Abstract
Peanut and its processed products are recurrently contaminated with aflatoxins (AFs) which are of potential public health concern. Among the different types of AFs, Aflatoxin B1 (B1) is the most frequently detected in peanuts over the maximum level (ML), and thus has warranted considerable research interest in the domain of food safety. In this study, we investigated the decontamination of B1 in three naturally-incurred lots (4, 12, and 40 µg/kg) of peanuts by a range of cooking treatments, including frying, pressure cooking, and roasting. B1 concentrations were determined by ultra-high performance liquid chromatography- fluorescence detection. The method provided a limit of quantification of 0.25 µg/kg for B1, which was much lower than any of its national and international MLs. The recoveries of B1 in fresh and cooked peanuts (positive-control) were in the range of 90–100%. Overall, all the cooking methods demonstrated a significant reduction in B1 loads. The degree to which the processing methods reduced the B1 content followed the pattern: roasting with a combination of NaCl and citric acid > pressure-cooking with a combination of NaCl and citric acid > frying. As the cooking procedures did not involve any complicated steps or sophisticated equipment, these could be readily adopted for decontamination or reduction in the level of B1 for a safer consumption of peanuts at the household level without affecting the organoleptic properties.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04761-3) contains supplementary material, which is available to authorized users.
Keywords: Aflatoxin B1, P eanut, Decontamination by cooking, Ultra-high performance liquid chromatography, Fluorescence detection
Introduction
Aflatoxins (AFs) are known to contaminate many agricultural products, food, animal feed, and the environment as a whole. Broadly classified as B1, B2, G1, and G2, AFs are highly hepatotoxic, hepatocarcinogenic, mutagenic, and teratogenic (Abdel-Wahhab et al. 2007). As reported by the International Agency for Research on Cancer, B1 is a type-1 carcinogen (IARC Monographs 2002). While many foods and feedstuffs are susceptible to AF contamination; peanuts, and maize are the most common substrates of B1 (Liskar et al. 1993). The monitoring of AFs in raw and processed peanuts in India (https://www.apeda.gov.in/apedawebsite/GroundNut/GroundNut.htm, unpublished monitoring data), as well as the European Union (https://webgate.ec.europa.eu/rasff-window/portal/?event=searchResultList) demonstrate the detection of only B1. And, as other AFs (e.g., B2, G1, and G2) are seldom found in this test matrix, they were not included in this study.
Worldwide, peanuts are consumed both in raw and processed forms including peanut butter, peanut based confectionary, and peanut oil. Indian recipes represent a wide range of culinary diversity originating from India’s multiculturalism. Although processing of peanuts in Indian homes might vary from region to region, they are broadly fried in oils, roasted, steamed with salt and cooked under pressure with spices, boiled with lime juice, treated with jaggery (e.g., chikki), or processed with a combination of these methods.
AF contamination had been a concern on many occasions with regard to international trade, as evident in the notifications released by the European Union (EU) (RASFF Annual report 2016) regarding exceeding levels of B1 in peanuts. Given the fact that serious health hazards related to AFs have become better known in recent times, the EU has set stringent regulations, according to which a product marketed for human consumption cannot have concentrations of B1, and the total AF greater than 2, and 4 µg/kg, respectively (EC 1881/2006). While the CODEX has set the ML for AF at 15 µg/kg (CODEX STAN 193–1995), the Food Safety Standards Authority of India has fixed it at 10 µg/kg for ready-to-eat nuts, and 15 μg/kg for the nuts for further processing (FSSAI 2011).
The strict food regulations have led to the need for using validated techniques, and thus have inspired analytical scientists to develop fit for purpose methodologies. In the past few decades, the use of high performance liquid chromatography (HPLC) with fluorescence detection (FLD) (Panalaks and Scott 1977) in the determination of AFs has greatly been compounded due to its selectivity and cost-effectiveness. Previously, we validated a high-throughput procedure for the direct analysis of AFs in fresh and processed peanuts using ultra-high performance LC (UHPLC) with FLD (Oulkar et al. 2017).
