Abstract
The present study compared the effects of corn starch coatings incorporated with Zataria multiflora essential oil (ZEO) and cinnamaldehyde (CIN) in conventional, nanoemulsion (NZEO) and fortified nanoemulsion (NZEOC) forms, on specific spoilage microorganisms of chicken meat and on the fate of inoculated Listeria monocytogenes during 20 days storage at 4 ± 1 °C. Based on the results of GC–MS analysis of ZEO, carvacrol (36.62%) was the most important compound of essential oil. Samples coated with the starch solution containing nanoemulsions had better antimicrobial activities than conventional forms. Also, NZEOC treatment had the best antimicrobial properties at the end of storage with the following results: Total viable count (7.96 log10 CFU/g), Psychrotrophic count (7.29 log10 CFU/g), Lactic acid bacteria (6.51 log10 CFU/g), Enterobacteriaceae count (6.98 log10 CFU/g), Mold and yeast count (5.16 log10 CFU/g) and inoculated L. monocytogenes (6.51 log10 CFU/g). Furthermore, the addition of CIN–ZEO during nanoemulsion formation (NZEOC) increased the antimicrobial properties of the samples compared to individual addition of NZEO and CIN (NZEO + CIN) to the starch solution. Therefore, corn starch coating containing NZEOC is recommended as a natural preservative to enhance the microbial stability of poultry meat.
Keywords: Starch coating, Nanoemulsion, Cinnamaldehyde, Zataria multiflora essential oil, Microbial quality
Introduction
Chicken meat consumption, as one of the most popular food around the world, has increased greatly in many countries in recent years (OECD/Food and Agriculture Organization of the United Nations 2016). Fresh chicken meat is highly susceptible to microbial spoilage due to its high levels of moisture and nutrients. Food packaging is one of the known ways to protect foods against microbial contamination and subsequently increase the shelf life of foods during storage time (Bazargani-Gilani et al. 2015).
Nowadays, in order to solve the environmental issues of application of plastic materials in industry, films and coatings based on lipids, proteins or polysaccharides have been used in food packaging systems. In addition, chemical contaminations caused by artificial packaging materials are a great threat for human life. Currently, there is an increasing interest in use of edible coatings and films as biodegradable packaging due to the presence of natural materials and absence of environmental pollution. Also, Edible coatings can improve the quality of foods by preventing physical damage, controlling the transfer of moisture and oxygen, and carrying antimicrobial agents. Starch is a polysaccharide made up from glucose molecules which is available at a low price and due to the nature of polymer, can be used to produce films and coatings (Molavi and Sedaghat 2013).
Alternative preservation techniques using naturally derived ingredients in order to overcome side effects of long-term usage of chemical preservatives, are attractive and their application in food products are being investigated. Essential oils are natural compounds that are added to the edible coatings to increase their antimicrobial properties. On the other hand, essential oil cannot easily escape from the coatings, so it remains at a high concentration for a long time around the product (Zarei et al. 2015).
Zataria multiflora Boiss is an aromatic shrub belonging to the Lamiaceae family and grows in different regions of Asia including southern Iran. This herb is known as antiseptic, antispasmodic, and anti-inflammatory in traditional medicine and is widely used in foods as a flavoring and antimicrobial agent (Basti et al. 2007; Hosseinzadeh et al. 2000). The antimicrobial activities of Z. multiflora essential oil (ZEO) depends on phenolic compounds such as thymol and carvacrol (Basti et al. 2007).
Cinnamaldehyde is a natural phenolic terpenoid compound with strong antimicrobial effects which is categorized as GRAS (Generally Recognized as Safe) and approved by FDA (Food and Drug Administration, USA) for use in food products. This compound is the most important constituent of cinnamon bark and has a variety of applications, including pharmaceutical, cosmetic and food industries. Antimicrobial properties of cinnamaldehyde (pure or as a component of cinnamon essential oils and extract) have been evaluated in several studies (Abdollahzadeh et al. 2018; Sheen et al. 2018).
Unfortunately, some characteristics of essential oils and their derived pure compounds, such as low solubility in water and volatility, have reduced the use of these natural compounds in food products. One of the best methods for solving this problem is encapsulating these compounds in oil-in-water (O/W) nanoemulsions (Chang et al. 2013). Using the inhibitory effect of nano-sized particles can improve the quality and shelf life of meat products (Ramachandraiah et al. 2015). Materials in the nanoscale dimension have greater surface area than larger particles, so increase their biological activity. Another important feature of nanoscale materials that distinguishes them from other groups is their quantum effects. These properties can lead to new changes such as effects on the reactions of nanoscale materials (Gholami-Shabani et al. 2012).
Many studies have been carried out on the antimicrobial effects of edible coatings incorporated with essential oils and phenolic compounds (Ramos et al. 2016; Sánchez-González et al. 2011). However, no studies have evaluated the antimicrobial properties of edible coatings containing essential oils fortified with pure compounds in the form of a nanoemulsion in a food model. Therefore, the major objective of the present study was to (1) determine the chemical composition of ZEO, (2) fabricate and characterize the nanoemulsions of ZEO alone and fortified with cinnamaldehyde, (3) compare antimicrobial activity of a corn starch coating incorporated with ZEO and cinnamaldehyde, in the conventional, nanoemulsion and fortified nanoemulsion forms on the microbial quality of chicken meat and on the fate of inoculated Listeria monocytogenes during 20 days of storage at 4 ± 1 °C.
Materials and methods
Materials
Corn starch, Ethanol, Tween 80, glycerol and Calcium chloride were purchased from Sigma Chemicals (Sigma-Aldrich, Steinheim, Germany). All medium cultures including BHI broth, peptone water (PW), plate count agar (PCA), potato dextrose agar (PDA), violet red bile glucose agar (VRBGA), de Man-Rogosa-Sharpe agar (MRSA) and Listeria Chrom agar (CHROM agar) as well as an anaerobic atmosphere generator (GasPak system type C) were provided by Merck company (Merck, Darmstadt, Germany). Lyophilized bacteria cultures of Listeria monocytogenes (ATCC 13,932) were provided by the Iranian Research Organization for Science and Technology, Tehran, Iran.
Isolation of essential oil
Flower shoots of Z. multiflora were bought from a local market in Zanjan, Iran and confirmed as such by the Institute of Medicinal Plants, Karaj, Iran. The essential oil was then extracted by a hydro-distillation method using the clevenger apparatus (Electro mental, Tehran, Iran) at 100 °C for 3 h. Finally, obtained essential oil was dried over anhydrous sodium sulfate, followed by sterilizing using a syringe filter (0.45 μm) and stored in dark sterilized vials at 4 °C prior to use.
