Abstract
The residue from chicken mechanically separated meat (MSM) is a potential source for the extraction of collagen. However, this process requires the removal of many covalent crosslinks, which makes it quite complex. Ultrasound has been successfully used to extract collagen; it reduces the process time and increases the yield. However, information regarding the effects of this treatment on the structural and functional properties of proteins is still very limited. Therefore, the aims of the present study were to obtain collagen from chicken MSM residue and to test the effects of pre-treatment with ultrasonic probe and enzymatic extraction with pepsin in its yield, as well as to evaluate the properties of extracted collagen using gel electrophoresis, Fourier-transform infrared spectroscopy, solubility, and differential scanning calorimetry. Both the ultrasound and the enzymatic extraction had a positive effect on the extraction yield of collagen from chicken MSM residue without affecting its integrity. Using ultrasound led to an increase of up to 40% in yield when compared to treatments without ultrasound application. Five extraction treatments were considered. The extracted collagen exhibited high thermal stability (43.9–47.0 °C) and mainly type I structure. The use of ultrasound as pre-treatment, together with enzymatic extraction with pepsin, were effective in increasing the extraction yield of collagen from chicken MSM residue, as well as preserving the triple helical structure of the native collagen.
Keywords: Electrophoresis, Differential exploration calorimetry, Infrared spectroscopy, Pepsin
Introduction
Collagen is a fibrous protein which is present in almost all tissues, including skin, bones, blood vessels and tendons. It is the most abundant body protein in mammals, accounting for 30% of all body protein (Damodaran et al. 2010). Generally extracted from cattle by-products; it is widely used in the pharmaceutical, cosmetic, photographic and food industries (Gómez-Guillén et al. 2011). However, due to the occurrence of bovine spongiform encephalopathy (BSE) and foot-and-mouth disease there has been increased interest in alternative sources of collagen (Jeevithan et al. 2014; Kim and Mendis 2006).
The residue from mechanically separated meat (MSM) of chicken is generated in large quantities in the poultry industry. The cooperative partner of the project, Cooperativa Central Aurora, generates a volume of approximately 1200 tons of MSM residue monthly; in addition the residue has low commercial value and is usually sent to manufacture animal feed. For being made up of bones, cartilage and scraps of meat and skin, this residue can be a potential source to obtain collagen, thus allowing its valorization. No studies were found in the literature regarding the use of chicken MSM residue as a raw material.
The methods commonly employed to extract collagen are based on its solubility in neutral salt solutions, acid solutions, and the use of proteases. Compared to the aforementioned methods, proteases (mainly pepsin) have resulted in higher yields (Li et al. 2013; Nagai et al. 2015; Wang et al. 2014). However, these conventional extraction methods are generally time consuming and can generate large amounts of waste.
To extract collagen it is necessary to remove many intra and intermolecular covalent crosslinks, mainly involving lysine and hydroxylysine residues, ester bonds and other connections with saccharides, which make the process quite complex (Ran and Wang 2014). In addition, the collagen extraction method itself may influence the length of the polypeptide chains and their functional properties (Gómez-Guillén et al. 2011).
Ultrasound has been successfully used to extract collagen, reducing the process time and increasing the yield (Kim et al. 2012; Li et al. 2009; Ran and Wang 2014). The effects of ultrasound are mainly due to the phenomenon of cavitation (Hu et al. 2013); during sonication, cavitation bubbles are rapidly formed and suffer a violent collapse, resulting in extreme temperatures and pressures. This leads to shear and turbulence in the cavitation zone (Chemat and Khan 2011).
Nevertheless, information regarding the effects of ultrasonic treatment on the structural and functional properties of proteins is still very limited. According to Ran and Wang (2014), the application of ultrasound over a long period of time can give rise to high temperatures and shear forces, as well as high pressures inside the medium. The breakdown of hydrogen bonds, as well as van der Waals interactions in the polypeptide chains, can also occur, leading to denaturation of the proteins.
The aims of the present study were to obtain collagen from chicken MSM residue and to test the effects of pre-treatment with ultrasonic probe and enzymatic extraction with pepsin in its yield, as well as to evaluate the properties of extracted collagen using gel electrophoresis (SDS-PAGE), Fourier-transform infrared spectroscopy (FTIR), solubility, and differential scanning calorimetry (DSC).
Materials and methods
The frozen raw material (residue from chicken MSM extraction process) was donated by the Cooperativa Central Aurora (Aurora Alimentos) in the city of Erechim, (RS), Brazil. It was obtained using a High Tech machine (model HT-6.0 SS) with a 6000 kg/h capacity, 36775 w power and weight of 1680 kg. The MSM residue was transported in refrigerated truck, inside polyethylene packages and stored for up to 1 year in a freezer (Metalfrio, VF50F) at − 22 °C.
Determination of the amino acid hydroxyproline of raw material
The determination of the amino acid hydroxyproline followed the Instituto Adolfo Lutz (2008) methodologies. The sample was hydrolyzed with 6 M hydrochloric acid at 110 °C for 8 h under reflux. Hydroxyproline was oxidized by chloramine T, the reddish complex formed after addition of 4-dimethylaminobenzaldehyde was measured in a spectrophotometer (Servylab, UV-M51, B, São Leopoldo, RS, Brazil) at 558 nm.