As AFs are thermally stable, numerous researchers over the past two decades have probed deep to understand how to effectively degrade AFs in peanuts using food processing techniques including brewing, frying, roasting, and chemical treatments (e.g., cooking with acidic/alkaline additives) (Jalili 2016). For instance, Saalia and Phillips (2011) reported that when naturally- and artificially-contaminated peanuts were subjected to extrusion cooking, the AFs were degraded by 59%, and 91%, respectively. In a review article, Kaushik 2015 provided a detailed discussion on the effect of processing techniques on mycotoxin contents in grains. In another research, Diedhiou et al. (2012) examined a number of African cooking processes, which are quite similar to Indian methods, in which they noted a considerable amount of reduction of AF (82.5%) when peanuts were roasted, made into peanut butter and subsequently steamed.
With globalization, Indian gastronomy has rapidly spread amongst cross-cultural consumers. As a result, the number of restaurant chains, which strive to offer authentic Indian cuisines, have been internationally growing. An efficient cooking method should be standardised to preserve the nutritive value, and gustatory appeal as far as possible. For a widespread implementation either in domestic, or food industrial settings, a method need not only to be efficacious, but also cost-effective, labour-saving, and time-saving. The dissipation study of AFs, more specifically, the fate of B1 in response to Indian cooking is still underestimated. To address this knowledge gap, an experiment was designed to evaluate the effectiveness of a range of widely used Indian cooking techniques in removing or breaking-down B1 in peanuts.
Materials and methods
Apparatus
The following apparatus were used for sample preparation: mixer/grinder (Philips, Amsterdam, Netherlands), orbital shaker (Scigenics Instruments, Mumbai, India), a high-speed homogenizer (DIAX-900, Heidolph, Schwabach, Germany), high-speed centrifuge (Kubota Corp., Tokyo, Japan), and vacuum manifold (Waters India Pvt. Ltd., Bengaluru, India). An aluminum pressure cooker of 3 L capacity (10,003 Prestige, Mumbai, India) was utilized for processing peanuts. The samples were roasted in an oven (LG Electronics Inc., model MS047GR, Korea) in the convection mode. Using a liquified petroleum gas burner (Surya, Mumbai, India), the peanut kernels were fried, and cooked under pressure.
Chemicals
The certified reference standard of B1 with > 95% purity was procured from Fluka (Bengaluru, India). A stock solution of 100 µg/kg was prepared in methanol, and the calibration levels were prepared by dilution with methanol: 0.1% acetic acid (1:1). All solvents were of HPLC grade (Merck, Bengaluru, India).
Immunoaffinity column
An AFLAPREP® immunoaffinity column (IAC, 3 mL, R-Biopharm AG, Darmstadt, Germany) was used for cleanup. It required phosphate buffered saline (PBS) solution for column washing and dilution, which was prepared by dissolving a PBS tablet (PH 7.3; Oxoid Ltd., Basingstoke, England) in water (100 mL).
Sample collection and pre-treatment
Twenty raw (skinned) peanut samples (10 kg of each) were randomly collated from different shops (located in Pune, India), and screened for B1. Out of these, three naturally-contaminated samples were chosen for testing the presence of the toxin: 1) Sample-A at 4 (± 1.2) μg/kg (twice the EU-ML), 2) Sample-B at 12 (± 3.2) µg/kg, and 3) Sample-C at 40 (± 4.8) µg/kg, which had the highest level of B1 among the collected sample lots. The samples were stored at 2ºC until further analysis. For acquiring a homogeneous distribution of B1, all samples were separately ground into powder by a mixer-grinder. To check the performance of the decontamination method in the slurry form, a part of sample-B was homogenized with 1:1 water, and designated as D. All the ground samples (A, B, and C) were separately fried, roasted, as well as cooked under pressure, but the slurry sample (D) was only subjected to cooking under pressure.
Cooking methods
Frying treatment
A part (50 g) of A, B, and C was fried in groundnut oil (50 mL) at 165-175ºC. In our preliminary experiments, we noted that the frying treatment for 10 min turned the color of the nuts to dark brown, and led to a burning smell, along with the development of a disagreeable taste. So, the samples were taken out from the pan at the intervals of 2, 5, and 7 min, cooled to room temperature (25 °C), and analyzed for B1.