Identification of chemical compounds of Zataria multiflora essential oil
Chemical compounds of ZEO were analyzed by GC–MS (Hewlett Packard 5890/5972, Palo Alto, CA, USA). The capillary column (30 m, inner diameter of 250 mm and film thickness of 0.25 μm) was applied where the temperature was programmed to rise from 50 to 265 °C at 2.5 °C/min then remain at 265 °C for 10 min. The temperature of the injection chamber was 250 °C and the helium gas rate was 1.5 mL/min. An EI detector with ionization energy of 70 eV and ionization temperature of 250 °C was applied for compound detection. Then, the spectra were identified using the Wiley-229 mass database retention time, the Kovats′ index calculation, the mass spectrum analysis of the compounds and finally, comparison with the standard mass spectra and valid sources from the National Institute of Standards and Technology (NIST).
Preparation of Zataria multiflora essential oil nanoemulsion alone and fortified with cinnamaldehyde
A modified method of Gahruie et al. (2017) was used for nanoemulsion preparation. The aqueous phase including Tween 80 in 5 mL distilled water (4.5% w/w) was stirred using a Heidolph MR Hei-Standard magnetic stirrer. (Heidolph Instruments, Schwabach, Germany) at 400 rpm for 10 min. Essential oil (6% w/w) was then added gradually into the mixture and stirred for 10 min. The obtained emulsion was homogenized at 10,000 rpm for 5, 10 and 15 min using a high speed homogenizer (SilentCrusher M, Heidolph Instruments, Germany).
Also, to prepare the nanoemulsion of ZEO fortified with cinnamaldehyde, equal amounts of ZEO and cinnamaldehyde (6% w/w) were added into the mixture of Tween 80 and distilled water, and the homogenization is performed according to the above method.
The dynamic light scattering (DLS) method was used to measure the particle size and zeta potential of dispersions by the Zetasizer Nano ZS device (Malvern Instruments Ltd., Malvern, UK) after appropriate dilution (Gahruie et al. 2017).
Preparation of edible coating solutions
Corn starch (3.4% w/v) and glycerol (1.8% v/v) were dissolved in sterile distilled water and heated at 90 °C with stirring for 10 min until gelatinization. The solution was then cooled to 40 °C and different treatments were added to the solution according to Table 1. Finally, the mixture was homogenized (IKA Ultra-Turrax T-25 Digital, Staufen, Germany) for 2 min at 2000 rpm.
Table 1.
List of chicken meat treatments
| No. | Treatment | Description |
|---|---|---|
| 1 | Control | Chicken meat coated with corn starch solution |
| 2 | ZEO | Chicken meat coated with corn starch solution containing 1% (W/V) Z. multiflora essential oil |
| 3 | NZEO | Chicken meat coated with corn starch solution containing 1% (W/V) nanoemulsion of Z. multiflora essential oil |
| 4 | CIN | Chicken meat coated with corn starch solution containing 1% (W/V) Cinnamaldehyde |
| 5 | ZEO + CIN | Chicken meat coated with corn starch solution containing 1% (W/V) Z. multiflora essential oil + 1% (W/V) Cinnamaldehyde |
| 6 | NZEO + CIN | Chicken meat coated with corn starch solution containing 1% (W/V) nanoemulsion of Z. multiflora essential oil + 1% (W/V) Cinnamaldehyde |
| 7 | NZEOC | Chicken meat coated with corn starch solution containing 1% (W/V) nanoemulsion of Z. multiflora essential oil fortified with Cinnamaldehyde |
Chicken samples
25-g pieces of fresh, skinless and boneless chicken breast meat were purchased and immediately transferred to the Food Microbiology Laboratory, under hygienic conditions using insulated polystyrene boxes containing ice bags. Subsequently, chicken breast meat pieces (25 g) were randomly divided into seven groups and immersed in starch solutions containing different treatments for 5 min, drained for 2 min and then immersed in CaCl2 solution (2% w/v) for 1 min to induce the crosslinking reaction (Table1). The coatings were dried on each side in a laminar-flow hood under ventilation for about 20 min. At the end, the samples were packed in sterile polyethylene pouches (Zipack, Tehran, Iran), stored at 4 °C and analyzed on days: 0, 5, 10, 15, and 20 (Raeisi et al. 2016).
Microbiological analysis
25 g of each sample was homogenized with 225 mL of 0.1% sterile peptone water using a stomacher (Seward Ltd, London, UK) with 400 strokes/min for 2.5 min at room temperature. For bacterial enumeration, 10 µl of serial dilutions of homogenates were transferred on to the specific agar plates according to the drop plate method. Plate count agar (PCA) was used for the total viable counts (TVC) after incubation at 37 °C for 24 h. Lactic acid bacteria (LAB) were determined by De Man, Rogosa and Sharpe (MRS) agar incubated at 25 °C for 5 days under anaerobic conditions (anaerobic jars with GasPak system type C). Enterobacteriaceae bacteria were counted using VRBG agar by the pour overlay method and the plates were incubated at 37 °C for 24 h. Psychrotrophic bacteria were determined on PCA and the plates were incubated at 7 °C for 10 days. For enumeration of molds and yeasts, 0.1 mL of homogenates was spread on to the potato dextrose agar (PDA) medium and colonies were counted after 5 days of incubation at 25 °C. All counts were reported as log10 CFU/g (Bazargani-Gilani et al. 2015; Raeisi et al. 2016).
Inoculation of L. monocytogenes
Inoculation of L. monocytogenes was done as described by Raeisi et al. (2016) method with some modifications. The chicken meat was initially divided into 25 g pieces and sprayed with ethanol 95% (v/v). Then, each sterile piece was inoculated with 250 μl of a 106 CFU/ml viable cells of L. monocytogenes, to reach a final concentration of ~ 104 CFU/g. Finally, the inoculated samples were immersed in coating solutions of the mentioned treatments and stored under aerobic conditions at 4 ± 1 °C. At the appropriate time intervals, each treated sample (25 g) was mixed with 225 mL of peptone water (0.1%) and serial dilution was performed as well. For counting Listeria monocytogenes, 10 μL of each dilution was cultured on Listeria Chrom agar (CHROM agar microbiology, France) using the drop plate method and plates were incubated at 37 °C for 24 h under aerobic conditions (Raeisi et al. 2016).
Statistical analysis
All experiments were performed in triplicate and data were statistically analyzed by One-way ANOVA using the SPSS software (SPSS Statistics Software, version 18). Significant difference among samples was determined by multiple comparisons using Tukey's test. Statistical significance level was indicated at P ≤ 0.05.