Obtaining the collagen
Pre-treatment
The pre-treatment of the chicken MSM residue was performed according to the methodology of Li et al. (2013) and Duan et al. (2009), with modifications. Initially, the non-collagenous proteins were removed with 0.1 M sodium hydroxide solution (NaOH) for 48 h, with a ratio of sample/alkaline solution of 1:20(w/v) and with a change of solution every 12 h. For the decalcification, a solution of 0.5 M EDTA-2Na (pH 7.5) was used for five days with a 1:10(w/v) sample/solvent ratio. The fat was extracted using 10% butyl alcohol for 48 h, with a solid/solvent ratio of 1:10(w/v), and with the solvent changed every 12 h. All the processes were performed using Shaker Incubator (Solab SL-223, Piracicaba, Brazil) equipment at 150 rpm and 4 °C.
After pre-treatment the samples were stored in a freezer (Metalfrio, VF50F) at − 22 °C for up to 15 days, for later extraction of collagen.
Extraction
To extract the collagen from the chicken MSM residue, the methodology of Ran and Wang (2014) was followed, with modifications regarding the time of ultrasonic pre-treatment and enzymatic extraction. A 22 factorial design with a central point of experimental space was used, which was performed in duplicate (N = 10). Table 1 shows the independent variables, which were: pre-treatment time with ultrasound (0, 15 and 30 min); and time of enzymatic extraction with pepsin (24, 36 and 48 h). The extraction time used was high because the material was very heterogeneous and complex.
Table 1.
Treatments tested based on complete 22 factorial design with central point: yields and protein content obtained for different treatments to extract collagen from chicken MSM residue
| Treatment | Ultrasound time (min) | Enzymatic extraction time (h) | Yield on wet basis (%) | Yield on dry basis (%) | Protein level (%) |
|---|---|---|---|---|---|
| T1 | 30 | 24 | 5.10b ± 0.26 | 11.44b ± 0.60 | 88.62a ± 1.58 |
| T2 | 30 | 48 | 6.74a ± 0.27 | 15.11a ± 0.62 | 88.53a ± 2.91 |
| T3 | 0 | 48 | 4.82b ± 0.23 | 10.80b ± 0.51 | 87.05a ± 2.21 |
| T4 | 0 | 24 | 3.92c ± 0.21 | 8.78c ± 0.47 | 88.34a ± 2.49 |
| PC | 15 | 36 | 5.37b ± 0.26 | 12.03b ± 0.58 | 86.32a ± 1.51 |
aAverages within the same column with the same superscript letters do not differ significantly from each other by Tukey’s test(p > 0.05). Values expressed as mean ± standard deviation.T1: 30 min of pre-treatment with ultrasound and 24 h of enzymatic extraction; T2: 30 min of pre-treatment with ultrasound and 48 h of enzymatic extraction; T3: 48 h of enzymatic extraction; T4: 24 h of enzymatic extraction; PC: 15 min of pre-treatment with ultrasound and 36 h of enzymatic extraction
The enzyme pepsin is an endopeptidase, capable of hydrolyzing peptide bonds between amino acids; it acts on inner portions of the polypeptide chains, giving rise to long polypeptides, such as proteases and peptones. Its optimum pH is between 1.5 and 2.0.
The ultrasound pre-treatment was performed in water, with a solid/solvent ratio of 1:15(w/v), in a jacketed cup linked to an ultra-thermostated bath (Solab SL-152/10, Piracicaba, Brazil) for temperature control (4 °C). A Hielscher UP200S ultrasonic probe (Ringwood, Germany) was used at its maximum power (400 W) and frequency (24 kHz) for the times shown in Table 1.
For the subsequent enzymatic extraction of the collagen, acetic acid was added at a concentration of 0.5 M, as well as the enzyme pepsin (Sigma, St. Louis, USA) at a ratio of 1:2.50 enzyme/protein. The mixtures were continuously shaken in a Shaker Incubator (Solab SL-223, Piracicaba, Brazil) at 150 rpm and 4 °C for the times shown in Table 1.
When the enzymatic extraction was finished, filtration was performed through two layers of gauze. The collagen was subsequently precipitated in the presence of 0.05 M tris (hydroxymethyl) aminomethane, at pH 7.5, by adding NaCl to a final concentration of 2.6 M. The precipitated collagen was centrifuged at 12,000 rpm for 30 min in a refrigerated centrifuge (Hitachi, CR22GIII) at 4 °C. After centrifugation, the collagen was re-dissolved in a small volume of 0.5 M acetic acid for subsequent cellulose membrane dialysis (typical molecular weight cut-off 14,000 KDa, Sigma, St. Louis, USA), which was performed for two days with 0.1 M acetic acid, and for 2 days with distilled water, both at 4 °C under stirring and a change of solution every 12 h. After dialysis, the collagen was frozen in a freezer (Metalfrio, VF50F) at − 22 °C and lyophilized in a lyophilizer (Terroni, LS 3000, São Carlos, Brazil).
Yield
The collagen yield was calculated based on the wet and dry weight of the starting material:
Characterization of collagen
Determination of protein content
The determination of the protein content (wet basis) followed the methodology of Lowry et al. (1951). The absorbance readings were performed at 660 nm in a spectrophotometer (Servylab, UV-M51, B, São Leopoldo, RS, Brazil); bovine serum albumin (Sigma, St. Louis, USA) was used as standard.
Fourier-transform infrared spectroscopy (FTIR)
The structural characteristics were evaluated using Fourier-transform infrared spectroscopy (FTIR) and Shimadzu IR Prestige-21 (Shimadzu Corporation, Japan) equipment using the attenuated total reflectance (ATR) technique in the range of 400–4000 cm−1. Sixteen scans per spectrum were performed at 2 cm−1 resolution.
