PURPOSE
Infant acute lymphoblastic leukemia (ALL) is characterized by a high incidence of KMT2A gene rearrangements and poor outcome. We evaluated the value of minimal residual disease (MRD) in infants with KMT2A-rearranged ALL treated within the Interfant-06 protocol, which compared lymphoid-style consolidation (protocol IB) versus myeloid-style consolidation (araC, daunorubicin, etoposide/mitoxantrone, araC, etoposide).
MATERIALS AND METHODS
MRD was measured in 249 infants by DNA-based polymerase chain reaction of rearranged KMT2A, immunoglobulin, and/or T-cell receptor genes, at the end of induction (EOI) and end of consolidation (EOC). MRD results were classified as negative, intermediate (< 5 × 10−4), and high (≥ 5 × 10−4).
RESULTS
EOI MRD levels predicted outcome with 6-year disease-free survival (DFS) of 60.2% (95% CI, 43.2 to 73.6), 45.0% (95% CI, 28.3 to 53.1), and 33.8% (95% CI, 23.8 to 44.1) for infants with negative, intermediate, and high EOI MRD levels, respectively (P = .0039). EOC MRD levels were also predictive of outcome, with 6-year DFS of 68.2% (95% CI, 55.2 to 78.1), 40.1% (95% CI, 28.1 to 51.9), and 11.9% (95% CI, 2.6 to 29.1) for infants with negative, intermediate, and high EOC MRD levels, respectively (P < .0001). Analysis of EOI MRD according to the type of consolidation treatment showed that infants treated with lymphoid-style consolidation had 6-year DFS of 78.2% (95% CI, 51.4 to 91.3), 47.2% (95% CI, 33.0 to 60.1), and 23.2% (95% CI, 12.1 to 36.4) for negative, intermediate, and high MRD levels, respectively (P < .0001), while for myeloid-style–treated patients the corresponding figures were 45.0% (95% CI, 23.9 to 64.1), 41.3% (95% CI, 23.2 to 58.5), and 45.9% (95% CI, 29.4 to 60.9).
CONCLUSION
This study provides support for the idea that induction therapy selects patients for subsequent therapy; infants with high EOI MRD may benefit from AML-like consolidation (DFS 45.9% v 23.2%), whereas patients with low EOI MRD may benefit from ALL-like consolidation (DFS 78.2% v 45.0%). Patients with positive EOC MRD had dismal outcomes. These findings will be used for treatment interventions in the next Interfant protocol.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) occurring in infants, defined as ≤ 365 days of age at diagnosis, is a rare and aggressive type of leukemia. Rearrangement of the lysine methyltransferase 2A gene (KMT2A, formerly known as the mixed lineage leukemia gene) occurs in 75% of infants with ALL and is associated with a poor outcome. Other high-risk factors on the Interfant-06 treatment protocol include age < 6 months at diagnosis and a high WBC count at diagnosis (≥ 300 × 109/L) and/or a poor prednisone response.1,2
CONTEXT
Key Objective
This study investigated the clinical relevance of minimal residual disease (MRD) in 249 infants with KMT2A-rearranged acute lymphoblastic leukemia (ALL) treated according to the Interfant-06 protocol.
Knowledge Generated
This study showed that MRD is of significant prognostic value for infants with KMT2A-rearranged ALL. Most important, our data show that patients with high MRD at the end of induction (EOI) have better outcome when treated with myeloid-like consolidation therapy, whereas patients with low MRD have better outcome when treated with lymphoid-type consolidation therapy. Patients with positive MRD at the end of consolidation (EOC) have dismal outcomes.
Relevance
Our study provides support that the use of MRD at EOI may potentially be of benefit to select patients who should be treated with either myeloid-style or lymphoid-style consolidation therapy. This finding will be integrated into the next worldwide Interfant protocol. Moreover, patients with positive MRD at EOC will be considered for additional, experimental therapies.
In older children (> 1 year) with ALL, minimal residual disease (MRD) has a strong prognostic value.3,4 Treatment protocols are therefore largely based on MRD-directed risk group stratification.5-8 In infant ALL, data on MRD are scarce.9-11 Previously, we have shown that MRD levels at different time points (TPs) have prognostic relevance for infants with ALL enrolled on the preceding Interfant-99 study.10 However, results were interpreted with caution due to low numbers and a heterogeneous population of KMT2A germline and rearranged cases.
The purpose of this study was to investigate the prognostic significance of MRD detection in infants with KMT2A-rearranged (KMT2Ar) ALL treated according to the Interfant-06 protocol and to determine whether MRD can be used to guide therapy. In this protocol, KMT2A-rearranged patients (allocated to either the medium-risk [MR] or the high-risk [HR] group) were eligible for random assignment to lymphoid-style consolidation chemotherapy or myeloid-style consolidation therapy. We recently showed that the type of consolidation treatment administered did not significantly influence outcome of the whole Interfant-06 population.2
MATERIALS AND METHODS
Patients and Treatment Protocol
Patients were included in this study if they were registered on Interfant-06, were KMT2A-rearranged, were documented to have morphological complete remission at the end of induction (EOI) (first complete remission [CR1]), and had availability of DNA material at diagnosis and at least one of the protocol-specified follow-up TPs up to the start of OCTADA(D). MRD analysis was not mandatory in this protocol except for MR patients at TP5 to evaluate eligibility to hematopoietic stem-cell transplantation (HSCT) in CR1. Patients were stratified as described by Pieters et al.2
All patients were enrolled into Interfant-06 protocol, and their parents gave written consent according to the Declaration of Helsinki. The study was approved by the Ethics Committee of all collaborating institutions and registered with the European Clinical Trials database (EudraCT 2005-004599-19) and with the National Cancer Institute (ClinicalTrials.gov identifier: NTC0550992).
