PURPOSE
A first-in-human study was performed with MP0250, a DARPin drug candidate. MP0250 specifically inhibits both vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) with the aim of disrupting the tumor microenvironment.
PATIENTS AND METHODS
A multicenter, open-label, repeated-dose, phase I study was conducted to assess the safety, tolerability, and pharmacokinetics of MP0250 in 45 patients with advanced solid tumors. In the dose-escalation part, 24 patients received MP0250 as a 3-hour infusion once every 2 weeks at five different dose levels (0.5-12 mg/kg). Once the maximum tolerated dose (MTD) was established, 21 patients were treated with a 1-hour infusion (n = 13, 8 mg/kg, once every 2 weeks and n = 8, 12 mg/kg, once every 3 weeks) of MP0250 in the dose confirmation cohorts.
RESULTS
In the dose-escalation cohort, patients treated with 12 mg/kg MP0250 once every 2 weeks experienced dose-limiting toxicities. Therefore, MTD was 8 mg/kg once every 2 weeks or 12 mg/kg once every 3 weeks. The most common adverse events (AEs) were hypertension (69%), proteinuria (51%), and diarrhea and nausea (both 36%); hypoalbuminemia was reported in 24% of patients. Most AEs were consistent with inhibition of the VEGF and HGF pathways. Exposure was dose-proportional and sustained throughout the dosing period for all patients (up to 15 months). The half-life was about 2 weeks. Signs of single-agent antitumor activity were observed: 1 unconfirmed partial response with a time to progression of 23 weeks and 24 patients with stable disease, with the longest duration of 72 weeks and a median duration of 18 weeks.
CONCLUSION
MP0250 is a first-in-class DARPin drug candidate with suitable tolerability and appropriate pharmacokinetic properties for further development in combination with other anticancer therapies.
INTRODUCTION
We report here the first-in-human study of MP0250, a DARPin drug candidate and novel biologic that specifically and simultaneously binds to and inhibits vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF).1
CONTEXT
Key Objective
DARPin molecules are a class of small, highly specific binding proteins that can be easily assembled to bind multiple molecular targets. MP0250 is a tri-specific molecule with individual domains binding vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and human serum albumin to increase its plasma half-life. This first-in-human study of intravenously administered MP0250 sought to determine the maximum tolerated dose (MTD) and assess its dose-limiting toxicities, pharmacokinetics, safety, and tolerability.
Knowledge Generated
The MTD of MP0250 was 8 mg/kg once every 2 weeks or 12 mg/kg once every 3 weeks. Drug half-life was about 2 weeks. Most adverse events were consistent with inhibition of the VEGF and HGF pathways, most commonly hypertension, proteinuria, nausea, diarrhea, and hypoalbuminemia. Single-agent antitumor activity was observed, including 1 patient with an unconfirmed partial response.
Relevance
MP0250 is a first-in-class DARPin drug candidate with a tolerability profile and pharmacokinetic properties, making it suitable for further development in combination with other anticancer therapies.
DARPin molecules are a class of small, highly specific binding proteins that can be easily assembled in a multispecific format; this is especially attractive for oncology drug development where simultaneous targeting of several pathways could be advantageous. Several other DARPin molecules are in the clinical and preclinical development stage.
The rationale for concomitant targeting of the VEGF- and HGF-driven signaling pathways is to produce greater disruption of the tumor cell-supporting microenvironment and thus overcome the clinical shortcomings of mono-targeted VEGF and HGF inhibitors.2-9
MP0250 is a tri-specific molecule with individual domains binding VEGF and HGF with picomolar affinity and two domains binding human serum albumin (HSA) to increase its plasma half-life (Fig 1). Preclinical studies have shown MP0250 to have greater effects on tumor growth and angiogenesis than individual VEGF- and HGF-blocking DARPin molecules and also demonstrated potentiation of the antitumor activities of cytotoxic and immunomodulatory agents.10 In addition, there is support from the literature for the potential to overcome treatment resistance, which is commonly caused by upregulation of the cMET pathway.11,12
FIG 1.
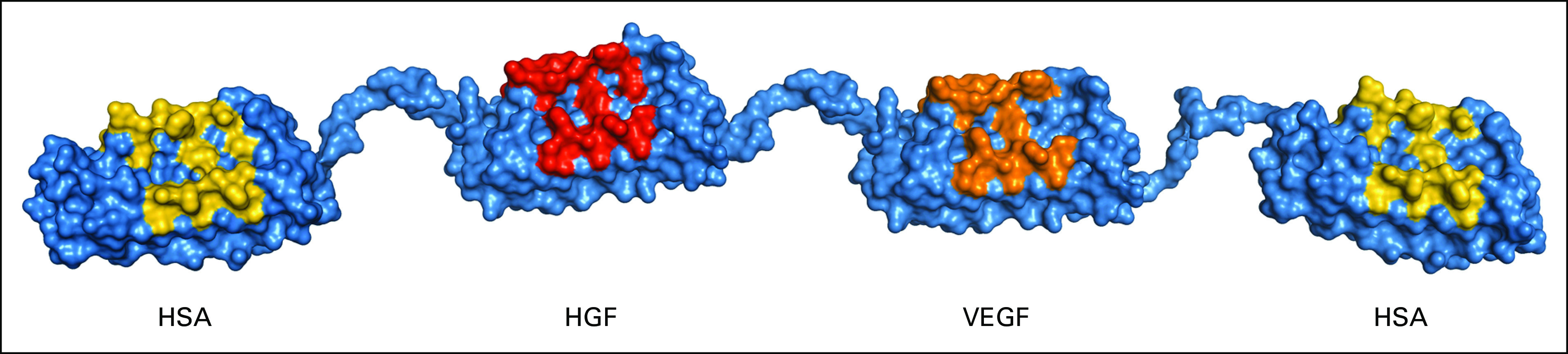
Structure of MP0250. HGF, hepatocyte growth factor; HSA, human serum albumin; VEGF, vascular endothelial growth factor.