Differential scanning calorimetry (DSC)
According to Damodaran et al. (2010), the denaturation of a protein is a phenomenon that involves the transformation of its three-dimensional structure and well defined to a disordered state. To evaluate the denaturation temperature of the collagens the melting point of the collagen gels was determined by DSC, for which a gel was initially prepared which was subsequently subjected to controlled heating. Approximately 3.0 mg of sample was weighed, mixed with deionized water at a ratio of 6:1 (water:collagen, m/m), and the mixtures were held for 120 min under refrigeration (7 °C) in order to balance the moisture content and to hydrate. The analyses were performed in sealed aluminum crucibles.
The DSC curves were obtained using DSC-Q200 (TA-Instruments, USA) equipment, which was pre-calibrated with 99.99% high purity indium, mp = 156.6 °C, ΔH = 28.56 J/g. The following parameters were used to record the enthalpy and the temperatures of the collagen denaturation process: air flow of 50 mL/min, heating rate of 10 °C/min and heating range from - 20 to 100 °C.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis (SDS-PAGE)
The gel electrophoresis analysis was based on the method proposed by Laemmli (1970), with modifications. The samples were diluted in water and then 40 μL of 60% trichloroacetic acid (TCA) and 100 μL of Milli-Q water in 200 μL of sample were added in Eppendorf centrifuge tubes. This solution was stored overnight in a freezer (12 h). After the samples were centrifuged at 10,000 rpm, 4 °C for 30 min, the supernatant was removed, taking care that the pellet was not broken. Then, 100 μl of iced 90% acetone solution was added in order to wash the pellet without breaking it. Further centrifugation was performed for 30 min. Once again, the supernatant was removed and another 100 μl of iced 90% acetone was added and centrifuged under the same conditions, again removing the supernatant. The precipitate was re-suspended with 50 μl of the sample buffer with 5% β-mercaptoethanol. The samples were then denatured in a water bath at 100 °C for 10 min.
Resolution gel (15%) and stacking gel (5%) were used: 20 μl of the samples to be analyzed and 15 μL of the molar mass standard were added and subjected to electrophoresis at 150 V and 30 mA for approximately 2 h in a vertical electrophoresis cell. The gel was subsequently stained with 0.1% Coomassie Brilliant Blue R-250 (Bio-Rad Laboratories Inc., Richmond, VA, USA) for approximately twelve hours. The gel was then discolored by heating in distilled water in microwaves until perfect visualization of the bands was achieved.
Solubility
Solubility was determined using the method of Montero et al. (1991), with modifications. The samples were dissolved in 0.5 M acetic acid to give a final concentration of 3 mg/mL and the pH was adjusted from 1.0 to 10.0 with NaOH or HCl. The solutions were slowly stirred at 4 °C for 30 min and centrifuged at 10,000 rpm and 4 °C for 30 min. The protein content in the supernatant was determined by the method of Lowry et al. (1951) using bovine serum albumin (Sigma, St. Louis, USA) as standard. The relative solubility of the collagen was calculated by comparing it with that which was obtained in the pH with the greatest solubility, taking this as 100%.
Statistical analysis
All the analyses were performed in triplicate, the results were subjected to analysis of variance (ANOVA) and Tukey's test, with a 5% significance level (p < 0.05), using Statistica® 8.0 (StatSoft Inc., Tulsa, OK, USA) software. The calculations of the effects performed on the extraction yield of the collagens were also obtained using the mentioned computer program.
After obtaining the FTIR spectra, each spectrum was treated with a normalized absorbance between 0 and 1, smoothed (15 points), corrected at the baseline, and the CO2 zone was removed using Shimadzu IRsolution 1.40 software. Then, a table was constructed with all the data, using the wavelength as the column and the samples as lines. The first and second derivatives of the spectra were then were tested using the EXPAND procedure in order to linearize them.
The data regarding the solubility of the different collagen extracted from the chicken MSM residue by different treatments were adjusted as a function of pH variation using the sigmoidal empirical function (logistic) according to the following statistical model:
where = the solubility (%) of the ith collagen in the ith repetition under pH γ; α = final solubility (%) at the post-transition baseline or plateau phase/stationary phase/steady state, when γ → ∞; = the initial solubility (%) at the pre-transition baseline or lag phase; = exponential; = rate of change of solubility; = pH value (1–10); = pH level where solubility reaches 50% of the transition zone, midpoint or inflection point; = experimental error associated with each observation, supposing The model parameters were estimated using the Gauss–Newton algorithm modified by the NLIN procedure of the SAS®.
The likelihood ratio test with the approximation by distribution is given by:
where = ; = total number of observations; = natural logarithm; = maximum likelihood estimate of under no constraint in the Ω parametric space (Complete model); = maximum likelihood estimate of under the linear constraints defined in (Reduced model); = sum of squares of the regression residue in the complete model; = sum of squares of the regression residue in the reduced model; = tabulated ; = level of significance; = number of degrees of freedom, given that , i.e. the number of estimated parameters in the complete and reduced models, respectively.
The likelihood ratio test with approximation by the F distribution is given by:
where = ; = sum of residual squares of the reduced model (under constraint); = sum of residual squares of the complete model; = number of degrees of freedom of the residual of the complete model; = number of degrees of freedom of the residue of the reduced model minus the number of degrees of freedom of the residue of the complete model; = ; = as previously defined.
The coefficient of determination (r2) of the regression models was expressed in relation to the source treatments (regression + lack of fit).
The statistical analyses were performed using SAS® System for Windows ™ version 9.4 (SAS Institute Inc., Cary, NC, USA) software with a 5% level of significance.
Results and discussion
Determination of the amino acid hydroxyproline of raw material
The crude chicken MSM residue, before pre-treatment, presented 2.67 g/100 g hydroxyproline, which, multiplied by fact 8, corresponds to 21.36 g/100 g of collagen in the residue. The collagen content was lower than that obtained by Araújo et al. (2018) for chicken feet, 56.31 g/100 g, this is probably because the MSM residue is a very heterogeneous sample containing bones, skin, meat and cartilage.
