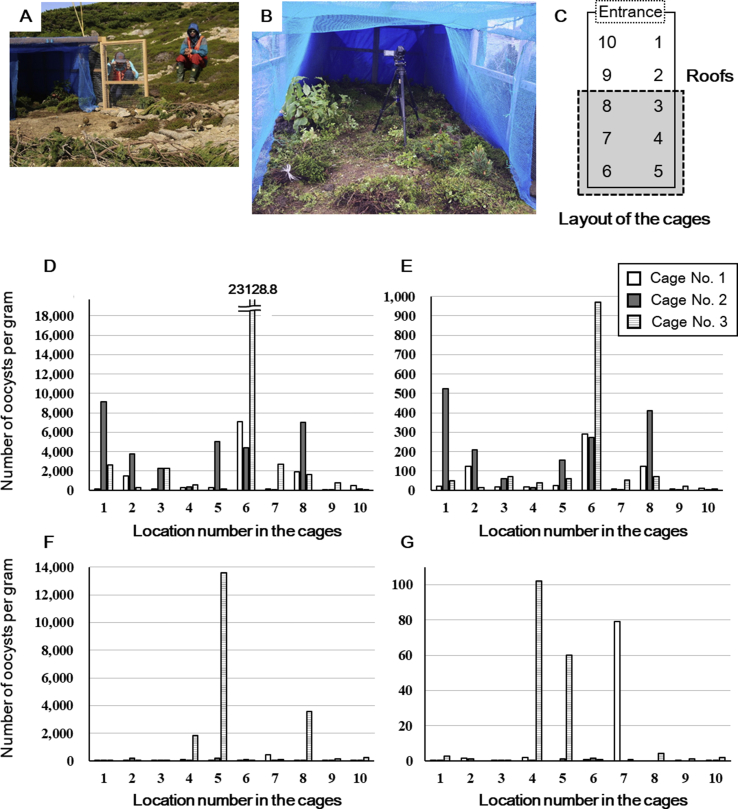
Fig. 2.
Internal and external appearance of a shelter cage used for cage protection of Japanese rock ptarmigan broods on Mt. Norikuradake (A and B, respectively) with location numbers to indicate the soil sample collection positions within the cages (C). OPG values for the soil samples within the cages at Mt. Norikuradake (D; E. uekii and E; E. raichoi) and Mt. Komagatake (F; E. uekii and G; E. raichoi).
Table 1.
Summary of cage protection in 2020 and schedules of collected samples.
The cages used in the present study (about 1.8 m wide by 3.6 m long without floors) were previously used for cage protection, were folded up and kept in a warehouse without any disinfectant treatment prior to reuse. They were set directly on the ground containing gravels and cobbles. Each brood was placed in a cage. Broods protected in the cages were permitted to graze freely outside of the cage during the daytime (≥2 times each day, for ≤3 h under observation) except for in cases of poor weather as previously reported (Kobayashi et al., 2019). The hens and chicks were also fed wild plants (e.g., Empetrum nigrum, Vaccinium ovalifolium, Stellaria nipponica, Aconogonon weyrichii), which were collected in alpine areas, and frozen V. vitis-idaea and E. nigrum fruits, which were collected the previous year. Mealworms (Tenebrio molitor) were used to replace the natural insects. The chicks consumed the cecal feces of their mothers as common coprophagy behavior.
2.3. Fecal samples
One to seven fresh fecal samples (collected within approximately 1 h after being shed) from chicks in each brood were collected daily for 3 days between 21 and 24 July, but the samples could not be identified to an individual. Fecal samples were placed in a cooler box, transported to the laboratory and stored at 4 °C until analyses were performed as described below.
2.4. Soil samples
Four areas in the Central Japanese Alps with alpine plants such as creeping pines (Pinus pumila) were identified as areas that would be potential habitats for Japanese rock ptarmigans, and a total of 43 soil samples were collected. Approximately 10–70 g samples of soil were collected at about 1–2 cm from the surface over an area of 5 cm square between 20 and May 22, 2020 (Table 2). The collection sites were designated as areas A (Mt. Komagatake; n = 4) (one female was found here in 2020), area B located about 100 m to the east of area A (n = 16) (the female was found here in 2019), areas C (Mt. Houkendake, 35°78′N, 137°81′E; n = 9) and area D (Mt. Inamaedake, 35°78′N, 137°82′E; n = 14).