This manuscript summarizes the phase I experience with MP0250 in patients with cancer and thus constitutes the basis of MP0250 clinical development as well as the development of other systemically administered DARPin molecules.
PATIENTS AND METHODS
Study Design and Treatment
This was a multicenter, open-label, phase I, dose-escalation, and expansion study of intravenously administered MP0250 (ClinicalTrials.gov identifier: NCT02194426). The primary objectives were to determine the maximum tolerated dose (MTD) and to assess dose-limiting toxicities (DLTs), pharmacokinetics (PKs), safety, and tolerability. The secondary objective was to characterize the immunogenicity of MP0250. Exploratory objectives included assessment of biomarkers and antitumor activity of MP0250.
The dose-escalation phase followed a traditional 3 + 3 study design including five dose cohorts (C1-C5) of 0.5, 1.5, 4, 8, and 12 mg/kg of MP0250 administered over a 3-hour infusion once every 2 weeks. The MTD was defined as the dose below the dose level that produces DLTs in ≥ 33% of patients. Once the MTD was established, two dose expansion cohorts were opened to further characterize the safety and biological activity of the selected dose. In the expansion cohorts, MP0250 was administered as a 1-hour infusion once every 2 weeks (cohort 6, C6, 13 patients at 8 mg/kg) or once every 3 weeks (cohort 7, C7, 8 patients at 12 mg/kg). The switch from once every 2 weeks to once every 3 weeks was supported by emerging PK data, and the infusion duration was reduced from 3 hours to 1 hour for patient convenience and to align with future once-every-3-week combination studies. Patients who benefited from treatment could continue treatment until tumor progression, unacceptable toxicity, or withdrawal from this study.
A DLT was defined as any drug-related adverse event (AE) meeting DLT criteria (provided in the Data Supplement, online only) that occurred from the time of first dose until completion of the DLT period (defined as 1 week after the third infusion in the once every 2 weeks schedule and 1 week after the second infusion in the once every 3 weeks schedule). A dose-escalation committee decided on dose escalation after all patients in a given cohort had completed the DLT period.
This study was approved by the ethics committees of participating institutions and appropriate regulatory authorities. All patients provided written informed consent. This study was conducted in compliance with the Declaration of Helsinki and guidelines for International Council for Harmonisation Good Clinical Practice.
Eligibility
Patients were at least 18 years old with advanced or metastatic solid tumor refractory to at least one line of prior standard treatment or for which no curative therapy was available. Patients had measurable or evaluable disease per RECIST version 1.1 and an Eastern Cooperative Oncology Group (ECOG) performance status of zero or one.
Safety Assessments
Routine clinical and laboratory assessments, including hematology, clinical chemistry, urinalysis, vital signs, and electrocardiography, were conducted at baseline and regularly throughout this study until up to 10 weeks after the last MP0250 administration.
AEs were continuously reported and coded using Medical Dictionary for Regulatory Activities version 17.0 terminology. AE intensity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
PKs, Antidrug Antibodies, and Pharmacodynamics
Plasma samples for PKs, antidrug antibody (ADA), and cytokine assessment were collected at predefined time intervals. More detailed information is given in the Data Supplement.
Antitumor Activity
Radiologic tumor response assessments were performed by computed tomography or magnetic resonance imaging scans at screening and at 8-week intervals thereafter. Tumor responses were assessed using RECIST v1.1.
Statistical Analyses
This was an open-label phase I study. Sample sizes of at least 15 patients in the dose-escalation part (ie, at least three patients for the five doses planned) and 16 patients in the expansion part (eight per dose group) were considered sufficient to adequately address the objectives of this study. All patients exposed to MP0250 were included in the safety analysis, and all patients for whom at least one PK, pharmacodynamic (PD), or antitumor activity parameter could be reasonably assessed were evaluated for these parameters. All data were descriptively summarized.
Details of methods are provided in the clinical study protocol (Data Supplement).