Results and discussion
Chemical composition of ZEO
The chemical compositions of ZEO are presented in Table 2. The GC–MS analysis of ZEO showed 25 different compounds that made up 95.89% of the essential oil. The dominant constituents of ZEO were carvacrol (36.62%), thymol (17.86%) and p-cymene (11.35%). This agreed with a previous study which reported 33.65% of carvacrol as the main compound of ZEO (Sharififar et al. 2007). In another study, Ziaee et al. (2018) reported that carvacrol (39.29%) and thymol (25.24%) were the main compounds of ZEO, respectively, which is in consistent with the results of present study. In another study, the main compounds of ZEO were thymol (51.2%), p-cymene (13.8%) and carvacrol (11.26%), which was similar to the present study (Mohammadi et al. 2016). These differences in the components of the ZEO could be due to differences in the geographical area, soil and climate changes, plant age, harvest season, essential oil extraction method and solvent used for extraction. Carvacrol and thymol are both phenolic compounds and have strong antimicrobial activities. These compounds are similar in structure with only a difference in the position of the hydroxyl group in the phenolic ring. However, the position of the hydroxyl group in the phenolic ring was not reported to affect the antimicrobial activity (Shahnia and Khaksar 2013). Phenolic compounds can penetrate the cell membrane and cause coagulation of cell contents (Donsì and Ferrari 2016).
Table 2.
Chemical composition of Zataria multiflora essential oil
| No. | Compound name | Area (%) | RT (min)* | KI** |
|---|---|---|---|---|
| 1 | α-Thujene | 0.15 | 11.30 | 927 |
| 2 | α-Pinene | 1.83 | 11.69 | 934 |
| 3 | β-Pinene | 0.45 | 14.03 | 981 |
| 4 | β-Myrcene | 0.87 | 14.61 | 992 |
| 5 | α-Phellandrene | 0.39 | 15.55 | 1010 |
| 6 | α-Terpinene | 0.48 | 16.09 | 1021 |
| 7 | p-Cymene | 11.35 | 16.66 | 1032 |
| 8 | D-Limonene | 0.88 | 16.78 | 1034 |
| 9 | 1,8-Cineole | 0.75 | 16.96 | 1036 |
| 10 | γ -Terpinene | 4.74 | 18.33 | 1064 |
| 11 | Linalol | 7.98 | 20.62 | 1108 |
| 12 | Terpinen-4-ol | 0.65 | 24.71 | 1190 |
| 13 | α-Terpineol | 0.80 | 25.51 | 1207 |
| 14 | Thymol methyl ether | 0.44 | 26.96 | 1238 |
| 15 | Carvacrol methyl ether | 2.02 | 27.40 | 1247 |
| 16 | 2-Methyl-3-phenyl-propanal | 0.69 | 27.93 | 1258 |
| 17 | Carvone | 0.50 | 28.17 | 1263 |
| 18 | Thymol | 17.86 | 30.23 | 1307 |
| 19 | Carvacrol | 36.62 | 30.75 | 1319 |
| 20 | Thymol acetate | 0.60 | 32.38 | 1356 |
| .21 | Carvacrol acetate | 2.01 | 33.24 | 1375 |
| 22 | β-Caryophyllene | 0.81 | 35.47 | 1427 |
| 23 | ( +)-Aromadendrene | 0.77 | 36.29 | 1447 |
| 24 | β-Spathulenol | 0.88 | 42.15 | 1592 |
| 25 | Caryophyllene oxide | 1.37 | 42.34 | 1596 |
| Total | 95.89 | |||
* Retention time
** Kovats indices
Particle size and polydispersity index (PDI) of nanoemulsions
The size of the nanoemulsion particles of Z. multiflora essential oil (NZEO) and nanoemulsion of Z. multiflora essential oil fortified with cinnamaldehyde (NZEOC) were 176.6 nm and 184.7 nm, respectively. The nano scale particle size is much less than the wavelength of the light, so causes no light distortion making the oil appear transparent or slightly foggy. In the study by Masoumi et al. (2016), the mean particle size of the nanoemulsion of ZEO was 66.5 nm; which was smaller than the results of the present study. However, in another study conducted by Gahruie et al. (2017), the particle size of O/W nanoemulsion of ZEO was 210.5 nm which was larger than the results of this study. The size of the nanoemulsion particle is an indicator of its stability, and stirring is known to reduce droplet size in an O/W emulsion. High energy inputs impose deforming forces that break droplets into smaller ones. The particle size of nanoemulsions obtained by sonication is affected by various factors such as amplitude, power, temperature and time (Gahruie et al. 2017). The type and polarity of essential oil, viscosity, type of surfactant, the physicochemical properties of the dispersed phase and the fixed phase are other important factors which determine the particle size of a nanoemulsion. The most important cause of emulsion instability is the Ostwald Ripening phenomenon, whereby single-phase droplets become larger in two-phase environments and smaller droplets are eliminated. As a result, heavier particles form and the emulsion is separated into two separate phases (Masoomi et al. 2016). However, the difference in droplet size leads to different functional properties between nanoemulsions (Chang et al. 2013). Also, the polydispersity index (PDI) value revealed a narrow size distribution of NZEO and NZEOC with 0.248 and 0.255 values, respectively. This assessment shows how the particle size distribution is spread and the small PDI values are related to narrow size distributions.
Microbiological changes
Total viable count (TVC)
The changes in TVC of chicken breast meat coated with starch solution during 20 days storage at 4 ± 1 °C are shown in Table 3. The initial TVC of samples ranged from 5.26 to 5.39 log10 CFU/g with no significant difference between treatments (P ≥ 0.05). The growth of TVC tended to increase in all experimental groups during the storage period and the highest level of bacterial count was observed in the control group with 12.58 log10 CFU/g at the end of storage time (P ≤ 0.05). This pattern was consistent with the results of a study conducted by Bazargani-Gilani et al. (2015), who reported an increase in TVC of chicken meat, after using chitosan coating containing ZEO. The combined use of ZEO and CIN in coatings (ZEO + CIN) was more effective than their individual use (P ≤ 0.05). Several studies have demonstrated the synergistic and/or additive antimicrobial effects between cinnamaldehyde with thymol or carvacrol as the major components of ZEO (Pei et al. 2009; Ye et al. 2013). Carvacrol and thymol can interact with the phospholipids of cell membranes and disturb their functionality, increasing the membrane permeability, breaking homeostasis, causing the leakage of ions and cytoplasmic content, as well as coagulation of cell contents (Donsì and Ferrari 2016). When carvacrol, thymol and cinnamaldehyde are used in combination, carvacrol or thymol increase the permeability of the cytoplasmic membrane, and possibly enable cinnamaldehyde to be more easily transported into the cell. On the other hand, carvacrol or thymol increase the number, size or duration of existence of the pores created by the binding of cinnamaldehyde to proteins in the cell membrane, thus creating the synergistic effects (Pei et al. 2009).
Table 3.
