Abstract
Polysaccharides a large chain of simple sugars covalently linked by glycosidic bonds which are obtained from living organisms and microbes commercially used in food and pharmaceutical industries. Marine macroalgae or seaweed is an unexploited natural source of polysaccharides, which contains many variant phytonutrients whose cells are enriched with sulfated polysaccharides which have been progressively read these days for their potential value in food and pharmaceutical applications. This review aims the exploration of these polysaccharides in food applications, with a focus on its types and biological properties in the view of food application.
Keywords: Sulfated polysaccharide, Macroalgae, Seaweed, Food packaging, Nutraceutical, Hydrocolloid
Introduction
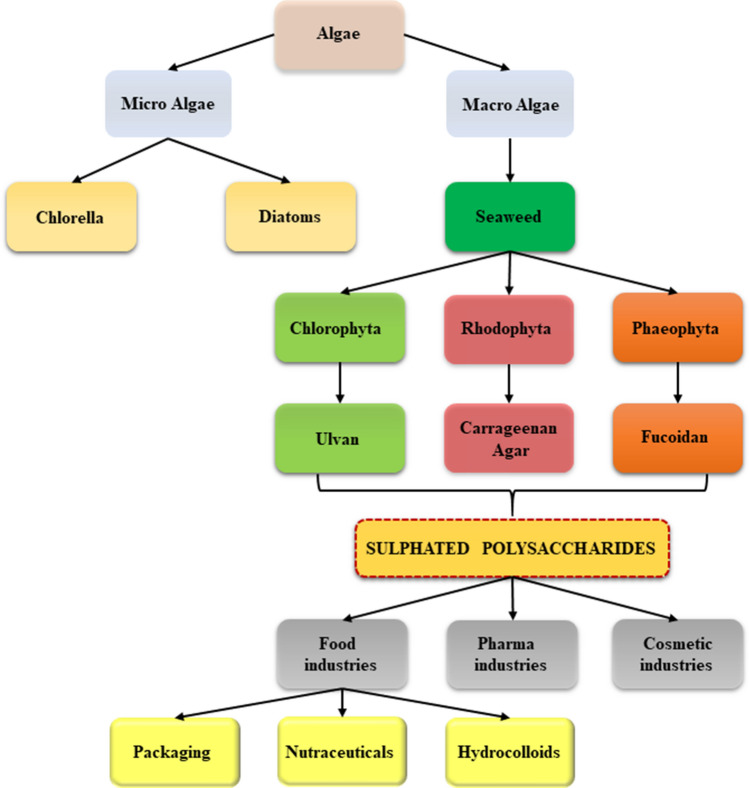
Our earth is occupied with 70% of water bodies containing diversified marine species such as planktons, nektons, benthos. They cover almost one half of the total existing global diversity. Since marine carbohydrates are loaded with many human health benefits, their usage is rising among people (Dittami et al. 2017). Aquatic plant-like macro algae called seaweeds are abundant origins of sulfate-containing poly sugars having commercial uses in food, cosmetic, pharma industries. They have peculiar tissue arrangements in their structure commonly lives in seawater as well as brackish water up to watermark level with air exposure. They exist either in free-floating or attached over the rock forms more than in lands or sands present near the littoral zone. Whereas some species grow quite below the water surface with no air and light exposure. Mostly, people consume seaweed as their regular food. In addition to that, they are considered as bio-resource having utilization in food (Fleurence et al. 2018), manure (Kiraci 2018), biorefineries (Balina et al. 2017), cosmetics (Nurjanah et al. 2016), medicine (Dillehay et al. 2008), biopolymer (Kadam et al. 2015), bioremediation (He et al. 2008), weather forecast (Díez et al. 2012), pollution controller (Ahmady-Asbchin et al. 2009) industries due to its non-toxic, edible, cheap and easy culturing properties. They contain specific macromolecule polysaccharides called sulfated polysaccharides (SP) comprising sulfate moieties in their structural carbohydrate backbone. They play a significant role in certain physiological processes in human health (De Jesus Raposo et al. 2013). Based on pigments that help in their photosynthesis process, marine algae are classified into three genres such as red, brown, and green, which are referred to as Rhodophyceae, Phaeophyceae, and Chlorophyceae, respectively (Pangestuti and Kim 2011). Some of the SP are fucoidans from brown algae, carrageenan, agar from red algae, and ulvans in green algae (Pereira 2018). Packaging plays a crucial role as a protective layer with a safety concern for the packed product. The food packaging material must have defense potentials such as enhanced anti-microbial and barrier properties for shielding the packed food materials from external factors like food-borne pathogens, storage, and transport environment as well as maintaining its quality and safety. Toxic-free materials are preferred to prevent their migration into food and avoiding health issues after consuming them. Biodegradable packaging is highly appreciated nowadays to reduce the usage of plastics and pollution. SP is a type of biopolymer preferred in bio food packaging these days including edible film production. They can be a potential blend or total replacement of synthetic polymers (Cazón et al. 2017). Besides, seaweeds are an excellent dietary fiber source with biochemical and nutritional proteins. Among varieties of seaweeds, SP from brown and red-pigmented seaweeds own high nutritional content which may replace the man-made nutritional supplements due to their sulfur contents aiding bioactivity and their unique modified anatomical structure among other sources of polysaccharides (Holdt and Kraan 2011). SP has an excellent hydro colloidal property, by creating viscous liquid while dissolving in water. Agar, carrageenan is some of the widely used commercial SP in the food processing industries owning excellent hydro colloidal properties. They are mostly heat reversible, forms hydrogels by themselves, and act as stabilizers, emulsifiers, etc. Agar and carrageenan aid the easy processing of gel-based food products like desserts, jams, jellies, bakery products, etc. (Bixler and Porse 2011). The brief description of SP and its commercial applications in food industries are discussed in the following article as well as in Fig. 1.
Fig. 1.
Overall schematic representation of the origin of sulfated polysaccharides from seaweeds and its applications in food industries
Sulfated polysaccharides
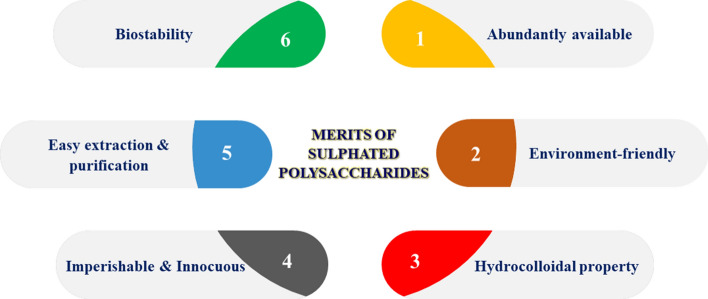
Sulfated polysaccharides (SP) are negatively charged polysaccharides present in the cell wall of marine algae or seaweeds constituted mostly by cellulose and hemicellulose with high carbohydrate content whereas low in calories and fat content. The negative charge is due to the cross-linkage of sulfate group ions with complex molecules of polysaccharides. Sulfate groups are present in their sugar structure's backbone to withhold the extreme marine conditions like high salinity, leads to the alterations in their polymeric structure turning into SP whose biological activity and commercial applications are very high (Kraan 2012). They are abundantly present in seaweeds, but also averagely in some mammals (invertebrates), fish skins, and few saline environment plants whereas it is absent in terrestrial plants. Brown, green, and red-colored species are the three types of seaweed whose sulfated polysaccharide contents range from 4 to 76%, where green seaweed alone yields nearly 65% dry weight. In the food industry, SP is generally extracted using hot water extraction and alcohol precipitation method. SP like fucoidans is extracted from brown algae, carrageenan from red seaweeds while green seaweeds yield ulvans. They are commercially used in food companies as biopolymers having the antimicrobial property in food packaging, supplements in nutraceutical foods, animal feeds as well as hydrocolloids like additives, emulsifiers, stabilizers, and thickening agent (De Jesus Raposo et al. 2013). In medical industries, SP is extracted using enzyme assisted extraction method to retain its biomedical properties by protecting the bio-active molecules and used widely as drug delivery assistants. As they are enriched with anti-coagulant, anti-inflammatory, anti-tumor, anti-viral, triglyceride, and cholesterol-reducing properties, they are highly preferred in the food fortification (Qin 2018). The schematic diagram of notable merits, organization entities of SP were shown in Fig. 2.