RESULTS
Patient Characteristics
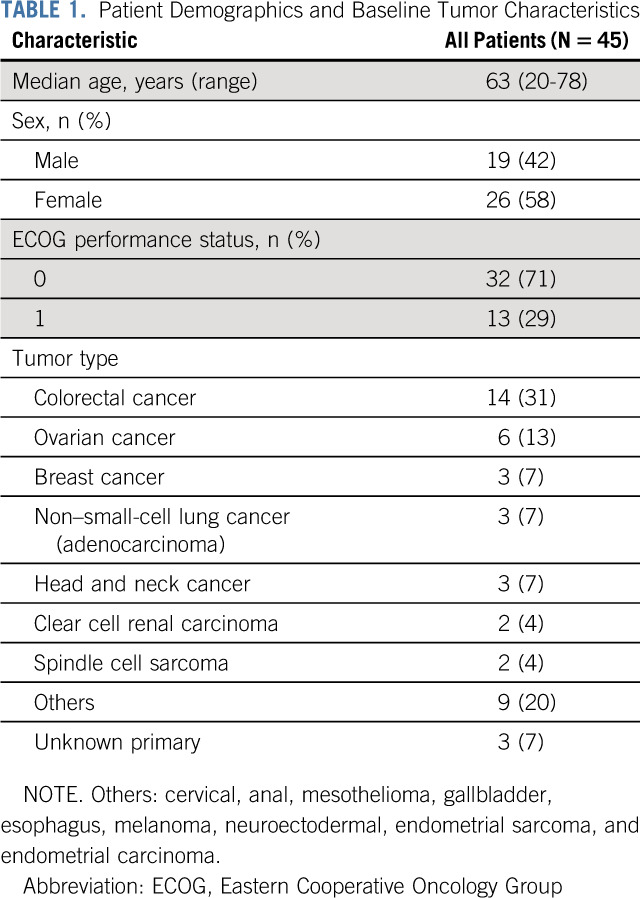
Twenty-four patients were enrolled in the dose-escalation phase, and 21 patients were enrolled in the expansion phase of this study. Patient characteristics of all 45 patients are summarized in Table 1. The most frequent tumor entities were colorectal cancer (31%) and ovarian cancer (13%). The ECOG performance status was 0 in 32 patients (71%) and 1 in 13 patients (29%). Twelve patients (27%) received previous anti-VEGF treatment. All patients completed at least one infusion of MP0250 (median, four infusions; range, 1-31 infusions).
TABLE 1.
Patient Demographics and Baseline Tumor Characteristics
MTD and DLT Evaluation
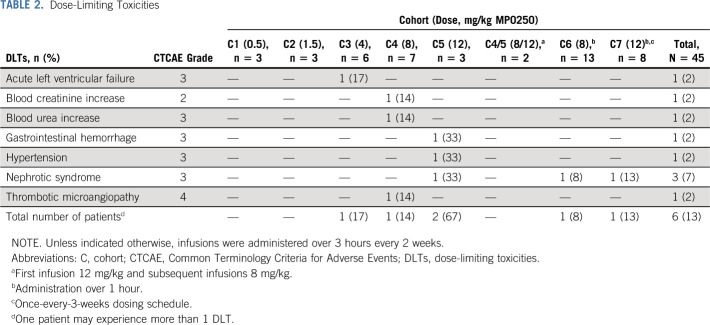
Treatment cohorts and DLTs are listed in Table 2. During the dose-escalation phase, four patients experienced DLTs. No DLTs were observed in the first two dose cohorts (0.5 and 1.5 mg/kg). In cohort 3 (4 mg/kg), one DLT occurred and this cohort was expanded to a total of six patients with no additional DLTs. In the next dose cohort (8 mg/kg), no DLT was reported in the first three treated patients. In cohort 5 (12 mg/kg), one of the three patients experienced two DLTs, leading to expansion of that cohort by two patients. After the second patient in this cohort experienced a DLT, the dose of the remaining patients was reduced to 8 mg/kg (two patients). Three more patients were enrolled at 8 mg/kg (cohort 4), of which one patient experienced three DLTs. As no more than 17% of patients experienced a DLT at this dose level, the administration of 8 mg/kg once every 2 weeks (and its dose intensity equivalent of 12 mg/kg once every 3 weeks) was established as MTD for MP0250 monotherapy in oncology patients and consequently used in the expansion phase. In the dose expansion phase, two of the 21 patients experienced DLTs.
TABLE 2.
Dose-Limiting Toxicities
Safety
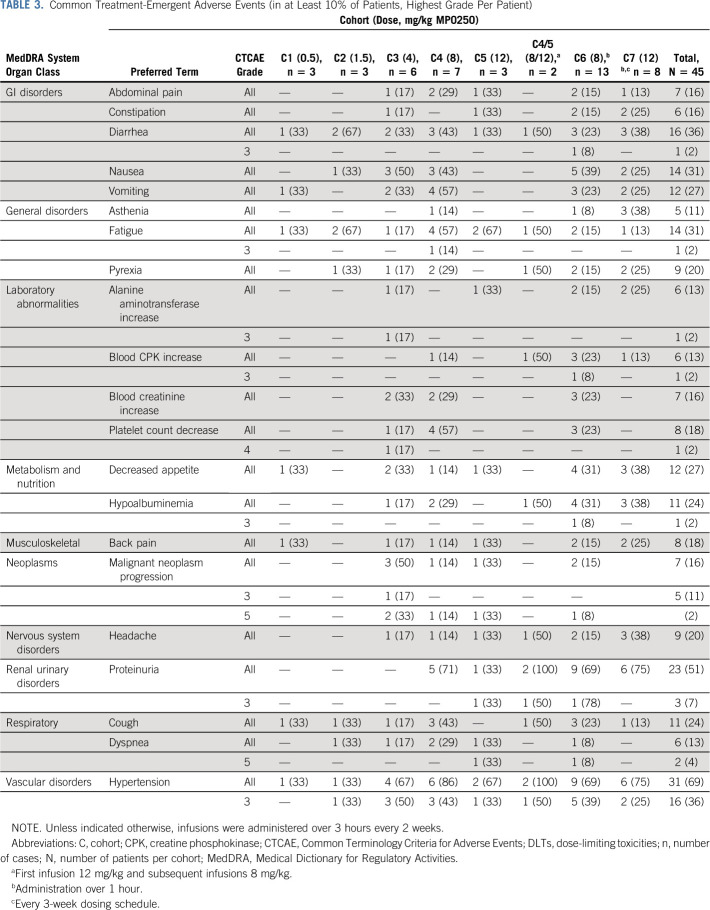
Treatment-emergent AEs that were reported in more than 10% of patients are shown in Table 3. The most frequent AEs were hypertension (69% of patients), proteinuria (51%), and diarrhea and nausea (both 36%). Hypoalbuminemia was reported in 24% of patients. Proteinuria was reported at a MP0250 dose ≥ 8 mg/kg once every 2 weeks, while hypertension was observed at all tested dose levels. The majority of AEs were of grade 1 or 2. An infusion-related reaction was reported in one patient.