Cooking under pressure
A portion (50 g) of A, B, and C was taken in different glass bottles (250 mL capacity), followed by the addition of 2 mL of citric acid (1%, 2%, and 5%) in each of them. The bottles were then kept in a pressure cooker containing sufficient amount of water, and heated for 20 min. Finally, the samples were brought down to room temperature, and subjected to the B1 testing.
In a separate set of experiment, citric acid was replaced with NaCl (2 mL, 2%), and processed as above. In a combined experiment, the samples were treated with a mixture of citric acid (2 mL, 5%) and NaCl (2 mL, 2%), cooked in a pressure cooker, followed by the analysis of the toxin.
Roasting
Earlier, studies have shown that roasting of peanuts using a varied range of temperature, time and energy using conventional ovens could effectively reduce the AF contents (Pluyer et al. 1987). For instance, peanuts roasted conventionally for inactivating AFs required a heating time cycle of 30–40 min at 150 °C (Arzandeh and Jinap 2011). As cooking using a convection oven has been gaining popularity in Indian households, it was also included here. The ground peanuts (A, B, C, 100 g each) were processed as follows: i) roasted without any additive; ii) roasted with citric acid (5%); iii) roasted with 2% NaCl; and iv) roasted with citric acid and NaCl. Roasting was done for 15, and 30 min at i) 120–130 °C, and ii) 180–190 °C. A continuous monitoring of temperature, and duration of roasting could avert undesirable effects on the taste, and color of peanuts. After roasting, the samples were cooled down, and the toxin was analyzed.
AF analysis
Extraction procedure
Due to its advantages, including obtaining satisfactory sensitivity at a trace level, and high throughput, our in-house validated method was chosen for the B1 analysis (Oulkar et al. 2017). According to this method, the samples (A, B, and C; 25 g each) were mixed with distilled water (1:1), and thoroughly homogenized. Unlike others, no water was added to sample-D. For extraction, 100 mL of methanol: water (8:2 v/v) was added, which contained 5 g of dissolved-NaCl. Thereafter, the bottle was placed on a shaker for 30 min at 150–170 revolutions per minute, and centrifuged at 1792 × g (10 min).
Immunoaffinity column cleanup
The supernatant (3 mL) was mixed with PBS (12 mL), and passed through an immunoaffinity column. After washing the column with PBS (10 mL), B1 was eluted with methanol (1 mL). By adding acidified water (0.1%), the eluent was diluted, and subjected to UHPLC-FLD analysis. As the samples were diluted by 6-folds, a dilution factor of 6 was applied for quantifying B1.
Chromatographic conditions
A UHPLC (Acquity H-Class, Waters Corporation, Manchester, UK) instrument was used which was connected with an FLD having a large volume (13 µL) flow cell. Using a bridged ethylene hybrid C18 column (2.1 × 100 mm, 1.7 µm), the chromatographic separation was carried out. The mobile phase comprising water:methanol:acetonitrile (64:18:18) was set at a flow rate of 0.4 mL/min.
Method validation
The single laboratory validation (SLV) was performed separately in fresh as well as processed peanuts. The method performance was evaluated in terms of calibration linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy (%), and precision (RSD, %). The LOD, and LOQ were the concentrations at which the signal to noise ratios (S/N) of B1 were≥ 3:1, and≥ 10:1, respectively.
In fresh (control) peanuts, the accuracy, and precision of the method were evaluated (n = 6) (Table S1) at 2 µg/kg, (EU-ML of B1), and three higher levels of B1, namely 4, 12, and 40 µg/kg, which matched with the incurred concentrations of the samples.
To ensure accuracy in estimating the effect of various cooking treatments on reduction in the B1 level, a positive quality-control sample was simultaneously analyzed (n = 6). The portions of a control sample were subjected to frying, pressure cooking, and roasting (Table S2). These samples were then spiked with B1 at 4, 12, and 40 µg/kg, and analyzed (n = 6) for recoveries, and precision.
Sensory analysis
The sensory characteristics were evaluated by a semi-trained panel of 10 judges, who were asked to score the peanut samples for color, texture, flavor, and overall acceptability, using a nine-point hedonic scale (Larmond 1970). A higher score indicated a higher degree of consumer acceptance (score 1: extremely-disliked; score 5: neither liked nor disliked; score 9: extremely-liked).