Microbial changes (log10 CFU/g) of chicken breast meat with starch coatings during 20 days storage at 4 ± 1 °C (Mean ± SD)
| Microorganisms | Treatment | Time (days) | ||||
|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | ||
| Total viable count | Control | 5.39 ± 0.01 Aa | 8.36 ± 0.08 Bd | 9.92 ± 0.06 Cd | 11.15 ± 0.12 Cd | 12.58 ± 1.08 Dd |
| ZEO | 5.31 ± 0.05 Aa | 6.43 ± 0.04 Bb | 7.65 ± 0.50 Cb | 8.87 ± 0.11 Dbc | 9.77 ± 0.40 Ebc | |
| NZEO | 5.31 ± 0.05 Aa | 5.67 ± 0.19 Aa | 6.57 ± 0.14 Ba | 7.85 ± 0.51 Ca | 8.43 ± 0.04 Cab | |
| CIN | 5.38 ± 0.04 Aa | 7.17 ± 0.19 Bc | 8.56 ± 0.25 Cc | 9.16 ± 0.08 Dc | 10.52 ± 0.28 Ec | |
| ZEO + CIN | 5.33 ± 0.02 Aa | 6.35 ± 0.01 Bb | 7.32 ± 0.08 Cb | 8.59 ± 0.32 Dabc | 9.01 ± 0.38 Dab | |
| NZEO + CIN | 5.29 ± 0.02 Aa | 5.74 ± 0.27 Ba | 6.57 ± 0.14 Ca | 8.09 ± 0.08 Dab | 7.98 ± 0.09 Da | |
| NZEOC | 5.26 ± 0.10 Aa | 5.71 ± 0.23 Aba | 6.44 ± 0.02 Ba | 7.76 ± 0.54 Ca | 7.96 ± 0.26 Ca | |
| Psychrotrophic bacteria | Control | 4.15 ± 0.04 Aa | 7.411 ± 0.07 Bc | 8.60 ± 0.31 Ce | 10.31 ± 0.08 Dd | 11.61 ± 0.41 Ed |
| ZEO | 4.16 ± 0.02 Aa | 5.91 ± 0.57 Bb | 6.84 ± 0.41 Bcd | 7.91 ± 0.41 Cbc | 8.67 ± 0.06 Cc | |
| NZEO | 4.14 ± 0.03 Aa | 4.85 ± 0.45 Aa | 5.98 ± 0.48 Babc | 6.40 ± 0.05 Ba | 7.61 ± 0.25 Cab | |
| CIN | 4.16 ± 0.02 Aa | 6.28 ± 0.06 Bb | 7.18 ± 0.06 Cd | 8.24 ± 0.05 Dc | 9.10 ± 0.13 Ec | |
| ZEO + CIN | 4.16 ± 0.02 Aa | 5.65 ± 0.46 Bab | 6.48 ± 0.32 Cbcd | 7.40 ± 0.02 Db | 7.99 ± 0.13 Db | |
| NZEO + CIN | 4.14 ± 0.03 Aa | 4.82 ± 0.34 Ba | 5.88 ± 0.17 Cab | 6.29 ± 0.07 Ca | 7.69 ± 0.20 Dab | |
| NZEOC | 4.15 ± 0.00 Aa | 4.75 ± 0.26 ABa | 5.33 ± 0.19 BCa | 5.93 ± 0.42 Ca | 7.29 ± 0.10 Da | |
| Lactic acid bacteria | Control | 4.11 ± 0.06 Aa | 5.71 ± 0.12 Bc | 7.44 ± 0.16 Cc | 9.06 ± 0.56 Dd | 11.03 ± 0.11Ed |
| ZEO | 4.05 ± 0.02 Aa | 4.59 ± 0.27 Aab | 5.65 ± 0.50 Bab | 6.61 ± 0.25 Cbc | 7.55 ± 0.30 Dbc | |
| NZEO | 4.05 ± 0.08 Aa | 4.19 ± 0.03 Aa | 5.11 ± 0.09 Ba | 5.92 ± 0.03 Ca | 6.60 ± 0.26 Da | |
| CIN | 4.11 ± 0.10 Aa | 4.71 ± 0.28 Ab | 5.89 ± 0.42 Bb | 6.98 ± 0.18 Cc | 8.00 ± 0.04 Dc | |
| ZEO + CIN | 4.04 ± 0.04 Aa | 4.50 ± 0.17 Bab | 5.37 ± 0.20 Cab | 6.15 ± 0.05 Dab | 7.12 ± 0.02 Eab | |
| NZEO + CIN | 4.01 ± 0.02 Aa | 4.30 ± 0.07 Aab | 5.13 ± 0.04 Bab | 5.97 ± 0.03 Cab | 6.86 ± 0.43 Da | |
| NZEOC | 4.02 ± 0.08 Aa | 4.15 ± 0.07 Aa | 4.99 ± 0.08 Ba | 5.86 ± 0.09 Ca | 6.51 ± 0.18 Da | |
| Enterobacteriaceae | Control | 4.30 ± 0.04 Aa | 6.38 ± 0.06 Bc | 8.08 ± 0.17 Ce | 9.80 ± 0.30 Dd | 10.94 ± 0.47 Ec |
| ZEO | 4.28 ± 0.02 Aa | 5.23 ± 0.07 Bb | 6.13 ± 0.05 Cc | 7.35 ± 0.08 Dc | 8.11 ± 0.06 Eb | |
| NZEO | 4.26 ± 0.07 Aa | 4.46 ± 0.02 Ba | 5.44 ± 0.03 Ca | 6.17 ± 0.07 Da | 7.22 ± 0.05 Ea | |
| CIN | 4.29 ± 0.03 Aa | 5.38 ± 0.06 Bb | 6.39 ± 0.06 Cd | 7.59 ± 0.43 Dc | 8.33 ± 0.03 Eb | |
| ZEO + CIN | 4.27 ± 0.05 Aa | 5.10 ± 0.14 Bb | 5.95 ± 0.05 Cbc | 6.96 ± 0.49 Dbc | 7.45 ± 0.03 Da | |
| NZEO + CIN | 4.27 ± 0.04 Aa | 4.61 ± 0.34 Aa | 5.71 ± 0.12 Bb | 6.44 ± 0.14 Cab | 7.37 ± 0.04 Da | |
| NZEOC | 4.25 ± 0.02 Aa | 4.35 ± 0.15 Aa | 5.24 ± 0.07 Ba | 6.24 ± 0.08 Cab | 6.98 ± 0.05 Da | |
| Mold & yeast | Control | 3.33 ± 0.02 Aa | 5.46 ± 0.01 Bf | 7.22 ± 0.03 Cf | 8.34 ± 0.01 Dg | 10.26 ± 0.01 Ec |
| ZEO | 3.32 ± 0.03 Aa | 4.19 ± 0.01 Bd | 4.98 ± 0.04 Cd | 5.44 ± 0.00 De | 6.31 ± 0.03 Eb | |
| NZEO | 3.27 ± 0.01 Aa | 3.89 ± 0.03 Bb | 4.34 ± 0.00 Cb | 5.03 ± 0.02 Dc | 5.45 ± 0.01 Ea | |
| CIN | 3.33 ± 0.07 Aa | 4.29 ± 0.01 Be | 5.23 ± 0.01 Ce | 6.28 ± 0.00 Df | 6.74 ± 0.59 Db | |
| ZEO + CIN | 3.25 ± 0.05 Aa | 4.04 ± 0.03 Bc | 4.43 ± 0.02 Cc | 5.22 ± 0.02 Dd | 5.65 ± 0.08 Ea | |
| NZEO + CIN | 3.26 ± 0.13 Aa | 3.86 ± 0.01 Bb | 4.28 ± 0.01 Cb | 4.95 ± 0.03 Db | 5.34 ± 0.01 Ea | |
| NZEOC | 3.28 ± 0.02 Aa | 3.64 ± 0.03 Ba | 4.14 ± 0.01 Ca | 4.76 ± 0.03 Da | 5.16 ± 0.01 Ea | |
Values followed by the same capital letter within the same row are not significantly different according the Tukey’s test (P ≥ 0.05)
Values followed by the same small letter within the same column of each microbial group, are not significantly different according the Tukey’s test (P ≥ 0.05)
Treatments containing ZEO in the form of nanoemulsion (NZEOC, NZEO + CIN, NZEO) showed better results than others and the best result belonged to NZEOC treatment with the count of 7.96 log10 CFU/g at the end day of storage (P ≤ 0.05). Also, the treatments containing ZEO in the form of nanoemulsion never allowed the maximum acceptable level of TVC for fresh chicken meat (7 log10 CFU/g) to be reached until day 10. This effect may be due to the fact that ZEO in the form of nanoemulsion, because of its smaller droplet size and higher surface charge, has a greater effect on the cell membrane and subsequently better interaction with multiple molecular sites in the microbial cell membrane (Donsì and Ferrari 2016). Our results agreed with those of Moghimi et al. (2016), who reported that the conversion of free essential oil to the nanoemulsion form significantly increased its antibacterial activity. Similar results have been reported in another study on the antibacterial effect of chitosan coatings incorporated with free or nano-encapsulated essential oil on the TVC of lamb during 20 days storage at 4 °C (Pabast et al. 2018). But the results disagreed with another study which showed that the antibacterial effect of ZEO nanoemulsion was similar to conventional ZEO (Shahabi et al. 2017). These contrasting results may be due to the differences in the composition of the essential oils and the preparation method of nanoemulsions, which affect their biological activity (Chang et al. 2013). Furthermore, the addition of CIN–ZEO during nanoemulsion formation and subsequent addition to coating solution (NZEOC) had a relatively better result than individual addition of NZEO and CIN to coating solution (NZEO + CIN), but this difference was not statistically significant (P ≥ 0.05). This phenomenon may be due to the entry of cinnamaldehyde into ZEO nanoparticles during sonication that facilitates the penetration of cinnamaldehyde across the cell wall.