TABLE 3.
Common Treatment-Emergent Adverse Events (in at Least 10% of Patients, Highest Grade Per Patient)
Grade 3 hypertension and proteinuria were reported in 16 and three patients (36% and 7%), respectively. The median onset was at day 21 for hypertension and day 62 for proteinuria. Median durations of the AEs were 8 days and 29 days for hypertension and proteinuria, respectively. No correlation between previous anti-VEGF treatment and the occurrence of these AEs was found. Treatment was discontinued due to proteinuria in six patients (13%), including one patient experiencing concomitant grade 2 hypertension.
Eleven patients (24%) experienced serious adverse reactions, as shown in the Data Supplement; those reported by more than one patient included nephrotic syndrome (four patients, 9%) and pulmonary embolism (three patients, 7%). Ten patients died during the treatment phase of this study, nine of them due to disease progression. The tenth patient died from cardiac failure where MP0250 could not be completely excluded as being a contributing factor; however, this patient had several preexisting cardiac risk factors: significant exposure to anthracycline chemotherapy and prior mediastinal irradiation, hypertension, left bundle branch block, and dyslipidemia. Later, atrial fibrillation was also reported as an AE (CTCAE severity grade 2), and the patient died 65 days after the first and last doses of MP0250. This event was judged retrospectively to be possibly related to MP0250 administration.
Treatment was discontinued due to AEs in 14 patients (31%). The most frequent AEs leading to treatment discontinuation were nephrotic syndrome (four patients, 9%), malignant neoplasm progression (two patients, 4%), and proteinuria (two patients, 4%).
PKs and ADAs
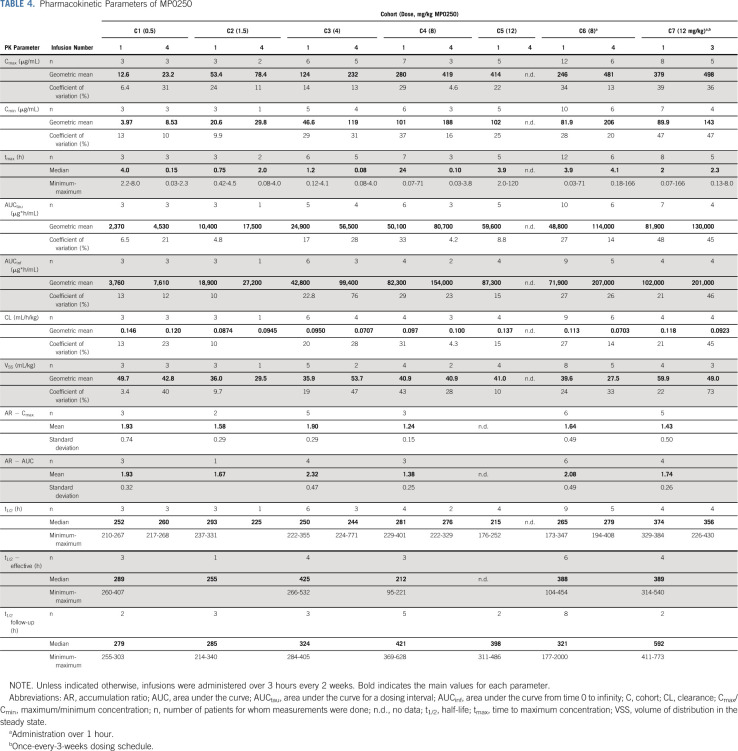
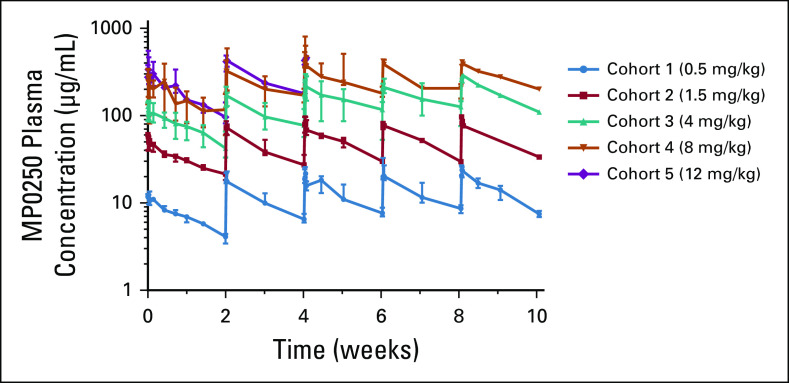
PK parameters are shown in Table 4, and median concentration-time profiles of cohorts in the dose-escalation part are depicted in Figure 2. After MP0250 infusion, the maximum concentration (Cmax) was followed by a decline of serum concentrations in a roughly monoexponential manner. The exposure-related parameters Cmax and area under the curve (AUC) increased in proportion to the dose. The estimated values of clearance, volume of distribution, and half-life were similar between dose levels, suggesting a linear PK of MP0250 in the dose range from 0.5 to 12 mg/kg. The estimated ranges of the geometric means of clearance and volume of distribution in the steady state were 0.070-0.146 mL/h/kg and 27.5-59.9 mL/kg, respectively.