Yield
The collagen yield (Table 1) ranged from 3.92 to 6.74% on wet basis and from 8.78 to 15.11% on dry basis. Treatment T2 (30 min of pre-treatment with ultrasound and 48 h of enzymatic extraction) presented the highest yield, and T4 (24 h of enzymatic extraction) presented the lowest yield. Treatments T1, T3 and PC did not differ between each other. Comparing treatments T2 and T3 (both with 48 h of enzymatic extraction), there was an increase in yield of around 40% when pre-treatment with ultrasound probe was performed. The treatments showed no significant difference in terms of protein content (Table 1), indicating that the increase in yield was due to a higher level of collagen extraction.
According to Li et al. (2009) this was due to an increase in enzymatic activity and dissolution of the substrate because the use of ultrasound results in a greater dispersion of the pepsin and opening of the collagen fibrils, facilitating the action of the enzyme. The use of ultrasound (40 kHz, 120 W) and pepsin to extract collagen from bovine tendon resulted in a considerable increase in yield compared to pepsin extraction alone; the yield was 55.4–80.6% in 24 h and 71.4–88.0% in 48 h.
The collagen from the chicken MSM residue showed lower yield (8.78%–12.03%) when compared to other residues. This was expected because the collagen extracted from the residue that comes from the entire carcass will present a lower yield than that obtained from a specific part. In a study by Araújo et al. (2018), the collagen yield of chicken feet ranged from 31.54 to 72.98 g/100 g, however the initial raw material had collagen content higher than the chicken MSM residue. Yet, the MSM residue collagen showed similar yields to other raw materials, such as Silver carp (Hypophthalmichthys molitrix) (5.9% dry weight) and rohu skin (Labeo rohita) (6.8% dry weight) (Savedboworn et al. 2017).
The effects of variables (pre-treatment with ultrasound probe, and enzymatic extraction with pepsin) and their interactions on the collagen yield from chicken MSM residue were studied. A first-order significant effect (p < 0.05) was found for the two tested variables, both of which showed a positive influence on the collagen yield. The effect of the interaction between the two variables also significantly increased the yield.
Longer pre-treatment times with ultrasound and enzymatic extraction were more effective in extracting collagen from the chicken MSM residue, under the tested conditions. Wang et al. (2008) also found a positive correlation between the extraction time with pepsin, and collagen yield from grass carp (Ctenopharyngodon idella). The extraction time ranged from 12 to 36 h; the optimum time to obtain the highest yield was 35.2 h.
Positive effects for the use of ultrasound on yield in relation to the extraction of collagen from bovine tendon were also found in a study by Ran and Wang (2014). According to Li et al. (2009), the use of ultrasound provides a better dissolution of the substrate because it opens the collagen fibers, thereby facilitating extraction.
Characterization of collagen
Fourier-transform infrared spectroscopy (FTIR)
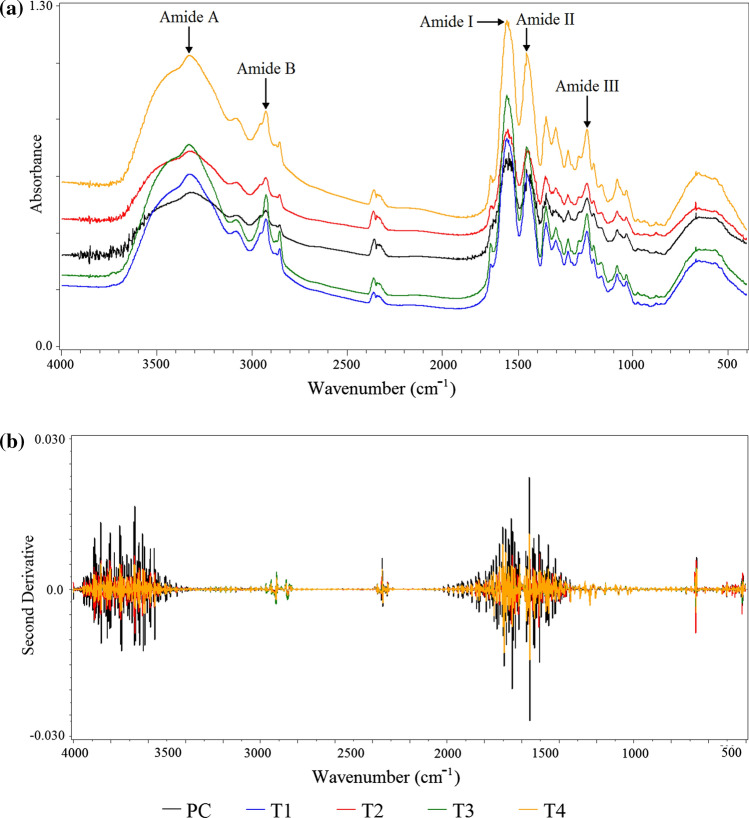
Figure 1a shows the main peaks of the infrared spectra of the chicken MSM residue collagen. The FTIR spectra found in this study resembled those found for collagen from other sources, such as collagen from chicken foot skin (Zhou et al. 2016), emu skin (Dromaius novaehollandiae) (Nagai et al. 2015) and squid skin (Doryteuthis singhalensis) (Veerurajet al. 2015). There were characteristic absorption peaks located in the region of the bands of amides A and B and I, II and III for all the collagen samples.