Table 2.
Examinations of eimerian oocysts in the soils collected in the Central Japanease Alps (Nagano Prefecture) on 20–22 May 2020.
| Locations sampled in the Central Japanease Alps | Number detected/number examined |
|
|---|---|---|
| E. uekii | E. raichoi | |
| A. Mt. Komagadake (35°79′N, 137°80′E) | 0/4 | 0/4 |
| B. Areas at 100 m to the east of area A | 0/16 | 0/16 |
| C. Mt. Houkendake (35°78′N, 137°81′E) | 0/9 | 0/9 |
| D. Mt. Inamaedake (35°78′N, 137°82′E) | 0/14 | 0/14 |
Within the three cages, 10 samples (approximately 10–80 g) were collected when the cages were at Mt. Norikuradake on 1 August and at Mt. Komagatake on 6 August as shown in Table 3 and Fig. 2A–C. Additionally, soil samples were collected at seven areas around the cages where the protected hens and chicks were released and fed more than 2 times within 2 h under observation and at three locations 300 m from the cages at Mt. Norikuradake. At Mt. Komagatake, three soil samples were collected in the area around the cages where birds were released every day. Collected soils were stored at 4 °C and examined in the laboratory as described below.
Table 3.
Examination of eimerian oocysts in soils collected in/around the cages at Mt. Norikuradake and Mt. Komagadake.
|
E. uekii |
E. raichoi |
|||||
|---|---|---|---|---|---|---|
| Number detected/number examined | Mean OPG (range) | Sporulation rate (range) (%) | Number detected/number examined | Mean OPG (range) | Sporulated rate (ranges) (%) | |
| Mt. Norikuradake in the Northern Japanese Alps | ||||||
| Cage No. 1 | 10/10 | 1208.2 (107.4–7122.2) | 53.5 (2–81) | 10/10 | 64.4 (107.4–7122.2) | 0a |
| Cage No. 2 | 10/10 | 3226.0 (24.6–9156.7) | 57.5 (13–72) | 10/10 | 165.7 (24.6–9156.7) | N.D. |
| Cage No. 3 | 10/10 | 3430.7 (112.0–23,128.8) | 48.0 (10–79) | 10/10 | 136.1 (112–23,128.8) | 0a |
| Areas around the cages | 3/7 | 2.4 (0.5–4.1) | 1/7 | 0.3a | N.D. | |
| Areas located 300 m from the cage | 0/3 | 0/3 | ||||
| Mt. Komagatake in the Central Alps | ||||||
| Cage No. 1 | 10/10 | 67.9 (0.7–454.2) | 75.5 (66–89) | 8/10 | 8.5 (0.2–79.2) | 65.0a |
| Cage No. 2 | 10/10 | 63.2 (3.5–209.4) | 79.0 (69–87) | 7/10 | 0.5 (0.2–1.5) | N.D. |
| Cage No. 3 | 10/10 | 1967.4 (9.8–13,568.0) | 90.9 (79–95) | 9/10 | 17.5 (0.6–102.1) | 74.0 (73–75) |
| Areas around the cages | 0/3 | 0/3 | ||||
N.D.; not determined.
; data from only one sample due to few oocysts.
2.5. Parasitic examinations
For the detection of parasites, the sugar flotation centrifuge method was conducted as described previously (Ekawasti et al., 2019, Matsubayashi et al., 2018b). Briefly, 1 g of fecal samples was filtrated, and sugar solution with a specific gravity of 1.2 was added. Floated parasites were placed onto a glass slide and the entire smear was examined under light microscopy. Oocysts (E. uekii or E. raichoi) were morphologically identified to species under a microscope (E200, Nikon, Tokyo, Japan) (Matsubayashi et al., 2018a), and the oocysts per gram (OPG) of Eimeria spp. was calculated. Soil samples were stirred in 300–500 ml of 0.05% Tween 20 (Nacalai Tesque) for 30 min, filtered through a steel mesh, and the oocysts were purified by the sugar flotation centrifuge method (Matsubayashi et al., 2020). After the purification, 5 μl of 1–3 ml oocyst solution in distilled water was put on slide glass, and OPG was calculated based on the number of oocysts at the entire smear.