Detection of MRD
Bone marrow samples were obtained at diagnosis, after induction (EOI, TP2; n = 217), after consolidation (EOC, TP4; n = 178), and after MARMA (TP5; n = 168) (see the Data Supplement, online only, for the treatment scheme of Interfant-06). Bone marrow samples were available at both EOI and EOC for 169 patients. Samples were shipped to the national reference laboratories. The KMT2A genomic breakpoint fusion sequence was used as the main MRD-polymerase chain reaction (PCR) target. Immunoglobulin and/or T-cell receptor (IG/TR) gene rearrangements were used as additional MRD-PCR targets. At diagnosis, the KMT2A genomic breakpoint fusion sequence was determined in Frankfurt (Burmeister et al12) or in Catania (Meyer et al13). IG/TR gene rearrangements were detected in the diagnostic specimens as previously described.14,15 On the basis of the junctional region of the identified genomic rearrangement, patient-specific primers were designed and tested for sensitivity and specificity at the national reference laboratories. Real-time quantitative PCR data were analyzed according to the EuroMRD guidelines.14 MRD results were classified as negative, intermediate (< 5 × 10−4), and high (≥ 5 × 10−4). In cases where MRD levels according to both KMT2A and IG/TR targets were identified, the highest result was used for analysis. Discrepant MRD levels were defined as ≥ 1 log difference in levels.16
Characterization of Patients with Immunophenotyping
To determine whether the leukemic blast cells of each patient also exhibited myeloid markers, the expression of CD117, CD13, CD33, and CD65/CD15 was analyzed according to the methods described by Dworzak et al17; each marker was scored positive if positive blast subset ≥ 10%.
Statistical Analysis
Fisher's exact tests were performed to compare patients with and without MRD evaluations with respect to known prognostic features. Disease-free survival (DFS) was defined as the time from CR1 to relapse, death in complete continuous remission, or second malignancy—whichever occurred first—or to last follow-up. DFS curves were computed according to the Kaplan-Meier estimator and their SEs according to Greenwood's formula. Outcome was compared by log-rank test. The cumulative incidence of relapse (CIR) was estimated adjusting for competing risks of other events and compared with Gray's test.18 Analysis of prognostic relevance of MRD on DFS was conducted with the Cox model (Wald test) with adjustment for risk group and postinduction treatment and adding the interaction between MRD and treatment. Analysis of impact of myeloid markers and other covariates on MRD was performed with a logistic regression model. KMT2A MRD measurements were compared with IG/TR MRD measurements using the Bland-Altman approach for analyses of agreement between two different assays.19 The hypothesis of zero mean difference (bias) was tested with a paired t test. All tests were two-sided. Analyses were performed using SAS version 9.4.
RESULTS
Patients
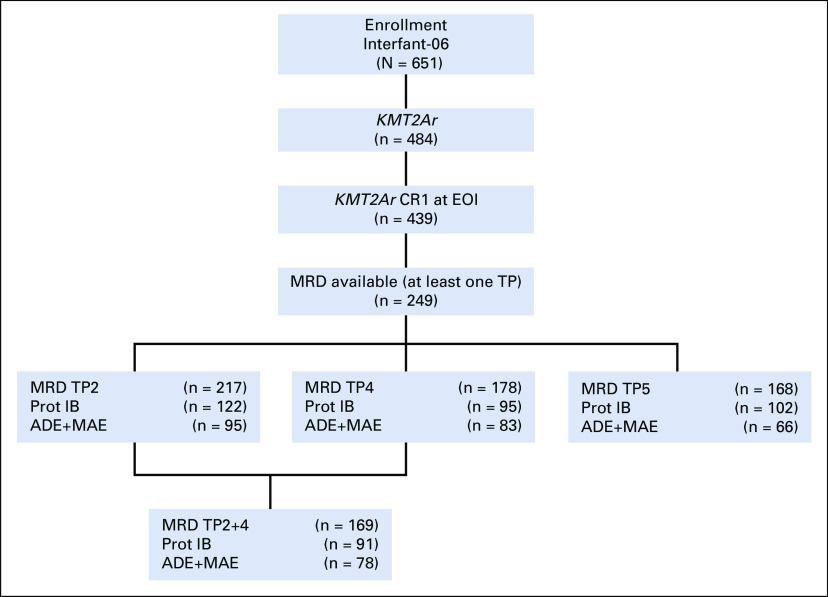
Of 439 KMT2Ar infants enrolled in the Interfant-06 protocol and in CR1 at EOI, MRD data were available for 249 (see the CONSORT diagram, Fig 3). No significant difference in sex, age at diagnosis, WBC at diagnosis, risk group, type of KMT2A rearrangement, immunophenotype (B-lineage CD10+ v CD10− v CD10 unknown), or prednisone response was detected between patients with and without available MRD data (Data Supplement). Patient characteristics are detailed in the Data Supplement. Of these 249 patients, 120 (48.2%) relapsed, 15 (6.0%) died in CCR, and 2 (0.8%) developed a second malignant neoplasm. The median follow-up time was 4.8 years (range 0.3-11.1).
FIG 3.
CONSORT diagram. ADE, araC, daunorubicin, etoposide; CR1, first complete remission; KMT2Ar, KMT2A-rearranged; MAE, mitoxantrone, araC, etoposide; MRD, minimal residual disease.