Fig. 1.
a FTIR spectra, and b second derivative of the spectra of the collagen from chicken MSM residue extracted using a 22 factorial design with a central point. T1: 30 min of pre-treatment with ultrasound and 24 h of enzymatic extraction; T2: 30 min of pre-treatment with ultrasound and 48 h of enzymatic extraction; T3: 48 h of enzymatic extraction; T4: 24 h of enzymatic extraction; PC: 15 min of pre-treatment with ultrasound and 36 h of enzymatic extraction
The amide A bands were found between 3323.35 (PC) and 3329.14 (T2) cm−1. They were associated with NH stretching vibrations, when the NH group of a peptide is involved in a hydrogen bond the position is shifted to lower frequencies (3300 cm−1) than an NH-free stretch vibration (3400–3440 cm−1) (Sai and Babu 2001).
The amide B bands of the collagen were found at 2926 cm−1, which was related to the asymmetric stretching of the stretching vibration of CH2, as well asthe absorption due to the CH2 alkyl chain (Hsuet al. 2005).
The amide I, II and III bands of the different collagen samples were found in the region of 1660, 1556 and 1240 cm−1, respectively. These bands are related to the structure of collagen (Heu et al 2010). The amide band I, with a characteristic wave number in the range of 1600 to 1700 cm−1, is mainly associated with the stretching vibrations of the carbonyl groups along the polypeptide backbone. The amide II and III bands represent N–H flexural vibrations coupled with C-N stretch vibration (Barth and Zscherp 2002; Jackson et al. 1995).
The ratio between the area of the amide III peak to the peak at 1454 cm−1 was close to 1.0 for all the collagen samples; this ratio generally indicates the collagen triple helix structure. This result demonstrated that the use of ultrasound and pepsin did not negatively affect the triple helix structures of the collagen (Wang et al. 2014).
A mathematical method to increase the resolution by using derivation was applied to the FTIR spectra of the collagen from the chicken MSM residue. Figure 1b presents the second derivative of the spectra of the collagen samples. This method of increasing resolution is based on the separation of the peaks that compose the bands, and on the correlation between their areas or intensities with their structures (Forato et al. 1998). The different collagen samples presented the same peaks, which were characteristic of collagen, but with different intensities, which indicates that the different treatments did not affect the integrity of the obtained collagens.
Differential scanning calorimetry (DSC)
Heating collagen causes the hydrogen bonds to break down between the polypeptide chains, transforming the triple helix structure of the collagen into a coiled random structure. Lower temperatures of denaturation of collagens indicate less thermal stability, and this is one of the factors that affects the subsequent application of these collagens. Completely denatured proteins can be used in the food industry only as an emulsifying agent and not as a source of nutritious fibers (Silva and Penna 2012). Table 2 shows the results of the DSC of the denaturation for the collagen samples from the chicken MSM residue.
Table 2.
Results of the DSC for denaturation of the collagen from chicken MSM residue extracted using a 22 factorial design with a central point
| Treatment | To (ºC) |
Tp (ºC) |
Tc (ºC) |
ΔH (J/g) |
|---|---|---|---|---|
| T1 | 43.14 cd ± 0.60 | 46.31c ± 0.17 | 54.97ª ± 0.55 | 15.19e ± 0.14 |
| T2 | 43.89c ± 0.34 | 47.62b ± 0.07 | 54.66ab ± 0.11 | 19.51b ± 0.33 |
| T3 | 44.67b ± 0.05 | 47.67b ± 0.04 | 50.27c ± 0.84 | 17.32d ± 0.33 |
| T4 | 46.94a ± 0.07 | 49.91ª ± 0.04 | 55.36ª ± 0.39 | 21.12ª ± 0.20 |
| PC | 42.91d ± 0.07 | 46.43c ± 0.32 | 53.55b ± 0.42 | 18.41c ± 0.24 |
a Averages within the same column with the same superscript letters do not differ significantly from each other by Tukey’s test (p > 0.05). Values expressed as mean ± standard deviation. To initial onset temperature, Tp peak temperature, Tc end set temperature,ΔH enthalpy of denaturation. T1: 30 min of pre-treatment with ultrasound and 24 h of enzymatic extraction; T2: 30 min of pre-treatment with ultrasound and 48 h of enzymatic extraction; T3: 48 h of enzymatic extraction; T4: 24 h of enzymatic extraction; PC: 15 min of pre-treatment with ultrasound and 36 h of enzymatic extraction
The collagen samples showed endothermic transitions corresponding to the denaturation of the collagen, whose initial temperature varied between 42.91 and 46.94º C, with enthalpies between 15.19 and 21.12 J/g. The T4 treatment, which was extracted with pepsin for 24 h, had the highest temperature and enthalpy of denaturation; in other words, it presented greater thermal stability compared to the other samples, which was probably due to this collagen having a more complete helical structure. The PC treatment (15 min pre-treatment with ultrasound and 36 h enzymatic extraction) had the lowest initial denaturation temperature (To) but it did not differ from the T1 treatment.
Zou et al. (2017) obtained a singular result when they extracted collagen from turtle; using ultrasound resulted in an increased denaturation temperature for the collagen compared to the conventional method. However, a study by Ali et al. (2018) suggested that ultrasound alone could not alter physical/chemical properties of collagen. Zeng et al. (2012) reported denaturation temperatures of between 33.97 and 34.62 °C, and enthalpy of 41.23–51.36 J/g for cobia (Rachycentron canadum) skin. Other studies have reported denaturation temperatures from 30 to 60 °C for collagen, depending on the origin and the type of collagen. Thermal denaturation temperatures are correlated with the content of the amino acids proline and hydroxyproline in collagen; the higher the content of these amino acids the more stable the helical structure of the molecules (Liu et al. 2014; Ran and Wang 2014; Sinthusamran et al. 2013).