TABLE 4.
Pharmacokinetic Parameters of MP0250
FIG 2.
Pharmacokinetic traces of MP0250. Plasma concentration versus time profiles of cohorts 1-5 with once every 2 weeks dosing intervals. Numbers of patients per data point vary due to different numbers of patients in each cohort and drop out of patients after various periods (n = 1-7, median, max/min). A dose level of 12 mg/kg in cohort 5 was given to patients only for the first two cycles depicted in the graph.
Following multiple-dose administration, MP0250 accumulated in the once-every-2-weeks and once-every-3-weeks dosing regimens. Steady-state conditions were established after approximately 6 weeks, corresponding to infusion 4 in the once-every-2-weeks scheme and infusion 3 in the once-every-3-weeks scheme.
Half-life was calculated using three different approaches (in the follow-up period, based on accumulation,13 and during the dosing intervals). In all cohorts, a half-life of around 2 weeks was calculated during the follow-up periods (overall median of all patients t1/2 follow-up 349 hours = 15 days) or based on the accumulation ratio (overall median of all patients t1/2 effective 377 hours = 16 days). A similar half-life was calculated for cohort 7 over the dosing intervals of 3 weeks (overall median for cohort 7 was 374 hours = 16 days). Generally, a lower half-life was calculated during the shorter once-every-2-weeks dosing intervals for cohort 1 through cohort 6 (overall median for cohort 1 to cohort 6 and combined dose 1 and dose 4 was 252 hours = 11 days).
In general, no differences in PK results between males and females were observed.
ADAs were detected in 20 of the 42 evaluable patients and had no impact on the PK of MP0250. The occurrence of ADAs was independent of the MP0250 dose. In 2 of the 20 ADA-positive patients, the titer ratio (titer sample post dose/titer sample predose 1) was higher than 16. In one patient of cohort 2 (1.5 mg/kg, once every 2 weeks, 3-hour infusion), the titer ratio reached 256 (the highest observed value). This may be related to a deviation from the study protocol in which this patient did not have a filter in the infusion line during the first MP0250 infusion. One patient of cohort 6 (8 mg/kg, once every 2 weeks, 1-hour infusion) showed a maximal titer ratio of 64 although this decreased at a later time point. There were no apparent side effects associated with the presence of ADAs.
Pharmacodynamics
Binding of MP0250 to both its targets (VEGF-A and HGF) in plasma was demonstrated. For VEGF, this was shown by a reduction of plasma levels of free VEGF-A to undetectable for the duration of treatment (Data Supplement), and for HGF, it was shown by total plasma HGF levels increasing in a dose- and time-dependent manner (Data Supplement). The increase in total HGF corresponded to an increase in MP0250-HGF complex in the circulation. Although the sample number was small, it appeared that MP0250-HGF complex formation might be plateauing around the dose of 4 mg/kg once every 2 weeks MP0250, thus possibly constituting the required dose for a maximum effect of MP0250 on the HGF biology.
PIGF (placental growth factor) levels decreased transiently in plasma after infusion of MP0250. There were no treatment-related changes observed for plasma levels of VEGF-C, angiopoietin-2, endoglin, fibroblast growth factor 2, interleukin (IL)-6, IL-8 and IL-10, tumor necrosis factor α, soluble VEGFR-2, or matrix metalloprotease 2 levels (data not shown).
Antitumor Activity
Among 40 patients evaluable for response, one patient (3%) had an unconfirmed partial response (uPR), 24 patients (60%) had stable disease (SD), and 15 patients (38%) had progressive disease.
The patient who had uPR had an anal cancer with lung metastases. The patient had received two prior lines of chemotherapy before entering into the trial. He received one infusion of 12 mg/kg followed by an infusion of 8 mg/kg once every 2 weeks and had PR at week 7 (30% reduction in target lesions), followed by SD at a subsequent tumor assessment with a time to progression of 23 weeks.
The median duration of SD was 18 weeks, with a maximum of 72 weeks in one patient with salivary gland carcinoma, who received MP0250 at the dose of 4 mg/kg (once every 2 weeks, 3-hour infusion).
The Kaplan-Meier analysis of progression-free survival (PFS) resulted in an overall median PFS of 15.1 weeks.
Of the 39 patients with a postdose assessment of the sum of longest diameters, 14 patients (36%) achieved some degree of tumor shrinkage (Data Supplement).