2.6. Oocyst sporulation time
Oocysts were purified from feces of infected chicks by the sugar flotation centrifuge method as previously described (Matsubayashi et al., 2020), and 100 μl of 4.0 × 104 oocysts of E. uekii and 1.7 × 104 oocysts of E. raichoi were orally inoculated into a 30-day-old Svalbard rock ptarmigan (Lagopus mutus hyperboreus) for five days. The fertilized eggs of Svalbard rock ptarmigans were kindly provided by the Nagano Chausuyama Zoo (Nagano, Japan) and a chick was successfully hatched. At 7 days after inoculation, feces were collected within 1.5 h after being shed for 12 h and stored at 4 °C. The passaged oocysts (E. uekii and E. raichoi) were purified in the laboratory on ice by the flotation method as described above and diluted in 1% potassium dichromate (Nacalai Tesque) and incubated at 15, 20, and 25 °C and observed at 1 h intervals to 24 h and then at 24-h intervals to 120 h to determine the timing of sporulation based on counts of 100 oocysts.
2.7. Examination of chicks
For the five chicks that were found dead during the brooding period, the appearance of clinical symptoms, such as diarrhea or weakness were not identified during our observations. The chicks were held at −20 °C in a mountain lodge adjacent to the cages for 1–2 months and then fixed in 10% neutral-buffered formalin (Nacalai Tesque, Kyoto, Japan) for a few weeks at the laboratory, necropsied, and their organs were sampled and examined as previously reported (Matsubayashi et al., 2020).
3. Results and discussion
Based on fecal examination of chicks for 3 days during cage protection, all or most of the samples were positive for E. uekii and/or E. raichoi (Table 4), and the maximum OPG exceeded 104. Histopathological examination revealed that developmental zoites of Eimeria spp. were found in the mucosa of intestinal organs in two chicks (chicks c and d) of the four chicks that died during cage protection (Table 5). However, most organs showed signs of degradation after death and pathological findings were not conclusive. Based on these findings, heavy infections of parasites (more than 104 of OPG and large numbers of zoites in the intestines) might have occurred around 21 July, which is in the latter part of cage protection, although the exact reason for death, including for chicks a and b, could not be clarified.
Table 4.
Fecal examinations of chicks in cage protection.
| Cage No.(Family) |
E. uekii |
E. raichoi |
||
|---|---|---|---|---|
| Number positive/number examined | Mean OPG (range) | Number positive/number examined | Mean OPG (range) | |
| 1 (Family A) | 13/13 | 5391.2 (66–26,620) | 11/13 | 143.5 (8-824) |
| 2 (Family B) | 7/7 | 5466.6 (10–11,454) | 7/7 | 408.3 (2-826) |
| 3 (Family C and D) | 4/4 | 9507.5 (108–20,016) | 4/4 | 4866.5 (1,032-12,794) |
Table 5.
Parasite development score by histopathological examination of four chicks that died during cage protection in 2020.
| Organ | Chick |
|||
|---|---|---|---|---|
| a | b | c | d | |
| Stomach | – | – | – | – |
| Duodenum | – | – | ++ | +++ |
| Jejunum | – | – | ++ | ++ |
| Ileum | – | – | – | +++ |
| Ceca (right and left) | −/− | −/− | −/− | −/+++ |
| Colon | – | – | + | +++ |
| Heart | – | – | – | – |
| Liver | – | – | – | – |
| Kidneys | – | – | – | – |
| Cage No. | 1 | 2 | 3 | 3 |
| Family | C | A | C or D | C or D |
| Date of death, 2020 | 12 July | 14 July | 28 July | 30 July |
-; no parasites, +; a few parasites in intestinal mucosa on some fields, ++; 1–5 parasites in intestinal mucosa per field, +++; >5 parasites per field.