Fig. 2.
Schematic diagram of notable merits, organization entities of sulfated polysaccharides
Sources of SP
Most of the seaweed cell wall comprises more than 40% of SP which is relatively above average when compared to other sources of SP.
Carrageenan
The cell wall of red seaweed consists of microfibrils (cellulose and ß-1, 3- xylans) and matrix, where matrix comprises 38% of SP in the form of carrageenan. Several Kappaphycus species yield carrageenan, among them a higher level of refined carrageenan was obtained from K. alvarezii, whose yield ranges between 20.4–28.4%. Carrageenan contains 15–40% of ester-sulfate content and its average relative molecular weight is 100 kDa or above. Other units of 3, 6-anhydrous-galactose (3, 6-AG) and d-galactose are linked by α-1, 3 and β-1, 4-glycosidic linkage form carrageenan (Bhowmick et al. 2015). The number and the position of ester sulfate groups and the content of 3, 6-AG influences in the chemical reaction property of carrageenan. Based on the solubility rate in potassium chloride, carrageenan is classified into λ, κ, ι, ε, μ carrageenan where all contains 22–35% of sulfate groups. Kappa carrageenan (κ) contains 25–30% of ester sulfate, 28–35% of 3, 6- AG contents; Iota (ι) carrageenan contains 28–30% of ester sulfate, and 25–30% of 3, 6-AG contents; Lambda carrageenan (λ) contains 32–39% of ester sulfate and no 3, 6-AG contents. The level of ester sulfate is indirectly proportional to the gel strength, solubility, and temperature stability. The increased ester sulfate level lowers the mechanical property of SP. The pH conditions influence the carrageenan activity where low pH disturbs its action (Cunha and Grenha 2016). They have wide applications in milk products, dietetic formulations, processed meats, toothpaste, laxatives, infant formula, skin preparations, cosmetics, and pesticides. More than 2% of carrageenan level in food products results in adverse health effects. Usage of degraded carrageenan is prohibited as it causes cancer in humans (Weiner 2014). SP like furcellaran is from highly obtained from red algae Laminaria species and lesser in Ascophyllum species. Fucus brown algae species and Undaria green seaweed species have less or no gelation property whereas they are served as food reserves in the form of prebiotics and dietary fibers. Porphyrin/nori species yield SP called porphyrin composed of structures like agarose whose biological properties including prebiotic properties are more efficient which are less exploited in food industries (De Jesus Raposo et al. 2013).
Agar
Agar is the compound present in the cell wall of red algae Rhodophyceae, responsible for constructing their structure. They are a blend of two polysaccharides namely agarose, the gelling portion, and agaropectin, the non-gelling part but aids in increasing the gel strength. Agarose is a heterogeneous polysaccharide consist of D galactose and 3, 6-anhydro-L-galactose sugars whereas agaropectin is a sulfated galactans containing D-glucuronic acid, sulfuric esters, and traces of pyruvic acid. They are linked by α and β bonds. They collectively represent as agar comprising substituent groups like sulfate and methyl esters as well as pyruvate acid ketals. Gelidium and Gracilaria species are the main sources of agar which generally occurs on the rocks beside shorelines (Jiao et al. 2011). The agar is usually extracted at high water temperature and pressure ranges between 100 and 130 °C with a pH between 5 and 6. During boiling, agar gets hydrated although they are not soluble in cold water. Cooling lower than 40 °C alters them into the firm and fragile gels. Since the aggregation of the double-helical structure of molecular chains results in the formation of a network, their gelation process usually occurs at aqueous solutions (Rhein-Knudsen et al. 2017). Their thermoreversible property depends on the species types and methyl ester contents along with the increased temperature condition and agar concentration with reducing cooling frequency. This thermoreversible property does not affect its mechanical properties. Their blending with other gums like guar and locust bean gums increases the gel strength and elastic property. The gel strength can be increased by the addition of glucose and any gelation inhibitory actions from other sources can be prevented by adding a little amount of glycerol. Firm texture, tolerating heat, great stability during acidic conditions, the high-level soluble ratio in strong sugar solutions, no or less reactivity with food components are the major advantages of agar usage in food industries (Lee et al. 2017).
Fucoidan
Though sea urchins and sea cucumbers produce fucoidan, higher yield and more bioactive property enhanced fucoidan was obtained from brown algae. Fucoidan molecular weight ranges from 20–200 kDa. Brown algae comprise nearly 40% w/v of SP to cell wall dry mass, in the form of Fucoidan. The fucoidan yielding seaweed species in India are Dictyota dichotoma, Padina boergesenii, P. gymnospora, Stoechospermum marginatum, Sargassum ilicifolium, S. marginatum, S. myriocystum, S. wightii, Turbinaria conoides, T. decurrens, T. ornate. Among these, Sargassum wightii yields 71.5 mg of fucoidan from 1 g of seaweed dry weight. The formation of the cell wall in brown seaweed is comprised of algin, cellulose, sulfated fucans in the ratio of 3:1:1 (Li et al. 2008). The simplest chemical form of fucoidan was obtained from Fucus vesiculosus containing 44.1% fucose, 26.3% sulfate, and 31.1% ash. Another variety of fucoidan comprises a polymer of l-fucose linked together by α-1, 2-linkage and sulfation occur mainly at O-4 position. Basic varieties incorporate α-1, 3-linkage, replicating the structure of substituting α-1, 3-and α-1, 4-glycosidic bonds, hexasaccharide replicating unit with some 2-O-sulfation and 2-O-acetylation (Leung et al. 2006). Sulfated fucans contain a trace amount of xylose, galactose, mannose, glucose, rhamnose, and d-glucuronic acid. The best method to extract fucoidan is hot water extraction and purified by ion-exchange or gel filtration chromatography. Due to its enhanced biomedical activity, fucoidan is mostly used in the medical industry as mentioned in Table 1 (Mustafa and Mobashir 2020). They are also used as a mucosal shielding and medically effective agent in the surgical procedure. As a nutraceutical or pharmaceutical agent, their dose ranges from 100–8,000 mg per day. Fucoidan can be taken in high concentration at a low dose level at regular time intervals. Their overdosage results in severe health hazards (Mukhamejanov and Kurilenko 2020).
Table 1.