Comparison Between KMT2A and IG/TR Targets
In samples with data on both KMT2A MRD-PCR and IG/TR MRD-PCR targets available (n = 223), results were concordant in 94% (n = 210/223) of samples. For all patients, both MRD-PCR targets used had a quantitative range of least 5 × 10−4. Results at EOI (n = 90) are shown in a scatterplot in the Data Supplement. There was no significant discrepancy between the two measurements (P = .60; Data Supplement). Only seven samples showed a difference of at least one log. In five samples, the MRD level was higher when measured by KMT2A, while in two samples, it was higher when measured by IG/TR. For EOC and TP5 samples, MRD measurements were concordant in 96% and 95% of cases, respectively. Scatterplots are shown in the Data Supplements.
In 19 patients, MRD detected using KMT2A as the target was either ≥ 5 × 10−4 at EOI or showed any positivity at EOC or TP5, while MRD detected using IG/TR was not. In 12 patients, the opposite occurred with MRD detected using IG/TR satisfying either of these cutoffs, while MRD detected using KMT2A targets did not. In these two groups of patients, there were 9/19 and 1/12 relapses, respectively (6-year CIR 48.7% [95% CI, 23.8 to 69.7] and 8.3% [95% CI, 0.4 to 32.6], respectively, P = .02).
Prognostic Significance of MRD at EOI
At EOI, 42 of 217 (19.4%) patients were MRD negative. MRD negativity at EOI was significantly associated with better outcome (P = .01). The 6-year DFS was 60.2% (95% CI, 43.2 to 73.6) for MRD-negative patients, 45.0% (95% CI, 28.3 to 53.1) for 82 patients with intermediate MRD levels, and 33.8% (95% CI, 23.8 to 44.1) for 93 patients with high MRD levels (P = .0039, Fig 1A). The proportion of MRD-negative patients increased with increasing age at diagnosis, from 10.6% (5 of 47) of patients of age 0-3 months to 34.3% (12 of 35) of patients of age 9-12 months, but this finding was not statistically significant (P = .16).
FIG 1.
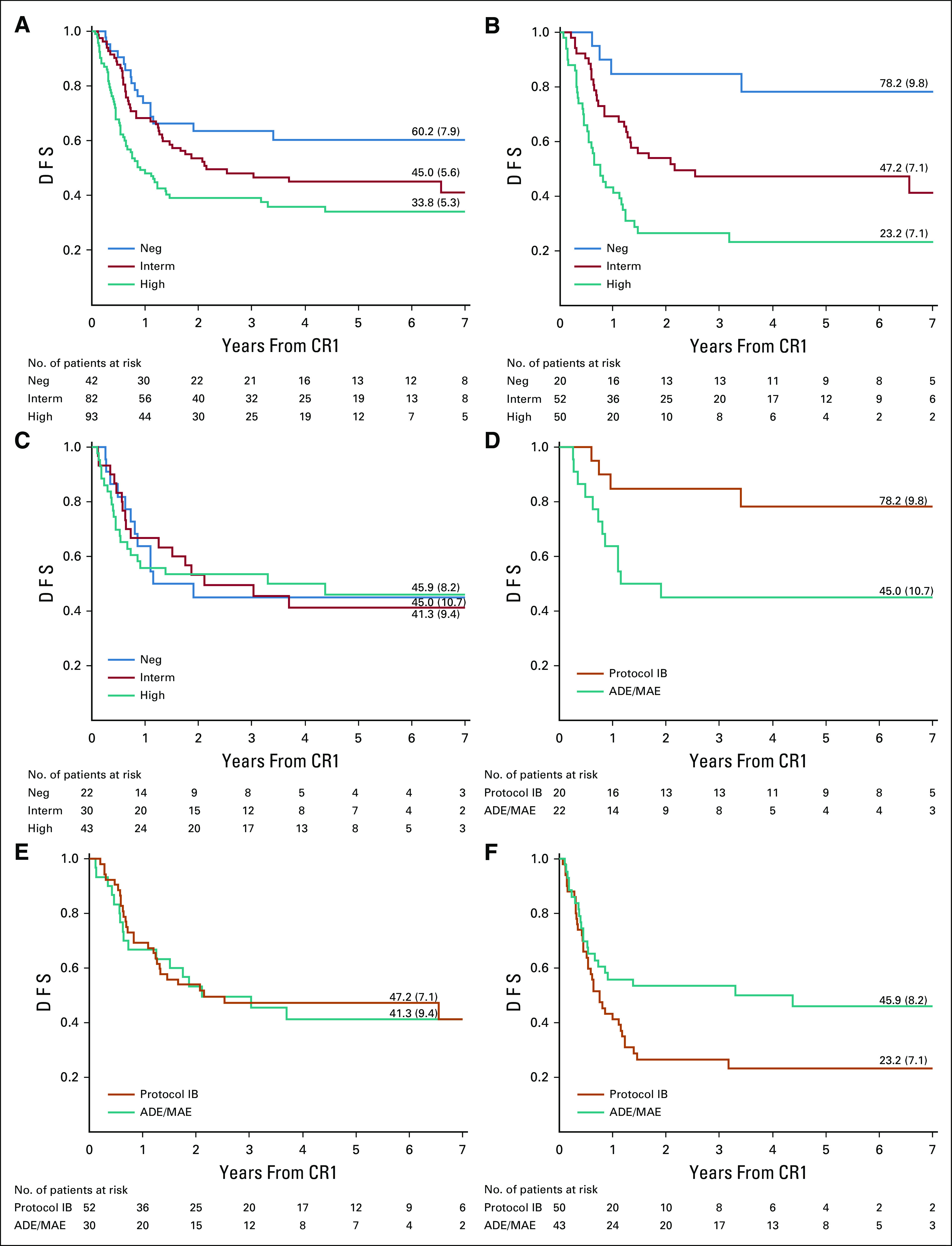
Prognostic impact of minimal residual disease (MRD) levels at the end of induction (EOI), as shown by Kaplan-Meier estimates of disease-free survival (DFS), for all patients (A), patients treated with protocol IB (B), and patients treated with araC, daunorubicin, etoposide (ADE)/mitoxantrone, araC, etoposide (MAE) (C). Outcome by treatment given of patients according to MRD at EOI, negative (D), intermediate (E), and high (F). Neg, MRD-negative; Interm, MRD-intermediate (< 5 × 10−4); High, MRD-high (≥ 5 × 10−4). CR1, first complete remission.