DISCUSSION
This study is the first clinical trial to evaluate the systemic administration of a new class of drugs, DARPin molecules, in patients with cancer. DARPin molecules are derived from protein binding domains found in ankyrin repeat proteins, a class of proteins widely used in nature for activities ranging from simple anchoring of proteins to enzyme inhibition.14 Binding domains with very high specificity and affinity can be selected and used as building blocks to create novel DARPin biologics that can combine multiple mechanisms of action in one molecule. MP0250 is a tri-specific molecule with four binding domains: one each for VEGF and HGF and two for HSA; albumin binding results in an extended plasma half-life, and the specific binding and neutralization of VEGF and HGF are responsible for therapeutic activity. The rationale to inhibit the VEGF and HGF pathways simultaneously is based on preclinical data that have shown that cMET can be upregulated to overcome resistance to VEGF inhibition and that simultaneous inhibition of these two pathways can overcome treatment resistance.9,11,12
The predominant side effects of MP0250 observed in this first-in-human study were hypertension and proteinuria, both side effects that are characteristic of VEGF targeting drugs.15-17 Both side effects were dose-related, which is suggestive of an increasing inhibition of VEGF signaling with increasing doses. Although grade 3 hypertension was reported in about one-third of the patients, this AE was generally manageable and not treatment limited. Severe proteinuria was reported at the highest tested dose and was dose limiting in three patients (7%), and proteinuria appeared on average after 2 months of therapy. This AE warrants close monitoring in future clinical trials of MP0250, and since dose interruption is required in some cases, this should be reflected in the trial design. The dose of MP0250 to be used in combination studies could well be lower than the single-agent recommended phase II dose, particularly with other therapies that may have renal toxicities. With respect to biochemical signs of HGF inhibition, hypoalbuminemia, a class effect of HGF inhibitors, was frequently observed.5 PD data also demonstrated that both components of the DARPin molecule were active, resulting in a reduction of circulating VEGF below the limit of detection as well as a dose-proportional increase in HGF-MP0250 complexes. Thus, several pieces of data support the hypothesis that both the VEGF targeting and the HGF targeting components of MP0250 maintain their ability to bind and inhibit their specific targets when administered to patients, and the dose range tested here is highly relevant for the MP0250 pharmacology. With the established recommended doses for further studies, MP0250 can now be tested in combination with other drugs to establish the role of concomitant VEGF/HGF inhibition as a potential remedy for patients with adaptive resistance.
The PK of MP0250 showed a dose-proportional increase in exposure (Cmax and AUC) and demonstrated a serum half-life of approximately 2 weeks. Clearance was low with a range similar to values reported for therapeutic antibodies.18,19 The half-life of 2 weeks is significantly longer than what would be expected for a molecule in the size of MP0250 (65 kDa) and indicates that the majority of the drug is bound as intended to serum albumin. Consequently, convenient dosing intervals of once every 2, 3, or even 4 weeks can be used, depending on the combination therapy desired. No alteration in exposure was observed in any patient.
Although conducted in a population of heavily pretreated patients, this study demonstrated signs of single-agent activity. One patient showed a uPR and over half of the patients demonstrated stabilization of disease with the longest duration of 72 weeks.
In summary, this is the first study that demonstrates that rationally designed DARPin molecules, a new class of agents built on naturally occurring ankyrin repeat proteins, can be safely administered to patients with cancer. The molecule, MP0250, which contains three different components addressing three different targets, showed clinical and PD evidence that all intended targets are bound and that a simultaneous inhibition of VEGF and HGF is achieved. Based on the results of the phase I study, MP0250 is currently in phase II clinical evaluation in combination with bortezomib and dexamethasone in patients with refractory multiple myeloma20 (ClinicalTrials.gov identifier: NCT03136653). Two additional DARPin drug candidates, MP0274, which binds to two different epitopes of the HER2 receptor and is capable of inducing apoptosis in HER2-expressing cell models, and MP0310 (AMG 506), a molecule that is designed to deliver 4-1BB activation to the tumor microenvironment via binding to fibroblast activation protein, have entered clinical testing (ClinicalTrials.gov identifiers: NCT03084926 and NCT04049903, respectively).
In a first-in-human clinical trial, MP0250, a rationally designed DARPin molecule, was found to have a manageable safety profile and showed signs of clinical activity supporting further clinical development especially in combination therapies. Furthermore, the data provide evidence that the DARPin platform could be a basis for a new class of drugs in which specific properties can be combined in a single molecule to provide innovative mechanisms of action.
ACKNOWLEDGMENT
We thank the patients who participated in this study and their families. In addition, we thank the coinvestigators, nurses, study coordinators at the sites, and the team working on MP0250 at Molecular Partners.