Sulfated polysaccharides and their biological and functional properties
| Sulfated polysaccharides | Sub-form | High yielding sources | Pigment of the seaweed source | Biological properties | Functional properties | References |
|---|---|---|---|---|---|---|
| Carrageenan | λ, κ, ι, ε, μ |
Kappaphycus Euchema Chondrus Iridaea Hypnea Porphyra Gigartina Palmaria |
Red - phycocyanin phycoerythrin |
Antioxidant, anti-microbial, anti-inflammatory, anti-cancer, anti-coagulant and oxygen, lipid barrier properties | Gelling, binding, thickening, emulsion, and protein stabilizing, suspending agent, viscosity controller and water retention |
Weiner (2014) Campo et al. (2009) |
| Agar | – |
Gelidium Gracilaria |
Red - phycocyanin phycoerythrin |
Anti-diabetic, anti-coagulant, antioxidant, anti-tumor, anti-viral, laxative properties, and alpha glucosidase inhibitor | Excellent gelling, thickening, clarifying, texturizing, emulsifying agent |
Lee et al. (2017) Rhein-Knudsen et al. (2017) |
| Fucoidan | F, U, L, G, GA |
Sargassum Padina Ascophyllum Macrocystis Undaria Laminaria Cladosiphon Fucus |
Brown- fucoxanthin |
Blood thinners, antioxidants, antiviral, antineoplastic, immunoregulatory, anticomplementary, anti-inflammatory, cholesterol-lowering and mucosal shielding agent | Gelling, chemical reactivity, foaming, suspension, improving quality, controlling moisture |
Wang et al. (2019) Mustafa and Mobashir (2020) |
| Ulvan | A3s, B3s, U3s, and U2′s,3 s |
Ulva Caulerpa Codium |
Green- chlorophyll a and b, carotene |
Anti-septic, anti-inflammatory, anti-cancerous, anti-viral and anti-microbial properties | Viscosity and thickening, suspension, caking, gelling, adhesion, encapsulation, form retention |
Yaich et al. (2014) Kidgell et al. (2019) |
Ulvan
Green seaweed consists of 9–36% w/v of SP in the form of Ulvan. Ulvan is a polyanionic heteropolysaccharide with the molecular weight ranges from 150–2000 kDa. Ulva species are the main sources of ulvans where U. conglobate and U. prolifera yield higher quantity. They contain monosaccharides such as rhamnose (45%), glucuronic acid (22.5%), xylose (9.6%), and iduronic acid (5%) (Lahaye and Robic 2007). These are α- and β-(1, 4)- linked monosaccharides bound to disaccharide units. Based on the type of disaccharides, ulvans are classified into A3s, B3s, U3s, and U2′s, 3 s types. In type A3s the β-D-glucuronic acid (1, 4) bound to α-L rhamnose 3-sulfate. In type B3s, α-L-iduronic acid (1, 4) bound to α-L-rhamnose 3-sulfate. In type U3s the β-D-xylose (1, 4) bound to α-L-rhamnose 3-sulfate. In type U2′s, 3 s the β-D-xylose 2-sulfate (1,4) bound to α-L-rhamnose 3-sulfate (Kidgell et al. 2019). The best way to extract ulvan without damaging its bioactive molecules is chelator assisted hot water extraction method followed by alcohol precipitation and purified by HPLC. The yield of purified ulvan ranges from 8–29% of the seaweed dry weight. Due to its non-adhesive nature, spontaneous supramolecular aggregation behavior, and antimicrobial property, it is used as antimicrobial coatings, micro/nano-formulations, and wound dressing. The therapeutic intake of ulvan should be less than 500 µg since it is not processed by the enzymes in our digestive system (Wells et al. 2017).
Isolation of SP from seaweeds
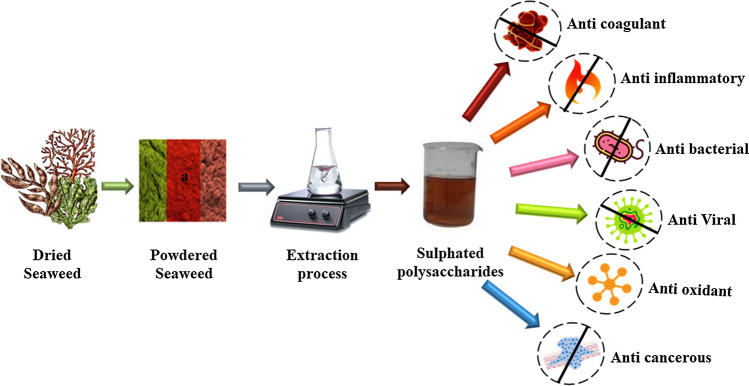
The molecular weight, structure, physicochemical properties, phytochemical constituents, and their composition solely depend on the species type and extraction process of SP. Every single variation in the above-mentioned factors modifies the SP properties and their final application is only based on them. The diagrammatic representation of extraction and bioactivity of SP were shown in Fig. 3.
Fig. 3.
Diagrammatic representation of extraction and bioactivity of sulfated polysaccharides
Extraction methods
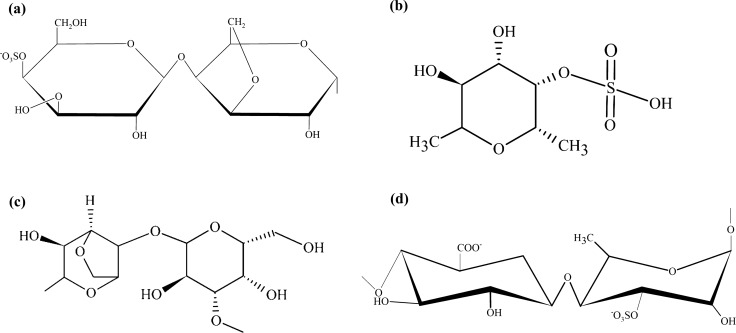
The extraction procedure differs by sources and application of SP. The highest yielding methods of each SP are described briefly, and their chemical structures were shown in Fig. 4.
Fig. 4.
Chemical structure of various sulfated polysaccharides such as (a) carrageenan (b) fucoidan (c) agar (d) ulvan
λ carrageenan
Craigie technique is employed along with the addition of a trace amount of 0.5 M NaHCO3 and 0.3 M KCl followed by precipitation using isopropanol. The obtained precipitate is washed with 3 M KCl to extract λ carrageenan. They do not form gel structure. They are purified by alkaline modification and long-term dialysis. Subsequently, they are dried under vacuum and characterized by infrared (IR) spectroscopy and C- 13 nuclear magnetic resonance (NMR) (De Lestang et al. 1987).
κ carrageenan
The extraction of κ carrageenan includes a hot water alkali method at 90 °C with pH 9 for 2 h followed by neutralization of filtrate with 1% HCl. It is precipitated using ethanol and KCl where a 2.5% concentration of KCl yields more economical κ carrageenan. They are later filtered using 80 mesh filter size. They are purified using 96% alcohol and dried at 70 °C for 24 h. The purified κ carrageenan is characterized using magnetic particle tester, gravimetric analysis, IR spectroscopy, and Fourier transform infrared radiation (FTIR) to confirm their functional groups (Tuvikene et al. 2010).
ι carrageenan
The extraction of ι carrageenan is a tedious and multi-step procedure when compared to other carrageenan extraction. The ι carrageenan gel is formed by calcium ions when their cations dissolve in hot water during hot water extraction with NaHCO3, homogenized with diatomaceous earth, pressure filtered, and cooled. Then the filtrate was subjected to α-amylase, the resultant was concentrated in the oven and purified by the dialysis process throughout the night against tap water and thrice with distilled water. They have a molecular weight of around 12,000 kDa approximately. Finally, they are lyophilized to acquire a purified form. FTIR is used to analyze some of the functional groups of ι carrageenan. They are sulfate, 3, 6-anhydrogalactose, sulfate ester at 4-position of galactose, and sulfate ester at 2-position of 3, 6-anhydrogalactose. C- 13 NMR is used for confirmation of ι carrageenan (Villanueva et al. 2009).
Epsilon carrageenan
Epsilon (ε) carrageenan is not characterized intensely and is not potentially used in any industrial applications.
Mu carrageenan
Mu (μ) is the semi-refined form of κ carrageenan and extracted using the above-mentioned procedure without including any alkaline salts. They cannot be completely purified as it contains lots of other residues including other carrageenan types. They are characterized by FTIR where the results obtained will be like κ carrageenan with additional sulfate ester group (Van de Velde 2008).
Agar
Agar is obtained using the hot water extraction method. Pretreatment results in improved yield rate and color can be improved by adjusting pH and addition of salts like phosphates. Seaweed is added to the boiling water in the extraction vessel and continued to boil for 1–3 h. It is then filtered and followed by bleaching with calcium hypochlorite and dewatering by the high-pressure press and the remaining water content is dried through the freeze-drying process or sun drying (Surender Reddy et al. 2018).
Fucoidan
The microwave-assisted method (Rodriguez-Jasso et al. 2011) and hot water extraction with alcohol precipitation are preferred for effective fucoidan extraction. The dry powdered sample is treated with an acid solution of 1% HCl for lysis of the seaweed cell wall followed by hot water treatment at 95 °C for 4 h. 0.5% NaOH was added to neutralize the solution and filtered. For precipitating polysaccharide, 95% ethanol is added in thrice the amount of filtrate and washed thrice with 70% ethanol. Ion exchange chromatography or HPLC is used for purifying fucoidan. It is characterized using IR spectroscopy, NMR, and FTIR (4000–400 wavelength) (Hahn et al. 2012).