Psychrotrophic bacteria
One of the main causes of meat spoilage at refrigeration temperatures is psychrotrophic bacteria. These Gram-negative bacteria decompose protein to produce several compounds such as ammonia and increase the pH of the meat (Raeisi et al. 2016). Gram-negative bacteria shows a higher resistance to herbal oils than Gram-positive bacteria because they limit the release of hydrophobic substances by the external lipopolysaccharide membrane (Sandri et al. 2007). The effect of treatments on the psychrotrophic counts of samples during storage is presented in Table 3. According to the results, there was no significant difference between the control and other treatments at the initial day of storage (P ≥ 0.05) and an increasing trend in microbial count can be observed over storage time (P ≤ 0.05). At the end of storage (day 20), the highest and lowest bacterial count belonged to control (11.61 log10 CFU/g) and NZEOC groups (7.29 log10 CFU/g), respectively (P ≤ 0.05). Comparing the results of TVC and psychrotrophic count indicates the close values between these two groups, because the psychrotrophic bacteria are the main spoilage microorganisms in fresh meat that are aerobically stored at refrigeration temperature (Bazargani-Gilani et al. 2015). Also, CIN treatment had weaker antibacterial effect on this group of bacteria than other treatments. This phenomenon may be due to the lack of disruptive action of this compound on the lipopolysaccharide membrane of Gram-negative bacteria and failure to evacuate intracellular ATP, unlike carvacrol and thymol as the major compounds of ZEO (Shahnia and Khaksar 2013). The antimicrobial effect of cinnamaldehyde is probably due to the binding of its carbonyl group to bacterial proteins and inhibition of amino acid decarboxylase activity (Burt 2004). Similar to the results for TVC, treatments containing ZEO nanoemulsions showed better results than others. One of the reasons for the increased antibacterial effects of essential oils in the form of nanoemulsions is their higher dispersion in the aqueous phase than in the conventional form, which leads to greater access of nanoemulsions to microbial cells (Donsì and Ferrari 2016). In addition, small nanoemulsion droplets can disrupt the cell membrane by altering the integration of phospholipid bilayers or by interfering with transport proteins (Moghimi et al. 2016). On the other hand, the electrostatic interaction of positively charged nanoemulsion droplets with the negatively charged microbial cell wall increases the concentration of essential oils at the site of action and results in their interference and complete destruction of the bacterial membrane. Also, the surface energy of the nanoparticles increases as compared to the conventional form, and will have more antibacterial properties due to increased passive cellular absorption mechanisms and their smaller size. In agreement with these results, Noori et al. (2018) reported the higher antimicrobial activity of sodium caseinate coating containing nanoemulsion of Zingiber officinale essential oil than coating containing conventional form of essential oil against psychrophilic bacteria in chicken breast fillets during storage at 4 °C. Moreover, in this bacterial group synergistic effects were observed between ZEO and cinnamaldehyde. The NZEOC treatment provided a better result than NZEO + CIN, with no statistical difference (P ≥ 0.05) at the end of storage time which could be due to cinnamaldehyde entry into the nanoparticles thus facilitating its passage through the cell wall.
Lactic acid bacteria (LAB)
Lactic acid-producing bacteria are the facultative anaerobic bacterial group that can be found in the microbial flora of chicken meat (Raeisi et al. 2016). Table 3 shows that the initial count of LAB in control group was 4.11 log10 CFU/g and increased to 11.03 log10 CFU/g during the storage period (P ≤ 0.05). NZEOC treatment had the greatest effect on the LAB count with a value about 4.5 log10 cycles less than the control group (P ≤ 0.05). The results indicate that the inhibitory effects of treatments on this group of bacteria were higher than on other bacterial groups, because most of the bacteria in this group are Gram-positive. In Gram-positive bacteria, the two-layer phospholipid membrane directly interacts with hydrophobic compounds of essential oils. As a result, it increases ion permeability, leakage of vital cell constituents and disruption of the bacterial enzyme system (Sandri et al. 2007).