Statistical analysis
Sigma Stat (version 3.0, Jandel Scientific, San Rafael, CA) software was utilized for estimating mean, and standard deviation. One-way analysis of variance (ANOVA) was performed, and the differences among the treatments were determined by calculating the p-value for each study, where p < 0.05 indicated a significant difference. The Student’s t-test was performed for the comparative evaluation of the cooking treatments.
Results and discussions
The cooking methods, namely frying, pressure cooking, and roasting showed variable decontamination effects. While evaluating the B1 levels in fresh and cooked nuts, the method of analysis was thoroughly validated to ensure that all measurements were sufficiently accurate, and precise.
Method validation
The UHPLC analysis had a runtime of 5 min. The peak of B1 eluted at the retention time of 2.7 min (Supplementary Fig. 1a, b, c). The calibration linearity of B1 was established in the range of 0.04–2 µg/kg (R2 > 0.99, weighting factor 1/×, residuals within 20%) levels. The LOQ, and LOD were achieved at 0.25, and 0.04 µg/kg, respectively. At the LOQ level, the recoveries in the fresh and processed peanuts were above 90%, with precision RSD of ~ 5% (Table S1). At the fortification levels of 2, 4, 12, and 40 µg/kg, the recoveries of B1 were between 90 and 97%, with precision-RSDs less than 5% (n = 6) (Table S1). The recoveries of B1 in the positive-control samples were in the range of 96–99% (Table S2, Fig. 1C). These results were in compliance with the requirements of the EU regulation 401/2006. The validation data revealed that any reduction in B1 concentration in the peanut kernels (post-cooking) might have happened due to their exposure to the decontamination treatments, and not for losing any recovery from the processed matrices.
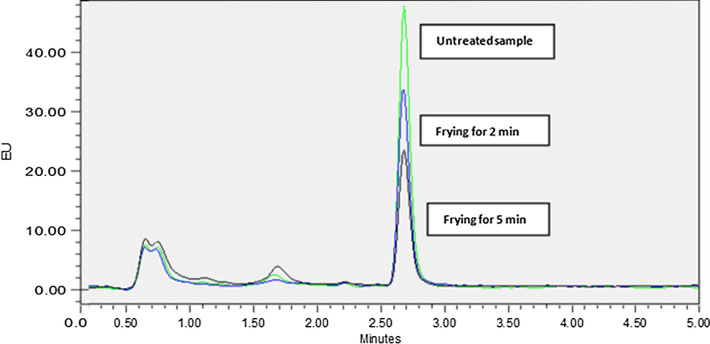
Fig. 1.
The chromatogram showing effect of frying (2 min and 5 min) on aflatoxin B1 in sample C
Influence of cooking methods on the fate of B1
Generally, AFs are stable to dry heat up to the melting point of 260 °C, however, while cooking at ~ 100–120 °C or even above they interact with the moisture content, which lead to their degradation (Samarazeewa, 1990). As noted, the addition of NaCl, and/or citric acid solution in cooking enhances the hydration effect, thereby resulting in a swifter degradation of AFs. In the next sections, the comparative effects of various cooking treatments are presented in details.
Frying
Often, high temperature processing produces heat-enhanced chemical reactions, which results in undesirable flavors, and darkened colors (Das and Mishra 2000). As the goal of frying was not only to achieve decontamination of B1, but also to retain the visual and sensory appeal, we optimized the treatments in such a way so that the quality of the nuts was retained. In this study, the extent of reduction of B1 was related to the cooking duration, and temperature. The frying of sample-A at 165-175ºC for 2 min resulted in a reduction by 30–40% (Table S3). However, the decontamination effect was significantly enhanced (p < 0.05) to 60–70% when the same sample was fried for 5 min (Fig. 1). The loss of the toxin was further increased to ~ 85%, when peanuts were fried for 7 min. This corresponds to a previous study (Lee et al. 1969), where an 83% reduction in B1 was recorded when the peanuts were fried for 7 min at 165-175ºC. However, with 7 min of frying, the nuts developed a slightly bitter-taste with organoleptic scores of 4–5; “disliked-moderately” to “neither liked nor disliked”. A rapid processing at ≥ 200ºC (4–5 min) although reduced the B1-concentration to less than EU-ML, it turned the nuts dark in color, bitter in taste with burning flavor, which was organoleptically least (score: 2–3; “disliked very much” to “disliked-moderately”) preferred among all.