Ulvan
The ulvan types such as (A3s, B3s, U3s, and U2′s, 3 s) are extracted based on the type of lyases used. Their extraction method follows alkaline hot water extraction above 70 °C and pH of above 7.5. Calcium salts are added for aiding the gel formation. Then the filtrate was extracted with 95% alcohol and the precipitate is recovered. For obtaining the pure form of desired Ulvan, a dialysis procedure is carried out for 3 days and lyophilized finally for storing and further applications. They are characterized by IR spectroscopy, FTIR, and NMR (Yaich et al. 2014).
Applications in food
SP is highly utilized in the food industries owing to their stabilizing, gelling, emulsifying, and viscosity-enhancing properties. Since it boosts and stabilizes the food structure, they are extensively employed in food preparations like jams, jellies, ice creams, other milk products as additives. Sulfated galactans like carrageenan, agar is the prominent commercially important SP used in food processing industries. The negative charge in SP is due to the presence of assorted groups in their structural backbone like sulfate, methoxy, etc. Subsequently, these groups will interface with cations present in food solvents like milk, water. This bonding develops the gelation process which fulfills the scope of reaching the industrially important thermo-mechanical range. They work as a vital tool for transforming the food surfaces, adjustment of colloids, decreasing fat contents, expansion of shelf life, etc. They assist in creating more innovative processed foods and creates wide applications over food enterprises (Alba and Kontogiorgos 2018).
Food packaging
Naturally extracted and decomposing polymers are preferred these days with a concern on the growing environmental downside related to the use of artificial polymers resulting in plastic waste (Sousa et al. 2014). SP like agar, fucoidan, carrageenan, and ulvan are biopolymers produced with a simple extraction process. They have adequate significance in nourishment, pharmaceutical, and biotechnological ventures where it is utilized as gelling, balancing out, and exemplifying specialists. They have the capability of forming both edible as well as non-edible film or wraps, bags, and covers with enhanced barrier properties by preventing the exchange of moisture, oxygen, enhancer as well as lipid content present in food and food products, the microbial culture medium as well as between various compounds present in mixed nourishment (Parreidt et al. 2018). They can be blended with commercial biopolymers like polylactic acid, polyolefins, polyhydroxy butyrate besides with nanoparticles and nanocrystals results in the formation of biocomposite nanofood packing. They are more like synthetic polymers with additional benefits like biodegradable capacity and toxic-free nature (Armentano et al. 2018).
The usage of SP along with nanoformulations like magnesium oxide, silver nanoparticles tends to increase antibacterial activity even at lower concentrations. The best source of SP to exhibit antibacterial activity includes Gracilaria sp., Sargassum sp., and Ulva. Ulvan is used as an anti-septic as it tends to have anti-microbial activity against several microbes. In the food industry, the SP is used to restrict the activity of foodborne pathogens namely Listeria monocytogenes, Enterococcus faecalis, Escherichia coli, Staphylococcus aureus, and Salmonella enterica. Anti-microbial activity depends on the SP source, extraction method, and dosage. The structure, molecular weight, chemical constituents, and bioactive factors vary by source and extraction method (Pérez et al. 2016).
Despite wide usage in the field of microbiology and food additives, the recent studies on agar are focusing broadly on the development of potential biodegradable food packaging applications (Salehi et al. 2019). They could form strong, clear, transparent, relatively flexible gels, characterized by their higher melting point and initial gelation temperature (Wang and Rhim 2015). It is known to soften on warming and set on cooling and this cycle will be persistent for an uncertain scope of times without trading off the mechanical characteristics of the gel. Such gel framing properties of agar manufacture it as a conventional possibility for blending with various biopolymers to fortify the mechanical properties of the homogenized hydrogels (Boral et al. 2010). Despite their great film-framing ability, there occur numerous disadvantages for its improvement as bundling material, its relatively significant expense, its brittle, and water-absorbing nature. Due to fragility, agar-based sheets cannot be prepared without the inclusion of plasticizers like glycerol, the renowned commonly used plasticizer in the current studies. Concerning their hydrophilic nature, numerous fillers like nano clays, carob gum, lignin, and cellulose nanocrystals are allied with agar to expand their water obstruction. Nevertheless, the mechanical properties of the sheets were collectively influenced by the nearness of the fillers with low loadings preferably under 5% may be joined without expanding the break ableness of the sheets (Martínez-Sanz et al. 2019).
From red edible seaweeds, carrageenan gels are extracted and possess better film-forming properties and are good carriers as antimicrobial agents. Their films are water-soluble, and their mechanical strength is generally weaker than other polysaccharide films. Because of their deliquescent characteristic properties, the least wet barrier properties are expected. However, they have sufficient oxygen and lipid barriers that may obstruct lipid oxidization in homogenous foods and lipid migration in heterogeneous foods. Carrageenan contains hydroxyl and sulfate groups which tend them to be hydrophilic. κ and λ have efficient gelling properties that develop excellent film properties (Sedayu et al. 2019). They are used in creating edible packaging, film coatings, and blends. This packaging material prevents discoloration, retaining moisture content, texture maintenance of food, and helps in the encapsulation of aroma products. They are used in the wrapping of meat and poultry foods. The addition of starch to carrageenan provides more efficacy in improving mechanical strength, gelling strength, and barrier properties. As hydrogel has the property of absorbing and retaining the water without dissolving in it, κ carrageenan is crosslinked to produce hydrogel to increase the stability of the film in the aqueous medium (Sanchez-García 2011). Since carrageenan has gas barrier properties, they are used as a food coat for the freshly diced fruits and vegetables. These coatings serve as a shield and help in delaying the discoloration of the food by reducing the respiration rate which supports to sustain its quality throughout their shelf life. Though packaging films produced from starch has sufficient dissolved oxygen-absorbing qualities, they lack in moisture barrier properties along with its undesirable compound properties compared with conventional polymers. Therefore, as a copolymer, they can be blended with hybrid carrageenan for manufacturing edible packaging film or wraps (Bico et al. 2009). Hybrid carrageenan containing a mixture of κ and ι carrageenan types has enhanced gelation ability than their pure individual forms. The relative water vapor and oxygen absorptivity, the barrier of radiations like ultraviolet rays. These advantageous properties result in enhanced hydrophobic coupled with hygroscopic nature as well as the sustainability of these eco-friendly bio packaging (Larotonda et al. 2016). Packaging films using carrageenan and nanomaterial blends boosts the food packaging necessities such water resistance and gas permeability together with increased elasticity.
Nutraceuticals
Nutraceuticals are the condensed form of the terms nutrition and pharmaceuticals signifying food and its additives with medicinal benefits. Nutraceutical prospective organic composites are commonly viewed as safe for human wellbeing and are typically utilized in traditional medications. In recent times, they attracted considerable interest in the suitable application of those composites for maximum health benefits (Gul et al. 2016). Recently, several investigations disclosed the pharmacological properties of SP obtained from several seaweed species. They signify that red and brown colored seaweed species have enriched nutritional quality and are employed as nourishing supplements along with the regular diet. This nourishing property is due to the presence of bioactive SP in their cell walls. They are the abundant source of soluble fiber containing more nutritional substances. It can be digested by enzymes present in the human digestive system (Cherry et al. 2019). The presence of sulfate anion in seaweed polysaccharide is due to the higher salt concentration in their existing marine environment, whereas they are absent in terrestrial plants. They act as an ionic regulator. To withstand the strenuous environment, they produce some self-defense strategies encountering the modification in their metabolic arrangements. Consequently, these changes lead to the enriched development of both major and minor phytochemical constituents. The major constituents of SP were illustrated in Fig. 5. Minor constituents include lipoproteins, vitamins, polyether, carotenoids, fatty acids, terpenoids, polyphenols, minerals, etc. Meanwhile, SP is enriched with unsaturated omega 3 fatty acids e.g. docosahexaenoic, eicosapentaenoic acid, omega 6 fatty acids e.g. linoleic, arachidonic acids and palmitic, myristic acids which are saturated fatty acids. They can be extracted easily by a simple process in less time and cost. 5–47% dry mass of seaweed contains protein and 42–48% of the essential amino acid (Wells et al. 2017). The biological activity of SP is enlisted in Table 1. These activities purely depend on their sulfate content and molecular weight.
Fig. 5.