Also, the treatments containing nanoemulsion of ZEO—NZEOC (6.51 log10 CFU/g), NZEO (6.60 log10 CFU/g) and NZEO + CIN (6.86 log10 CFU/g)—exhibited the lowest values on the final day of storage, with no significant difference between them (P ≥ 0.05). In a study by Pabast et al. (2018), the treatment with edible chitosan coatings enhanced with nanoparticle of Satureja khuzestanica essential oil, had a greater inhibitory effect than free essential oil on LAB in lamb meat which in consistent with the results of the present study (Pabast et al. 2018). Because of the smaller size of nanoemulsion droplets and their superficial load, they can be transported through the cell wall. The nanoparticles can be attached to the bacterial membrane through an electrostatic reaction and cause complete destruction of the bacterial membrane. The energy of the nanoparticle surface is increased in comparison with the conventional form, and will have more antibacterial properties due to the increased passive cellular absorption mechanisms and their smaller size (Nasseri et al. 2017).
Enterobacteriaceae count
Enterobacteriaceae bacteria are a large family of Gram-negative bacteria that naturally live in the intestines of humans and animals and form an important part of the microbial flora of chicken meat. The Enterobacteriaceae count can be considered as an indicator of hygiene and possible enteric contamination in foods including chicken meat (Bazargani-Gilani et al. 2015). According to Table 3, the initial count for the control group was 4.30 log10 CFU/g with no statistically significant difference to other groups (P ≥ 0.05) and increased to the final value of 10.94 log10 CFU/g on 20th day of storage. ZEO treatment reduced the Enterobacteriaceae count about 2.8 log10 cycles at the end of storage (P ≤ 0.05). A study by Bazargani-Gilani et al. (2015) about the antimicrobial effects of chitosan coatings containing ZEO on the Enterobacteriaceae count of chicken meat during refrigerated storage confirms the results of the present study. This is probably due to the synergistic effects between thymol and carvacrol as the main compounds of ZEO against this group of bacteria. The synergistic effects of thymol and carvacrol on the Enterobacteriaceae population of poultry patties have been previously demonstrated (Mastromatteo et al. 2009). Also, the antimicrobial effects of CIN showed no significant difference compared to ZEO at the end of storage (P ≥ 0.05). It has been proven that cinnamaldehyde can inhibit the growth of bacteria belonging to the Enterobacteriaceae family (Dhara and Tripathi 2013). Moreover, ZEO + CIN treatment had a greater antimicrobial effect than individual CIN and ZEO treatments which shows the synergistic effect between CIN and ZEO in starch coating (P ≤ 0.05). In agreement with our findings, many studies have proven synergistic antibacterial effects of thymol and/or carvacrol (as the main components of ZEO) with cinnamaldehyde against foodborne pathogens belonging to the Enterobacteriaceae family (Pei et al. 2009; Ye et al. 2013). Also, the effect of nanoemulsion treatments against Enterobacteriaceae counts was less effective than conventional treatments (P ≤ 0.05) and the best antibacterial effect belonged to NZEOC treatment with the value of 6.98 log10 CFU/g at the end of storage. The results of the study by Khanzadi et al. (2020) showed that the antimicrobial activity of alginate nanoemulsions coatings containing Zataria multiflora essential oil was higher than a coarse coating on the Enterobacteriaceae count of trout fillet, which is consistent with the present results. In another study, the inhibitory effect of encapsulated chitosan nanoparticles containing cinnamon essential oil was higher than free nanoparticles against Enterobacteriaceae bacteria in beef patties (Ghaderi-Ghahfarokhi et al. 2017).
For this bacterial group, as in other groups, NZEOC exhibited a higher synergistic antibacterial effect compared with adding NZEO and CIN individually to the coating solution (NZEO + CIN). This phenomenon may be due to the entry of cinnamaldehyde into ZEO nanoemulsion during sonication that facilitates penetration of cinnamaldehyde through the cell wall.
Mold and yeast count
Several studies have shown that mold and yeast species are involved in chicken meat spoilage (Bazargani-Gilani et al. 2015; Noori et al. 2018). As shown in Table 3, the initial molds and yeasts count of samples ranged from 3.25 log10 CFU/g to 3.33 log10 CFU/g with no significant difference between them (P ≥ 0.05). The results indicate that all antimicrobial treatments significantly reduced the molds and yeasts count of the chicken samples compared to the control group during storage (P ≤ 0.05). Also, the inhibitory effects of treatments on molds and yeasts were higher than on the bacterial groups. The antifungal activity of ZEO and cinnamaldehyde have been reported for various types of molds and yeasts (Mohajeri et al. 2018; OuYang et al. 2019). The results of the present study agreed with those of Bazargani-Gilani et al. (2015) about the effects of chitosan coating containing pomegranate juice and ZEO on yeast/mold counts of chicken meat during 20 days of refrigerated storage. Cinnamaldehyde and ZEO have several different effects on cell membranes, cell walls, and cytoplasmic contents of fungi that cause the destruction, scraping and squashing of hyphae and structural destruction of the cytoplasm (OuYang et al. 2019). On the other hand, the mechanism of the inhibitory effects on the growth of antimicrobial compounds containing an aldehyde structure (e.g. cinnamaldehyde) against fungi is mainly due to the reaction of aldehydes with the -SH groups affecting the growth of this group of microorganisms (Shahnia and Khaksar 2013). The nano-treatments which include NZEOC, NZEO + CIN and NZEO had better antifungal activities than conventional treatments with 5.16, 5.34 and 5.45 log10 CFU/g at the end of storage, respectively (P ≤ 0.05). In agreement with these findings, Nasseri et al. (2016) reported that ZEO nanoparticles at low doses had stronger antifungal effects than free ZEO (Nasseri et al. 2016). In another study, the edible sodium caseinate coatings containing nanoemulsion of ginger essential oil had greater inhibitory effects than emulsion forms against molds and yeasts of chicken breast fillets during storage at 4 °C (Noori et al. 2018).
