Abstract
The study explores the antioxidant activity, volatile chemical profile and antifungal potential of Pimenta dioica leaf essential oil (EO) against toxin producing Aspergillus flavus. GC–MS analysis of EO revealed the presence of 41 compounds with eugenol (54%), as the major compound followed by myrcene (16.0%) and chavicol (12.5%). It exhibited the strong antioxidant activity with IC50 value of 19.40 µg/ml and polyphenolic content of 526.9 mg g−1 gallic acid equivalent. The aflatoxin producing IISRaf1strain from nutmeg (Myristica fragrans) was identified by 18S rRNA sequencing as Aspergillus flavus (MH345939). MIC of P. dioica leaf EO against A. flavus was found to be at 0.04%. The changes in hyphae growth and architecture after treatment with EO were observed under light microscopy. Antifungal compounds eugenol which got separated at the particular spot caused the clear zone at the TLC plate by agar overlay bioassay. The mode of action of antifungal activity of EO was recorded in terms of its effect on ergosterol content of plasma membrane and malate dehydrogenases activity (MDH) of A. flavus. Thus P. dioica leaf EO might be viable alternative as plant based preservative in perspective on its antioxidant, antifungal activity and efficacy in food system.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04756-0) contains supplementary material, which is available to authorized users.
Keywords: Aflatoxin, Agar overlay bioassay, Eugenol, Ergosterol, Malate dehydrogenase activity
Introduction
Fungi are ubiquitous plant pathogens that are that are principle deterioration operators of foods and feedstuffs. The fungal infection influences crop yield and quality as well as defile sustenances with harmful mycotoxins. The admission of such mycotoxin contaminated foodstuffs by animals and human beings has vast health implications, as these toxins are able to develop disorders in human and animals (Bhat and Vasanthi 2003).
Mycotoxins can be produced either previously or after harvesting the produce and levels may increase during processing and storage. Accordingly prevention of fungal growth and development is an efficient method for managing the mycotoxin contamination. Suppression of fungal growth in plants, vegetables, fresh fruits and spices is essential to reduce the health risk to man and animal. Recently, significant demands from consumers to ease or eradicate the synthetic additives in their food stuffs have lead to a revival of scientific attention in plant based compounds. Also, it is essential to find a simple, non toxic and financially savvy strategy to check fungal infection of food and feed stuffs (Udomkun et al. 2017). In this point of view, plant essential oils (EOs), are getting attraction as herbal additives as a result of their strong antioxidant, antifungal, antibacterial and nematicidal activity (Bakkali et al. 2008).
Allspice (Pimenta dioica) is an exotic plant whose fruits or leaves are used as a spice and its flavour and aroma is a mix of clove, nutmeg and cinnamon and hence the name “allspice”. It is an evergreen tree with moderate canopy belonging to the family Myrtaceae (Rao et al. 2012). Although there are scattered information available on its phytochemicals constituents and its antifungal activity, many more aspects are yet to be studied (Bhat and Vasanthi 2003; Miyajima et al. 2004). Since the tree is commonly used in traditional drug as antiseptic and antimicrobial, it has been selected for scientific validation in the present study. Hence the present study was designed to assess the chemical and biochemical mechanism of action of P. dioica leaf EO on germination, growth and morphology of aflatoxin producing A. flavus.
Materials and methods
Test plant
The P. dioica leaves were collected from a fully grown tree (7 year old) in a farm at ICAR-Indian Institute of Spices Research (11.29395°N, 75.82038°E), Kozhikode, India located at 50 m above MSL. Leaves were washed in a running tap water, dried in shade, powdered using mixer grinder, and stored in sealed container at 4 °C until used.
Extraction of P. dioica leaf EO
The essential oil was extracted by hydro-distillation using modified clevenger apparatus (ASTA 1997). Forty gram of powdered leaves were mixed with 400–500 ml of water and distilled for 4 h at 100 °C. At the end, essential oil was collected and residual water particles were eliminated by addition of anhydrous sodium sulphate and stored at 4 °C till use.
Chemical profile of P. dioica leaf EO
Qualitative profiling of volatile constituents in essential oil was carried out using a Shimadzu GC–MS 2010 (Shimadzu corporation, Kyoto, Japan) apparatus attached to a mass selective detector fitted with a Rtx-wax column (30 m × 0.25 mm × 0.25 µm). The column temperature were as follows: 60 °C, for 5 min, then gradient 5 °C min−1 to 110 °C, then gradient 3 °C min−1 to 200 °C then 5 °C min−1 to 240 °C and hold 1 min at this temperature. Temperature of the interface was 250 °C, ion source 220 °C. Other operational parameters are as follows: Injection volume − 0.1 µl; Injection mode—split (1:160 split ratio); Acquisition mode—scan; Helium flow rate — 1.0 ml/min; Scan range, 40–650 m/z with the scan speed of 1250. The composition of EO sample was detected by the comparison of their mass spectra with those of NIST/WILEY Mass Spectral Library (Adams 2007).