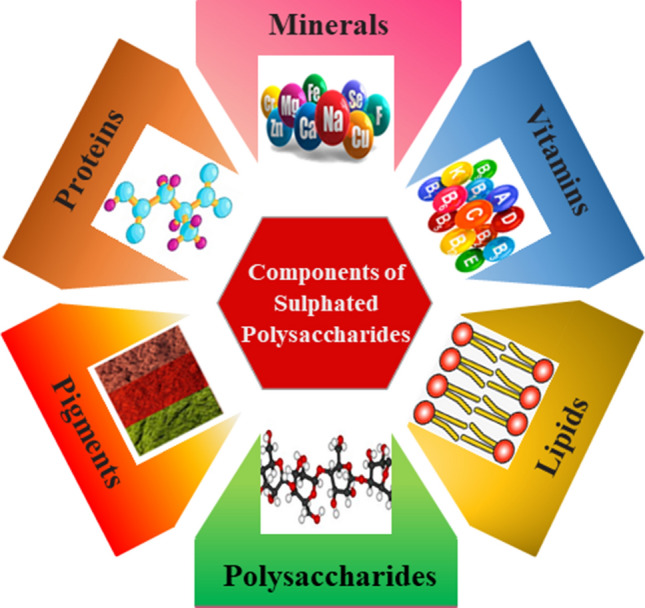
Major constituents of sulfated polysaccharides
SP extracted from the rhodophytes group, Porphyridium cruentum showed significant anti-viral activity during a study on the Hela cell lines against African swine fever virus, vaccinia virus (VACV) as well as vesicular stomatitis virus (VSV). Similarly, SP from P. purpureum shows significant anti-viral activity against ectromelia virus, VACV via green fluorescent protein studied on HepG2 and Vero C1008 cell lines (Ibrahim et al. 2017). Porphyridium sp. shows anti-viral activity against murine leukemia virus (MuLV), Herpes simplex virus 1 and 2, viral hemorrhagic septicemia virus, hepatitis B virus (HBV), Murine sarcoma virus-124 (MuSV-124), and varicella-zoster virus (VZV) which are examined at fibroblast cell lines like NIH/3T3. SP extracted from Rhodella reticulata show anti-viral activity against MuLV-124 and MuSV type virus. Herpes simplex virus (HSV) 1 and 2 and VZV which is tested on the NIH/3T3 cell line (Talyshinsky et al. 2002). Ulvans reveals the strong viral infection inhibitory power on some human infecting viruses like influenza A virus, human metapneumovirus, HSV. This inhibitory action is dependent on dosage exposure and specific to selective viral strains. In addition to this, fucoidan has a strong viral replication inhibitory effect against human immunodeficiency virus, herpes simplex virus (HSV), human cytomegalovirus, influenza virus, and bovine viral diarrhea virus (Wang et al. 2019). Carrageenan shows potential inhibitory effects on several viruses like human papillomavirus, dengue virus, vaccinia, VACV, VSV, measles, HSV 1 and 2, etc., by inhibiting the early infection as well as their replication process. ι-carrageenan has effective antiviral property than λ- and κ-carrageenan. High molecular weight carrageenan has a low cell-penetrating capacity (Wang et al. 2012). From these investigations, it has been shown that SP from seaweed has great potentials as anti-viral agents in the food.
The broad polar association between negatively charged SP and cationic protease proteins forms anticoagulant activity. This mechanism forms heparin cofactor II-mediated thrombin inhibition, the effectiveness differs from the compound. Agar and carrageenan are collectively called as sulfated galactans from red seaweed. They show greater anti-coagulant property by increasing the production of plasma cofactor. Their anti-coagulant activity differs on the extraction of sulfated galactans, structure, charge density, and interaction complex types. Longer chain sulfated galactans with molecular size above 45 kDa is preferred as it strongly binds to antithrombin factors (Glauser et al. 2009). Sulfated d-galactan enhances thrombin and factor Xa inhibition by binding with heparin cofactor II or directly binds with the antithrombin factor. A high potent anticoagulant is produced when factor Xa is the target instead of thrombin. Strong protease interaction is produced when sulfated galactans have 2, 3-disulfate alpha units, this results in high coagulant activity compared to other SP (Ciancia et al. 2010). SP from green seaweed species like Ulva, Codium, Monostroma has greater potential anticoagulant activity than that of heparin (Mao et al. 2006).
Anti-inflammation activity is triggered by complement cascade, enzyme inhibition, and selectin blockage. Fucoidans, ulvan as well as galactans activate nitric oxide and cytokine production in macrophages and prevent the leukocyte movement to the injury site which leads to an increase in excessive cytokine production and inflammation takes place (Senni et al. 2006). The lower molecular weight SP generates the immunostimulant activity against S180 tumors which is proved in mice (Chen et al. 2008).
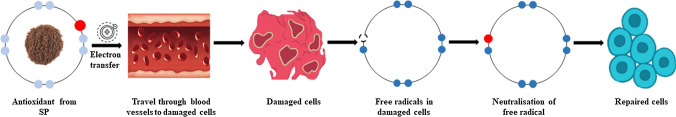
One of the major reasons for developing it as nutraceutical food is due to its antioxidant property. Food nutritional quality and drug safety are affected by reactive oxygen species such as superoxide anion, hydrogen peroxide, and hydroxyl radicals. The aggregation and the action of reactive oxygen/chemical species are prevented by SP. Lipid peroxidation through ascorbic acid, linoleic acid, FeSO4 is prohibited by SP by preventing the oxidative damage to fibroblasts (Ibrahim et al. 2017). Lipid peroxidation through ascorbic acid and FeSO4 proved in mouse cells (De Jesus Raposo et al. 2013). The SP from Rhodella species from the red seaweed family has more antioxidant properties when compared to other SP sources. They have strong antioxidant activity against superoxide anion radical scavenging, and 2X stronger than vitamins like α-tocopherol. For higher efficiency, the SP within 6.55–256 kDa are preferred whereas SP having high molecular weight has no antioxidant property (Chen et al. 2010). The mechanism of the antioxidant activity of SP is demonstrated in Fig. 6.
Fig. 6.
Mechanism of antioxidant activity of sulfated polysaccharides
Among the species, red-colored species have the highest protein content whereas green-colored species serve next, and brown colored species have the least content. Generally, on a scoring basis of 0–1, protein content in the egg secure a score of 1, seaweeds like Undaria pinnatifida scores 1.0, Pyropia/Porphyra scores 0.91 and Laminaria saccharina scores 0.82. This shows that seaweed has a score greater than or equal to proteins from plant-based sources except for soy which has a 1.0 score (Shannon and Abu-Ghannam 2019). Though agar has wide applications in food industries as cost-effective hydrocolloids, they do not enrich the nutritional content in the food. Despite this, they assist in lowering the blood sugar level, inhibiting the erythrocytes agglutination as well as absorbing harmful radiations like ultraviolet rays. They efficiently suppress the formation of pro-inflammatory chemical indicators like cytokines along with enzymes like nitric oxide synthases. In addition to this, they are dynamic against enzymatic activities accountable for the antioxidant and its resulting antitumor activities. The carrageenan can be extracted in both high and low molecular weight fractions where both can be used in functional as well as biomedical foods, respectively. Ulvan serves as a great nutritional fiber due to its high-water retaining abilities. Studies have shown that they are effective against free radical formation, lipid disorder, pathogenic fungus, proliferative activity of neoplastic cells (Mišurcová et al. 2012). The biological properties of each SP are enlisted in Table 1.
Hence seaweeds can be used as the replacement of animal-sourced proteins for vegans in the regular diet. Iron and iodine are another two most abundant elements present in seaweed. Some species of seaweed contains fat and water-soluble vitamins and essential minerals nearly hundreds of times more than the terrestrial plants per unit of dry mass. SP from many seaweed species performs better than animal and plant-based nutraceuticals referred according to adult nutrient intake rate. Due to these reasons, they achieved increased attention as nutraceutical supplementary foods for humans along with medicinal applications (Shannon and Abu-Ghannam 2019).