All soil samples from the four areas on Mt. Komagatake in the Central Japanese Alps were negative for oocysts on 20 to 22 May before the brood families were transported (Table 2). However, most soil samples within cages contained oocysts with a maximum OPG of more than 104 at both Mt. Norikuradake and Mt. Komagatake (Table 3). Compared to Mt. Komagatake, the OPG at Mt. Norikuradake was mostly higher. The longer duration of cage protection at Mt. Norikuradake than at Mt. Komagatake may explain the difference in oocyst load. E. uekii oocysts showed a higher prevalence than E. raichoi. A higher prevalence and OPG was reported for E. uekii in wild Japanese rock ptarmigans than for E. raichoi (Matsubayashi et al., 2018a, 2018b, 2020), and the present results in soils corroborate previous findings.
The mean sporulation rates in soils within the cages ranged from 48.0 to 90.9% (Table 3). Higher sporulation rates at Mt. Komagatake may be due to higher temperature or better weather than encountered at Mt. Norikuradake during the cage protection period. Sporulated oocysts clearly formed sporocysts and sporozoites, and these were not morphologically degraded. Oocysts were detected in some soil samples around the cages but with an extremely low OPG. Consequently, soil at the back side in the cages and around the entrances was highly contaminated with oocysts. Hens and chicks spent a relatively longer time and slept at night at the back side of the cage, resulting in a higher number of oocysts accumulating in these soils and a greater potential for these soils to be a source of infection to the chicks.
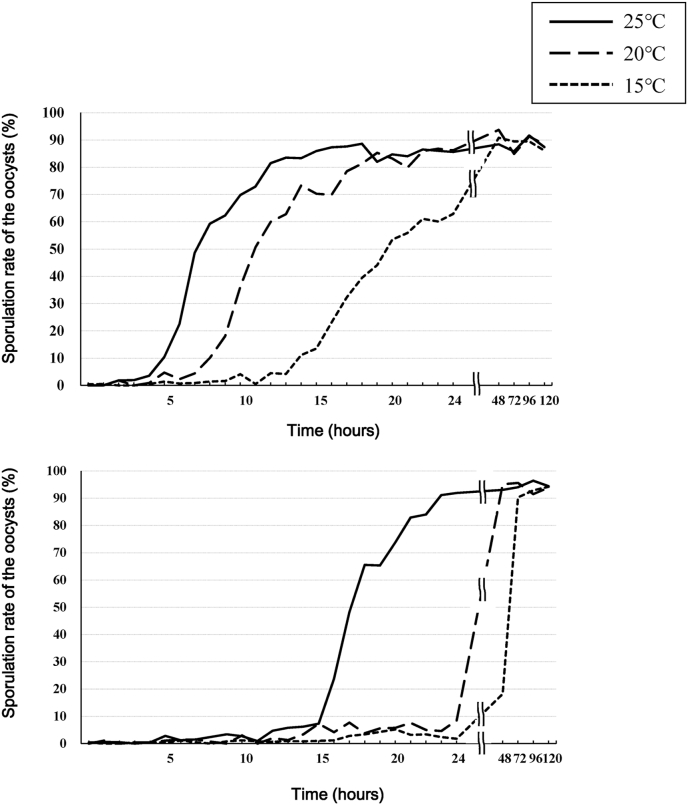
Sporulation rates increased depending on temperature, and E. uekii more rapidly sporulated than E. raichoi (Fig. 3). At 25, 20 and 15 °C, it took 8, 11, and 20 h, respectively, for more than 50% of the oocysts of E. uekii to sporulate and 18, 48, and 72 h, respectively, for E. raichoi. Lower detection of E. raichoi in feces and soils might be due to the delayed time to sporulation. Although we hypothesize that coprophagy of feces is the route of infection, there is little possibility that oocysts in fresh feces would be sporulated in the short time before ingestion by chicks. In other animal species, the bovine Eimeria spp. sporulation rate was 32% by 6 days at 18 °C, 18% by 12 days at 23 °C, and 90% at 3 days at 28 °C (Pyziel and Demiaszkiewicz, 2015). These results indicate that the life cycle of Eimeria spp. from Japanese rock ptarmigans, especially E. uekii, could be adapted to environments such as timber regions.