In general terms, all the different treatments in the present study presented good thermal stability, indicating that the use of ultrasound and pepsin in the extraction was not detrimental to the stability of the collagen within the tested conditions.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis (SDS-PAGE)
Figure 2 shows the SDS-PAGE for the different treatments of collagen from chicken MSM residue using 15% gel. Two distinct bands, close to 125 kDa and corresponding to the α1 and α2 chains, were identified, indicating that the collagen samples were mainly composed of type I collagen, which generally consists of two α1 chains and one α2 chain. It was possible to verify some proteins of lower molecular weight, between 100 and 30 kDa, for all the treatments.
Fig. 2.
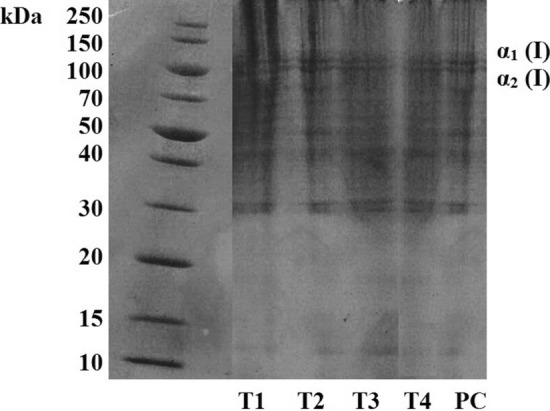
Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis (SDS-PAGE) of collagen from chicken MSM residue extracted using a 22 factorial design with a central point. T1: 30 min of pre-treatment with ultrasound and 24 h of enzymatic extraction; T2: 30 min of pre-treatment with ultrasound and 48 h of enzymatic extraction; T3: 48 h of enzymatic extraction; T4: 24 h of enzymatic extraction; PC: 15 min of pre-treatment with ultrasound and 36 h of enzymatic extraction
Previous studies have shown that avian collagen is composed of α chains (α1 (I) and α2 (I)) and β-chains (Araújo et al. 2018; Nagai et al. 2015). However, in the SDS-PAGE analysis of the collagen from the chicken MSM residue the β band was not identified.
The use of pre-treatment with ultrasonic probe in the T1, T2 and PC treatments did not lead to the appearance of low molecular weight peptides, proving that the use of ultrasound did not negatively affect the integrity of the collagen samples, which maintained the triple helix structure intact; a result that was also confirmed by the FTIR spectra (Fig. 1). The fact that the structure was preserved was probably due to the ultrasound being used at a controlled temperature (4º C) and for a short period of time (15 or 30 min).
Solubility
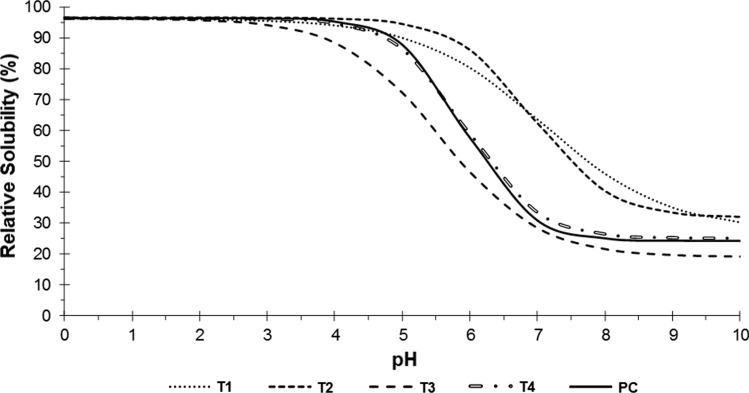
The data regarding solubility for the different treatments of collagen from chicken MSM residue were adjusted using the sigmoidal empirical function; the relative solubility curves as a function of pH that were obtained are presented in Fig. 3. The initial solubility (β) did not differ (p > 0.05) between the treatments; a mean of 96.5% was obtained, which remained constant until close to pH 3.0, indicating that all the collagen samples had good solubility at acid pH. The treatments presented significant differences in terms of the values of final solubility (α), pH at the inflection point (δ), and rate of change of solubility (κ).
Fig. 3.
Relative solubility curves as a function of the pH (0 to 10) of the collagen from chicken MSM residue extracted according to 22 factorial design. T1: 30 min of pre-treatment with ultrasound and 24 h of enzymatic extraction; T2: 30 min of pre-treatment with ultrasound and 48 h of enzymatic extraction; T3: 48 h of enzymatic extraction; T4: 24 h of enzymatic extraction; PC: 15 min of pre-treatment with ultrasound and 36 h of enzymatic extraction
All the collagen samples showed a marked decrease in solubility with increasing pH (between 5 and 8) but the rate of change of solubility (κ) was higher in the T2, T4 and PC treatments. Chi et al. (2014) also observed maximum solubility at pH of 1 to 4, as well as a severe drop in pH from 5 to 7 for acid-soluble collagen from the skin of hammerhead shark (Sphyrna lewini).
Treatments T1 and T2 (both with 30 min of ultrasonic pre-treatment) had a higher pH at the inflection point (close to 7.0), indicating the higher solubility of these treatments, since this point indicates the pH at which the solubility reaches 50%. Treatments T1 and T2 also had a final solubility (α) higher than the other treatments.
Collagen from chicken MSM residue showed similar behavior to bovine tendon collagen, extracted with pepsin after ultrasonic treatment. Tendon collagen presented high solubility in pH of 2–4. The solubility was higher than 92% and there was a marked decrease close to neutrality; the level was 21% at pH 6 and it remained low, but relatively stable, to pH 9 (Ran and Wang 2014).