Hydrocolloids
The material which forms gelatinous or viscous dispersions when they are dispersed in water is termed as hydrocolloids. The name hydrocolloid is derived from the Greek words hydro (water) and Kolla (gum). They are long hydrophilic polysaccharide chains containing OH groups responsible for effective water-binding aids in gel formations. They exist either in single as well as the reversible state. Among all seaweeds, SP from red and brown species such as carrageenan and agar are commercially available hydrocolloids. They serve as stabilizers, thickeners, emulsifiers, and fillers in food, pharmaceutical, cosmetics, and many other industries, with their unique physiochemical properties (Milani and Maleki 2012). Their functional properties are enlisted in Table 1.
Agar is the oldest exploited commercial hydrocolloid since the sixteenth century. It is the first hydrocolloid endorsed by the Food and Drug Administration as GRAS (Generally Recognized as Safe) which utilized it as food additives with European enlistment number of E406. It is comprised of two glycans sugars, such as agarose and agaropectin which are liable for gelling and thickening properties, respectively. 3/4th of its production is generally used for food applications whilst the remaining are used in medicinal as well as biotech companies. The red seaweeds species such as Gelidium and Gracilaria yield the best quality agar (Gioele et al. 2017). They have excellent gelling properties with a resistance to high temperatures and are thermoreversible on cooling. Generally, agar can be extracted easily from seaweed by boiling it in hot water and gelation occurs at 32–45 °C. To produce native agar, alkali treatment is mostly preferred to enhance its gel strength and fulfill commercial agar requirements. Agar extracted from species like Gracilaria chilensis have enriched sugar contents responsible for boosting the gel strength. These agars are sugar active i.e. during the encounter of sucrose, they become sturdy and increases the gel strength. In addition to this, salts such as potassium, calcium enhance the gel potency. These agars are used for candies as well as other foodstuffs preparation (Bertasa et al. 2020).
Agar has wide application in the processing of food products like pastry fillings, jam and jellies, confections, beverages, spreads, garnishes, puddings, desserts, ice-cream, meat, and poultry products. Their applications generally differ based on the quality of its functional property. They are extensively used in the preparation of jellies where food colors and flavors are added to hot agar extract followed by mold casting and cooling (Khalil et al. 2018). Agar utilized in the baking industry has more improved functional properties than other hydrocolloids like carrageenan, gelatins, etc. Minimum traces of agar i.e. below 2% concentration, are used in gelling the meat and fish products for canning purposes. They are more suitable for water and milk-based desserts since they have efficient gelling activity with them. It helps in the formulation of non-sticky aesthetic food coatings, glazing, icing based on sugar with concentration ranging from low to high. Furthermore, they are used as stabilizers for retaining ice cream texture as well as a clarifying agent in fermented beverages such as wine, beer, vinegar, juices, etc. by creating flocculation for separating the suspended solid particles from liquid. Beside all these food applications, they are used in the microbiological studies for the large-scale preparations for culture media for microbial growth (Saha and Bhattacharya 2010).
The aspects that propel the development and scope of carrageenan in the market include the alternative to animal-based hydrocolloids like gelatin by fulfilling the additives demand in the leading commercial processed foods. Even though carrageenan has difficulty in characterization, they have more commercial importance on comparing with other hydrocolloids since each species produces it in different proportions and types (Gul et al. 2016). For commercial purposes, three types of carrageenan such as κ, λ, and ι carrageenan should be isolated separately, since their combined form decreases the gel strength which is not ideal for industrial purposes. They produce thermoreversible products. Due to their greater functional properties, on binding with water they serve as thickener, stabilizer, and texture modifier. They enhance the appearance as well as the quality of the food in a commercial aspect. They are extensively used in dairy, baking, and food processing industries in the production of foods such as puddings, milkshakes, nutritional milk drinks, tofu, frozen yogurt, chocolate milk, vegan option in contrast to gelatin, prepared meat, pastries, creams, organic product juices, brew, dry food powders such as instant soups, sauces mixes, and flavors, jam, spreads, canned food enhancer, pet nourishment (Krempel et al. 2019). Generally, fat contents in processed meat disturb the water retention capacity. Since carrageenan has water withstanding capacities, it is included as a fat replacement to maintain its tenderness and is used in hamburger as well as canned meats preparations. In addition to this, they are used as a stabilizer for cooked meat by upholding the infused water contents to maintain its juicy nature. They are used as clarifiers as well as refiners in beer and wine production, respectively. On comparing with other gelling agents, carrageenan has gelation capacity in milk even at very low concentrations, thus they have wide applications in the dairy industry. The bonds are created between the positive charge of the milk protein casein with the negative charge of the sulfur group containing carrageenan and thus formation of gel takes place. On combining with calcium salts, carrageenan maintains the softy texture of tofu (Yousefi and Jafari 2019). Since carrageenan gels have more transparency and gelation occurs at high temperatures, they are used as glazers in cakes as well as water-based jelly desserts. As they form strong and firm gels with rapid setting nature, they are used in cheese processing. Meanwhile, during dessert preparation, carrageenan has more benefits than pectin because carrageenan dissolves directly in sugar solution whereas, pectin must be pre-dissolved in water and then added into sugar solution (Porse and Rudolph 2017).
Concluding remarks
The oceanic habitat contains several thousand species where marine phytoplankton like macroalgae called seaweed have their metabolic and structural modifications to withstand the critical environment for survival purposes. These alterations help in the production of several secondary metabolites enriched with biological properties. Subsequently, they are inexhaustible sources containing enhanced macro and micro compounds widely used for both commercials as well as medicinal purposes. In this current world, people started seeking organic products in all fields on concern with healthy and pollution-free aspects. Thus, the usage of seaweed SP is in the inclined stage since many researchers have started exploring its entire beneficial outlook. They have extensive applications in the food industries to boost the quality, nutrition as well as retaining the characteristic property of the processed food. They are easily available, extractable, product convertible at low cost and time effectively. As their enriched nourishing phytochemical constituents with various biological properties increase immune power, they are used as nutraceuticals. On encountering with milk or water, they perform great gelation activity and are used as additives in maintaining, stabilizing, and structuring the processed food. In the food packaging section, more kinds of ongoing studies are focusing on the usage of SP as a biopolymer and serve as a potential alternative for synthetic polymers. Feasibly in the future, there will be more utilization of seaweed SP and their byproducts in food, medicine, and cosmetic industries. Their systematic application will reduce pollution, increases the usage of natural resources, helps in creating innovative products for highly profitable commercial purposes, reducing the usage of synthetic polymers, etc. After all, SP usage is emerging in various sectors of food industries and there is substantial interest in their further development in food processing and packaging sectors. Hence further investigations must be focused more on the partial blending or replacement of SP over the conventional synthetic or animal-derived compounds in food industries and promoting the eco- friendly and healthy products.