Fig. 3.
Sporulation rates for E. uekii (A) and E. raichoi (B) at temperatures of 15, 20, and 25 °C.
Cage protection is thought to be effective for the conservation of Japanese rock ptarmigans (Kobayashi, 2020). In a previous report, the cage-protected chicks shed higher numbers of oocysts in feces (less than 105-106 of E. uekii and E. raichoi at maximum number of OPG in wild chicks, but more than 106 of E. uekii and E. raichoi for several days in caged chicks) (Matsubayashi et al., 2018b, 2020). In the wild, Japanese rock ptarmigans move around to different areas after the chicks hatch without fixed nests and, thus, wild birds might not be exposed to frequent risk for heavy infections. However, soils of the vast habitats cannot be fully examined, and there is little information on the dynamics of Eimeria spp., including transmission routes in the wild. Coprophagy by chicks might be considered as the main route for infection, although consuming fresh feces does not allow much possibility for infection as oocysts do not have enough time to sporulate. The ecological behavior of hens warming the chicks in their feathers or other unknown factors could alternatively be associated with infection.
In the present study, the back sides of the cages where families frequently stayed became heavily contaminated. In the cages, the chicks could be infected directly or indirectly by the routes of soils highly contaminated with feces containing oocysts. As one conservation strategy, Japanese rock ptarmigans could be transported to zoos and artificially bred and protected in cages. Therefore, it is necessary to prevent the chicks from becoming heavily infected through methods such as using slatted floors or removing feces, although the pathogenicity of Eimeria spp. remains unknown. Our results suggest that large populations of the birds, as are encountered in artificial breeding programs, could increase the risk for infection because the oocysts have a high resistance to antibiotics and survive in many environments (Daugschies et al., 2013; Lassen et al., 2013; Das et al., 2015).
Ethics statement
All experiments in the wild were carried out without using live animals. Thus, ethical approval for animal experimentation was not necessary. All examinations in the field study were permitted by the Ministry of the Environment Government of Japan. Fecal collection was performed in a non-invasive manner. No animals were sacrificed for the purpose of the field study. In the experimental infection study, the animals were treated in accordance with the protocols approved by the Animal Care and Use Committee in accordance with the Animal Experimentation Guidelines of Osaka City University (approval No. 15003). Human participants were not involved in this study.
Certification of declaration of interests
All the authors confirm that we declare that they have no conflict of interest.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge Daichi Iijima (Chiba University, Japan) for assistance with collecting feces, Masaru Kobayashi, Megumi Shimada, Shinya Tokutake, and Satomi Doai (National Institute of Animal Health, Japan) for assistance with histopathology, and Rika Sekiguchi and Noriko Asama (Osaka Prefecture University, Japan) for help with fecal and soil examinations. The kind support of the Chojo Sanso Lodge and the Houken Sanso Lodge, and of Nagano Chausuyama Zoo is acknowledged. This study was supported in part by grants from JSPS KAKENHI (Grant No. 19H04319) and the Environment Research and Technology Development Fund of the Ministry of the Environment (No. 4-1604).
Contributor Information
Makoto Matsubayashi, Email: matsubayashi@vet.osakafu-u.ac.jp.
Kazunari Ushida, Email: k_ushida@isc.chubu.ac.jp.