Determination of total phenolic contents in P. dioica leaf EO
Phenolic constitutents were quantified using Folin–Ciocalteu (FC) reagent following Gholivind et al. (2010). Methanolic solution of EO at varying concentrations and FC reagent (1 ml) and 1 ml of 20% Na2CO3 were added into the reaction mixture. Blank was concomitantly prepared comprised of 1 ml methanol, 1 ml Folin–Ciocalteu’s reagent and 1 ml of 20% Na2CO3. The reaction mixtures were kept in a water bath at 45 °C for 30 s. The absorbance was analysed in a spectrophotometer (Shimadzu-UV1800, Shimadzu corporation, Kyoto, Japan) at 650 nm. For each analysis triplicate samples were prepared. Total phenolic content of EO was calculated by comparing with standard curve equation obtained and was expressed as mg gallic acid equivalents g−1 of the oil.
Antioxidant activity P. dioica leaf EO
The ability of the P. dioica leaf EO to scavenge DPPH (2, 2-Diphenyl-picrylhydrazil) free radicals was assessed according to Blois (2002). Methanol diluted EO of each concentration (1 ml each) were mixed with methanolic solution of DPPH (1 ml). The contents were mixed and incubated at room temperature for 30 min in dark condition. After incubation, the absorbance of the solution was recorded at 517 nm against methanol blank. Control tube contained all reagents apart from the EO. The DPPH radical scavenging activity was estimated from the % inhibition versus concentration plot, using a non –linear regression algorithm and expressed as IC50 value
whereas Ab = absorption of blank sample; Aa = absorption of tested EO.
Selection of Aspergillus strain
Aspergillus strain (IISRaf1) was obtained from market a sample of nutmeg collected through the survey was used as a test strain for all in vitro studies. It was cultured using potato dextrose agar (PDA) media and incubated at 28 ± 2 °C for 7 days. Identification of fungal isolate was achieved morphologically based on their microscopic and macroscopic features according to Samson et al. (2007).
Detecting the ability of Aspergillus isolate for aflatoxin production
Qualitative method
To test the ability of Aspergillus strain (IISRaf1) for aflatoxin production, Aspergillus differentiation medium (ADM) containing chloramphenicol was used (Fakruddin et al. 2015). ADM were inoculated with isolated Aspergillus strain (IISRaf1) and the culture plates were incubated at 28 °C for 5 days. Development of orange coloration in reverse side of the plates were indication of aflatoxin production.
Quantitative method
Single spore culture of Aspergillus strain (IISRaf1) were grown on PDA media and the spores were obtained from the agar plates surface with sterile distilled water. The spore concentration was determined with the help of haemocytometer and was in tune to 106 conidia/ml as a final concentration. This spores was used for inoculation to nutmeg mace samples and uninoculated sample was maintained as control. After 7 days incubation, the sample was dried using hot air oven at 45 °C for 48 h and powdered using mixer grinder. Twenty five grams of powdered samples were transferred into 250 ml conical flask. To this, 5 g of sodium chloride and 100 ml of aflatoxin extraction solution (containing 70% methanol) was added. After mixing, the flasks were kept aside for 5 min to settle down the suspended particles. The contents were filtered through a fluted filter paper (Whatman, grade 4 V) into a beaker. From this, 15 ml of the filtrate was transferred to a 50 ml graduated measuring cylinder and 30 ml of distilled water was added. Aflatoxins in reaction mixture were extracted with 50 ml of methanol: water (8:2, v/v) by ultrasonication after 5 days of incubation. The contents were filtered and collected by AflaTest column. The methanol elute from the column was evaporated under a nitrogen stream and quantified using a Waters Acquity UPLC H-Class system attached with auto sampler, quaternary solvent delivery pump, and fluorescence detector, connected to Waters Empower data software. The mobile phase consisted of methanol/water (50/50, v/v) at a flow rate of 0.2 ml/min at the determination wavelength of kex 360 nm and kem 440 nm (AOAC 2005). The aflatoxin production by Aspergillus strain was determined in comparison with the amount of aflatoxin in unincoulated control samples.
Based on the aflatoxin production ability, isolate was selected and used for further molecular identification and antifungal studies.