Acknowledgements
The authors are thankful to the management of the Vellore Institute of Technology, Vellore, Tamil Nadu, India for providing constant support and encouragement.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
Authors declare that this work has not been published previously and there are no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmady-Asbchin S, Andres Y, Gerente C, Le CP. Natural seaweed waste as sorbent for heavy metal removal from solution. Environ Technol. 2009;30(7):755–762. doi: 10.1080/09593330902919401. [DOI] [PubMed] [Google Scholar]
- Alba K, Kontogiorgos V. Seaweed polysaccharides (agar, alginate carrageenan) In: Shahidi F, Melton L, Varelis P, editors. Encyclopedia of food chemistry. Amsterdam: Elsevier; 2018. pp. 240–250. [Google Scholar]
- Armentano I, Puglia D, Luzi F, Arciola CR, Morena F, Martino S, Torre L. Nanocomposites based on biodegradable polymers. Materials. 2018;11(5):795. doi: 10.3390/ma11050795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balina K, Romagnoli F, Blumberga D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia. 2017;128:504–511. doi: 10.1016/j.egypro.2017.09.067. [DOI] [Google Scholar]
- Bertasa M, Dodero A, Alloisio M, et al. Agar gel strength: a correlation study between chemical composition and rheological properties. EurPolym J. 2020;123:109442. doi: 10.1016/j.eurpolymj.2019.109442. [DOI] [Google Scholar]
- Bhowmick B, Sarkar G, Rana D, Roy I, Saha NR, Ghosh S, Bhowmik M, Chattopadhyay D. Effect of carrageenan and potassium chloride on an in situ gelling ophthalmic drug delivery system based on methylcellulose. RSC Adv. 2015;5(74):60386–60391. doi: 10.1039/c5ra06858d. [DOI] [Google Scholar]
- Bico SLS, Raposo MFJ, Morais RMSC, Morais AMMB. Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh-cut banana. Food Control. 2009;20:508–514. doi: 10.1016/j.foodcont.2008.07.017. [DOI] [Google Scholar]
- Bixler HJ, Porse H. A decade of change in the seaweed hydrocolloids industry. J ApplPhycol. 2011;23(3):321–335. doi: 10.1007/s10811-010-9529-3. [DOI] [Google Scholar]
- Boral S, Saxena A, Bohidar HB. Syneresis in agar hydrogels. Int J BiolMacromol. 2010;46(2):232–236. doi: 10.1016/j.ijbiomac.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Campo VL, Kawano DF, da Silva DB, Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis - A review. Carbohydr Polym. 2009;77:167–180. doi: 10.1016/j.carbpol.2009.01.020. [DOI] [Google Scholar]
- Cazón P, Velazquez G, Ramírez JA, Vázquez M. Polysaccharide-based films and coatings for food packaging: a review. Food Hydrocoll. 2017;68:136–148. doi: 10.1016/j.foodhyd.2016.09.009. [DOI] [Google Scholar]
- Chen B, You W, Huang J, Yu Y, Chen W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J MicrobiolBiotechnol. 2010;26(5):833–840. doi: 10.1007/s11274-009-0240-y. [DOI] [Google Scholar]
- Chen D, Wu XZ, Wen ZY. Sulfated polysaccharides and immune response: promoter or inhibitor? Panminerva Med. 2008;50:177–183. [PubMed] [Google Scholar]
- Cherry P, O’Hara C, Magee PJ, McSorley EM, Allsopp PJ. Risks and benefits of consuming edible seaweeds. Nutr Rev. 2019;77(5):307–329. doi: 10.1093/nutrit/nuy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancia M, Quintana I, Cerezo AS. Overview of anticoagulant activity of sulfated polysaccharides from seaweeds in relation to their structures, focusing on those of green seaweeds. Curr Med Chem. 2010;17(23):2503–2529. doi: 10.2174/092986710791556069. [DOI] [PubMed] [Google Scholar]
- Cunha L, Grenha A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar Drugs. 2016;14:42. doi: 10.3390/md14030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus Raposo MF, De Morais RMSC, De Morais AMMB. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs. 2013;11(1):233–252. doi: 10.3390/md11010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lestang BG, Quillet M, Bremond M. λ-carrageenan in the gametophytes of Chondrus crispus. Phytochemistry. 1987;26(6):1705–1707. doi: 10.1016/S0031-9422(00)82272-4. [DOI] [Google Scholar]
- Díez I, Muguerza N, Santolaria A, Ganzedo U, Gorostiaga JM. Seaweed assemblage changes in the eastern Cantabrian Sea and their potential relationship to climate change. Estuar Coast Shelf Sci. 2012;99:108–120. doi: 10.1016/j.ecss.2011.12.027. [DOI] [Google Scholar]
- Dillehay TD, Ramír C, Pino M, Collins MB, Rossen J, Pino-Navarro JD. Monte Verde: seaweed, food, medicine, and the peopling of South America. Science. 2008;325(5877):1287–1289. doi: 10.1126/science.1156533. [DOI] [PubMed] [Google Scholar]
- Dittami SM, Heesch S, Olsen JL, Collén J. Transitions between marine and freshwater environments provide new clues about the origins of multicellular plants and algae. J Phycol. 2017;53(4):731–745. doi: 10.1111/jpy.12547. [DOI] [PubMed] [Google Scholar]
- Fleurence J, Morançais M, Dumay J. Seaweed proteins. In: Yada RY, editor. Proteins in food processing. 2. Amsterdam: Elsevier; 2018. pp. 245–262. [Google Scholar]
- Gioele C, Marilena S, Valbona A, et al. Gracilaria gracilis, source of agar: a short review. Curr Org Chem. 2017;21:380–386. doi: 10.2174/1385272820666161017164605. [DOI] [Google Scholar]
- Glauser BF, Rezende RM, Melo FR, Pereira MS, Francischetti IMB, Monteiro RQ, Rezaie AR, Mourão PAS. Anticoagulant activity of a sulfatedgalactan: serpin-independent effect and specific interaction with factor Xa. ThrombHaemost. 2009;102(6):1183–1193. doi: 10.1160/TH09-04-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul K, Singh AK, Jabeen R. Nutraceuticals and functional foods: the foods for the future world. Crit Rev Food SciNutr. 2016;56:2617–2627. doi: 10.1080/10408398.2014.903384. [DOI] [PubMed] [Google Scholar]
- Hahn T, Lang S, Ulber R, Muffler K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012;47(12):1691–1698. doi: 10.1016/j.procbio.2012.06.016. [DOI] [Google Scholar]
- He P, Xu S, Zhang H, Wen S, Dai Y, Lin S, Yarish C. Bioremediation efficiency in the removal of dissolved inorganic nutrients by the red seaweed, Porphyra yezoensis, cultivated in the open sea. Water Res. 2008;42(4–5):1281–1289. doi: 10.1016/j.watres.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J ApplPhycol. 2011;23(3):543–597. doi: 10.1007/S10811-010-9632-5. [DOI] [Google Scholar]
- Ibrahim M, Salman M, Kamal S, Rehman S, Razzaq A, Akash SH. Algae-based biologically active compounds. In: Zia KM, Zuber M, Ali M, editors. Algae based polymers, blends, and composites: chemistry biotechnology and materials science. Amsterdam: Elsevier; 2017. pp. 155–271. [Google Scholar]
- Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9:196–233. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam SU, Pankaj SK, Tiwari BK, Cullen PJ, O’Donnell CP. Development of biopolymer-based gelatin and casein films incorporating brown seaweed Ascophyllum nodosum extract. Food Packag Shelf Life. 2015;6:68–74. doi: 10.1016/j.fpsl.2015.09.003. [DOI] [Google Scholar]
- Khalil A, Tye YY, NurulFazita MR. A review of extractions of seaweed hydrocolloids: properties and applications. Express Polym Lett. 2018;12:296–317. doi: 10.3144/expresspolymlett.2018.27. [DOI] [Google Scholar]
- Kidgell JT, Magnusson M, de Nys R, Glasson CRK. Ulvan: a systematic review of extraction, composition and function. Algal Res. 2019;39:101422. doi: 10.1016/j.algal.2019.101422. [DOI] [Google Scholar]
- Kiraci S. Effects of seaweed and different farm manures on growth and yield of organic carrots. J Plant Nutr. 2018;41(6):716–721. doi: 10.1080/01904167.2018.1425435. [DOI] [Google Scholar]
- Kraan S. Algal polysaccharides novel applications and outlook. In: Chang C-F, editor. Carbohydrates-comprehensive studies on glycobiology and glycotechnology. Croatia: InTech; 2012. pp. 489–532. [Google Scholar]
- Krempel M, Griffin K, Khouryieh H. Hydrocolloids as emulsifiers and stabilizers in beverage preservation. In: Grumezescu AM, Holban AM, editors. Preservatives and preservation approaches in beverages: volume 16: the science of beverages. Amsterdam: Elsevier; 2019. pp. 427–465. [Google Scholar]