When samples A, and B were fried for 5 min at 165–175ºC, the residue levels were reduced to 1.4 (65%-reduction, below the EU-ML), and 4.7 (60.5%-reduction) µg/kg, respectively. When frying was conducted for 7 min, the levels in both A and B reduced to below the EU-ML. In sample-C, the B1 level was degraded to 18 (55%-reduction), and 6 (85%-reduction) μg/kg, when the nuts were fried for 5 min, and 7 min, respectively.
In this study, the organoleptic properties of the samples did not alter until the pan was kept on a medium flame for 5 min at the above-mentioned temperatures. But, when the material was subjected to frying for a longer duration (10 min), the samples not only changed its colour to dark brown, or black, but also its taste turned bitter. Again, rapid processing at higher temperatures (above 250ºC) for 4–5 min spoiled the organoleptic appeal of the nuts. Fried peanuts are used in the preparation of traditional recipes, say poha (a popular breakfast delicacy made up with flattened rice, fried peanuts, and spices), and in various types of fried rice preparations. Based on the current experimentation results, our recommendation would be to fry peanuts for ~ 5 min at a medium flame (160–180ºC) as they will turn relatively safer for consumption and still regarded as of the correct quality by consumers (organoleptic score: 8–9, “liked very much” to “extremely-liked”). Only Sample A catered to our gustatory need and at the same time was safe for consumption. As in the above-mentioned rice preparations the fried peanuts are processed in a pressure cooker, it can be assumed that this would further enhance the degradation of B1 during cooking, however, it needs further investigations.
Cooking under pressure
Pressure-cooking, a processing technique that uses water in a sealed vessel at a higher pressure, has been worldwide adopted due to its time-effectiveness. Previously, Park and Kim (2006) demonstrated a substantial reduction of B1 in Korean rice following pressure cooking. Elsewhere, Diedhiou et al. (2004) reported ~ 50% reduction of B1 in the samples of African peanut flour, although it was subjected to boiling for 30 min.
In the current study, the extent of decontamination of B1 in samples A, B, and D was in the range of 30–40%, while the reduction was slightly lower in C (25–35%). A more substantial effect was recorded when pressure cooking was carried out in the presence of citric acid and NaCl. When the nuts were cooked for 20 min either with citric acid, or NaCl, the effect was almost similar. The extent of decontamination of B1 was 30–40% in sample-A, 50–60% in B, 30–35% in C, and 55–65% in D (Table S4). With a combined treatment of citric acid and NaCl, A, B, C, and D showed a significant (p < 0.05) reduction in B1 in the range of 70–80%, 60–70%, 55–65%, and 60–70%, respectively (Fig. 2, Table S4). In all cases, the contamination level was reduced below the CODEX and FSSAI MLs. However, the levels were still above the EU-ML except for sample-A. Notably, pressure cooking with citric acid and NaCl did not affect the quality of nuts (organoleptic score: 7.4–8.2; “moderately-liked” to “liked very much”).
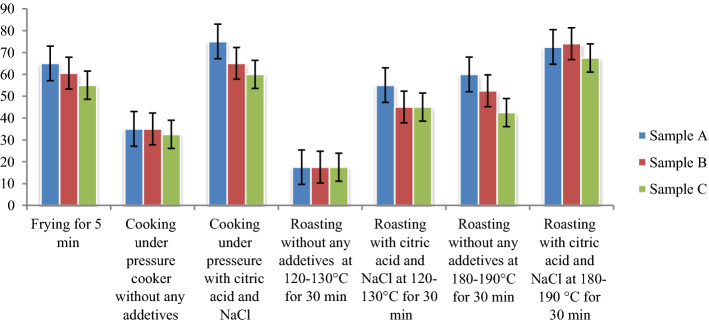
Fig. 2.