Richard D. Baird
Honoraria: Molecular Partners
Consulting or Advisory Role: Shionogi, Daiichi Sankyo, Molecular Partners, Roche/Genentech, Novartis
Research Funding: AstraZeneca, Genentech, Shionogi, Molecular Partners, Sanofi, Boehringer Ingelheim, Roche, Biomarin, G1 Therapeutics, Carrick Therapeutics
Travel, Accommodations, Expenses: Shionogi, AstraZeneca, Molecular Partners, Daiichi Sankyo
Mark Middleton
Consulting or Advisory Role: Merck, Bristol-Myers Squibb, Immunocore, BiolineRx, Johnson & Johnson, Vista Pharmacueticals, Vaccitech
Research Funding: Immunocore, Novartis, AstraZeneca, Roche, Amgen, Millennium, Bristol-Myers Squibb, Vertex, Merck, Pfizer, Replimune, Array BioPharma, Regeneron
Uncompensated Relationships: GenesisCare
Simon Lord
Stock and Other Ownership Interests: Mitox Therapeutics
Honoraria: Eisai, Prosigna
Consulting or Advisory Role: Shionogi
Research Funding: Pathios Therapeutics
Travel, Accommodations, Expenses: Roche
Adrian Harris
Employment: Curve Therapeutics
Consulting or Advisory Role: Abingworth
Jordi Rodón
Consulting or Advisory Role: Peptomyc, Kelun Pharmaceuticals, Merck Sharp & Dohme, Spectrum Pharmaceuticals, Pfizer, Roche/Genentech, Ellipses Pharma, Certera, Bayer, Ionctura, Novartis, Lilly, Orion Corporation, Servier Pharmaceuticals, Molecular Partners, NovellusDx
Research Funding: Novartis, Bayer, Blueprint Pharmaceuticals, Spectrum Pharmaceuticals, Tocagen, Symphogen, BioAltla, Pfizer, Genmab, CytomX Therapeutics, Kelun-Biotech, Millennium, GlaxoSmithKline, Ipsen
Travel, Accommodations, Expenses: ESMO, Department of Defense, Merck Sharp & Dohme, Louisiana State University, Kelun Pharmaceuticals/Klus Pharma, Huntsman Cancer Institute, Cancer Core Europe, Karolinska Cancer Institute, King Abdullah International Medical Research Center, Bayer, WIN Consortium, Janssen, Molecular Partners
Other Relationship: VHIO/Ministero De Empleo Y Seguridad Social, European Journal of Cancer, Chinese University of Hong Kong, SOLTI, GlaxoSmithKline
Christof Zitt
Employment: Molecular Partners
Stock and Other Ownership Interests: Molecular Partners
Keith M. Dawson
Stock and Other Ownership Interests: Molecular Partners
Nicolas Leupin
Stock and Other Ownership Interests: Molecular Partners
Travel, Accommodations, Expenses: Molecular Partners
Michael T. Stumpp
Employment: Molecular Partners
Leadership: Molecular Partners
Stock and Other Ownership Interests: Molecular Partners
Patents, Royalties, Other Intellectual Property: I am a co-inventor of the DARPin technology
Andreas Harstrick
Employment: Affimed Therapeutics, Molecular Partners
Leadership: Affimed Therapeutics
Stock and Other Ownership Interests: Molecular Partners, Affimed Therapeutics
Honoraria: Orion Corporation, ICON Clinical Research
Consulting or Advisory Role: Orion Corporation
Analía Azaro
Consulting or Advisory Role: Orion Corporation, Amcure
Research Funding: AMCURE
Stefanie Fischer
Research Funding: Astellas Pharma
Travel, Accommodations, Expenses: Bayer, Astellas Pharma
Aurelius Omlin
Consulting or Advisory Role: Astellas Pharma, Bayer, Sanofi, Roche, Janssen, MSD, Molecular Partners
Speakers' Bureau: Bayer, Astellas Pharma, Janssen-Cilag
Research Funding: Teva, Janssen
Travel, Accommodations, Expenses: Sanofi, Bayer, Astellas Pharma, Janssen
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2018.
SUPPORT
Support in Cambridge is acknowledged from Cancer Research UK, the NIHR Biomedical Research Centre, the NIHR Clinical Research Facility, and the Experimental Cancer Medicine Centre.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Richard D. Baird, Adrian Harris, Jordi Rodón, Christof Zitt, Michael T. Stumpp, Andreas Harstrick, Aurelius Omlin
Financial support: Nicolas Leupin
Administrative support: Nicolas Leupin
Collection and assembly of data: Richard D. Baird, Constanza Linossi, Mark Middleton, Simon Lord, Jordi Rodón, Stefanie Fischer, Aurelius Omlin
Data analysis and interpretation: Richard D. Baird, Mark Middleton, Adrian Harris, Jordi Rodón, Christof Zitt, Ulrike Fiedler, Keith M. Dawson, Nicolas Leupin, Michael T. Stumpp, Andreas Harstrick, Analía Azaro, Stefanie Fischer, Aurelius Omlin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
First-in-Human Phase I Study of MP0250, a First-in-Class DARPin Drug Candidate Targeting VEGF and HGF, in Patients With Advanced Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Richard D. Baird
Honoraria: Molecular Partners
Consulting or Advisory Role: Shionogi, Daiichi Sankyo, Molecular Partners, Roche/Genentech, Novartis
Research Funding: AstraZeneca, Genentech, Shionogi, Molecular Partners, Sanofi, Boehringer Ingelheim, Roche, Biomarin, G1 Therapeutics, Carrick Therapeutics
Travel, Accommodations, Expenses: Shionogi, AstraZeneca, Molecular Partners, Daiichi Sankyo
Mark Middleton
Consulting or Advisory Role: Merck, Bristol-Myers Squibb, Immunocore, BiolineRx, Johnson & Johnson, Vista Pharmacueticals, Vaccitech
Research Funding: Immunocore, Novartis, AstraZeneca, Roche, Amgen, Millennium, Bristol-Myers Squibb, Vertex, Merck, Pfizer, Replimune, Array BioPharma, Regeneron
Uncompensated Relationships: GenesisCare
Simon Lord
Stock and Other Ownership Interests: Mitox Therapeutics
Honoraria: Eisai, Prosigna
Consulting or Advisory Role: Shionogi
Research Funding: Pathios Therapeutics
Travel, Accommodations, Expenses: Roche
Adrian Harris
Employment: Curve Therapeutics
Consulting or Advisory Role: Abingworth
Jordi Rodón
Consulting or Advisory Role: Peptomyc, Kelun Pharmaceuticals, Merck Sharp & Dohme, Spectrum Pharmaceuticals, Pfizer, Roche/Genentech, Ellipses Pharma, Certera, Bayer, Ionctura, Novartis, Lilly, Orion Corporation, Servier Pharmaceuticals, Molecular Partners, NovellusDx