- Lahaye M, Robic A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromol. 2007;8(6):1765–1774. doi: 10.1021/bm061185q. [DOI] [PubMed] [Google Scholar]
- Larotonda FDS, Torres MD, Gonçalves MP, et al. Hybrid carrageenan-based formulations for edible film preparation Benchmarking with kappa carrageenan. J ApplPolym. 2016 doi: 10.1002/app.42263. [DOI] [Google Scholar]
- Lee WK, Lim YY, Leow ATC, et al. Factors affecting yield and gelling properties of agar. J ApplPhycol. 2017;29:1527–1540. doi: 10.1007/s10811-016-1009-y. [DOI] [Google Scholar]
- Leung MYK, Liu C, Koon JCM, Fung KP. Polysaccharide biological response modifiers. ImmunolLett. 2006;105(2):101–114. doi: 10.1016/j.imlet.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao W, Zang X, Li Y, Zhang H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J ApplPhycol. 2006;18:9–14. doi: 10.1007/s10811-005-9008-4. [DOI] [Google Scholar]
- Martínez-Sanz M, Martínez-Abad A, López-Rubio A. Cost-efficient bio-based food packaging films from unpurified agar-based extracts. Food Packag Shelf Life. 2019;21:100367. doi: 10.1016/j.fpsl.2019.100367. [DOI] [Google Scholar]
- Milani J, Maleki G. Hydrocolloids in food industry. In: Valdez B, editor. Food industrial processes-methods and equipment. Croatia: InTech; 2012. pp. 2–37. [Google Scholar]
- Mišurcová L, Škrovánková S, Samek D, et al. Health benefits of algal polysaccharides in human nutrition. In: Henry J, et al., editors. Advance in food and nutrition research. Amsterdam: Elsevier; 2012. pp. 75–145. [DOI] [PubMed] [Google Scholar]
- Mukhamejanov E, Kurilenko V. Fucoidan: a nutraceutical for metabolic and regulatory systems homeostasis maintenance. World J Adv Res Rev. 2020;6:255–264. doi: 10.30574/wjarr.2020.6.1.0106. [DOI] [Google Scholar]
- Mustafa S, Mobashir M. LC–MS and docking profiling reveals potential difference between the pure and crude fucoidan metabolites. Int J BiolMacromol. 2020;143:11–29. doi: 10.1016/j.ijbiomac.2019.11.232. [DOI] [PubMed] [Google Scholar]
- NurjanahNurilmala M, Hidayat T, Sudirdjo F. Characteristics of seaweed as raw materials for cosmetics. AquatProcedia. 2016;7:177–180. doi: 10.1016/j.aqpro.2016.07.024. [DOI] [Google Scholar]
- Pangestuti R, Kim SK. Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods. 2011;3(4):255–266. doi: 10.1016/j.jff.2011.07.001. [DOI] [Google Scholar]
- Parreidt TS, Müller K, Schmid M. Alginate-based edible films and coatings for food packaging applications. Foods. 2018;7(10):170. doi: 10.3390/FOODS7100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L. Biological and therapeutic properties of the seaweed polysaccharides. Int Biol Rev. 2018 doi: 10.18103/ibr.v2i2.1762. [DOI] [Google Scholar]
- Pérez MJ, Falqué E, Domínguez H. Antimicrobial action of compounds from marine seaweed. Mar Drugs. 2016;14(3):52. doi: 10.3390/md14030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porse H, Rudolph B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J ApplPhycol. 2017;29:2187–2200. doi: 10.1007/s10811-017-1144-0. [DOI] [Google Scholar]
- Qin Y. Seaweed hydrocolloids as thickening, gelling, and emulsifying agents in functional food products. In: Qin Y, editor. Bioactive seaweeds for food applications: natural ingredients for healthy diets. Amsterdam: Elsevier; 2018. pp. 135–152. [Google Scholar]
- Rhein-Knudsen N, Ale MT, Ajalloueian F, et al. Rheological properties of agar and carrageenan from Ghanaian red seaweeds. Food Hydrocoll. 2017;63:50–58. doi: 10.1016/j.foodhyd.2016.08.023. [DOI] [Google Scholar]
- Rodriguez-Jasso RM, Mussatto SI, Pastrana L, Aguilar CN, Teixeira JA. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. CarbohydrPolym. 2011;86(3):1137–1144. doi: 10.1016/j.carbpol.2011.06.006. [DOI] [Google Scholar]
- Saha D, Bhattacharya S. Hydrocolloids as thickening and gelling agents in food: a critical review. J Food SciTechnol. 2010;47(6):587–597. doi: 10.1007/s13197-010-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Sharifi-Rad J, Seca AML, Pinto DCGA, Michalak I, Trincone A, Prakash Mishra A, Nigam M, Zam W, Martins N. Current trends on seaweeds: looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules. 2019;24(22):4182. doi: 10.3390/molecules24224182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-García MD. Carrageenan polysaccharides for food packaging. In: Lagarón J-M, editor. Multifunctional and nanoreinforced polymers for food packaging. Amsterdam: Elsevier; 2011. pp. 594–609. [Google Scholar]
- Sedayu BB, Cran MJ, Bigger SW. A Review of property enhancement techniques for carrageenan-based films and coatings. CarbohydrPolym. 2019;216:287–302. doi: 10.1016/j.carbpol.2019.04.021. [DOI] [PubMed] [Google Scholar]
- Senni K, Gueniche F, Foucault-Bertaud A, Igondjo-Tchen S, Fioretti F, Colliec-Jouault S, Durand P, Guezennec J, Godeau G, Letourneur D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch Biochem Biophys. 2006;445:56–64. doi: 10.1016/j.abb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Shannon E, Abu-Ghannam N. Seaweeds as nutraceuticals for health and nutrition. Phycologia. 2019;58(5):563–577. doi: 10.1080/00318884.2019.1640533. [DOI] [Google Scholar]
- Sousa AMM, Sereno AM, Hilliou L, Gonçalves MP. Biodegradable agar extracted from gracilaria vermiculophylla : film properties and application to edible coating. Trans Tech Publ. 2014;636:739–744. doi: 10.4028/www.scientific.net/MSF.636-637.739. [DOI] [Google Scholar]
- Surender Reddy K, Abraham A, Afewerki B, et al. Extraction of agar and alginate from marine seaweeds in red sea region. Int J Mar Biol Res. 2018;3:1–8. doi: 10.15226/24754706/3/2/00126. [DOI] [Google Scholar]
- Talyshinsky MM, Souprun YY, Huleihel MM. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002;2(1):8. doi: 10.1186/1475-2867-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvikene R, Truus K, Robal M, Volobujeva O, Mellikov E, Pehk T, Kollist A, Kailas T, Vaher M. The extraction, structure, and gelling properties of hybrid galactan from the red alga Furcellaria lumbricalis (Baltic Sea, Estonia) J ApplPhycol. 2010;22(1):51–63. doi: 10.1007/s10811-009-9425-x. [DOI] [Google Scholar]
- Van de Velde F. Structure and function of hybrid carrageenans. Food Hydrocoll. 2008;22(5):727–734. doi: 10.1016/j.foodhyd.2007.05.013. [DOI] [Google Scholar]
- Villanueva RD, Montaño MNE, Romero JB. Iota-carrageenan from a newly farmed, rare variety of eucheumoid seaweed-"endong". J ApplPhycol. 2009;21(1):27–30. doi: 10.1007/s10811-008-9356-y. [DOI] [Google Scholar]
- Wang LF, Rhim JW. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int J BiolMacromol. 2015;80:460–468. doi: 10.1016/j.ijbiomac.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang S-X, Guan H-S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xing M, Cao Q, et al. Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar Drugs. 2019;17:183. doi: 10.3390/md17030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner ML. Food additive carrageenan: part II: a critical review of carrageenan in vivo safety studies. Crit Rev Toxicol. 2014;44:244–269. doi: 10.3109/10408444.2013.861798. [DOI] [PubMed] [Google Scholar]
- Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH. Algae as nutritional and functional food sources: revisiting our understanding. J ApplPhycol. 2017;29(2):949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaich H, Garna H, Besbes S, Barthélemy JP, Paquot M, Blecker C, Attia H. Impact of extraction procedures on the chemical, rheological and textural properties of ulvan from Ulva lactuca of Tunisia coast. Food Hydrocoll. 2014;40:53–63. doi: 10.1016/j.foodhyd.2014.02.002. [DOI] [Google Scholar]
- Yousefi M, Jafari SM. Recent advances in application of different hydrocolloids in dairy products to improve their techno-functional properties. Trends Food SciTechnol. 2019;88:468–483. doi: 10.1016/j.tifs.2019.04.015. [DOI] [Google Scholar]