Molecular identification
DNA Isolation
The mycelial mass of Aspergillus strain (IISRaf1) was harvested from 48 h potato dextrose broth by filtering the content by sterile filter paper (Whatman No. 2). Fungal mycelia 0.1 g was added to an eppendorf tube. To this tube, 750 µl of extraction buffer, 50 mg PVP and 20 mg glass powder was added and grounded using a micro pestle. The reaction mixture was vortexed and centrifuged for 5 min at 13,000 rpm. After centrifugation, the supernatant was taken in a sterile eppendorf tube and an equal volume of phenol: chloroform: isoamylalcohol (25:24:1) was added and inverted gently for 2 min and centrifuged for 5 min at 13,000 rpm at 4 °C. The supernatant was taken with equal volumes of chloroforms isoamylalcohol (24:1). The contents were gently mixed for 5 min and centrifuged again for 5 min at 13,000 rpm at 4 °C. The aqueous layers were transferred to a sterile eppendorf tube and equal volume of ice-cold ethanol was added, centrifuged for 10 min at 1300 rpm at 4 °C. 70% ethanol were used for washing the pellets at 13,000 rpm for 2 min at 4 °C. The pellets were air dried for 15 min and minimum volume of sterile double distilled water was resuspended. 5 µl of RNAase (10 mg/ml) was added and incubated at 37 °C for overnight at 4 °C (Cooke and Duncan 1997).
PCR of internal transcribed spacer (ITS) regions
The PCR assay was carried out in a total reaction volume of 20 µl consisting of template DNA 2 µl, Taq PCR reaction mix 10 µl (0.2 mM dNTPs, 1.5 units of Taq DNA polymerase, 1.5 mM MgCl2, 10 mM Tris–HCl and 50 mM KCl, 2 µl of the DNA template (100 ng), 2 µl of each primers [forward primer: (ITS-1)5′-TCC GTA GGT GAA CCT GCG G-3′, reverse primer (ITS-4) 5′-TCC TCC GCT TAT TGAT TAT GC-3′] (Sigma, Aldrich) and 2 µl distilled water. PCR amplification was performed in a thermocycler (Invitrogen Proflex PCR system) consisted of initial denaturation at 92 °C for 4 min followed by 35 cycles consisting of denaturation at 92 °C for 40 s, annealing at 55 °C for 90 s, and elongation at 72 °C for 2 min with final elongation at 72 °C for 5 min following the last cycle. The reaction was completed with incubation for 10 min at 72 °C (White et al. 1990). The amplicons were separated in agarose gel (1%) and stained with ethidium bromide were visualized under UV light (Sambrook and Russell 2001).
Sequence analysis and phylogenetic tree construction
For the Aspergillus strain (IISRaf1) identification, 18 s rDNA sequences were compared with already available in the data bank of the National Center for Biotecnology and Information (NCBI), using BLAST search tool (Altschul et al. 1997). The species identification was done based on the best score. The sequence of Aspergillus strain (IISRaf1) along with already reported gene sequences of A. flavus from the GenBank were aligned using MUSCLE included in MEGA 6.0 for phylogenetic tree construction.
Antifungal activity measurements
Poisoned food technique
The inhibitory effect of the P. dioica leaf EO was assessed on the test fungi by the poisoned food technique of Mohana and Raveesha (2007). Melted potato dextrose agar media with required quantity of EO was added to provide preferred concentrations of 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09 and 1.0%. For even distribution of the oil in the growth medium, 0.05% of Tween-20 was added. For control, same amount of distilled water instead of EO and 0.05% Tween-20 was added with the medium. After that, a fungal disc (5 mm dia) of Aspergillus strain (IISRaf1) was placed in the middle of the media plate and were incubated at 28 ± 1 °C. Percent inhibition of the test fungi was recorded for 7 days and was calculated after comparison with the control. Fungi toxicity was expressed as percentage inhibition of mycelia (Naz et al. 2006).
where dc = average diameter of fungal colony in control and dt = average diameter of fungal colony in treatment.
Determination of mycelial weight
Different concentrations of EO (0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, and 0.08%) in potato dextrose broth (PDB) were prepared and inoculated with 106 spores/ml of Aspergillus strain (IISRaf1). EO was replaced with sterile distilled water in the control samples. After 7 days of incubation, dry weight of mycelium was computed. Mycelia was filtered through Whatman No. 1 filter paper and washed with sterile distilled water. Then it was incubated to dry at 60 °C for 6 h and then at 40 °C over night in hot air oven. The filter paper containing dry mycelia was weighted. Growth inhibition percentage on the dry weight basis was calculated as
Spore germination assay
Eight different concentrations of EO (0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07 and 0.08%) were tried for spore germination of Aspergillus strain (IISRaf1). Spores collected from 10-day old fungal cultures was placed on 2% sucrose solution over glass slides and incubated in a moist chamber for 24 h at 25 ± 2 °C. Each slide was then fixed in lactophenol cotton blue and observed under 20 × magnification of using the light microscope (Leica DM 5000B) for spore germination. Number of germinated spores was scored using haemocytometer to calculated the percentage of spore germination (Slawecki et al. 2002).
Effect of P. dioica leaf EO volatiles on Aspergillus strain (IISRaf1)
The agar-free lid of each Petri dish was placed upside down and a paper disc (8 mm, whatman) treated with P. dioica leaf essential oils different concentrations (1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9% and 10%) dissolved in ethanol (20 µl) were placed on the lid. Aspergillus strain (IISRaf1) mycelial disc (8 mm dia) was placed on PDA media at the centre of the Petri plate. For control, single colony of IISRaf1 alone was placed on the centre of PDA media and sealed. After evaporating ethanol, Petri dishes were sealed with parafilm to prevent EOs and their compounds from leaking, and the plates were incubated at 28 °C in the dark for 4 days (Sindhu et al. 2011).