Fate of inoculated L. monocytogenes
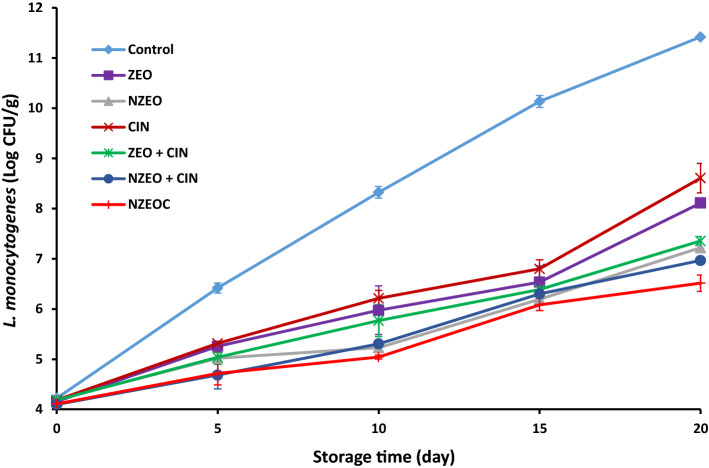
L. monocytogenes is considered as one of the most important foodborne pathogens that can infect humans through consumption of chicken meat. As a result, controlling the growth of this bacterium is a vital concept for the safety of chicken meat. Counts of inoculated L. monocytogenes in the control and treated samples during 20 days storage at 4 ± 1 °C are shown in Fig. 1. The average count of inoculated L. monocytogenes was 4.16 log10 CFU/g at initial day of storage (P ≥ 0.05). L. monocytogenes in the control samples increased significantly more than the other treatments and finally reached 11.42 log10 CFU / g (P ≤ 0.05). The antibacterial effects of the EOs and pure components derived from them against L. monocytogenes depend on several factors including the major components of EOs, type and purity of components, extraction method, bacterial strain and growth phase, inoculum volume, storage condition and structure of food (Abdollahzadeh et al., 2018). The antibacterial activity of ZEO is probably due to its main constituents, carvacrol and thymol. Both of these aromatic phenolic compounds appear to make the cell membrane of L. monocytogenes permeable. Also, cinnamaldehyde probably exerts its anti-listerial properties through inhibiting the glucose uptake by L. monocytogenes and subsequently rapid inhibition of the energy metabolism as well as the effect on cell membrane permeability (Gill and Holley 2004). In the present study, a single strain of L. monocytogenes was investigated, but the antimicrobial agents may have different effects on other strains of this bacterial species (Abdollahzadeh et al. 2018). The results showed the greater antimicrobial activity of ZEO + CIN in comparison with CIN and ZEO individually, which confirms the synergistic effect between CIN and ZEO in starch coating (P ≤ 0.05). These results are consistent with the results of Raeisi et al. (2016) which showed that the combined use of cinnamon and rosemary EOs and other antibacterial agent (nisin) in edible coating had a greater inhibitory effect than individual usage on L. monocytogenes inoculated in chicken meat (Raeisi et al. 2016). According to Fig. 1, samples treated with coatings containing nanoemulsions had better anti-listerial activities than conventional treatments, and the NZEOC sample had the lowest final count (6.51 log10 CFU/g) between all treatments (P ≤ 0.05). Sani et al. (2017) reported the noticeable inhibitory effect of whey protein nanocomposite films containing rosemary essential oil against L. monocytogenes inoculated in lamb meat which is consistent with the results of this study. This feature is due to the increased contact of the essential oil with bacteria as a result of particle size reduction (Masoomi et al. 2016). A noteworthy finding in the present study was the comparison between NZEO + CIN and NZEOC. The results showed that NZEOC treatment had significantly higher antimicrobial effects than the separate addition of nanoemulsion of Z. multiflora essential oil and cinamaldehyde to the starch solution (NZEO + CIN) (P ≤ 0.05). The greater antibacterial properties of NZEOC treatment compared with NZEO + CIN may be due to cinnamaldehyde entering the nanoparticles and facilitating its passage through the cell membrane, in addition to their synergistic effects.
Fig. 1.
Effect of different corn starch coatings on inoculated L. monocytogenes in chicken breast meat during storage at 4 ± 1 °C for 20 days (Mean ± SD)
Conclusion
The findings of this study showed that starch coatings containing ZEO nanoemulsions had greater antimicrobial effects than coatings containing conventional form of ZEO on specific spoilage microorganisms and pathogenic bacteria of chicken meat during 20 days storage at 4 ± 1 °C. Moreover, chicken meats coated with a starch solution containing a nanoemulsion of ZEO fortified with cinnamaldehyde (NZEOC) had the best antimicrobial properties. Also, the addition of CIN to ZEO during the formation of nanoemulsion (NZEOC) increased the antimicrobial effects of coating solutions compared with the individual addition of NZEO and CIN to starch solution (NZEO + CIN). In general, the starch solution containing NZEOC as an example of edible coatings containing essential oils enriched with pure compounds derived from them in the form of nanoemulsions, can be recommended for the meat packaging industry as an effective and natural antimicrobial preservation method.
Acknowledgement
This paper was extracted from the MSc thesis of Zahra Abbasi and financially supported by the School of Public Health, Zanjan University of Medical Science, Zanjan, Iran (project code: A-12-964-5). The Ethics Committee of Zanjan University of Medical Sciences has approved this project (code of ethics: ZUMS.REC.1395.297)
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdollahzadeh E, Ojagh SM, Hosseini H, Ghaemi EA, Irajian GH. Quantitative and qualitative evaluation of antibacterial activity of cinnamon essential oil and ZnO nanoparticles against Listeria monocytogenes. J Fish Sci Technol. 2018;7:49–55. [Google Scholar]
- Basti AA, Misaghi A, Khaschabi D. Growth response and modelling of the effects of Zataria multiflora Boiss essential oil, pH and temperature on Salmonella typhimurium and Staphylococcus aureus. LWT-Food Sci Technol. 2007;40:973–981. doi: 10.1016/j.lwt.2006.07.007. [DOI] [Google Scholar]
- Bazargani-Gilani B, Aliakbarlu J, Tajik H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov Food Sci Emerg Technol. 2015;29:280–287. doi: 10.1016/j.ifset.2015.04.007. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chang Y, McLandsborough L, McClements DJ. Physicochemical properties and antimicrobial efficacy of carvacrol nanoemulsions formed by spontaneous emulsification. J Agric Food Chem. 2013;61:8906–8913. doi: 10.1021/jf402147p. [DOI] [PubMed] [Google Scholar]
- Dhara L, Tripathi A. Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing enterobacteriaceae by in vitro and molecular docking analysis. Eur J Integr Med. 2013;5:527–536. doi: 10.1016/j.eujim.2013.08.005. [DOI] [Google Scholar]
- Donsì F, Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Gahruie HH, Ziaee E, Eskandari MH, Hosseini SMH. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohyd Polym. 2017;166:93–103. doi: 10.1016/j.carbpol.2017.02.103. [DOI] [PubMed] [Google Scholar]
- Ghaderi-Ghahfarokhi M, Barzegar M, Sahari M, Gavlighi HA, Gardini F. Chitosan-cinnamon essential oil nano-formulation: application as a novel additive for controlled release and shelf life extension of beef patties. Int J Biol Macromol. 2017;102:19–28. doi: 10.1016/j.ijbiomac.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Gholami-Shabani MH, Imani A, Chamani M, Razzaghi-Abyaneh M, Riazi GH, Chian M, et al. Evaluation of the antibacterial properties of silver nanoparticles synthesized with Fusarium Oxysporum and Escherichia coli. New Cell Mol Biotechnol J. 2012;6:27–33. [Google Scholar]
- Gill AO, Holley RA. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl Environ Microbiol. 2004;70:5750–5755. doi: 10.1128/AEM.70.10.5750-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol. 2000;73:379–385. doi: 10.1016/S0378-8741(00)00238-5. [DOI] [PubMed] [Google Scholar]
- Khanzadi S, Keykhosravy K, Hashemi M, Azizzadeh M. Alginate coarse/nanoemulsions containing Zataria multiflora Boiss essential oil as edible coatings and the impact on microbial quality of trout fillet. Aquac Res. 2020;51:873–881. doi: 10.1111/are.14418. [DOI] [Google Scholar]
- Masoomi V, Tajik H, Moradi M, Forough M, Shahabi N. Antimicrobial effects of Zataria multiflora boiss. essential oil nanoemulsion against Escherichia coli O157: H7. J Urmia Univ Med Sci. 2016;27:608–617. [Google Scholar]
- Mastromatteo M, Lucera A, Sinigaglia M, Corbo MR. Combined effects of thymol, carvacrol and temperature on the quality of non-conventional poultry patties. Meat Sci. 2009;83:246–254. doi: 10.1016/j.meatsci.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Moghimi R, Ghaderi L, Rafati H, Aliahmadi A, McClements DJ. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016;194:410–415. doi: 10.1016/j.foodchem.2015.07.139. [DOI] [PubMed] [Google Scholar]
- Mohajeri FA, Misaghi A, Gheisari H, Basti AA, Amiri A, Ghalebi SR, Derakhshan Z, Tafti RD. The effect of Zataria multiflora Boiss essential oil on the growth and citrinin production of Penicillium citrinum in culture media and cheese. Food Chem Toxicol. 2018;118:691–694. doi: 10.1016/j.fct.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Hashemi M, Hosseini SM. Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innov Food Sci Emerg Technol. 2016;33:580–588. doi: 10.1016/j.ifset.2015.10.015. [DOI] [Google Scholar]
- Molavi H., Sedaghat N. 2013. Biodegradable films based on starch 2nd National Conference on Food Sci Technol, Islamic Azad University, Quchan Branch, 28–30 (in Persian).