Strikingly, when analyzing MRD results according to the type of consolidation treatment given, EOI MRD levels were only predictive of treatment outcome for patients treated with lymphoid-style consolidation (protocol IB). In 122 patients treated with lymphoid-style consolidation, the 6-year DFS was 78.2% (95% CI, 51.4 to 91.3), 47.2% (95% CI, 33.0 to 60.1), and 23.2% (95% CI, 12.1 to 36.4) for negative (n = 20, four events), intermediate (n = 52, 28 events), and high (n = 50, 37 events) MRD levels, respectively (P < .0001, Fig 1B). For 95 patients treated with myeloid-style consolidation (ADE/MAE), the corresponding numbers were 45.0% (95% CI, 23.9 to 64.1; n = 22, 12 events), 41.3% (95% CI, 23.2 to 58.5; n = 30, 17 events), and 45.9% (95% CI, 29.4 to 60.9; n = 43, 22 events; P = .99, Fig 1C). Most events were relapses (Data Supplement, p) with CIR curves showing comparable data (Data Supplement).
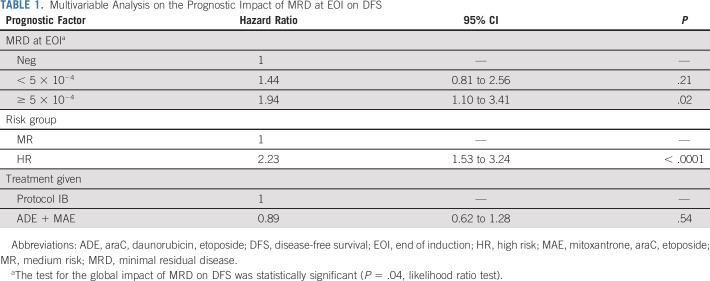
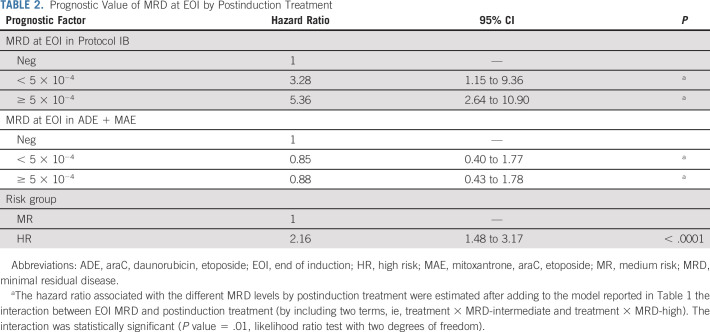
To evaluate the prognostic impact of EOI MRD on DFS, we applied a Cox model adjusting for risk stratification (HR v MR) and treatment given (araC, daunorubicin, etoposide [ADE]/mitoxantrone, araC, etoposide [MAE] v protocol IB). In this model, high MRD level at EOI was associated with lower DFS (hazard ratio, 1.94; 95% CI, 1.10 to 3.41; P = .02, Table 1). Risk group also showed a significant impact on DFS, while the type of consolidation treatment given did not. When the interaction between MRD at EOI and treatment was added to the model, significance was attained (P = .01), indicating that, as shown in Figures 1B-1C, the prognostic impact of MRD depends on the type of consolidation treatment. According to this model, MRD at EOI was prognostic for DFS only for patients treated with lymphoid-style consolidation, and the estimated hazard ratio for these patients who had high EOI MRD increased to 5.36 (95% CI, 2.64 to 10.9, Table 2).
TABLE 1.
Multivariable Analysis on the Prognostic Impact of MRD at EOI on DFS
TABLE 2.
Prognostic Value of MRD at EOI by Postinduction Treatment
This implies that patients with negative EOI MRD (n = 42) had a better outcome when treated with lymphoid-style consolidation (n = 20; 6-year DFS, 78.2% [95% CI, 51.4 to 91.3]) compared with patients treated with myeloid-style consolidation (n = 22; 6-year DFS, 45.0% [95% CI, 23.9 to 64.1], Fig 1D). In contrast, patients with high EOI MRD levels (n = 93) had a better outcome when treated with myeloid-style consolidation (n = 43; 6-year DFS, 45.9% [95% CI, 29.4 to 60.9]) compared with patients treated with lymphoid-style consolidation (n = 50; 6-year DFS, 23.2% [95% CI, 12.1 to 36.4], Fig 1F). Censoring 67 patients who received HSCT in CR1 did not change these results (Data Supplement).