Reduction (%) of aflatoxin B1 by various treatments
Roasting
Researchers have reported a series of studies that depicted how oven heating affected the level of mycotoxins. In a study on peanuts (at different contamination levels), Lee et al. (1969) reported an effective removal of B1 by ~ 80% on roasting for 30 min at 150 °C. In another study, Pluyer et al. (1987) showed that roasting of naturally-contaminated peanuts in an oven at 150 °C for 30 min caused 30–45% removal of B1. Arzandeh and Jinap (2011) reported a reduction of B1 by 78.4% in artificially-contaminated peanuts those were treated at 150 °C for a longer duration (120 min). Elsewhere, Mobeen et al. (2011) reported 50–60% decontamination of B1 when peanuts were roasted in a microwave oven. However, all the above-mentioned studies were performed without adding any organic acid, or salt. In our experiment, however, the extent of decontamination of B1 was in the range of 30–40% in all samples (without any additives).
Roasted peanuts add flavor to cuisines, and so they are commonly used in homemade salads, and sauce (made with spinach, peanuts, and spices). As crispy and brown peanuts are preferred in the above recipes, the convection mode of the oven was chosen. Today, dosa and idli (South Indian fermented food items), which are served with chutney (a dip prepared by mixing dry roasted peanuts, herbs, yoghurt, salt, and spices), have become globalized cuisines. For this, we drew inspiration from a previous study by Rastegar et al. (2017) who demonstrated how roasting of pistachio nuts (50 g) with lemon juice (30 mL), and/or citric acid (6 g) at 120 °C for 1 h drastically reduced the B1-levels (93.1%).
In the present study, with a rise in roasting-temperature, a higher elimination of B1 was recorded. The level of B1 in A, B, and C was reduced by 10–12% when roasted at 120–130 °C (15 min). When the same treatment was performed for 30 min, the extent of decontamination was slightly increased (15–25%), which was much lower than the results shared by Ogunsanwo et al. (2004), who recorded a 58.8% reduction in B1 when the Nigerian peanuts were roasted at 140 °C for 40 min. When roasting was performed at a higher temperature (180–190 °C, 15 min), the B1 levels in A, B, and C reduced by 50–60%, 50–55%, and 15–30%, respectively (Table S5). An increase in the duration (30 min) of roasting at 180–190 °C did not result in any further reduction of B1 in A, and B. In sample C, however, the extent of decontamination was improved to 40–45%. Even though roasting time, temperature, and initial concentration of B1 are considered as the key factors affecting the decline of AF, the current results indicate the roasting-temperature to be the major factor, also mentioned in earlier studies (Arzandeh and Jinap 2011; Lee et al. 1969). Roasting with either NaCl, or citric acid (180–190 °C, 15 or 30 min) had a similar effect, 70–78% decontamination for A and B, and 65–70% for C. This effect was higher than the extent of decontamination achieved by Diedhiou et al. (2012), who reported 64% reduction in B1 when peanuts were roasted at 140 °C for 1 h. In the case of Nigerian peanut seeds, however, roasting at 150 °C for 30 min resulted a 70% reduction in B1 (Ogunsanwo 2005). As noted, this study establishes a significant improvement in decontamination as a consequence of Indian style of cooking where the nuts were roasted after adding NaCl, or citric acid.
A secondary point of concern is that chemical additives (e.g., common salt, citric acid etc.) are abundantly used in many recipes, but they do not have any specific tolerance limit in the CODEX, or other quality standards. From the organoleptic viewpoint, their addition should, however, be judiciously controlled for gratifying one’s palate. When treatment with 5% citric acid was given, the taste of the roasted peanuts in all samples under investigation remained unaffected. On the contrary, the addition of the same concentration to the raw samples had altered their organoleptic status. Depending on the findings, we recommend 5% of citric acid treatment for the common recipes depending upon the culture of the place.
When the samples were roasted with a combination of citric acid and NaCl at 120–130 °C for 15 min, the B1 level was degraded by 35–40%, 42–48%, and 32–38% in samples A, B, and C, respectively (Table S5). A rise in the roasting-temperature to 180–190 °C led to a considerable increase in decontamination as follows: 62–67%, 55–60%, and 50–55% in samples A, B, and C, respectively (p < 0.05).