Conclusion
Pre-treatment with ultrasound, as well as the time of enzymatic extraction with pepsin, had a positive effect on the yield of collagen from chicken MSM residue without affecting the integrity of the collagen. This was verified by the FTIR spectra, which showed peaks characteristic of bands A and B and I, II and III, and the electrophoresis analysis. Using ultrasound resulted in an increase in yield of about 40% in the T2 treatment (pre-treated for 30 min with ultrasound and extracted with pepsin for 48 h) compared to the T3 treatment (extracted with pepsin for 48 h).
All the treatments exhibited high thermal stability. The treatment extracted with pepsin for 24 h (T4) showed the highest denaturation temperature, 46.96º C. Analysis by SDS-PAGE showed that all the collagen samples were mainly composed of type I collagen; the use of ultrasound did not lead to the appearance of low molecular weight peptides.
In view of the above, it can be concluded that the use of ultrasound as pre-treatment, together with enzymatic extraction using pepsin, were effective in increasing the extraction yield of collagen from chicken MSM residue and also preserved the triple helical structure of native collagen.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 and Programa de Apoio aos Pólos Tecnológicos—Secretaria de Desenvolvimento Econômico, Ciência e Tecnologia do Rio Grande do Sul.
To FAPERGS, CNPQ and Cooperativa Central Aurora for the financial support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michele M. Schmidt, Email: mixi.schmidt@gmail.com
Alessandra R. Vidal, Email: alee.alessandra@yahoo.com.br
Renius O. Mello, Email: reniusmello@gmail.com
Marcio A. Mazutti, Email: marciomazutti@gmail.com
Rogério L. Cansian, Email: cansian@uricer.edu.br
Rosa C. P. Dornelles, Email: rosacrisprestesdornelles@outlook.com
Ernesto H. Kubota, Email: ernehk2008@yahoo.com.br
References
- Abdollahi M, Rezaei M, Jafarpour A, Undeland I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food chem. 2018;242:568–578. doi: 10.1016/j.foodchem.2017.09.045. [DOI] [PubMed] [Google Scholar]
- Ali AMM, Kishimura H, Benjakul S. Extraction efficiency and characteristics of acid and pepsin soluble collagens from the skin of golden carp (Probarbus Jullieni) as affected by ultrasonication. Process Biochem. 2018;66:237–244. doi: 10.1016/j.procbio.2018.01.003. [DOI] [Google Scholar]
- Araújo ÍBDS, Bezerra TKA, Nascimento ESD, Gadelha CADA, Santi-Gadelha T, Madruga MS. Optimal conditions for obtaining collagen from chicken feet and its characterization. Food Sci Technol. 2018;38:167–173. doi: 10.1590/fst.27517. [DOI] [Google Scholar]
- Barth A, Zscherp C. What vibrations tell about proteins. Q Rev Biophys. 2002;35:369–430. doi: 10.1017/S003358350200381. [DOI] [PubMed] [Google Scholar]
- Chemat F, Khan MK. Applications of ultrasound in food technology:processing, preservation and extraction. Ultrason Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Chi CF, Wang B, Li ZR, Luo HY, Ding GF, Wu CW. Characterization of acid-soluble collagen from the skin of hammerhead shark (Sphyrna lewini) J Food Biochem. 2014;38:236–247. doi: 10.1111/jfbc.12042. [DOI] [Google Scholar]
- Damodaran S, Parkin K, Fennema OR. Química de alimentos de fennema. 4ª. São Paulo: Artmed; 2010. [Google Scholar]
- Duan R, Zhang J, Du X, Yao X, Konno K. Properties of collagen from skin, scale and bone of carp (Cyprinus carpio) Food Chem. 2009;112:702–706. doi: 10.1016/j.foodchem.2008.06.020. [DOI] [Google Scholar]
- Forato LA, Bernardes Filho R, Colnago LA. Estudo de métodos de aumento de resolução de espectros de FTIR para análise de estruturas secundárias de proteínas. Quím Nova. 1998;21:146–150. doi: 10.1590/S0100-40421998000200008. [DOI] [Google Scholar]
- Gómez-Guillén MC, Giménez B, Lopéz-Caballero ME, Montero MP. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 2011;25:1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- Heu MS, Lee JH, Kim HJ, Jee SJ, Lee JS, Jeon YJ, Shahidi F, Kim JS. Characterization of acid-and pepsin-soluble collagens from flatfish skin. Food Sci Biotechnol. 2010;19:27–33. doi: 10.1007/s10068-010-0004-3. [DOI] [Google Scholar]
- Hsu BL, Weng YM, Liao YH, Chen W. Structural investigation of edible zein films/coatings and directly determining their thickness by FT-Raman spectroscopy. J Agric Food Chem. 2005;53:5089–5095. doi: 10.1021/jf0501490. [DOI] [PubMed] [Google Scholar]
- Hu H, Fan X, Zhou Z, Xu X, Dan G, Wang L, Huang X, Pan S, Zhu L. Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrason Sonochem. 2013;20:187–195. doi: 10.1016/j.ultsonch.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Instituto Adolfo Lutz (2008) Ministério da Saúde. Agência Nacional de Vigilância Sanitária (ANVISA). Métodos Físico-Químicos para Análises de Alimentos, Ministério da Saúde, Agência Nacional de Vigilância Sanitária, Brasília, Ministério da Saúde.