Characteristics of Patients Benefiting From Myeloid-Style Consolidation
To characterize patients who benefit from myeloid-style consolidation therapy, we investigated whether these patients displayed a more myeloid immunophenotype. Of the 122 patients with data available for both MRD EOI and assessment of myeloid immunophenotype, 84 (68.9%) had expression of at least one myeloid marker. For patients with negative MRD at EOI, only 50% had expression of one or more myeloid markers (n = 10/20) versus 63.3% in patients with intermediate MRD (n = 31/49) and 81.1% in patients with high MRD (n = 43/53) (P = .0186). These data indicate that patients with high MRD levels after induction chemotherapy more frequently co-expressed myeloid markers than those with lower MRD values. In a logistic regression model investigating the risk of high MRD at EOI, the presence of both at least one positive myeloid marker and prednisone poor response showed a statistically significant impact, with an estimated odds ratio of 2.54 (95% CI, 1.03 to 6.22, P = .042) and 2.90 (95% CI, 1.03 to 8.15, P = .043), respectively (Data Supplement). There were however no specific markers associated with MRD levels (Data Supplement). In most patients, only one marker was positive and the number of positive markers was not associated with MRD levels (P = .8407, Data Supplement).
Prognostic Significance of MRD at EOC
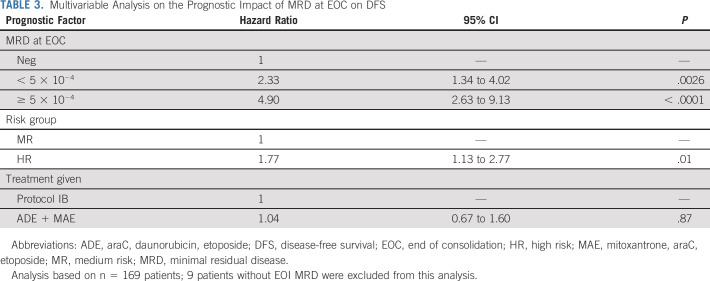
At EOC, 72 of 178 (40.4%) patients were MRD-negative. Patients who were MRD-negative at EOC had significantly better DFS (P < .001). The 6-year DFS was 68.2% (95% CI, 55.2 to 78.1) for MRD-negative patients at EOC, while patients with intermediate (n = 73) and high (n = 33) MRD levels had a 6-year DFS of 40.1% (95% CI, 28.1 to 51.9) and 11.9% (2.6 to 29.1), respectively (P < .0001, Fig 2A and Data Supplement). The prognostic value of EOC MRD was identified in patients treated with lymphoid-type consolidation as well as in patients treated with myeloid-type consolidation (Figs 2B-2C). In a Cox model, both MRD at EOC and risk group had a significant effect on DFS (Table 3).
FIG 2.
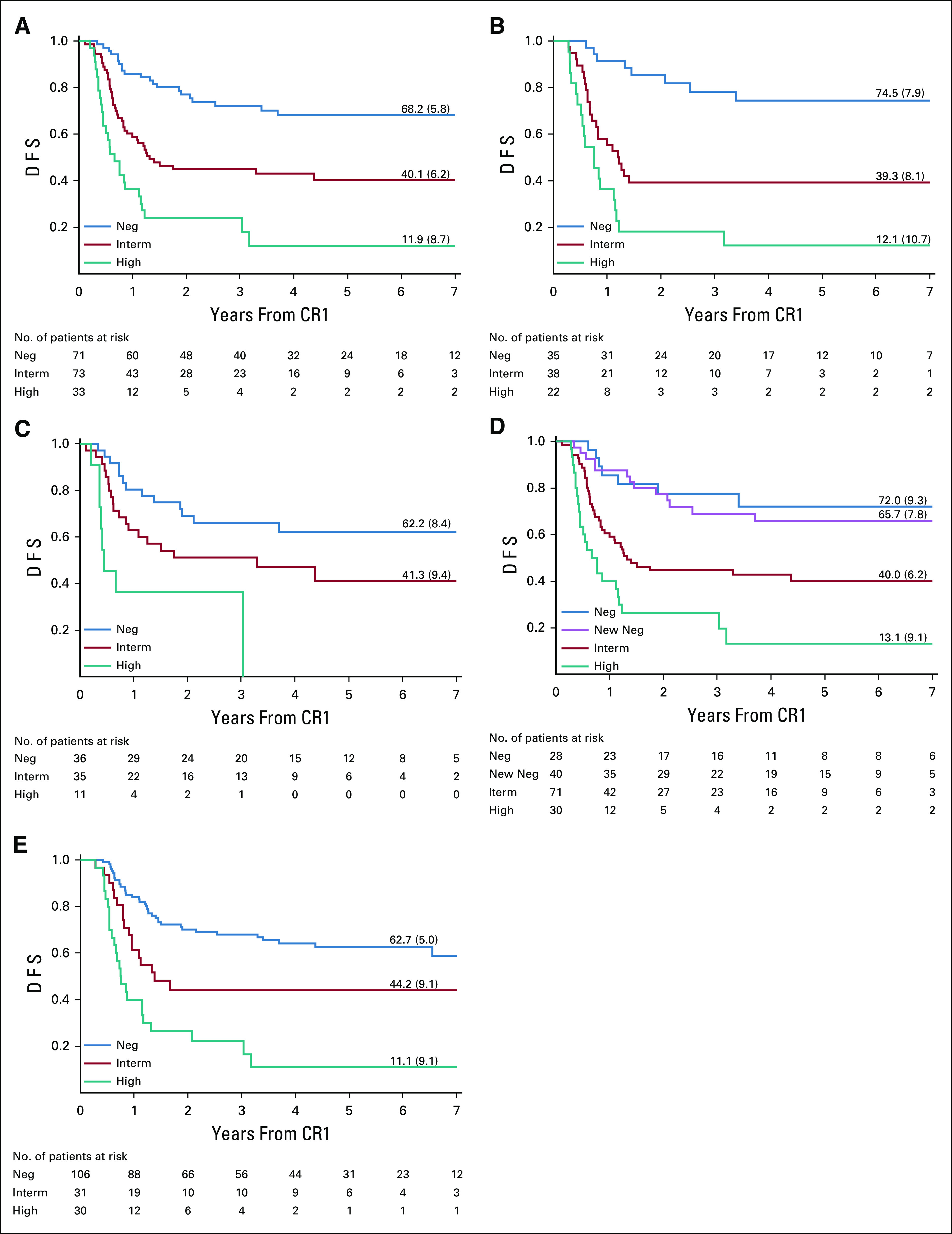
Prognostic impact of minimal residual disease (MRD) levels at the end of consolidation (EOC) (A-D) and after MARMA (E), as shown by Kaplan-Meier estimates of disease-free survival (DFS): EOC MRD for all patients (A), patients treated with protocol IB (B), patients treated with araC, daunorubicin, etoposide (ADE)/mitoxantrone, araC, etoposide (MAE) (C), and for all patients with MRD at EOI and EOC (D) and MRD after MARMA for all patients (E). Neg, MRD-negative; Interm, MRD-intermediate (< 5 × 10−4); High, MRD-high (≥ 5 × 10−4). (D): Neg, MRD-negative at EOI and EOC; New Neg, MRD-positive at EOI and MRD-negative at EOC. For (A-D), One MRD-negative patient treated with ADE/MAE was excluded from these analyses due to missing data on first complete remission (CR1). For (E), One MRD-intermediate patient was excluded from this analysis due to missing data on CR1.
TABLE 3.
Multivariable Analysis on the Prognostic Impact of MRD at EOC on DFS
MRD data for both EOI and EOC were available for 169 patients. Patients who were MRD-positive at EOI and became negative at EOC were defined as new negatives. These patients (n = 40) had almost the same outcome as patients who were already MRD-negative at EOI (n = 28): 6-year DFS was 65.7% (95% CI, 48.1 to 78.6) and 72.0% (95% CI, 49.3 to 85.8), respectively (Fig 2D). In a Cox model that considered MRD levels at both TPs, the hazard ratio of patients who became MRD-negative at EOC was not significantly different from that of patients negative at both TPs, while MRD positivity at EOC was significantly associated with a three-fold increase in the risk of treatment failure, as compared with patients who were MRD-negative at both TPs (Table 4).