Light microscopy
Aspergillus strain (IISRaf1) mycelium was collected after 4 days of incubation from the margin of the colony grown on PDA with 0 and 0.03% concentration of EO. The samples were examined under the microscope attached camera (Leica DM 5000B) at 40 × to examine morphological abnormalities. Samples without EO (control) were also recorded.
Detection of antifungal compound
Separation of essential oil in TLC plate and detection of its anti-fungal activity with agar overlay bioassay
The leaf EO of P. dioica, which showed significant antifungal activity was subjected to TLC separation of its components. Various combinations of the polar and non-polar solvents were tried to determine the best mobile phase solvent system to achieve a clear separation of the bands. The Retention factor (Rf) values of the separated bands were calculated by using the formula:
P. dioica leaf EO diluted with ethanol at 10% concentration and their components were partially separated in TLC. This TLC plate was used for agar overlay bioassay. Two sets of TLC plates were prepared for the run. One of them was used to visualize the separated compounds using anisaldehyde reagent. The other TLC plate was placed in a Petriplate and covered with PDA medium containing 106 spores/ml of Aspergillus strain (IISRaf1). To facilitate the good diffusion of the tested compounds into the agar medium, the plates were incubated at 32 °C for 72 h. After the incubation tetrazolium dye (0.1%) was sprayed over the plates for detecting the living cells and incubated overnight (Dewanjee et al. 2015).
Mode of action of P. dioica leaf EO against Aspergillus strain (IISRaf1)
Determination of ergosterol content in plasma membrane
Ergosterol content in the plasma membrane of Aspergillus strain (IISRaf1) was determined by the method of Tian et al. (2012). Spore suspension (106 conidia ml−1) of IISRaf1was inoculated in PDA medium with 0, 0.01, 0.02, 0.03% and 0.04% of EO at 28 °C for 5 days. After sufficient growth mycelia were collected by filtration and washed twice with sterile distilled water. To each mycelial sample, 5 ml of 25% alcoholic potassium hydroxide solution was added and vortexed for 5 min and then incubated at 85 °C for 4 h. Sterols were extracted from each sample by adding mixture of 2 ml sterile distilled water and 5 ml n-heptane and vortexed for 2 min. The n-heptane layer was obtained and scanned between 230 and 300 nm by UV-1800. The presence of ergosterol (at 282 nm) and dehydroergosterol, the intermediate of ergosterol, (at 230 and 282 nm) in the n-heptane layer led to a characteristic absorbance curve. Samples without EO treatment were treated as controls. The ergosterol content was calculated as follows:
where 290 and 518 are the E values (in percentages per cm) determined for crystalline ergosterol and dehydroergosterol, respectively. E value means the absorbance of samples (a concentration of 1 mol L−1) at a certain wavelength via an optical path of 1 cm.
Determination of malate dehydrogenases (MDH) activity
The MDH activity in EO treated Aspergillus strain (IISRaf1) were detected using Shonk and Boxer (1964) method with minor modifications. The assay recorded the decrease in absorbance of oxidation of NADH to NAD by the enzyme at 340 nm. The reaction mixture comprised of 2.7 ml of 100 mM phosphate buffer with pH 7.5, 0.2% of NADH and 50 µl of culture homogenate. The contents were mixed well and incubated at room temperature for 20 min. The reaction was started by adding 0.1 ml oxaloacetate (1 mg ml−1). The decrease in the absorbance was measured for 3 min at 340 nm in a UV–VIS spectrophotometer (Shimadzu corporation, Kyoto, Japan) and specific activity of the enzyme is expressed as µmoles of NADH consumed/min/mg of protein. The protein concentration of homogenized fungal culture was analysed by the protocol of Lowry et al (1951).
Data analysis
Data were analysed using Microsoft Excel for Windows 2007 add-ins with XLSTAT Version 2010.5.05 (XLSTAT 2010). All the treatments were replicated three times. Significant differences between the treatments were determined using analysis of variance (ANOVA) with 5% level of significance.
Results and discussion
P. dioica leaf EO and its components
Plant EOs are excellent sources for the management of Aspergillus species because of its bioactive compounds with antifungal, nematicidal, and insecticidal traits (Park et al. 2006; Lee et al. 2008). Furthermore, EOs has low-residue concern and extremely volatile, when they are used on grain or stored food stuffs.