References
- Das M., Deka D.K., Sarmah P.C., Islam S., Sarma S. Diversity of Eimeria spp. in dairy cattle of Guwahati, Assam, India. Vet. World. 2015;8:941–945. doi: 10.14202/vetworld.2015.941-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugschies A., Bangoura B., Lendner M. Inactivation of exogenous endoparasite stages by chemical disinfectants: current state and perspectives. Parasitol. Res. 2013;112:917–932. doi: 10.1007/s00436-013-3324-4. [DOI] [PubMed] [Google Scholar]
- del Hoyo J., Elliott A., Sargatal J. vol. 2. Lynx Edicions; Barcelona: 1994. Hand-book of the Birds of the World; p. 638. (New World Vul-Tures to Guineafowl). [Google Scholar]
- Ekawasti F., Nurcahyo W., Wardhana A.H., Shibahara T., Tokoro M., Sasai K., Matsubayashi M. Molecular characterization of highly pathogenic Eimeria species among beef cattle on Java Island, Indonesia. Parasitol. Int. 2019;72:101927. doi: 10.1016/j.parint.2019.101927. [DOI] [PubMed] [Google Scholar]
- Fuglei E., Henden J.A., Callahan C.T., Gilg O., Hansen J., Ims R.A., Isaev A.P., Lang J., McIntyre C.L., Merizon R.A., Mineev O.Y., Mineev Y.N., Mossop D., Nielsen O.K., Nilsen E.B., Pedersen Å.Ø., Schmidt N.M., Sittler B., Willebrand M.H., Martin K. Circumpolar status of Arctic ptarmigan: population dynamics and trends. Ambio. 2020;49:749–761. doi: 10.1007/s13280-019-01191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsgard P.A. The University of Nebraska Press; USA: 1983. The Grouse of the World; p. 413. [Google Scholar]
- Kobayashi A. Results of Japanese rock ptarmigan (Lagopus muta japonica) conservation and future prospects. Bull. Omachi Alpine Museum. 2020;5:7–10. [in Japanese] [Google Scholar]
- Kobayashi A., Nakamura H. Chick and juvenile survival of Japanese rock ptarmigan Lagopus muta japonica. Wildl. Biol. 2013;19:58–367. [Google Scholar]
- Kobayashi A., Tsuchida S., Ueda A., Yamada T., Murata K., Nakamura H., Ushida K. Role of coprophagy in the cecal microbiome development of an herbivorous bird Japanese rock ptarmigan. J. Vet. Med. Sci. 2019;81:1389–1399. doi: 10.1292/jvms.19-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen B., Lepik T., Bangoura B. Persistence of Eimeria bovis in soil. Parasitol. Res. 2013;112:2481–2486. doi: 10.1007/s00436-013-3413-4. [DOI] [PubMed] [Google Scholar]
- Matsubayashi M., Tsuchida S., Kobayashi A., Shibahara T., Nakamura H., Murata K., Ushida K. Molecular identification of two Eimeria species, E. uekii and E. raichoi as type B, in wild Japanese rock ptarmigans, Lagopus muta japonica. Int. J. Parasitol. Parasites Wildl. 2018;7:243–250. doi: 10.1016/j.ijppaw.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi M., Tsuchida S., Ushida K., Murata K. Surveillance of Eimeria species in wild Japanese rock ptarmigans, Lagopus muta japonica, and insight into parasitic seasonal life cycle at timberline regions of the Japanese Alps. Int. J. Parasitol. Parasites Wildl. 2018;7:134–140. doi: 10.1016/j.ijppaw.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi M., Kinoshita M., Kobayashi A., Tsuchida S., Shibahara T., Hasegawa M., Nakamura H., Sasai K., Ushida K. Parasitic development in intestines and oocyst shedding patterns for infection by Eimeria uekii and Eimeria raichoi in Japanese rock ptarmigans, Lagopus muta japonica, protected by cages in the Southern Japanese Alps. Int. J. Parasitol. Parasites Wildl. 2020;12:19–24. doi: 10.1016/j.ijppaw.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of the Environment, Government of Japan . 2012. National Conservation Program for Japanese Rock Ptarmigans.https://www.env.go.jp/nature/kisho/hogozoushoku/pdf/jigyoukeikaku/raicho.pdf (Accessed on 25 July 2018); Available online: [Google Scholar]
- Nakamura H. Rock ptarmigan Lagopus mutus japonicus. Jap. J. Ornithol. 2007;56:93–114. [in Japanese] [Google Scholar]
- Pyziel A.M., Demiaszkiewicz A.W. Observations on sporulation of Eimeria bovis (Apicomplexa: Eimeriidae) from the European bison Bison bonasus: effect of temperature and potassium dichromate solution. Folia Parasitol (Praha) 2015;62:2015. doi: 10.14411/fp.2015.020. 020. [DOI] [PubMed] [Google Scholar]