- Jackson M, Watson PH, Halliday WC, Mantsch HH. Beware of connective tissue proteins: assignment and implications of collagen absorptions in infrared spectra of human tissues. Biochim Biophys Acta. 1995;270:1–6. doi: 10.1016/0925-4439(94)00056-V. [DOI] [PubMed] [Google Scholar]
- Jeevithan E, Wu W, Nanping W, Lan H, Bao B. Isolation, purification and characterization of pepsin soluble collagen isolated from silvertip shark (Carcharhinus albimarginatus) skeletal and head bone. Process Biochem. 2014;49:1767–1777. doi: 10.1016/j.procbio.2014.06.011. [DOI] [Google Scholar]
- Kim HK, Kim YH, Kim YJ, Park HJ, Lee NH. Effects of ultrasonic treatment on collagen extraction from skins of the sea bass Lateolabrax japonicus. Fish Sci. 2012;78:485–490. doi: 10.1007/s12562-012-0472-x. [DOI] [Google Scholar]
- Kim SE, Mendis E. Bioactive compounds from marine processing byproducts–a review. Food Res Int. 2006;39:383–393. doi: 10.1016/j.foodres.2005.10.010. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat. 1970;22:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li D, Mu C, Cai S, Lin W. Ultrasonic irradiation in the enzymatic extraction of collagen. Ultrason Sonochem. 2009;16:605–609. doi: 10.1016/j.ultsonch.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Li ZR, Wang B, Chi CF, Zhang QH, Gong YD, Tang JJ, Luo HY, Ding GF. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius) Food Hydrocoll. 2013;31:103–113. doi: 10.1016/j.foodhyd.2012.10.001. [DOI] [Google Scholar]
- Liu W, Tian Z, Li C, Li G. Thermal denaturation of fish collagen in solution: a calorimetric and kinetic analysis. Thermochim Acta. 2014;581:32–40. doi: 10.1016/j.tca.2014.02.012. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:256–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Montero P, Jimenez-Colmenero F, Borderias J. Effect of pH and the presence of NaCl on some hydration properties of collagenous material from trout (Salmoirideus Gibb) muscle and skin. J Sci Food Agric. 1991;54:137–146. doi: 10.1002/jsfa.2740540115. [DOI] [Google Scholar]
- Nagai T, Tanoue Y, Kai N, Suzuki N. Characterization of collagen from emu (Dromaius novaehollandiae) skins. J Food Sci Technol. 2015;52:2344–2351. doi: 10.1007/s13197-014-1266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran XG, Wang LY. Use of ultrasonic and pepsin treatment in tandem for collagen extraction from meat industry by-products. J Sci Food Agric. 2014;94:585–590. doi: 10.1002/jsfa.6299. [DOI] [PubMed] [Google Scholar]
- Regazzi AJ, Silva CHO. Teste para verificar a igualdade de parâmetros e a identidade de modelos de regressão não-linear I dados no delineamento inteiramente casualizado. Revista de Matemática e Estatística. 2004;22:33–45. [Google Scholar]
- Sai KP, Babu M. Studies on rana tigerina skin collagen. Comp Biochem Physiol Part B: Biochem Mol Biol. 2001;128:81–90. doi: 10.1016/S1096-4959(00)00301-8. [DOI] [PubMed] [Google Scholar]
- Savedboworn W, Kittiphattanabawon P, Benjakul S, Sinthusamran S, Kishimura H. Characteristics of collagen from rohu (Labeo rohita) skin. J Aquat Food Prod Technol. 2017;26:248–257. doi: 10.1080/10498850.2015.1133747. [DOI] [Google Scholar]
- Silva TF, Penna ALB (2012) Colágeno: características químicas e propriedades funcionais. Rev Inst Adolfo Lutz 71:530–539
- Sinthusamran S, Benjakul S, Kishimura H. Comparative study on molecular characteristics of acid soluble collagens from skin and swim bladder of seabass (Lates calcarifer) Food Chem. 2013;138:2435–2441. doi: 10.1016/j.foodchem.2012.11.136. [DOI] [PubMed] [Google Scholar]
- Veeruraj A, Arumugam M, Ajithkumar T, Balasubramanian T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis) Food Hydrocoll. 2015;43:708–716. doi: 10.1016/j.foodhyd.2014.07.025. [DOI] [Google Scholar]
- Wang L, Liang Q, Wang Z, Xu J, Liu Y, Ma H. Preparation and characterization of type I collagens from skin of Amur sturgeon (Acipenser schrenckii) Food Chem. 2014;148:410–414. doi: 10.1016/j.foodchem.2013.10.074. [DOI] [PubMed] [Google Scholar]
- Wang L, Yang B, Wang R, Du X. Extraction of pepsin-soluble collagen from grass carp (Ctenopharyngodon idella) skin using an artificial neural network. Food Chem. 2008;111:683–686. doi: 10.1016/j.foodchem.2008.04.037. [DOI] [Google Scholar]
- Wang L, Yang B, Du X. Extraction of acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. J Food Process Eng. 2009;32:743–751. doi: 10.1111/j.1745-4530.2008.00242.x. [DOI] [Google Scholar]
- Zeng S, Yin J, Yang S, Zhang C, Yang P, Wu W. Structure and characteristics of acid and pepsin-solubilized collagens from the skin of cobia (Rachycentron canadum) Food Chem. 2012;135:1975–1984. doi: 10.1016/j.foodchem.2012.06.086. [DOI] [PubMed] [Google Scholar]
- Zhou C, Li Y, Yu X, Yang H, Ma H, Yagoub AEA, Cheng Y, Hu J, Out PNY. Extraction and characterization of chicken feet soluble collagen. LWT-Food Sci Technol. 2016;74:145–153. doi: 10.1016/j.lwt.2016.07.024. [DOI] [Google Scholar]
- Zou Y, Wang L, Cai P, Li P, Zhang M, Sun Z, Sun C, Xu W, Wang D. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. Int J Biol Macromol. 2017;105:1602–1610. doi: 10.1016/j.ijbiomac.2017.03.011. [DOI] [PubMed] [Google Scholar]