In the present study, P. dioica leaf EO was yielded with 1.8% by hydro-distillation method. GC–MS analysis revealed the major components of the EO were eugenol (54.0%), myrcene (16.0%), chavicol (12.5%), limonene (4.6%), 1-octen-3-ol (2.7%), linalool (1%), terpinen-4-ol (0.7%) and β-phellendrane (0.7%) (Table 1). The main constituents identified in our anlaysis were very similar to previously reported results (Toni-Moy et al. 2016). Similarly, Zabka et al. (2009) analyzed allspice EO by GC–MS and found eugenol, β-caryophyllene and methyl eugenol as major constituents. However the chemical constituents of EOs varies widely with production circumstances, such as date of harvesting, storage time, as well as soil and climate factors (Galambosi and Peura 1996). The variation in the constituents of our analyzed results could be attributed to these factors.
Table 1.
GC–MS profile of P. dioica leaf EO
| SN | Compound | Retention time | % |
|---|---|---|---|
| 1 | Myrcene | 5.90 | 15.96 |
| 2 | Limonene | 6.77 | 4.62 |
| 3 | β-Phellendrene | 6.99 | 0.71 |
| 4 | 1-octen-3-ol | 14.23 | 2.69 |
| 5 | Linalool | 16.88 | 1.0 |
| 6 | β-Cadinene | 22.92 | 1.5 |
| 7 | Cinnamaldehyde | 30.86 | 0.77 |
| 8 | Terpinen-4-ol | 18.28 | 0.69 |
| 9 | Eugenol | 35.62 | 54.04 |
| 10 | Chavicol | 40.44 | 12.54 |
| 11 | Other minor compounds | – | 6.8 |
Total phenolic content of essential oil was found to be 526.9 ± 41.4 mg gallic acid equivalent (GAE) g−1. Stored food products are spoiled by the oxidation which leads to nutritional decrement, rancidity and development of toxic compounds (Hsieh and Kinsella 1989). Oxidative stress stimulates A. flavus to produce more aflatoxin (AFB1) during storage (Prakash et al. 2015) which leads to reduce their shelf-life and quantitative as well as qualitative loss of food stuffs. Compared to the total phenolic contents of some spices (de Soysa et al. 2016) in dry basis, such as clove (310.4 mg GAE g−1), cinnamon (67.5 mg GAE g−1), cardamom (3.5 mg GAE g−1), nutmeg mace (28.4 mg GAE g−1), and nutmeg seed (34.8 mg GAE g−1) a higher level was found in the analyzed sample. Our results recorded the high radical scavenging activity with IC50 value of 19.40 µg ml−1 which is higher than earlier reported other EOs (Mohamed et al. 2007).
Confirmation of aflatoxin production by IISRaf1
A strain of high aflatoxin producing Aspergillus sp (IISRaf1) was randomly selected according to the orange colour pigmentation in differentiation medium. Ability of aflatoxins production by this strain in nutmeg mace samples was quantified using HPLC. HPLC analysis showed that aflatoxin B1 content in inoculated and control (uninoculated) samples were 3.4 ± 0.26 µg kg−1 and < 0.5 ± 0.03 µg kg−1, respectively. From our results, aflatoxin production potential of Aspergillus strain IISRaf1 in nutmeg mace samples was proved.
Molecular identification of Aspergillus strain (IISRaf1) and phylogenetic tree construction
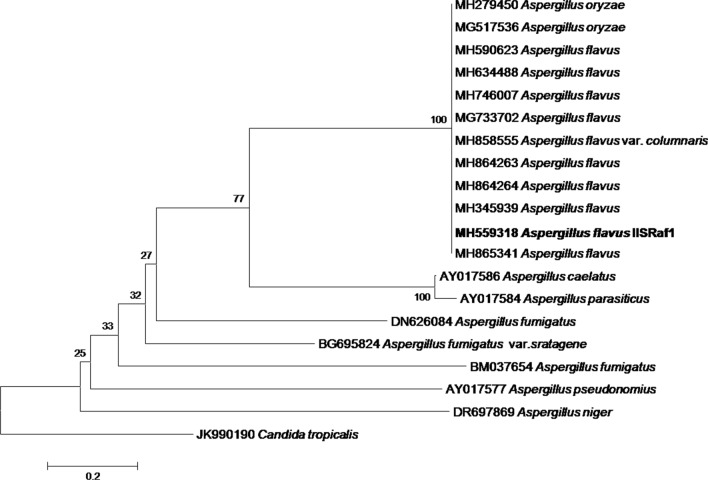
The amplicons of ITS1-5.8S-ITS2 region of rDNA produced approximately 600 bp, using the universal primers ITS1 and ITS4. Similarly, amplicons sizes varying between 565 and 613 bp reported by Henry et al. (2000). The sequence results of IISRaf1 was submitted in GenBank and accession number was MH559318. Neighbour-joining tree was constructed based on partial 18S rRNA nucleotide sequences of Aspergillus flavus (Fig. 1). Based on the phylogenetic tree, it was found to be the close relationship among IISRaf1 and other reported aflatoxin producing A. flavus in the data base.
Fig. 1.