TABLE 4.
Multivariable Analysis on the Prognostic Impact of the Combination of MRD Levels at EOI and EOC on DFS
Prognostic Significance of MRD at the End of MARMA (TP5)
At the end of MARMA (TP5), 106 of 168 (63.1%) patients were MRD-negative. MRD at TP5 was significantly related to DFS (Fig 2E and Data Supplement): the 6-year DFS was 62.7% (95% CI, 52.1 to 71.5) for MRD-negative patients, while patients with intermediate (n = 32) and high (n = 30) MRD levels had a 6-year DFS of 44.2% (95% CI, 26.2 to 60.8) and 11.1% (95% CI, 2.3 to 28.0), respectively (P < .0001). MRD had prognostic impact for patients treated with lymphoid-type consolidation as well as for patients treated with myeloid-type consolidation.
DISCUSSION
In this study, we have shown that MRD diagnostics in infants with KMT2A-rearranged ALL treated according to the Interfant-06 protocol predict outcome and can be used for risk stratification and treatment allocation. Negative MRD at EOI was a very strong prognostic factor in patients treated with lymphoid-style consolidation (protocol IB) but not in infants given myeloid-type consolidation (ADE/MAE). At the EOC, negative MRD was a very strong prognostic factor irrespective of the type of consolidation treatment given. Most importantly, induction therapy seems to select patients for subsequent therapy; infants with high EOI MRD benefit from AML-like consolidation, whereas infants with low MRD benefit from ALL-like consolidation.
This is the largest multicenter prospective study on MRD in infant ALL. This large-scale international treatment protocol (Interfant-06) allowed analysis of similarly treated infants with KMT2Ar ALL. Given that this protocol included a randomized question on consolidation therapy for KMT2Ar infants, molecular responses could be analyzed separately.
The prognostic relevance of MRD in patients given protocol IB therapy is in line with MRD results of the Interfant-99 MRD study and of a recent joint mixed-lineage leukemia–baby/Interfant study.10,11 However, in the Interfant-99 MRD study, numbers were limited and a mix of KMT2A germline and rearranged cases was studied. In the study of Popov et al,11 MRD was measured by flow cytometry in two cohorts, and also a mix of KMT2A germline and rearranged cases was studied. In this analysis, we included only infants with KMT2Ar due to their very poor outcome.2 The identification of new prognostic factors, such as MRD, in addition to current characteristics used for risk stratification, is of significant value for guiding treatment and improving outcome for infants with ALL.
In the Interfant-06 study, the genomic KMT2A fusion breakpoints were the preferred PCR target given that earlier studies showed that more reliable MRD data were obtained with this marker compared with the occasionally absent, oligoclonal, or less stable IG/TR rearrangements.10,12 We show that for samples where both targets were available, there was no significant discrepancy between the measurements. However, when we applied a cutoff for TP2 (MRD ≥ 5 × 10−4) and TP4/TP5 (any positive MRD level), use of KMT2A targets predicted relapse better than IG/TR. False positivity for IG/TR PCR targets in regenerating BM has been described.20,21 Our results indicate that KMT2A genomic breakpoint fusion sequence is the preferred target for measurement of MRD in infants with KMT2A-rearranged ALL.