The extent of decontamination of AF was similar in all replicates. Broadly, the levels of B1 in samples A, B, and C fell below the CODEX and FSSAI MLs. Hence, roasting was quite effective in degrading B1 over a wide-range of contaminations. It is worthy of consideration that NaCl and citric acid are widely used in Indian recipes, and they do not have any toxicological concerns. In the literature, Méndez-Albores (2005) reported conversion of B1 to a less toxic form B2a on exposure to aqueous citric acid. However, we did not detect the signals pertaining to B2a or any other metabolites in the chromatograms of the processed samples, indicating a faster degradation of this toxin to non-detectable fragments. The organoleptic scores (~ 8.5–8.9) also suggest the highest acceptability (“extremely-liked”).
Comparing the treatments
Roasting was noted as the most effective treatment to achieve a reduction of B1 levels in peanuts, followed by the other treatments such as pressure cooking, and frying (Fig. 2). According to the Student's t-test results, the absolute t value was higher than the critical value for the samples subjected to roasting at 5% level of significance. This value was the lowest for the frying treatment, indicating that frying was the least effective treatment for degrading the toxin.
Conclusion
The UHPLC-FLD-based study investigated the samples of peanuts and revealed a variable decontamination effect of B1 in various home-based cooking methods of India. The results revealed that roasting in the presence of NaCl and citric acid had the most pronounced effect for the removal of B1. Cooking under pressure with addition of citric acid and NaCl also reduced the level of this toxin to a considerable extent. After processing, the concentrations of B1 in the studied samples were mostly within the regulatory maximum limits prescribed by the national and international regulatory authorities. The recommended cooking procedures are easy to learn and perform, and do not affect the quality and consumer acceptability of the nuts. They can also be practiced without difficulty, as the consumables required for these methods are readily available almost in every household kitchen. The procedures also did not involve any extra or complicated steps. Importantly, there were no sophisticated instruments or expensive chemicals required, which make these decontamination procedures cost-effective. Based on the findings, these cooking methods are recommended to limit the intake and exposure to B1, thereby safeguarding overall consumer health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr Suparna Banerjee for offering us the content development, and writing support. We wish to extend our thankfulness to Dr Ankita Lakade, and Dr Anindita Paul for their technical comments.
Complaince with ethical standards
Conflict of interest
The authors have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Wahhab MA, et al. Zizyphusspina-christi extract protects against aflatoxin B1-intitiated hepatic carcinogenicity. Afr J Tradit Complement Altern Med. 2007;4:248–256. [PMC free article] [PubMed] [Google Scholar]
- Arzandeh S, Jinap S. Effect of initial aflatoxin concentration, heating time and roasting temperature on aflatoxin reduction in contaminated peanuts and process optimisation using response surface modelling. Int J Food Sci Technol. 2011;46:485–491. doi: 10.1111/j.1365-2621.2010.02514.x. [DOI] [Google Scholar]
- CODEX STAN 193–1995. General standard for contaminants and toxins in food and feed (CODEX STAN 193–1995). www.fao.org/input/download/standards/17/CXS_193e_2015.pdf
- Das C, Mishra H. Effects of aflatoxin B1 in detoxification on the physiochemical properties and quality of ground nut meal. Food Chem. 2000;7:483–487. doi: 10.1016/S0308-8146(00)00120-5. [DOI] [Google Scholar]
- Diedhiou PM, Ba F, Kane A, Mbaye N. Effect of different cooking methods on aflatoxin fate in peanut products. Afr J Food Sci Technol. 2012;3:53–58. [Google Scholar]