Neighbour-joining tree analysis of partial 18S rRNA sequences of Aspergillus flavus (IISRaf1 MH559318) showing similarity between other Aspergillus strains
Inhibitory effect of P. dioica leaf EO on A. flavus (IISRaf1)
Results of growth inhibition of A. flavus (IISRaf1) by P. dioica leaf EO in agar medium are presented in (Fig. 2). After the 7 days of incubation, development of fungi was completely inhibited by 0.04% EO. Radial growth and biomass of A. flavus (IISRaf1) was drastically reduced in response to different concentrations of P. dioica leaf EO ranging from 0.02 to 0.03% (Fig. 2a). Insignificant mycelial dry weight was recorded at 0.02% concentration (Fig. 2b). The EO at 0.04% concentration able to inhibit the 100% of spore germination (Fig. 2b) and this range is fungicidal for A. flavus (IISRaf1) (Bhat and Raveesha 2016). EO is hydrophobic, which alters integrity of cell membrane and leads to the loss of cytoplasmic components. The vaporization experiment of the P. dioica leaf EO on A. flavus evidently shows the antifungal property of the compound. The majority of studies on EO were carried out in liquid phase. However it is more suitable to use the oils in their gaseous phase for post-harvest usage and transport.
Fig. 2.

Effect of various concentrations of P. dioica leaf EO on a) percent radial growth inhibition, b) dry weight (mg) and spore germination (%) (after 7 days) of A. flavus (IISRaf1) at 28 ± 1 ˚C. Data are presented as mean ± SE (n = 3)
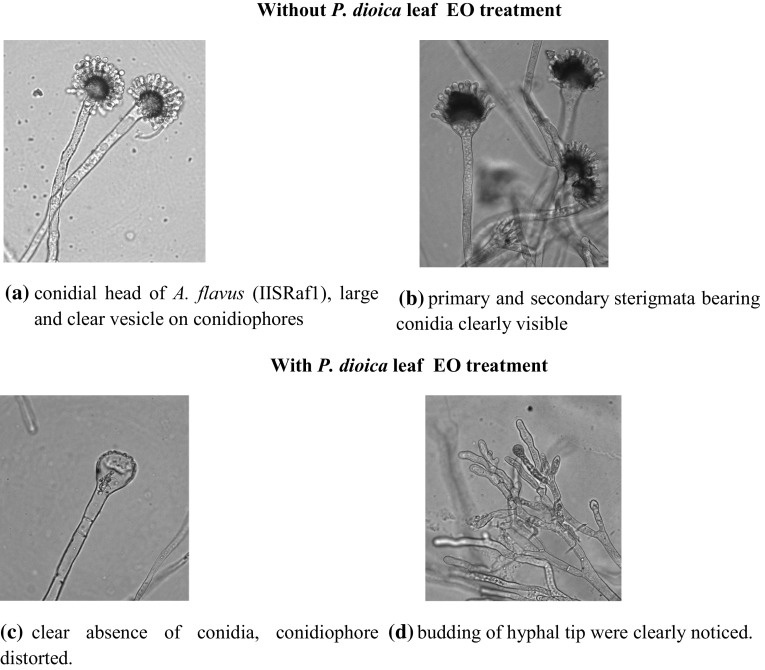
In addition to growth inhibition, distinctive morphological changes were noticed in the presence of essential oil when compared to untreated control. It includes visible loss of pigmentation, lack of sporulation and irregular maturity of conidiophores. Whereas untreated mycelium had clear, homogenous cytoplasm, and profuse conidiation on conidial heads were large and sterigmata bearing conidia were clearly noticed under microscopic examination (Fig. 3). Based on the present study, essential oil from P. dioica leaf possesses fungicidal properties for suppressing the growth of A. flavus (IISRaf1) which results of irrevocable morphological changes. Antifungal activity of EOs of 25 medicinal plants, including that of P. dioica, against two species each of Fusarium, Penicillium and Aspergillus were assessed by Zabka et al. (2009). The occurrence of the EO in the growth medium might have induced noticeable changes in the morphology of the hyphae. Such alteration due to interference of cell wall synthesis by EO, which influences fungal growth and morphogenesis.
Fig. 3.
Microphotographs of A. flavus (IISRaf1) mycelium grown on PDA without or with P. dioica leaf EO
Detection of antifungal compound
The TLC separation of P. dioica leaf EO showed the presence of seven bands when eluted with solvent system comprising of toluene and ethyl acetate in the ratio 9.3:0.7. The Rf values of the bands were calculated, and they were as follows: 0.15, 0.32, 0.43, 0.52, 0.70, 0.76 and 0.80 (Fig. 4). Appearance of a clear colourless inhibition zone against pink backgrounds around the place where eugenol was separated in Agar overlay assay as shown in Fig. 4. Separation and localization of the bioactive compounds by TLC and bioautography were again proved presence of antifungal compounds. However, further separation would be needed to characterize the compounds and assess their potency in suppressing the growth of the test fungus. Moreover, Bhat and Raveesha et al. (2016) mentioned the bioautography of the petroleum ether extract of P. dioica showed small clear zones around the TLC spots at Rf 0.52 and 0.59 against Candida albicans, Microsporum canis and M. gypseum.