Research Funding: Novartis, Bayer, Blueprint Pharmaceuticals, Spectrum Pharmaceuticals, Tocagen, Symphogen, BioAltla, Pfizer, Genmab, CytomX Therapeutics, Kelun-Biotech, Millennium, GlaxoSmithKline, Ipsen
Travel, Accommodations, Expenses: ESMO, Department of Defense, Merck Sharp & Dohme, Louisiana State University, Kelun Pharmaceuticals/Klus Pharma, Huntsman Cancer Institute, Cancer Core Europe, Karolinska Cancer Institute, King Abdullah International Medical Research Center, Bayer, WIN Consortium, Janssen, Molecular Partners
Other Relationship: VHIO/Ministero De Empleo Y Seguridad Social, European Journal of Cancer, Chinese University of Hong Kong, SOLTI, GlaxoSmithKline
Christof Zitt
Employment: Molecular Partners
Stock and Other Ownership Interests: Molecular Partners
Keith M. Dawson
Stock and Other Ownership Interests: Molecular Partners
Nicolas Leupin
Stock and Other Ownership Interests: Molecular Partners
Travel, Accommodations, Expenses: Molecular Partners
Michael T. Stumpp
Employment: Molecular Partners
Leadership: Molecular Partners
Stock and Other Ownership Interests: Molecular Partners
Patents, Royalties, Other Intellectual Property: I am a co-inventor of the DARPin technology
Andreas Harstrick
Employment: Affimed Therapeutics, Molecular Partners
Leadership: Affimed Therapeutics
Stock and Other Ownership Interests: Molecular Partners, Affimed Therapeutics
Honoraria: Orion Corporation, ICON Clinical Research
Consulting or Advisory Role: Orion Corporation
Analía Azaro
Consulting or Advisory Role: Orion Corporation, Amcure
Research Funding: AMCURE
Stefanie Fischer
Research Funding: Astellas Pharma
Travel, Accommodations, Expenses: Bayer, Astellas Pharma
Aurelius Omlin
Consulting or Advisory Role: Astellas Pharma, Bayer, Sanofi, Roche, Janssen, MSD, Molecular Partners
Speakers' Bureau: Bayer, Astellas Pharma, Janssen-Cilag
Research Funding: Teva, Janssen
Travel, Accommodations, Expenses: Sanofi, Bayer, Astellas Pharma, Janssen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Binz HK Bakker TR Phillips DJ, et al. : Design and characterization of MP0250, a tri-specific anti-HGF/anti-VEGF DARPin® drug candidate. MAbs 9:1262-1269, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy N: VEGF suppresses invasion. Nat Rev Cancer 12:581, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Shen H, McDonald KL: The complexities of resistance to bevacizumab. J Cancer Ther 3:491-503, 2012 [Google Scholar]
- 4.Cunningham D Tebbutt NC Davidenko I, et al. : Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Cancer Ther 33:4000, 2015 [Google Scholar]
- 5.Spigel D Edelman MJ O'Byrne KJ, et al. : Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-small-cell lung cancer: METLung. J Clin Oncol 35:412-420, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA: Hallmarks of cancer: The next generation. Cell 144:646-674, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P: Angiogenesis in life, disease and medicine. Nature 438:932-936, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Gherardi E Birchmeier W Birchmeier C, et al. : Targeting MET in cancer: Rationale and progress. Nat Rev Cancer 12:89-103, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Chen TT Filvaroff E Peng J, et al. : MET suppresses epithelial VEGFR2 via intracrine VEGF-induced endoplasmic reticulum-associated degradation. EBioMedicine 2:406-420, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiedler U Ekawardhani S Cornelius A, et al. : MP0250, a VEGF and HGF neutralizing DARPin® molecule shows high anti-tumor efficacy in mouse xenograft and patient-derived tumor models. Oncotarget 8:98371-98383, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascone T Xu L Lin HY, et al. : The HGF/c-MET pathway is a driver and biomarker of VEGFR-inhibitor resistance and vascular remodeling in non-small cell lung cancer. Clin Cancer Res 23: 5489-5501, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahangiri A Lay MD Miller LM, et al. : Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res 9:1773-1783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boxenbaum H, Battle M: Effective half-life in clinical pharmacology. J Clin Pharmacol 35:763-766, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Stumpp MT, Dawson KM, Binz HK: Beyond antibodies: The DARPin® drug platform. BioDrugs 34:423-433, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S Kim C Baer L, et al. : Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol 21: 1381-1389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roche. Bevacizumab summary of product characteristics (EMA). https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf
- 17.Roche. Bevacizumab prescribing information (FDA). www.accessdata.fda.gov/drugsatfda_docs/label/2017/125085s319lbl.pdf
- 18.Dostalek M Gardner I Gurbaxani BM, et al. : Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet 52:83-124, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Ryman JT, Meibohm B: Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol 6:576-588, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grzasko N Knop S Goldschmidt H, et al. : The MP0250-CP201 Mirror study: A phase 2 study update of MP0250 plus bortezomib and dexamethasone in relapse/refractory multiple myeloma (RRMM) patients previously exposed to proteasome inhibitors and immunomodulatory drugs, ASH poster 2019. https://investors.molecularpartners.com/∼/media/Files/M/Molecular-Partners/documents/mp0250-cp201-mirror-study-poster-1899.pdf