- Nasseri M, Arouiee H, Golmohammadzadeh S, Jaafari M, Neamati H. Effect of solid lipid nanoparticle containing essential oil of Zataria multiflora on the inhibitory growth of Aspergillus ochraceus, Aspergillus niger and Aspergillus flavus. J Appl Res Plant Prot. 2017;5:161–174. [Google Scholar]
- Nasseri M, Golmohammadzadeh S, Arouiee H, Jaafari MR, Neamati H. Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition. Iran J Basic Med Sci. 2016;19:1231–1237. [PMC free article] [PubMed] [Google Scholar]
- Noori S, Zeynali F, Almasi H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control. 2018;84:312–320. doi: 10.1016/j.foodcont.2017.08.015. [DOI] [Google Scholar]
- OECD/Food and Agriculture Organization of the United Nations “Meat” in OECD-FAO agricultural outlook 2016–2025. OECD Publishing Paris. 2016 doi: 10.1787/agr_outlook-2016-10-en. [DOI] [Google Scholar]
- OuYang O, Duan X, Li L, Tao N. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of geotrichum citri-aurantii. Front Microbiol. 2019;10:55. doi: 10.3389/fmicb.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabast M, Shariatifar N, Beikzadeh S, Jahed G. Effects of chitosan coatings incorporating with free or nano-encapsulated satureja plant essential oil on quality characteristics of lamb meat. Food Control. 2018;91:185–192. doi: 10.1016/j.foodcont.2018.03.047. [DOI] [Google Scholar]
- Pei, R.s., Zhou, F., Ji, B.p., Xu, J., Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J Food Sci. 2009;74:M379–M383. doi: 10.1111/j.1750-3841.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- Raeisi M, Tabaraei A, Hashemi M, Behnampour N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int J Food Microbiol. 2016;238:139–145. doi: 10.1016/j.ijfoodmicro.2016.08.042. [DOI] [PubMed] [Google Scholar]
- Ramachandraiah K, Han SG, Chin KB. Nanotechnology in meat processing and packaging: potential applications—a review. Asian-Aust J Anim Sci. 2015;28:290. doi: 10.5713/ajas.14.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Jiménez A, Garrigós M (2016) Carvacrol-based films: usage and potential in antimicrobial packaging, Antimicrobial food packaging. Elsevier Ltd, Academic Press, UK; USA, pp 329–338
- Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M. Use of essential oils in bioactive edible coatings: a review. Food Eng Rev. 2011;3:1–16. doi: 10.1007/s12393-010-9031-3. [DOI] [Google Scholar]
- Sandri I, Zacaria J, Fracaro F, Delamare A, Echeverrigaray S. Antimicrobial activity of the essential oils of Brazilian species of the genus cunila against foodborne pathogens and spoiling bacteria. Food Chem. 2007;103:823–828. doi: 10.1016/j.foodchem.2006.09.032. [DOI] [Google Scholar]
- Sani MA, Ehsani A, Hashemi M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int J Food Microbiol. 2017;251:8–14. doi: 10.1016/j.ijfoodmicro.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Shahabi N, Tajik H, Moradi M, Forough M, Ezati P. Physical, antimicrobial and antibiofilm properties of Zataria multiflora Boiss essential oil nanoemulsion. Int J Food Sci Technol. 2017;52:1645–1652. doi: 10.1111/ijfs.13438. [DOI] [Google Scholar]
- Shahnia M, Khaksar R. Antimicrobial effects and determination of minimum inhibitory concentration (MIC) methods of essential oils against pathogenic bacteria. Iran J Nutr Sci Food Technol. 2013;7:949–955. [Google Scholar]
- Sharififar F, Moshafi M, Mansouri S, Khodashenas M, Khoshnoodi M. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control. 2007;18:800–805. doi: 10.1016/j.foodcont.2006.04.002. [DOI] [Google Scholar]
- Sheen S, Huang CY, Ramos R, Chien SY, Scullen OJ, Sommers C. Lethality prediction for Escherichia coli O157: H7 and uropathogenic E. coli in ground chicken treated with high pressure processing and trans-cinnamaldehyde. J Food Sci. 2018;83:740–749. doi: 10.1111/1750-3841.14059. [DOI] [PubMed] [Google Scholar]
- Ye H, Shen S, Xu J, Lin S, Yuan Y, Jones GS. Synergistic interactions of cinnamaldehyde in combination with carvacrol against food-borne bacteria. Food Control. 2013;34:619–623. doi: 10.1016/j.foodcont.2013.05.032. [DOI] [Google Scholar]
- Zarei M, Ramezani Z, Ein-Tavasoly S, Chadorbaf M. Coating effects of orange and pomegranate peel extracts combined with chitosan nanoparticles on the quality of refrigerated silver carp fillets. J Food Process Preserv. 2015;39:2180–2187. doi: 10.1111/jfpp.12462. [DOI] [Google Scholar]
- Ziaee E, Razmjooei M, Shad E, Eskandari MH. Antibacterial mechanisms of Zataria multiflora Boiss. essential oil against Lactobacillus curvatus. LWT-Food Sci Technol. 2018;87:406–412. doi: 10.1016/j.lwt.2017.08.089. [DOI] [Google Scholar]