Fig. 4.
Thin layer chromatography with agar overlay assay of the EO of P. dioica leaf against A. flavus (IISRaf1)
Action of P. dioica leaf EO on ergosterol production and MDH activity
The ergosterol content of A. flavus in response to allspice leaf EO treatment was recorded to be 96.7 ± 8.7%, 72.5 ± 6.9%, 43.6 ± 6.1%, 22.7 ± 4.2% at control, 0.01%, 0.02%, 0.03% respectively (Fig. 5a). MDH are vital enzyme for energy metabolism of A. flavus, which is the essential metabolic enzyme in tricarboxylic acid (TCA) cycle. The efficacy of P. dioica leaf EO on MDH were shown in Fig. 5b. Each concentrations of P. dioica leaf EO could inhibit the MDH activity with a dose-dependent mode. Plasma membrane is an important organelle and ergosterol’s presence in the plasma membrane of the fungus is pivotal for its growth and development. Our results suggest that anti-fungal activity of P. dioica leaf EO could be attributed to reduction in the synthesis of ergosterol or its degradation by EO constituents (Tian et al. 2012). Nevertheless, further investigations are necessary to indentify the correct mode of action of EO. Since EOs are composed of highly lipophilic, low-molecular weight components which could easily pass through fungal cell wall membranes to interrupt the cell integrity mainly with the mitochondrial membrane (Tian et al. 2011). Our finding showed that P. dioica leaf EO could inhibit the mitochondrial dehydrogenases, as a result that regular metabolic function of A. flavus could be interrupted.
Fig. 5.

The content of ergosterol (a) and Malate dehydrogenase (MDH) activity (b) in A. flavus (IISRaf1) after treated with different concentrations of P. dioica leaf EO. Data are presented as mean ± SE (n = 3)
Conclusion
Based on the in vitro studies, antioxidant, antifungal efficacy of P. dioica leaf EO was observed on A. flavus (IISRaf1). These studies showed that this EO may be applied as a food additive because its suppressive action on fungus and its ability to inhibit the ergosterol production would enhance the storage life of post harvest products. Phenol rich chemical profile is the key reason for the significant antioxidant activity allspice leaf EO. Further studies are needed to find out the P. dioica leaf EO cost–benefit ratio, determine the toxicity and development of oil components into environmentally viable bioprotectants.
Conflict of interest
Authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors wish to thank Dr. R. Suseela Bhai and Dr. P. Umadevi for technical support in fungal characterization.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. 4. Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Spice Trade Association (ASTA) Official analytical methods. New York: American Spice Trade Association; 1997. p. 53. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) (2005) Official method of analysis. 18th edn. Association of Officiating Analytical Chemists, Washington DC (Method 935.14 and 992.24)
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bhat A, Raveesha KA. Antifungal activity of Pimenta dioica (L.) Merril an aromatic medicinal tree. Int J Pharm Pharm Sci. 2016;8:92–95. doi: 10.22159/ijpps.2016v8i10.9924. [DOI] [Google Scholar]
- Bhat RV, Vasanthi S. (2003). Mycotoxin food safety risk in developing countries, 2020 vision Briefs 10 No. 3. International Food Policy Research Institute (IFPRI), Washington, DC
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 2002;26:1199–1200. [Google Scholar]
- Cooke DEL, Duncan JM. Phylogenetics analysis of Phytophthora species based on ITS1 and ITS2 sequences of the ribosomal RNA gene repeat. Mycol Res. 1997;101:667–677. doi: 10.1017/S0953756296003218. [DOI] [Google Scholar]
- Dewanjee S, Gangopadhyay M, Bhattacharya N, Khanra RD, Tarun K. Bioautography and its scope in the field of natural product chemistry. J Pharm Anal. 2015;5(2):75–84. doi: 10.1016/j.jpha.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Soysa EJS, Abeysinghe DC, Dharmadasa RM. Comparison of phytochemicals antioxidant activity and essential oil content of Pimenta dioica (L.) Merr. (Myrtaceae) with four selected spice crop species. World J Agric Res. 2016;4:158–161. [Google Scholar]
- Fakruddin M, Chowdhury A, Hossain MN, Ahmed MM. Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. Springer Plus. 2015;4:159. doi: 10.1186/s40064-015-0947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambosi B, Peura P. Agrobotanical features and oil content of wild and cultivated forms of caraway (Carum carvi L.) JEOR. 1996;8:389–397. [Google Scholar]