Using MRD diagnostics, we could identify a subgroup of patients with a relatively good outcome; patients with MRD negativity at EOI treated with lymphoid-style consolidation had a 6-year DFS of 78.2%. In addition, patients who became MRD-negative at EOC (new negatives) also had a relatively good outcome with a 6-year DFS of 65.7%, irrespective of the consolidation treatment given. Nevertheless, outcomes of this relatively favorable group remain inferior to that of children with ALL who are older than 1 year of age at diagnosis (5-year event-free survival 93%).5,22-24 Therefore, there seems no place for therapy reduction in this specific subgroup.
MRD assessment at EOI also identified a subgroup of infants with a very poor prognosis. Patients with high MRD levels at EOI had a very poor outcome when treated with lymphoid-style consolidation (6-year DFS of 23.2%).
Our data indicate that ALL-based induction leads to selection of patients who respond well to lymphoid-type treatment (those with low EOI MRD) and are likely to benefit from lymphoid-style consolidation therapy IB. On the other hand, poor response to the ALL-based induction (high EOI MRD) suggests more myeloid-like leukemia that should be offered a myeloid-style consolidation therapy ADE/MAE. This hypothesis is further supported by the more pronounced expression of myeloid markers in patients with high EOI MRD levels. An interesting hypothesis would be whether a subset of infant ALL patients would benefit from a myeloid-like induction course. However, our study only shows that the response to a lymphoid-type induction course discriminated between patients who thereafter benefitted from lymphoid or myeloid consolidation therapy. Our study cannot answer the question whether a myeloid induction therapy would be of benefit for some patients.
MRD levels after consolidation therapy was a strong prognostic factor. Patients who were MRD-positive at EOC or after MARMA had a poor outcome with 6-year DFS < 45%. For these infants, new agents directed against novel targets related to KMT2A rearrangements or immunotherapeutic approaches such as CD19-directed antibodies or CART cells may be required to achieve molecular remission prior to consolidation with allogeneic HSCT.
We conclude that MRD assessment has independent prognostic value for infants with KMT2A-rearranged ALL. Our study supports the idea that induction therapy selects patients for subsequent therapy; infants with high EOI MRD may benefit from AML-like consolidation, whereas infants with low EOI MRD may benefit from ALL-like consolidation. These findings will be used for improved risk stratification and treatment selection in the next Interfant protocol.
EQUAL CONTRIBUTION
M.G.V. and R.P. shared senior authorship of this work.
SUPPORT
Supported by the Czech Ministry of Health (NV19-07-00329), Italian Ministry of University and Research, PRIN 2017 - N. 20178S4EK9 (MGV), and Italian Association for Cancer Research (AIRC), grants IG2015-17593 to G.C. and IG2017-20564 to A.B.
CLINICAL TRIAL INFORMATION
The study was approved by the Ethics Committee of all collaborating institutions and registered with the European Clinical Trials Database (EudraCT 2005-004599-19) and with the National Cancer Institute (ClinicalTrials.gov identifier: NTC0550992).
AUTHOR CONTRIBUTIONS
Conception and design: Inge M. van der Sluis, Myriam Campbell, Gabriele Escherich, Rishi S. Kotecha, Martin Schrappe, Maria Grazia Valsecchi, Rob Pieters
Provision of study materials or patients: Inge M. van der Sluis, Andishe Attarbaschi, Benoit Brethon, Myriam Campbell, Gabriele Escherich, Rishi S. Kotecha, Chi Kong Li, Luca Lo Nigro, Franco Locatelli, Rolf Marschalek, Jan Zuna, Tomasz Szczepanski
Collection and assembly of data: Janine Stutterheim, Inge M. van der Sluis, Paola de Lorenzo, Julia Alten, Philip Ancliffe, Andishe Attarbaschi, Benoit Brethon, Myriam Campbell, Gabriele Escherich, Alina Ferster, Rishi S. Kotecha, Birgitte Lausen, Chi Kong Li, Luca Lo Nigro, Franco Locatelli, Rolf Marschalek, Claus Meyer, Martin Schrappe, Jan Stary, Ajay Vora, Jan Zuna, Vincent H. J. van der Velden
Data analysis and interpretation: Janine Stutterheim, Inge M. van der Sluis, Paola de Lorenzo, Philip Ancliffe, Andishe Attarbaschi, Andrea Biondi, Myriam Campbell, Giovanni Cazzaniga, Gabriele Escherich, Alina Ferster, Rishi Sury Kotecha, Chi Kong Li, Franco Locatelli, Rolf Marschalek, Martin Schrappe, Ajay Vora, Vincent H. J. van der Velden, Tomasz Szczepanski, Maria Grazia Valsecchi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Clinical Implications of Minimal Residual Disease Detection in Infants With KMT2A-Rearranged Acute Lymphoblastic Leukemia Treated on the Interfant-06 Protocol
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Inge M. van der Sluis
Consulting or Advisory Role: Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Andishe Attarbaschi
Honoraria: Jazz Pharmaceuticals, Amgen, Novartis
Consulting or Advisory Role: Jazz Pharmaceuticals, Amgen, Novartis
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Benoit Brethon
Honoraria: Amgen SAS, Jazz Pharmaceutical
Consulting or Advisory Role: Amgen SAS
Travel, Accommodations, Expenses: Amgen SAS, Astellas Pharma
Andrea Biondi
Consulting or Advisory Role: Novartis, Celgene, Incyte
Chi Kong Li
Consulting or Advisory Role: Amgen Asia
Franco Locatelli
Honoraria: Bellicum Pharmaceuticals, Miltenyi Biotec, Bluebird Bio, Medac
Consulting or Advisory Role: Amgen, Novartis, Pfizer
Rob Pieters
Honoraria: ERYTECH Pharma, Jazz Pharmaceuticals, Kite Pharma, Novartis, Servier/Pfizer
Consulting or Advisory Role: ERYTECH Pharma, Kite Pharma, Jazz Pharmaceuticals, Servier
Travel, Accommodations, Expenses: ERYTECH Pharma, Kite Pharma, Jazz Pharmaceuticals, Servier
Martin Schrappe
Consulting or Advisory Role: Novartis, Servier, Jazz Pharmaceuticals
Speakers' Bureau: Servier, Jazzpharma
Research Funding: Shire, Novartis, Servier
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, Servier
Ajay Vora
Consulting or Advisory Role: Jazz Pharmaceuticals, Pfizer, Medac, Amgen, Janssen Oncology, Novartis Pharmaceuticals UK Ltd
Travel, Accommodations, Expenses: Medac
Vincent H. J. van der Velden
Research Funding: BD Biosciences, Pfizer, Janssen
Patents, Royalties, Other Intellectual Property: Patent EuroFlow MRD antibody tubes. No financial relationship.