- EC 1881/2006 (2006) Official Journal of the European Union, L364, pp 5–24. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&from=EN
- EC 401/2006 (2006). Official Journal of the European Union, L 70,pp 12–34. https://www.fsvps.ru/fsvps-docs/ru/usefulinf/files/es401-2006.pdf
- FSSAI (2011). Food safety and standards, contaminants, toxins and residues regulations, 2011 Available at https://fssai.gov.in/home/fss-legislation/fss-regulations.html
- International Agency for Research on Cancer, (IARC) (2002). Some traditional herbal medicines, some mycotoxins, naphthalene, and styrene. IARC Monographs. Vol. 82, IARC, Lyon [PMC free article] [PubMed]
- Jalili M. A review on aflatoxins reduction in food. Iran J Health Saf Environ. 2016;3:445–459. [Google Scholar]
- Kaushik G. Effect of processing on mycotoxin content in grains. Crit Rev Food Sci Nutr. 2015;55:1672–1683. doi: 10.1080/10408398.2012.701254. [DOI] [PubMed] [Google Scholar]
- Larmond E (1970) Methods of sensory evaluation of food. Can Deptt Agric Pubs: 1284–1290
- Lee LS, Cucullu AF, Franz AO, Jr, Pons WA., Jr Destruction of aflatoxins in peanuts during dry and oil roasting. J Agric Food Chem. 1969;17:451–453. doi: 10.1021/jf60163a010. [DOI] [Google Scholar]
- Lisker N, Michaeli R, Frank ZR. Mycotoxigenic potential of Aspergillus flavus strains isolated from groundnuts in Israel. Mycopathologia. 1993;122:177–183. doi: 10.1007/BF01103479. [DOI] [PubMed] [Google Scholar]
- Méndez-Albores A, Arambula-Villa G, Loarca-Piña MGF, Castano-Tostado E, Moreno-Martínez E. Safety and efficacy evaluation of aqueous citric acid to degrade B-aflatoxins in maize. Food Chem Toxicol. 2005;43(2):233–238. doi: 10.1016/j.fct.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Mobeen AK, Aftab A, Asif A, Zuzzer AS. Aflatoxins B1 and B2 contamination of peanut and peanut products and subsequent microwave detoxification. J Pharm. 2011;1(1):1–3. doi: 10.1111/j.2042-7158.1949.tb12365.x. [DOI] [Google Scholar]
- Ogunsanwo BM, Faboya OOP, Idowu OR, Lawal OS, Bankole SA. Effect of roasting on the aflatoxin contents of Nigerian peanut seeds. Afr J Biotechnol. 2005;3:451–455. doi: 10.5897/AJB2004.000-2096. [DOI] [Google Scholar]
- Oulkar D, Goon A, Dhanshetty M, Khan Z, Satav S, Banerjee K. High-sensitivity direct analysis of aflatoxins in peanuts and cereal matrices by ultra-performance liquid chromatography with fluorescence detection involving a large volume flow cell. J Environ Sci Health Part B. 2017;53:255–260. doi: 10.1080/03601234.2017.1410416. [DOI] [PubMed] [Google Scholar]
- Panalaks T, Scott PM. Sensitive silica gel-packed flow cell for fluorometric detection of aflatoxins by high pressure liquid chromatography. J Assoc Off Anal Chem. 1977;60:583–592. [PubMed] [Google Scholar]
- Park JW, Kim YB. Effect of pressure cooking on aflatoxin B1 in rice. J Agric Food Chem. 2006;54:2431–2435. doi: 10.1021/jf053007e. [DOI] [PubMed] [Google Scholar]
- Pluyer HR, Ahmed EM, Wei CI. Destruction of aflatoxins on peanuts by oven-and microwave-roasting. J Food Prot. 1987;50:504–508. doi: 10.4315/0362-028X-50.6.504. [DOI] [PubMed] [Google Scholar]
- Rapid Alert System for Food and Feed. Available at https://ec.europa.eu/food/sites/food/files/safety/docs/rasff_annual_report_2016.pdf
- Rastegar H, Shoeibi S, Yazdanpanah H, Amirahmadi M, Khaneghah AM, Campagnollo FB, Sant’Ana AS, Removal of aflatoxin B1 by roasting with lemon juice and/or citric acid in contaminated pistachio nuts. Food Cont. 2017;71:279–284. doi: 10.1016/j.foodcont.2016.06.045. [DOI] [Google Scholar]
- Saalia FK, Phillips RD. Degradation of aflatoxins by extrusion cooking: effects on nutritional quality of extrudates. LWT - Food Sci Technol. 2011;44:1496–1501. doi: 10.1016/j.lwt.2011.01.021. [DOI] [Google Scholar]
- Samarajeewa U, Sen AC, Cohen MD, Wei CI. Detoxification of aflatoxins in foods and feeds by physical and chemical methods. J Food Prot. 1990;53(6):489–501. doi: 10.4315/0362-028X-53.6.489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.