- Gholivind MB, Rahimi-Nasrabadi M, Batooli H, Ebrahimabadi AH. Chemical composition and antioxidant activities of the essential oil and methanol extracts of Psammogeton canescens. Food Chem Toxicol. 2010;48:24–28. doi: 10.1016/j.fct.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Henry T, Iwen PC, Hinrichs SH. Identification of Aspergillus species using Internal Transcribed Spacer (ITS) regions 1 and 2. J Clin Microbiol. 2000;38:1510–1515. doi: 10.1128/JCM.38.4.1510-1515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh RJ, Kinsella JE. Oxidation of polyunsaturated fatty acids: Mechanisms, products, and inhibition with emphasis on fish. Adv Food Nutr Res. 1989;33:233–341. doi: 10.1016/S1043-4526(08)60129-1. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim J, Shin SC, Lee SG, Park IK. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Fragr J. 2008;23:23–28. doi: 10.1002/ffj.1850. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Marzouk A, Fatma A, Mona A, Amira MG, Elsayed AA. Anticancer and antioxidant tannins from Pimenta dioica leaves. Zeitschrift für Naturforschung. 2007;62:526–536. doi: 10.1515/znc-2007-7-811. [DOI] [PubMed] [Google Scholar]
- Mohana DC, Raveesha KA. Anti-fungal evaluation of some plant extracts against some plant pathogenic field and storage fungi. IJAT. 2007;4(1):119–137. [Google Scholar]
- Miyajima Y, Kikuzaki H, Hisamoto M, Nikatani N. Antioxidative polyphenols from berries of Pimenta dioica. BioFactors. 2004;21:301–303. doi: 10.1002/biof.5520220159. [DOI] [PubMed] [Google Scholar]
- Naz F, Rauf CA, Haque IU, Ahmad I. Management of Rhizoctonia solani with plant diffusates and chemicals. Pak J Phytopathol. 2006;18(1):36–43. [Google Scholar]
- Park K, Choi KS, Kim DH, Choi IH, Kim LS, Bak WC, Choi JW, Shin SC. Fumigant activity of plant essential oils and components from horse radish, anise and garlic oils against Lycoriella ingenua (Diptera: Sciaridae) Pest Manag Sci. 2006;62:723–728. doi: 10.1002/ps.1228. [DOI] [PubMed] [Google Scholar]
- Prakash B, Kedia A, Mishra PK, Dubey NK. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities-potentials and challenges. Food Control. 2015;47:381–391. doi: 10.1016/j.foodcont.2014.07.023. [DOI] [Google Scholar]
- Rao PS, Navinchandr S, Jayaveera KN. An important spice, Pimenta dioica (Linn.) Merill: a review. ICPJ. 2012;1(8):221–225. [Google Scholar]
- Sambrook J, Russell D. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Samson RA, Noonim P, Meijer M, Houbraken J, Frisvad JC, Varga J. Diagnostic tools to identify black Aspergilli. Stud Mycol. 2007;59:129–145. doi: 10.3114/sim.2007.59.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonk CE, Boxer GE. Enzyme patterns in human tissues. Methods for the determination of glycolytic enzymes. Cancer Res. 1964;24:709. [PubMed] [Google Scholar]
- Sindhu S, Chempakam B, Leela NK, Suseela Bhai R. Chemoprevention by essential oil of turmeric leaves (Curcuma longa L.) on the growth of Aspergillus flavus and aflatoxin production. Food Chem Toxicol. 2011;49:1188–1192. doi: 10.1016/j.fct.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Slawecki RA, Ryan EP, Young DH. Novel fungitoxicity assays for inhibition of germination-associated adhesion of Botrytis cinerea and Puccinia recondita spores. Appl Environ Microbiol. 2002;68(2):597–601. doi: 10.1128/AEM.68.2.597-601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Ban X, Zeng H, He J, Huang B, Youwei W. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int J Food Microbiol. 2011;145:464–470. doi: 10.1016/j.ijfoodmicro.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Tian J, Huang B, Luo X, Zeng H, Ban X, He J. The control of Aspergillus flavus with Cinnamomum jensenianum essential oil and its potential use as a food preservative. Food Chem. 2012;130:520–527. doi: 10.1016/j.foodchem.2011.07.061. [DOI] [Google Scholar]
- Toni-Moy AS, Henry ICL, Watson CT. Quantification and characterization of Pimenta dioica (Allspice) essential oil extracted via hydrodistillation, solvent and super critical fluid extraction methodologies. Am J Essent Oil. 2016;4(3):27–30. [Google Scholar]
- Udomkun P, Nimo WA, Nagle M, Müller J, Bernard V, Bandyopadhyay R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application: a review. Food Control. 2017;76:127–138. doi: 10.1016/j.foodcont.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Burns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press Inc; 1990. pp. 315–322. [Google Scholar]
- Zabka M, Pavela R, Slezakova L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxicogenic fungi. Ind Crops Prod. 2009;30:250–253. doi: 10.1016/j.indcrop.2009.04.002. [DOI] [Google Scholar]
- XLSTAT. (2010). Addinsoft SARL, Paris. Available at https://www.xlstat.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.