Tomasz Szczepanski
Honoraria: Gilead Sciences, Novartis, Amgen, Servier
Consulting or Advisory Role: Novartis, Takeda, Roche
Travel, Accommodations, Expenses: Servier, Jazz Pharmaceuticals, Amgen, Roche, Gilead Sciences, SOBI, CSL Behring, Octapharm, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pieters R Schrappe M De Lorenzo P, et al. : A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): An observational study and a multicentre randomised trial. Lancet 370:240-250, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Pieters R De Lorenzo P Ancliffe P, et al. : Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the interfant-06 protocol: Results from an international phase III randomized study. J Clin Oncol 37:2246-2256, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen JJ Seriu T Panzer-Grümayer ER, et al. : Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 352:1731-1738, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Faderl S Kantarjian HM Talpaz M, et al. : Clinical significance of minimal residual disease in leukemia. Int J Oncol 17:1277-1287, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Pieters R de Groot-Kruseman H Van der Velden V, et al. : Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol 34:2591-2601, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Conter V Bartram CR Valsecchi MG, et al. : Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 115:3206-3214, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Schrappe M Bleckmann K Zimmermann M, et al. : Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: Results of an international randomized trial (AIEOP-BFM ALL 2000). J Clin Oncol 36:244-253, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Schrappe M Valsecchi MG Bartram CR, et al. : Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood 118:2077-2084, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Li A Goldwasser MA Zhou J, et al. : Distinctive IGH gene segment usage and minimal residual disease detection in infant acute lymphoblastic leukaemias. Br J Haematol 131:185-192, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Van der Velden VHJ Corral L Valsecchi MG, et al. : Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia 23:1073-1079, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Popov A Buldini B De Lorenzo P, et al. : Prognostic value of minimal residual disease measured by flow-cytometry in two cohorts of infants with acute lymphoblastic leukemia treated according to either MLL-baby or interfant protocols. Leukemia 34:3042-3046, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Burmeister T Marschalek R Schneider B, et al. : Monitoring minimal residual disease by quantification of genomic chromosomal breakpoint sequences in acute leukemias with MLL aberrations. Leukemia 20:451-457, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Meyer C Schneider B Reichel M, et al. : Diagnostic tool for the identification of MLL rearrangements including unknown partner genes. Proc Natl Acad Sci U S A 102:449-454, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Velden VHJ Cazzaniga G Schrauder A, et al. : Analysis of minimal residual disease by Ig/TCR gene rearrangements: Guidelines for interpretation of real-time quantitative PCR data. Leukemia 21:604-611, 2007 [DOI] [PubMed] [Google Scholar]
- 15.van der Velden VHJ, van Dongen JJM: MRD detection in acute lymphoblastic leukemia patients using IG/TR gene rearrangements as targets for real-time quantitative PCR. Methods Mol Biol 538, 115-150, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Cazzaniga G De Lorenzo P Alten J, et al. : Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ABL1 methodologies. Haematologica 103:107-115, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dworzak MN Buldini B Gaipa G, et al. : AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of pediatric acute lymphoblastic leukemia. Cytometry 94:82-93, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 19.Bland JM; Altman D: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307-310, 1986 [PubMed] [Google Scholar]
- 20.van der Velden VHJ, Wijkhuijs JM, van Dongen JJM: Non-specific amplification of patient-specific Ig/TCR gene rearrangements depends on the time point during therapy: Implications for minimal residual disease monitoring. Leukemia 22:641-644, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Kotrova M van der Velden VHJ van Dongen JJM, et al. : Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant 52:962-968, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Enshaei A O’Connor D Bartram J, et al. : A validated novel continuous prognostic index to deliver stratified medicine in pediatric acute lymphoblastic leukemia. Blood 135:1438-1446, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Maloney KW Devidas M Wang C, et al. : Outcome in children with standard-risk B-cell acute lymphoblastic leukemia: Results of Children's Oncology Group Trial AALL0331. J Clin Oncol 38:602-612, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burke MJ Salzer WL Devidas M, et al. : Replacing cyclophosphamide/cytarabine/mercaptopurine with cyclophosphamide/etoposide during consolidation/delayed intensification does not improve outcome for pediatric B-cell acute lymphoblastic leukemia: A report from the COG. Haematologica 104:986-992, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]